- 1 Department of Plant Sciences, University of California, Davis, CA, USA
- 2 Department of Horticulture, Faculty of Agriculture, University of Suleyman Demirel, Isparta, Turkey
- 3 Instituto Tecnologico del Valle de Oaxaca, Oaxaca, Mexico
Grafting has been used in agriculture for over 2000 years. Disease resistance and environmental tolerance are highly beneficial traits that can be provided through use of grafting, although the mechanisms, in particular for resistance, have frequently been unknown. As information emerges that describes plant disease resistance mechanisms, the proteins, and nucleic acids that play a critical role in disease management can be expressed in genetically engineered (GE) plant lines. Utilizing transgrafting, the combination of a GE rootstock with a wild-type (WT) scion, or the reverse, has the potential to provide pest and pathogen resistance, impart biotic and abiotic stress tolerance, or increase plant vigor and productivity. Of central importance to these potential benefits is the question of to what extent nucleic acids and proteins are transmitted across a graft junction and whether the movement of these molecules will affect the efficacy of the transgrafting approach. Using a variety of specific examples, this review will report on the movement of organellar DNA, RNAs, and proteins across graft unions. Attention will be specifically drawn to the use of small RNAs and gene silencing within transgrafted plants, with a particular focus on pathogen resistance. The use of GE rootstocks or scions has the potential to extend the horticultural utility of grafting by combining this ancient technique with the molecular strategies of the modern era.
Introduction
In agriculture today, the ancient technique of plant grafting is an indispensable tool that offers an opportunity for combining beneficial root and shoot characteristics for the production of high-value horticultural crops. Under natural conditions, plants can undergo inosculation, the union between plant parts growing in close proximity whose cambial surfaces are breached through abrasion allowing separate vasculature systems to merge. For example, California black oaks are known to share root systems through natural root grafts and fortuitous contacts between strawberry or potato plants can result in stem grafts. Given that “natural grafting” occurs without human intervention, it is likely the art of grafting arose through discovery rather than by innovation, although it is unclear when or where grafting originated (Mudge et al., 2009). Chinese writings mention grafting of peach varieties as early as 1560 BC, and grafting was knowledgeably discussed by Aristotle, Theophrastus, Cato, and Varro from the fourth to first centuries BC (Roberts, 1949).
Grafting provides a number of critical horticultural benefits. Grafting two woody species, each with desirable traits, was instrumental in the domestication of a variety of tree species (e.g., apples, pears, and plums) that, otherwise, were recalcitrant to asexual propagation techniques (Mudge et al., 2009). In perennial species, grafting is used for clonal propagation and production of specialized ornamental trees. Grafting provides the means to repair or bypass damaged trunks, hasten development of fruiting varieties, or accentuate useful vigor or dwarfing characteristics. Rootstocks also provide resistance to pests and pathogens, including insects and soil-borne diseases, and tolerance of abiotic stress conditions, such as thermal shock, low root temperature, boron toxicity, and salinity (Bulder et al., 1991a,b; Ahn et al., 1999; Rivero et al., 2003; Edelstein et al., 2005, 2007; Dolgov and Hanke, 2006; Venema et al., 2008). Currently, almost every commercial fruit or nut tree production system uses grafting to increase yields or avoid disease (Kubota et al., 2008).
In many parts of the world, grafting is used in vegetable production (Edelstein et al., 1999; Romano and Paratore, 2001; Fernandez-Garcia et al., 2004; Khah et al., 2006; Balliu et al., 2008; Davis et al., 2008; King et al., 2008; Kubota et al., 2008; Misovic et al., 2009; Di Gioia et al., 2010). Grafting is used widely with Solanaceae and Cucurbitaceae crops to reduce infections by soil-borne pathogens and to enhance the tolerance of abiotic stresses (Colla et al., 2010; Justus and Kubota, 2010; Lee et al., 2010; Rouphael et al., 2010; Savvas et al., 2010; Schwarz et al., 2010). In the Middle East, grafted vegetable rootstocks are used in order to utilize poorer soils, and in Japan, almost 95% of the watermelons, oriental melons, eggplants, cucumbers, and tomatoes are grafted before transplantation to fields or greenhouses (Lee, 1994). Grafting vegetable and fruit plants became increasingly important for disease control in Europe and Israel after the soil fumigant methyl bromide was banned under the Montreal Protocol in 2005 (Cohen et al., 2007; Davis et al., 2008). In Israel, approximately 20% of the cultivated tomato plants are grafted. Grafting scions to vigorous, disease-resistant rootstocks is an alternative to chemical control methods, a particularly appealing feature for organic cultivation of crops. An economic advantage of grafting is that a few rootstock lines may be utilized with multiple scion varieties, although the success of particular rootstock:scion combinations may be variable.
For farmers who grow heirloom tomato varieties organically, grafting is an emerging option, since, while tasteful and attractive, these varieties often lack many of the disease resistance and vigor traits of conventionally produced varieties. The use of grafted tomatoes in the US has grown (Kubota, 2008; Kubota et al., 2008) as the demand for elite and heirloom varieties from small organic farms has increased. In North America, >95% of the grafted tomatoes are for greenhouse and high tunnel production, reflecting the markets for high-value fresh tomatoes. While the cost per grafted tomato seedling in the US is 1.5–2 times greater than for conventional seedlings, the possibility of introducing advantageous horticultural traits without compromising fruit characteristics offers significant value. Currently, most propagators supplying grafted seedlings to the North American market are in Canada and Mexico, although there are some specialty market suppliers in Ohio and North Carolina. At least 40 million tomato plants now are grafted in North America, with the US being the largest user (Kubota et al., 2008).
While conventional breeding has developed disease-resistant rootstock genotypes for grafting, the additional approach of transgrafting resistant genetically engineered (GE) rootstocks with WT scions provides a potentially valuable merger of ancient and modern technologies. In this paper, we discuss how transgrafting can increase the options to provide agricultural solutions. Our specific “mechanistic” focus is on the mobilization across graft junctions of transgenes and the products they encode.
Transgrafting
Transgrafting (the use of a GE rootstock with a WT-scion, or the use of a WT rootstock with a GE scion) has the potential to expand the traits provided by grafting since the benefits derived from transgenes can be harnessed. A transgrafted plant, with a WT scion, may allow agricultural industries to benefit from a transgenic trait expressed in a rootstock while addressing consumer concerns about food derived from GE-crops, because the scion would not have been GE (Haroldsen et al., 2012). Transgrafting also offers advantages for the environment, since under proper orchard maintenance, pollen flow concerns would be minimized because the non-engineered scion would be the only source of pollen (Lev-Yadun and Sederoff, 2001; COGEM, 2006). In addition, deregulation of one or a small number of rootstocks that could be used with multiple scion cultivars or varieties would be preferable over engineering each scion genotype, especially given that the cost to deregulate each GE line is $6–15 million in the US (Kalaitzandonakes et al., 2007). A deregulated rootstock could be utilized with different scion cultivars and, in some cases, multiple genera. Accomplishing these benefits while maintaining a scion that is free of transgenic DNA could facilitate the entry of GE specialty crops into commercial production since deregulation of each scion cultivar would likely not be necessary, lowering the burden placed on specialty crop producers. We focus this review primarily on the benefits that can be provided by a GE rootstock; it is also possible that transgrafting can provide benefits to root crops, such as potato or cassava, if non-GE rootstocks are grafted with GE scions.
One consideration for the use of transgrafted plants is the identification of transgene product(s) that have the potential to move between rootstocks and scions. Systemic acquired resistance (SAR), mediated by salicylic acid, in grafts made with plants that are challenged with a pathogen demonstrate that molecules or signals can move within plants and subsequently provide resistance in anticipation of pathogen contact (Gaffney et al., 1993; Conrath, 2011). With GE rootstocks, the potential movement of DNA and RNA genetic or epigenetic factors and translocation of proteins can be evaluated because of their identifiable and unique characteristics. While grafted scions and rootstocks are generally assumed to conserve their own genetic identity, it is becoming evident that certain transcription factors, mRNAs, regulatory micro RNAs (miRNAs), small interfering RNAs (siRNAs), peptides, and proteins are mobile in the plant vascular system and thus, may cross the graft union. Potentially, delivery of any of these products from a GE rootstock can be advantageous for the scion, as is the case with SAR, where the plant experiences enhancement of pathogen and pest resistance (Gaffney et al., 1993).
Mobility of Genetic Components
Historically, nucleic acids were believed to be cell-autonomous (i.e., contained in the cell of origin), unable to move beyond the point of synthesis. However, this paradigm has evolved as sensitive analytical methods have become available and been used to demonstrate that nucleic acids are present and functional outside of the cells where they are synthesized. Proteins are also known to cross cellular barriers and exert developmental control beyond their site of synthesis. The possibility that these molecules can move shaping the way we think about transgenic rootstocks and their potential applications.
