- Genetics, Reproduction and Development Laboratory (GReD) Genetics, Reproduction and Development Laboratory, Institut National de la Santé et de la Recherche Médicale U1103, Centre National de la Recherche Scientifique UMR6293, Clermont University, Clermont-Ferrand, France
The formation of the musculoskeletal system is a remarkable example of tissue assembly. In both vertebrates and invertebrates, precise connectivity between muscles and skeleton (or exoskeleton) via tendons or equivalent structures is fundamental for movement and stability of the body. The molecular and cellular processes underpinning muscle formation are well-established and significant advances have been made in understanding tendon development. However, the mechanisms contributing to proper connection between these two tissues have received less attention. Observations of coordinated development of tendons and muscles suggest these tissues may interact during the different steps in their development. There is growing evidence that, depending on animal model and muscle type, these interactions can take place from progenitor induction to the final step of the formation of the musculoskeletal system. Here, we briefly review and compare the mechanisms behind muscle and tendon interaction throughout the development of vertebrates and Drosophila before going on to discuss our recent findings on the coordinated development of muscles and tendon-like structures in Drosophila leg. By altering apodeme formation (the functional Drosophila equivalent of tendons in vertebrates) during the early steps of leg development, we affect the spatial localization of subsequent myoblasts. These findings provide the first evidence of the developmental impact of early interactions between muscle and tendon-like precursors, and confirm the appendicular Drosophila muscle system as a valuable model for studying these processes.
In vertebrates, the progenitors of axial tendons arise from a dorsal subdomain of the sclerotome, called syndetome, that is immediately adjacent to the myotome from which myogenic cells originate (Brent et al., 2003). Crucial here is the fact that FGF signals emanating from the myotome are directly responsible for inducing the syndetome (Brent and Tabin, 2004; Brent et al., 2005; Chen and Galloway, 2014). Interactions between tendon and muscle progenitors thus take place in the very early steps of axial tendon development. On the other hand, the progenitors of limb and craniofacial tendons emerge independently of muscle progenitors (Kardon, 1998; Schweitzer et al., 2010). However, the muscles are subsequently required for further tendon development and maintenance (Edom-Vovard et al., 2002; Grenier et al., 2009). Thus, at either early or later stages of development, musculoskeletal formation in vertebrates is reliant on interactions between muscles and tendons.
The myogenesis process has been remarkably well preserved throughout the evolution. Various different models have been developed to study muscle development and muscle physiology, including insect models such as grasshopper and fly (Ho et al., 1983; Ball et al., 1985a; de Joussineau et al., 2012; Dobi et al., 2015). Drosophila melanogaster is the dominant genetic model used in studies of insect development, as transgenic flies are relatively easy to generate and there is a large range of genetic tools now available. Most of our knowledge of Drosophila muscle development stems from studies conducted on larval muscles (see Dobi et al., 2015 and de Joussineau et al., 2012 for review). Larval somatic muscles are set up during embryogenesis and have a very simplified pattern of 30 muscles repeated in each hemisegment. They originate from a subdivision of the mesoderm from which segregate three types of myoblasts, including the so-called Founder Cells (FC) and Fusion Competent Myoblasts (FCM) (Bate, 1990; Leptin, 1991; Baylies et al., 1998). Each FC fuses with several FCM to build a syncytial myotube (Rochlin et al., 2010; Abmayr and Pavlath, 2012; Kim et al., 2015). The expression of a distinct set of identity genes by the original FC determines the characteristic shape, size, position and innervation of each myofiber (Tixier et al., 2010; de Joussineau et al., 2012). Adult muscle precursors (AMP) arethe third type of myoblasts that are set aside during embryogenesis. AMPs are maintained quiescent and undifferentiated during embryogenesis and larval life, before proliferating then differentiating into adult muscles during metamorphosis (Bate et al., 1991; Broadie and Bate, 1991; Currie and Bate, 1991; Roy and VijayRaghavan, 1999). At both larval and adult stages, muscles are only effective when they are properly anchored to the exoskeleton through Muscle Attachment Sites (MAS). These tendon-like cells have been well investigated for larval muscles, yet only a few studies have focused on adult “tendogenesis” (Volk, 1999; Ghazi et al., 2000, 2003; Schnorrer and Dickson, 2004; Soler et al., 2004; Schweitzer et al., 2010).
