- 1Department of Medicine and Surgery, University of Salerno, Salerno, Italy
- 2Center for Translational Medicine, Department of Pharmacology, Temple University of Philadelphia, PA, USA
- 3Department of Physiology and Cellular Biophysics, Columbia University in the City of New York, New York, NY, USA
- 4Department of Advanced Biomedical Sciences“Federico II” University of Naples, Italy
- 5IRCCS, Multimedica Research Hospital, Milan, Italy
Activation of the adrenergic system has a profound effects on metabolism. Increased circulating catecholamine and activation of the different adrenergic receptors deployed in the various organs produce important metabolic responses which include: (1) increased lipolysis and elevated levels of fatty acids in plasma, (2) increased gluconeogenesis by the liver to provide substrate for the brain, and (3) moderate inhibition of insulin release by the pancreas to conserve glucose and to shift fuel metabolism of muscle in the direction of fatty acid oxidation. These physiological responses, typical of the stress conditions, are demonstrated to be detrimental for the functioning of different organs like the cardiac muscle when they become chronic. Indeed, a common feature of many pathological conditions involving over-activation of the adrenergic system is the development of metabolic alterations which can include insulin resistance, altered glucose and lipid metabolism and mitochondrial dysfunction. These patterns are involved with a variably extent among the different pathologies, however, they are in general strictly correlated to the level of activation of the adrenergic system. Here we will review the effects of the different adrenergic receptors subtypes on the metabolic variation observed in important disease like Heart Failure.
Introduction
Heart function relies to a great extent on cardiac muscle oxidative metabolism. Given the high mitochondrial content of this tissue, cardiac muscle generates ATP almost exclusively (about 90%) through oxidative phosphorylation (Stanley et al., 2005) by using different metabolic substrates. Indeed, cardiac muscle possesses a metabolic flexibility or plasticity, allowing it to maintain its function during stressful conditions. In the adult heart the major pathway for ATP production is fatty acid oxidation while the relative contribution of glucose increases during stress or injury, such as exercise or ischemia (Bing et al., 1954; Wisneski et al., 1987). Thus, it is not surprising that an impairment of cardiac muscle energy metabolism represents an important risk factor for the development of cardiac diseases (Stanley et al., 2005; Neubauer, 2007). Indeed, under pathological conditions, the heart exhibits a severe malfunction of different metabolic pathways, such as the tricarboxylic acid (TCA) cycle and b-oxidation (Stanley et al., 2005; Neubauer, 2007). This metabolic remodeling is characterized by a lower oxidative capacity, contractile dysfunction and cardiac muscle insulin resistance (Stanley et al., 2005; Neubauer, 2007). Different therapeutic strategies have been undertaken to modulate metabolic pathways in the failing heart, though it remains controversial whether targeting glucose vs. fatty acid metabolism individually or combined represents a better approach to improve metabolic flexibility and cardiac function (Kolwicz and Tian, 2009; Ardehali et al., 2012). Surely, the importance of a preserved metabolism includes not only an efficient energy supply needed for myocardium to accomplish his contractile function but also protection against oxidative stress (Neubauer, 2007), which is involved in the remodeling and progression of the disease.
