- 1 School of Medicine, University of Western Sydney, Sydney, NSW, Australia
- 2 Neuroscience Research Australia, Sydney, NSW, Australia
Because the cardiovascular system and respiration are so intimately coupled, disturbances in respiratory control often lead to disturbances in cardiovascular control. Obstructive Sleep Apnea (OSA), Chronic Obstructive Pulmonary Disease (COPD), and Bronchiectasis (BE) are all associated with a greatly elevated muscle vasoconstrictor drive (muscle sympathetic nerve activity, MSNA). Indeed, the increase in MSNA is comparable to that seen in congestive heart failure (CHF), in which the increase in MSNA compensates for the reduced cardiac output and thereby assists in maintaining blood pressure. However, in OSA – but not COPD or BE – the increase in MSNA can lead to hypertension. Here, the features of the sympathoexcitation in OSA, COPD, and BE are reviewed in terms of the firing properties of post-ganglionic muscle vasoconstrictor neurons. Compared to healthy subjects with low levels of resting MSNA, single-unit recordings revealed that the augmented MSNA seen in OSA, BE, COPD, and CHF were each associated with an increase in firing probability and mean firing rates of individual neurons. However, unlike patients with heart failure, all patients with respiratory disease exhibited an increase in multiple within-burst firing which, it is argued, reflects an increase in central sympathetic drive. Similar patterns to those seen in OSA, COPD, and BE were seen in healthy subjects during an acute increase in muscle vasoconstrictor drive. These observations emphasize the differences by which the sympathetic nervous system grades its output in health and disease, with an increase in firing probability of active neurons and recruitment of additional neurons being the dominant mechanisms.
The respiratory and cardiovascular systems are tightly coupled in order to maximize oxygen delivery to, and removal of carbon dioxide from, the tissues of the body. It is not surprising, then, that diseases that affect the respiratory system may have cardiovascular consequences. Indeed, diseases that compromise gas exchange – such as obstructive sleep apnea (OSA), chronic obstructive pulmonary disease (COPD), and bronchiectasis (BE) – are all associated with an increase in vasoconstrictor drive to the skeletal muscle vascular beds. This increase in muscle sympathetic nerve activity (MSNA), as recorded directly from muscle fascicles of the common peroneal nerve via intraneural microelectrodes, is comparable in magnitude to that seen in congestive heart failure (CHF). It is known that sustained hypoxemia causes a long-lasting increase in MSNA and blood pressure, and that this persists following the return to normoxia (Morgan et al., 1995; Hansen and Sander, 2003; Tamisier et al., 2005). It is also known that episodes of airway obstruction in sleep cause repeated bouts of hypoxemia and an increase in MSNA (Somers et al., 1995; Narkiewicz et al., 1999) that persists in the awake state, leading to the development of hypertension (Hedner et al., 1988; Carlsson et al., 1993, 1996; Narkiewicz et al., 1998, 1999; Elam et al., 2002). The sympathoexcitation associated with OSA has been shown to normalize following nocturnal treatment with continuous positive airway pressure (Waradekar et al., 1996; Narkiewicz et al., 1999). However, unlike OSA, in which airflow limitation is acute, COPD is a disease characterized by chronic airflow limitation, especially in expiration (McKenzie et al., 2003). The compromised gas exchange and resultant chronic hypoxemia increases the load on the cardiovascular system. Three studies have shown that MSNA is increased in COPD (Heindl et al., 2001; Raupach et al., 2008; Ashley et al., 2010). Bronchiectasis, another respiratory disease associated with chronic airflow limitation, has also been shown to be associated with increased MSNA (Ashley et al., 2010). BE is due to collapse of large airways and the production of large volumes of mucus secretions; unlike COPD, there is neither hypoxemia nor hypercapnia in BE.