DNA
While there is no current evidence that would support the movement of genomic DNA through the vascular system of a grafted plant (apart from DNA-based plant viruses), movement of plastid DNA across cellular barriers immediately adjacent to the graft junction has been demonstrated (Stegemann and Bock, 2009). In this study, two cultivars of tobacco were each transformed with antibiotic-resistance selectable and visual markers. One cultivar was transformed with a kanamycin resistance gene and the nuclear-encoded yellow fluorescent protein (YFP) and another cultivar was transformed with a spectinomycin resistance gene and a plastid-encoded green fluorescent protein (GFP) marker. Explants taken from tissue immediately adjacent to the graft junction were able to grow on selective media for both constructs and fluorescence from nuclei and plastids was detected. This outcome was not due to cellular fusion but rather to the exchange of large sections of plastid (but not nuclear) DNA. However, the study did not exclude the possibility that entire organelles were transferred. While this effect was restricted to a few cell layers near the graft junction, it, nevertheless, challenges the idea that the rootstock and scion strictly maintain their individual genetic identities. It has been suggested that exchange of genetic material might occur during graft healing as cell walls and vascular systems are being remodeled. The formation of new plasmodesmata could allow the rootstock and scion cells to become symplastic and, perhaps, exchange organelles (i.e., chloroplasts in this example); this would thus accomplish transfer of organellar genes. It is important to emphasize that the resulting chimera was not due to cellular fusion, because through single nucleotide polymorphism (SNP) genotyping and partial sequencing, scion cells were shown to have incorporated only a large piece of the rootstock plastid DNA.
While it is extremely unlikely that genomic or organellar DNA would be mobile over long-distances, as suggested by some researchers (Ohta, 1991), it is possible that heritable changes induced by epigenetic modifications of genomic DNA may occur as a result of movement. Heritable changes can result from RNA-mediated silencing mechanisms; siRNA can induce epigenetic effects such as sequence-specific DNA methylation (Jones et al., 2001). Our more recent understanding of heritable epigenetic influences might explain earlier claims of graft hybridization that alleged phenotypic changes in grafted pepper progeny due to mobility of DNA through the graft junction and into the seeds (Taller et al., 1998; Liu et al., 2010). Although grafting applications that take advantage of epigenetic modifications have not been developed, epigenetic changes present an opportunity to endow progeny with characteristics that result from transcriptional down-regulation or gene silencing without introduction of heritable transgenic DNA. Furthermore, based on previous epigenesis experiments (Jones et al., 2001), subsequent generations could revert back to non-silenced phenotypes, thereby limiting the duration of the original modification to the plant of interest, while providing a potential containment against the spread of transcriptionally modified progeny.
mRNA
Evidence of a highly regulated and selective process involving long-distance trafficking of mRNA has been demonstrated. Observations have been made of differential localization and accumulation of transcripts in sink tissues, presence of mRNA-binding proteins in phloem sap, and sequence-specific motifs of mobile mRNAs that interact with transcript-binding proteins. Messenger RNAs encoding transcriptional regulators and cell fate/cycle-related, hormone response, and metabolic genes have been identified in pumpkin and tomato sieve tube elements (SE) (Ruiz-Medrano et al., 1999; Kim et al., 2001; Haywood et al., 2005).
For example, the transcripts of pumpkin CmNACP, a member of the family of NAC transcription factors that are involved in apical meristem development and leaf senescence, have been identified in scion tissues from pumpkin rootstock–cucumber scion (i.e., heterografted) plants. This observation supports the idea of long-distance transport and accumulation of CmNACP RNA in vegetative, floral, and root meristematic tissues. Data for this experiment were gathered using in situ RT-PCR and confirmed by in situ hybridization studies. Further experiments with seven other phloem sap-localized transcripts gave similar results, demonstrating the existence of delivery systems of specific transcripts to shoot and root apices (Ruiz-Medrano et al., 1999).
In another pumpkin rootstock/cucumber scion heterograft experiment, a phloem-mobile pumpkin RNA, CmPP16, was found in stems, leaves, and floral tissues of the scion. It was determined that the translated protein product CmPP16 bound sense and antisense CmPP16 transcripts and, thus, mediated the transport of its own mRNA into the phloem translocation stream (Xoconostle-Cazares et al., 1999). Due to this self-mobility characteristic, the protein was termed a “plant paralog to viral movement protein.”
In a grafted tomato example, a line carrying the dominant mutation, Mouse ears (Me), which causes rounded and unlobed leaflets, was used as the rootstock and grafted to a semi-dominant Xanthophyllic (Xa) mutant scion with yellow, lobed leaves. Eleven of 13 grafted plants demonstrated the Me phenotype in the scion. Interestingly, the Me gene is a fusion of two separate genes, PFP and LeT-6, that produces two transcript splice variants, but only the longer transcript is in-frame with the Let-6 homeodomain and only this transcript was detectable in the scion. Fluorescent in situ RT-PCR confirmed accumulation of the longer Me transcript that had been detected in scion phloem sieve tubes and associated companion cells (CC) by Northern blots and confocal imaging. It was concluded that the Me phenotype of the scion was caused by movement of the Me transcript from the rootstock. The authors suggested that patterns of transcript accumulation observed by in situ experiments may not be entirely due to promoters expressing locally, but also may be attributed to transport of transcripts (Kim et al., 2001). In a follow up experiment, the Me tomato genotype was used as a heterografted rootstock with potato as the scion. Again, leaf morphological changes in the scion were observed and DNA gel blot analysis of the RT-PCR products demonstrated translocation of the Me transcript across the graft junction (Kudo and Harada, 2007).
Two mutant transcripts from the GRAS gene family, CmGAIP and GAI, were used to examine processes underlying mRNA mobility in pumpkin and Arabidopsis. In pumpkin these genes influence responses to gibberellin hormones. The pumpkin CmGAIP transcript, with a deleted DELLA domain, and the equivalent Arabidopsis mutant gai, with a non-functional DELLA domain, were analyzed because the DELLA domain mutations offer an easily trackable semi-dominant, dark-leafed, dwarf phenotype. The CmGAIP transcripts were found in stem, leaf, and floral tissues of heterografted plants with pumpkin rootstocks, particularly in the stem CC and SE. Long-distance trafficking of these transcripts influenced development and leaf morphology in the scion. While the CmGAIP transcripts could be found in floral tissues, they were never detected in maturing fruit tissue; thus, it was concluded that tissue sink strength did not necessarily affect localization and delivery. To confirm specificity and selectivity and to rule out promoter effects, enhanced GFP (eGFP) was transformed into rootstocks under the companion cell-specific SUC2 promoter. Although fluorescent signal from the eGFP protein could be detected in grafted scions, the eGFP transcript was not detected, suggesting that inherent properties of particular transcripts were likely responsible for their mobility or lack of mobility. That is, CC were able to retain eGFP transcript, but allowed the eGFP protein product to enter the phloem and the CC did not retain the CmGAIP transcripts. The observations suggested a complex, regulated, and cell/tissue-specific process underlying mRNA phloem mobility (Haywood et al., 2005). Furthermore, the 3′ untranslated region (UTR) of the GAI transcript was shown to be necessary and sufficient to target GFP RNA for long-distance movement (Haywood et al., 2005). A mutated, movement-defective GAI transcript could be partially rescued by restoring nucleotides involved in the formation of predicted stem-loop structures. Thus, in addition to the nucleic acid sequence, the macromolecular structure of the mRNA may also contribute to its ability to be mobilized (Huang and Yu, 2009).
Aside from studies of individual transcripts, large scale experiments have identified families of mobile RNAs. Transcripts within the extracellular apoplastic compartments would be candidate mobile RNAs, particularly in the vascular fluids. Out of 1830 expressed sequence tags (ESTs) isolated from melon phloem exudate and sequenced, 986 were shown to be unique and many transcripts associated with biotic responses, stress and defense responses, metal-ion binding, and signal transduction were detected. Only three of the 1830 ESTs were identified as encoding Rubisco or chlorophyll-related proteins. Thus, the authors of this experiment concluded that the results were not due to contamination from surrounding cells. Heterografting with cucumber rootstocks revealed that 43 of the 986 unique transcripts were mobile and translocated through the vascular system into the pumpkin scion, perhaps suggesting conservation among these RNA trafficking motifs, at least within the Cucurbits (Omid et al., 2007). Despite specific experimental examples, the general mechanism behind RNA trafficking motifs is not well understood. However, studies using non-protein-coding viroids offer evidence that the tertiary structure of viroid RNA is a requirement for mobility across cellular boundaries as well as through the phloem (Zhong et al., 2007; Takeda et al., 2011).
In addition to the heterografting studies, there is evidence for cross species mRNA mobility in the parasite–host interaction between Cuscuta and tomato (Roney et al., 2007). RT-PCR and microarray analyses showed the presence of over 400 tomato transcripts in Cuscuta tissue. Earlier studies had shown that one of the transcripts, LeGAI, was mobile in tomato phloem (Haywood et al., 2005).
