- 1Institute of Biomedical Problems, Russian Academy of Sciences, Moscow, Russia
- 2Faculty of Fundamental Medicine, Lomonosov Moscow State University, Moscow, Russia
The main focus of the current review is the nitric oxide (NO)-mediated signaling mechanism in unloaded skeletal. Review of the published data describing muscles during physical activity and inactivity demonstrates that NO is an essential trigger of signaling processes, which leads to structural and metabolic changes of the muscle fibers. The experiments with modulation of NO-synthase (NOS) activity during muscle unloading demonstrate the ability of an activated enzyme to stabilize degradation processes and prevent development of muscle atrophy. Various forms of muscle mechanical activity, i.e., plantar afferent stimulation, resistive exercise and passive chronic stretch increase the content of neural NOS (nNOS) and thus may facilitate an increase in NO production. Recent studies demonstrate that NO-synthase participates in the regulation of protein and energy metabolism in skeletal muscle by fine-tuning and stabilizing complex signaling systems which regulate protein synthesis and degradation in the fibers of inactive muscle.
Recent studies have highlighted a critical role of NO in the complex signaling regulation in functional muscle fibers. In the present review we summarize role of NO and NOS in anabolic and catabolic signaling processes in skeletal muscle under conditions of elevated and decreased contractile activity.
NO interacts with cell components using three main mechanisms. First, NO reacts with molecular oxygen and superoxide anions, producing a number of low-molecular NO derivatives (Nox) which possess redox activity and are capable of participation in electron transportation reactions (Stamler et al., 1992). The reaction between NO and superoxide anions leads to the production of peroxynitrites, the most reactive free-radical molecules in biological systems which are often used as indicators of oxidative stress (Freeman, 1994). Skeletal muscle produces small amounts of NO and superoxide anions under normal physiological conditions. Secondly, NO derivatives react with metal-transporters such as haem iron and iron-sulfide centers producing NO-metal complexes. NO can also regulate the function of metalloproteins. For example, the NO-haem bond increases soluble guanylate cyclase activity in muscle, raising the concentration of cyclic guanosine monophosphate (cGMP) (Kobzik et al., 1994). Third, reduced thiols are the main targets of NO. NO reacts with thiols of proteins (RSH, RS−) through S-nitrosylation. Thus, S-nitrosylation of glutathione and other non-regulatory thiols represents another mechanism of NO buffering by the cells. This reaction can also regulate protein function through conformational changes by increasing disulfide formation, or through reacting with the nearest centers containing metal atoms (Stamler, 1994). Activation of the two main NO-mediated signaling processes depends on guanylate cyclase activation and protein S-nitrosylation.
Isoforms of NOS and Regulation of nNOS Activity
Synthesis of NO is catalyzed by NOS. Three NOS isoforms are known: type I or neuronal NOS (nNOS) is continuously expressed in different neuronal structures and in muscles; type II is inducible NOS (iNOS) which usually is not present in muscle cells; type III endothelial NOS (eNOS) is expressed in endothelial cells (Lancaster and Hibbs, 1990; Forstermann et al., 1994). nNOS and eNOS are calcium-dependent, iNOS is calcium-independent and is transcriptionally regulated by different cytokines (Hevel et al., 1991; Stuehr et al., 1991). nNOS and eNOS are regulated by intracellular Ca2+ levels through Ca2+-dependent binding of NOS to the calcium-calmodulin complex (CaM). nNOS and eNOS initiate activation at a Ca2+ concentration of about 100 nmol/L, and are fully activated at 500 nmol/L of Ca2+ (Förstermann et al., 1990, 1991; Pollock et al., 1991; Schmidt et al., 1991).
Several isoforms/splice-variants of nNOS are known. nNOSμ is the most well-studied splice variant of nNOS in rat skeletal muscle (Alderton et al., 2001). The main bulk of NO is produced by the nNOSμ isoform in skeletal muscle. It is localized to the subsarcolemmal zone and is associated with dystrophin-sarcoglican complex of cytoskeletal proteins. nNOSμ can also be associated with the cytoskeletal complex at the nuclear membrane (Aquilano et al., 2014). nNOS activity, i.e., its ability to produce NO, is regulated by phosphorylation on the serine residue by insulin-activated protein kinases (Hinchee-Rodriguez et al., 2013) or AMP-dependent protein kinase (AMPK) (Chen et al., 2000). A decrease in NO production is, most likely, caused by calpain-dependent degradation of nNOS or its export out of the subsarcolemmal complex (Lainé and de Montellano, 1998; Crosbie et al., 2002). Heat shock protein 90 (HSP90) plays a critical role in nNOS activation and stability (Bender et al., 1999; Kone et al., 2003; Piech et al., 2003; Papapetropoulos et al., 2004). HSP90 regulates nNOS activity through positive allosteric modulation, leading to the formation of active nNOS conformation or by an increase in nNOS affinity for the calcium-calmodulin complex (CaM) (Song et al., 2001). It was previously reported that nNOS interaction with HSP90 regulates insertion of a haem-group (Minami et al., 1994; Billecke et al., 2002). Blockade of HSP90 causes inhibition of nNOS activity and generation of monomer haem-deficit nNOS which undergoes rapid proteasome degradation (Osawa et al., 2003; Dunbar et al., 2004). Moreover, HSP90 regulates degradation of nNOS by calpain (Averna et al., 2007). NOS substrate, the precursor of NO—L-arginine is reported to be the NOS activator while L-NAME (N-nitro-L-arginine methyl ester hydrochloride) is its competitive inhibitor. Both of these compounds regulate nNOS and eNOS isoforms expressed in skeletal muscle.