Development of the Tendon-like Structures in Drosophila
Mechanisms of Interaction between Muscles and MAS during Embryogenesis
Much as in vertebrates, Drosophila muscles have to be properly attached to the (exo-)skeleton in order to transmit the force generated by fiber contraction.
Invertebrate model organisms lack an internal skeleton, but somatic muscles interact with Epidermal Muscle Attachment (EMA) cells that are singled out from a cluster of exoskeleton cells called the apodeme (Ball et al., 1985b; Radnikow and Bässler, 1991; Volk, 1999). As muscles and apodemes connect through a secreted extracellular matrix, forming the equivalent of the Myo-Tendinous Junction (MTJ) in vertebrates, they are widely referred to as “tendons” in Drosophila studies (Volk, 1999; Schweitzer et al., 2010). Therefore, although invertebrate apodemes and vertebrate tendon cells do not share the same origin (ectodermal and mesodermal origin, respectively), they do ensure the same function.
As stated earlier, MAS formation has been well described in Drosophila for larval muscles (Volk, 1999; Schnorrer and Dickson, 2004; Schweitzer et al., 2010) in which studies report how the apodemes derive from specialized ectodermal cells. The initial differentiation of these cells is muscle-independent and requires ectodermal signals such as Wg, Hh, and EGF (Piepenburg et al., 2000; Hatini and DiNardo, 2001) to induce the expression of Stripe (Sr). Sr is the earliest known marker of tendon-like precursors. It encodes a transcription factor with a zinc-finger domain and shares sequence homologies with members of the vertebrate Egr family (Volk and VijayRaghavan, 1994; Frommer et al., 1996). Interestingly, EGR1 and EGR2 are involved in tendon-cell differentiation in vertebrate limbs (Lejard et al., 2011). In Drosophila, Sr is both necessary and sufficient for MAS induction by activating the expression of most of the MAS-specific genes (Becker et al., 1997; Vorbrüggen and Jäckle, 1997). The Sr gene encodes for two isoforms: SrA and SrB (Frommer et al., 1996). A low level of SrB isoform is required for induction of tendon-like precursors during early embryonic development (Becker et al., 1997; Vorbrüggen and Jäckle, 1997). These precursors then secrete signaling molecules that are involved in muscle guidance and attachment. For example, under Sr regulation, Slit ligand is expressed by tendon-like precursors and interacts with its receptors Robo 1 and 2 that are located on the membrane of specific muscles migrating toward their attachment sites (Kramer et al., 2001; Volohonsky et al., 2007). In turn, muscle fibers produce Vein, a neuregulin-like ligand that activates the EGFR pathway in tendon-like cells (Yarnitzky et al., 1997). EGFR pathway activation leads to an increase in SrB expression, which subsequently promotes splicing of the SrA isoform and leads to the terminal differentiation of the MAS and the establishment of the MTJ (Nabel-Rosen et al., 2002; Volohonsky et al., 2007). The tendon-like precursors that do not receive EGF signal from muscles lose MAS marker expression and eventually dedifferentiate. Thus, in a similar way to the craniofacial and limb tendons described in vertebrate studies, tendon-like precursors in Drosophila embryo are specified independently of muscle cells whereas the terminal phase of differentiation is muscle-dependent (Volk, 1999; Schnorrer and Dickson, 2004; Schweitzer et al., 2010).
Adult Muscles Anchoring to Cuticle within the Thorax Allows Fly Locomotion
The MAS development during the adult myogenesis in Drosophila has paid much less attention than in embryo. In adults, the main thoracic muscles are the flight muscles [including Direct Flight Muscles (DFM) and large Indirect Flight Muscles (IFM)] and the leg muscles (Miller, 1950; Fernandes et al., 1991; Soler et al., 2004) developing from AMP that are associated with wing and leg discs, respectively. Theses myoblasts are characterized by the expression of Twist that persists until metamorphosis (Bate et al., 1991; Broadie and Bate, 1991). The MAS of flight muscles develop from distinct groups of Sr-expressing cells from the wing disc epithelium (Fernandes et al., 1996). The pattern of flight muscle MAS is defined by the integration of several molecular signals including Notch, Wnt, and Dpp, and is regulated by the transcription factors Apterous and Achaete scute (Ghazi et al., 2000, 2003; Usui et al., 2004). In the leg, apodemes adopt their own particular shape. Sr-expressing epithelial cells invaginate inside the developing leg to form long internal structures (Soler et al., 2004). Remarkably, Twist-expressing myoblasts accumulate around the apodeme precursors long before they form syncytial fibers. This observation strongly suggests that muscle and tendon-like precursors interact with each other at an early stage in the developing leg (Soler et al., 2004). Similar long internal apodemes have been described in appendages of other insects (Ball et al., 1985b; Radnikow and Bässler, 1991) and more generally in several groups of arthropods, including crustaceans (Medler and Mykles, 2015). Despite their value as models for histological and physiological studies, the lack of genetic tools for these organisms precludes any attempt at systematic molecular or genetic analysis.
