- 1Department of Physical Education and Sport Sciences (DPESS), School of Physical Education (PE), University of Thessaly, Trikala, Greece
- 2Institute for Research and Technology-Centre for Research and Technology Hellas, Trikala, Greece
- 3Department of Surgery, Faculty of Medicine, University of Thessaly, Larissa, Greece
- 4Department of Nephrology, Faculty of Medicine, University of Thessaly, Larissa, Greece
Renal failure is accompanied by progressive muscle weakness and premature fatigue, in part linked to hypokinesis and in part to uremic toxicity. These changes are associated with various detrimental biochemical and morphological alterations. All of these pathological parameters are collectively termed uremic myopathy. Various interventions while helpful can't fully remedy the pathological phenotype. Complex mechanisms that stimulate muscle dysfunction in uremia have been proposed, and oxidative stress could be implicated. Skeletal muscles continuously produce reactive oxygen species (ROS) and reactive nitrogen species (RNS) at rest and more so during contraction. The aim of this mini review is to provide an update on recent advances in our understanding of how ROS and RNS generation might contribute to muscle dysfunction in uremia. Thus, a systematic review was conducted searching PubMed and Scopus by using the Cochrane and PRISMA guidelines. While few studies met our criteria their findings are discussed making reference to other available literature data. Oxidative stress can direct muscle cells into a catabolic state and chronic exposure to it leads to wasting. Moreover, redox disturbances can significantly affect force production per se. We conclude that oxidative stress can be in part responsible for some aspects of uremic myopathy. Further research is needed to discern clear mechanisms and to help efforts to counteract muscle weakness and exercise intolerance in uremic patients.
Introduction
Among the clinical entities affecting thousands of patients, chronic kidney disease (CKD) is a silent epidemic expected to influence more than 50% of the Americans born today (Grams et al., 2013) and approximately 40% of the population in Europe (Zoccali et al., 2010). Muscular weakness, muscle wasting, limited endurance, exercise intolerance, and fatigue are components of the functional and morphological abnormalities collectively termed uremic myopathy, which often also includes uremic cardiomyopathy (Campistol, 2002). While the pathogenesis of uremic myopathy is not clear, it is thought that an interplay of uremic toxicity and hypokinesis guide these abnormalities in patients with CKD and especially in end-stage renal disease (ESRD) patients undergoing hemodialysis (HD) therapy. Observations of a significant correlation between glomerular filtration rate (GFR) and exercise tolerance (e.g., Clyne et al., 1987, 1994) led to studies revealing the very low activity levels and poor functional capacity of renal patients (Kouidi et al., 1998; Johansen et al., 2003; Sakkas et al., 2003a). Moreover, various groups turned their attention to exercise and other interventions to remedy or halt muscle deterioration in pre-dialysis (e.g., Clyne et al., 1991) and dialysis patients (e.g., Sakkas et al., 2003b; Johansen et al., 2006). Despite the evident improvements in exercise capacity and muscle morphology (Sakkas et al., 2003b, 2008a), in increasing muscle mass with steroid supplementation (Topp et al., 2003), in improving sleep and overall quality of life (Sakkas et al., 2008c) it appears that interventions so far cannot restore muscle functionality in ESRD patients to the level of age-matched healthy sedentary individuals (Sakkas et al., 2003b, 2008a; Giannaki et al., 2011).
Components of Uremic Myopathy
Loss of skeletal muscle strength in renal patients, contributes to easy fatigability, and can be linked to loss of muscle fibers and atrophy of the remaining fibers (Porter et al., 1995; Sakkas et al., 2003a). In cross-sectional studies, comparing age-matched controls and end-stage patients, atrophy and loss of type IIα and IIx fibers, reduced muscle fiber capillarization and peripheral activation (Sakkas et al., 2003a), and a significant decrease in the mean diameter of both fiber types (Crowe et al., 2007) has been observed. However, not all functional consequences can be attributed to atrophy. Interventions to improve muscle mass indicate that there is a functional deficit in the existing muscle mass. Dialysis patients present with rapid and large accumulation of inorganic phosphate during submaximal exercise, lower oxidative potential, larger phosphocreatine reduction with slower recovery but also with evidence of central activation failure, all these factors contributing to early and excess fatigue (Johansen et al., 2005). Abnormal mitochondria respiratory capacity, is also a factor responsible for easily fatigability in CKD patients, as mitochondrial morphology is disturbed in patients with CKD (Kouidi et al., 1998), while alterations in respiratory chain proteins likely enhance reactive oxygen species (ROS) production which has been seen in a rat uremia model (Yazdi et al., 2013).