The purpose of this review is to highlight the different mechanisms by which the increase in MSNA in OSA, COPD, and BE is brought about, comparing these to the sympathoexcitation seen in CHF and during acute increases in MSNA in healthy subjects. Accordingly, I have brought together previously published data I have obtained in each of these conditions (Macefield and Wallin, 1999; Macefield et al., 1999; Elam and Macefield, 2001; Elam et al., 2002; Ashley et al., 2010), the current review serving as a means of synthesizing the state of knowledge about the sympathoexcitation in chronic respiratory disease, and comparing this with that of CHF – largely considered the “gold standard” of a pathological increase in MSNA. As will become apparent, the material I present is largely sourced from single-unit recordings of muscle sympathetic (vasoconstrictor) nerve activity. Although more difficult to obtain, unitary recordings provide a more sensitive measure of central sympathetic drive – i.e., the intensity of central sympathetic outflow – than standard multi-unit recordings (Macefield et al., 1994, 2002). In multi-unit recordings the absolute strength (intensity) of the recorded activity cannot be determined because of the dependence on the proximity of the electrode tip to the active nerve fibers, but this is not a limitation with single-unit recordings. Differences in the strength of multi-unit sympathetic activity (i.e., the number and the amplitude of multi-unit bursts) may be brought about in two ways: by differences in (i) the number of active neurons or (ii) the mean firing frequency of the neurons. A combination of both may also occur. A difference in mean firing frequency can be brought about by (a) a difference in firing probability of active neurons, i.e., the percentage of heart beats in which a given neuron is active or (b) a difference in the number of spikes a given neuron discharges within the bursts (or a combination of both). As explored by Lambert et al. (2012) in different cardiovascular and anxiety diseases, single-unit recordings allow one to tease out the mechanisms by which the sympathoexcitation is brought about in different pathophysiological states.
Clinical Features in Respiratory Disease
Three groups of patients with respiratory disease were compared: one group with acute respiratory insufficiency – OSA – and two with chronic respiratory insufficiency, COPD and bronchiectasis (BE). All of the patients were elderly. Although the OSA patients were – as a group – obese (body mass index >30), the mean BMI of the COPD patients was normal, while the BE patients were classified as being overweight but not obese. Spirometric data for the OSA patients were normal, but both the COPD and BE patients had clear evidence of severe respiratory insufficiency: forced vital capacity was significantly below normal – 1.80 ± 0.15 and 1.89 ± 0.22 l for the two groups – with forced expiratory volume in 1 s (FEV1) being only 0.82 ± 0.07 l in the COPD patients and 1.03 ± 0.15 l in the BE patients. Blood gas analysis revealed evidence of significant hypoxemia (69.3 ± 5.3 mmHg) and hypercapnia (59.0 ± 3.3 mmHg) in the patients with COPD, but not in those with BE or OSA. Table 1 provides clinical details from the patients, together with data from a group of older healthy control subjects. Mean heart rates were within the normal range in all patients, although heart rates in the patients were significantly higher than in the older healthy subjects. As a group, those with OSA had hypertension: resting systolic and diastolic blood pressures were 145 ± 5 and 96 ± 5 mmHg, respectively – significantly higher than those of the healthy older subjects. Conversely, neither the patients with COPD or bronchiectasis (BE) had elevated blood pressures.
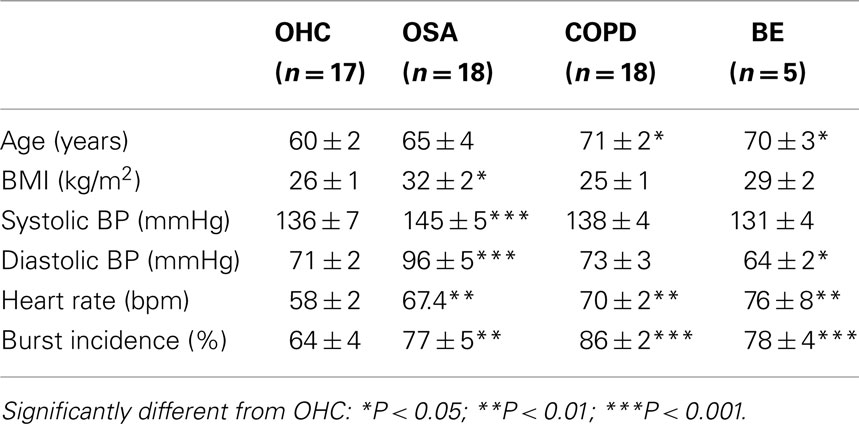
Table 1. Clinical details of the patients with obstructive sleep apnea (OSA; Elam et al., 2002), obstructive pulmonary disease (COPD; Ashley et al., 2010), or bronchiectasis (BE; Ashley et al., 2010), relative to a group of older healthy control subjects (OHC; Hart et al., 2009).
MSNA in Respiratory Disease: Multi-Unit Recordings
Compared with healthy subjects with low levels of MSNA, who had an average burst incidence of 21 ± 2%, MSNA was high (burst incidence >75%) in all patients with respiratory disease. While it is known that MSNA is higher in older subjects, the levels expressed by the patients were significantly higher than those of a group of older healthy subjects (64 ± 4%). Moreover, the levels of activity were comparable to those seen in patients with CHF. Mean data are provided in Table 1. However, high resting levels of MSNA can also be found in young healthy subjects. Indeed, a group of healthy subjects with a burst incidence of 75 ± 1% had levels that were statistically identical to those recorded in patients with OSA (77 ± 5%) or BE (78 ± 4%). As seen in Table 1, burst incidence was significantly higher in COPD (85 ± 2%) and CHF (88 ± 5%).