Perspectives
Drosophila Appendicular Myogenesis as a Model for Early Interactions between Muscle and Muscle Attachment Site Progenitors
The leg muscle system of Drosophila is a complex structure that counts 14 distinct muscles (Miller, 1950; Soler et al., 2004). Unlike larval muscles, each leg muscle is composed of several fibers organized around a specific long internal apodeme and attached from one side to this tendon-like structure and from the other side to the cuticle (via embryonic-like apodemes) (Soler et al., 2004). This particular pattern of multifiber muscles enables precise and coordinated movements of all nine articulated segments of the leg. In addition to being functionally comparable to vertebrate limb muscles, there are other morphological parallels to draw with the musculoskeletal system of the vertebrate limb. For example, in vertebrates, long tendons of the limb extend from the most distal region (paw) to more proximal segments (arm/leg) where they are associated with their corresponding muscles, allowing articulation of the distal limb part (Kardon, 1998; Huang et al., 2015). Similar schemes have been developed in insect legs, with some long internal apodemes running through several leg segments (Ball et al., 1985b; Radnikow and Bässler, 1991; Soler et al., 2004). In Drosophila, the leg muscles derive from adepithelial cells that are located at the surface of leg imaginal discs while the tendon-like precursors are groups of Stripe-positive cells belonging to the disc epithelium. Both tendon-like and muscle precursors are set aside before metamorphosis and leg development. More strikingly, a specialized subpopulation of muscle precursors identified as Founder Cells (FCs) are specified as early as the third larval instar (L3) near these tendon-like precursors (Soler et al., 2004; Maqbool et al., 2006). We have previously reported (Soler et al., 2004) that at the early pupa stage when a disc evaginates to form a 3D leg structure, the tendon-like precursors invaginate inside the developing leg and specific FC myoblasts follow this pattern (Figures 1A,B). This observation suggested that invaginating tendon-like precursors of the leg could interact with FCs to accurately localize them, and could thus play a crucial role during the early steps of leg muscle development. This hypothesis is further supported by our recent data indicating that tendon-like precursors are indeed required for proper patterning of appendicular myoblasts (Figure 1).
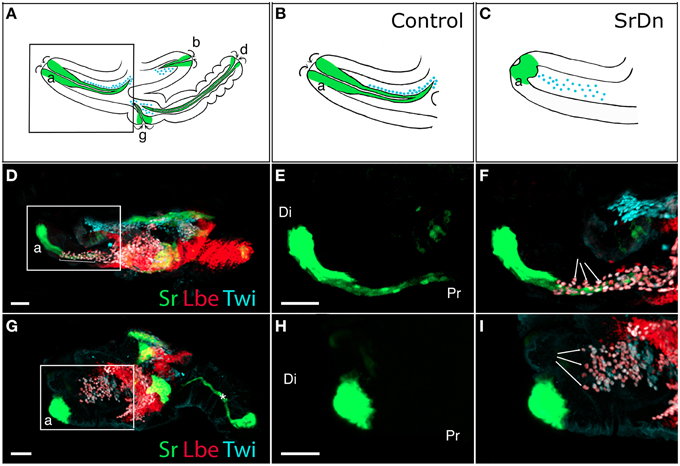
Figure 1. Disrupting apodeme development affects myoblast spatial organization. (A–C) Drawings of sagittal views at 5 h After Pupae Formation (APF) of a whole wild-type leg disc (A) with a focus on the tibia levator tendon/apodeme (a) of the dorsal femur segment (B) and a leg disc for which apodeme invagination in the dorsal femur was affected (C). Only some apodemes (green) and their associated myoblasts (blue) are represented. Note that their invagination (curved arrows) goes on to form a lumen. (D–I) Leg discs at 5 h APF from Tub-Gal80TS;Stripe-gal4>UASGFP (D–F) and Tub-Gal80TS;Stripe-gal4>UASGFP, UAS-StripeDN (G–I). Apodemes are visualized by GFP and myoblasts stained for Twist (in cyan) and Lbe (in red). At this stage, the leg disc elongates along the proximo-distal axis (Pr-Di). (D) Control leg disc showing tibia levator tendon (a) invaginating from distal to proximal ends of the dorsal femur (insert), the myoblasts are aligned along this apodeme (brackets). (E,F) Enlargements