- 1 Department of Botany, University of British Columbia, Vancouver, BC, Canada
- 2 Department of Biology, McGill University, Montreal, QC, Canada
Arabidopsis seed coat epidermal cells produce a large quantity of mucilage that is extruded upon exposure to water. Chemical analyses and cell biological techniques suggest that this mucilage represents a specialized type of secondary cell wall composed primarily of pectin with lesser amounts of cellulose and xyloglucan. Once extruded, the mucilage capsule has a distinctive structure with an outer non-adherent layer that is easily removed by shaking in water, and an inner adherent layer that can only be removed with strong acid or base. Most of the cellulose in the mucilage is present in the inner layer and is responsible at least in part for its adherence to the seed. There are also differences in the pectin composition between the two layers that could contribute to the difference in adherence. The Arabidopsis seed coat epidermis and its mucilage are not essential for seed viability or germination. This dispensability, combined with the fact that the epidermal cells synthesize an accessible pectin-rich cell wall at a specific time in development, makes them well suited as a genetic model for studying cell wall biogenesis, function, and regulation. Mutants defective in seed mucilage identified by both forward and reverse genetic analyses are proving useful in establishing connections between carbohydrate structure and cell wall properties in vivo. In the future, genetic engineering of seed coat mucilage carbohydrates should prove useful for testing hypotheses concerning cell wall structure and function.
Introduction
When wetted, seeds of Arabidopsis thaliana (Arabidopsis), like those of many angiosperm species, become surrounded by a gelatinous capsule called mucilage (Figure 1A).
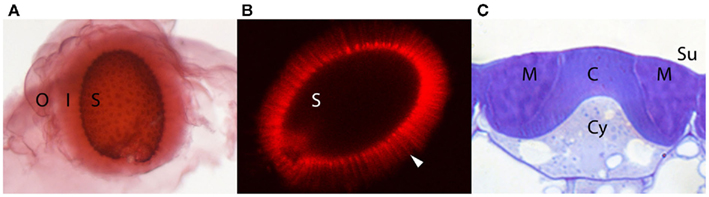
Figure 1. Mature Arabidopsis seeds with extruded mucilage and a developing seed coat epidermal cell. (A) Mature seed (S) following exposure to water and staining with ruthenium red without shaking. The extruded mucilage capsule is comprised of an outer, loosely attached, lightly stained layer (O), and an inner adherent layer (I) with faint more darkly staining “rays.” (B) Mature seed (S) following exposure to water and staining with pontamine fast scarlet. The cellulose network of the extruded mucilage including the more densely stained “rays” (arrowhead) extending outward from the top of each columella can be seen. (C) Cross section of a single seed coat epidermal cell on the outer surface (Su) of a seed at 11 days post anthesis stained with toluidine blue O. The mucilage (M) between the columella (C) and the outer primary wall can be seen. At this stage cellulose for the columella is still being synthesized and will continue until the cytoplasm (Cy) is completely displaced.
The mucilage originates from the specialized epidermal cells of the seed coat. During seed coat differentiation following cessation of cell growth, mucilage is synthesized and secreted to a region of the apoplast adjacent to the radial and outer tangential cell walls, forming a donut-shaped pocket between the membrane and the cell wall. Later, a thick cellulose-rich cell wall (columella) is synthesized beneath the mucilage pocket, along the outer tangential and radial sides of the cell that completely displaces the cytoplasm by the end of seed development. At maturity, therefore, the epidermal cells consist of a volcano-shaped cellulosic cell wall surrounded by a donut-shaped mucilage pocket, all of which are contained within the primary cell wall (Figure 1C). Upon exposure to water, the expanding hydrophilic mucilage ruptures the primary wall and expands to encapsulate the seed, presumably for dispersal, protection, and/or hydration (reviewed in Arsovski et al., 2010; Western, 2012). Over the past decade, research using chemical analyses and cell biological techniques has revealed that the mucilage has a complex composition and structure and as we argue below, could be considered a specialized pectin-rich secondary cell wall. Because the seed coat is so amenable to molecular–genetic manipulations and the mucilage so accessible, seed coat mucilage provides a unique system for studying many aspects of cell wall structure and function. Below, we review the current literature pertaining to mucilage composition and structure and discuss its potential for enhancing cell wall research.
Composition of Arabidopsis Seed Coat Mucilage
Pectins
Pectins consist of a heterogeneous group of acidic polysaccharides characterized by the presence of galacturonic acid (GalA). Two key pectins include rhamnogalacturonan I (RG I) and homogalacturonan (HG). RG I has a backbone of alternating (1 → 2)-α-L-rhamnose (Rha) and (1 → 4)-β-D-GalA that can be substituted on the Rha residues with side-chains consisting of arabinans, galactans, type I arabinogalactans and terminal galactose (t-Gal) residues. HG consists of an unbranched chain of (1 → 4)-β-D-GalA residues that can be methylesterified or acetylated. Enzymatic removal of the methyl esters can allow Ca2+-based ionic bonding between HG molecules; the degree of gel formation of pectins is largely determined by the extent and pattern of HG de-esterification and disruption of HG Ca2+-bonding by other, branched pectins such as RG I (Harholt et al., 2010).
