- 1Department of Biomedicine, Aarhus University, Aarhus, Denmark
- 2Centre for Membrane Pumps in Cells and Disease-PUMPKIN, Danish National Research Foundation, Department of Molecular Biology and Genetics, Aarhus University, Aarhus, Denmark
- 3Aarhus Institute of Advanced Studies, Aarhus University, Aarhus, Denmark
A functional Na+/K+-ATPase consists of a catalytic α subunit and a regulatory β subunit. Four α isoforms of the Na+/K+-ATPase are found in mammals, each with a unique expression pattern and catalytic activity. The α2 isoform, encoded by the ATP1A2 gene, is primarily found in the central nervous system (CNS) and in heart-, skeletal- and smooth muscle tissues. In the CNS, the α2 isoform is mainly expressed in glial cells. In particular, the α2 isoform is found in astrocytes, important for astrocytic K+ clearance and, consequently, the indirect uptake of neurotransmitters. Both processes are essential for proper brain activity, and autosomal dominantly mutations in the ATP1A2 gene cause the neurological disorder Familial hemiplegic migraine type 2 (FHM2). FHM2 is a severe subtype of migraine with aura including temporary numbness or weakness, and affecting only one side of the body. FHM2 patients often suffer from neurological comorbidities such as seizures, sensory disturbances, cognitive impairment, and psychiatric manifestations. The functional consequences of FHM2 disease mutations leads to a partial or complete loss of function of pump activity; however, a clear phenotype-genotype correlation has yet to be elucidated. Gene-modified mouse models targeting the Atp1a2 gene have proved instrumental in the understanding of the pathology of FHM2. Several Atp1a2 knockout (KO) mice targeting different exons have been reported. Homozygous Atp1a2 KO mice die shortly after birth due to respiratory malfunction resulting from abnormal Cl− homeostasis in brainstem neurons. Heterozygous KO mice are viable, but display altered behavior and neurological deficits such as altered spatial learning, decreased motor activity and enhanced fear/anxiety compared to wild type mice. FHM2 knock-in (KI) mouse models carrying the human in vivo disease mutations W887R and G301R have also been reported. Both models display altered cortical spreading depression (CSD) and point to deficits in the glutamatergic system as the main underlying mechanism of FHM2.
The Na+/K+-ATPase: Expression and Function
The Na+/K+-ATPase is a transmembrane ion-pump located at the plasma membrane of all mammalian cells. It's function moves three Na+ ions out of the cell and two K+ ions into the cell utilizing energy from ATP hydrolysis (Skou, 1957).
A functional pump consists of a catalytic α-subunit and a regulatory β-subunit (Kaplan, 2002). Four α-subunit isoforms, designated as α1-α4, encoded by different genes are found in mammals. Each α-subunit isoform has its own unique expression pattern and catalytic activity, which can be modulated by the β-subunit (Larsen et al., 2014; Hilbers et al., 2016). The ATP1A1 gene encodes the α1isoform, which is considered to be the isoform that maintains basic cellular functions and is accordingly expressed almost ubiquitously in all tissue and cell types (Lingrel et al., 2007). The α2 isoform, encoded by the ATP1A2 gene, is primarily expressed in the central nervous system (CNS) and in heart-, skeletal,- and smooth muscle tissues. In the CNS, the α2 isoform is expressed primarily in astrocytes but also in other glial cells (McGrail et al., 1991). During embryonic development, the α2 isoform is expressed primarily in neurons, which gradually changes to the glial cell expression pattern in the adult, murine brain (McGrail et al., 1991; Cameron et al., 1994; Cholet et al., 2002; Moseley et al., 2003).
The α3 isoform, encoded by the ATP1A3 gene, is also expressed in the CNS and nervous tissue, but expresses specifically in the neurons of the basal ganglia, cerebellum, and hippocampus (McGrail et al., 1991; Bøttger et al., 2011; Li et al., 2013).
General Na+/K+-ATPase activity is crucial for multiple cellular functions such as maintaining resting membrane potential, regulating cellular volume, regulating pH, and driving the secondary active transport (Dobretsov and Stimers, 2005). Regulated and specialized pump activity is important for normal brain function. This is also reflected in the expressive relationship between the α1, α2, and α3 isoforms in the CNS and the complex neurological disorders caused by mutations in the ATP1A2 and ATP1A3 genes (reviewed in Bøttger et al., 2012; Heinzen et al., 2014). Furthermore, increased evidence suggests roles for the Na+/K+-ATPase in regulating signaling pathways, such as: the membrane-associated non-receptor tyrosine kinase Src: activation of Ras/Raf/ERK1,2, phosphatidylinositol 3-kinase (PI3K): PI3K-dependent protein kinase B, phospholipase C, ()i oscillations (Aperia, 2007; Schoner and Scheiner-Bobis, 2007; Liu and Xie, 2010): and gene transcription (Egr-1, Fos, Jun, Nr4a2, Hes1, and Gabre; Tupler et al., 2001).
The α2 Isoform and Its Function in Astrocytes
Astrocytes are some of the most abundant cells in the CNS of mammals, with a ratio of 1.4 astrocytes for every neuron in the human cortex (Nedergaard et al., 2003). Through their physical properties and neuron-glial signaling pathways, they play an essential role in neuronal homeostasis through their physical properties and neuron-glial signaling pathways. During neuronal activity, a vast efflux of K+ ions into the extracellular space occurs. The extracellular concentration of K+ ranges from 2.5 to 3.5 mM in normal conditions but can increase to 50–80 mM under ischemic and cortical spreading depression events (Walz, 2000).
Since imbalances between extracellular K+ and K+ clearance can affect neuronal excitability and abnormal brain function as well as cause significant neuronal death. Even under conditions of abundant glucose supply, extracellular K+ homeostasis must be tightly regulated to support normal brain activity (Walz, 2000).