MSNA in Respiratory Disease: Single-Unit Recordings
A characteristic feature of the firing pattern of human muscle vasoconstrictor neurons is that they tend to fire only once in a multi-unit burst of impulses, i.e., only once per cardiac interval. And while they can fire multiple times, this is relatively rare. The same is true in patients with respiratory disease. An example of a unitary recording from a 65-year-old patient with COPD is shown in Figure 1. Typical of what we find in COPD, this unit was active in many cardiac intervals, i.e., it possessed a fairly high firing probability. However, in bronchiectasis – despite the high overall level of MSNA – the average firing probability was not significantly different from that of individual neurons recorded in healthy subjects with normally high levels of MSNA (37.8 ± 6.8 vs. 34.9 ± 3.6%); the same was true for burst incidence (77.6 ± 4.3 vs. 74.9 ± 0.5%). Despite this, mean firing rates in BE were more than double those seen in the healthy subjects (0.72 ± 0.11 vs. 0.33 ± 0.04 Hz). How can this be? Well, if one compares the firing distribution in BE and the healthy subjects with high levels of MSNA, it can be seen that there was a significant shift away from individual neurons firing only once per cardiac interval in the healthy subjects to firing two or more spikes per burst in the patients with BE. The same trend was seen in patients with OSA and COPD: the percentage of solitary spikes was 60.0 ± 6.2, 58.7 ± 2.8, and 63.4 ± 3.3% in BE, OSA, and COPD, compared to 77.6 ± 3.8% in the healthy subjects with high levels of MSNA. However, there was a difference between BE on the one hand and OSA and COPD on the other: while firing probability was low in BE, it was elevated (>50%) in OSA and COPD. Indeed, firing probabilities in OSA and COPD were comparable to those seen in CHF.
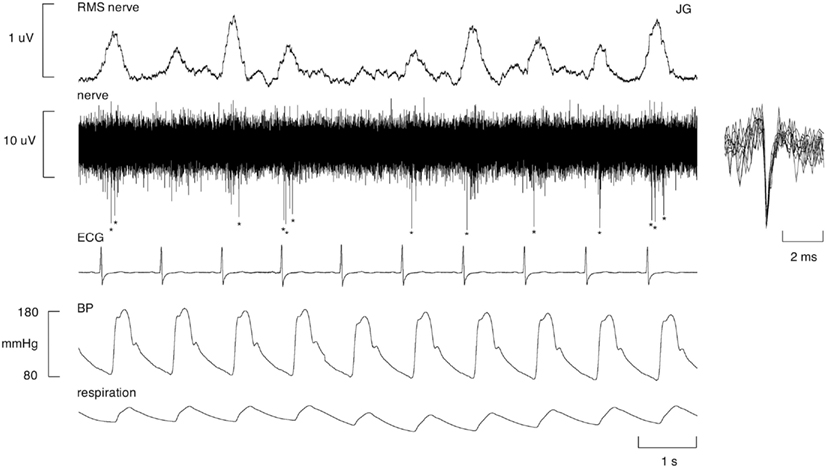
Figure 1. Unitary recording from a single muscle vasoconstrictor neuron in an awake female patient with COPD. This unit generally fired only one spike (indicated by asterisks) per cardiac interval, but occasionally fired multiple spikes within a burst of MSNA. Superimposed spikes show a uniform spike morphology, indicating that the action potentials originated from the same single sympathetic axon. The RMS-processed nerve signal primarily reflects far-field activity of many sympathetic neurons firing.
Histograms of the firing distributions for patients with BE, OSA, and COPD are shown in Figure 2, together with comparison data for healthy subjects and patients with CHF. Data for the healthy subjects have been pooled from those with low levels of resting MSNA (Macefield et al., 1994) and those with high levels (Macefield and Wallin, 1999). It can be seen that the spike firing distribution in patients with CHF was not different to that of the healthy subjects, whereas there was a clear shift away toward multiple firing in each of the respiratory diseases shown.
