- Department of Plant Biology, Carnegie Institution for Science, Stanford, CA, USA
Most metazoa use hexose transporters to acquire hexoses from their diet and as a transport form for distributing carbon and energy within their bodies; insects use trehalose, and plants use sucrose as their major form for translocation. Plant genomes contain at least three families of mono- and disaccharide transporters: monosaccharide/polyol transporters that are evolutionary closely related to the yeast and human glucose transporters, sucrose transporters of the SUT family, which similar to the hexose transporters belong to the major facilitator superfamily, but share only minimal amino acid sequence homology with the hexose transporters, and the family of SWEET sugar transporters conserved between animals and plants. Recently, the genome sequence of the spikemoss Selaginella has been determined. In order to study the evolution of sugar transport in plants, we carefully annotated of the complement of sugar transporters in Selaginella. We review the current knowledge regarding sugar transport in spikemoss and provide phylogenetic analyses of the complement of MST and SUT homologs in Selaginella (and Physcomitrella).
Introduction
A hallmark of vascular plants is the transport of sugars and nitrogen from places of synthesis to sites that depend on external supply (Giaquinta, 1983). Green tissues engage in photosynthesis, produce sugars and assimilate inorganic nitrogen, and export excess to support non-photosynthetic tissues such as roots and reproductive organs. Key features include roots or root-like structures necessary for nutrient acquisition and vascularization of the sporophyte to bring nutrients from the root or root-like structures to the leaves and to transport photoassimilates to the root.
While most animals use glucose as the major transport form for carbon skeletons and energy between organs, many higher plants use the disaccharide sucrose for long distance translocation. In addition, plants also contain homologs of the human and yeast hexose transporters, most probably for either transport in and out of the vacuole as well as import of hexoses derived from extracellular metabolism via invertases. Moreover, members of the hexose transporter families in both plants and animals (solute transporter family SLC2) specialize in the transport of polyols. Importantly, as far as it has been tested, both animal and plant sugar transporters can transport a wide spectrum of other compounds such as secondary metabolites as well (for recent reviews, cf. Kühn and Grof, 2010; Ayre, 2011). Interestingly, insects have specific trehalose transporters that are used to transport this disaccharide between cells (Kikawada et al., 2007; Kanamori et al., 2010). Animals also have SUT sucrose transporter homologs (solute transporter family SLC45). The Drosophila homolog SCRT transports sucrose, is potentially located in intracellular melanosomes, and mutation leads to increased lethality. SRCT has been implicated in an essential role in transporting sucrose as an osmolytes as or as a nutrient (Meyer et al., 2011). Mutations in the mammalian SLC45 homologs lead to oculocutaneous albinism suggesting a critical role in melanosomes (Newton et al., 2001).
Here we will focus on the complement of sugar transporters of the SUT and MST (STP/ERD6) families of sugar transporters in one of the earlier land plants, Selaginella, the genome sequence of which has recently been published (Banks et al., 2011). The Selaginella genome also contains homologs of the recently identified SWEET transporter family, which will be reviewed independently (Chen et al., 2010, 2012; Sosso et al., in preparation).
Selaginella as a member of the lycophytes is part of an ancient lineage of vascular plants that had arisen ∼400 million years ago (Banks, 2009). In angiosperms, which have attracted most of the attention for nutrient distribution so far, we have been able to identify many of the key transporters for organic carbon and reduced nitrogen. Sucrose is a dominant transport form for carbon and energy in many angiosperms, and the transporters for loading the phloem and for import of sucrose into the seed have been identified (Riesmeier et al., 1992). Interestingly, plants also have transporters for monosaccharides and sugar alcohols (Lalonde et al., 2004; Büttner, 2010). Their role is not always fully clear, thus an analysis of Selaginella as a representative of an ancient vascular plant may help shedding light on the evolution and function of these diverse transporters. Still not all parts of the angiosperms sugar transport engine are identified; major missing components include the efflux transporters required for nectar production, sugar efflux from roots into the rhizosphere, and efflux from the seed coat.
Besides sucrose, nitrogen is quantitatively the most relevant mineral nutrient. We also analyzed the Selaginella complement of genes for transporters involved in cellular uptake of ammonium, urea, and the translocation of amino acids (DeMichele et al., submitted; Wipf et al., submitted).
Sugar Transport in Selaginella
Bryophytes and Pteridophytes have been shown to contain glucose, fructose, and sucrose (Allsopp, 1951). Selaginella kraussiana and Selaginella caulescens do not seem to contain significant amounts of sucrose but rather trehalose. Interestingly, the genome of Selaginella moellendorffii contains homologs of both the monosaccharide and sucrose transporter families from Arabidopsis known today.