Spatial buffering plays a critical role in the mechanism of K+ clearance (Karwoski et al., 1989; Walz, 2000; Kofuji and Newman, 2004). This principle is based on the influx of K+ into astrocytes though K+ channels, with the central channel being Kir4.1, a member of the inward rectifier-type potassium channel family. This positive influx then spreads electronically through the cytoplasm of the astrocyte and exits again as K+ at locations distant from the active neurons (Karwoski et al., 1989; Kofuji and Newman, 2004; Macaulay and Zeuthen, 2012). In addition to Kir4.1, both the Na+/K+-ATPase and the Na+/K+/2Cl− cotransporter 1 (NKCC1) are necessary for the import and export of K+ in the astrocytes (Galvan et al., 1979; D'Ambrosio et al., 2002; MacVicar et al., 2002; reviewed in Walz, 2000; Hertz et al., 2015). This is further described elsewhere in this special issue by MacAulay and colleagues.
In the Na+/K+-ATPase, the α2 isoform combined with the β2 subunit have K+ affinity and voltage-sensitivity. As a result it is specifically geared to control extracellular K+ concentration during intense neuronal firing (Larsen et al., 2014). Furthermore, the α2 and β2 subunit isoforms are co-expressed and appears to co-localize in astrocytes in the rodent brain (McGrail et al., 1991; Fink et al., 1996; Knapp et al., 2000; Cholet et al., 2002). Thus, the α2/β2 complex appears to be the main Na+/K+-ATPase constellation involved in the astrocytic K+ clearance (Larsen et al., 2014).
Controlling the neurotransmitter levels present in the synapse is required for functional neuronal signaling, plasticity and neuroprotection. Removal of neurotransmitters occurs through diffusion, enzymatic degradation, and the reuptake by neurons and glial cells. The importance of astrocytic neurotransmitter uptake is highlighted in mice deficient of the astrocytic glutamate transporter excitatory amino acid transporter 2 (EAAT2), where Eatt2-deficiency causes lethal spontaneous seizures and increased susceptibility to acute cortical injury in mice (Tanaka et al., 1997). The α2 isoform localizes predominately in astrocytes and other glial cells (McGrail et al., 1991; Fink et al., 1996; Knapp et al., 2000; Cholet et al., 2002) and has been shown to co-distribute with both astrocytic glutamate transporters excitatory amino acid transporter 1 (EAAT1) and EAAT2 (Cholet et al., 2002). Additionally, the α2 isoform immunoprecipitated with both EAAT1 and EAAT2 in rat cerebellar and forebrain tissues, respectively (Rose et al., 2009). In the same study, it was shown that the ouabain-mediated inhibition of Na+/K+-ATPase activity had an inhibitory effect on glutamate uptake. Another study similarly found the pump combination α2/β2 in a protein complex with glutamate and lactate transports, thereby sustaining glutamate-dependent lactate transport (Kleene et al., 2007). The uptake of glutamate via glutamate transporters is driven by the Na+-gradient. Astrocytes, therefore, rely on Na+,K+-ATPases to pump out accumulated Na+ (Grewer and Rauen, 2005). The direct interaction between the α2 isoform and astrocytic glutamate transporters highlights this dependency, suggesting the existence of local regulation and crosstalk between Na+/K+-ATPases activity and glutamate uptake. Based on the dependency of many other neurotransmitter transporters on the Na+-gradient, it is likely that the Na+/K+-ATPase is also functionally linked to these transporters. Numerous studies have shown a coupling between the α3 subunit and neurotransmitter transport such as the glycine transporter GlyT2 (de Juan-Sanz et al., 2013).
Familial Hemiplegic Migraine
Familial hemiplegic migraine (FHM) is an autosomal dominant inherited migraine with aura and typically includes episodes of temporary numbness or weakness affecting one side of the body (hemiparesis). There are three recognized subtypes of FHM (FHM1-3), each caused by distinctive mutations in a specific gene. FHM Type 1, which accounts for around 50% of all FHM cases, is caused by mutations in the CACNA1A gene encoding the Cav2.1 P/Q voltage-dependent calcium channel (Ophoff et al., 1996). FHM Type 2 is caused by mutations in the ATP1A2 gene (De Fusco et al., 2003) and FHM type 3 is caused by mutations in the SCN1A gene encoding the Na+ channel, voltage-gated, type I, α subunit (Dichgans et al., 2005). Thus, all three types of FHM are related to alterations in ion-transport and ion-homostasis.
FHM patients in general share common migraine associated symptoms such as nausea, vomiting, and increased sensitivity to stimuli such as sound and light. In addition, diverse comorbidities have in addition been observed for each subtype and also for different mutations in a given subtype. In FHM2, observed neurological symptoms include sensory disturbance, impaired vision during aura episodes, speech difficulties, varied forms of epilepsy and seizures, developmental disabilities, cognitive impairments and cerebellar defects, which cause ataxia, dysarthria, and nystagmus. Rarer manifestations such as psychiatric disorders (depression, borderline personality, and obsessive-compulsive disorder), obesity, dystonic posturing, and anxiety have also been reported (Barrett et al., 2008; Bøttger et al., 2012; Ferrari et al., 2015).
The genetic linkage between ATP1A2 and FHM2 was first made in 2003 (Marconi et al., 2003). In this study of two large Italian families with extensive history of FHM2, the FHM2 locus was narrowed to a 0.9 Mb region on chromosome 1q23 wherein mutations in the ATP1A2 gene were first identified. Subsequent sequence analysis of candidate genes revealed specific FHM2-associated mutations in the ATP1A2 gene in each of the families (De Fusco et al., 2003). Numerous new mutations in the ATP1A2 gene have been reported post-analysis, indicating that there are more than 35 FHM2-associated mutations, spanning most of the ATP1A2 coding region (Bøttger et al., 2012). Approximately half of the FHM2 mutations tested in vitro show complete loss of pump function, while a majority of the remainder showed reduced pump activity relative to normal α2 pump activity. A small number of mutations either prevent α2 expression or prevent the transport of α2 to the plasma membrane (Bøttger et al., 2012).