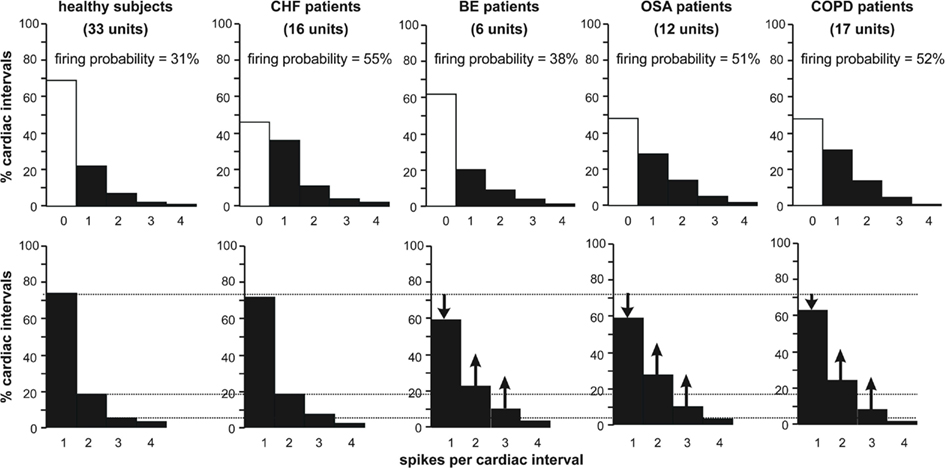
Figure 2. Firing distributions of single muscle vasoconstrictor neurons in healthy subjects [data combined from subjects with low (Macefield et al., 1994) or high (Macefield and Wallin, 1999) resting levels of MSNA], and in patients with congestive heart failure (CHF; Macefield et al., 1999), bronchiectasis (BE; Ashley et al., 2010), obstructive sleep apnea (OSA; Elam et al., 2002), or chronic obstructive pulmonary disease (COPD; Ashley et al., 2010). The histograms showing pooled data on the percentage of cardiac intervals in which units were quiescent, fired a single spike or 2, 3, or 4 spikes. In the top panel, all cardiac intervals have been included: the open columns represent those cardiac intervals in which the neurons were silent. The lower panels show data in which only those cardiac intervals were included in which a unit was active.
Acute Increases in MSNA
In five healthy subjects with high levels of resting MNSA, unitary recordings were made from nine neurons during quiet breathing and during a manouevre that causes an acute increase in MSNA – an inspiratory-capacity apnea (Macefield and Wallin, 1995). Using paired data the mean firing probabilities of the 9 units increased significantly from 32.5 ± 6.2% at rest to 56.3 ± 3.1% during the static phase of a maximal inspiratory breath-hold. As seen in Table 1, this firing probability is comparable to that seen in OSA, COPD, and CHF. Moreover, during this acute increase in muscle vasoconstrictor drive the percentage of cardiac intervals in which a solitary spike was generated decreased from 85.1 ± 4.5% at rest to 61.3 ± 5.6% during the apnea (p < 0.01). Correspondingly, the percentage of cardiac intervals in which a unit fired twice increased from 11.5 ± 2.7 to 26.7 ± 2.2% (p < 0.01). The percentage of intervals in which a unit generated three or four spikes also increased during the apnea, although these changes failed to reach statistical significance. It is notable that the spike distribution was comparable to that seen in BE, OSA, and COPD (Table 2).
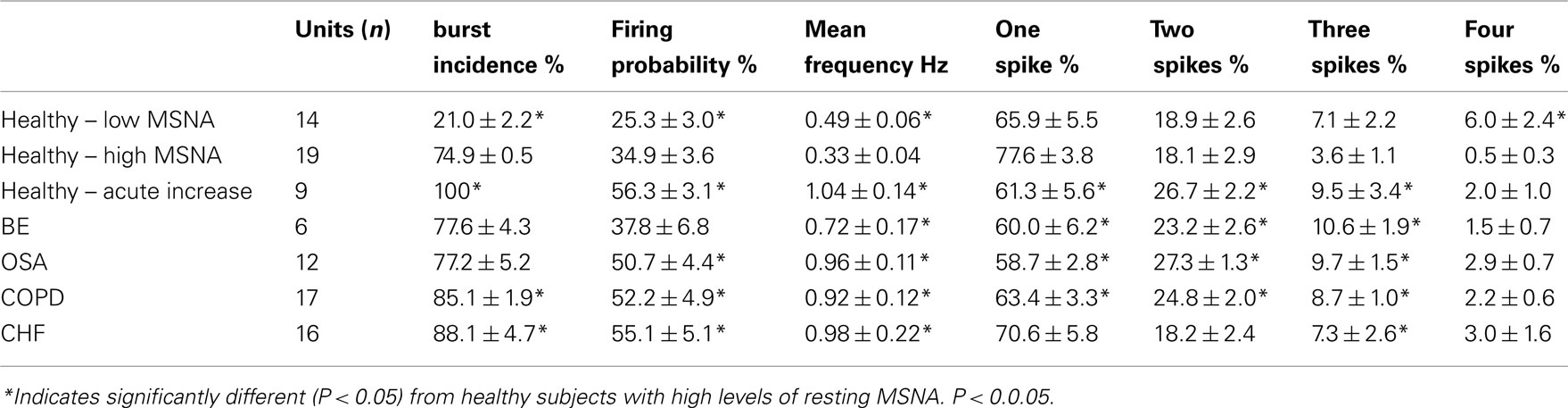
Table 2. Firing properties (mean ± SE) of muscle vasoconstrictor neurons in healthy subjects with low (Macefield et al., 1994) or high (Macefield and Wallin, 1999) resting levels of MSNA, during an acute increase in MSNA caused by a maximal inspiratory breath-hold (Macefield and Wallin, 1999), and in patients with bronchiectasis (BE; Ashley et al., 2010), obstructive sleep apnea (OSA; Elam et al., 2002), chronic obstructive pulmonary disease (COPD; Ashley et al., 2010), or congestive heart failure (CHF; Macefield et al., 1999).