CKD patients, especially the end-stage ones, lead a very sedentary lifestyle. Morphological abnormalities however have been observed in both locomotory and non-locomotory muscles (Sakkas et al., 2003a) thus not all of the dysfunction can be attributed to inactivity.
Biochemical and nutritional changes occurring through the progression of CKD can stimulate protein losses and can contribute to the development of muscle wasting. This has grave significance as catabolic conditions increase the risk of morbidity and mortality (Griffiths, 1996; Gordon et al., 2007).
Pro-dialysis and dialysis patients face increasing dietary restrictions. Malnutrition is associated with hypoalbuminemia, which is inversely correlated with mortality in uremic patients (Lowrie and Lew, 1990), and it is also used as a marker of depleted protein stores (Carrero et al., 2008).
Metabolic acidosis, which is commonly associated with CKD, stimulates the breakdown of muscle proteins resulting in loss of muscle mass (Hu et al., 2013). Furthermore, the observation that insulin resistance, is common in patients with CKD, suggests that impaired insulin signaling could also contribute to protein losses (Sakkas et al., 2008b; Zhang et al., 2009). Moreover, CKD is associated with an increase in circulating levels of inflammatory cytokines. Specifically, levels of circulating IL-6, TNF-α, serum amyloid A, and C-reactive protein are increased in patients with CKD (Zhang et al., 2009, 2011; Cheung et al., 2010). Notably, it is contested that in well-dialyzed patients, circulating proinflammatory markers are the main cause for hypoalbuminemia rather than malnutrition (Kaysen et al., 2004). The possibility of an accelerated protein degradation in CKD mediated by the ubiquitin-proteasome system (UPS) (Wang and Mitch, 2013) should also be considered.
Apart from a compounded or accelerated muscle loss, a reduction in the ability to anabolize muscle could be an issue in CKD. Still, interventions with nandrolone decanoate were successful in increasing muscle mass, albeit without improving muscle strength (Topp et al., 2003), pointing to an available anabolic response. However, there are suggestions that CKD may dampen the function of satellite cells. Zhang et al. (2010) using a mouse model of CKD reported a delayed regeneration of damaged muscle and reductions in MyoD protein and the myogenin expression, indicating a decreased satellite cell proliferation and differentiation (Zhang et al., 2010).
To compound the above, in dialyzed patients, the HD procedure per se stimulates protein degradation and reduced protein synthesis with the effect persisting for 2 h following dialysis (Ikizler et al., 2002). Thus, while blunting of anabolic responses can't be excluded, a multitude of factors can promote protein loss, especially in the end-stage patients.
Is there a Role for Oxidative Stress in Uremic Muscle Dysfunction?
Oxidative stress promotes catabolic state and accelerates muscle atrophy (Moylan and Reid, 2007). But it can also affect contractility of the available muscle and sarcomeric protein expression.
Many studies have found that oxidative stress can cause long-term effects and acute effects (Lamb and Westerblad, 2011) on contractility. Long-term effects include altered gene and protein expression or damages in lipids and proteins that are irreversible, while acute effects are reversible. The decrease in Ca2+ sensitivity which contributes to muscle fatigue is considered as an acute effect of oxidative stress (Lamb and Westerblad, 2011).
A key mechanism that has been proposed to explain the ROS contribution in muscle fatigue is the reduced myofibrillar Ca2+ sensitivity and/or sarcoplasmic reticulum Ca2+ release (Allen et al., 2008). Moreover, an increase in NO during fatigue in fast twitch muscle fibers contribute in decreased myofibrillar Ca2+ sensitivity (Lamb and Westerblad, 2011). However, in slow-twitch fibers NO donors, did not affect myofibrillar Ca2+ sensitivity (Spencer and Posterino, 2009). Also, a study by Reardon and Allen (2009), showed that iron can increase ROS production at high temperature in the skeletal muscle cells, accelerating muscle fatigue.
