- 1Paris Laboratory of Molecular Oral Pathophysiology, Centre de Recherche des Cordeliers, Institut National de la Santé et de la Recherche Médicale UMRS 1138, Université Paris-Descartes, Université Pierre et Marie Curie-Paris, Paris, France
- 2Université Paris-Diderot, Unité de Formation et de Recherche d'Odontologie, Paris, France
- 3Centre de Référence des maladies rares de la face et de la cavité buccale MAFACE hôpital Rothschild, AP-HP, Paris, France
Endocrine disrupting chemicals (EDCs) play a part in the modern burst of diseases and interfere with the steroid hormone axis. Bisphenol A (BPA), one of the most active and widely used EDCs, affects ameloblast functions, leading to an enamel hypomineralization pattern similar to that of Molar Incisor Hypomineralization (MIH). In order to explore the molecular pathways stimulated by BPA during amelogenesis, we thoroughly investigated the receptors known to directly or indirectly mediate the effects of BPA. The expression patterns of high affinity BPA receptors (ERRγ, GPR30), of ketosteroid receptors (ERs, AR, PGR, GR, MR), of the retinoid receptor RXRα, and PPARγ were established using RT-qPCR analysis of RNAs extracted from microdissected enamel organ of adult rats. Their expression was dependent on the stage of ameloblast differentiation, except that of ERβ and PPARγ which remained undetectable. An additional large scale microarray analysis revealed three main groups of receptors according to their level of expression in maturation-stage ameloblasts. The expression level of RXRα was the highest, similar to the vitamin D receptor (VDR), whereas the others were 13 to 612-fold lower, with AR and GR being intermediate. Immunofluorescent analysis of VDR, ERα and AR confirmed their presence mainly in maturation- stage ameloblasts. These data provide further evidence that ameloblasts express a specific combination of hormonal receptors depending on their developmental stage. This study represents the first step toward understanding dental endocrinology as well as some of the effects of EDCs on the pathophysiology of amelogenesis.
Introduction
The environment has become increasingly contaminated by various pollutants which may have a role in the modern burst of diseases. Among environmental toxicants, endocrine-disrupting chemicals (EDCs) have been associated over these past 50 years with many existing or emerging diseases including hormone-dependent cancers, diabetes, obesity, and decreased fertility (De Coster and van Larebeke, 2012; Maqbool et al., 2016). This is supported by numerous epidemiological surveys (De Coster and van Larebeke, 2012; Grindler et al., 2015; Ehrlich et al., 2016) and experimental studies (Brieño-Enrı́quez et al., 2015; Chevalier et al., 2015; Robinson and Miller, 2015; Maqbool et al., 2016; Palanza et al., 2016; Ziv-Gal and Flaws, 2016 for recent reviews). Among the thousands of EDCs, bisphenol A (BPA) is one of the most active and ubiquitous due to its wide use by the plastic industry. The consequences of exposure to BPA have been studied in detail in the development and pathophysiology of multiple organs including gonads, brain, pancreas, liver, heart, and adipose tissue, acting on different effectors of the steroid axis (Chevalier et al., 2015; Robinson and Miller, 2015; Palanza et al., 2016; Seachrist et al., 2016; Ziv-Gal and Flaws, 2016 for recent reviews). BPA has also been shown to induce enamel hypomineralization in rats (Jedeon et al., 2013). Amelogenesis follows a well-known sequence of cell proliferation, differentiation, maturation, and death characterized by specific gene-expression patterns (Nanci, 2012). Ameloblasts sequentially secrete enamel matrix proteins (amelogenin, enamelin, ameloblastin) and proteases (KLK4 and MMP20). The proteases degrade the enamel matrix allowing subsequent mineral crystal growth under the correct pH and ionic conditions [aided by several solute carriers (SLCs) and ion-handling proteins]. BPA modulates the expression of at least one enamel key gene at each stage of amelogenesis, including enamelin, KLK4, and SLC26A4 (Jedeon et al., 2013, 2016a). The resulting rat enamel defects may be scored as those observed in human Molar Incisor Hypomineralization (MIH; Jedeon et al., 2013), a recently described enamel pathology (Weerheijm et al., 2001; Weerheijm and Merjare, 2003). The teeth of rats exposed to BPA and those of humans affected by MIH share similar structural and biochemical abnormalities. Thus, exposure of rats to BPA is a good experimental model of MIH (Jedeon et al., 2013). MIH mostly affects permanent first molars and incisors which are the first teeth to mineralize, from the third trimester of fetal life to four-5 years after birth (Weerheijm et al., 2001), corresponding to the window of the highest susceptibility to EDCs. This enamel disease presents a similar epidemiological evolution to EDC-related diseases. It was almost non-existent before the 80s', but now affects ~15–18% of 6 to 9-year-old children (Jälevik, 2010; Jedeon et al., 2015). It may therefore constitute a marker of exposure to pollutants that disrupt amelogenesis. The mechanism of action of BPA is still unclear but seem to modulate directly or indirectly the activity of multiple receptors (Acconcia et al., 2015). Among them, BPA has been shown to bind the estrogen receptors (ERα and ERβ) (Delfosse et al., 2012), GPR30 (or GPER) (Pupo et al., 2012) and ERRγ with a high affinity (Liu et al., 2012). It also directly or indirectly interferes with the activity of the androgen receptor (AR), the progesterone receptor (PGR), the glucocorticoid receptor (GR), and the PPARγ (Acconcia et al., 2015; Rehan et al., 2015). The mechanism of action of BPA in dental cells is even less evident as its putative receptors are poorly defined in dental tissues, except for ERα (Jedeon et al., 2014a).