It is clear that RNA sequences specify their mobility. Both the 3′ and 5′ UTRs appear to contain cis-acting sequences termed “zip codes” that provide competence for mRNA vascular transport, transcript stability, and translational regulation (Bassell et al., 1999; Jansen, 2001; Lucas et al., 2001; Banerjee et al., 2009). It is known that mobile mRNAs can influence phenotypes (Kim et al., 2001; Kudo and Harada, 2007) and, at least in one case, this was demonstrated to be the direct result of translation of the mobile mRNA (Schmelzer et al., 2005). The experimental evidence makes it clear that mRNAs are present in the vascular stream and can be transported with a high degree of specificity. There have been no studies yet that demonstrate the non-regulated mobility or diffusion of mRNA into the vasculature. Given the relative instability of nascent mRNAs (Shyu et al., 2008) and the identification of protein binding regions in mobile mRNAs (Gomez et al., 2005), it is generally believed that mRNA transport is mediated via a ribonuclear protein complex (RNP) and movement of isolated single-stranded RNA (ssRNA) transcripts has not been reported (Lucas et al., 2001; Gomez et al., 2005; Lough and Lucas, 2006). Aside from providing protection against endogenous ribonucleases, RNP proteins may provide additional information for targeting functions. Utilization of the mRNA transit mechanisms with specific anti-pathogen transcripts may be a viable strategy for improving pathogen resistance of scions, although no specific examples for this approach have been described at present.
Future applications will likely involve the addition of “zip codes” to target rootstock-generated transcripts to specific scion tissues or organs. Under the control of temporal, developmental, or inducible promoters in a rootstock, the effects of the transgene in the scion would be evident while maintaining the shoot, as well as its seed and pollen, free from transgenic DNA. In Arabidopsis, the mean and median half-lives of mRNAs are 5.9 and 3.8 h, respectively, but this varies with mRNA function and sub-cellular localization (Narsai et al., 2007). Given the relatively short half-lives of RNA transcripts, it is possible that once fruit and other products are harvested by removal from the plant, any transgenic RNAs in the scion tissues would degrade because the conduits from the sites of RNA synthesis, the source rootstocks, have been severed. Much remains to be discovered in the field of nucleic acid movement and associations before applications that can utilize mobile, scion-targeted mRNAs are sufficiently defined to permit their exploitation.
Small Non-Coding RNAs
Small double stranded RNAs [sRNAs, less than 200 nucleotides (nt)] that participate in gene silencing can be divided into two major groups: siRNA and miRNA. siRNAs are generated from perfect double stranded RNAs produced by RNA-dependent RNA polymerase and can be induced by viruses, genetic constructs, or experimentally introduced. miRNAs are derived from non-coding, imperfect stem-loop RNAs and transcribed from their own promoters by RNA polymerase II. Both are processed by the RNA-induced silencing complex, but while siRNAs have a strictly silencing or quenching effect on gene expression, miRNAs are able to regulate gene expression in a much more tunable manner (Vazquez et al., 2010).
The silencing effect can be cell-autonomous or non-autonomous, the latter indicating that silencing effects can be exerted over long-distances from the site of synthesis. With endogenous miRNAs, evidence indicates that most appear to be cell-autonomous (Parizotto et al., 2004; Alvarez et al., 2006). There are exceptions; for instance, the gradual spreading and accumulation of miRNA166 in phloem tissue has been observed during leaf development (Juarez et al., 2004). In addition, regulation of transcription factors in roots and xylem patterning due to crosstalk between miRNA166 and miRNA165 and transcription factors has been observed (Carlsbecker et al., 2010). Additionally, long-distance movement of miRNA399 is essential for inorganic phosphate uptake in the roots of phosphate-stressed Arabidopsis, rapeseed, and pumpkin (Lin et al., 2008; Pant et al., 2008).
Non-cell-autonomous gene silencing was first shown in tobacco with the nitrate reductase gene (Palauqui and Vaucheret, 1995). Subsequent grafting experiments confirmed that, in addition to its non-cell-autonomous nature, the effect could spread unidirectionally from the tobacco rootstock to tobacco scions across a 30-cm WT-grafted “bridge” (Palauqui et al., 1997). Similar results were later reported in grafted sunflower using a GUS marker gene (Hewezi et al., 2005). In both tobacco and sunflower, the silencing effect was unidirectional, from rootstock to scion. Three-week-old embryos (seeds) derived from self-fertilized graft-silenced scions in sunflower did not show the silencing effect, demonstrating that, at least in this case, the silencing signal was not transmitted to the progeny through the graft. Both of these examples used sense transgenes, therefore this type of silencing effect commonly is referred to as co-suppression. Several other groups working with similar systems have reported analogous results (Voinnet et al., 1998; Sonoda and Nishiguchi, 2000; Crete et al., 2001; Mallory et al., 2003; Tournier et al., 2006).
In contrast, antisense silencing was shown in tobacco to be not graft-transmissible regardless of whether the signal originated in the scion or rootstock (Crete et al., 2001). In tomato grafting experiments with the ACC oxidase gene, antisense silencing of scion ACC oxidase was not seen early after graft establishment, however after several weeks a graft-transmissable silencing was observed (Shaharuddin et al., 2006). This time lag may account for why the earlier experiments concluded that there was no silencing in grafted antisense lines. A high level of expression of the target gene in the scion was necessary for the detection of silencing by Northern hybridization, as a result of expression of the antisense construct in the rootstock, a situation similar to the nitrate reductase experiments discussed earlier (Palauqui et al., 1997). Thus, experimental time lines, the levels of target gene(s) expression, and the model organisms used may be important determinants of the efficacy of antisense silencing in grafted systems.
It has also been shown that even when target gene(s) are not present in the recipient graft, transgenic siRNAs (in addition to endogenous sRNAs) can accumulate from donor grafts (Molnar et al., 2010). Arabidopsis containing a GFP inverted-repeat silencing construct as the donor was grafted with WT or GFP-expressing scions as recipients. The sRNAs identified in scion tissues included siRNAs generated as a result of the GFP construct and a substantial population of endogenous sRNAs from the rootstock donor as well. Size classes ranging from 21 to 25 nt were most abundant, and the 24-nt class directed epigenetic modification of the GFP signal in the scion. The massively parallel deep sequencing methods used by this group showed that if a silencing target was not present in the recipient (i.e., completely WT-scion without GFP), then siRNAs generated from hairpin-GFP in the rootstock were still present in the scion, albeit at levels several of orders of magnitude lower. This could be why previous experiments using less sensitive detection techniques, such as Northern blots, did not detect mobility of the signal. A recent report has shown that beyond the 24-nt siRNAs mentioned above, all size classes of siRNAs can trigger homologous sequence-specific methylation of targets at long-distances, at least in Arabidopsis (Dunoyer et al., 2010).
What facilitates the movement of sRNAs? sRNAs (∼15 kDa) and associated RNPs are small enough to be translocated based on their size, since experiments have shown that a 27-kDa GFP is able to diffuse into the vascular system (Imlau et al., 1999; Kim et al., 2005). Results of experiments where movement proteins are included indicate that spreading of the silencing signal is at least partially dependent on the size of the plasmodesmatal apertures (Kobayashi and Zambryski, 2007). Alternatively, movement of the silencing signal might be selective, perhaps requiring protein–protein, or protein–nucleic acid interactions in order to obviate the apparent plasmodesmatal aperture size exclusion limit. This view is supported by experiments involving mutants defective or deficient in the ability to move signals (Dunoyer et al., 2005, 2007; Yelina et al., 2010). Regardless of uncertainties related to the mechanism(s) of sRNA movement, the evidence demonstrates that movement does indeed occur through the phloem component of the vascular system and is mediated by plasmodesmata, at least to some degree.
Many experiments have been performed regarding the mobility of RNAs, both large and small, but whether the same pathways that are used for the movement of mRNA are used for miRNA or siRNA movement has not been determined. The emerging idea that sRNAs are involved in physiology, defense, and development, both cell autonomously and for long-distance signaling, is becoming more widely accepted (Buhtz et al., 2010). Given the variability in mobility detected across several studies, it seems that plasmodesmata-based transport of sRNAs is a regulated process. However, the molecular mechanisms that mediate sRNA mobility and whether they are cis or trans-acting are unknown.
Researchers have successfully employed strategies that utilize the expression of siRNAs in order to protect the plant root zone from pests and pathogens (Escobar et al., 2002; Klink and Matthews, 2009). For example, in soybean, resistance strategies that target soybean cyst nematode genes, including those associated with stimulating root growth in infected plants, sperm production, and female development have been tested (Huang et al., 2006; Steeves et al., 2006; Klink et al., 2009). By grafting these plants to WT scions, systemic protection may be achieved in a manner similar to the virus resistance reported in tobacco (Smirnov et al., 1997) and more recently in cassava (Manihot esculenta) in experiments demonstrating control of the devastating Cassava brown streak Uganda virus (Yadav et al., 2011). Aside from pathogen resistance, down–regulation, and/or epigenetic modification of transcripts and genetic networks in the scion or the rootstock also appear to be possible through the use of siRNAs and could influence scion-specific characteristics, such as flowering time, fruit production or quality, or root characteristics, such as tuberization in potatoes (Martin et al., 2009).