Discussion
Standard multi-unit recordings have shown that elevated levels of MSNA feature in many diseases. In addition to CHF, OSA, COPD, and bronchiectasis, MSNA is increased in many different forms of hypertension: essential (Grassi et al., 1998; Schlaich et al., 2004), pregnancy-induced (Schobel et al., 1996; Fischer et al., 2004), renovascular (Johansson et al., 1999; Miyajima et al., 2001), and that associated with chronic kidney disease (Hausberg et al., 2002; Tuncel et al., 2002; Schlaich et al., 2009). However, it should be emphasized that a high level of MSNA does not, on its own, mean there is any underlying pathology: many healthy young individuals have high MSNA at rest yet normal blood pressures and no evidence of cardiovascular disease (Macefield and Wallin, 1999). This is one of the advantages of single-unit recordings: because of the quantal nature of unitary recordings, the firing properties of individual neurons can be compared across subjects and diseases. This means that by providing evidence of increases in firing probability, mean firing rate or multiple firing one can see whether the elevated level of MSNA reflects an increase in central sympathetic drive that may be being driven by some underlying pathology. Individual muscle vasoconstrictor neurons in healthy subjects with elevated resting levels of MSNA have low firing probabilities, low firing rates, and a low incidence of multiple firing (Macefield and Wallin, 1999). Conversely, pathophysiological increases in MSNA are associated with at least one of the following features: high firing probabilities, high firing rates, and a high incidence of multiple firing of individual neurons. Moreover, the patterns of increase differ according to the type of pathology.
The overall burst incidence in COPD (86%) was comparable to that seen in CHF (88%), which for both conditions was greater than either the patients with bronchiectasis (78%) or OSA (77%). Indeed, COPD and CHF represent two of the highest pathophysiological causes of augmented spontaneous MSNA ever recorded. The firing probability of muscle vasoconstrictor neurons was also as high in COPD (52%) as in CHF (55%), as was also the case for OSA (51%). It is interesting that the firing probability of single neurons in BE (38%) was no different from that of the healthy subjects with high levels of MSNA (35%). We know that hypoxemia and hypercapnia are not clinical features of bronchiectasis, so perhaps this accounts for the low firing probabilities seen in this disease. Nevertheless, given that the overall burst incidence were comparable in the two groups, one can conclude that, unless one of the firing properties is elevated, a high level of MSNA does not equate to an increase in central sympathetic drive.