The aim of this study was to systematically investigate the expression pattern of the putative BPA receptors and members of their family during amelogenesis in order to understand the effects of BPA on enamel as well as those of other EDCs acting through these receptors. These data may thus help to decipher the physiological endocrine-mediated regulations of amelogenesis and enamel pathologies resulting from endocrine disruption. To date, only the vitamin D pathway has been investigated in dental cells (Berdal et al., 1993; Descroix et al., 2010; Woo et al., 2015). Dental endocrinology needs to be explored in depth to understand the pathways of hormones effects on dental growth and enamel quality.
Materials and Methods
Animals and Biological Samples
Two month-old Wistar rats were purchased from Janvier France Sarl (Le Genest Saint Isle, France) and bred in our animal house. All animals were fed ad libitum, and maintained in accordance with the guidelines for the care and use of laboratory animals from the French Ministry of Agriculture (A-75-06-12).
Three groups of three 30 day-old male and three other similar groups of female rats were constituted and used in this study. Rats were anesthetized by isoflurane inhalation, killed, and their mandibles immediately dissected. The incisors were extracted and soft dental tissues microdissected as previously described (Jedeon et al., 2013). Briefly, dental epithelial cells from the secretion stage and the maturation stage were separately dissected using the molar reference line for isolation, removing the underlying 2 mm-tissue corresponding to the transition stage (Smith and Nanci, 1989). The incisor wasn't opened during enamel organ dissection thus avoiding contamination by the mesenchyme. The anatomically distinguishable cervical loop was dissected from the apical end of the incisor. Microdissection quality was validated by RT-PCR using Enamelin primers for the secretion stage, and KLK4 or SLC26A4 for the maturation stage; Jedeon et al., 2016a). The absence of contamination by the mesenchyme and bone was verified using osteocalcin primers.
RNA Extraction and Gene Expression Profiling
RNAs were extracted from microdissected cervical loop, and secretion- and maturation-stage cells of rat enamel organ using the RNeasy® Protect Mini Kit (Qiagen-France) according to the manufacturer's procedure. Spectrophotometry was used to assess the concentration and purity of RNA by measuring absorbance at 260 nm with a NanoDrop 1000ănd RNA Integrity Number (RIN) (threshold > 9.5) with an Agilent Bioanalyzer, respectively. Reverse transcription was carried out with 1 μg total RNA for 50 min at 42°C, using a random primer oligodT primer mix, according to the manufacturer's instructions (Superscript II®—Invitrogen). Real-time quantitative PCR was performed using the CFX96 device (Bio-Rad Laboratories, Hercules, CA, United States). SYBER green fluorescence corresponding to neosynthesized amplicons was quantified at the end of each of the 45 PCR cycles corresponding to a denaturation step of 2 s at 95°C followed by a polymerization step of 30 s at 60°C. Each PCR was independently repeated in triplicate and the results normalized against those for the three selected reference genes, RS15, GAPDH, and TBP1, for which the expression did not vary under our experimental conditions. Details of the primers and the corresponding amplicon sizes are presented in Table 1. The standard curve method was used to calculate the values corresponding to the relative amounts of test and reference RNAs. Mean ratios of test RNA/standard RNA were calculated for each sample. Similar data were obtained using the ΔΔCt method.
RNAs extracted from microdissected maturation-stage cells of male rat enamel organ were used for microarray experiments performed with Affymetrix RatGene1.0 ST chip probes at the Genom'IC platform of Cochin Institute (Paris, France) to measure the relative level of each (steroid) receptor.
Immunofluorescence Assays
Dental tissues were fixed by immersion in a 4% paraformaldehyde solution for 4 h. After washing in PBS, the samples were dehydrated in ethanol, rinsed in clearene (Leica-France) and paraffin-embedded (Paraplast plus, Sigma). Serial 8 μm sections were cut using a microtome (RM 2145, Leica, France). Sections were deparaffinized and rehydrated in decreasing concentrations of ethanol. Slices were microwaved for 20 min, and the tissues permeabilized with 0.5% Triton X-100 for 10 min. Sections were then washed in PBS and blocked with 10% normal goat serum in PBS for 1 h at room temperature. Slices were incubated overnight at 4°C with primary rabbit polyclonal anti-AR (N-20:sc-816, Santa Cruz) (1:200), anti-VDR (ab3508, Abcam) (1:500), or anti-ERα (sc-542, Santa Cruz) (1:50) antibodies. Sections were incubated with secondary goat anti-IgG coupled to Alexa Fluor 594 antibody (A-11072, Life Technologies) (1:500) at room temperature for one h in the dark. After rinsing with PBS, sections were immersed in DAPI (010M4003-Sigma) (1:100000) for 5 min and finally mounted with Fluoromount (Southern Biotech, Clinisciences).
Statistical Analysis
RT-qPCR data resulting from three independent analyses of three RNA samples of each tissue (loop, secretion, maturation, mesenchyme, and other tissues used as references) are presented as means ± SD. and were analyzed with GraphPad Prism Software Version 5.0 (GraphPad Software Inc., La Jolla, CA) using One way Analysis of Variance followed by Bonferroni's correction. Compared values were considered to be significantly different when *p <0.05, **p <0.01, or ***p <0.001.
Results
Expression Patterns of BPA Putative Receptors during Amelogenesis
We determined the specific pattern of expression for each high-affinity BPA receptor ERRγ, GPR30, ERα, and ERβ, and the other members of the ERR family, ERRα and ERRβ during amelogenesis by qPCR analysis of the enamel organ RNAs (Figure 1A).