To date, genotype-phenotype correlations have yet to be made, yet it is likely that the severity of the main symptoms and the spectra of comorbidities are linked to the effect of the mutation.
Due to the heterogeneity of human patients, however, it may be impossible to correlate genotype-phenotype by clinical patients studies. Therefore animal models are important tools for understanding the spectrum of FHM2 phenotypes and to elucidate the mechanisms behind FHM2.
Gene-Modified α2 Mouse Models
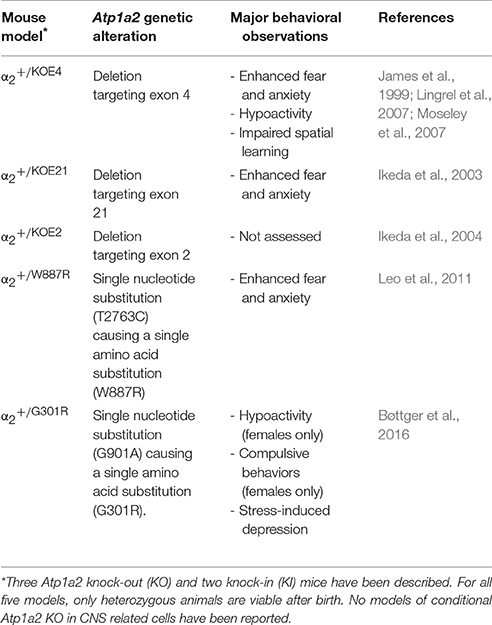
Several gene-modified mouse models targeting the Atp1a2 gene have been reported, and extensively used to study the in vivo functions of the α2 isoform (Table 1).
Only heterozygous α2 knock-out (KO) and knock-in (KI) mice are viable, as homozygous mice dies immediately after birth (James et al., 1999; Ikeda et al., 2003, 2004; Leo et al., 2011). These immature deaths of homozygous mice were reportedly caused by respiratory failure in α2KOE2/KOE2 mice (Ikeda et al., 2004). The activity of respiratory motor neurons in the fourth cervical ventral root were altered in α2KOE2/KOE2 fetuses, as the response of these neurons to electrical stimulation in the ventrolateral medulla was significantly slower in homozygous KO fetuses compared to wild type fetuses (Ikeda et al., 2004). A higher intracellular Cl− concentration was found in these ventrolateral medulla neurons in α2KOE2/KOE2 fetuses. Furthermore, the α2 isoform co-immunoprecipitated with the K+-Cl− cotransporter KCC2, suggesting a functional coupling (Ikeda et al., 2004; Pedersen et al., 2006). This is supported by the fact that homozygous Kcc2 KO mice also die immediately after birth due to severe motor deficits that abolish respiration (Hübner et al., 2001). Thus, homozygous α2KOE2/KOE2 mice with a complete loss of the α2 isoform function, exhibit severe deficits in Cl− homeostasis in vulnerable cells including the respiratory center neurons, thereby causing abnormal neuronal activity and resulting in respiratory failure upon birth.
α2 Isoform Knock-Out Mice
Relative to WT littermates, heterozygous α2+/KOE21 and α2+/KOE4 mice showed a 50% reduction in the α2 isoform protein levels (Ikeda et al., 2003; Moseley et al., 2007). The α1 isoform protein levels were comparable between the two groups of mice, whereas α3 isoform protein levels in α2+/KOE4 mice were reduced to 80% relative to WT (Moseley et al., 2007).
Both α2+/KOE4 and α2+/KOE21 mice models displayed increased fear and anxiety behavior as the main abnormal behavioral phenotype (Ikeda et al., 2003; Lingrel et al., 2007; Moseley et al., 2007). Characteristic of these behavioral phenotypes, the α2+/KOE21 mice had fewer entries and spent less time in the open arms in the elevated plus maze test (Ikeda et al., 2003). In a similar zero maze test screening for fear and anxiety, the same pattern was observed in in α2+/KOE4 mice (Moseley et al., 2007). Another common anxiety behavioral test is the light/dark test, which consists of one illuminated room connected to one dark room. The mouse to be tested is placed in the dark room. The time of first entry into the illuminated room and all subsequent periods of time spent in this room are recorded. Compared to WT littermates, α2+/KOE21 mice not only spent significantly less time in the illuminated room, but the latency to enter the illuminated room was also significantly higher (Ikeda et al., 2003), indicating increased fear and anxiety behavior phenotypes.
In the open field test, α2+/KOE4 mice were found to be overall less active compared to WT mice (Moseley et al., 2007). In contrast, no altered spontaneous activity in home cage was observed for the α2+/KOE21 mice (Ikeda et al., 2003). This suggests that the hypolocomotion observed in the open field test was induced by the enhanced stress and anxiety response in α2+/KOE4 mice, caused by the unfamiliar and open environment of the open field setup. This correlates with the observation that α2+/KOE4 mice spend more time in the periphery region instead of the center region during the open field test, which is a commonly used indicator of enhanced fear behavior (Moseley et al., 2007).
Utilizing the Morris water maze test, α2+/KOE4 mice were also tested for spatial learning and memory (Moseley et al., 2007). The latency to find the hidden platform was higher for α2+/KOE4 mice compared to WT, which normally indicates impaired learning. However, the distance traveled to reach the platform was comparable for α2+/KOE4 and WT mice, suggesting no impairment of spatial memory (Moseley et al., 2007). Thus, the increased latency could be a consequence of the enhanced fear and anxiety related behavior observed in α2+/KOE4 mice, as the immersion in water is highly stressful to the mouse. In support of this, the α2+/KOE21 mice display an increased freezing time in conditioned fear stimuli, which suggests that learning and memory capability is not impaired (Ikeda et al., 2003).