Regulation of the Expression and Localization of Neuronal NOS in Skeletal Muscle
nNOS is expressed in skeletal muscle fibers and in the axons of motoneurons (Nakane et al., 1993). Immunohistochemistry reveals higher concentrations of nNOS in fast, when compared with slow skeletal muscle fibers (Kobzik et al., 1994). nNOS is usually located near the sarcolemma, and its content is very high at the neuromuscular junctions (Brenman et al., 1996). However, it can also be found in the cytoplasm of muscle fibers as an unbound molecule. In the sarcolemmal zone, nNOS is associated with α1-syntrophin of the dystrophin-sarcoglycan complex (Brenman et al., 1995; Wakayama et al., 1997). This association depends on the presence of thePDZ domain of syntrophin (Adams et al., 2001). Sarcolemmal localization of several other proteins such as aquaporin-4 and mechano-sensitive calcium channel TRPC-1 also depends on the presence of the PDZ domain of syntrophin (Adams et al., 2001; Vandebrouck et al., 2007). nNOSμ, bound to the dystrophin-sarcoglycan complex, was also found inside the inner membrane of the myonuclear envelope (Aquilano et al., 2014).
Function of NO in a Contracting Muscle
During increased muscle contractile activity NO production in muscle (Perez et al., 2002; Vassilakopoulos et al., 2003) as well as NO content in blood (with verified muscle origin) is increased (Perez et al., 2002). Rue and colleagues (2007) reported a 48% increase in the NO content during contraction of a single muscle fiber (Pye et al., 2007). This increase of NO content was not observed in the presence of L-NAME, a NOS inhibitor. Zhang et al. (2004) found, that NO is produced in C2C12 skeletal muscle cells during static or dynamic stretching (Zhang et al., 2004). Repeated physical exercises lead to elevated nNOS expression (Tatchum-Talom et al., 2000; McConell et al., 2007). However, the nature of the mechano-dependent nNOS activation still remains unknown. The signaling role of NO during increased muscles contractile activity has not been well studied. L-NAME (NOS inhibitor) prevented a fast-to-slow fiber type transition during chronic electrostimulation of muscles in rats (Martins et al., 2012). This suggests the existence of synergetic effects of NO and calcineurin/NFAT signaling pathways on the regulation of myosin heavy chain expression. Indeed, the authors demonstrated that NO suppresses GSK3β via activation of the guanylate cyclase pathway and that this prevents nuclear export of NFATc1. As a result, increased NO production in the contracting muscle promotes a high level of expression of slow myosin heavy chain isoform.
NO is known to stimulate AMPK (5′-AMP-dependent protein kinase) in skeletal muscle. This increases energy supplies in muscle during aerobic exercise; including biogenesis of mitochondria, activation of β-oxidation of fatty acids, as well as insulin-dependent and insulin-independent glucose transport (Lira et al., 2007, 2010; Deshmukh et al., 2010). Moreover, similar to AMPK, NO is an effective inhibitor of histone deacetylases (Watson and Riccio, 2009) promoting expression of many muscle-specific proteins critical for the contractile activity.
Roles of NO in Activity-Dependent Muscle Hypertrophy
Most of scientists support the idea that two known mechanisms underlying development of activity-dependent muscle hypertrophy are: (1) activation of translation mediated by the protein kinase complex mTORC1, and (2) activation of satellite cell proliferation followed by their differentiation and fusion with neighboring muscle fibers, increasing the number of myonuclei (Bodine, 2006; Bruusgaard et al., 2010). The role of NOS activity in activity-dependent muscle hypertrophy is supported by the muscle compensatory loading in synergist removal experiments. L-NAME administration decreases the level of muscle hypertrophy in these experiments (Zhang et al., 2004). A complete block of the increase in slow myosin heavy chains and α-actin expression was also observed under these conditions (Sellman et al., 2006).
Increased NO production was recently shown to activate signaling pathways promoting mTORC1 activity (Ito et al., 2013). According to the data obtained by several research groups, NOS activation during muscle stretching or resisting exercise initiates proliferation of G0-satellite cells (Anderson and Wozniak, 2004; Yamada et al., 2006, 2008; Tatsumi, 2010). Released NO passes through the sarcolemma, and activates hepatocyte growth factor (HGF) through the activity of matrix metalloproteinases. Activated HGF interacts with c-met receptors of satellite cells, promoting their entrance into the cell cycle. Therefore, during resistance exercises NO works as an activator and as a regulator of signaling, and sometimes as a trigger of muscle hypertrophy.
Regulation of the Calpain-Mediated Cytoskeletal Proteolysis
Michetti et al. (1995) described an inhibition of proteolytic activity of μ-calpain of skeletal muscle by iNOS in vitro. NO can also prevent multiple effects of calcium ionophores on C2C12 muscle cells, which include degradation of vinculin, intercellular junction proteins, and decrease of total protein content. These effects of NO are mediated by the inhibition of μ-calpain activity via the cysteine S-nytrosylation. Experiments using cell cultures showed that NO also regulates calpain-dependent degradation of cytoskeletal proteins (Koh and Tidball, 2000). Zhang et al. (2004) reproduced these experiments with some modifications. They found that 10% stretching in C2C12 cells increases NO content and nNOS activity, increased synthesis of talin, vinculin, and desmin, and to concomitant fiber stiffness (Zhang et al., 2004). These effects are enhanced by the administration NO precursor L-arginine and by calpain inhibitors, and are blocked by the NO-synthase inhibitor L-NAME. In summary, these data suggest the existence of a significant crosstalk between NOS activity and NO production on one side, and the status of cytoskeletal proteins on the other.