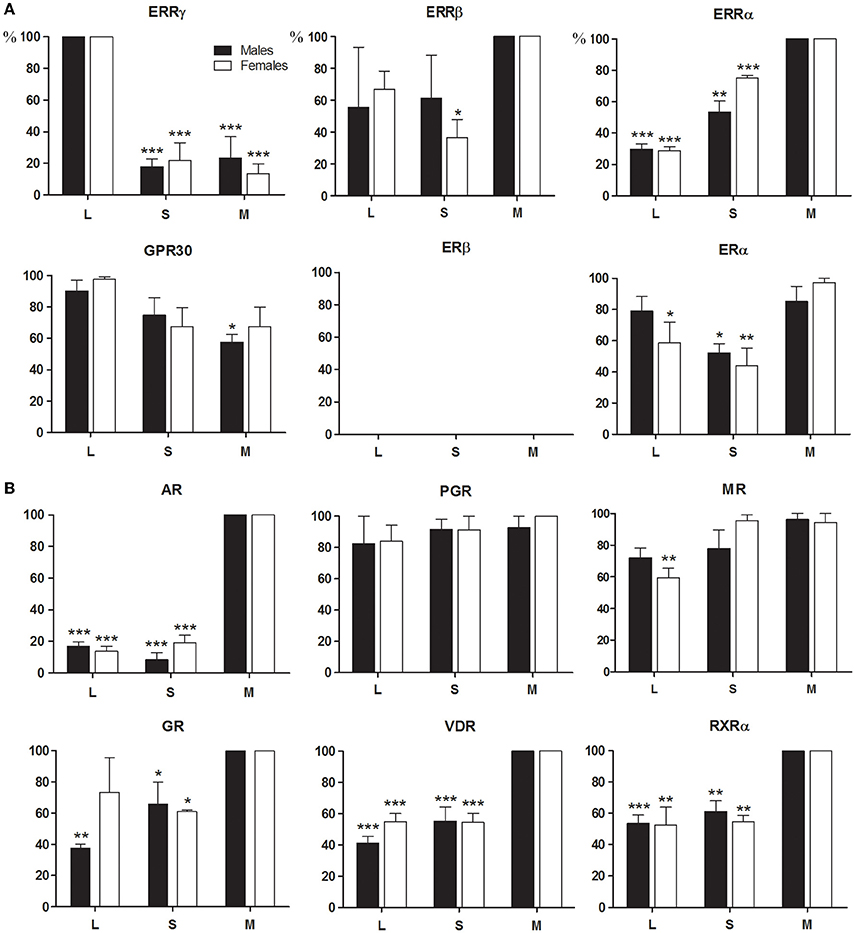
Figure 1. Expression profiles of steroid receptors during amelogenesis. RNAs extracted from microdissected rat enamel organ were analyzed by RT-qPCR after verifying the absence of mesenchymal and bone contamination. Dental cells from the secretion stage (S) and the maturation stage (M) were separately dissected using the molar reference line for isolation (See Materials and Methods). The cervical loop (L) that contains dental precursor cells, was anatomically distinguishable. The highest expression level ratio calculated for each studied and reference gene, using the standard curve method was set to 100% to compare data from the three independent experiments. Males (black bars) and females (white bars) were treated separately. The compared values were considered to be significantly different when *p <0.05, **p <0.01, ***p <0.001. (A) BPA receptors, ERRγ, and to a lesser extent GPR30 and ERα, were mainly expressed in the cervical loop, whereas ERRα and ERRβ were mostly expressed in the maturation stage. ERα and ERRβ expression pattern varied considerably between samples. ERβ was undetectable. (B) The other receptors able to mediate the effects of BPA were also expressed in the rat enamel organ, especially during the maturation stage. VDR and RXRα, two key receptors in amelogenesis, were also mostly expressed during the maturation stage.
Rat enamel organ cells expressed all the tested receptors except the ERβ, which was undetectable at all stages of amelogenesis (Figure 1A). The BPA receptors ERRγ, and to a lesser extent GPR30, were primarily expressed in early-stage ameloblasts (secretory and pre-ameloblasts). ERRγ expression was 5.0 to 6.7-fold higher in the cervical loop containing the precursors than in secretion and maturation stages containing differentiated ameloblasts. The other two members of the ERR family, the ERRα and ERRβ, were expressed throughout amelogenesis with a 3.6- and 1.3-fold accumulation in the maturation stage ameloblasts, respectively. The ERα presented a variable profile depending on the animal. Some animals expressed the ERα essentially in the cervical loop, whereas it was mostly in the maturation-stage ameloblasts in others.
Both males and females expressed similar levels of all receptors measured.
Expression Pattern of Additional Steroid Receptors, GR, AR, MR, PGR, VDR, and Retinoid Receptors during Amelogenesis
We also measured the expression of all receptors known to be involved in the action of BPA, including the AR, PGR and GR/MR (Figure 1B). The AR exhibited the highest difference of expression which was 7.3-fold higher in maturation-stage than in early-stage ameloblasts. AR mRNA was mostly detected in maturation-stage epithelium where its level of expression was 3.6- and 5.7-fold higher than in the mesenchyme and in testis, respectively (Figure 2A). Immunofluorescence assays also showed the presence of the AR protein in dental epithelium, exclusively in maturation-stage ameloblasts, but not in secretion-stage ameloblasts, nor in cells of the papillary layer (Figure 2B). Among the different receptors investigated, its localization was the most specific, restricted to maturation-stage ameloblasts.