Physcomitrella growth seems unaffected by the presence of 0.5% glucose (Allsopp, 1951). However, this sugar could not be used by the moss as an alternative carbon source when photosynthesis was blocked by DCMU, nor did they grow in glucose in darkness, while Ceratodon showed some growth on glucose and sucrose (Allsopp, 1951). The ligule of Selaginella kraussiana has been shown to take up 3H-glucose (Sigee, 1976). Trehalose appears to be a dominant sugar in many Selaginella species (cf. ref. in Allsopp, 1951). The desiccation-tolerant Selaginella lepidophylla contains very high trehalose levels and a very high activity trehalose-6-phosphate synthase as compared to other plants, potentially suggesting that trehalose accumulation may be related to the resurrection phenotype (Van Dijck et al., 2002). Trehalose transporters have been identified in insects, where they function as facilitators and belong to the hexose transporter family (MFS; Kikawada et al., 2007). Plant trehalose transporters have not yet been characterized, however since the monosaccharide transporter family has not been analyzed systematically for trehalose transport activity, members of this family are prime candidates for such a function.
Putative Selaginella Monosaccharide/Sugar Alcohol Transporters
Analysis of the ubiquitous monosaccharide or hexose transporters as well as sugar alcohol transporters in Selaginella moellendorffii revealed the presence of at least 26 alleles (Figures 1A–G; Figure S1 in Supplementary Material provides full tree), while 54 homologs were found in Arabidopsis (Lalonde et al., 2004; Büttner, 2010) and 60 in rice. Moreover, the Selaginella monosaccharide transporter proteins are highly similar to their homologs in Physcomitrella patens (Johnson and Thomas, 2007). To be able to perform a direct comparison, the annotation in Physcomitrella needs to be improved, since in many cases the start codon is not correctly assigned, putative sequencing errors have lead to apparent frameshifts, and intron locations are in several cases probably not correctly assigned. The Selaginella annotation carried out in this project and the resulting improved gene models available for the Selaginella sugar transporters will be useful to improve the Physcomitrella annotation. Close homologs are also found in green algae such as Chlorella and Chlamydomonas, as well as in cyanobacteria, fungi, and in the animal kingdom including the important human GLUT glucose facilitators (HGNC Solute Carrier Family Series SLC2)1.
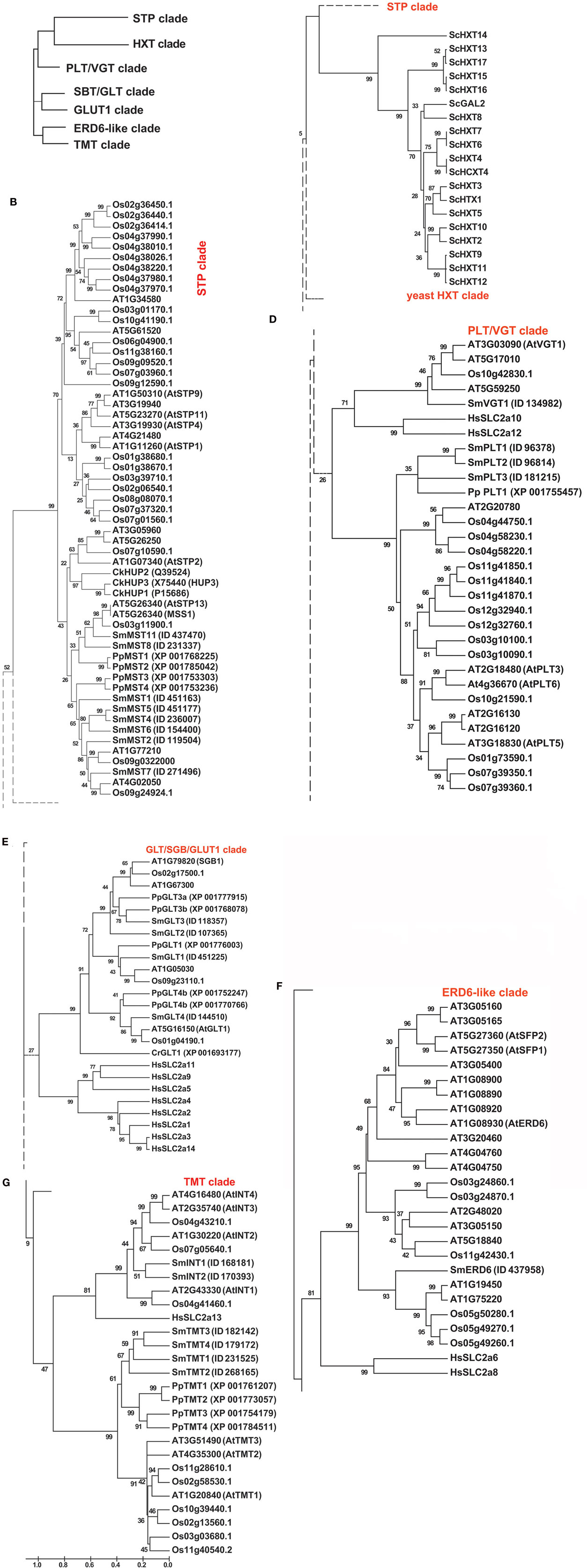
Figure 1. Phylogenetic tree of the monosaccharide transporter family. (A) Clade overview, (B) STP clade, (C) Hxt clade, (D) PLT–VGT clade, (E) SBG–GLT–GLUT1 clade, (F) ERD6-like clade, (G) TMT clade. The tree was obtained by aligning all protein sequences using ClustalW and then build using the software Molecular Evolutionary Genetics Analysis (MEGA v5, Tamura et al., 2011). The bootstrap consensus tree is inferred from 1000 replicates. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 185 amino acid sequences and a total of 277 positions. At, Arabidopsis thaliana; Ck, Chlorella kessleri; Cr, Chlamydomonas reinhardtii; Hs, Homo sapiens; Os, Oryza sativa; Pp, Physcomitrella patens; Sc, Saccharomyces cerevisiae; Sm, Selaginella moellendorffii.