The enhanced fear and anxiety behavior was investigated further in α2+/KOE21 mice and homozygous α2KOE21/KOE21 fetuses. It was found that homozygous α2KOE21/KOE21 fetuses displayed selective neuronal apoptosis in the amygdala and piriform cortex, both regions involved in fear, anxiety and basic defense mechanism behavior (Davis et al., 1994; Ikeda et al., 2003). Furthermore, c-Fos expression was significantly elevated in these regions in α2KOE21/KOE21 fetuses and also in adult α2+/KOE21 mice after conditioned fear stimuli, which indicate neuronal hyperactivity in the areas (Ikeda et al., 2003). An increase in the excitability of amygdala output neurons increased aversive fear conditioning (Davis et al., 1994), thus the enhanced fear and anxiety behavior of α2 KO mice probably arise from abnormal function of these brain regions.
Uptake of glutamate and GABA into crude synaptosome preparations from α2KOE21/KOE21 fetuses was impaired compared to WT preparations and consequently, both glutamate and GABA levels were increased in brains from α2KOE21/KOE21 fetuses relative to WT littermates (Ikeda et al., 2003). A dysfunction in the removal of neurotransmitters from synapse could be the underlying cause of neuronal hyperactivity and of the observed neurodegeneration in the amygdala and piriform cortex. This is further supported by other studies showing co-localization, molecular interaction and functional interaction between α2 and glutamate transporters (Cholet et al., 2002; Kleene et al., 2007; Rose et al., 2009). It is reasonable to suggest that heterozygous mice also share these abnormalities, which cause the observed behavioral phenotypes in α2+/KOE21 mice. Another plausible explanation, as observed in α2KOE2/KOE2 fetuses, is the α2 isoform interaction with KCC2 and altered intracellular Cl− concentration, which appears to be the cause of respiratory failure in α2-/- mice (Ikeda et al., 2004; Pedersen et al., 2006). This Cl− homeostasis deficit could result in enhanced fear and anxiety behavior for all α2+/- mice. Similar to the α2+/KOE4 and α2+/KOE21 mice, hypomorphic mice with 17% KCC2 levels display increased anxiety-like behavior in several behavioral tests, including the elevated-plus maze test (Ikeda et al., 2003; Tornberg et al., 2005; Moseley et al., 2007).
While most neurological studies involving the α2 subunit focus on its effect in FHM2, several recent studies have also linked the Na+/K+-ATPase to other critical neurological disorders such as Amyotrophic lateral sclerosis (ALS), Huntington's disease and Alzheimer's disease (Acuña et al., 2013; Valencia et al., 2013; Gallardo et al., 2014; Ohnishi et al., 2015). In the ALS study, the α2+/KOE4 model was used to study the pathology of this disease. A protein complex consisting of α2 and α-adducin was found enriched in cultured astrocytes expressing mutant superoxide dismutase 1 (SOD1), the most common genetic factor for ALS (Gallardo et al., 2014). Interestingly, knock down of α2 in cultured astrocytes protected co-cultured motor neurons from degeneration, and mating α2+/KOE4 and SOD1G93A mutant mice significantly reduced neurodegeneration, thereby increasing the lifespan of α2+/KOE4- SOD1G93A mice compared to SOD1G93A mice (Gallardo et al., 2014).
α2 Isoform Knock-In Mice
Western blotting for α2 isoform protein in brain lysates showed reduced α2 protein levels to around 60% in heterozygous adult α2+/W887R (Leo et al., 2011) and α2+/G301R mice (Bøttger et al., 2016) and to very minimal levels in homozygous fetuses of α2G301R/G301R. Specific brain regions were additionally probed in the α2+/G301R mice with similar results (Bøttger et al., 2016). No alteration in the α1 and α3 subunit isoform protein levels was found in either of the KI mouse models (Leo et al., 2011; Bøttger et al., 2016), suggesting that these isoforms are not upregulated to compensate for the haploinsufficiency of the α2 subunit in the α2+/W887R and α2+/G301R mice.
Mutated α2-W887R transfected into HeLa cells was expressed but did not properly transport to the cell surface. Instead, it was found confined to the endoplasmic reticulum and proteasome inhibition with MG132 increased α2-W887R levels significantly (Leo et al., 2011). This suggests improper folding and subsequently proteasomal degradation as the fate of α2-W887R. However, transfection of α2-W887R into COS7 cells, showed α2-W887R at the plasma membrane, by both immunofluorescence staining and by subcellular fractionation (De Fusco et al., 2003). Interestingly, α2-W887R located to the plasma membrane when expressed in Xenopus laevis oocytes (Koenderink et al., 2005). On measuring ion flux and ATPase activity, this W887R mutation was shown to be inactive (Koenderink et al., 2005).
In vitro studies on α2-G301R found that this mutation did not cause significant changes in mRNA stability as mRNA levels in HeLa cells transfected with either α2 or α2-G301R, respectively, were comparable (Santoro et al., 2011). However, immunofluorescence staining for transfected α2-G301R showed that the mutated version did not express properly. Thus, it was concluded that this loss of function mutation prompts deficits in folding, maturation, or intracellular trafficking of the α2-G301R that causes it to be degraded (Santoro et al., 2011). From these studies and the reduced α2 levels in α2+/W887R and α2+/G301R mice, it appears reasonable to consider both mutated pumps expressed but display no functional pump activity due to improper transport and/or degradation. This is an important difference to the KO models, since mutated α2 protein (α2-W887R and α2-G301R) might still be able to interact with important factors such as the β subunits that could have an effect on the unmutated α2 isoform in these models.
The phenotypic behavioral consequences of introducing the W887R mutation were assessed using a modified SHIRPA protocol (Leo et al., 2011). In this protocol, few behavioral differences were observed between α2+/W887R and WT mice. The only significant alteration between α2+/W887R mice compared to WT littermates was an elevated fear response and increased anxiety (Leo et al., 2011).