Cardiac Metabolic Dysfunction During HF
Animal models of HF have evidenced excessive uptake and myocardial free fatty acid (FFA) accumulation with reduced glucose utilization (Lommi et al., 1998; Taylor et al., 2001) and in both animal models of HF and in human disease these metabolic alterations reduce myocardial oxygen efficiency and lead to a depletion of intracellular ATP (Neubauer et al., 1992; Taylor et al., 2001). The importance of a preserved glucose cardiac utilization may be related to its higher efficiency in terms of ATP production and molecule of oxygen consumed, with consequently reduced oxygen wastage and reactive oxygen species (ROS) production, as compared to FFA. Insulin receptor signaling is critically involved in increasing glucose uptake in the myocardium and cardiac insulin resistance contributes to the development of left ventricular (LV) dysfunction by reducing cardiac efficiency through metabolic shift toward fatty acids utilization (Peterson et al., 2004b). Indeed, a profound state of insulin resistance has been found in the hearts of ob/ob mice and the ability of these hearts to modulate substrate utilization in response to insulin and changes is altered (Mazumder et al., 2004). Accordingly, normalization of cardiac metabolism by overexpressing a human GLUT4 transgene in mice with cardiac insulin resistance recovered the altered cardiac function observed in these animals (Belke et al., 2000; Semeniuk et al., 2002). Therefore, these studies indicate that cardiac insulin resistance reduces the metabolic efficiency of the heart, which leads to a contractile dysfunction. Moreover, insulin resistance is a known and recognized phenomenon leading to HF (Boudina et al., 2009) as seen in positron emission tomographic (PET) studies showing that the failing human myocardium has reduced glucose uptake in favor of FFA uptake (Witteles et al., 2004). Several hypotheses have been proposed to explain the association between altered cardiac metabolism, insulin resistance, and HF, and among these there is a strong correlation with neurohormonal activation (Kostis and Sanders, 2005; Zucker, 2006), which increases plasma FFA levels, inhibits insulin receptor signaling and causes the loss of myocyte glucose uptake (Opie and Sack, 2002). As known neurohormonal activation includes over-activation of the adrenergic and RAAS system, and we will here specifically focus on the adrenergic mechanisms that underlie altered myocardial metabolism and insulin resistance in HF.
Sympathetic Nervous System and Cardiac Metabolism: Role of the β Adrenergic Receptors and Related Signaling
The Sympathetic Nervous System (SNS) is maladaptively activated in response to a chronic reduction in cardiac output and it is characterized by an increased secretion and reduced cardiac catecholamine reuptake (Eisenhofer et al., 1996). The effects of the catecholamine incretion on the cardiac metabolism are mediated by both central and peripheral mechanisms. For example, increased catecholamines have directly detrimental effects on the heart, which cause marked enzyme loss as an index of diffuse myocardial damage, and substantial oxygen-wastage even in the absence of FFA in the perfusate (Opie et al., 1979). Furthermore, norepinephrine promotes both coronary vasoconstriction and increased plasma FFA levels (Paolisso et al., 1991), which further promote oxygen-wastage (Sasaoka et al., 2006). Infusion studies in volunteers support a role for increased norepinephrine levels in HF as a cause of elevated plasma FFA (Sasaoka et al., 2006). In turn, FFAs reciprocally augment SNS activity, at least in normal controls. In human skeletal muscle, a dose-response relationship exists between plasma FFA (Santomauro et al., 1999; Peterson et al., 2004a; Banerjee and Peterson, 