The putative monosaccharide transporter genes can be divided into seven subclades (Figures 1A–G; Table 1; Figure S1 in Supplementary Material). The MSTs (STP/ERD6-like transporters) typically function as high affinity monosaccharide proton cotransporters in plants, here called MST (monosaccharide transporters) since they transport pentoses and various hexoses (Büttner, 2010). Three groups of transporters have been identified that play roles in vacuolar transport: (i) vacuolar ERD6-like transporters that function in facilitative transport of monosaccharides across the tonoplast membrane (Yamada et al., 2010); (ii) VGT-like transporters, that in Arabidopsis also localize to vacuolar membranes and function as a hexose transporters (Büttner, 2010); and (iii) TMTs, characterized by extended loops, that also function as vacuolar monosaccharide transporters (Wormit et al., 2006). pGLTs are plastidic glucose transporters (Weber et al., 2000), while INT-like transporters transport inositol (Schneider et al., 2008) and PLTs transport polyols (Reinders et al., 2005).
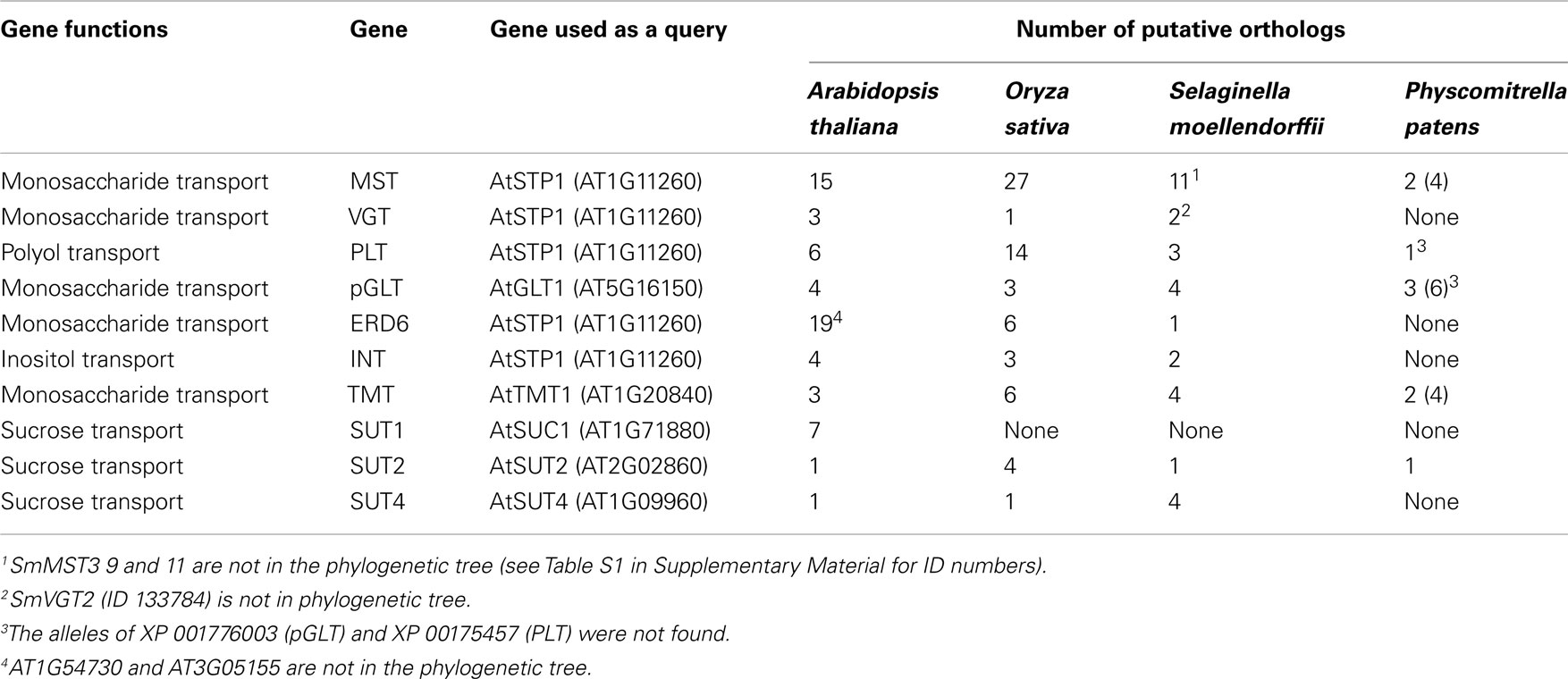
Table 1. Number of genes of the different monosaccharide (MST, ERD6, pGLT, VGT, TMT, INT, and PLT) and sucrose transporters (SUT/SUC) found in Arabidopsis, rice, Selaginella, and Physcomitrella.