of the box region in (D) showing apodeme developing in the femur (E) and the myoblasts organized around it (arrows in F). (G) When StripeDN is expressed in apodemes, they fail to develop correctly, with the result that the apodeme (a) in the dorsal femur is unable to invaginate to form a long internal structure (insert). (H,I) Enlargements of the box region in (G) showing aborted apodeme in femur (H). Myoblasts in this region do not appear to align in the proximo-distal axis and seem to distribute in random directions into the femur segment (arrows in I). Note that in (G), the main apodeme in the tarsus (star) does invaginate despite expressing UAS-StripeDN at 5h APF. This first apodeme invaginates as early as L3, at which stage we shifted the larvae from 18 to 29°C to allow Gal4 expression, which thus makes it very likely that it undergoes invagination before StripeDN protein accumulation could have any effect. Myoblasts associated with this apodeme are not in focus. Scale bar = 30 μm. Apodeme and muscle annotations: (a) tibia levator tendon in dorsal femur (associated muscle: tibia levator muscle), (b) tibia depressor tendon in ventral femur (associated muscle: tibia depressor muscle), (d) long tendon in tarsus (associated muscles: long tendon muscle 1, tarsus reductor muscles 1 and 2), (g) tarsus levator tendon in dorsal tibia (associated muscle: tarsus levator muscle). See Soler et al. (2004) for more detailed annotations.
How Could Disruption of Tendon-Like Precursors Affect Muscle FCs in the Developing Leg?
Our earlier work (Maqbool et al., 2006) showed that ladybird early (lbe), an ortholog of Lbx1, which is a key regulator of appendicular myogenesis in vertebrates (Buckingham et al., 2003), is expressed in different sub-populations of myoblasts characterized by Twist expression (Bate et al., 1991; Broadie and Bate, 1991). From the third instar larval stage, the spatial distribution of these myoblasts revealed a highly stereotyped pattern that underpins the formation of defined muscles in the adult leg. We also showed that lbe and its paralog ladybird late (lbl) genes are required for proper patterning of leg muscles and that different levels of Lbe protein contribute to myoblast diversity within the leg (Maqbool et al., 2006). As these myoblasts also express the Dumbfounded-lacZ reporter gene, they are very likely the equivalent of embryonic FC in embryo (Soler et al., 2004; Maqbool et al., 2006). More strikingly, in each segment, Lbe-positive groups of myoblasts lie close to Sr-positive tendon-like precursors. This distribution is particularly obvious in the dorsal femur where Lbe and Twist-expressing myoblasts accumulate next to the tibia levator tendon (tilt). At the beginning of metamorphosis, these myoblasts remain associated with the tilt as it begins to invaginate. Five hours After Pupae Formation (APF), they progressively align all along this internal apodeme. Figures 1A,B illustrates this spatial distribution of myoblasts and their association with invaginating apodemes. In dissected leg discs, apodemes are visualized by GFP expression driven by Sr-Gal4 driver and myoblasts by immunostaining against Lbe and Twist (Figures 1D–F). In order to determine whether invaginating apodeme could influence myoblast behavior, we challenged apodeme development by over-expressing a dominant-negative form of Sr (SrDN) (Vorbrüggen and Jäckle, 1997) using the Sr-Gal4 driver. As Sr is also involved in MAS development in embryos, we used a ubiquitous