Protein
In addition to RNAs, proteins may be transported over long-distances in a regulated fashion. Certain motifs, reminiscent of nuclear localization signals, allow protein entry into CC and subsequently into the phloem for long-distance movement. Despite the evidence for selective and regulated processes for protein long-distance translocation, there is also evidence that shows non-specific “leakage” of supposedly cell-autonomous proteins into sieve tubes and subsequently into sink tissues. Xylem vessels, which mainly transport water and low molecular weight inorganic and organic solutes, have been shown to contain proteins, although at lower concentrations than in phloem sap (Aguero et al., 2008; Buhtz et al., 2010). Proteins targeted to the apoplast may inadvertently enter xylem or phloem vasculature and subsequently be transported to and unloaded in sink tissues.
Examples of movement of proteins include exogenous viral movement proteins, endogenous transcription factors and xylem/phloem proteins (P-proteins). Some of the first studies of xylem protein transport involved viral movement proteins (Wolf et al., 1989), but as knowledge has progressed, more researchers have been able to demonstrate mobility of endogenous plant proteins. For many years, proteins had been observed in the phloem, but the idea of a coordinated, selective, and regulated process of trafficking, influencing not only development, but plant responses to environmental cues is a more recent idea that has gained support (Kehr, 2009). Mobile proteins or non-cell-autonomous proteins (NCAPs) may be encoded by as many as 20% of the genes in Arabidopsis (Lee et al., 2006). A comprehensive analysis of phloem sap proteins in pumpkin and cucumber using high resolution 2-D gel electrophoresis and partial sequencing by mass spectrometry identified several hundred proteins in the phloem, and the majority of these proteins may have roles in stress and defense reactions (Walz et al., 2004).
Models of the mechanics underlying protein mobility in the vasculature include the structures associated with the vascular tissue. Within the phloem, SE, which lack a nucleus, ribosomes, and a vacuole, depend on neighboring CC for maintenance of their metabolic tasks (Fisher et al., 1992). Because mature SE cells cannot synthesize proteins, the likely origins of proteins in the phloem are immature SE or CC. Structurally different from the plasmodesmata that connect mesophyll cells, specialized plasmodesmata between CC and SE are branched with all of the branches on the CC side funneling to a single opening on the SE membrane side. The requirements for specificity of transport between CC and SE are not completely known but accumulating evidence points to the importance of these branched plasmodesmata. Reviews from two research groups establish plasmodesmata as the “gatekeepers” of macromolecular transport into the SE (Zambryski and Crawford, 2000; Lough and Lucas, 2006). The specific mechanisms governing the regulation of plasmodesmatal apertures are still a mystery, but fluorescently labeled dextrans and GFP expression have been used to study plasmodesmatal size exclusion limits and their function under differing conditions. Through grafting, the vascular networks (phloem and xylem) of both rootstock and scion become connected and what is mobile in the rootstock vascular networks is likely to become mobile in the vascular networks of the scion.
In a thorough heterografting experiment involving 11 interspecific and intergeneric Cucurbit graft combinations, several structural P-proteins appeared in the recipient phloem exudate, as shown by SDS-PAGE and Coomassie staining. The results effectively demonstrated the direction of transmission was dependent on the combination of heterograft used, with some graft partners taking the role of donor or acceptor, and some able to perform both roles (Golecki et al., 1998). This has clear implications for choosing of graft partners for GE-modified rootstocks. Fluorescence microscopy of graft junctions has shown sieve tube bridges connecting scion external bundle phloem to internal bundle rootstock phloem when mobility was demonstrated. This observation identified physical continuity within the phloem as a prerequisite for mobility of proteins, but did not resolve the selective directionality observed (Golecki et al., 1999). When two Cucurbit structural P-proteins, PP1 and PP2 were examined in intergeneric grafts, RT-PCR and Northern blots demonstrated that protein products rather than mRNA transcripts were translocated across the graft junctions.
In addition to structural proteins, RNA-binding proteins appear to be abundant in the phloem translocation stream. Phloem sap collected and analyzed from four different sources (cucumber, lupine, castor bean, and yucca) all contained sRNAs of 18–25 nt sizes with various abundance profiles for each species. Fractionation of the phloem sap from pumpkin, cucumber, and lupine also identified a small ∼27 kDa protein (PSPR1) that bound strongly to 18–24 nt ssRNA. After cloning the pumpkin PSPR1 gene, microinjection studies demonstrated that PSPR1 specifically shuttled a high percentage of the ssRNAs across cell boundaries. In these studies, co-injection and subsequent movement of a 20-kDa fluorescent dextran showed that plasmodesmatal aperture was at least 20 kDa. Apparently, dilated plasmodesmata alone were not sufficient to allow the movement of ssRNAs between cells, since use of another protein shown to increase plasmodesmatal apertures (KN1) was not sufficient to allow the movement of the ssRNAs (Yoo et al., 2004). Given that the ssRNAs were approximately 8 kDa, their lack of movement when KN1 was provided suggested a sequestration mechanism or a more complex ssRNA-binding protein interaction than is currently presumed.
In an informative experiment, rice thioredoxin (RPP13-1) a major phloem sieve tube protein with basic antioxidant functions, was expressed in E. coli and fluorescently labeled with FITC (Ishiwatari et al., 1995). In tobacco, the labeled, heterologously expressed RPP13-1 protein was observed to migrate beyond the site of injection. However, the similarly purified and labeled E. coli homolog of RPP13-1 was not phloem-mobile under duplicate conditions, suggesting significant sequence or structure requirements for movement. Co-injection of rice RPP13-1 and FITC-labeled dextrans established that RPP13-1 increased the plasmodesmatal size exclusion limit to 9–20 kDa, from ∼1 kDa. Furthermore, two mutants of RPP13-1 that were deficient for mobility were identified and crystal structure prediction studies suggested that charged clusters of residues on the outer surface were responsible for binding and/or transport of RPP13-1 through the companion cell-plasmodesmata complex (Ishiwatari et al., 1998).
Aoki et al. (2002) demonstrated the importance of protein structure for mobility using two heat shock proteins (HSPs), CmHsc70-1 and CmHsc70-2, that had been isolated from pumpkin phloem sap. In microinjection experiments, CmHsc70-1 and CmHsc70-2, interacted with plasmodesmata, increasing the size exclusion limit and thereby, enhanced their own cell-to-cell transport. The C-terminal region of these HSPs potentiated their non-cell-autonomous mobility through the plasmodesmata. A gain-of-function experiment in which the C-terminal cucumber HSP motif was fused to a human Hsp70 protein established that the fusion protein, but not WT human Hsp70, could move from cell-to-cell following microinjection into pumpkin cotyledons, much like the movement of injected intact CmHsc70-1 and CmHsc70-2. Interestingly, fusing the HSP C-terminal motif to GFP did not result in cell-to-cell migration, suggesting that at least in this case, the targeting motif was only active in the context of highly conserved HSPs (Aoki et al., 2002). Unlike nuclear localization signals or ER-targeting peptides, vascular system targeting peptides may have several different motifs, perhaps suggesting specialized interactions with different families of proteins, and/or selective import/export mechanisms.
While targeting motifs appear to be important in regulating mobility, a non-regulated diffusion-based mechanism in the symplast from one cell to another is supported by the observation that protein size influences non-targeted movement of GFP but differences appear to be species- and developmental stage-dependent. Earlier studies indicated that non-regulated diffusion is limited to ∼50 kDa proteins in mature leaves and 60 kDa proteins in developing leaves (Oparka et al., 1999; Crawford and Zambryski, 2000).
Unregulated diffusion-based movement across the sieve tube element–companion cell complex has been observed when CC-specific promoters (e.g., AtSUC2) regulate 27 kDa GFP expression. GFP was detected in the SE and carried to sink tissue in the translocation stream (Imlau et al., 1999). While it was perhaps not surprising to detect the GFP in the vascular system due to the porous end plates of the SE, unloading of the GFP into the mesophyll sink cells was unexpected. Using the same promoter, GFP-fusions as large as 67 kDa subsequently were shown to traffic from CC to SE in root tips, although larger variants were restricted to a zone of cells adjacent to the mature protophloem. Only the smaller GFP variants (27–36 kDa) moved beyond this zone (Stadler et al., 2005).
To add further complexity to protein trafficking and regulation, phosphorylation, and glycosylation are required for pumpkin CmPP16 to interact and form a stable complex with the mobility-endowing protein, Nt-NCAPP1, prior its phloem trafficking (Taoka et al., 2007). Discrepancies in observed mobility from one study to another could be attributed to phosphorylation and glycosylation since earlier studies did not take these post-translational, covalent modifications into consideration.