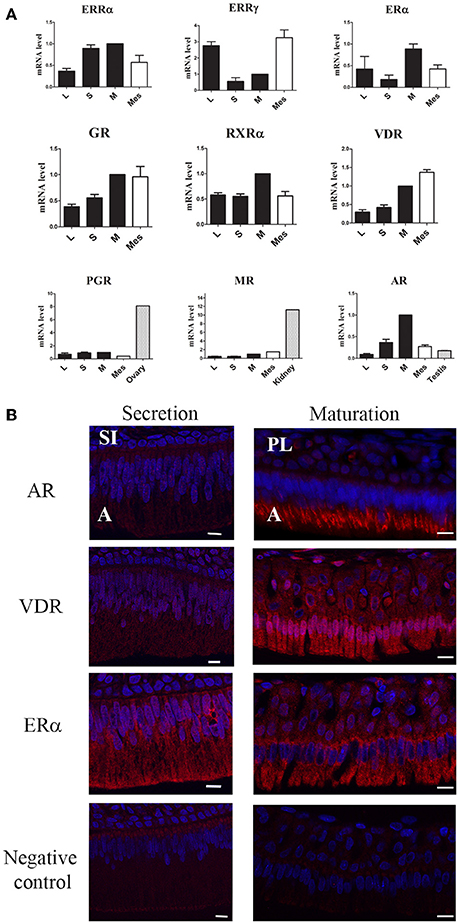
Figure 2. Specificity of steroid hormone and VD receptor expression in maturation-stage ameloblasts. (A) Expression levels calculated by the ΔΔCt method were compared between the cervical loop (L), secretion-stage cells (S), maturation-stage cells (M), mesenchymal cells (Mes) and other tissues used as references: testis for AR, kidney for MR, and ovary for PGR. The AR showed the most preferential expression in maturation-stage enamel tissue relative to all the other receptors tested with a level of expression even higher than that found in testis, used as the androgen responsive tissue. Results are from three independent analyses of three RNA samples of each tissue and are presented as the means ± SD. (B) Immunofluorescent assays for the AR, ERα, and VDR, three receptors involved in amelogenesis. The ER signal was very low in all cells of the enamel organ. The signals corresponding to the AR and VDR were clearly localized in maturation-stage ameloblasts (involved in enamel terminal mineralization). The AR and VDR were also slightly detected in the secretion-stage. A, ameloblasts; PL, papillary layer; SI, stratum intermedium. Scale bars, 10 μm.
The other receptors were mostly expressed in maturation-stage ameloblasts with a 2.6-fold higher level of the GR than in the cervical loop (Figure 1B). The MR and PGR were also mostly expressed during the maturation stage, but with only small differences relative to other stages. The level of MR expression in the maturation-stage cells was 11.2-fold lower than in the kidney, and the level of PGR 8.1-fold lower than in the ovary used as positive controls (Figure 2A).
We also examined the expression patterns of the VDR and its partner the RXRα. Both VDR and RXRα mRNAs accumulated in the maturation-stage ameloblasts with a mean two-fold higher level than in the other compartments of enamel organ (Figure 1B). Immunohistological assays, showing the localization of the corresponding proteins, confirmed the RT-qPCR data with a signal for the VDR throughout the enamel organ, but stronger in mature ameloblasts (Figure 2B).
We observed no major differences between males and females (Figure 1B).
Comparison of Relative Expression Levels of Steroid, BPA, Retinoid, and Vitamin D Receptors In Maturation Stage Ameloblasts
We determined the relative expression levels of the studied receptors in maturation-stage ameloblasts by microarray analysis. The most highly expressed receptors were RXRα, RARα, and VDR (Figures 3A,B). Maturation-stage ameloblasts also expressed all members of the ketosteroid receptors, GR, MR, AR, and PGR. GR and AR levels of expression were similar whereas MR and PGR were 5.9- and 7.9-fold lower, respectively.