temperature-sensitive Gal80ts allele to repress SrDN expression until mid-L3 stage. Figures 1G–I shows that SrDN expression affects apodeme formation at 5 h APF. In particular, compared to controls (Figure 1E) tendon-like cells appear unable to form a long internal structure in the dorsal femur (Figure 1H). Even after disrupting apodeme development, Lbe expression was still detected in associated myoblasts, indicating that the occurrence of invaginating apodeme is not required to maintain the expression of this muscle identity gene (compare panels F and I in Figure 1). However, these myoblasts appeared disorganized within the everting segment when the apodeme is affected (Figure 1I) yet well aligned on the developing apodeme of the control leg disc (Figure 1F). This observation indicates that in the absence of invaginating tendon-like precursors, myoblasts are no longer correctly distributed within the observed segment, even though they still follow the segmental subdivision of the leg disc. To compare the spatial distribution of dorsal femur myoblasts between control leg discs and SrDN leg discs in which tilt apodeme is significantly affected, 3D reconstructions of several early pupa discs (5 h APF) were built and visualized using Imaris™ Software (Figures 2A,B). These 3D reconstructions were then used to measure the distance between the Most Distal myoblast (MD myoblast) and the Site of Tendon-like Invagination (STI) at the epithelium surface (distal femur) (Figure 2). This MD myoblast-to-STI distance was the parameter that showed least variation across Sr-DN samples. Our data show that, mean MD myoblast-to-STI distance is significantly higher in control leg discs (46,9 μm, n = 8) than after SrDN expression in tendon-like precursors (shortened to 31,67 μm, n = 11; Figure 2C). This result demonstrates that apodeme alteration leads to aberrant myoblast positioning in the leg discs of early pupa and may therefore impact the morphological and functional properties of the corresponding adult leg muscles. It remains to be elucidated whether muscle precursor distribution is controlled by direct interactions with the developing apodemes or whether their positioning is guided by physical constraints during leg disc evagination. It is reasonable to assume that in absence of apodeme, myoblasts would have a wider area in which to spread within the segment cavity. In both hypotheses, the data reported here show that in the early steps of leg development, apodemes are directly or indirectly required for proper patterning/organization of myoblasts that have previously been identified as FC (Soler et al., 2004; Maqbool et al., 2006).
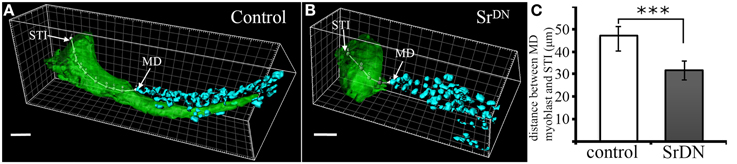
Figure 2. Spatial distribution of myoblasts after SrDN expression. (A–B) Confocal 3D rendering of tibia levator tendon (in green) and associated myoblasts (in cyan) in the dorsal femur of a leg disc at 5 h APF. (A) shows invaginating apodeme for the control sample with the spatial distribution of myoblasts to be compared against myoblast distribution after affecting apodeme development (B). Distance from the Most Distal myoblast (MD) to the Site of Tendon Invagination (STI) was measured using Imaris MeasurementPro through the 3D volume of the apodeme. Scale bar = 10 μm. (C) Quantification of MD-to-STI distance. Mean distance is significantly reduced in SrDN samples (31, 67 μm; n = 11) compared to control samples (46, 95 μm; n = 8). Error bars represent standard deviation, *** p-value < 0.001 using the Student's t-test.