Two groups have demonstrated that non-endogenous proteins are retained in the rootstock. The Gastrodia antifungal protein (GAFP-1, a lectin) expressed by transgenic plum rootstocks under the control of the constitutive CaMV35S promoter was identified in roots by immunoblot, but not in the soft shoot or leaf tissues of grafted, WT scions. This suggested that GAFP-1 was not moving into the WT-scion tissues of transgrafted plum trees (Nagel et al., 2010). In the other example, transgenic watermelon rootstocks over-expressing a cucumber mottle mosaic virus coat protein (CGMMV-CP) gene were transgrafted with WT watermelon. Protein expression and mRNA levels were detected in the transgenic rootstock but not in the non-transgenic scion (Youk et al., 2009). Detection limits of the techniques utilized were not reported in either of these studies. A pokeweed (Phytolacca americana) antiviral protein was expressed in transgenic Nicotiana tabacum rootstocks and provided resistance to potato virus X in NN and nn grafted non-transgenic scions. However, the antiviral protein was detected only in the rootstocks and not in the grafted scion tissues (Smirnov et al., 1997). The basis for resistance expression in this situation is not clear.
Protein translocation from a transgenic rootstock to a WT-scion will likely depend on the species and/or type of protein in the transgene construct. Should proteins encoded by transgenes manage to migrate to the scion, their longevity is a consideration. For example, NPTII and GUS proteins have estimated half-lives of 6–7 min and 36 h, respectively, in planta (Lo et al., 2005). If NPTII were translocated to scions it would be lost rapidly, but the GUS protein would not be reduced to 1% of the initial level accumulated in scions for 10 days.
Research on the production of proteins encoded by transgenes in rootstocks for delivery to scions arguably is more advanced than analogous work with the use of nucleic acids. For example, researchers at the University of Florida have engineered grape rootstocks that deliver hybrid lytic peptides to control bacterial and fungal diseases (Dutt et al., 2007; Gray et al., 2007). Work in our lab has shown that delivery of a protein that inhibits microbial maceration of plant cell walls is possible (below). While advances to date have focused on delivery of single gene products with specific functions to scions, future advances may target transport of transcription factors that influence expression of multiple genes, which could coordinate concerted scion responses to complex challenges such as pathogens, pests, or abiotic stresses.
Delivery of Anti-Pathogen Proteins from Rootstocks to Scion: The pPGIP Example
Proteins that are delivered to and function in the apoplast can provide protection against pathogens, particularly those pathogens that target the cell wall. The plant cell wall is the site where the molecular conversations that determine the host plant’s fate are begun (Cantu et al., 2008a,b). In many plant–microbe or plant–pathogen interactions, the plant cell walls are a major obstacle to colonization or expansion within plant tissues. To overcome this barrier, most fungal pathogens produce a variety of enzymes, which degrade the host cell wall. Polygalacturonases (PGs) (EC 3.2.1.15) are often the first enzymes secreted during the infections (Collmer and Keen, 1986). PGs cleave α-(1 → 4) linkages between D-galacturosyl residues in pectic homogalacturonan, causing cell separation and tissue maceration. Botrytis cinerea expresses six PGs during infection and growth on plant hosts (Wubben et al., 1999) and the PG-inhibiting protein (PGIP) produced in pear fruit (pPGIP), inhibits some but not all of these PGs (Sharrock and Labavitch, 1994).
Given the importance of PGs in pest and pathogen interactions with plants, it is not surprising that PGIPs are components of the defenses against invasion by pathogens and pests (Powell et al., 2000; Ferrari et al., 2003; Aguero et al., 2005; Shackel et al., 2005; Celorio-Mancera et al., 2008). Tomato foliar and ripe fruit resistance to the fungal pathogen, B. cinerea, is improved about 40% by the constitutive over-expression of pPGIP in tomatoes (Powell et al., 2000). The Miridae insect, Lygus hesperus, produces PGs that cause damage to alfalfa and cotton florets (Shackel et al., 2005) and PGIPs can inhibit these PGs and may, therefore, reduce the damage to plant tissues (Celorio-Mancera et al., 2008). The nematode, Meloidogyne incognita) causing root knot disease expresses PGs (McCarter et al., 2003), but it is not known if they can be inhibited by PGIPs. PGIPs expressed in rootstocks, therefore, are potential anti-pathogen proteins that could be delivered from the rootstock to the scion in transgrafted plants.
Our work has shown that pPGIP expression reduces the effects of Pierce’s Disease in grapevines, caused by the bacterium, Xylella fastidiosa (Aguero et al., 2005) because it inhibits the X. fastidiosa virulence factor, PG (Roper et al., 2007; Perez-Donoso et al., 2010). As with other vascular pathogens, the X. fastidiosa PG contributes to disease development by digesting the polysaccharides in the pit membranes of the xylem network. When intact, these so-called “membranes” (they are actually primary cell wall structures) help to prevent the pathogen’s vessel-to-vessel spread from the initial sites of infection of grapevines (Sun et al., 2011). Because pPGIP inhibits the X. fastidiosa PG and because pPGIP can enter the xylem, PGIPs in the xylem of both the rootstock and the scion could provide protection against other PG-utilizing pathogens in the water transport system.
We have observed that when pPGIP-expressing transgenic plants are used as rootstocks onto which non-expressing scions are grafted, the pPGIP protein, but not the pPGIP-encoding nucleic acids, are exported to the scion, crossing the graft union via the xylem system (Aguero et al., 2005). In grafted tomato plants expressing pPGIP in the rootstock, pPGIP protein has been detected in scion leaves (Figure 1). Similarly, in grafted grapevines, we have observed the pPGIP protein in the wild-type scion tissue grafted onto pPGIP-expressing rootstocks (Figure 1).
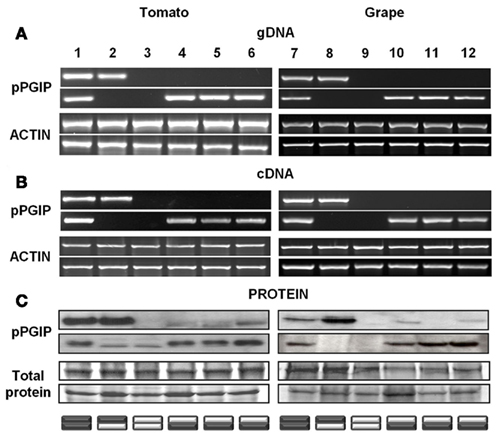
Figure 1. PCR products from tomato and grape leaf tissues using (A) genomic DNA and (B) reverse transcribed mRNA (cDNA). Actin gene from each respective species was used as a positive control. Western blots (C) of protein extracts taken from scion and rootstock leaf tissues. Coomassie-stained total protein is used as a loading control. pPGIP is visualized with a polyclonal antibody whose limit of detection is 40 ng of protein. The pPGIP protein detected in the scion leaves has moved from transgenic rootstocks into wild-type (WT) scion tissue (lanes 4–6, 10, 12). This movement is not seen in the reciprocal graft (lanes 2, 8). Movement was not detected in grape scion (lane 11), though present in the rootstock. Cross-reactivity of the pPGIP antibody to wild-type tomato tissue can be observed in lanes 2 and 3 (rootstock), but these bands do not match the size of the transgenic pPGIP. Graft combinations are represented by stacked rectangles (bottom = rootstock; top = scion) with dark boxes representing transgenic portions of the plant and light boxes representing WT.
Furthermore, we have observed that expression of pPGIP in rootstocks reduces pathogen damage in scion tissues (Figure 2). Thus, defense factors in roots (e.g., pPGIP) can be made available to scions via grafting, improving the vigor, quality, and pathogen/pest resistance of the food-producing scion and its crop.
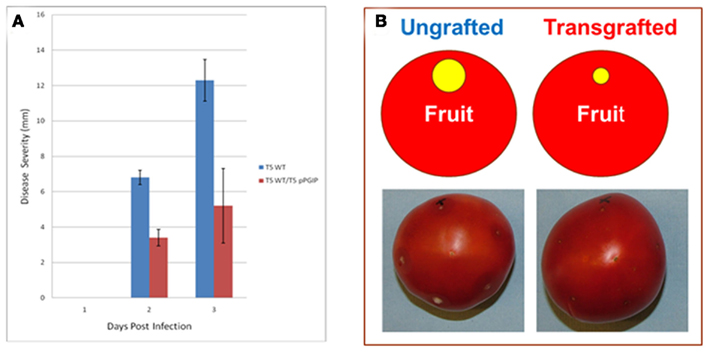
Figure 2. Disease severity of B. cinerea infections of T5 control (ungrafted) and transgrafted (T5:pPGIP rootstock with T5 scion) light red fruit. (A) Disease severity is measured as the diameter of the macerated tissue around the sites of inoculation. Each fruit was inoculated at five puncture sites with 1000 B. cinerea spores in 10 μl water. Fruit were maintained in moist crispers at 20°C and lesion diameter was measured daily (Cantu et al., 2008a, 2009). SE of the mean (SEM) are indicated. (B) Image of infected fruit 3 days post infection.