Consistent with sugar alcohols being involved in resurrection, there are both inositol and polyol transporter homologs in Selaginella. All three types of vacuolar monosaccharide transporters are found as well as plastidic glucose and Mex1-like maltose transporters (unpublished results). The overall composition is similar in Selaginella as in monocots such as rice, although we find a lower number of MSTs in Selaginella. Arabidopsis is characterized by a larger number of ERD6-like transporters compared to monocots and Selaginella.
Sucrose Transport and SUT Family Members
To our knowledge, sucrose transport has not been studied in detail in mosses. Yet, sucrose can be used in media for axenic culture of mosses. For many higher plants, sucrose is the dominant form for sugar translocation. Sucrose, a disaccharide composed of glucose and fructose [α-D-glucopyranosyl-(1 ↔ 2)-β-D-fructofuranoside] has a low viscosity even at high concentrations (soluble to several molar), has no reducing end, and is thus considered more inert than glucose, the major transport form in animals. Sucrose is produced in the mesophyll cells of plant leaves (as well as other organs) by the combined activity of sucrose phosphate synthase and sucrose phosphate phosphatase or by sucrose synthase. Sucrose is exported into the cell wall space by SWEET sucrose uniporters (Chen et al., 2012) (Giaquinta, 1983). Extracellular sucrose is then loaded into the long distance transport system of higher plants, the phloem, with the help of secondary active transporters in the membrane of the sieve element companion cell complex. The first sucrose transporter gene SUT1 was identified by expression cloning from spinach and potato leaf cDNA libraries in an engineered yeast strain (Riesmeier et al., 1992, 1993). Interestingly, the sucrose transporter also mediates transport of the disaccharide maltose and a variety of glucosides (Sivitz et al., 2005). SUT1 has an affinity for sucrose in the low micromolar range (around 1 mM). SUT1 is essential for sucrose export from the leaves of potato and tobacco as shown by antisense repression (Riesmeier et al., 1994; Bürkle et al., 1998). The Arabidopsis homolog was called SUC2 and null mutation in SUC2 is lethal (Gottwald et al., 2000). Two more distantly related sucrose transporter homologs SUT2 and SUT4 were functionally characterized in the yeast expression system and shown to also transport sucrose and maltose (Barker et al., 2000; Weise et al., 2000). All three transporters have been localized to enucleate sieve elements (Kühn et al., 1997; Barker et al., 2000; Weise et al., 2000). A recent proteomic analysis identified the barley SUT4 homolog in the tonoplast membrane (Endler et al., 2006). AtSUT4/SUC4-fusions also localize to the tonoplast (Schneider et al., 2012) and contribute to export of sucrose from the vacuole (Schulz et al., 2011). SUT2 and SUT4 are low affinity sucrose transporters in Solanaceae and Arabidopsis. SUT2 is characterized by an extended central loop that contains conserved domains CCB1 and CCB2 (Lalonde et al., 2004). In tomato, LeSUT1 and LeSUT2 inhibition affects tomato fruit development, while the potato SUT4 appears to be important for a variety of functions flowering, tuberization, and shade avoidance response (Hackel et al., 2006; Chincinska et al., 2008). In poplar, RNAi (RNA interference) inhibition of the SUT4 paralog PtaSUT4 has been shown to affect the leaf/stem biomass ratio, implicating the vacuolar sucrose transporter SUT4 in sucrose partitioning, and biomass allocation (Payyavula et al., 2011). Such a evolutionary conserved role in biomass allocation is supported by similar findings in rice for the SUT4 family member OsSUT2 (which belongs to the SUT4 clade; Eom et al., 2011). Note that the nomenclature of sucrose transporters in Arabidopsis may differ. SUT1 and SUT3 are paralogs in tobacco (Lemoine et al., 1999) the corresponding genes in Arabidopsis are called SUC2 as well as SUC1, 5, 6, 7, 8, and 9. SUT2 has been called SUC3 and SUT4 has been called SUC4.