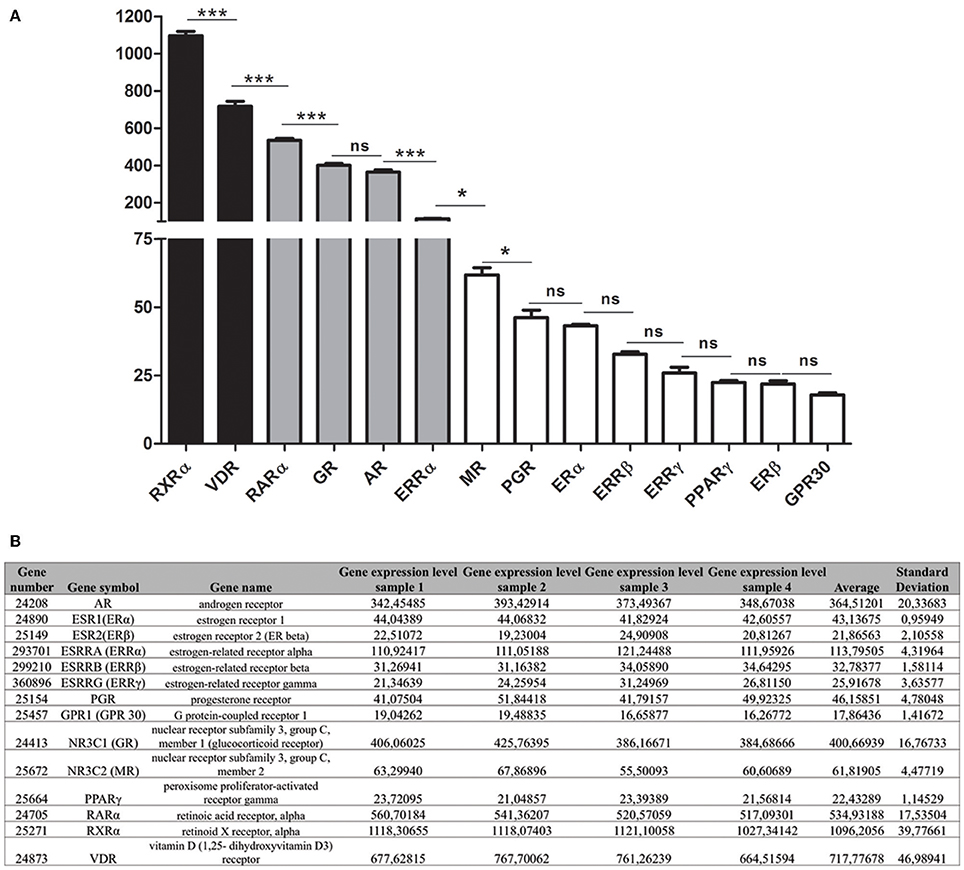
Figure 3. Relative level of expression of steroid hormone and vitamin receptors during the maturation stage. (A) The relative level of expression of each mRNA was determined by microarray analysis of RNAs extracted from maturation-stage enamel organ. Three main groups of receptors were distinguished: The RXRα and VDR were the most highly expressed (black bars); the GPR30, MR, PGR, ERα, ERRβ, and ERRγ weakly (white bars); and the RARα, GR, AR, and ERRα expression levels were intermediate (gray bars). PPARγ, GPR30 and ERβ were at the limit of the detection. Data resulted from microarray analyses of four RNA samples were presented as means ± SD and were compared using One way Analysis of Variance followed by Bonferroni's correction. The compared values were considered to be significantly different when *p <0.05, ***p <0.001. ns, non significant. (B) Raw microarray data and statistical analysis for the calculated mean levels of expression of the studied receptors.
The other receptors (ERα, ERRβ, ERRγ) were weakly expressed in maturation-stage ameloblasts: ERRγ was one of the least expressed, with mRNA level that was 27.7-fold less than the VDR (Figure 3). The ERβ, GPR30, and PPARγ, three other putative BPA receptors were almost undetectable.
Discussion
The effects of vitamin D (VD) on bone and enamel mineralization are well-known, but little is known about all other endocrine regulations of dental growth and mineralization. Recent reports showing the effects of EDCs on enamel mineralization (Bloch-Zupan et al., 1994; Alaluusua et al., 2004; Jan et al., 2007; Jedeon et al., 2014b) suggest that amelogenesis may be regulated by endogenous steroid hormones. The present study shows that many steroid receptors are expressed by ameloblasts with a specific pattern depending on cell proliferation and differentiation, making ameloblasts responsive cells to steroid hormones. The VDR, which binds VD and forms active heterodimers with the RXRα, was the most highly expressed nuclear receptor along with the RXRα throughout amelogenesis. This is in accordance with previous data showing the presence of VDR (Berdal et al., 1993) and RARα/RXRα (Bloch-Zupan et al., 1994) in enamel organ cells, reflecting the importance of VD and vitamin A/retinol in tooth development reported many years ago. VDR/RXR heterodimers control ameloblast differentiation and the expression of key enamel genes such as amelogenin and calbindin D 28k (Berdal et al., 1993; Papagerakis et al., 1999). They were also the most highly expressed nuclear receptors in mesenchymal cells, including odontoblasts, in accordance with previously published data showing the effects of VD on dentin (Davideau et al., 1996). We also detected the GR, AR, and ERRα, among the most highly expressed steroid hormone receptors, throughout amelogenesis with the highest level of expression in maturation-stage ameloblasts. The role of ERRα, and more generally of ERRs, in amelogenesis is unknown. Corticoids affect enamel hardness and mineralization (Pawlicki et al., 1992), and a responsive element for GR (GRE) has been found in the amelogenin promoter (Gibson et al., 1997). Concerning the AR, it has already been detected in dental pulp cells (Dale et al., 2002; Inaba et al., 2013). In addition, our past work, as well as the present study, show that AR expression in the maturation-stage ameloblasts which is higher than in the secretion-stage and mesenchymal cells, suggesting a selective role of androgens in enamel final mineralization (Jedeon et al., 2016b). Testosterone is able to modulate the expression of enamel key genes present in maturation-stage ameloblasts such as SLC26A4 (or pendrin) and KLK4 (Jedeon et al., 2016b). Moreover, the higher level of AR expression in dental epithelium than in testis suggests that ameloblasts are responsive to plasmatic testosterone and thus androgen regulation of final enamel mineralization. This is likely not the case for the PGR and MR which levels of expression in enamel organ were 10 to 20-fold lower than in ovary and kidney, respectively.