Concluding Remarks
Grafting has been used extensively to improve productivity, mainly in woody perennial horticultural crops like fruits and nuts, but is increasingly used to enhance the productivity and disease resistance of high-value vegetable crops. Transgrafting should extend the utility and value of the grafting strategy, enabling the utilization, in rootstocks, or scions, of transgenes whose products serve novel, potentially powerful functions. Obvious examples are the introduction of genes with demonstrated efficacy in disease resistance (e.g., PGIPs) and pest control, but also may include traits that target developmental events, metabolic pathways, or fruit quality. In designing and utilizing these strategies it will be important to consider the mechanisms that regulate long-distance translocation of DNA, RNA, sRNAs, and proteins to assess the durability and efficacy of alternative strategies. Key questions regarding regulatory consideration also must be assessed as this technology matures and research projects approach commercial reality. With increasing understanding of the mobilization of transgene-encoded molecules, researchers continue to expand the ability to deliver agronomic improvements to food products, extending the utility of horticultural grafting and providing a modern arsenal of options to an ancient art.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding of portions of this work was provided by Research Grant Award No. US-4309-10 from BARD, the United States – Israel Binational Agricultural Research and Development Fund.
References
Aguero, C. B., Thorne, E. T., Ibanez, A. M., Gubler, W. D., and Dandekar, A. M. (2008). Xylem sap proteins from Vitis vinifera L. Chardonnay. Am. J. Enol. Vitic. 59, 306–311.
Aguero, C. B., Uratsu, S. L., Greve, C., Powell, A. L. T., Labavitch, J. M., Meredith, C. P., and Dandekar, A. M. (2005). Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol. Plant Pathol. 6, 43–51.
Ahn, S. J., Im, Y. J., Chung, G. C., Cho, B. H., and Suh, S. R. (1999). Physiological responses of grafted-cucumber leaves and rootstock roots affected by low root temperature. Sci. Hortic. 81, 397–408.
Alvarez, J. P., Pekker, I., Goldshmidt, A., Blum, E., Amsellem, Z., and Eshed, Y. (2006). Endogenous and synthetic microRNAs stimulate simultaneous, efficient, and localized regulation of multiple targets in diverse species. Plant Cell 18, 1134–1151.
Aoki, K., Kragler, F., Xoconostle-Cazares, B., and Lucas, W. J. (2002). A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl. Acad. Sci. U.S.A. 99, 16342–16347.
Balliu, A., Vuksani, G., Kaciu, S., Nasto, T., and Haxhinasto, L. (2008). Grafting effects on tomato growth rate, yield and fruit quality under saline irrigation water. Acta Hortic. 801, 1161–1166.
Banerjee, A. K., Lin, T., and Hannapel, D. J. (2009). Untranslated regions of a mobile transcript mediate RNA metabolism. Plant Physiol. 151, 1831–1843.
Bassell, G. J., Oleynikov, Y., and Singer, R. H. (1999). The travels of mRNAs through all cells large and small. FASEB J. 13, 447–454.
Buhtz, A., Pieritz, J., Springer, F., and Kehr, J. (2010). Phloem small RNAs, nutrient stress responses, and systemic mobility. BMC Plant Biol. 10, 64. doi: 10.1186/1471-2229-10-64
Bulder, H. A. M., DenNijs, A. P. M., Speek, E. J., Van Hasselt, P. R., and Kuiper, P. J. C. (1991a). The effect of low root temperature on growth and lipid composition of low temperature tolerant rootstock genotypes for cucumber. J. Plant Physiol. 138, 661–666.
Bulder, H. A. M., Speek, E. J., Van Hasselt, P. R., and Kuiper, P. J. C. (1991b). Growth temperature and lipid composition of cucumber genotypes differing in adaptation to low energy conditioning. J. Plant Physiol. 138, 655–660.
Cantu, D., Blanco-Ulate, B., Yang, L., Labavitch, J. M., Bennett, A. B., and Powell, A. L. T. (2009). Ripening-regulated susceptibility of tomato fruit to Botrytis cinerea requires NOR but not RIN or ethylene. Plant Physiol. 150, 1434–1449.
Cantu, D., Vicente, A. R., Greve, L. C., Dewey, F. M., Bennett, A. B., Labavitch, J. M., and Powell, A. L. (2008a). The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. U.S.A. 105, 859–864.
Cantu, D., Vicente, A. R., Labavitch, J. M., Bennett, A. B., and Powell, A. L. T. (2008b). Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 13, 610–617.
Carlsbecker, A., Lee, J. Y., Roberts, C. J., Dettmer, J., Lehesranta, S., Zhou, J., Lindgren, O., Moreno-Risueno, M. A., Vaten, A., Thitamadee, S., Campilho, A., Sebastian, J., Bowman, J. L., Helariutta, Y., and Benfey, P. N. (2010). Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 465, 316–321.
Celorio-Mancera, M. D. L. P., Allen, M., Powell, A., Ahmadi, H., Salemi, M., Phinney, B., Shackel, K., Greve, L., Teuber, L., and Labavitch, J. (2008). Polygalacturonase causes Lygus-like damage on plants: cloning and identification of western tarnished plant bug (Lygus hesperus) polygalacturonases secreted during feeding. Arthropod Plant Interact. 2, 215–225.
COGEM. (2006). New Techniques in Plant Biotechnology. COGEM Report CGM/061024-02, Vol. 2011, Bilthoven: Commissie Genetische Modificatie.
Cohen, R., Burger, Y., Horev, C., Koren, A., and Edelstein, M. (2007). Introducing grafted cucurbits to modern agriculture: the Israeli experience. Plant Dis. 91, 916–923.
Colla, G., Rouphael, Y., Leonardi, C., and Bie, Z. (2010). Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 127, 147–155.
Collmer, A., and Keen, N. T. (1986). The role of pectic enzymes in plant pathogenesis. Annu. Rev. Phytopathol. 24, 383–409.
Crawford, K. M., and Zambryski, P. C. (2000). Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10, 1032–1040.
Crete, P., Leuenberger, S., Iglesias, V. A., Suarez, V., Schob, H., Holtorf, H., van Eeden, S., and Meins, F. (2001). Graft transmission of induced and spontaneous post-transcriptional silencing of chitinase genes. Plant J. 28, 493–501.
Davis, A. R., Perkins-Veazie, P., Hassell, R., Levi, A., King, S. R., and Zhang, X. (2008). Grafting effects on vegetable quality. HortScience 43, 1670–1672.
Di Gioia, F., Serio, F., Buttaro, D., Ayala, O., and Santamaria, P. (2010). Influence of rootstock on vegetative growth, fruit yield and quality in ‘Cuore di Bue,’ an heirloom tomato. J. Hortic. Sci. Biotechnol. 85, 477–482.
Dolgov, S. V., and Hanke, M. V. (2006). “Transgenic temperate fruit tree rootstocks,” in Tree Transgenesis: Recent Developments, eds M. Fladung, and D. Ewald (Heidelberg: Springer), 335–350.
Dunoyer, P., Brosnan, C. A., Schott, G., Wang, Y., Jay, F., Alioua, A., Himber, C., and Voinnet, O. (2010). An endogenous, systemic RNAi pathway in plants. EMBO J. 29, 1699–1712.
Dunoyer, P., Himber, C., Ruiz-Ferrer, V., Alioua, A., and Voinnet, O. (2007). Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat. Genet. 39, 848–856.
Dunoyer, P., Himber, C., and Voinnet, O. (2005). DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 37, 1356–1360.
Dutt, M., Li, Z. T., Kelley, K. T., Dhekney, S. A., Van Aman, M., Tattersall, J., and Gray, D. J. (2007). Transgenic rootstock protein transmission in grapevines. Acta Hortic. 738, 749.
Edelstein, M., Ben-Hur, M., Cohen, R., Burger, Y., and Ravina, I. (2005). Boron and salinity effects on grafted and non-grafted melon plants. Plant Soil 269, 273–284.
Edelstein, M., Ben-Hur, M., and Plaut, Z. (2007). Grafted melons irrigated with fresh or effluent water tolerate excess boron. J. Am. Soc. Hortic. Sci. 132, 484–491.
Edelstein, M., Cohen, R., Burger, Y., Shriber, S., Pivonia, S., and Shtienberg, D. (1999). Integrated management of sudden wilt of melons, caused by Monosporascus cannonballus, using grafting and reduced rate of methyl bromide. Plant Dis. 83, 1142–1145.
Escobar, M. A., Leslie, C. A., McGranahan, G. H., and Dandekar, A. M. (2002). Silencing crown gall disease in walnut (Juglans regia L.). Plant Sci. 163, 591–597.
Fernandez-Garcia, N., Martinez, V., Cerda, A., and Carvajal, M. (2004). Fruit quality of grafted tomato plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 79, 995–1001.
Ferrari, S., Vairo, D., Ausubel, F. M., Cervone, F., and De Lorenzo, G. (2003). Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15, 93–106.
Fisher, D. B., Wu, Y., and Ku, M. S. (1992). Turnover of soluble proteins in the wheat sieve tube. Plant Physiol. 100, 1433–1441.
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H., and Ryals, J. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756.
Golecki, B., Schulz, A., Carstens-Behrens, U., and Kollmann, R. (1998). Evidence for graft transmission of structural phloem proteins or their precursors in heterografts of Cucurbitaceae. Planta 206, 630–640.
Golecki, B., Schulz, A., and Thompson, G. A. (1999). Translocation of structural P proteins in the phloem. Plant Cell 11, 127–140.