The genome of Selaginella was analyzed for the occurrence of sucrose transporter homologs (Table 1; Figure 2; Figure S2 in Supplementary Material provides full tree; Table S1 in Supplementary Material). Close homologs were identified in Physcomitrella; the haploid Selaginella genome encodes five sucrose transporter genes. The Selaginella SUT genes fall into two clades, SUT2 and a branch close, but clearly distinct from the SUT1 and SUT4-like clades found in higher plants (Lalonde et al., 2004). One member falls into the SUT2 clade and was named SmSUT2L1-1 (the allele from the second haploid line was called SmSUT2L1-2), as well as four genes in the SUT1/SUT4 branch (here named SUT4L1, SUT4L2, SUT4L3, SUT4L4). This classification is supported by similarities in the intron structure and the presence of an extended central loop containing a conserved CCB2 domain in the Selaginella and higher plant SUT2s (the CCB1 box is not conserved between Selaginella and Physcomitrella and all the other candidates containing this longer central loop). Monocots also do not have a SUT1 homolog, which is interesting since this transporter is essential for several dicots. Monocots have two types of SUT2, one with and one without a central loop. While it is conceivable that the mosses/spikemosses had lost one of the two. However, based on the distance to the higher plant SUT1 and SUT4 clades, it is more likely that this is an ancestral clade and that SUT1 and 4 evolved from this ancestral clade. We therefore named the clade moss SUT1/SUT4 clade.
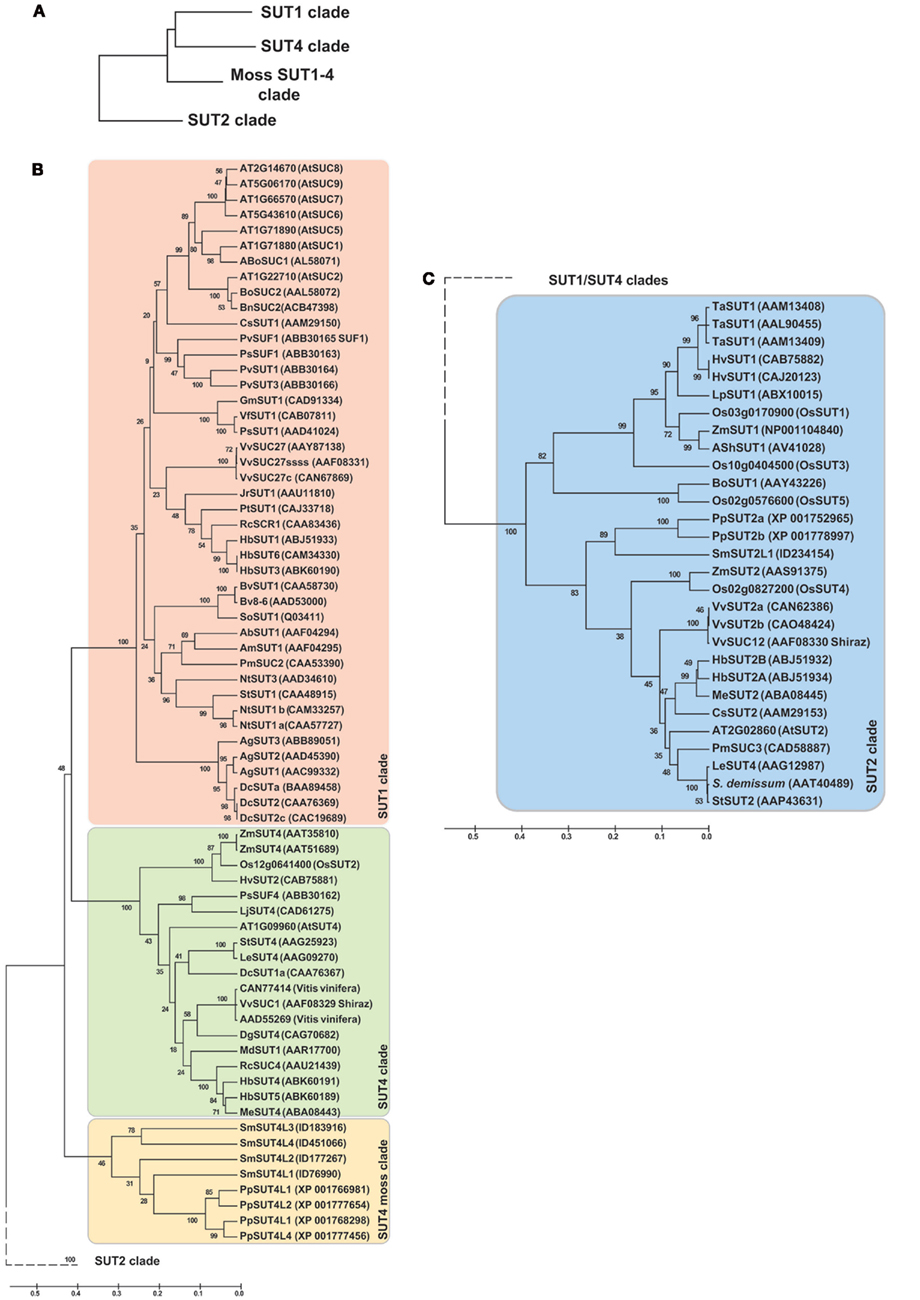
Figure 2. Phylogenetic tree of the sucrose transporter (SUT) family. (A) Clade overview, (B) SUT1–SUT4 clade, (C) SUT2 clade. The tree was obtained by aligning all protein sequences using ClustalW and then build using the software Molecular Evolutionary Genetics Analysis (MEGA v5, Tamura et al., 2011). The bootstrap consensus tree is inferred from 1000 replicates. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The analysis involved 99 amino acid sequences and a total of 401 positions. Ab, Asarina barclaiana; Ag, Apium graveolens; Am, Alonsoa meridionalis; At, Arabidopsis thaliana; Bn, Brassica napus; Bo, Brassica oleracea; Bv, Beta vulgaris; Cs, Citrus sinensis; Dc, Daucus carota; Dg, Datisca glomerata; Hb, Hevea brasiliensis; Hv, Hordeum vulgaris; Jr, Juglans regia; Gm, Glycine max; Le, Lycopersicon esculentum; Lj, Lotus japonicum; Lp, Lolium perenne; Md, Malus x domestica; Me, Manihot esculenta; Nt, Nicotiana tabacum; Os, Oryza sativa; Pm, Plantago major; Pp, Physcomitrella patens; Ps, Pisum sativum; Pt, Populus tremula × Populus tremuloides; Pv, Phaseolus vulgaris; Rc, Ricinus communis; Sh, Saccharum hybrid; So, Spinacia oleracea; Sm, Selaginella moellendorffii; St, Solanum tuberosum; Ta, Triticum aestivum; Vf, Vicia faba; Vv, Vitis vinifera; Zm, Zea mays.