2007) and defects in insulin signaling (Belfort et al., 2005). This may in part be caused by FFA-mediated activation of protein kinase C, which phosphorylates insulin receptors and results in reduced capillary opening and reduced myocyte glucose import (Itani et al., 2002; Wagenmakers et al., 2006). Moreover, also locally activated SNS appear to be relevant in the altered cardiac metabolism. With use of PET with a norepinephrine analog and 18F-fluorodeoxyglucose, myocardial segments with LV dysfunction have reduced presynaptic norepinephrine reuptake and myocardial glucose uptake in relation to less impaired myocardial segments in the same patients (Mongillo et al., 2007). Thus, after control for confounding variables, altered metabolism and IR directly relate to local SNS activity. As known, the adverse effects of the SNS on the heart are mediated by the adrenergic receptors (AR), however, extensive research has indicated that the various subtypes, in particular β1 and β2 ARs, are differently involved in pathophysiology of HF and so it is likely to be the same for modifications of cardiac metabolism observed during disease. Indeed, β1- and β2-AR regulate different signal pathways, resulting in different outcomes on cardiac function. Stimulation of β1- and β2-adrenoceptors can induce the activation of the stimulatory G protein (Gαs)/adenylylcyclase (AC)/cAMP/cAMP-dependent protein kinase A (PKA) signaling pathway, which consequently leads to the phosphorylation of several target proteins within the cardiac myocyte, such as phospholamban, L-type calcium channel and troponin I (Woo and Xiao, 2012). Nonetheless, this signal pathway is the main mechanism by which β1- rather than β2 adrenoceptors regulates cardiac contractility/relaxation and rate (Baruscotti et al., 2010; Woo and Xiao, 2012). In contrast, the β2 AR has been shown to regulate an alternative signaling pathway through activation of the inhibitory G protein (Gαi) and the heterodimer formed by the β and γ subunits of the G protein (Gβγ) (Zhu et al., 2011). Besides the inhibition of AC, the main signal pathway regulated by β2-AR through Gαi/Gβγ appears to be the phosphatidylinositol-3kinase (PI3K) signaling cascade, although other proteins such as the AMP-dependent protein kinase (AMPK), mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase1and 2 (ERK1/2) have recently been proposed as novel targets of β2-AR (Zhang et al., 2011). About the effects of adrenergic system on metabolism, it is known that sustained beta adrenergic stimulation induce insulin resistance (Cipolletta et al., 2009) and in this context the β2 adrenergic receptor appears to have a main role in overall glucose homeostasis by acting on pancreatic islet hormone secretion, liver and muscle glucose transport metabolism (Usui et al., 2005; Shahid and Hussain, 2007; Garcia-Guerra et al., 2010). At cardiac level several studies have raised the possibility of using selective β2-agonists as potential modulators of cardiac muscle energy metabolism. Short- and long-term stimulation of the β2-AR has been associated with the modulation of fatty acid and glucose metabolism (Philipson, 2002). Indeed, acute treatment of myocytes in vitro or skeletal muscle ex vivo with β2 agonists induces a significant increase in glucose uptake, reaching comparable levels to insulin stimulation (Nevzorova et al., 2002, 2006; Ngala et al., 2008). A putative mechanism for these evidence would involve the activation of PI3K and its downstream signal pathway (Zhu et al., 2001; Jo et al., 2002; Perez-Schindler et al., 2011; Zhang et al., 2011) and in particular the phosphorylation and inactivation of TBC1D4 (also known as Akt substrate of 160 kDa, AS160) by Akt (Sakamoto and Holman, 2008). TBC1D4 inhibit the translocation of the glucose