Mean firing rates in the COPD patients (0.92 Hz) were similar to those found in OSA (0.96 Hz) and CHF patients (0.98 Hz), but higher than those in the bronchiectasis patients (0.72 Hz) and the healthy subjects (0.33 Hz). However, unlike the CHF patients, who showed no decrease in the proportion of solitary spikes generated within a burst, the patients with COPD, OSA, and BE each showed a significant trend toward multiple firing. We had already demonstrated that a shift toward multiple firing can occur in CHF, but only during the prolonged cardiac intervals following ventricular ectopic beats (Elam and Macefield, 2001). While the shift toward multiple firing can be explained by the elevated chemical drive in COPD and OSA, it is difficult to account for the increase seen in BE; perhaps it is related to the increased work of breathing in this disease. Regardless of the underlying mechanisms by which multiple within-burst firing occurs, it is not unreasonable to posit that a shift toward multiple firing will increase the liberation of noradrenaline (and co-localized neurotransmitters) from the vasoconstrictor nerve terminals – thereby increasing the degree of neurally mediated vasoconstriction. In addition, as discussed previously (Macefield et al., 1994), the generation of intermittently high instantaneous frequencies associated with multiple firing may have physiological consequences: for instance, it is known that electrical stimulation of muscle vasoconstrictor neurons in the cat with an irregular pattern is more effective at contracting arterioles than is a regular pattern of stimulation (Andersson, 1983), and similar results have been found for the mesenteric (Nilsson et al., 1985) and gastric (Polenov et al., 1991) vessels in the rat. In humans, electrical stimulation of sudomotor neurons with irregular stimulus trains is more effective at liberating sweat than is stimulation with regular trains (Kunimoto et al., 1991, 1992), and it has been argued that – compared to sudomotor neurons active in thermally induced sweating – the firing patterns of sudomotor neurons in patients with hyperhidrosis show a significant shift toward multiple firing which would be expected to increase the release of acetylcholine from the nerve terminals and hence the liberation of sweat from the skin (Macefield et al., 2008). Similar arguments have been made by Murai et al. (2006, 2009) with respect to the firing of single muscle vasoconstrictor neurons in heart failure. Morever, Lambert et al. (2011) recently showed that cardiac noradrenaline spillover is higher when muscle vasoconstrictor neurons (and presumably, in parallel, cardiac sympathetic neurons) exhibit an increase in multiple firing, arguing that multiple firing does indeed cause an increase in neurotransmitter release.
Pharmacological treatment was not withdrawn prior to the study, so as to avoid rebound cardiovascular responses and associated effects on MSNA. Nevertheless, despite the ongoing treatment MSNA remained high in all patients. Unlike the high incidence of OSA with obesity, the COPD subjects had, on average, a normal BMI. It is known that obesity itself increases the resting level of MSNA, but the levels typically encountered (burst incidence 53%) are much lower than those we see in COPD, OSA, BE, or CHF; moreover, individual muscle vasoconstrictor neurons do not show an increased incidence of multiple firing in obesity (Lambert et al., 2007), so we do not believe that the firing characteristics observed in OSA, COPD, or BE can be accounted for by body weight. Interestingly, despite the elevated levels of central sympathetic drive evident in COPD and BE, none of these patients had high blood pressure. So, why does the increase in MSNA in OSA lead to hypertension? Perhaps, it is because the hypoxia and hypercapnia seen in COPD act directly on blood vessels to cause vasodilatation, thereby counteracting the neutrally mediated vasoconstriction. Indeed, it is known that in brain-dead patients, who have no central sympathetic drive, asphyxia causes vasodilatation, and hypotension (Lahana et al., 2005). However, why patients with BE do not develop hypertension is unknown, given that they are not chronically asphyxic.
Conclusion
In conclusion, each of the respiratory diseases herein examined showed evidence of an elevated central sympathetic outflow – as judged by the firing properties of single muscle vasoconstrictor neurons. While the overall level of sympathoexcitation seen in OSA, COPD, and BE, as measured from multi-unit burst incidence, is comparable to that seen in CHF, the unitary firing properties observed in OSA, COPD, and BE are more closely aligned with each other than with the firing properties seen in CHF.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andersson, P. O. (1983). Comparative vascular effects of stimulation continuously and in bursts of the sympathetic nerves to cat skeletal muscle. Acta Physiol. Scand. 118, 343–348.
Ashley, C., Burton, D., Sverrisdottir, Y. B., Sander, M., McKenzie, D. K., and Macefield, V. G. (2010). Firing probability and mean firing rates of human muscle vasoconstrictor neurons are elevated during chronic asphyxia. J. Physiol. (Lond.) 588, 701–711.
Carlsson, J. T., Hedner, J., Elam, M., Ejnell, H., Sellgren, J., and Wallin, B. G. (1993). Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103, 1763–1768.
Carlsson, J. T., Hedner, J., Sellgren, J., Elam, M., and Wallin, B. G. (1996). Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 154, 1490–1496.
Elam, M., and Macefield, V. G. (2001). Multiple firing of single muscle vasoconstrictor neurons during cardiac dysrhythmias in human heart failure. J. Appl. Physiol. 91, 717–724.
Elam, M., Macefield, V. G., and McKenzie, D. (2002). The firing pattern of single muscle vasoconstrictor neurons in awake patients with the obstructive sleep apnea syndrome. J. Appl. Physiol. 93, 297–303.
Fischer, T., Schobel, H. P., Frank, H., Andreae, M., Schneider, K. Y., and Heusser, K. (2004). Pregnancy-induced sympathetic hyperactivity: a precursor of preeclampsia. Eur. J. Clin. Invest. 34, 443–448.