Behavioral phenotypes were likewise addressed in the α2+/G301R mice. During the tail suspension test, for example, the α2+/G301R mice exhibited depression-like behavior, such as increased immobility, compared to WT mice (Bøttger et al., 2016). In a sucrose preference test, α2+/G301R mice displayed stress-induced anhedonia and increased acoustic startle responses, implying abnormal levels of fear and anxiety (Bøttger et al., 2016). Female α2+/G301R mice were found to have additional abnormal behavioral phenotypes compared to male α2+/G301R mice. Unlike WT females and male α2+/G301R mice, α2+/G301R females were hypoactive in open field test (Bøttger et al., 2016). Excessive grooming behaviors were also observed in the open field, suggesting compulsive behavior in female α2+/G301R mice. The marble-burying test is one of the standard behavioral tests for rodents to assess repetitive compulsive-like behaviors. Female α2+/G301R mice were found to display obsessive compulsive disorder (OCD)-like traits as they buried significantly more marbles compared to both WT and to male α2+/G301R mice.
Interestingly, these female-specific phenotypes were reverted by a single progestin treatment, implying that the female hormone cycle contributes to the altered behaviors noted in α2+/G301R females compared to WT females and male α2+/G301R mice. Intriguingly, this coincides with the influence of ovarian hormones on migraines, and the higher prevalence of migraines in women compared to men (Vos et al., 2012).
N-Methyl-D-aspartic acid or N-Methyl-D-aspartate (NMDA)-receptor antagonists also reverted specific α2+/G301R phenotypes including hypoactivity, OCD-like behaviors and increased acoustic stimuli startle response. This indicates that the glutamatergic system is affected by the mutated α2 isoform. In support of this, α2G301R/G301R mice had elevated glutamate levels in all major brain regions. Mixed astrocyte-neuron cultures from α2G301R/G301R mice displayed a significant reduction in glutamate uptake compared to similar cultures from WT mice (Bøttger et al., 2016). This further links the female sex hormone cycle and the glutamate system to the comorbid psychiatric manifestations of FHM2.
Cortical Spreading Depression
A key characteristic of FHM2 is the appearance of the aura phenomenon prior to the onset of the hemiplegic migraine. It is widely accepted that cortical spreading depression (CSD) is the molecular mechanism behind aura (Lauritzen et al., 2011). CSD is a short-lasting wave of neuronal and glial cell hyperactivity followed by a wave of long-lasting depression of neuronal activity (Pietrobon and Moskowitz, 2013). During the intense neuronal activity, excess K+ ions and glutamate are released into the extracellular space. The inefficient removal of K+ and glutamate by astrocytes can lead to wide-reaching depolarization that depresses further neuronal firing (Lauritzen et al., 2011; Pietrobon and Moskowitz, 2013). The CSD usually begins in the visual cortex and propagates slowly over the cortex at a rate of 2–5 mm/min (Pietrobon and Moskowitz, 2013).
In vivo CSD recordings revealed that α2+/W887R mice were more susceptible to CSD. This is based on a 35% decrease in induction threshold relative to WT littermates (Leo et al., 2011). Furthermore, induced CSD events in the α2+/W887R mice had a higher propagation velocity compared to WT littermates (Leo et al., 2011). The duration of triggered CSD events, however, was not altered between α2+/W887R mice and littermates (Leo et al., 2011). Electrocorticography in α2+/G301R mice, compared to WT littermates, revealed a prolonged recovery phase following CSD (Bøttger et al., 2016). Further CSD assays are necessary to conclude the effects of the G301R mutation on CSD.
It is difficult to compare the CSD studies of the two α2-KI mouse models, due to differing parameter and assay conditions used in each study. Potassium acetate, for example, was used for CSD induction in the right somatosensory cortex in α2+/G301R mice, whereas incremental electrical current stimuli were delivered in the occipital cortex for the α2+/W887R mice. Moreover, the different use of anesthetics (α-chloralose versus urethane) affects the data collection, as previous studies of other anesthetics were shown to influence the CSD induction threshold and to propagate speed differently (Kudo et al., 2013). Furthermore, only male mice were used in the α2+/G301R study (Bøttger et al., 2016), whereas a mixed sex population was used for the α2+/W887R study (Leo et al., 2011). In particular, considering the female specific phenotypes of the α2+/G301R, it appears that sex and specific sex hormones play an important role in FHM2. To sufficiently compare these two models and the CSD underlying FHM2, a comparable study using the same CSD induction method, same anesthetics and a focus on sex-specific phenotypes is required.
The CSD abnormalities observed in both FHM2 models are comparable with two FHM1 knock-in mouse models of which revealed increased susceptibility to CSD compared to WT mice. This further supports CSD as an important symptom of migraines and FHM in general (van den Maagdenberg et al., 2004, 2010). To further emphasize the importance of sex and CSD, it was found that female FHM1 KI mice were more susceptible to CSD than male KI mice (Eikermann-Haerter et al., 2009). This sex difference was abolished with ovariectomy and senescence, further solidifying the role of the female sex hormone cycle, besides genetics, in the susceptibility to CSD (Eikermann-Haerter et al., 2009).
Perspectives on the Use of FHM2 Mouse Models
Migraines affect nearly 15% of the world population, and while FHM2 is a relatively rare migraine form, further insight into FHM2 will lead to a broader understanding of migraines in general (Vos et al., 2012). Animal models of human genetic disorders are important tools for investigators in elucidating the molecular and physiological deficits leading to the disorder. As discussed in this review, much has already been learned from existing Atp1a2 animal models (Ikeda et al., 2003, 2004; Lingrel et al., 2007; Moseley et al., 2007; Leo et al., 2011; Bøttger et al., 2016). The continued work with existing models and generating of new models with different FHM2 mutations will expand our understanding of FHM2. Further study of different FHM2 mouse models together with clinical descriptions of comparable FHM2 patients will shed light on any genotype-phenotype correlations, a critical aspect when studying a disorder with varying symptoms, severity, and different functional consequences of mutations.