The generally higher expression of steroid hormone receptors in the maturation-stage ameloblasts suggests a hormonal control of final enamel mineralization, and thus of enamel quality rather than enamel quantity. This has been experimentally demonstrated in rodent models for the VD/VDR. The deletion of the VDR leads to enamel hypomineralization even in the presence of normal levels of calcium and phosphate (Descroix et al., 2010). Indeed, low serum levels of VD during infancy is associated to caries (Schroth et al., 2014). Dental decay is a complex process involving many factors such as saliva, oral microbiota, and lifestyle, but enamel quality is also an important parameter. Elevated VD serum levels are negatively correlated to MIH (Kühnisch et al., 2015) and to EDC contamination (Johns et al., 2016), suggesting that MIH may be due, at least in part, to endocrine disruption. Epidemiological data have shown that contamination by PCBs and dioxin, two different classes of EDCs, may be associated with enamel hypomineralization (Alaluusua et al., 2004; Jan et al., 2007). Our previous experimental data showed that rats exposed to low-dose genistein and vinclozolin, as well as BPA, present enamel hypomineralization similar to human MIH (Jedeon et al., 2013, 2014b), which is both a hypomineralizing and hypoplasic enamel pathology (Jedeon et al., 2013). The selective affection of MIH suggests disruption during a narrow time window compatible with the steroid hormone secretion pattern during enamel mineralization. The clinical characteristics of enamel defects in MIH also suggest that BPA disrupts amelogenesis throughout the process. It may directly or indirectly modulate receptor activities, not only in maturation-stage ameloblasts, but also in pre-secretory and proliferating cells of the cervical loop. The ERα has already been shown to mediate, at least in part, the short-term mitogenic effects of BPA in pre-ameloblastic cells, but not genomic effects (Jedeon et al., 2014a). Similar non-genomic effects of BPA involving GPR30 activation has been shown in prostate cancer cells (Prins et al., 2014). The three high affinity BPA receptors, ERRγ, GPR30, and ERs, were very weakly expressed in the maturation-stage ameloblasts. They are mainly detected in proliferating epithelial and mesenchymal precursor cells of the loop, especially the ERRγ, which is the highest affinity receptor for BPA (Okada et al., 2008; Acconcia et al., 2015). The ERRγ is the in vivo receptor of BPA involved in the mineralization process of otoliths in zebrafish (Tohmé et al., 2014).
Despite the preferential impact of BPA in males, we detected no major differences between males and females in the hormone receptor expression patterns, or their expression levels. One possible explanation is that this sexual discrepancy may be due to disrupted levels of estrogens or androgens (Scinicariello and Buser, 2016). BPA exerts its anti-androgenic effects by preventing AR activation and lowering the levels of endogenous testosterone. BPA and other anti-androgenic EDCs may exert their anti-androgenic effects on final enamel mineralization through the AR expressed in maturation-stage ameloblasts (Jedeon et al., 2016b). Thus, the high testosterone levels in males following the birth, concomitant with amelogenesis, may cause a sexual dimorphism in enamel quality.
In conclusion, our data show that dental cells express many steroid receptors, of which the expression pattern depends on their stage of differentiation. This study provides clues for further studies of dental endocrinology which needs to be developed in depth to understand the effects of steroid hormone receptors and EDCs acting through such receptors on dental growth and enamel quality.
Author Contributions
SH, SL, and KJ: Contribution to the acquisition, analysis and interpretation of data, interpretation of data for the work, drafting the work, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AB and SB: Substantial contributions to the conception and design of the work, interpretation of data for the work, drafting the work, writing the paper, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was funded by the University Paris-Diderot, the French National Institute of Health and Medical Research (INSERM), the Institut Benjamin Delessert, and the Institut Français pour la Recherche Odontologique (IFRO).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Georges Zadigue for animal breeding. We thank Manon Le Normand and Khaled Salhi for their experimental contribution.
References
Acconcia, F., Pallottini, V., and Marino, M. (2015). Molecular mechanisms of action of BPA. Dose Response 13:1559325815610582. doi: 10.1177/1559325815610582
Alaluusua, S., Calderara, P., Gerthoux, P. M., Lukinmaa, P. L., Kovero, O., Needham, L., et al. (2004). Developmental dental aberrations after the dioxin accident in Seveso. Environ. Health Perspect. 112, 1313–1318. doi: 10.1289/ehp.6920
Berdal, A., Hotton, D., Pike, J. W., Mathieu, H., and Dupret, J. M. (1993). Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev. Biol. 155, 172–179. doi: 10.1006/dbio.1993.1016
Bloch-Zupan, A., Mark, M. P., Weber, B., and Ruch, J. V. (1994). In vitro effects of retinoic acid on mouse incisor development. Arch. Oral Biol. 39, 891–900. doi: 10.1016/0003-9969(94)90021-3
Brie-o-Enríquez, M. A., García-López, J., Cárdenas, D. B., Guibert, S., Cleroux, E., Děd, L., et al. (2015). Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells. PLoS ONE 10:e0124296. doi: 10.1371/journal.pone.0124296
Chevalier, N., Fénichel, P., and Bisphenol, A. (2015). Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 16, 299–309. doi: 10.1007/s11154-016-9333-8
Dale, J. B., Sarich, S. L., Bretz, T. M., Hatton, J. F., and Zachow, R. J. (2002). Hormonal regulation of androgen receptor messenger ribonucleic acid expression in human tooth pulp. J. Dent. Res. 81, 360–365. doi: 10.1177/154405910208100514
Davideau, J. L., Papagerakis, P., Hotton, D., Lezot, F., and Berdal, A. (1996). In situ investigation of vitamin D receptor, alkaline phosphatase, and osteocalcin gene expression in oro-facial mineralized tissues. Endocrinology 137, 3577–3585. doi: 10.1210/endo.137.8.8754789
De Coster, S., and van Larebeke, N. (2012). Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J. Environ. Public Health 2012:713696. doi: 10.1155/2012/713696
Delfosse, V., Grimaldi, M., Pons, J. L., Boulahtouf, A., le Maire, A., Cavailles, V., et al. (2012). Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. U.S.A. 109, 14930–14935. doi: 10.1073/pnas.1203574109
Descroix, V., Kato, S., Lézot, F., and Berdal, A. (2010). Physiopathology of dental rickets in vitamin D receptor-ablated mice. J. Dent. Res. 89, 1427–1432. doi: 10.1177/0022034510379603
Ehrlich, S., Lambers, D., Baccarelli, A., Khoury, J., Macaluso, M., and Ho, S. M. (2016). Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am. J. Perinatol. 33, 1313–1318. doi: 10.1055/s-0036-1586500
Gibson, C. W., Collier, P. M., Yuan, Z. A., Chen, E., Adeleke-Stainback, P., Lim, J., et al. (1997). Regulation of amelogenin gene expression. Ciba Found Symp. 205, 187–197. discussion: 197–199.