Moreover, ROS generation can acutely affect contractile function and disturbs structural transition within the actomyosin complex which is crucial for force generation. Exposure to low or high concentrations of peroxide (5 or 50 mM) reduces maximum force and velocity of contraction, with the high peroxide resulting in irreversible loss of calcium regulation of force mediated by oxidation of methionines in the heavy and essential light chains (Prochniewicz et al., 2008a). More elegant work from same group, examining structural dynamics of actin and myosin pointed to an effect of oxidation on weak-to-strong structural transition and by using site-directed mutagenesis of Dictyostelium (Dicty) myosin II oxidation, a redistribution of existing structural states of the actin-binding cleft was implicated (Prochniewicz et al., 2008b; Klein et al., 2011). Alterations in myosin heavy chain expression in uremic animals have also been reported (Taes et al., 2004).
Many studies have observed increased levels of oxidative stress biomarkers in blood samples of CKD patients (Samouilidou and Grapsa, 2003; Filiopoulos et al., 2009). In the literature, there are sufficient studies with different technical approaches in which the activity and role of ROS and reactive nitrogen species (RNS) in skeletal muscle has been studied using both in vivo and in vitro methods in a variety of contexts (Powers et al., 2011). Thus, based on recent advances in our understanding of how ROS and RNS affect muscle function, this mini-review aimed to examine if oxidative stress can contribute to muscle dysfunction in ESRD.
Methods
A systematic review was conducted searching PubMed and Scopus by using the Cochrane and PRISMA guidelines. A comprehensive literature search was conducted from September 2014 until November 2014. We used PubMed, ScienceDirect and Scopus or Google Scholar to search for studies that investigated the relationship among (i) oxidative stress and uremic myopathy in humans, and (ii) markers of oxidative stress in the skeletal muscle of uremic patients on HD. Eligibility of the studies based on titles, abstracts and full-text articles was determined by two reviewers. Studies were selected using inclusion and exclusion criteria. We included only those studies that met the following criteria: they assessed oxidative stress markers in the skeletal muscle of patients on HD; they used human biopsies; they addressed randomized control trials, controlled trials, or clinical trials designed to evaluate oxidative stress in skeletal muscle in uremic patients on HD therapy; they were written in English.
Results and Discussion
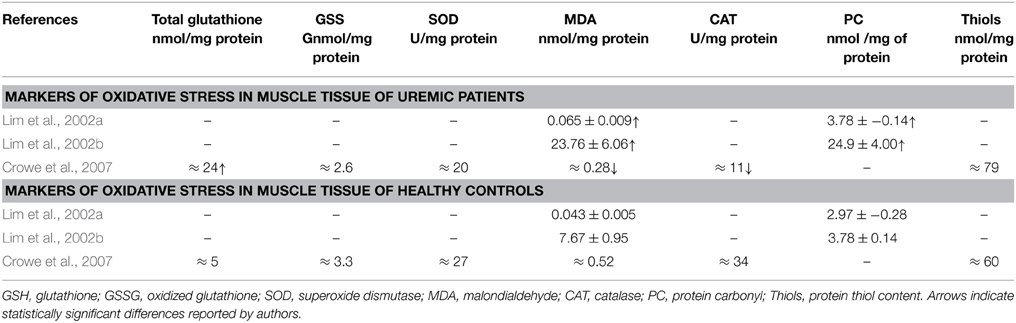
Only three studies have examined the oxidative damage in human skeletal muscle of uremic patients on HD (Table 1). Their findings are discussed with reference to renal human blood findings and/or animal muscle findings either models of CKD or models of other conditions.
Lim et al. (2002a) found increased malondialdehyde (MDA) and protein carbonyls (PC) levels reflecting extensive oxidative damage to total protein content and lipids, in muscle suggested by the authors to be due to increased levels of inflammatory cytokines and to increased protein degradation. Increased levels of lipid peroxidation in blood samples of CKD patients during HD treatment has also been found elsewhere (Varan et al., 2010) and could enhance the susceptibility of LDL oxidation which is a major contributor in the genesis of atherosclerosis. The above observations, together with animal findings in the role of carbonyl stress in vascular injury (Chen et al., 2013) concur to a role of protein oxidation in long-term vascular damage which could impact overall vessel functionality and thus striated muscle's bioenergetics and function.
The same group also reported increased mitochondrial protein and lipid oxidative damage in skeletal muscle of uremic patients compared to age-matched controls (Lim et al., 2002b). The authors also reported mitochondrial DNA mutations, and overall oxidative damage to total cellular DNA, supporting a notion of attenuating regenerative and bioenergetics capacities of the skeletal muscles of renal patients. Also, mitochondrial DNA deletions have been observed in the skeletal muscle of ESRD patients similar with these found in the skeletal muscle of elderly subjects due to oxidative damage which probably contribute to the impaired mitochondrial energy metabolism that characterizes uremic patients (Lim et al., 2000).