transporter type4 (GLUT4) from intracellular vesicles to the plasmamembrane, hence an increase in TBC1D4 phosphorylation would consequently enhance glucose uptake (Sakamoto and Holman, 2008). Moreover, TBC1D4 is also targeted by AMPK, which represent a key mechanism in the regulation of insulin-independent glucose uptake (Sakamoto and Holman, 2008; Maarbjerg et al., 2011). Consistent with the potential role of β2-AR in glucose metabolism, high levels of AMPK phosphorylation and activity in response to β2-AR stimulation (Li et al., 2010; Perez-Schindler et al., 2011) have been found in cardiomyocytes, potentially as consequence of an increase in the AMP/ATP ratio, or activation of upstream AMPK kinases (Hardie et al., 2012). Different results, however, have been found in presence of an enhanced β2-AR signaling by means of stable overexpression in non-cardiac cells, where, in vitro studies shows impaired glucose extraction later on insulin stimulation (Cipolletta et al., 2009). Moreover “in vivo” studies demonstrated a greater efficiency of carvedilol, a non-selective β-AR antagonist, in ameliorating myocardial insulin sensitivity and glucose extraction in animal model of HF as compared to metoprolol, a selective β1-AR antagonist, indicating that antagonism of β2AR positively regulates cardiac glucose metabolism in an animal model HF (Nikolaidis et al., 2006). These conflicting results may rely on the different models used for studies and/or to differences in acute and chronic stimulation of the β2AR. While acute activation of the receptor can favor glucose uptake by increasing GLUT4 translocation to the plasmamembrane, chronic adrenergic stimulation, as seen during HF, would be rather detrimental by up or down regulation of specific intracellular molecules. Recent discoveries have found in the kinase of the G protein coupled receptor type 2 (GRK2) a potential molecular link between chronic adrenergic stimulation and development of altered myocardial metabolism observed during HF (Ciccarelli et al., 2011). So far, the implications of GRK2 upregulation during HF have been exclusively considered for the detrimental effects on β AR signaling and cardiac inotropism (Koch et al., 1995), however, studies in non-cardiac cells have shown ability to interact with different substrates belonging to different cellular pathways such as insulin signaling, and, of note, to mediate insulin resistance produced by enhanced β2-AR receptor signaling (Cipolletta et al., 2009). In the failing heart GRK2 is upregulated (Iaccarino et al., 2005) and affects myocardial glucose uptake at the early stages of the disease, before cardiac dilation and reduced function are evident, indicating that metabolic modifications are relevant in the progression of HF and to be the molecular link between over-activation of the adrenergic system and the altered glucose uptake during HF (Ciccarelli et al., 2011). Recent studies have also indicated that GRK2 is also able to localize into mitochondria, but this role in the global cellular physiology is not completely clear. Indeed, different cellular models have shown different behaviors of GRK2 when it localizes into mitochondria. Specifically, Fusco et al. have indicated that GRK2 is protective for the cell by increasing ATP production and promoting mitogenesis (Fusco et al., 2012). As known, GRK2 shuttles from cytosol to plasmamembrane, by anchoring to free Gβγ through its pleckstrin homology and binding domains within the carboxyl terminus, following both catecholamine and insulin stimulation. Similarly, ischemia causes acute cellular and mitochondrial accumulation of GRK2 (Fusco et al., 2012), an effect reverted by oxygen restoration, but, surprisingly it preserves ATP loss and induces mitochondria biogenesis after ischemia/reperfusion, indicating a protective effect of GRK2 for mitochondria after acute stress.