FHM2 mouse models will be essential for drug development, drug screening and drug testing for treatment of FHM2 patients. A potential drug target for FHM2 is the Na+/K+-ATPase itself because lost pump function and/or decreased/altered pump activity is the main cause of FHM2 (Bøttger et al., 2012). Since all FHM2 patients are heterozygous, targeting the unmutated α2 isoform with activators could potentially overcome this haplodeficiency. In support of this therapeutic strategy, a recent report showed that crossing a Atp1a3 mouse model (Myskin) for Alternating Hemiplegia of Childhood (AHC) with a mouse model overexpressing a WT Atp1a3 allele could rescue the mutant phenotype through increased α3 pump activity (Kirshenbaum et al., 2016). Modulating Na+/K+-ATPase is also a desirable therapeutic strategy, due to this molecules' localization in the plasma membrane and well-regulated function. The mechanism of regulation is via factors (e.g., the cardiac glycoside ouabain) binding to the extracellular part of the pump.
An important aspect of Atp1a2 deficits appears to be the resulting changes to the glutamatergic system. These changes are manifested through molecular and histological interactions between the mutant α2-subunit isoform and a glutamate transporter (i.e., EAAT1 or EAAT2) or through reduced glutamate uptake in cells with limited pump activity. Our data suggests that future studies of mouse models with elevated glutamate levels (e.g., α2-KO and -KI mice), which exhibit reversion of key specific α2-KI phenotypes when targeted with NMDA receptor antagonists (Cholet et al., 2002; Ikeda et al., 2003; Rose et al., 2009; Bøttger et al., 2016), might prove beneficial as a preclinical model to test drug targets within the glutamatergic system in FHM2 patients.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
TI and KL were supported by a grant from the Danish National Research Foundation (DNRF) (PUMPKIN DNRF85). TI was further supported by the Health Faculty, Aarhus University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acuña, A. I., Esparza, M., Kramm, C., Beltrán, F. A., Parra, A. V., Cepeda, C., et al. (2013). A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington's disease in mice. Nat. Commun. 4, 2917. doi: 10.1038/ncomms3917
Aperia, A. (2007). New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 261, 44–52. doi: 10.1111/j.1365-2796.2006.01745.x
Barrett, C. F., van den Maagdenberg, A. M., Frants, R. R., and Ferrari, M. D. (2008). Familial hemiplegic migraine. Adv. Genet. 63, 57–83. doi: 10.1016/S0065-2660(08)01003-1
Bøttger, P., Doganli, C., and Lykke-Hartmann, K. (2012). Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci. Biobehav. Rev. 36, 855–871. doi: 10.1016/j.neubiorev.2011.10.005
Bøttger, P., Glerup, S., Gesslein, B., Illarionova, N. B., Isaksen, T. J., Heuck, A., et al. (2016). Glutamate-system defects behind psychiatric manifestations in a familial hemiplegic migraine type 2 disease-mutation mouse model. Sci. Rep. 6:22047. doi: 10.1038/srep22047
Bøttger, P., Tracz, Z., Heuck, A., Nissen, P., Romero-Ramos, M., and Lykke-Hartmann, K. (2011). Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J. Comp. Neurol. 519, 376–404. doi: 10.1002/cne.22524
Cameron, R., Klein, L., Shyjan, A. W., Rakic, P., and Levenson, R. (1994). Neurons and astroglia express distinct subsets of Na,K-ATPase alpha and beta subunits. Brain Res. Mol. Brain Res. 21, 333–343. doi: 10.1016/0169-328X(94)90264-X
Cholet, N., Pellerin, L., Magistretti, P. J., and Hamel, E. (2002). Similar perisynaptic glial localization for the Na+,K+-ATPase alpha 2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cereb. Cortex 12, 515–525. doi: 10.1093/cercor/12.5.515
D'Ambrosio, R., Gordon, D. S., and Winn, H. R. (2002). Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J. Neurophysiol. 87, 87–102. doi: 10.1152/jn.00240.2001
Davis, M., Rainnie, D., and Cassell, M. (1994). Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 17, 208–214. doi: 10.1016/0166-2236(94)90106-6
De Fusco, M., Marconi, R., Silvestri, L., Atorino, L., Rampoldi, L., Morgante, L., et al. (2003). Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 33, 192–196. doi: 10.1038/ng1081
de Juan-Sanz, J., Nunez, E., Villarejo-Lopez, L., Perez-Hernandez, D., Rodriguez-Fraticelli, A. E., Lopez-Corcuera, B., et al. (2013). Na+/K+-ATPase is a new interacting partner for the neuronal glycine transporter GlyT2 that downregulates its expression in vitro and in vivo. J. Neurosci. 33, 14269–14281. doi: 10.1523/JNEUROSCI.1532-13.2013
Dichgans, M., Freilinger, T., Eckstein, G., Babini, E., Lorenz-Depiereux, B., Biskup, S., et al. (2005). Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 366, 371–377. doi: 10.1016/S0140-6736(05)66786-4
Dobretsov, M., and Stimers, J. R. (2005). Neuronal function and alpha3 isoform of the Na/K-ATPase. Front. Biosci. 10, 2373–2396. doi: 10.2741/1704
Eikermann-Haerter, K., Dileköz, E., Kudo, C., Savitz, S. I., Waeber, C., Baum, M. J., et al. (2009). Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J. Clin. Invest. 119, 99–109. doi: 10.1172/JCI36059
Ferrari, M. D., Klever, R. R., Terwindt, G. M., Ayata, C., and van den Maagdenberg, A. M. (2015). Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 14, 65–80. doi: 10.1016/S1474-4422(14)70220-0
Fink, D., Knapp, P. E., and Mata, M. (1996). Differential expression of Na,K-ATPase isoforms in oligodendrocytes and astrocytes. Dev. Neurosci. 18, 319–326. doi: 10.1159/000111422
Gallardo, G., Barowski, J., Ravits, J., Siddique, T., Lingrel, J. B., Robertson, J., et al. (2014). An α2-Na/K ATPase/α-adducin complex in astrocytes triggers non-cell autonomous neurodegeneration. Nat. Neurosci. 17, 1710–1719. doi: 10.1038/nn.3853