It is considered that mitochondrial membranes are more likely to develop oxidative damage due to the relatively high amounts of lipid containing polyunsaturated fatty acids that they possess (Laganiere and Yu, 1993) and this would explain their increased oxidative damage. Given that mitochondria are considered the predominant source of ROS in muscle fibers (Davies et al., 1982; McArdle et al., 2001; Jackson, 2009), due to the elevated oxygen consumption that occurs with increased mitochondrial activity, especially during exercise (Powers et al., 2011) it is conceivable that damage to the mitochondria membrane might further compound their function as a ROS source causing more leaking. Moreover, it has been suggested that mitochondrial ROS leaking depends on fiber type both at resting basal respiration and at an increased respiration (as in exercise). In an animal saponin-treated muscle study of mitochondrial respiration, type IIb skeletal muscle fibers showed significantly higher free radical leaking compared to type IIa and I fibers at basal respiration (Anderson and Neufer, 2006).
If indeed the ROS load in the late stages renal skeletal muscle is high, that might in part explain the higher susceptibility of type II fibers to atrophy observed in end stage patients, where not only generalized muscle atrophy was observed but more prominent atrophy was seen in type II (especially IIx) vs type I fibers either in non-locomotory (Sakkas et al., 2003a) or locomotory muscle samples. Furthermore, on the possible role of the mitochondrial dysfunction in renal muscle atrophy it should be noted that de-innervation studies show that denervated muscle mitochondria release fatty acid hydro peroxides, mediated by calcium dependent phospholipase A2 (Bhattacharya et al., 2009). Such observations together with observations of an increased sensitivity of human aged mitochondria to apoptosis (Gouspillou et al., 2014), and the findings of Lim et al. (2002b) reviewed above, can collectively substantiate an important role for mitochondrial dysfunction, in the pathogenesis of uremic myopathy.
In contrast to the findings of Lim et al. and Crowe et al. (2007), reported decreased MDA content, increased total glutathione and no change in protein thiols content, superoxide dismutase (SOD), oxidized glutathione (GSSG) and catalase activity and concluded that there is no evident connection between oxidative stress and muscle atrophy in uremia. However, if one considers the age difference in the subjects of the conflicting studies one cannot discount the possibility that the much younger subjects, which also had spent less time on dialysis, of the Crowe et al. study had better preserved mitochondrial status. Adaptation of various antioxidant mechanisms can also explain some of the above differences. It should also be noted that in transgenic mice studies, the model overexpressing phospholipid hydroperoxide glutathione peroxidase, Gpx4-Tg (which is associated with mitochondrial and other membranes) protected against denervation atrophy and not the manganese superoxide dismutase (Sod2-Tg) or copper-zinc superoxide dismutase (Sod1-Tg) models, suggesting that the release of fatty acid hydroperoxides from mitochondria may be a more important factor in denervation-induced atrophy than superoxide and hydrogen peroxide (Bhattacharya et al., 2009).
The above discourse further highlights the difficulty faced by researchers in renal patient studies. Many confounding factors such as years in dialysis, nutrition, physical activity levels, level of treatment, and comorbidities can affect muscle status and accelerate or decelerate disease and aging effects (Figure 1).