These data are supported by the importance of GRK2 for embryonic cardiac development (Jaber et al., 1996) and, in adult life, by the early eccentric dilation of the heart and the vascular inflammation induced by selective GRK2 removal in the myocardium and endothelium, respectively (Brunn et al., 2006; Ciccarelli et al., 2013). On the contrary, Chen et al. have also shown that elevated level of GRK2 activates a pro-death signal through its localization into mitochondria during stress conditions (Chen et al., 2013).
This discrepancy could be related to the model and timing used in different studies, however, in a synthetic view of the latest GRK2 studies, we could advance the idea that the inhibitory property of GRK2 could be useful in acute phase of stress/stimulation, when the cytosolic molecule shuttles to the plasmamembrane and mitochondria, setting cellular activity in a resting phase and less susceptible to the stress and/or excess of signaling. However, in a perpetuating situation, the increased activity/level of GRK2 and chronic reduction of β adrenergic and insulin signaling becomes detrimental for cell function and survival (Figures 1A,B). Further studies will be needed to evaluate this hypothesis; nevertheless, several reports already indicate GRK2 as a potential target for treatment of both altered metabolism and contractility during HF.
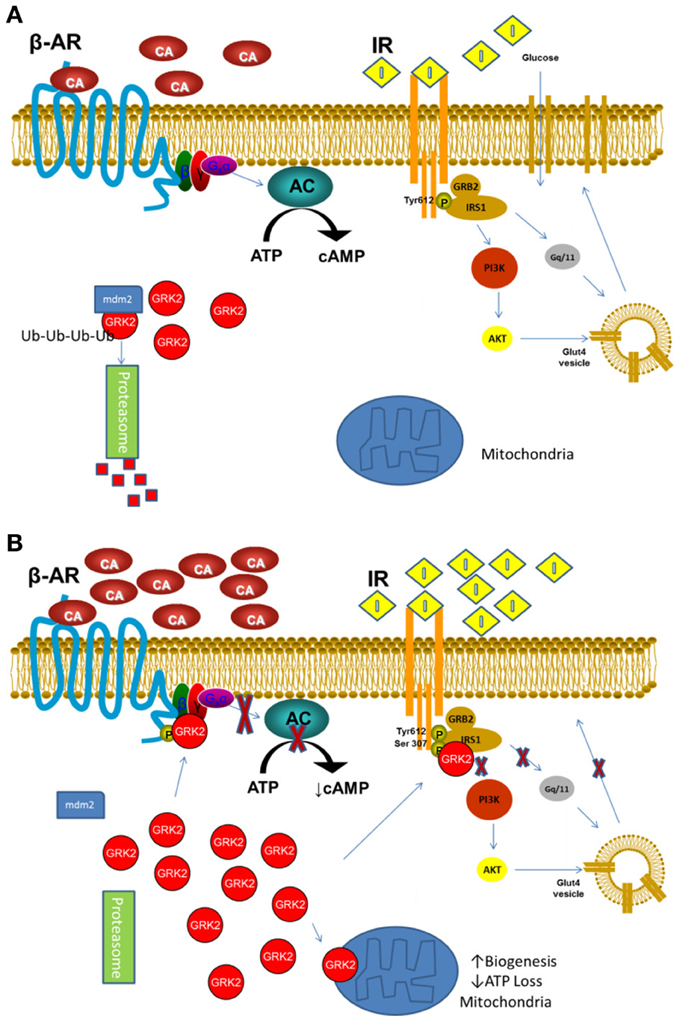
Figure 1. (A) In basal or low stimulation conditions GRK2 is mostly cytosolic and its level are tightly regulated by mdm2 ubiquitination and degradation through the Proteasome. (B) Following acute or excessive stimulation by catecholamines or Insulin or hypoxia, GRK2 detaches from mdm2 and quickly accumulates in the cell, moving to membranes where desensitizes β-AR and IR. Moreover, it can translocates to the mitochondria, reducing ATP loss and inducing mitochondria biogenesis (β-AR, β Adrenergic Receptor; IR, Insulin Receptor; CA, Catecholamine; I, Insulin).
Conclusions and Perspectives
Modification of cardiac metabolism is a fascinating field of investigation for development of new strategies for treatment of myocardial dysfunction. This consideration is based on the evidence that an efficient metabolic supply is needed for the single cardiomyocyte to accomplish its contractile function and efficient utilization is also important to counteract oxygen wastage and increased ROS on which relies progression of the disease. Current available compounds acting on the β ARs seem to be effective for these goals, however, identification of specific target, as GRK2, clearly involved in regulation of cardiac contractility, remodeling and metabolism would prepare the ground for a more efficient therapy aimed to ameliorate patients prognosis and outcome.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ardehali, H., Sabbah, H. N., Burke, M. A., Sarma, S., Liu, P. P., Cleland, J. G., et al. (2012). Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur. J. Heart Fail 14, 120–129. doi: 10.1161/CIRCRESAHA.112.300754
Banerjee, S., and Peterson, L. R. (2007). Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr. Cardiol. Rep. 9, 143–149. doi: 10.1007/BF02938341
Baruscotti, M., Barbuti, A., and Bucchi, A. (2010). The cardiac pacemaker current. J. Mol. Cell Cardiol. 48, 55–64. doi: 10.1016/j.yjmcc.2009.06.019
Belfort, R., Mandarino, L., Kashyap, S., Wirfel, K., Pratipanawatr, T., Berria, R., et al. (2005). Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes 54, 1640–1648. doi: 10.2337/diabetes.54.6.1640
Belke, D. D., Larsen, T. S., Gibbs, E. M., and Severson, D. L. (2000). Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am. J. Physiol. Endocrinol. Metab. 279, E1104–E1113.