Grassi, G., Colombo, M., Seravalle, G., Spaziani, D., and Mancia, G. (1998). Dissociation between muscle and skin sympathetic nerve activity in essential hypertension, obesity, and congestive heart failure. Hypertension 31, 64–67.
Hansen, J., and Sander, M. (2003). Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J. Physiol. (Lond.) 546, 921–929.
Hart, E. C., Joyner, M. J., Wallin, B. G., Johnson, C. P., Curry, T. B., Eisenach, J. H., and Charkoudian, N. (2009). Age-related changes in the sympathetic-hemodynamic balance in men. Hypertension 54, 127–133.
Hausberg, M., Kosch, M., Harmelink, P., Barenbrock, M., Hohage, H., Kisters, K., Dietl, K. H., and Rahn, K. H. (2002). Sympathetic nerve activity in end-stage renal disease. Circulation 106, 1974–1979.
Hedner, J., Ejnell, H., Sellgren, J., Hedner, T., and Wallin, B. G. (1988). Is high and fluctuating muscle nerve sympathetic activity in the sleep apnea syndrome of pathogenetic importance for the development of hypertension? J. Hypertens. Suppl. 6, S529–S531.
Heindl, S., Lehnert, M., Criée, C. P., Hasenfuss, G., and Andreas, S. (2001). Marked sympathetic activation in patients with chronic respiratory failure. Am. J. Respir. Crit. Care Med. 164, 597–601.
Johansson, M., Elam, M., Rundqvist, B., Eisenhofer, G., Herlitz, H., Lambert, G., and Friberg, P. (1999). Increased sympathetic nerve activity in renovascular hypertension. Circulation 18, 2537–2542.
Kunimoto, M., Kirnö, K., Elam, M., Karlsson, T., and Wallin, B. G. (1991). Neuroeffector characteristics of sweat glands in the human hand activated by regular neural stimuli. J. Physiol. (Lond.) 442, 391–411.
Kunimoto, M., Kirnö, K., Elam, M., Karlsson, T., and Wallin, B. G. (1992). Neuro-effector characteristics of sweat glands in the human hand activated by irregular stimuli. Acta Physiol. Scand. 146, 261–269.
Lahana, A., Constantopulos, S., and Nakos, G. (2005). The local component of the acute cardiovascular response to simulated apneas in brain-dead humans. Chest 128, 634–639.
Lambert, E., Hering, D., Schlaich, M., and Lambert, G. (2012). Advances in sympathetic nerve recording in humans. Front. Physiol. 3:11. doi:10.3389/fphys.2012.00011
Lambert, E., Straznicky, N., Schlaich, M., Esler, M., Dawood, T., Hotchkin, E., and Lambert, G. (2007). Differing pattern of sympathoexcitation in normal-weight and obesity-related hypertension. Hypertension 50, 862–868.
Lambert, E. A., Schlaich, M. P., Dawood, T., Sari, C., Chopra, R., Barton, D. A., Kaye, D. M., Elam, M., Esler, M. D., and Lambert, G. W. (2011). Single-unit muscle sympathetic nervous activity and its relation to cardiac noradrenaline spillover. J. Physiol. (Lond.) 589, 2597–2605.
Macefield, V. G., Elam, M., and Wallin, B. G. (2002). Firing properties of single postganglionic sympathetic neurons recorded in awake human subjects. Auton. Neurosci. 95, 146–159.
Macefield, V. G., Rundqvist, B., Sverrisdottir, Y. B., Wallin, B. G., and Elam, M. (1999). Firing properties of single muscle vasoconstrictor neurons in the sympathoexcitation associated with congestive heart failure. Circulation 100, 1708–1713.
Macefield, V. G., Sverrisdottir, Y. B., Elam, E., and Harris, J. (2008). Firing properties of sudomotor neurons in hyperhidrosis and thermal sweating. Clin. Auton. Res. 18, 325–330.
Macefield, V. G., and Wallin, B. G. (1995). Effects of static lung inflation on sympathetic activity in human muscle nerves at rest and during asphyxia. J. Autonom. Nerv. Sys. 53, 148–156.
Macefield, V. G., and Wallin, B. G. (1999). Firing properties of single vasoconstrictor motoneurones in subjects with high levels of muscle sympathetic activity. J. Physiol. (Lond.) 516, 293–301.
Macefield, V. G., Wallin, B. G., and Vallbo, Å. B. (1994). The discharge behaviour of single vasoconstrictor motoneurones in human muscle nerves. J. Physiol. (Lond.) 481, 799–809.