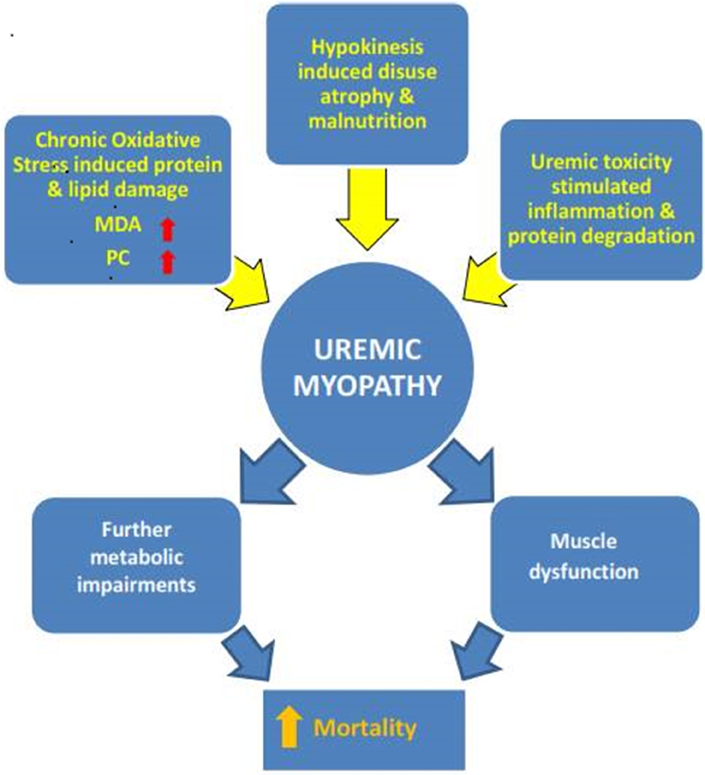
Figure 1. The multifactorial nature of uremic myopathy. Many specific disease-related but also lifestyle factors (e.g., physical inactivity) contribute to the pathological muscle state. Exactly when one factor reaches critical importance cannot be surmised so far. The results of this systematic mini review do point to oxidative stress as a contributor to the development of uremic myopathy. MDA, malondialdehyde; PC, protein carbonyls.
Conclusions
CKD has a high and increasing prevalence not only in the old retirees but also in the middle-aged Europeans (Zoccali et al., 2010). Skeletal muscle dysfunction is a ubiquitous finding in CKD patients on advanced stages of the disease. The impact is grave as the statistics are implacable. Muscle loss and weakness contribute to the high morbidity and mortality of these patients, especially at the end-stage renal failure. Many specific disease-related but also lifestyle factors (such as physical inactivity) can be seen as contributors to the pathological muscle state. Exactly when one factor reaches critical importance cannot be surmised so far. The few studies meeting our search criteria while not agreeing, do point to a possibly important role for oxidative stress in uremic myopathy. It is not known if hypothesized oxidative stress mediated effects on muscle function are more of an acute or a chronic nature. In vitro studies however show clearly that oxidative stress does have a role whether via chronic, protein and other modifications or acute contractility effects. If anything the three studies and the peripheral literature highlight the need for a systematic study of the disease mechanisms affecting skeletal muscle performance in renal disease.
As long as great unknowns remain on the mechanisms and modulation of uremic myopathy, which leads to debilitation and premature death, progress in the management of this new epidemic is the least slowed down. We suggest that more muscle research, human and animal, should be done on pro-dialysis stages including work on the role of oxidative stress. This would allow researchers to decipher early changes, and perhaps identify susceptible individuals for accelerated muscle loss, before moving into the end-stage situation which on its own has detrimental effects on muscle status.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Educational and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Thales (MuscleFun Project-MIS 377260) Investing in knowledge society through the European Social Fund.
References
Allen, D. G., Lamb, G. D., and Westerblad, H. (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88, 287–332. doi: 10.1152/physrev.00015.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anderson, E. J., and Neufer, P. D. (2006). Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am. J. Physiol. Cell Physiol. 290, C844–C851. doi: 10.1152/ajpcell.00402.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bhattacharya, A., Muller, F. L., Liu, Y., Sabia, M., Liang, H., Song, W., et al. (2009). Denervation induces cytosolic phospholipase A2-mediated fatty acid hydroperoxide generation by muscle mitochondria. J. Biol. Chem. 284, 46–55. doi: 10.1074/jbc.M806311200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Campistol, J. M. (2002). Uremic myopathy. Kidney Int. 62, 1901–1913. doi: 10.1046/j.1523-1755.2002.00614.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carrero, J. J., Chmielewski, M., Axelsson, J., Snaedal, S., Heimburger, O., Barany, P., et al. (2008). Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin. Nutr. 27, 557–564. doi: 10.1016/j.clnu.2008.04.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chen, X., Mori, T., Guo, Q., Hu, C., Ohsaki, Y., Yoneki, Y., et al. (2013). Carbonyl stress induces hypertension and cardio-renal vascular injury in Dahl salt-sensitive rats. Hypertens. Res. 36, 361–367. doi: 10.1038/hr.2012.204
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheung, W. W., Paik, K. H., and Mak, R. H. (2010). Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 25, 711–724. doi: 10.1007/s00467-009-1427-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clyne, N., Ekholm, J., Jogestrand, T., Lins, L. E., and Pehrsson, S. K. (1991). Effects of exercise training in predialytic uremic patients. Nephron 59, 84–89. doi: 10.1159/000186524