Discussion
Taken together, the data reported here show that in developing Drosophila legs, the invaginating tendon-like precursors orchestrate the spatial positioning of tightly associated appendicular founder myoblasts. Such early interactions between apodeme and muscle precursors have never before been observed during embryonic or flight muscle development. This study demonstrates that appendicular myogenesis is an attractive model for studying early interactions between tendon-like and muscle progenitors. In vertebrate limbs, muscle, and tendon induction occur independently, but the specification of tendon progenitors of the axial musculoskeletal system is directly dependent on the FGF ligand emanating from the adjacent myotome (Brent et al., 2003; Brent and Tabin, 2004). Thus, at least for certain muscles, early muscle/tendon interactions are required in both Drosophila and vertebrates and similar mechanisms may control certain aspects of these interactions. Note too that long internal apodemes have already been described in appendages of many invertebrates such as crustaceans (Medler and Mykles, 2015) and insects undergoing hemimetabolous development (incomplete metamorphosis with no pupal stage), as is the case of grasshoppers for which leg muscle system development around a long internal apodeme has been well described (Ball and Goodman, 1985; Ball et al., 1985b). In this model, muscle pioneers (equivalent of FC cells) are associated with ectodermal sites where the invagination begins (Ball et al., 1985b). However, the lack of specific markers precludes any attempt to determine whether these sites were already specified as tendon-like precursors and whether physical contacts were made at this stage. Using our Drosophila leg model, we showed that the presumptive leg muscle founders segregate close to the Sr-expressing apodeme, long before they start invaginating (Soler et al., 2004). Moreover, our most recent observations (CS, unpublished data) indicate that cell–cell contact indeed occurs as early as the third larval stage through cytoplasmic projections. The role of these connections has yet to be elucidated, but one possibility is that they are required to promote the segregation of FC and their identity. This hypothesis could be tested by abolishing the specification of the tendon-like precursors.
Author Contributions
CS, conception and design of the work, analysis and interpretation of data, drafting the work, final approval, and agreement for all aspects of the work; LL, acquisition, analysis, and interpretation of data, revising the work, final approval, and agreement for all aspects of the work; KJ, interpretation of data, revising the work, final approval, and agreement for all aspects of the work.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), the Association Française contre les Myopathies (AFM), the Fondation pour la Recherche Medicale (FRM) and the national grant IDE-CELL-SPE from the Agence Nationale de la Recherche (ANR).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abmayr, S. M., and Pavlath, G. K. (2012). Myoblast fusion: lessons from flies and mice. Development 139, 641–656. doi: 10.1242/dev.068353
Ball, E. E., and Goodman, C. S. (1985). Muscle development in the grasshopper embryo: II. Syncytial origin of the extensor tibiae muscle pioneers. Dev. Biol. 111, 399–416. doi: 10.1016/0012-1606(85)90493-2
Ball, E. E., Ho, R. K., and Goodman, C. S. (1985a). Development of neuromuscular specificity in the grasshopper embryo: guidance of motoneuron growth cones by muscle pioneers. J. Neurosci. 5, 1808–1819.
Ball, E. E., Ho, R. K., and Goodman, C. S. (1985b). Muscle development in the grasshopper embryo: I. Muscles, nerves, and apodemes in the metathoracic leg. Dev. Biol. 111, 383–398. doi: 10.1016/0012-1606(85)90492-0
Bate, M. (1990). The embryonic development of larval muscles in Drosophila. Development 110, 791–804.
Bate, M., Rushton, E., and Currie, D. A. (1991). Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development 113, 79–89.
Baylies, M. K., Bate, M., and Gomez, M. R. (1998). Myogenesis: a view from Drosophila. Cell 93, 921–927. doi: 10.1016/S0092-8674(00)81198-8
Becker, S., Pasca, G., Strumpf, D., Min, L., and Volk, T. (1997). Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124, 2615–2622.
Brent, A. E., Braun, T., and Tabin, C. J. (2005). Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse. Development 132, 515–528. doi: 10.1242/dev.01605
Brent, A. E., Schweitzer, R., and Tabin, C. J. (2003). A Somitic compartment of tendon progenitors. Cell 113, 235–248. doi: 10.1016/S0092-8674(03)00268-X
Brent, A. E., and Tabin, C. J. (2004). FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131, 3885–3896. doi: 10.1242/dev.01275
Broadie, K. S., and Bate, M. (1991). The development of adult muscles in Drosophila: ablation of identified muscle precursor cells. Development 113, 103–118.