Distant SUT homologs have been identified in fungi (Reinders and Ward, 2001). The Schizosaccharomyces pombe homolog was shown to function primarily as a maltose transporter, but also can transport sucrose (Reinders and Ward, 2001). Homologs also exist in the animal kingdom, e.g., medaka and mouse, which are directly or indirectly involved in melanin accumulation (Fukamachi et al., 2001). Interestingly, no SUT homologs have been found in green algae, while glucose transporters are well conserved in algae (Caspari et al., 1994).
Despite the importance of trehalose for plants and its occurrence in Selaginella, we have not found homologs of the insect-specific trehalose transporter family in Selaginella or Arabidopsis (Kikawada et al., 2007; Kanamori et al., 2010).
Summary
Taken together, the haploid genome of the spikemoss Selaginella contains 5 sucrose transporter homologs as well as 26 hexose/polyol transporter homologs. In addition, Selaginella also contains homologs of the recently identified SWEET transporter family (Chen et al., 2010, 2012). Unfortunately, at present it is not possible to assess the physiological function of these transporters in Selaginella due to the lack of tools for gene replacement and transformation. However the genome of the moss Physcomitrella appears to contain a similar complement of transporters in the two families, and all necessary tools for analyzing the physiological function of these genes are available. It will however be necessary to carefully annotate the respective gene annotation in Physcomitrella before entering in to such an endeavor. The careful annotation performed here for the Selaginella genes can serve as guidance for improving the Physcomitrella annotation.
Materials and Methods
Selaginella moellendorffii genes for monosaccharide and sucrose transporters were obtained by blasting a set of Arabidopsis transporter homologs (see Table 1) against the Selaginella genome sequence at the JGI website2. When necessary (and if possible), gene models were modified according to the available information (i.e., ESTs) and a new gene model was promoted. A list containing protein identifier numbers, sequences, and links to the JGI database of all Selaginella proteins included is presented as Table S1 in Supplementary Material.
Monosaccharide and sucrose transporter families were treated separately. Multiple sequence alignments were generated using MEGA 5.0 software (Tamura et al., 2011). The quality of the alignments was assessed and non-homologous sequences were removed at beginning and end of the sequences. The phylogenetic analysis was done using the maximum likelihood method and the consensus tree was inferred from 1000 bootstrap replicates. The analysis was done using only one Selaginella allele.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the Department of Energy (DE-FG02-04ER15542) and NIH (NIDDK; 1RO1DK079109) for supporting this work.
Footnotes
References
Allsopp, A. (1951). The sugars and non-volatile acids of some Archegoniates: a survey using paper chromatography. J. Exp. Bot. 2, 121–124.
Ayre, B. G. (2011). Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 4, 377–394.
Banks, J. A. (2009). Selaginella and 400 million years of separation. Annu. Rev. Plant Biol. 60, 223–238.