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clyne, N., Jogestrand, T., Lins, L. E., and Pehrsson, S. K. (1994). Progressive decline in renal function induces a gradual decrease in total hemoglobin and exercise capacity. Nephron 67, 322–326. doi: 10.1159/000187987
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clyne, N., Jogestrand, T., Lins, L. E., Pehrsson, S. K., and Ekelund, L. G. (1987). Factors limiting physical working capacity in predialytic uraemic patients. Acta Med. Scand. 222, 183–190. doi: 10.1111/j.0954-6820.1987.tb10657.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Crowe, A. V., McArdle, A., McArdle, F., Pattwell, D. M., Bell, G. M., Kemp, G. J., et al. (2007). Markers of oxidative stress in the skeletal muscle of patients on haemodialysis. Nephrol. Dial. Transplant. 22, 1177–1183. doi: 10.1093/ndt/gfl721
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Davies, K. J., Quintanilha, A. T., Brooks, G. A., and Packer, L. (1982). Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 107, 1198–1205. doi: 10.1016/S0006-291X(82)80124-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Filiopoulos, V., Hadjiyannakos, D., Takouli, L., Metaxaki, P., Sideris, V., and Vlassopoulos, D. (2009). Inflammation and oxidative stress in end-stage renal disease patients treated with hemodialysis or peritoneal dialysis. Int. J. Artif. Organs 32, 872–882.
Giannaki, C. D., Stefanidis, I., Karatzaferi, C., Liakos, N., Roka, V., Ntente, I., et al. (2011). The effect of prolonged intradialytic exercise in hemodialysis efficiency indices. ASAIO J. 57, 213–218. doi: 10.1097/MAT.0b013e318215dc9e
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gordon, P. L., Sakkas, G. K., Doyle, J. W., Shubert, T., and Johansen, K. L. (2007). Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Ren. Nutr. 17, 397–407. doi: 10.1053/j.jrn.2007.06.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gouspillou, G., Bourdel-Marchasson, I., Rouland, R., Calmettes, G., Biran, M., Deschodt-Arsac, V., et al. (2014). Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 13, 39–48. doi: 10.1111/acel.12147
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grams, M. E., Chow, E. K. H., Segev, D. L., and Coresh, J. (2013). Lifetime Incidence of CKD Stages 3-5 in the United States. Am. J. Kidney Dis. 62, 245–252. doi: 10.1053/j.ajkd.2013.03.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Griffiths, R. D. (1996). Muscle mass, survival, and the elderly ICU patient. Nutrition 12, 456–458. doi: 10.1016/S0899-9007(96)00141-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hu, W. T., Shelnutt, M., Wilson, A., Yarab, N., Kelly, C., Grossman, M., et al. (2013). Behavior Matters-Cognitive Predictors of Survival in Amyotrophic Lateral Sclerosis. PLoS ONE 8:e57584. doi: 10.1371/journal.pone.0057584
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ikizler, T. A., Pupim, L. B., Brouillette, J. R., Levenhagen, D. K., Farmer, K., Hakim, R. M., et al. (2002). Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 282, E107–E116.
Jackson, M. J. (2009). Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic. Biol. Med. 47, 1267–1275. doi: 10.1016/j.freeradbiomed.2009.09.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johansen, K. L., Doyle, J., Sakkas, G. K., and Kent-Braun, J. A. (2005). Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R805–R813. doi: 10.1152/ajpregu.00187.2005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johansen, K. L., Painter, P. L., Sakkas, G. K., Gordon, P., Doyle, J., and Shubert, T. (2006). Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J. Am. Soc. Nephrol. 17, 2307–2314. doi: 10.1681/ASN.2006010034
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johansen, K. L., Shubert, T., Doyle, J., Soher, B., Sakkas, G. K., and Kent-Braun, J. A. (2003). Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 63, 291–297. doi: 10.1046/j.1523-1755.2003.00704.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaysen, G. A., Dubin, J. A., Muller, H. G., Rosales, L., Levin, N. W., and Mitch, W. E. (2004). Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int. 65, 1408–1415. doi: 10.1111/j.1523-1755.2004.00520.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Klein, J. C., Moen, R. J., Smith, E. A., Titus, M. A., and Thomas, D. D. (2011). Structural and functional impact of site-directed methionine oxidation in myosin. Biochemistry 50, 10318–10327. doi: 10.1021/bi201279u