Gravitational Unloading of Skeletal Muscle: NO Production, nNOS Content and Localization
Experiments using rat tail suspension (Tidball et al., 1998; Suzuki et al., 2007), prolonged bed-rest or immersion of human volunteers (Moukhina et al., 2004; Rudnick et al., 2004) showed that total nNOS content is decreased during gravitational unloading. A decrease of nNOS content in muscle fibers also was observed in mice subjected to weightlessness onboard the International Space Station during a 90-day flight (Sandonà et al., 2012). nNOS mRNA expression was also decreased during simulated gravitational unloading (Lomonosova et al., 2011). We previously reported a decrease of NO levels in rat soleus muscle after 2 weeks of unloading (Lomonosova et al., 2011). This result is in a disagreement with the data from Suzuki et al. (2007) who demonstrated increases in NO in combination with a pronounced decrease of total nNOS content in mouse soleus muscle after 14 days of tail suspension. At the same time, levels of NO in nNOS knockout mice and in mice treated with nNOS inhibitor were the same as in control wild type mice (Suzuki et al., 2007). Suzuki and colleagues explained the increase of NO concentration in muscle fibers in their experiments by a translocation of NOS from the membrane to the cytoplasm. This phenomenon was observed on multiple occasions, but currently there are no published data documenting higher NO production by non-membrane-bound NOS. Since both studies (Suzuki et al., 2007; Lomonosova et al., 2011) used the same experimental approach measuring of NO levels the observed discrepancies need further examination.
Sarcolemmal nNOS content was recently shown to be decreased during gravitational unloading (Sandonà et al., 2012). This was associated with protein degradation and with translocation of a part of the nNOS to the cytosolic compartment (Suzuki et al., 2007; Sandonà et al., 2012; Sun et al., 2013; Liu et al., 2014). Lawler and colleagues demonstrated, that administration of EUK-134 blocked nNOS dissociation from the sarcolemma and its translocation to the cytosolic compartment during 54-h unloading experiments (Lawler et al., 2014). This protected pFOXO3a (phospho-Forkhead transcription factor) from de-phosphorylation, and as a result prevented atrophy of soleus muscle and slow-to-fast shift in muscle fiber type (Lawler et al., 2014). These effects could be attributed to prevention of the decrease of total nNOS content due to the changes in nNOS activity related to its release from the sarcolemma. Moreover, nNOS is translocated not only to the cytoplasm, but also to the nucleus along with α-syntrophin (Aquilano et al., 2014). It was noted that altered nNOS splicing and nuclear localization could be a contributing factor in pathology of muscular diseases in humans. Data from Blottner's group demonstrated that after prolonged hypokinesia, not only did nNOS content decrease but also total levels of protein nitrosylation were diminished in human soleus and vastus lateralis muscles (Salanova et al., 2008).
Gravitational Unloading of Skeletal Muscle: Effects of NO on Degradation of Cytoskeletal Proteins
Unloading leads to the destruction of cytoskeletal and contractile proteins. These changes are usually attributed to increased activity of calcium-dependent proteases calpains. Increased concentration of calcium ions in the sarcoplasm was recently demonstrated at the early stages of gravitational unloading (Ingalls et al., 1999; Shenkman and Nemirovskaya, 2008) along with increased expression of μ-calpain and decreased expression of calpostatin, an endogenous inhibitor of calpains (Ma et al., 2011; Lomonosova et al., 2012b). Yet, Tidball's group (Samengo et al., 2012) found sufficient content of calpain in muscle fibers of young mice to support active proteolysis. In their experiments calpain molecules in muscles of young mice were nitrosylated and did not possess their typical level of proteolytic activity. To the contrary, old animals had lowered levels of nitrosylation, correlated with increased calpain proteolytic activity and decreased content of cytoskeletal proteins. The described experiments clarify possible mechanisms of calpain activation during conditions of decreased nNOS activity.
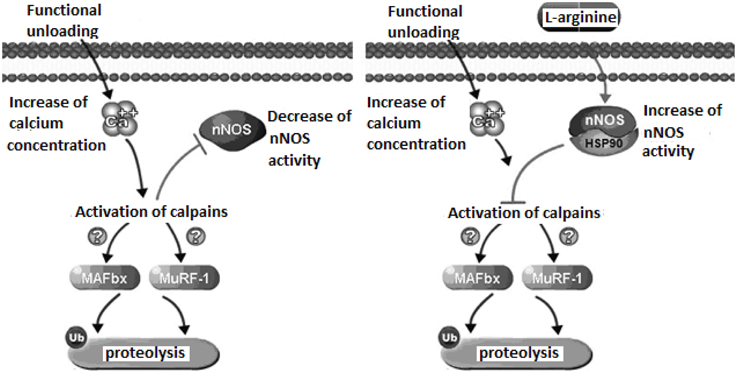
Our experiments with administration of the natural NO precursor L-arginine demonstrated prevention of calpain-dependent hydrolysis of cytoskeletal proteins during unloading. In Figure 1, we presented the proposed mechanism of nNOS-induced effects on protein degradation in skeletal muscle during unloading. We hypothesize that lowered NO concentration in muscle during unloading can cause reduced S-nitrosylation of calpains. This leads to calpain activation and to the degradation of cytoskeletal and contractile proteins, resulting in muscle atrophy. To test our hypothesis we administered L-arginine to rats during unloading experiments. Two weeks of unloading decreased NO concentration in muscle, while L-arginine administration prevented any NO decrease and diminished atrophy of soleus muscle. Moreover, L-arginine supplementation prevented the decrease of several cytoskeletal proteins (desmin and dystrophin) in rat soleus muscle during unloading (Lomonosova et al., 2011). Administration of the NO precursor also prevented an increase in the mRNA expression levels of E3-ubiquitin ligases (atrogin-1/MAFbx, MuRF-1—muscle RING-finger protein-1) during unloading.