Bing, R. J., Siegel, A., Ungar, I., and Gilbert, M. (1954). Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am. J. Med. 16, 504–515. doi: 10.1016/0002-9343(54)90365-4
Boudina, S., Bugger, H., Sena, S., O'Neill, B. T., Zaha, V. G., Ilkun, O., et al. (2009). Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119, 1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101
Brunn, G. J., Saadi, S., and Platt, J. L. (2006). Differential regulation of endothelial cell activation by complement and interleukin 1alpha. Circ. Res. 98, 793–800. doi: 10.1161/01.RES.0000216071.87981.16
Chen, M., Sato, P. Y., Chuprun, J. K., Peroutka, R. J., Otis, N. J., Ibetti, J., et al. (2013). Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal-regulated kinase-dependent heat shock protein 90-mediated mitochondrial targeting. Circ. Res. 112, 1121–1134. doi: 10.1161/CIRCRESAHA.112.300754
Ciccarelli, M., Chuprun, J. K., Rengo, G., Gao, E., Wei, Z., Peroutka, R. J., et al. (2011). G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation 123, 1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642
Ciccarelli, M., Sorriento, D., Franco, A., Fusco, A., Del Giudice, C., Annunziata, R., et al. (2013). Endothelial G Protein-Coupled Receptor Kinase 2 regulates vascular homeostasis through the control of free radical oxygen species. Arterioscler. Thromb. Vasc. Biol. 33, 2415–2424. doi: 10.1161/ATVBAHA.113.302262
Cipolletta, E., Campanile, A., Santulli, G., Sanzari, E., Leosco, D., Campiglia, P., et al. (2009). The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc. Res. 84, 407–415. doi: 10.1093/cvr/cvp252
Eisenhofer, G., Friberg, P., Rundqvist, B., Quyyumi, A. A., Lambert, G., Kaye, D. M., et al. (1996). Cardiac sympathetic nerve function in congestive heart failure. Circulation 93, 1667–1676. doi: 10.1161/01.CIR.93.9.1667
Fusco, A., Santulli, G., Sorriento, D., Cipolletta, E., Garbi, C., Dorn, G. W. 2nd., et al. (2012). Mitochondrial localization unveils a novel role for GRK2 in organelle biogenesis. Cell Signal. 24, 468–475. doi: 10.1016/j.cellsig.2011.09.026
Garcia-Guerra, L., Nieto-Vazquez, I., Vila-Bedmar, R., Jurado-Pueyo, M., Zalba, G., Diez, J., et al. (2010). G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes 59, 2407–2417. doi: 10.2337/db10-0771
Hardie, D. G., Ross, F. A., and Hawley, S. A. (2012). AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262. doi: 10.1038/nrm3311
Iaccarino, G., Barbato, E., Cipolletta, E., De Amicis, V., Margulies, K. B., Leosco, D., et al. (2005). Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur. Heart J. 26, 1752–1758. doi: 10.1093/eurheartj/ehi429
Itani, S. I., Ruderman, N. B., Schmieder, F., and Boden, G. (2002). Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase, C, and IkappaB-alpha. Diabetes 51, 2005–2011. doi: 10.2337/diabetes.51.7.2005
Jaber, M., Koch, W. J., Rockman, H., Smith, B., Bond, R. A., Sulik, K. K., et al. (1996). Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. U.S.A. 93, 12974–12979. doi: 10.1073/pnas.93.23.12974
Jo, S. H., Leblais, V., Wang, P. H., Crow, M. T., and Xiao, R. P. (2002). Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent G(s) signaling during beta2-adrenergic stimulation. Circ. Res. 91, 46–53. doi: 10.1161/01.RES.0000024115.67561.54
Koch, W. J., Rockman, H. A., Samama, P., Hamilton, R. A., Bond, R. A., Milano, C. A., et al. (1995). Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science 268, 1350–1353. doi: 10.1126/science.7761854
Kolwicz, S. C. Jr., and Tian, R. (2009). Metabolic therapy at the crossroad: how to optimize myocardial substrate utilization? Trends Cardiovasc. Med. 19, 201–207. doi: 10.1016/j.tcm.2009.12.005
Kostis, J. B., and Sanders, M. (2005). The association of heart failure with insulin resistance and the development of type 2 diabetes. Am. J. Hypertens. 18, 731–737. doi: 10.1016/j.amjhyper.2004.11.038
Li, J., Yan, B., Huo, Z., Liu, Y., Xu, J., Sun, Y., et al. (2010). beta2- but not beta1-adrenoceptor activation modulates intracellular oxygen availability. J. Physiol. 588, 2987–2998. doi: 10.1113/jphysiol.2010.190900
Lommi, J., Kupari, M., and Yki-Jarvinen, H. (1998). Free fatty acid kinetics and oxidation in congestive heart failure. Am. J. Cardiol. 81, 45–50. doi: 10.1016/S0002-9149(97)00804-7