Buckingham, M., Bajard, L., Chang, T., Daubas, P., Hadchouel, J., Meilhac, S., et al. (2003). The formation of skeletal muscle: from somite to limb. J. Anat. 202, 59–68. doi: 10.1046/j.1469-7580.2003.00139.x
Chen, J. W., and Galloway, J. L. (2014). The development of zebrafish tendon and ligament progenitors. Development 141, 2035–2045. doi: 10.1242/dev.104067
Currie, D. A., and Bate, M. (1991). The development of adult abdominal muscles in Drosophila: myoblasts express twist and are associated with nerves. Development 113, 91–102.
de Joussineau, C., Bataillé, L., Jagla, T., and Jagla, K. (2012). Diversification of muscle types in Drosophila: upstream and downstream of identity genes. Curr. Top. Dev. Biol. 98, 277–301. doi: 10.1016/B978-0-12-386499-4.00011-2
Dobi, K. C., Schulman, V. K., and Baylies, M. K. (2015). Specification of the somatic musculature in Drosophila. Wiley Interdiscip. Rev. Dev. Biol. 4, 357–375. doi: 10.1002/wdev.182
Edom-Vovard, F., Schuler, B., Bonnin, M.-A., Teillet, M.-A., and Duprez, D. (2002). Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 247, 351–366. doi: 10.1006/dbio.2002.0707
Fernandes, J., Bate, M., and Vijayraghavan, K. (1991). Development of the indirect flight muscles of Drosophila. Dev. Camb. Engl. 113, 67–77.
Fernandes, J. J., Celniker, S. E., and VijayRaghavan, K. (1996). Development of the indirect flight muscle attachment sites in Drosophila: role of the PS integrins and the stripe gene. Dev. Biol. 176, 166–184. doi: 10.1006/dbio.1996.0125
Frommer, G., Vorbrüggen, G., Pasca, G., Jäckle, H., and Volk, T. (1996). Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 15, 1642–1649.
Ghazi, A., Anant, S., and VijayRaghavan, K. (2000). Apterous mediates development of direct flight muscles autonomously and indirect flight muscles through epidermal cues. Development 127, 5309–5318.
Ghazi, A., Paul, L., and VijayRaghavan, K. (2003). Prepattern genes and signaling molecules regulate stripe expression to specify Drosophila flight muscle attachment sites. Mech. Dev. 120, 519–528. doi: 10.1016/S0925-4773(03)00042-X
Grenier, J., Teillet, M.-A., Grifone, R., Kelly, R. G., and Duprez, D. (2009). Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS ONE 4:e4381. doi: 10.1371/journal.pone.0004381
Hatini, V., and DiNardo, S. (2001). Distinct signals generate repeating striped pattern in the embryonic parasegment. Mol. Cell 7, 151–160. doi: 10.1016/S1097-2765(01)00163-0
Ho, R. K., Ball, E. E., and Goodman, C. S. (1983). Muscle pioneers: large mesodermal cells that erect a scaffold for developing muscles and motoneurones in grasshopper embryos. Nature 301, 66–69. doi: 10.1038/301066a0
Huang, A. H., Lu, H. H., and Schweitzer, R. (2015). Molecular regulation of tendon cell fate during development. J. Orthop. Res. 33, 800–812. doi: 10.1002/jor.22834
Kardon, G. (1998). Muscle and tendon morphogenesis in the avian hind limb. Development 125, 4019–4032.
Kim, J. H., Ren, Y., Ng, W. P., Li, S., Son, S., Kee, Y.-S., et al. (2015). Mechanical tension drives cell membrane fusion. Dev. Cell 32, 561–573. doi: 10.1016/j.devcel.2015.01.005
Kramer, S. G., Kidd, T., Simpson, J. H., and Goodman, C. S. (2001). Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science 292, 737–740. doi: 10.1126/science.1058766
Lejard, V., Blais, F., Guerquin, M.-J., Bonnet, A., Bonnin, M.-A., Havis, E., et al. (2011). EGR1 and EGR2 involvement in vertebrate tendon differentiation. J. Biol. Chem. 286, 5855–5867. doi: 10.1074/jbc.M110.153106
Leptin, M. (1991). Twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5, 1568–1576. doi: 10.1101/gad.5.9.1568
Maqbool, T., Soler, C., Jagla, T., Daczewska, M., Lodha, N., Palliyil, S., et al. (2006). Shaping leg muscles in Drosophila: role of ladybird, a conserved regulator of appendicular myogenesis. PLoS ONE 1:e122. doi: 10.1371/journal.pone.0000122
Medler, S., and Mykles, D. L. (2015). “Muscle structure, fiber types, and physiology,” in Physiology, the Natural History of the Crustacean, eds E. S. Chang and M. Thiel, (Oxford: Oxford University Press), 103–133.