Recent experiments demonstrated a role for NO as an endogenous inhibitor of 26S proteasomes (Liu et al., 2014). The main function of the proteasome is to degrade unnecessary or damaged proteins by proteolysis. Therefore, lack of increase in the expression of E3-ubiquitin ligases after administration of L-arginine during unloading can be related to a decrease of calpain activity. There are several explanations for the increased expression of ubiquitine ligases due to the activation of μ-calpain (Smith and Dodd, 2007) and calpain P94 (Kramerova et al., 2005). nNOS activity can be also modulated through crosstalk with other regulatory proteins, including heat shock protein 90 (HSP90). Interaction with HSP90 increases nNOS activity and protects cytoskeletal proteins from ubiquitin proteasome degradation (Peng et al., 2009). Under normal conditions skeletal muscle contains high amounts of HSP90 proteins which become significantly decreased during skeletal muscle atrophy (Sakurai et al., 2005; Sõti et al., 2005; Seo et al., 2006). In our study on the administration NO precursor L-arginine during unloading we have not observed a loss of HSP90 content in skeletal muscles of rats administered L-arginine (Lomonosova et al., 2011). Therefore, the decrease of soleus muscle atrophy in our experiments could be in part, the result of HSP90 protection of muscle proteins from ubiquitin proteasome degradation. Thus, activation of NO-dependent pathways can inhibit the signaling systems regulating protein degradation in muscle during unloading, supporting a critical role of NO signaling in these conditions.
Gravitational Unloading of Skeletal Muscle: Effects of NO on Fiber Type Composition
Unloading is known to stimulate expression of the fast isoforms of myosin heavy chains (MHC) leading to changes in contractile properties of soleus muscle. Unexpectedly, in our study we found that L-arginine administration also prevented a decrease of slow MHC expression levels in soleus muscle during unloading (Lomonosova et al., 2011). This data demonstrates a critical role of nNOS in the regulation of contractile protein expression. Based on the data on inhibition of the calcineurin/NFAT by GSK3β and its regulation by the NO-induced cyclic guanosine monophosphate pathway (Martins et al., 2012), we hypothesized that increases in NO content after L-arginine administration in our experiments prevents slow-to-fast transition in soleus muscles of rats.
Gravitational Unloading of Skeletal Muscle: Effects of Mechanical Activities on nNOS
Most of scientists link alterations in skeletal muscles following unloading with a lack of muscle contractile activity or with decreased resistive components of muscle contraction (Falempin and Mounier, 1998). The same can be said about changes in the NO-dependent signaling pathways. Interestingly, mechanical stimulation of a plantar supporting zone with concurrent increases of electric activity of soleus (Grigoriev et al., 2004) prevents a loss of nNOS during immersion experiments. These data demonstrate the dependence of NOS content on muscle contractile activity (Moukhina et al., 2004). Resistance physical exercise during hypokinesia also blocks decreases of protein nitrosylation levels (Rudnick et al., 2004). Therefore, decreased muscle contractile activity leads to a loss of nNOS content and activity. Chronic passive stretching is often used to simulate the loading component of muscle contraction. We hypothesized that nNOS can participate in atrophy prevention in soleus muscle under conditions of stretching combined with rat tail suspension.
To test our hypothesis, we blocked NOS with L-NAME during stretching combined with unloading (Lomonosova et al., 2012a). Muscle membrane stretching induces nNOS activity (Deshmukh et al., 2010) preventing an increase in the activity of calpains, expression of E3-ubiqutin ligases, and protein ubiquitination during unloading. The content of nNOS is not decreased during stretching combined with unloading (Lomonosova et al., 2012a; Xu et al., 2012). If our hypothesis is correct, administration of the nNOS blocker L-NAME during stretching, combined with unloading would result in muscle atrophy. However, in our study, NOS inhibition in stretched soleus muscle during rat tail suspension does not cause muscle atrophy. In fact, the level of E3-ubiquitin ligases was not increased in any of the tail suspended rats in our experiments with stretched soleus muscle with or without inhibition of NOS activity. We concluded that NO-dependent signaling is not critical for the maintenance of muscle mass in stretched soleus muscle during unloading. At the same time, muscle stretching during unloading completely prevented the decrease of slow and increase of fast MHC expression (Falempin and Mounier, 1998). L-NAME administration neutralized this effect of stretching, supporting a role of NO in preserving MHC expression during muscle stretching, combined with unloading.
Some authors have linked increased muscle mass with the higher content of myonuclei and activation of the satellite cells in muscle (Wang et al., 2006; Shenkman et al., 2010). A decrease in NO concentration was previously reported to lower the ability of satellite cells to proliferate. An increase in the number of satellite cells was prevented by administration of L-NAME in stretched muscle (Shenkman et al., 2010). This suggests that satellite cell activation and proliferation is a NO-dependent process (Kartashkina et al., 2010).
Conclusion
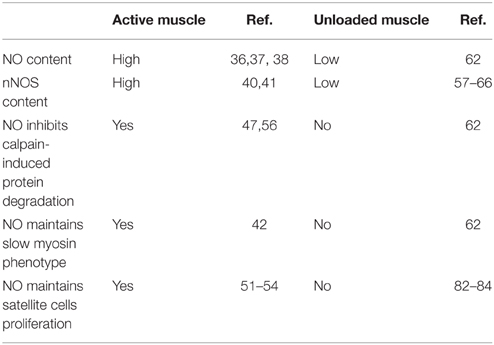
Exogenous activation of nNOS and probably other NOS isoforms leads to an increase in NO production. This can block damage and atrophic processes in skeletal muscle, as well as changes in the expression of myosin isoforms under unloading conditions. The expression and content of NOS is decreased during unloading and this coincides with decrease in NO production. Based on these findings, we hypothesize that a reduction of NOS and NO production promotes muscle atrophy. Our hypothesis is supported by recent studies demonstrating that NO-synthase participates in the regulation of protein turnover in skeletal muscle by fine-tuning and stabilizing signaling pathways that regulate protein synthesis and degradation in the muscle fibers of inactive muscle (Table 1).