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kouidi, E., Albani, M., Natsis, K., Megalopoulos, A., Gigis, P., Guiba-Tziampiri, O., et al. (1998). The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol. Dial. Transplant. 13, 685–699. doi: 10.1093/ndt/13.3.685
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Laganiere, S., and Yu, B. P. (1993). Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology 39, 7–18. doi: 10.1159/000213509
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lamb, G. D., and Westerblad, H. (2011). Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 589, 2119–2127. doi: 10.1113/jphysiol.2010.199059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, P. S., Cheng, Y. M., and Wei, Y. H. (2000). Large-scale mitochondrial DNA deletions in skeletal muscle of patients with end-stage renal disease. Free Radic. Biol. Med. 29, 454–463. doi: 10.1016/S0891-5849(00)00334-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, P. S., Cheng, Y. M., and Wei, Y. H. (2002a). Increase in oxidative damage to lipids and proteins in skeletal muscle of uremic patients. Free Radic. Res. 36, 295–301. doi: 10.1080/10715760290019318
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, P. S., Ma, Y. S., Cheng, Y. M., Chai, H., Lee, C. F., Chen, T. L., et al. (2002b). Mitochondrial DNA mutations and oxidative damage in skeletal muscle of patients with chronic uremia. J. Biomed. Sci. 9, 549–560. doi: 10.1007/BF02254982
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lowrie, E. G., and Lew, N. L. (1990). Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am. J. Kidney Dis. 15, 458–482. doi: 10.1016/S0272-6386(12)70364-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McArdle, A., Pattwell, D., Vasilaki, A., Griffiths, R. D., and Jackson, M. J. (2001). Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am. J. Physiol. Cell Physiol. 280, C621–C627.
Moylan, J. S., and Reid, M. B. (2007). Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35, 411–429. doi: 10.1002/mus.20743
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Porter, M. M., Vandervoort, A. A., and Lexell, J. (1995). Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sci. Sports 5, 129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Powers, S. K., Ji, L. L., Kavazis, A. N., and Jackson, M. J. (2011). Reactive oxygen species: impact on skeletal muscle. Compr. Physiol. 1, 941–969. doi: 10.1002/cphy.c100054
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prochniewicz, E., Lowe, D. A., Spakowicz, D. J., Higgins, L., O'conor, K., Thompson, L. V., et al. (2008a). Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 294, C613–C626. doi: 10.1152/ajpcell.00232.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prochniewicz, E., Spakowicz, D., and Thomas, D. D. (2008b). Changes in actin structural transitions associated with oxidative inhibition of muscle contraction. Biochemistry 47, 11811–11817. doi: 10.1021/bi801080x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reardon, T. F., and Allen, D. G. (2009). Time to fatigue is increased in mouse muscle at 37 degrees C; the role of iron and reactive oxygen species. J. Physiol. 587, 4705–4716. doi: 10.1113/jphysiol.2009.173005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakkas, G. K., Ball, D., Mercer, T. H., Sargeant, A. J., Tolfrey, K., and Naish, P. F. (2003a). Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol. Dial. Transplant. 18, 2074–2081. doi: 10.1093/ndt/gfg325
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakkas, G. K., Hadjigeorgiou, G. M., Karatzaferi, C., Maridaki, M. D., Giannaki, C. D., Mertens, P. R., et al. (2008a). Intradialytic aerobic exercise training ameliorates symptoms of restless legs syndrome and improves functional capacity in patients on hemodialysis. ASAIO J. 54, 185–190. doi: 10.1097/MAT.0b013e3181641b07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakkas, G. K., Karatzaferi, C., Zintzaras, E., Giannaki, C. D., Liakopoulos, V., Lavdas, E., et al. (2008b). Liver fat, visceral adiposity, and sleep disturbances contribute to the development of insulin resistance and glucose intolerance in nondiabetic dialysis patients. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1721–R1729. doi: 10.1152/ajpregu.00935.2007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakkas, G. K., Liakopoulos, V., Karatzaferi, C., and Stefanidis, I. (2008c). Sleep quality and dialysis efficacy affect functional capacity in patients receiving haemodialysis therapy. Nephrol. Dial. Transplant. 23, 2703–2704. doi: 10.1093/ndt/gfn048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sakkas, G. K., Sargeant, A. J., Mercer, T. H., Ball, D., Koufaki, P., Karatzaferi, C., et al. (2003b). Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol. Dial. Transplant. 18, 1854–1861. doi: 10.1093/ndt/gfg237