Banks, J. A., Nishiyama, T., Hasebe, M., Bowman, J. L., Gribskov, M., dePamphilis, C., Albert, V. A., Aono, N., Aoyama, T., Ambrose, B. A., Ashton, N. W., Axtell, M. J., Barker, E., Barker, M. S., Bennetzen, J. L., Bonawitz, N. D., Chapple, C., Cheng, C., Correa, L. G., Dacre, M., DeBarry, J., Dreyer, I., Elias, M., Engstrom, E. M., Estelle, M., Feng, L., Finet, C., Floyd, S. K., Frommer, W. B., Fujita, T., Gramzow, L., Gutensohn, M., Harholt, J., Hattori, M., Heyl, A., Hirai, T., Hiwatashi, Y., Ishikawa, M., Iwata, M., Karol, K. G., Koehler, B., Kolukisaoglu, U., Kubo, M., Kurata, T., Lalonde, S., Li, K., Li, Y., Litt, A., Lyons, E., Manning, G., Maruyama, T., Michael, T. P., Mikami, K., Miyazaki, S., Morinaga, S., Murata, T., Mueller-Roeber, B., Nelson, D. R., Obara, M., Oguri, Y., Olmstead, R. G., Onodera, N., Petersen, B. L., Pils, B., Prigge, M., Rensing, S. A., Riano-Pachon, D. M., Roberts, A. W., Sato, Y., Scheller, H. V., Schulz, B., Schulz, C., Shakirov, E. V., Shibagaki, N., Shinohara, N., Shippen, D. E., Sorensen, I., Sotooka, R., Sugimoto, N., Sugita, M., Sumikawa, N., Tanurdzic, M., Theissen, G., Ulvskov, P., Wakazuki, S., Weng, J. K., Willats, W. W., Wipf, D., Wolf, P. G., Yang, L., Zimmer, A. D., Zhu, Q., Mitros, T., Hellsten, U., Loque, D., Otillar, R., Salamov, A., Schmutz, J., Shapiro, H., Lindquist, E., Lucas, S., Rokhsar, D., and Grigoriev, I. V. (2011). The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 332, 960–963.
Barker, L., Kühn, C., Weise, A., Schulz, A., Gebhardt, C., Hirner, B., Hellmann, H., Schulze, W., Ward, J. M., and Frommer, W. B. (2000). SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12, 1153–1164.
Bürkle, L., Hibberd, J. M., Quick, W. P., Kühn, C., Hirner, B., and Frommer, W. B. (1998). The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol. 118, 59–68.
Büttner, M. (2010). The Arabidopsis sugar transporter (AtSTP) family: an update. Plant Biol. 12, 35–41.
Caspari, T., Will, A., Opekarova, M., Sauer, N., and Tanner, W. (1994). Hexose/H+ symporters in lower and higher plants. J. Exp. Biol. 196, 483–491.
Chen, L. Q., Hou, B. H., Lalonde, S., Takanaga, H., Hartung, M. L., Qu, X. Q., Guo, W. J., Kim, J. G., Underwood, W., Chaudhuri, B., Chermak, D., Antony, G., White, F. F., Somerville, S. C., Mudgett, M. B., and Frommer, W. B. (2010). Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468, 527–532.
Chen, L. Q., Qu, X. Q., Hou, B. H., Sosso, D., Osorio, S., Fernie, A. R., and Frommer, W. B. (2012). Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335, 207–211.
Chincinska, I. A., Liesche, J., Krugel, U., Michalska, J., Geigenberger, P., Grimm, B., and Kuhn, C. (2008). Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol. 146, 515–528.
Endler, A., Meyer, S., Schelbert, S., Schneider, T., Weschke, W., Peters, S. W., Keller, F., Baginsky, S., Martinoia, E., and Schmidt, U. G. (2006). Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 141, 196–207.
Eom, J. S., Cho, J. I., Reinders, A., Lee, S. W., Yoo, Y., Tuan, P. Q., Choi, S. B., Bang, G., Park, Y. I., Cho, M. H., Bhoo, S. H., An, G., Hahn, T. R., Ward, J. M., and Jeon, J. S. (2011). Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 157, 109–119.
Fukamachi, S., Shimada, A., and Shima, A. (2001). Mutations in the gene encoding B, a novel transporter protein, reduce melanin content in medaka. Nat. Genet. 28, 381–385.
Gottwald, J. R., Krysan, P. J., Young, J. C., Evert, R. F., and Sussman, M. R. (2000). Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. U.S.A. 97, 13979–13984.
Hackel, A., Schauer, N., Carrari, F., Fernie, A. R., Grimm, B., and Kuhn, C. (2006). Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J. 45, 180–192.
Johnson, D. A., and Thomas, M. A. (2007). The monosaccharide transporter gene family in Arabidopsis and rice: a history of duplications, adaptive evolution, and functional divergence. Mol. Biol. Evol. 24, 2412–2423.
Kanamori, Y., Saito, A., Hagiwara-Komoda, Y., Tanaka, D., Mitsumasu, K., Kikuta, S., Watanabe, M., Cornette, R., Kikawada, T., and Okuda, T. (2010). The trehalose transporter 1 gene sequence is conserved in insects and encodes proteins with different kinetic properties involved in trehalose import into peripheral tissues. Insect Biochem. Mol. Biol. 40, 30–37.
Kikawada, T., Saito, A., Kanamori, Y., Nakahara, Y., Iwata, K., Tanaka, D., Watanabe, M., and Okuda, T. (2007). Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 104, 11585–11590.
Kühn, C., Franceschi, V. R., Schulz, A., Lemoine, R., and Frommer, W. B. (1997). Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275, 1298–1300.