Miller, A. (1950). “The internal anatomy and histology of the imago of Drosophila melanogaster,” in Biology of Drosophila ed M. Demerec, (New York, NY: John Wiley & Sons), 420–531.
Nabel-Rosen, H., Volohonsky, G., Reuveny, A., Zaidel-Bar, R., and Volk, T. (2002). Two isoforms of the Drosophila RNA binding protein, how, act in opposing directions to regulate tendon cell differentiation. Dev. Cell 2, 183–193. doi: 10.1016/S1534-5807(01)00118-6
Piepenburg, O., Vorbrüggen, G., and Jäckle, H. (2000). Drosophila segment borders result from unilateral repression of hedgehog activity by wingless signaling. Mol. Cell 6, 203–209. doi: 10.1016/S1097-2765(05)00011-0
Radnikow, G., and Bässler, U. (1991). Function of a muscle whose apodeme travels through a joint moved by other muscles: why the retractor unguis muscle in stick insects is tripartite and has no antagonist. J. Exp. Biol. 157, 87–99.
Rochlin, K., Yu, S., Roy, S., and Baylies, M. K. (2010). Myoblast fusion: when it takes more to make one. Dev. Biol. 341, 66–83. doi: 10.1016/j.ydbio.2009.10.024
Roy, S., and VijayRaghavan, K. (1999). Muscle pattern diversification in Drosophila: the story of imaginal myogenesis. BioEssays 21, 486–498.
Schnorrer, F., and Dickson, B. J. (2004). Muscle building: mechanisms of myotube guidance and attachment site selection. Dev. Cell 7, 9–20. doi: 10.1016/j.devcel.2004.06.010
Schweitzer, R., Zelzer, E., and Volk, T. (2010). Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807–2817. doi: 10.1242/dev.047498
Soler, C., Daczewska, M., Da Ponte, J. P., Dastugue, B., and Jagla, K. (2004). Coordinated development of muscles and tendons of the Drosophila leg. Development 131, 6041–6051. doi: 10.1242/dev.01527
Tixier, V., Bataillé, L., and Jagla, K. (2010). Diversification of muscle types: recent insights from Drosophila. Exp. Cell Res. 316, 3019–3027. doi: 10.1016/j.yexcr.2010.07.013
Usui, K., Pistillo, D., and Simpson, P. (2004). Mutual exclusion of sensory bristles and tendons on the notum of dipteran flies. Curr. Biol. 14, 1047–1055. doi: 10.1016/j.cub.2004.06.026
Volk, T. (1999). Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 15, 448–453. doi: 10.1016/S0168-9525(99)01862-4
Volk, T., and VijayRaghavan, K. (1994). A central role for epidermal segment border cells in the induction of muscle patterning in the Drosophila embryo. Development 120, 59–70.
Volohonsky, G., Edenfeld, G., Klämbt, C., and Volk, T. (2007). Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development 134, 347–356. doi: 10.1242/dev.02735
Vorbrüggen, G., and Jäckle, H. (1997). Epidermal muscle attachment site-specific target gene expression and interference with myotube guidance in response to ectopic stripe expression in the developing Drosophila epidermis. Proc. Natl. Acad. Sci. U.S.A. 94, 8606–8611. doi: 10.1073/pnas.94.16.8606
Keywords: tendon, muscle development, leg disc, tissue interactions, Drosophila
Citation: Soler C, Laddada L and Jagla K (2016) Coordinated Development of Muscles and Tendon-Like Structures: Early Interactions in the Drosophila Leg. Front. Physiol. 7:22. doi: 10.3389/fphys.2016.00022
Received: 16 September 2015; Accepted: 15 January 2016;
Published: 04 February 2016.
Edited by:
Elzbieta M. Pyza, Jagiellonian University, PolandReviewed by:
Peter Bräunig, Rheinisch-Westfälische Technische Hochschule, GermanyXiaojing J. Gao, Stanford University, USA
Michael Taylor, Cardiff University, UK
Copyright © 2016 Soler, Laddada and Jagla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cedric Soler, cedric.soler@udamail.fr;
Krzysztof Jagla, christophe.jagla@udamail.fr
 Cedric Soler
Cedric Soler Lilia Laddada
Lilia Laddada Krzysztof Jagla
Krzysztof Jagla