McKenzie, D. K., Frith, P. A., Burdon, J. G. W., and Town, G. I. (2003). The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2003. Med. J. Aust. 178(Suppl. 6), S1–S40.
Miyajima, E., Yamada, Y., Yoshida, Y., Matsukawa, T., Shionoiri, H., Tochikubo, O., and Ishii, M. (2001). Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension 17, 1057–1062.
Morgan, B. J., Crabtree, D. C., Palta, M., and Skatrud, J. B. (1995). Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J. Appl. Physiol. 79, 205–213.
Murai, H., Takamura, M., Maruyama, M., Nakano, M., Ikeda, T., Kobayashi, D., Otowa, K., Ootsuji, H., Okajima, M., Furusho, H., Takata, S., and Kaneko, S. (2009). Altered firing pattern of single-unit muscle sympathetic nerve activity during handgrip exercise in chronic heart failure. J. Physiol. (Lond.) 587, 2613–2622.
Murai, H., Takata, S., Maruyama, M., Nakano, M., Kobayashi, D., Otowa, K., Takamura, M., Yuasa, T., Sakagami, S., and Kaneko, S. (2006). The activity of a single muscle sympathetic vasoconstrictor nerve unit is affected by physiological stress in humans. Am. J. Physiol. Heart Circ. Physiol. 290, H853–H860.
Narkiewicz, K., Kato, M., Phillips, B. G., Pesek, C. A., Davison, D. E., and Somers, V. K. (1999). Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100, 2332–2335.
Narkiewicz, K., Pesek, C. A., Kato, M., Phillips, B. G., Davison, D. E., and Somers, V. K. (1998). Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 32, 1039–1043.
Nilsson, H., Ljung, B., Sjöblom, N., and Wallin, B. G. (1985). The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol. Scand. 123, 303–309.
Polenov, S. A., Lensman, M. V., Zasorin, K. V., and Kolobanov, S. V. (1991). “The effect of continuous and burst stimulation of sympathetic nerves on gastric submucosal microvessels in rats,” in Resistance Arteries, Structure and Function, eds M. J. Mulvany, C. Aalkjaer, and A. M. Heagerty (Amsterdam: Elsevier Science Publishers), 169–172.
Raupach, T., Bahr, F., Herrmann, P., Luethje, L., Heusser, K., Hasenfuss, G., Bernardi, L., and Andreas, S. (2008). Slow breathing reduces sympathoexcitation in COPD. Eur. Respir. J. 32, 387–392.
Schlaich, M. P., Lambert, E., Kaye, D. M., Krozowski, Z., Campbell, D. J., Lambert, G., Hastings, J., Aggarwal, A., and Esler, M. D. (2004). Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and angiotensin neuromodulation. Hypertension 43, 169–175.
Schlaich, M. P., Socratous, F., Hennebry, S., Eikelis, N., Lambert, E. A., Straznicky, N., Esler, M. D., and Lambert, G. W. (2009). Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 20, 933–939.
Schobel, H. P., Fischer, T., Heuszer, K., Geier, H., and Schmeider, R. E. (1996). Preeclampsia – a state of sympathetic hyperactivity. N. Engl. J. Med. 335, 1480–1485.
Somers, V. K., Dyken, M. E., Clary, M. P., and Abboud, F. M. (1995). Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 95, 1897–1904.
Tamisier, R., Anand, A., Nieto, L. M., Cunnington, D., and Weiss, J. W. (2005). Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J. Appl. Physiol. 98, 343–349.
Tuncel, M., Augustyniak, R., Zhang, W., Toto, R. D., and Victor, R. G. (2002). Sympathetic nervous system function in renal hypertension. Curr. Hypertens. Rep. 4, 229–236.
Keywords: bronchiectasis, chronic obstructive pulmonary disease, obstructive sleep apnea, microneurography, single-unit, sympathoexcitation
Citation: Macefield VG (2012) Firing patterns of muscle vasoconstrictor neurons in respiratory disease. Front. Physio. 3:153. doi: 10.3389/fphys.2012.00153
Received: 24 February 2012; Accepted: 03 May 2012;
Published online: 23 May 2012.
Edited by:
Elisabeth Lambert, Baker IDI Heart and Diabetes Institute, AustraliaReviewed by:
Gavin W. Lambert, Baker IDI Heart and Diabetes Institute, AustraliaKarie Scrogin, Loyola University Chicago, USA
Copyright: © 2012 Macefield. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Vaughan G. Macefield, School of Medicine, University of Western Sydney, Locked Bag 1797, Penrith NSW 2751, Australia. e-mail: v.macefield@uws.edu.au