Funding
The work was supported by RFBR grants 13-04-00888, 11-04-00788, 14-04-01632.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adams, M. E., Mueller, H. A., and Froehner, S. C. (2001). In vivo requirement of the α-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J. Cell Biol. 155, 113–122. doi: 10.1083/jcb.200106158
Alderton, W. K., Cooper, C. E., and Knowles, R. G. (2001). Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357, 593–615. doi: 10.1042/bj3570593
Anderson, J. E., and Wozniak, A. C. (2004). Satellite cell activation on fibers: modeling events in vivo—an invited review. Can. J. Physiol. Pharmacol. 82, 300–310. doi: 10.1139/y04-020
Aquilano, K., Baldelli, S., and Ciriolo, M. R. (2014). Nuclear recruitment of neuronal nitric-oxide synthase by α-syntrophin is crucial for the induction of mitochondrial biogenesis. J. Biol. Chem. 289, 365–378. doi: 10.1074/jbc.M113.506733
Averna, M., Stifanese, R., De Tullio, R., Salamino, F., Bertuccio, M., Pontremoli, S., et al. (2007). Proteolytic degradation of nitric oxide synthase isoforms by calpain is modulated by the expression levels of HSP90. FEBS J. 274, 6116–6127. doi: 10.1111/j.1742-4658.2007.06133.x
Bender, A. T., Silverstein, A. M., Demady, D. R., Kanelakis, K. C., Noguchi, S., Pratt, W. B., et al. (1999). Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J. Biol. Chem. 274, 1472–1478. doi: 10.1074/jbc.274.3.1472
Billecke, S. S., Bender, A. T., Kanelakis, K. C., Murphy, P. J., Lowe, E. R., Kamada, Y., et al. (2002). Hsp90 is required for heme binding and activation of apo-neuronal nitric-oxide synthase: geldanamycin-mediated oxidant generation is unrelated to any action of hsp90. J. Biol. Chem. 277, 20504–20509. doi: 10.1074/jbc.M201940200
Bodine, S. C. (2006). mTOR signaling and the molecular adaptation to resistance exercise. Med. Sci. Sports Exerc. 38, 1950–1957. doi: 10.1249/01.mss.0000233797.24035.35
Brenman, J. E., Chao, D. S., Gee, S. H., McGee, A. W., Craven, S. E., Santillano, D. R., et al. (1996). Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84, 757–767. doi: 10.1016/S0092-8674(00)81053-3
Brenman, J. E., Chao, D. S., Xia, H., Aldape, K., and Bredt, D. S. (1995). Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82, 743–752. doi: 10.1016/0092-8674(95)90471-9
Bruusgaard, J. C., Johansen, I. B., Egner, I. M., Rana, Z. A., and Gundersen, K. (2010). Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc. Natl. Acad. Sci. U.S.A. 107, 15111–15116. doi: 10.1073/pnas.0913935107
Chen, Z. P., McConell, G. K., Michell, B. J., Snow, R. J., Canny, B. J., and Kemp, B. E. (2000). AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am. J. Physiol. Endocrinol. Metab. 279, 1202–1206.
Crosbie, R. H., Barresi, R., and Campbell, K. P. (2002). Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J. 16, 1786–1791. doi: 10.1096/fj.02-0519com
Deshmukh, A. S., Long, Y. C., de Castro Barbosa, T., Karlsson, H. K., Glund, S., Zavadoski, W. J., et al. (2010). Nitric oxide increases cyclic GMP levels, AMP-activated protein kinase (AMPK)α1-specific activity and glucose transport in human skeletal muscle. Diabetologia 53, 1142–1150. doi: 10.1007/s00125-010-1716-x
Dunbar, A. Y., Kamada, Y., Jenkins, G. J., Lowe, E. R., Billecke, S. S., and Osawa, Y. (2004). Ubiquitination and degradation of neuronal nitric-oxide synthase in vitro: dimer stabilization protects the enzyme from proteolysis. Mol. Pharmacol. 66, 964–969. doi: 10.1124/mol.104.000125
Falempin, M., and Mounier, Y. (1998). Muscle atrophy associated with microgravity in rat: basic data for countermeasures. Acta Astronaut. 42, 489–501. doi: 10.1016/S0094-5765(98)00141-6
Forstermann, U., Closs, E. I., Pollock, J. S., Nakane, M., Schwarz, P., Gath, I., et al. (1994). Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 23, 1121–1131. doi: 10.1161/01.HYP.23.6.1121
Förstermann, U., Gorsky, L. D., Pollock, J. S., Ishii, K., Schmidt, H. H., Heller, M., et al. (1990). Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Mol. Pharmacol. 38, 7–13.
Forstermann, U., Pollock, J. S., Schmidt, H. H., Heller, M., and Murad, F. (1991). Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 88, 1788–1792. doi: 10.1073/pnas.88.5.1788
Freeman, B. (1994). Free radical chemistry of nitric oxide. Looking at the dark side. Chest 105, 79–84. doi: 10.1378/chest.105.3_supplement.79S
Grigoriev, A. I., Kozlowskaya, I. B., and Shenkman, B. S. (2004). Role of support afferentation in the tonic muscle system. I. M. Sechenov. Ross. Physiol. J. 90, 508–521.