Grindler, N. M., Allsworth, J. E., Macones, G. A., Kannan, K., Roehl, K. A., and Cooper, A. R. (2015). Persistent organic pollutants and early menopause in U.S. women. PLoS ONE 10:e0116057. doi: 10.1371/journal.pone.0116057
Inaba, T., Kobayashi, T., Tsutsui, T. W., Ogawa, M., Uchida, M., and Tsutsui, T. (2013). Expression status of mRNA for sex hormone receptors in human dental pulp cells and the response to sex hormones in the cells. Arch. Oral Biol. 58, 943–950. doi: 10.1016/j.archoralbio.2013.02.001
Jälevik, B. (2010). Prevalence and diagnosis of Molar-Incisor- Hypomineralisation (MIH): a systematic review. Eur. Arch. Paediatr. Dent. 11, 59–64. doi: 10.1007/BF03262714
Jan, J., Sovcikova, E., Kocan, A., Wsolova, L., and Trnovec, T. (2007). Developmental dental defects in children exposed to PCBs in eastern Slovakia. Chemosphere 67, S350–S354. doi: 10.1016/j.chemosphere.2006.05.148
Jedeon, K., Berdal, A., and Babajko, S. (2015). “The tooth, target organ of Bisphenol A, could be used as a biomarker of exposure to this agent,” in Sources, Risks of Environmental Exposure and Human Health Effects, eds YG, and A. Bisphenol (New York, NY: Nova Science Publishers), 205–225.
Jedeon, K., De la Dure-Molla, M., Brookes, S. J., Loiodice, S., Marciano, C., Kirkham, J., et al. (2013). Enamel defects reflect perinatal exposure to bisphenol A. Am. J. Pathol. 83, 108–118. doi: 10.1016/j.ajpath.2013.04.004
Jedeon, K., Houari, S., Loiodice, S., Thuy, T. T., Le Normand, M., Berdal, A., et al. (2016a). Chronic exposure to bisphenol A exacerbates dental fluorosis in growing rats. J. Bone Miner. Res. [Epub ahead of print]. doi: 10.1002/jbmr.2879
Jedeon, K., Loiodice, S., Le Normand, M., Houari, S., Chaloyard, J., Salhi, K., et al. (2016b). Androgen receptor involvement in rat amelogenesis: an additional way for endocrine disrupting chemicals to affect enamel synthesis. Endocrinology. [Epub ahead of print]. doi: 10.1210/en.2016-1342
Jedeon, K., Loiodice, S., Marciano, C., Vinel, A., Canivenc Lavier, M. C., Berdal, A., et al. (2014a). Estrogen and bisphenol A affect male rat enamel formation and promote ameloblast proliferation. Endocrinology 155, 3365–3375. doi: 10.1210/en.2013-2161
Jedeon, K., Marciano, C., Loiodice, S., Boudalia, S., Canivenc Lavier, M.-C., Berdal, A., et al. (2014b). Enamel hypomineralization due to endocrine disruptors. Connective Tissue Res. 55, 1–5. doi: 10.3109/03008207.2014.923857
Johns, L. E., Ferguson, K. K., and Meeker, J. D. (2016). Relationships between urinary phthalate metabolite and bisphenol A concentrations and vitamin D levels in U.S. Adults: National Health and Nutrition Examination Survey (NHANES), 2005-2010. J. Clin. Endocrinol. Metab. jc20162134. [Epub ahead of print]. doi: 10.1210/jc.2016-2134
Kühnisch, J., Thiering, E., Kratzsch, J., Heinrich-Weltzien, R., Hickel, R., and Heinrich, J. (2015). GINIplus study group; LISAplus study group. Elevated serum 25(OH)-vitamin D levels are negatively correlated with molar-incisor hypomineralization. J. Dent. Res. 94, 381–387. doi: 10.1177/0022034514561657
Liu, X., Matsushima, A., Nakamura, M., Costa, T., Nose, T., and Shimohigashi, Y. (2012). Fine spatial assembly for construction of the phenol pocket to capture bisphenol A in the human nuclear receptor estrogen related receptor γ. J. Biochem. 151, 403–415. doi: 10.1093/jb/mvs008
Maqbool, F., Mostafalou, S., Bahadar, H., and Abdollahi, M. (2016). Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 145, 265–273. doi: 10.1016/j.lfs.2015.10.022
Nanci, A. (2012). “Enamel: Composition, Formation, and Structure,” in Ten Cate's. Oral Histology Development, Structure, and Function, 8th Edn, (Saint Louis: Elsevier Mosby), 122–164.