Maarbjerg, S. J., Sylow, L., and Richter, E. A. (2011). Current understanding of increased insulin sensitivity after exercise—emerging candidates. Acta Physiol. (Oxf.) 202, 323–335. doi: 10.1111/j.1748-1716.2011.02267.x
Mazumder, P. K., O'Neill, B. T., Roberts, M. W., Buchanan, J., Yun, U. J., Cooksey, R. C., et al. (2004). Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53, 2366–2374. doi: 10.2337/diabetes.53.9.2366
Mongillo, M., John, A. S., Leccisotti, L., Pennell, D. J., and Camici, P. G. (2007). Myocardial pre-synaptic sympathetic function correlates with glucose uptake in the failing human heart. Eur. J. Nucl. Med. Mol. Imaging 34, 1172–1177. doi: 10.1007/s00259-007-0371-z
Neubauer, S. (2007). The failing heart—an engine out of fuel. N. Engl. J. Med. 356, 1140–1151. doi: 10.1056/NEJMra063052
Neubauer, S., Krahe, T., Schindler, R., Horn, M., Hillenbrand, H., Entzeroth, C., et al. (1992). 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86, 1810–1818. doi: 10.1161/01.CIR.86.6.1810
Nevzorova, J., Bengtsson, T., Evans, B. A., and Summers, R. J. (2002). Characterization of the beta-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br. J. Pharmacol. 137, 9–18. doi: 10.1038/sj.bjp.0704845
Nevzorova, J., Evans, B. A., Bengtsson, T., and Summers, R. J. (2006). Multiple signalling pathways involved in beta2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br. J. Pharmacol. 147, 446–454. doi: 10.1038/sj.bjp.0706626
Ngala, R. A., O'Dowd, J., Wang, S. J., Agarwal, A., Stocker, C., Cawthorne, M. A., et al. (2008). Metabolic responses to BRL37344 and clenbuterol in soleus muscle and C2C12 cells via different atypical pharmacologies and beta2-adrenoceptor mechanisms. Br. J. Pharmacol. 155, 395–406. doi: 10.1038/bjp.2008.244
Nikolaidis, L. A., Poornima, I., Parikh, P., Magovern, M., Shen, Y. T., and Shannon, R. P. (2006). The effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathy. J. Am. Coll. Cardiol. 47, 1871–1881. doi: 10.1016/j.jacc.2005.11.082
Opie, L. H., and Sack, M. N. (2002). Metabolic plasticity and the promotion of cardiac protection in ischemia and ischemic preconditioning. J. Mol. Cell. Cardiol. 34, 1077–1089. doi: 10.1006/jmcc.2002.2066
Opie, L. H., Thandroyen, F. T., Muller, C., and Bricknell, O. L. (1979). Adrenaline-induced “oxygen-wastage” and enzyme release from working rat heart. Effects of calcium antagonism, beta-blockade, nicotinic acid and coronary artery ligation. J. Mol. Cell. Cardiol. 11, 1073–1094. doi: 10.1016/0022-2828(79)90395-X
Paolisso, G., De Riu, S., Marrazzo, G., Verza, M., Varricchio, M., and D'Onofrio, F. (1991). Insulin resistance and hyperinsulinemia in patients with chronic congestive heart failure. Metabolism 40, 972–977. doi: 10.1016/0026-0495(91)90075-8
Perez-Schindler, J., Philp, A., Baar, K., and Hernandez-Cascales, J. (2011). Regulation of contractility and metabolic signaling by the beta2-adrenergic receptor in rat ventricular muscle. Life Sci. 88, 892–897. doi: 10.1016/j.lfs.2011.03.020
Peterson, L. R., Herrero, P., Schechtman, K. B., Racette, S. B., Waggoner, A. D., Kisrieva-Ware, Z., et al. (2004a). Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109, 2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8
Peterson, L. R., Waggoner, A. D., Schechtman, K. B., Meyer, T., Gropler, R. J., Barzilai, B., et al. (2004b). Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue doppler imaging. J. Am. Coll. Cardiol. 43, 1399–1404. doi: 10.1016/j.jacc.2003.10.062
Philipson, L. H. (2002). beta-Agonists and metabolism. J. Allergy Clin. Immunol. 110:S313–S317. doi: 10.1067/mai.2002.129702
Sakamoto, K., and Holman, G. D. (2008). Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 295, E29–E37. doi: 10.1152/ajpendo.90331.2008
Santomauro, A. T., Boden, G., Silva, M. E., Rocha, D. M., Santos, R. F., Ursich, M. J., et al. (1999). Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48, 1836–1841. doi: 10.2337/diabetes.48.9.1836
Sasaoka, T., Wada, T., and Tsuneki, H. (2006). Lipid phosphatases as a possible therapeutic target in cases of type 2 diabetes and obesity. Pharmacol. Ther. 112, 799–809. doi: 10.1016/j.pharmthera.2006.06.001
Semeniuk, L. M., Kryski, A. J., and Severson, D. L. (2002). Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am. J. Physiol. Heart Circ. Physiol. 283, H976–H982.