Gomez, G., Torres, H., and Pallas, V. (2005). Identification of translocatable RNA-binding phloem proteins from melon, potential components of the long-distance RNA transport system. Plant J. 41, 107–116.
Gray, D., Li, Z., Dhekney, S., Dutt, M., Hopkins, D., and Zimmerman, T. (2007). Field testing of transgenic grapevine for bacterial and fungal disease resistance. HortScience 42, 858–858.
Haroldsen, V. M., Paulino, G., Chi-Ham, C. L., and Bennett, A. B. (2012). Survey of fruit and nut crops finds that grape, citrus and walnut lead in biotech research. Calif Agr. (in press).
Haywood, V., Yu, T. S., Huang, N. C., and Lucas, W. J. (2005). Phloem long-distance trafficking of GIBBERELLIC acid-insensitive RNA regulates leaf development. Plant J. 42, 49–68.
Hewezi, T., Alibert, G., and Kallerhoff, J. (2005). Local infiltration of high- and low-molecular-weight RNA from silenced sunflower (Helianthus annuus L.) plants triggers post-transcriptional gene silencing in non-silenced plants. Plant Biotechnol. J. 3, 81–89.
Huang, G., Allen, R., Davis, E. L., Baum, T. J., and Hussey, R. S. (2006). Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. U.S.A. 103, 14302–14306.
Huang, N.-C., and Yu, T.-S. (2009). The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J. 59, 921–929.
Imlau, A., Truernit, E., and Sauer, N. (1999). Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11, 309–322.
Ishiwatari, Y., Fujiwara, T., McFarland, K. C., Nemoto, K., Hayashi, H., Chino, M., and Lucas, W. J. (1998). Rice phloem thioredoxin h has the capacity to mediate its own cell-to-cell transport through plasmodesmata. Planta 205, 12–22.
Ishiwatari, Y., Honda, C., Kawashima, I., Nakamura, S., Hirano, H., Mori, S., Fujiwara, T., Hayashi, H., and Chino, M. (1995). Thioredoxin-h is one of the major proteins in rice phloem sap. Planta 195, 456–463.
Jones, L., Ratcliff, F., and Baulcombe, D. C. (2001). RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr. Biol. 11, 747–757.
Juarez, M. T., Twigg, R. W., and Timmermans, M. C. (2004). Specification of adaxial cell fate during maize leaf development. Development 131, 4533–4544.
Justus, I., and Kubota, C. (2010). Effects of low temperature storage on growth and transplant quality of non-grafted and grafted cantaloupe-type muskmelon seedlings. Sci. Hortic. 125, 47–54.
Kalaitzandonakes, N., Alston, J., and Bradford, K. (2007). Compliance costs for regulatory approval of new biotech crops. Nat. Biotechnol. 25, 209–211.
Kehr, J. (2009). Long-distance transport of macromolecules through the phloem. F1000 Biol. Rep. 1, 1–5.
Khah, E. M., Kakava, E., Mavromatis, A., Chachalis, D., and Goulas, C. (2006). Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 8, 3–7.
Kim, I., Kobayashi, K., Cho, E., and Zambryski, P. C. (2005). Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 102, 11945–11950.
Kim, M., Canio, W., Kessler, S., and Sinha, N. (2001). Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293, 287–289.
King, S. R., Davis, A. R., Liu, W., and Levi, A. (2008). Grafting for disease resistance. HortScience 43, 1673–1676.
Klink, V., Kim, K.-H., Martins, V., MacDonald, M., Beard, H., Alkharouf, N., Lee, S.-K., Park, S.-C., and Matthews, B. (2009). A correlation between host-mediated expression of parasite genes as tandem inverted repeats and abrogation of development of female Heterodera glycines cyst formation during infection of Glycine max. Planta 230, 53–71.
Klink, V. P., and Matthews, B. F. (2009). Emerging approaches to broaden resistance of soybean to soybean cyst nematode as supported by gene expression studies. Plant Physiol. 151, 1017–1022.
Kobayashi, K., and Zambryski, P. (2007). RNA silencing and its cell-to-cell spread during Arabidopsis embryogenesis. Plant J. 50, 597–604.
Kubota, C. (2008). Use of grafted seedlings for vegetable production in North America. Acta Hortic. 770, 21–28.
Kubota, C., McClure, M., Kokalis-Burelle, N., Bausher, M., and Rosskopf, E. (2008). Vegetable grafting: history, use and current technology status in North America. HortScience 43, 1664–1669.
Kudo, H., and Harada, T. (2007). A graft-transmissible RNA from tomato rootstock changes leaf morphology of potato scion. HortScience 42, 225–226.
Lee, J. M. (1994). Cultivation of grafted vegetables.1. Current status, grafting methods, and benefits. Hortscience 29, 235–239.
Lee, J.-M., Kubota, C., Tsao, S. J., Bie, Z., Echevarria, P. H., Morra, L., and Oda, M. (2010). Current status of vegetable grafting: diffusion, grafting techniques, automation. Sci. Hortic. 127, 93–105.
Lee, J. Y., Colinas, J., Wang, J. Y., Mace, D., Ohler, U., and Benfey, P. N. (2006). Transcriptional and posttranscriptional regulation of transcription factor expression in Arabidopsis roots. Proc. Natl. Acad. Sci. U.S.A. 103, 6055–6060.
Lev-Yadun, S., and Sederoff, R. (2001). Grafting for transgene containment. Nat. Biotechnol. 19, 1104.
Lin, S. I., Chiang, S. F., Lin, W. Y., Chen, J. W., Tseng, C. Y., Wu, P. C., and Chiou, T. J. (2008). Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 147, 732–746.
Liu, Y. S., Wang, Q. L., and Li, B. Y. (2010). New insights into plant graft hybridization. Heredity 104, 1–2.
Lo, C., Wang, N., and Lam, E. (2005). Inducible double-stranded RNA expression activates reversible transcript turnover and stable translational suppression of a target gene in transgenic tobacco. FEBS Lett. 579, 1498–1502.
Lough, T. J., and Lucas, W. J. (2006). Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 57, 203–232.
Lucas, W. J., Yoo, B. C., and Kragler, F. (2001). RNA as a long-distance information macromolecule in plants. Nat. Rev. Mol. Cell Biol. 2, 849–857.
Mallory, A. C., Mlotshwa, S., Bowman, L. H., and Vance, V. B. (2003). The capacity of transgenic tobacco to send a systemic RNA silencing signal depends on the nature of the inducing transgene locus. Plant J. 35, 82–92.
Martin, A., Adam, H., Díaz-Mendoza, M., Żurczak, M., González-Schain, N. D., and Suárez-López, P. (2009). Graft-transmissible induction of potato tuberization by the microRNA miR172. Development 136, 2873–2881.
McCarter, J., Dautova Mitreva, M., Martin, J., Dante, M., Wylie, T., Rao, U., Pape, D., Bowers, Y., Theising, B., Murphy, C., Kloek, A., Chiapelli, B., Clifton, S., Bird, D. M., and Waterston, R. (2003). Analysis and functional classification of transcripts from the nematode Meloidogyne incognita. Genome Biol. 4, R26.
Misovic, A., Ilin, Z., and Markovic, V. (2009). Effect of different rootstock type on quality and yield of tomato fruits. Acta Hortic. 807, 619–624.
Molnar, A., Melnyk, C. W., Bassett, A., Hardcastle, T. J., Dunn, R., and Baulcombe, D. C. (2010). Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 328, 872–875.
Mudge, K., Janick, J., Scofield, S., and Goldschmidt, E. E. (2009). A history of grafting. Hortic. Rev. (Am. Soc. Hortic. Sci.) 35, 437–494.
Nagel, A. K., Kalariya, H., and Schnabel, G. (2010). The Gastrodia antifungal protein (GAFP-1) and its transcript are absent from scions of chimeric-grafted Plum. HortScience 45, 188–192.
Narsai, R., Howell, K. A., Millar, A. H., O’Toole, N., Small, I., and Whelan, J. (2007). Genome-wide analysis of mRNA decay rates and their determinants in Arabidopsis thaliana. Plant Cell 19, 3418–3436.
Ohta, Y. (1991). Graft-transformation, the mechanism for graft-induced genetic changes in higher plants. Euphytica 55, 91–99.
Omid, A., Keilin, T., Glass, A., Leshkowitz, D., and Wolf, S. (2007). Characterization of phloem-sap transcription profile in melon plants. J. Exp. Bot. 58, 3645–3656.
Oparka, K. J., Roberts, A. G., Boevink, P., Santa Cruz, S., Roberts, I., Pradel, K. S., Imlau, A., Kotlizky, G., Sauer, N., and Epel, B. (1999). Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754.
Palauqui, J. C., Elmayan, T., Pollien, J. M., and Vaucheret, H. (1997). Systemic acquired silencing: transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16, 4738–4745.
Palauqui, J. C., and Vaucheret, H. (1995). Field trial analysis of nitrate reductase co-suppression: a comparative study of 38 combinations of transgene loci. Plant Mol. Biol. 29, 149–159.