Hevel, J. M., White, K. A., and Marletta, M. A. (1991). Purification of the inducible murine macrophage nitric oxide synthase: identification as a flavoprotein. J. Biol. Chem. 266, 22789–22791.
Hinchee-Rodriguez, K., Garg, N., Venkatakrishnan, P., Roman, M. G., Adamo, M. L., Masters, B. S., et al. (2013). Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochem. Biophys. Res. Commun. 435, 501–505. doi: 10.1016/j.bbrc.2013.05.020
Ingalls, C. P., Warren, G. L., and Armstrong, R. B. (1999). Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J. Appl. Physiol. 87, 386–390.
Ito, N., Ruegg, U. T., Kudo, A., Miyagoe-Suzuki, Y., and Takeda, S. (2013). Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 19, 101–106. doi: 10.1038/nm.3019
Kartashkina, N. L., Turtikova, O. V., Kuznetsov, S. L., Kalamkarov, G. R., Bugrova, A. E., Orlov, O. I., et al. (2010). Effect of NO on myosatellites multiplication during functional loading and stretching. Dokl. Biol. Sci. 432, 267–170. doi: 10.1134/S0012496610030014
Kobzik, L., Reid, M. B., Bredt, D. S., and Stamler, J. S. (1994). Nitric oxide in skeletal muscle. Nature 372, 546–548. doi: 10.1038/372546a0
Koh, T. J., and Tidball, J. G. (2000). Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 279, 806–812.
Kone, B. C., Kuncewicz, T., Zhang, W., and Yu, Z. Y. (2003). Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am. J. Physiol. Renal. Physiol. 285, 178–190. doi: 10.1152/ajprenal.00048.2003
Kramerova, I., Kudryashova, E., and Venkatraman, G. (2005). Calpain 3 participates in sarcomere remodeling by acting upstream of the ubiquitin–proteasome pathway. Hum. Mol. Genet. 14, 2125–2134. doi: 10.1093/hmg/ddi217
Lainé, R., and de Montellano, P. R. (1998). Neuronal nitric oxide synthase isoforms alpha and mu are closely related calpain-sensitive proteins. Mol. Pharmacol. 54, 305–312.
Lancaster, J. R., and Hibbs, J. B. (1990). EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc. Natl. Acad. Sci. U.S.A. 87, 1223–1227. doi: 10.1073/pnas.87.3.1223
Lawler, J. M., Kunst, M., Hord, J. M., Lee, Y., Joshi, K., Botchlett, R. E., et al. (2014). EUK-134 ameliorates nNOSμ translocation and skeletal muscle fiber atrophy during short-term mechanical unloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, 470–482. doi: 10.1152/ajpregu.00371.2013
Lira, V. A., Brown, D. L., Lira, A. K., Kavazis, A. N., Soltow, Q. A., Zeanah, E. H., et al. (2010). Nitric oxide and AMPK cooperatively regulate PGC-1α in skeletal muscle cells. J. Physiol. 588, 3551–3566. doi: 10.1113/jphysiol.2010.194035
Lira, V. A., Soltow, Q. A., Long, J. H., Betters, J. L., Sellman, J. E., and Criswell, D. S. (2007). Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 293, 1062–1068. doi: 10.1152/ajpendo.00045.2007
Liu, H., Yu, S., Zhang, H., and Xu, J. (2014). Identification of nitric oxide as an endogenous inhibitor of 26S proteasomes in vascular endothelial cells. PLoS ONE 9:e98486. doi: 10.1371/journal.pone.0098486
Lomonosova, Y. N., Kalamkarov, G. R., Bugrova, A. E., Shevchenko, T. F., Kartashkina, N. L., Lysenko, E. A., et al. (2011). Protective effect of L-arginine administration on proteins of unloaded m. soleus. Biochemistry (Mosc.). 76, 571–580. doi: 10.1134/S0006297911050075
Lomonosova, Y. N., Kalamkarov, G. R., Bugrova, A. E., Shevchenko, T. F., Kartashkina, N. L., Lysenko, E. A., et al. (2012a). Role of NO-synthase in regulation of protein metabolism of stretched rat m. soleus muscle during functional unloading. Biochemistry (Mosc.). 77, 208–216. doi: 10.1134/S0006297912020137
Lomonosova, Y. N., Shenkman, B. S., and Nemirovskaya, T. L. (2012b). Attenuation of unloading-induced rat soleus atrophy with the heat-shock protein inducer 17-(allylamino)-17-demethoxygeldanamycin. FASEB J. 26, 4295–4301. doi: 10.1096/fj.12-204412
Ma, X. W., Li, Q., Xu, P. T., Zhang, L., Li, H., and Yu, Z. B. (2011). Tetanic contractions impair sarcomeric Z-disk of atrophic soleus muscle via calpain pathway. Mol. Cell. Biochem. 354, 171–180. doi: 10.1007/s11010-011-0816-3
Martins, K. J. B., St-Louis, M., Murdoch, G. K., MacLean, I. M., McDonald, P., Dixon, W. T., et al. (2012). Nitric oxide synthase inhibition prevents activity-induced calcineurin–NFATc1 signaling and fast-to-slow skeletal muscle fibre type conversions. J. Physiol. 590, 1427–1442. doi: 10.1113/jphysiol.2011.223370
McConell, G. K., Bradley, S. J., Stephens, T. J., Canny, B. J., Kingwell, B. A., and Lee-Young, R. S. (2007). Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, 821–828. doi: 10.1152/ajpregu.00796.2006
Michetti, M., Salamino, F., Melloni, E., and Pontremoli, S. (1995). Reversible inactivation of calpain isoforms by nitric oxide. Biochem. Biophys. Res. Commun. 207, 1009–1014. doi: 10.1006/bbrc.1995.1285
Minami, Y., Kimura, Y., Kawasaki, H., Suzuki, K., and Yahara, I. (1994). The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol. Cell. Biol. 14, 1459–1464. doi: 10.1128/MCB.14.2.1459
Moukhina, A. M., Shenkman, B. S., Blottner, D., Nemirovskaya, T., Lemesheva, Y., Püttmann, B., et al. (2004). Effects of support stimulation on human soleus fiber characteristics during exposure to dry immersion. J. Gravit. Physiol. 11, 137–138.