Okada, H., Tokunaga, T., Liu, X., Takayanagi, S., Matsushima, A., and Shimohigashi, Y. (2008). Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ. Health Perspect. 116, 32–38. doi: 10.1289/ehp.10587
Palanza, P., Nagel, S. C., Parmigiani, S., and Vom Saal, F. S. (2016). Perinatal exposure to endocrine disruptors: sex, timing and behavioral endpoints. Curr. Opin. Behav. Sci. 7, 69–75. doi: 10.1016/j.cobeha.2015.11.017
Papagerakis, P., Hotton, D., Lezot, F., Brookes, S., Bonass, W., Robinson, C., et al. (1999). Evidence for regulation of amelogenin gene expression by 1,25-dihydroxyvitamin D(3) in vivo. J. Cell. Biochem. 76, 194–205. doi: 10.1002/(SICI)1097-4644(20000201)76:2<194::AID-JCB4>3.0.CO;2-U
Pawlicki, R., Knychalska-Karwin, Z., Stankiewicz, D., Jakób-Dolezal, K., and Karwan, T. (1992). Disturbances of mineral metabolism in teeth of rats receiving corticosteroids for 3 generations. Folia Histochem. Cytobiol. 30, 75–78.
Prins, G. S., Hu, W. Y., Shi, G. B., Hu, D. P., Majumdar, S., Li, G., et al. (2014). Bisphenol A promotes human prostate stem progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155, 805–817. doi: 10.1210/en.2013-1955
Pupo, M., Pisano, A., Lappano, R., Santolla, M. F., De Francesco, E. M., Abonante, S., et al. (2012). Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ. Health Perspect. 120, 1177–1182. doi: 10.1289/ehp.1104526
Rehan, M., Ahmad, E., Sheikh, I. A., Abuzenadah, A. M., Damanhouri, G. A., Bajouh, O. S., et al. (2015). Androgen and progesterone receptors are targets for bisphenol A (BPA), 4-Methyl-2,4-bis-(P-Hydroxyphenyl)Pent-1-Ene–a potent metabolite of BPA, and 4-Tert-Octylphenol: a computational insight. PLoS ONE 10:e0138438. doi: 10.1371/journal.pone.0138438
Robinson, L., and Miller, R. (2015). The impact of bisphenol A and phthalates on allergy, asthma, and immune function: a review of latest findings. Curr. Environ. Health Rep. 2, 379–387. doi: 10.1007/s40572-015-0066-8
Schroth, R. J., Lavelle, C., Tate, R., Bruce, S., Billings, R. J., and Moffatt, M. E. (2014). Prenatal vitamin D and dental caries in infants. Pediatrics 133, e1277–e1284. doi: 10.1542/peds.2013-2215
Scinicariello, F., and Buser, M. C. (2016). Serum testosterone concentrations and urinary bisphenol A, Benzophenone-3, triclosan, and paraben levels in male and female children and adolescents: NHANES 2011-2012. Environ. Health Perspect. [Epub ahead of print]. doi: 10.1289/EHP150
Seachrist, D. D., Bonk, K. W., Ho, S. M., Prins, G. S., Soto, A. M., and Keri, R. A. (2016). A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 59, 167–182. doi: 10.1016/j.reprotox.2015.09.006
Smith, C. E., and Nanci, A. (1989). A method for sampling the stages of amelogenesis on mandibular rat incisors using the molars as a reference for dissection. Anat. Rec. 225, 257–266. doi: 10.1002/(SICI)1097-0185
Tohmé, M, Prud'homme, S. M., Boulahtouf, A., Samarut, E., Brunet, F., Bernard, L., et al. (2014). Estrogen-related receptor γ is an in vivo receptor of bisphenol A. FASEB J. 28, 3124–3133. doi: 10.1096/fj.13-240465
Weerheijm, K. L., Jälevik, B., and Alaluusua, S. (2001). Molar-incisor hypomineralisation. Caries Res. 35, 390–391. doi: 10.1159/000047479
Weerheijm, K. L., and Merjare, I. (2003). Molar incisor hypomineralisation: a questionnaire inventory on its occurrence in member countries of the European Academy of Paediatric Dentistry (EAPD). Int. J. Paediatr. Dent. 13, 411–416. doi: 10.1046/j.1365-263X.2003.00498.x
Woo, S. M., Lim, H. S., Jeong, K. Y., Kim, S. M., Kim, W. J., and Jung, J. Y. (2015). Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Mol. Cells 38, 604–609. doi: 10.14348/molcells.2015.2318
Keywords: amelogenesis, steroid receptors, steroid hormones, endocrine disrupting chemicals, enamel mineralization
Citation: Houari S, Loiodice S, Jedeon K, Berdal A and Babajko S (2016) Expression of Steroid Receptors in Ameloblasts during Amelogenesis in Rat Incisors. Front. Physiol. 7:503. doi: 10.3389/fphys.2016.00503
Received: 01 August 2016; Accepted: 13 October 2016;
Published: 02 November 2016.
Edited by:
Thimios Mitsiadis, University of Zurich, SwitzerlandReviewed by:
Javier Catón, CEU San Pablo University, SpainVictor E. Arana-Chavez, University of São Paulo, Brazil
Supawadee Sukseree, Medical University of Vienna, Austria
Copyright © 2016 Houari, Loiodice, Jedeon, Berdal and Babajko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sylvie Babajko, sylvie.babajko@crc.jussieu.fr
†These authors have contributed equally to this work.
 Sophia Houari
Sophia Houari Sophia Loiodice1,2†
Sophia Loiodice1,2† Katia Jedeon
Katia Jedeon Ariane Berdal
Ariane Berdal Sylvie Babajko
Sylvie Babajko