Shahid, G., and Hussain, T. (2007). GRK2 negatively regulates glycogen synthesis in mouse liver FL83B cells. J. Biol. Chem. 282, 20612–20620. doi: 10.1074/jbc.M700744200
Stanley, W. C., Recchia, F. A., and Lopaschuk, G. D. (2005). Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 85, 1093–1129. doi: 10.1152/physrev.00006.2004
Taylor, M., Wallhaus, T. R., Degrado, T. R., Russell, D. C., Stanko, P., Nickles, R. J., et al. (2001). An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with congestive heart failure. J. Nucl. Med. 42, 55–62.
Usui, I., Imamura, T., Babendure, J. L., Satoh, H., Lu, J. C., Hupfeld, C. J., et al. (2005). G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol. Endocrinol. 19, 2760–2768. doi: 10.1210/me.2004-0429
Wagenmakers, A. J., van Riel, N. A., Frenneaux, M. P., and Stewart, P. M. (2006). Integration of the metabolic and cardiovascular effects of exercise. Essays Biochem. 42, 193–210. doi: 10.1042/bse0420193
Wisneski, J. A., Gertz, E. W., Neese, R. A., and Mayr, M. (1987). Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J. Clin. Invest. 79, 359–366. doi: 10.1172/JCI112820
Witteles, R. M., Tang, W. H., Jamali, A. H., Chu, J. W., Reaven, G. M., and Fowler, M. B. (2004). Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J. Am. Coll. Cardiol. 44, 78–81. doi: 10.1016/j.jacc.2004.03.037
Woo, A. Y., and Xiao, R. P. (2012). beta-Adrenergic receptor subtype signaling in heart: from bench to bedside. Acta Pharmacol. Sin. 33, 335–341. doi: 10.1038/aps.2011.201
Zhang, W., Yano, N., Deng, M., Mao, Q., Shaw, S. K., and Tseng, Y. T. (2011). beta-Adrenergic receptor-PI3K signaling crosstalk in mouse heart: elucidation of immediate downstream signaling cascades. PLoS ONE 6:e26581. doi: 10.1371/journal.pone.0026581
Zhu, W., Petrashevskaya, N., Ren, S., Zhao, A., Chakir, K., Gao, E., et al. (2011). Gi-biased beta2AR signaling links GRK2 upregulation to heart failure. Circ. Res. 110, 265–274. doi: 10.1161/CIRCRESAHA.111.253260
Zhu, W. Z., Zheng, M., Koch, W. J., Lefkowitz, R. J., Kobilka, B. K., and Xiao, R. P. (2001). Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 98, 1607–1612. doi: 10.1073/pnas.98.4.1607
Keywords: cardiac metabolism, beta adrenergic system, heart failure, GRKs, mitochondria
Citation: Ciccarelli M, Santulli G, Pascale V, Trimarco B and Iaccarino G (2013) Adrenergic receptors and metabolism: role in development of cardiovascular disease. Front. Physiol. 4:265. doi: 10.3389/fphys.2013.00265
Received: 05 August 2013; Paper pending published: 14 August 2013;
Accepted: 08 September 2013; Published online: 03 October 2013.
Edited by:
Giuseppe Rengo, Salvatore Maugeri Foundation, ItalyReviewed by:
Anastasios Lymperopoulos, Nova Southeastern University, USAWalter J. Koch, Temple University, USA
Copyright © 2013 Ciccarelli, Santulli, Pascale, Trimarco and Iaccarino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Ciccarelli, Department of Medicine and Surgery, University of Salerno, Salerno, Italy e-mail: mciccarelli@unisa.it