Nakane, M., Schmidt, H. H., Pollock, J. S., Förstermann, U., and Murad, F. (1993). Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 316, 175–180. doi: 10.1016/0014-5793(93)81210-Q
Osawa, Y., Lowe, E. R., Everett, A. C., Dunbar, A. Y., and Billecke, S. S. (2003). Proteolytic degradation of nitric oxide synthase: effect of inhibitors and role of hsp90-based chaperones. J. Pharmacol. Exp. Ther. 304, 493–497. doi: 10.1124/jpet.102.035055
Papapetropoulos, A., Fulton, D., Lin, M. I., Fontana, J., McCabe, T. J., Zoellner, S., et al. (2004). Vanadate is a potent activator of endothelial nitric-oxide synthase: evidence for the role of the serine/threonine kinase Akt and the 90-kDa heat shock protein. Mol. Pharmacol. 65, 407–415. doi: 10.1124/mol.65.2.407
Peng, H.-M., Morishima, Y., Clapp, K. M., Lau, M., Pratt, W. B., and Osawa, Y. (2009). Dynamic cycling with Hsp90 stabilizes neuronal nitric oxide synthase through calmodulin-dependent inhibition of ubiquitination. Biochemistry 48, 8483–8490. doi: 10.1021/bi901058g
Perez, A. C., de Oliveira, C. C., Prieto, J. G., Ferrando, A., Vila, L., and Alvarez, A. I. (2002). Quantitative assessment of nitric oxide in rat skeletal muscle and plasma after exercise. Eur. J. Appl. Physiol. 88, 189–191. doi: 10.1007/s00421-002-0693-2
Piech, A., Dessy, C., Havaux, X., Feron, O., and Balligand, J. L. (2003). Differential regulation of nitric oxide synthases and their allosteric regulators in heart and vessels of hypertensive rats. Cardiovasc. Res. 57, 456–467. doi: 10.1016/S0008-6363(02)00676-4
Pollock, J. S., Förstermann, U., Mitchell, J. A., Warner, T. D., Schmidt, H. H., Nakane, M., et al. (1991). Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 88, 10480–10484. doi: 10.1073/pnas.88.23.10480
Pye, D., Palomero, J., and Kabayo, T. (2007). Real-time measurement of nitric oxide in single mature mouse skeletal muscle fibres during contractions. J. Physiol. 581, 309–318. doi: 10.1113/jphysiol.2006.125930
Rudnick, J., Püttmann, B., Tesch, P. A., Alkner, B., Schoser, B. G., Salanova, M., et al. (2004). Differential expression of nitric oxide synthases (NOS 1-3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. FASEB J. 18, 1228–1230. doi: 10.1096/fj.03-0792fje
Sakurai, T., Fujita, Y., Ohto, E., Oguro, A., and Atomi, Y. (2005). The decrease of the cytoskeleton tubulin follows the decrease of the associating molecular chaperone alphaB-crystallin in unloaded soleus muscle atrophy without stretch. FASEB J. 19, 1199–1201.
Salanova, M., Schiffl, G., Rittweger, J., Felsenberg, D., and Blottner, D. (2008). Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem. Cell Biol. 130, 105–118. doi: 10.1007/s00418-008-0399-6
Samengo, G., Avik, A., Fedor, B., Whittaker, D., Myung, K. H., Wehling-Henricks, M., et al. (2012). Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell 11, 1036–1045. doi: 10.1111/acel.12003
Sandonà, D., Desaphy, J. F., Camerino, G. M., Bianchini, E., Ciciliot, S., Danieli-Betto, D., et al. (2012). Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 7:e33232. doi: 10.1371/journal.pone.0033232
Schmidt, H. W., Pollock, J. S., Nakane, M., Gorsky, L. D., Förstermann, U., and Murad, F. (1991). Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc. Natl. Acad. Sci. U.S.A. 88, 365–369. doi: 10.1073/pnas.88.2.365
Sellman, J. E., DeRuisseau, K. C., Betters, J. L., Lira, V. A., Soltow, Q. A., Selsby, J. T., et al. (2006). In vivo inhibition of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J. Appl. Physiol. 100, 258–265. doi: 10.1152/japplphysiol.00936.2005
Seo, Y., Lee, K., Park, K., Bae, K., and Choi, I. (2006). A proteomic assessment of muscle contractile alterations during unloading and reloading. J. Biochem. 139, 71–80. doi: 10.1093/jb/mvj007
Shenkman, B. S., and Nemirovskaya, T. L. (2008). Calcium-dependent signaling mechanisms and soleus fiber remodeling under gravitational unloading. J. Muscle Res. Cell Motil. 29, 221–230. doi: 10.1007/s10974-008-9164-7
Shenkman, B. S., Turtikova, O. V., Nemirovskaya, T. L., and Grigoriev, A. I. (2010). Skeletal muscle activity and the fate of myonuclei. Acta Nat. 2, 59–66.