Galvan, M., Bruggencate, G. T., and Senekowitsch, R. (1979). The effects of neuronal stimulation and ouabain upon extracellular K+ and Ca2+ levels in rat isolated sympathetic ganglia. Brain Res. 160, 544–548. doi: 10.1016/0006-8993(79)91084-9
Grewer, C., and Rauen, T. (2005). Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J. Membr. Biol. 203, 1–20. doi: 10.1007/s00232-004-0731-6
Heinzen, E. L., Arzimanoglou, A., Brashear, A., Clapcote, S. J., Gurrieri, F., Goldstein, D. B., et al. (2014). Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol. 13, 503–514. doi: 10.1016/S1474-4422(14)70011-0
Hertz, L., Song, D., Xu, J., Peng, L., and Gibbs, M. E. (2015). Role of the astrocytic Na(+), K(+)-ATPase in K(+) homeostasis in brain: K(+) uptake, signaling pathways and substrate utilization. Neurochem. Res. 40, 2505–2516. doi: 10.1007/s11064-014-1505-x
Hilbers, F., Kopec, W., Isaksen, T. J., Holm, T. H., Lykke-Hartmann, K., Nissen, P., et al. (2016). Tuning of the Na,K-ATPase by the beta subunit. Sci. Rep. 6:20442. doi: 10.1038/srep20442
Hübner, C. A., Stein, V., Hermans-Borgmeyer, I., Meyer, T., Ballanyi, K., and Jentsch, T. J. (2001). Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30, 515–524. doi: 10.1016/S0896-6273(01)00297-5
Ikeda, K., Onaka, T., Yamakado, M., Nakai, J., Ishikawa, T. O., Taketo, M. M., et al. (2003). Degeneration of the amygdala/piriform cortex and enhanced fear/anxiety behaviors in sodium pump alpha2 subunit (Atp1a2)-deficient mice. J. Neurosci. 23, 4667–4676.
Ikeda, K., Onimaru, H., Yamada, J., Inoue, K., Ueno, S., Onaka, T., et al. (2004). Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase alpha2 subunit-deficient mice is attributable to abnormal Cl- homeostasis in brainstem neurons. J. Neurosci. 24, 10693–10701. doi: 10.1523/JNEUROSCI.2909-04.2004
James, P. F., Grupp, I. L., Grupp, G., Woo, A. L., Askew, G. R., Croyle, M. L., et al. (1999). Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol. Cell 3, 555–563. doi: 10.1016/S1097-2765(00)80349-4
Kaplan, J. H. (2002). Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535. doi: 10.1146/annurev.biochem.71.102201.141218
Karwoski, C. J., Lu, H. K., and Newman, E. A. (1989). Spatial buffering of light-evoked potassium increases by retinal Muller (glial) cells. Science 244, 578–580. doi: 10.1126/science.2785716
Kirshenbaum, G. S., Dachtler, J., Roder, J. C., and Clapcote, S. J. (2016). Transgenic rescue of phenotypic deficits in a mouse model of alternating hemiplegia of childhood. Neurogenetics 17, 57–63. doi: 10.1007/s10048-015-0461-1
Kleene, R., Loers, G., Langer, J., Frobert, Y., Buck, F., and Schachner, M. (2007). Prion protein regulates glutamate-dependent lactate transport of astrocytes. J. Neurosci. 27, 12331–12340. doi: 10.1523/JNEUROSCI.1358-07.2007
Knapp, P. E., Itkis, O. S., and Mata, M. (2000). Neuronal interaction determines the expression of the alpha-2 isoform of Na, K-ATPase in oligodendrocytes. Brain Res. Dev. Brain Res. 125, 89–97. doi: 10.1016/S0165-3806(00)00125-5
Koenderink, J. B., Zifarelli, G., Qiu, L. Y., Schwarz, W., De Pont, J. J., Bamberg, E., et al. (2005). Na,K-ATPase mutations in familial hemiplegic migraine lead to functional inactivation. Biochim. Biophys. Acta. 1669, 61–68. doi: 10.1016/j.bbamem.2005.01.003
Kofuji, P., and Newman, E. A. (2004). Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. doi: 10.1016/j.neuroscience.2004.06.008
Kudo, C., Toyama, M., Boku, A., Hanamoto, H., Morimoto, Y., Sugimura, M., et al. (2013). Anesthetic effects on susceptibility to cortical spreading depression. Neuropharmacology 67, 32–36. doi: 10.1016/j.neuropharm.2012.10.018
Larsen, B. R., Assentoft, M., Cotrina, M. L., Hua, S. Z., Nedergaard, M., Kaila, K., et al. (2014). Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia 62, 608–622. doi: 10.1002/glia.22629
Lauritzen, M., Dreier, J. P., Fabricius, M., Hartings, J. A., Graf, R., and Strong, A. J. (2011). Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J. Cereb. Blood Flow Metab. 31, 17–35. doi: 10.1038/jcbfm.2010.191
Leo, L., Gherardini, L., Barone, V., De Fusco, M., Pietrobon, D., Pizzorusso, T., et al. (2011). Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 7:e1002129. doi: 10.1371/journal.pgen.1002129
Li, B., Hertz, L., and Peng, L. (2013). Cell-specific mRNA alterations in Na+, K+-ATPase alpha and beta isoforms and FXYD in mice treated chronically with carbamazepine, an anti-bipolar drug. Neurochem. Res. 38, 834–841. doi: 10.1007/s11064-013-0986-3
Lingrel, J. B., Williams, M. T., Vorhees, C. V., and Moseley, A. E. (2007). Na,K-ATPase and the role of alpha isoforms in behavior. J. Bioenerg. Biomembr. 39, 385–389. doi: 10.1007/s10863-007-9107-9
Liu, J., and Xie, Z. J. (2010). The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta. 1802, 1237–1245. doi: 10.1016/j.bbadis.2010.01.013
Macaulay, N., and Zeuthen, T. (2012). Glial K(+) clearance and cell swelling: key roles for cotransporters and pumps. Neurochem. Res. 37, 2299–2309. doi: 10.1007/s11064-012-0731-3
MacVicar, B. A., Feighan, D., Brown, A., and Ransom, B. (2002). Intrinsic optical signals in the rat optic nerve: role for K(+) uptake via NKCC1 and swelling of astrocytes. Glia 37, 114–123. doi: 10.1002/glia.10023
Marconi, R., De Fusco, M., Aridon, P., Plewnia, K., Rossi, M., Carapelli, S., et al. (2003). Familial hemiplegic migraine type 2 is linked to 0.9Mb region on chromosome 1q23. Ann. Neurol. 53, 376–381. doi: 10.1002/ana.10464
McGrail, K. M., Phillips, J. M., and Sweadner, K. J. (1991). Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J. Neurosci. 11, 381–391.