Pant, B. D., Buhtz, A., Kehr, J., and Scheible, W. R. (2008). MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 53, 731–738.
Parizotto, E. A., Dunoyer, P., Rahm, N., Himber, C., and Voinnet, O. (2004). In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev. 18, 2237–2242.
Perez-Donoso, A. G., Sun, Q., Roper, M. C., Greve, L. C., Kirkpatrick, B., and Labavitch, J. M. (2010). Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected Grapevines. Plant Physiol. 152, 1748–1759.
Powell, A. L. T., van Kan, J., ten Have, A., Visser, J., Greve, L. C., Bennett, A. B., and Labavitch, J. M. (2000). Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol. Plant Microbe Interact. 13, 942–950.
Rivero, R. M., Ruiz, J. M., and Romero, L. (2003). Can grafting in tomato plants strengthen resistance to thermal stress? J. Sci. Food Agric. 83, 1315–1319.
Romano, D., and Paratore, A. (2001). Effects of grafting on tomato and eggplant. Acta Hortic. 559, 149–153.
Roney, J. K., Khatibi, P. A., and Westwood, J. H. (2007). Cross-species translocation of mRNA from host plants into the parasitic plant dodder. Plant Physiol. 143, 1037–1043.
Roper, M. C., Greve, L., Labavitch, J. M., and Kirkpatrick, B. C. (2007). Xylella fastidosa requires polygalacturonase for colonization and pathogenicity on Vitis vinifera grapevines. Mol. Plant Microbe Interact. 20, 411–419.
Rouphael, Y., Schwarz, D., Krumbein, A., and Colla, G. (2010). Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 127, 172–179.
Ruiz-Medrano, R., Xoconostle-Cazares, B., and Lucas, W. J. (1999). Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126, 4405–4419.
Savvas, D., Colla, G., Rouphael, Y., and Schwarz, D. (2010). Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci. Hortic. 127, 156–161.
Schmelzer, K. R., Kubala, L., Newman, J. W., Kim, I. H., Eiserich, J. P., and Hammock, B. D. (2005). Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. U.S.A. 102, 9772–9777.
Schwarz, D., Rouphael, Y., Colla, G., and Venema, J. H. (2010). Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci. Hortic. 127, 162–171.
Shackel, K. A., Celorio-Mancera, M. D. L. P., Ahmadi, H., Greve, L. C., Teuber, L. R., Backus, E. A., and Labavitch, J. M. (2005). Micro-injection of Lygus salivary gland proteins to simulate feeding damage in alfalfa and cotton flowers. Arch. Insect Biochem. Physiol. 58, 69–83.
Shaharuddin, N. A., Han, Y., Li, H., and Grierson, D. (2006). The mechanism of graft transmission of sense and antisense gene silencing in tomato plants. FEBS Lett. 580, 6579–6586.
Sharrock, K. R., and Labavitch, J. M. (1994). Polygalacturonase inhibitors of Bartlett pear fruits: differential effects on Botrytis cinerea polygalacturonase isozymes, and influence on products of fungal hydrolysis of pear cell walls and on ethylene induction in cell culture. Physiol. Mol. Plant Pathol. 45, 305–319.
Shyu, A. B., Wilkinson, M. F., and van Hoof, A. (2008). Messenger RNA regulation: to translate or to degrade. EMBO J. 27, 471–481.
Smirnov, S., Shulaev, V., and Tumer, N. E. (1997). Expression of Pokeweed antiviral protein in transgenic plants induces virus resistance in grafted wild-type plants independently of salicylic acid accumulation and pathogenesis-related protein synthesis. Plant Physiol. 114, 1113–1121.
Sonoda, S., and Nishiguchi, M. (2000). Graft transmission of post-transcriptional gene silencing: target specificity for RNA degradation is transmissible between silenced and non-silenced plants, but not between silenced plants. Plant J. 21, 1–8.
Stadler, R., Wright, K. M., Lauterbach, C., Amon, G., Gahrtz, M., Feuerstein, A., Oparka, K. J., and Sauer, N. (2005). Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 41, 319–331.
Steeves, R. M., Todd, T. C., Essig, J. S., and Trick, H. N. (2006). Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct. Plant Biol. 33, 991–999.
Stegemann, S., and Bock, R. (2009). Exchange of genetic material between cells in plant tissue grafts. Science 324, 649–651.
Sun, Q., Greve, C. L., and Labavitch, J. M. (2011). Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiol. 155, 1976–1987.
Takeda, R., Petrov, A. I., Leontis, N. B., and Ding, B. (2011). A three-dimensional RNA motif in potato spindle tuber viroid mediates trafficking from palisade mesophyll to spongy mesophyll in Nicotiana benthamiana. Plant Cell 23, 258–272.
Taller, J., Hirata, Y., Yagishita, N., Kita, M., and Ogata, S. (1998). Graft-induced genetic changes and the inheritance of several characteristics in pepper (Capsicum annuum L.). Theor. Appl. Genet. 97, 705–713.
Taoka, K., Ham, B. K., Xoconostle-Cazares, B., Rojas, M. R., and Lucas, W. J. (2007). Reciprocal phosphorylation and glycosylation recognition motifs control NCAPP1 interaction with pumpkin phloem proteins and their cell-to-cell movement. Plant Cell 19, 1866–1884.
Tournier, B., Tabler, M., and Kalantidis, K. (2006). Phloem flow strongly influences the systemic spread of silencing in GFP Nicotiana benthamiana plants. Plant J. 47, 383–394.
Vazquez, F., Legrand, S., and Windels, D. (2010). The biosynthetic pathways and biological scopes of plant small RNAs. Trends Plant Sci. 15, 337–345.
Venema, J. H., Dijk, B. E., Bax, J. M., van Hasselt, P. R., and Elzenga, J. T. M. (2008). Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solanum habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot. 63, 359–367.
Voinnet, O., Vain, P., Angell, S., and Baulcombe, D. C. (1998). Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95, 177–187.
Walz, C., Giavalisco, P., Schad, M., Juenger, M., Klose, J., and Kehr, J. (2004). Proteomics of curcurbit phloem exudate reveals a network of defence proteins. Phytochemistry 65, 1795–1804.
Wolf, S., Deom, C. M., Beachy, R. N., and Lucas, W. J. (1989). Movement protein of tobacco mosaic-virus modifies plasmodesmatal size exclusion Limit. Science 246, 377–379.
Wubben, J. P., Mulder, W., ten Have, A., van Kan, J. A. L., and Visser, J. (1999). Cloning and partial characterization of endopolygalacturonase genes from Botrytis cinerea. Appl. Environ. Microbiol. 65, 1596–1602.
Xoconostle-Cazares, B., Xiang, Y., Ruiz-Medrano, R., Wang, H. L., Monzer, J., Yoo, B. C., McFarland, K. C., Franceschi, V. R., and Lucas, W. J. (1999). Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283, 94–98.
Yadav, J. S., Ogwok, E., Wagaba, H., Patil, B. L., Bagewadi, B., Alicai, T., Gaitan-Solis, E., Taylor, N. J., and Fauquet, C. M. (2011). RNAi-mediated resistance to Cassava brown streak Uganda virus in transgenic cassava. Mol. Plant Pathol. 12, 677–687.
Yelina, N. E., Smith, L. M., Jones, A. M., Patel, K., Kelly, K. A., and Baulcombe, D. C. (2010). Putative Arabidopsis THO/TREX mRNA export complex is involved in transgene and endogenous siRNA biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 107, 13948–13953.
Yoo, B. C., Kragler, F., Varkonyi-Gasic, E., Haywood, V., Archer-Evans, S., Lee, Y. M., Lough, T. J., and Lucas, W. J. (2004). A systemic small RNA signaling system in plants. Plant Cell 16, 1979–2000.
Youk, E. S., Pack, I. S., Kim, Y.-J., Yoon, W. K., Kim, C.-G., Ryu, S. B., Harn, C. H., Jeong, S.-C., and Kim, H. M. (2009). A framework for molecular genetic assessment of a transgenic watermelon rootstock line. Plant Sci. 176, 805–811.
Zambryski, P., and Crawford, K. (2000). Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. 16, 393–421.
Keywords: genetically engineered, protein, mRNA, siRNA, rootstock, scion, transgrafting
Citation: Haroldsen VM, Szczerba MW, Aktas H, Lopez-Baltazar J, Odias MJ, Chi-Ham CL, Labavitch JM, Bennett AB and Powell ALT (2012) Mobility of transgenic nucleic acids and proteins within grafted rootstocks for agricultural improvement. Front. Plant Sci. 3:39. doi: 10.3389/fpls.2012.00039
Received: 27 December 2011; Accepted: 17 February 2012;
Published online: 02 March 2012.
Edited by:
Vitaly Citovsky, State University of New York at Stony Brook, USACopyright: © 2012 Haroldsen, Szczerba, Aktas, Lopez-Baltazar, Odias, Chi-Ham, Labavitch, Bennett and Powell. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ann L. T. Powell, Department of Plant Sciences, MS5, University of California, Davis, Davis, CA 95616, USA. e-mail: alpowell@ucdavis.edu