Kühn, C., and Grof, C. P. L. (2010). Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 13, 288–298.
Lalonde, S., Wipf, D., and Frommer, W. B. (2004). Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 55, 341–372.
Lemoine, R., Bürkle, L., Barker, L., Sakr, S., Kühn, C., Regnacq, M., Gaillard, C., Delrot, S., and Frommer, W. B. (1999). Identification of a pollen-specific sucrose transporter-like protein NtSUT3 from tobacco. FEBS Lett. 454, 325–330.
Meyer, H., Vitavska, O., and Wieczorek, H. (2011). Identification of an animal sucrose transporter. J. Cell Sci. 124, 1984–1991.
Newton, J. M., Cohen-Barak, O., Hagiwara, N., Gardner, J. M., Davisson, M. T., King, R. A., and Brilliant, M. H. (2001). Mutations in the human orthologue of the mouse underwhite gene (uw) underlie a new form of oculocutaneous albinism, OCA4. Am. J. Hum. Genet. 69, 981–988.
Payyavula, R. S., Tay, K. H., Tsai, C. J., and Harding, S. A. (2011). The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J. 65, 757–770.
Reinders, A., Panshyshyn, J. A., and Ward, J. M. (2005). Analysis of transport activity of Arabidopsis sugar alcohol permease homolog AtPLT5. J. Biol. Chem. 280, 1594–1602.
Reinders, A., and Ward, J. M. (2001). Functional characterization of the alpha-glucoside transporter Sut1p from Schizosaccharomyces pombe, the first fungal homologue of plant sucrose transporters. Mol. Microbiol. 39, 445–454.
Riesmeier, J. W., Hirner, B., and Frommer, W. B. (1993). Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5, 1591–1598.
Riesmeier, J. W., Willmitzer, L., and Frommer, W. B. (1992). Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 11, 4705–4713.
Riesmeier, J. W., Willmitzer, L., and Frommer, W. B. (1994). Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J. 13, 1–7.
Schneider, S., Beyhl, D., Hedrich, R., and Sauer, N. (2008). Functional and physiological characterization of Arabidopsis inositol transporter1, a novel tonoplast-localized transporter for myo-inositol. Plant Cell 20, 1073–1087.
Schneider, S., Hulpke, S., Schulz, A., Yaron, I., Holl, J., Imlau, A., Schmitt, B., Batz, S., Wolf, S., Hedrich, R., and Sauer, N. (2012). Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol. doi: 10.1111/j.1438-8677.2011.00506.x. [Epub ahead of print].
Schulz, A., Beyhl, D., Marten, I., Wormit, A., Neuhaus, E., Poschet, G., Buttner, M., Schneider, S., Sauer, N., and Hedrich, R. (2011). Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 68, 129–136.
Sigee, D. C. (1976). Structure and function in the ligule of Selaginella kraussiana. 3. The uptake of tritiated glucose. Protoplasma 90, 333–341.
Sivitz, A. B., Reinders, A., and Ward, J. M. (2005). Analysis of the transport activity of barley sucrose transporter HvSUT1. Plant Cell Physiol. 46, 1666–1673.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739.
Van Dijck, P., Mascorro-Gallardo, J. O., De Bus, M., Royackers, K., Iturriaga, G., and Thevelein, J. M. (2002). Truncation of Arabidopsis thaliana and Selaginella lepidophylla trehalose-6-phosphate synthase unlocks high catalytic activity and supports high trehalose levels on expression in yeast. Biochem. J. 366, 63–71.
Weber, A., Servaites, J. C., Geiger, D. R., Kofler, H., Hille, D., Groner, F., Hebbeker, U., and Flugge, U. I. (2000). Identification, purification, and molecular cloning of a putative plastidic glucose translocator. Plant Cell 12, 787–802.
Weise, A., Barker, L., Kühn, C., Lalonde, S., Buschmann, H., Frommer, W. B., and Ward, J. M. (2000). A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12, 1345–1355.
Wormit, A., Trentmann, O., Feifer, I., Lohr, C., Tjaden, J., Meyer, S., Schmidt, U., Martinoia, E., and Neuhaus, H. E. (2006). Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18, 3476–3490.
Keywords: sucrose, hexose, glucose, carrier, transporter, plasma membrane, vacuole, polyol
Citation: Lalonde S and Frommer WB (2012) SUT sucrose and MST monosaccharide transporter inventory of the Selaginella genome. Front. Plant Sci. 3:24. doi: 10.3389/fpls.2012.00024
Received: 01 November 2011; Accepted: 20 January 2012;
Published online: 07 February 2012.
Edited by:
Angus S. Murphy, Purdue University, USAReviewed by:
Rosario Vera-Estrella, Universidad Nacional Autonoma de Mexico, MexicoJohn M. Ward, University of Minnesota, USA
Copyright: © 2012 Lalonde and Frommer. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Wolf B. Frommer, Department of Plant Biology, Carnegie Institution for Science, Stanford, CA 94305, USA. e-mail: wfrommer@carnegiescience.edu