Moseley, A. E., Lieske, S. P., Wetzel, R. K., James, P. F., He, S., Shelly, D. A., et al. (2003). The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J. Biol. Chem. 278, 5317–5324. doi: 10.1074/jbc.M211315200
Moseley, A. E., Williams, M. T., Schaefer, T. L., Bohanan, C. S., Neumann, J. C., Behbehani, M. M., et al. (2007). Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J. Neurosci. 27, 616–626. doi: 10.1523/JNEUROSCI.4464-06.2007
Nedergaard, M., Ransom, B., and Goldman, S. A. (2003). New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530. doi: 10.1016/j.tins.2003.08.008
Ohnishi, T., Yanazawa, M., Sasahara, T., Kitamura, Y., Hiroaki, H., Fukazawa, Y., et al. (2015). Na, K-ATPase alpha3 is a death target of Alzheimer patient amyloid-beta assembly. Proc. Natl. Acad. Sci. U.S.A. 112, E4465–E4474. doi: 10.1073/pnas.1421182112
Ophoff, R. A., Terwindt, G. M., Vergouwe, M. N., van Eijk, R., Oefner, P. J., Hoffman, S. M., et al. (1996). Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87, 543–552. doi: 10.1016/S0092-8674(00)81373-2
Pedersen, S. F., O'Donnell, M. E., Anderson, S. E., and Cala, P. M. (2006). Physiology and pathophysiology of Na+/H+ exchange and Na+ -K+ -2Cl- cotransport in the heart, brain, and blood. Am. J. Physiol. 29, R1–R25. doi: 10.1152/ajpregu.00782.2005
Pietrobon, D., and Moskowitz, M. A. (2013). Pathophysiology of migraine. Annu. Rev. Physiol. 75, 365–391. doi: 10.1146/annurev-physiol-030212-183717
Rose, E. M., Koo, J. C., Antflick, J. E., Ahmed, S. M., Angers, S., and Hampson, D. R. (2009). Glutamate transporter coupling to Na,K-ATPase. J. Neurosci. 29, 8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009
Santoro, L., Manganelli, F., Fortunato, M. R., Soldovieri, M. V., Ambrosino, P., Iodice, R., et al. (2011). A new Italian FHM2 family: clinical aspects and functional analysis of the disease-associated mutation. Cephalalgia 31, 808–819. doi: 10.1177/0333102411399351
Schoner, W., and Scheiner-Bobis, G. (2007). Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 293, C509–C536. doi: 10.1152/ajpcell.00098.2007
Skou, J. C. (1957). The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta. 23, 394–401. doi: 10.1016/0006-3002(57)90343-8
Tanaka, K., Watase, K., Manabe, T., Yamada, K., Watanabe, M., Takahashi, K., et al. (1997). Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276, 1699–1702. doi: 10.1126/science.276.5319.1699
Tornberg, J., Voikar, V., Savilahti, H., Rauvala, H., and Airaksinen, M. S. (2005). Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur. J. Neurosci. 21, 1327–1337. doi: 10.1111/j.1460-9568.2005.03959.x
Tupler, R., Perini, G., and Green, M. R. (2001). Expressing the human genome. Nature 409, 832–833. doi: 10.1038/35057011
Valencia, A., Sapp, E., Kimm, J. S., McClory, H., Ansong, K. A., Yohrling, G., et al. (2013). Striatal synaptosomes from Hdh140Q/140Q knock-in mice have altered protein levels, novel sites of methionine oxidation, and excess glutamate release after stimulation. J. Huntingtons Dis. 2, 459–475. doi: 10.3233/JHD-130080
van den Maagdenberg, A. M., Pietrobon, D., Pizzorusso, T., Kaja, S., Broos, L. A., Cesetti, T., et al. (2004). A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 41, 701–710. doi: 10.1016/S0896-6273(04)00085-6
van den Maagdenberg, A. M., Pizzorusso, T., Kaja, S., Terpolilli, N., Shapovalova, M., Hoebeek, F. E., et al. (2010). High cortical spreading depression susceptibility and migraine-associated symptoms in Ca(v)2.1 S218L mice. Ann. Neurol. 67, 85–98. doi: 10.1002/ana.21815
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2163–2196. doi: 10.1016/S0140-6736(12)61729-2
Keywords: α2 sodium ion pump, astrocytes, familial hemiplegic migraine type 2 (FHM2), mouse models
Citation: Isaksen TJ and Lykke-Hartmann K (2016) Insights into the Pathology of the α2-Na+/K+-ATPase in Neurological Disorders; Lessons from Animal Models. Front. Physiol. 7:161. doi: 10.3389/fphys.2016.00161
Received: 15 February 2016; Accepted: 15 April 2016;
Published: 04 May 2016.
Edited by:
Olga Vagin, University California, Los Angeles, USAReviewed by:
Leif Hertz, China Medical University, ChinaRebecca Lam, Max Planck Institute of Biophysics, Germany
Michael Gordon Harrington, Huntington Medical Research Institutes, USA
Copyright © 2016 Isaksen and Lykke-Hartmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin Lykke-Hartmann, kly@biomed.au.dk
 Toke J. Isaksen
Toke J. Isaksen Karin Lykke-Hartmann
Karin Lykke-Hartmann