Smith, I. J., and Dodd, S. L. (2007). Calpain activation causes a proteasome-dependent increase in protein degradation and inhibits the Akt signalling pathway in rat diaphragm muscle. Exp. Physiol. 92, 561–573. doi: 10.1113/expphysiol.2006.035790
Song, Y., Zweier, J. L., and Xia, Y. (2001). Heat-shock protein 90 augments neuronal nitric oxide synthase activity by enhancing Ca2+/calmodulin binding. Biochem. J. 355, 357–360. doi: 10.1042/bj3550357
Sõti, C., Nagy, E., Giricz, Z., Vígh, L., Csermely, P., and Ferdinandy, P. (2005). Heat shock proteins as emerging therapeutic targets. Br. J. Pharmacol. 146, 769–780. doi: 10.1038/sj.bjp.0706396
Stamler, J. S. (1994). Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78, 931–936. doi: 10.1016/0092-8674(94)90269-0
Stamler, J. S., Singel, D. J., and Loscalzo, J. (1992). Biochemistry of nitric oxide and its redox-activated forms. Science. 258, 1898–1902. doi: 10.1126/science.1281928
Stuehr, D. J., Cho, H. J., Kwon, N. S., Weise, M. F., and Nathan, C. F. (1991). Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc. Natl. Acad. Sci. U.S.A. 88, 7773–7777. doi: 10.1073/pnas.88.17.7773
Sun, L. W., Blottner, D., Luan, H. Q., Salanova, M., Wang, C., Niu, H. J., et al. (2013). Bone and muscle structure and quality preserved by active versus passive muscle exercise on a new stepper device in 21 days tail-suspended rats. J. Musculoskelet. Neuronal. Interact. 13, 166–177.
Suzuki, N., Motohashi, N., Uezumi, A., Fukada, S., Yoshimura, T., Itoyama, Y., et al. (2007). NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Invest. 117, 2468–2476. doi: 10.1172/JCI30654
Tatchum-Talom, R., Schulz, R., McNeill, J. R., and Khadour, F. H. (2000). Upregulation of neuronal nitric oxide synthase in skeletal muscle by swim training. Am. J. Physiol. Heart Circ. Physiol. 279, 1757–1766.
Tatsumi, R. (2010). Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim. Sci. J. 81, 11–20. doi: 10.1111/j.1740-0929.2009.00712.x
Tidball, J. G., Lavergne, E., Lau, K. S., Spencer, M. J., Stull, J. T., and Wehling, M. (1998). Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am. J. Physiol. 275, 260–266.
Vandebrouck, A., Sabourin, J., Rivet, J., Balghi, H., Sebille, S., Kitzis, A., et al. (2007). Regulation of capacitative calcium entries by 1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of α1-syntrophin. FASEB J. 21, 608–617. doi: 10.1096/fj.06-6683com
Vassilakopoulos, T., Deckman, G., Kebbewar, M., Rallis, G., Harfouche, R., and Hussain, S. N. (2003). Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, 452–457. doi: 10.1152/ajplung.00270.2002
Wakayama, Y., Inoue, M., Murahashi, M., Shibuya, S., Jimi, T., Kojima, H., et al. (1997). Ultrastructural localization of α1-syntrophin and neuronal nitric oxide synthase in normal skeletal myofiber and their relation to each other and to dystrophin. Acta Neuropathol. 94, 455–464. doi: 10.1007/s004010050733
Wang, X. D., Kawano, F., Matsuoka, Y., Fukunaga, K., Terada, M., Sudoh, M., et al. (2006). Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am. J. Physiol. Cell Physiol. 290, 981–989. doi: 10.1152/ajpcell.00298.2005
Watson, P. M., and Riccio, A. (2009). Nitric oxide and histone deacetylases: a new relationship between old molecules. Commun. Integr. Biol. 2, 11–13. doi: 10.4161/cib.2.1.7301
Xu, P. T., Li, Q., Sheng, J. J., Song, Z., and Yu, Z. B. (2012). Passive stretch reduces calpain activity through nitric oxide pathway in unloaded soleus muscles. Mol. Cell. Biochem. 367, 113–124. doi: 10.1007/s11010-012-1325-8
Yamada, M., Sankoda, Y., Tatsumi, R., Mizunoya, W., Ikeuchi, Y., Sunagawa, K., et al. (2008). Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int. J. Biochem. Cell Biol. 40, 2183–2191. doi: 10.1016/j.biocel.2008.02.017
Yamada, M., Tatsumi, R., Kikuiri, T., Okamoto, S., Nonoshita, S., Mizunoya, W., et al. (2006). Matrix metalloproteinases are involved in mechanical stretch–induced activation of skeletal muscle satellite cells. Muscle Nerve 34, 313–319. doi: 10.1002/mus.20601
Keywords: skeletal muscle, unloading, nitric oxide (II), NO-synthase, protective function of NO
Citation: Shenkman BS, Nemirovskaya TL and Lomonosova YN (2015) No-dependent signaling pathways in unloaded skeletal muscle. Front. Physiol. 6:298. doi: 10.3389/fphys.2015.00298
Received: 24 July 2015; Accepted: 09 October 2015;
Published: 31 October 2015.
Edited by:
Catherine Coirault, Institut National de la Santé et de la Recherche Médicale, FranceReviewed by:
Stewart Ian Head, University of New South Wales, AustraliaKunihiro Sakuma, Toyohashi University of Technology, Japan
Copyright © 2015 Shenkman, Nemirovskaya and Lomonosova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tatiana L. Nemirovskaya, nemirovskaya@bk.ru
 Boris S. Shenkman1
Boris S. Shenkman1 Tatiana L. Nemirovskaya
Tatiana L. Nemirovskaya Yulia N. Lomonosova
Yulia N. Lomonosova