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Samouilidou, E., and Grapsa, E. (2003). Effect of dialysis on plasma total antioxidant capacity and lipid peroxidation products in patients with end-stage renal failure. Blood Purif. 21, 209–212. doi: 10.1159/000070691
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Spencer, T., and Posterino, G. S. (2009). Sequential effects of GSNO and H2O2 on the Ca2+ sensitivity of the contractile apparatus of fast- and slow-twitch skeletal muscle fibers from the rat. Am. J. Physiol. Cell Physiol. 296, C1015–C1023. doi: 10.1152/ajpcell.00251.2008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Taes, Y. E. C., Speeckaert, M., Bauwens, E., De Buyzere, M. R., Libbrecht, J., Lameire, N. H., et al. (2004). Effect of dietary creatine on skeletal muscle myosin heavy chain isoform expression in an animal model of uremia. Nephron Exp. Nephrol. 96, E103–E110. doi: 10.1159/000077376
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Topp, K. S., Painter, P. L., Walcott, S., Krasnoff, J. B., Adey, D., Sakkas, G. K., et al. (2003). Alterations in skeletal muscle structure are minimized with steroid withdrawal after renal transplantation. Transplantation 76, 667–673. doi: 10.1097/01.TP.0000076096.45542.1B
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Varan, H. I., Dursun, B., Dursun, E., Ozben, T., and Suleymanlar, G. (2010). Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: comparison of two dialysis membranes. Int. J. Nephrol. Renovasc. Dis. 3, 39–45.
Wang, X. H., and Mitch, W. E. (2013). Muscle wasting from kidney failure-a model for catabolic conditions. Int. J. Biochem. Cell Biol. 45, 2230–2238. doi: 10.1016/j.biocel.2013.06.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yazdi, P. G., Moradi, H., Yang, J. Y., Wang, P. H., and Vaziri, N. D. (2013). Skeletal muscle mitochondrial depletion and dysfunction in chronic kidney disease. Int. J. Clin. Exp. Med. 6, 532–539.
Zhang, L., Du, J., Hu, Z., Han, G., Delafontaine, P., Garcia, G., et al. (2009). IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 20, 604–612. doi: 10.1681/ASN.2008060628
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, L., Rajan, V., Lin, E., Hu, Z., Han, H. Q., Zhou, X., et al. (2011). Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 25, 1653–1663. doi: 10.1096/fj.10-176917
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, L., Wang, X. H., Wang, H., Du, J., and Mitch, W. E. (2010). Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 21, 419–427. doi: 10.1681/ASN.2009060571
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zoccali, C., Kramer, A., and Jager, K. J. (2010). Epidemiology of CKD in Europe: an uncertain scenario. Nephrol. Dial. Transplant. 25, 1731–1733. doi: 10.1093/ndt/gfq250
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: oxidative stress, uremia, muscle dysfunction, uremic myopathy, premature fatigue, muscle weakness
Citation: Kaltsatou A, Sakkas GK, Poulianiti KP, Koutedakis Y, Tepetes K, Christodoulidis G, Stefanidis I and Karatzaferi C (2015) Uremic myopathy: is oxidative stress implicated in muscle dysfunction in uremia? Front. Physiol. 6:102. doi: 10.3389/fphys.2015.00102
Received: 01 December 2014; Accepted: 13 March 2015;
Published: 30 March 2015.
Edited by:
Brian McDonagh, University of Liverpool, UKReviewed by:
Laszlo Csernoch, University of Debrecen, HungaryDavid Sheehan, University College Cork, Ireland
Brian McDonagh, University of Liverpool, UK
Copyright © 2015 Kaltsatou, Sakkas, Poulianiti, Koutedakis, Tepetes, Christodoulidis, Stefanidis and Karatzaferi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christina Karatzaferi, Muscle Physiology and Mechanics Group, Department of Physical Education and Sport Sciences (DPESS), School of Physical Education (PE), University of Thessaly, Tmima Epistimis Fysikis Agogis kai Athlitismou - Panepistimio Thessalias, Karyes 42100, Trikala, Thessaly, Greece ck@pe.uth.gr
 Antonia Kaltsatou
Antonia Kaltsatou Giorgos K. Sakkas
Giorgos K. Sakkas Konstantina P. Poulianiti
Konstantina P. Poulianiti Yiannis Koutedakis1
Yiannis Koutedakis1 Christina Karatzaferi
Christina Karatzaferi