The pectinaceous nature of Arabidopsis seed coat mucilage is evident from staining with ruthenium red and the metachromatic dye toluidine blue O (Figure 1; Western et al., 2000). Chemical analysis of extracted mucilage using a variety of techniques (GC/MS with and without sugar linkage analysis, HPLC, Fourier transform-infrared spectroscopy) confirms the presence of pectins, primarily unbranched RG I (Goto, 1985; Western et al., 2000; Penfield et al., 2001; Dean et al., 2007; Macquet et al., 2007a). The predominance of RG I as the main component of Arabidopsis mucilage is also apparent using antibody staining for unbranched RG I with CCRC-M36 and INRA-RU2 (Young et al., 2008; Arsovski et al., 2009a; Pattathil et al., 2010; Sullivan et al., 2011). A significant reduction of overall mucilage production is also seen in mutants for the UDP-L-Rha synthase MUCILAGE-MODIFIED 4/RHAMNOSE SYNTHASE 2 that is specifically upregulated during mucilage synthesis (Usadel et al., 2004; Western et al., 2004; Oka et al., 2007). Small amounts of typical RG I side-chain polysaccharides, arabinans and galactans, as well as arabinogalactan type I, have been identified in mucilage with whole seed immunofluorescence (LM6, LM5, and JIM13 antibodies, respectively) and confirmed through linkage analysis, which also detects the number of branch points on the RG I backbone (Dean et al., 2007; Macquet et al., 2007a; Arsovski et al., 2009a; HE McFarlane and TL Western, unpublished data). HG of varying degrees of methyl esterification has also been identified using the JIM5 (low–medium esterified HG) and JIM7 (medium–highly esterified HG) antibodies and confirmed with chemical analysis (Willats et al., 2001; Rautengarten et al., 2008; Walker et al., 2011).
Cellulose
The presence of cellulose microfibrils has been recognized in many seed coat mucilages and Arabidopsis mucilage is no exception. Crystalline cellulose fibrils radiating out from the seed coat mucilage cells have been detected through their birefringent properties in polarized light (“Maltese-cross effect”; Vaughan and Whitehouse, 1971; Sullivan et al., 2011), by staining for dyes that detect β-glucans such as calcofluor white and pontamine fast scarlet S4B, and with fluorescently labeled prokaryotic cellulose binding domains (Willats et al., 2001; Blake et al., 2006; Harpaz-Saad et al., 2011; Mendu et al., 2011). Changes to mucilage structure are also observed in mutants for the cellulose synthase subunit CELLULOSE SYNTHASE 5 (CESA5; previously identified as MUM3; see below Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011).
Hemicellulose
Linkage analysis of Arabidopsis mucilage has suggested the presence of small quantities of the cross-linking glycan (hemicellulose) arabinoxylan (Naran et al., 2008; Arsovski et al., 2009a). While xylans have not been detected in mucilage with immunofluorescence with the monoclonal antibody LM10, a polyclonal antibody to another hemicellulose, xyloglucan (XG), detected epitopes throughout the mucilage as well as in the underlying columella (Young et al., 2008). If XG is indeed present, it may associate with the cellulose in the mucilage similar to its proposed role in primary cell walls.
Protein
While there is no direct evidence for a significant amount of protein in Arabidopsis mucilage (Macquet et al., 2007a), mutations in three cell wall-associated proteins lead to changes in mucilage structure. MUM2 encodes a secreted β-galactosidase that is required to remove t-Gal and (arabino)galactan side-chains from RG I to enable correct mucilage hydration and release from the seed coat (Dean et al., 2007; Macquet et al., 2007b). The β-xylosidase/α-arabinofuranosidase BETAXYLOSIDASE 1 (BXL1) also affects mucilage release, and is required for trimming of arabinan side-chains (Arsovski et al., 2009a). Conversely, SOS5 is a fasciclin-like arabinogalactan protein (AGP) with a glycophosphatidylinositol (GPI) tail that anchors it to the outer leaflet of the plasma membrane (Shi et al., 2003). The heavily glycosylated AGP domains of SOS5 may interact with cell wall polysaccharides, while the fasciclin repeats may lead to protein interaction; cleavage of the GPI anchor (Shi et al., 2003) may also allow movement of SOS5 into the mucilage. Both linkage analysis and immunofluorescence results demonstrate the presence of type I arabinogalactans in Arabidopsis mucilage that may act as side-chains on RG I or represent the glycosylated portions of AGPs such as SOS5 (Dean et al., 2007; Arsovski et al., 2009a; HE McFarlane and TL Western, unpublished data).
Structure of Arabidopsis Seed Coat Mucilage
Ruthenium red staining reveals two domains in Arabidopsis mucilage – a cloudy, diffuse outer layer surrounding a dense, inner layer (Figure 1A). The inner mucilage layer is marked by the appearance of darkly staining “rays” that radiate out from the columella of each epidermal cell (Western et al., 2000). Below we discuss the structural characteristics and composition of these two layers, as well as comment on their functions and relationship.
Outer Mucilage
The outer mucilage layer of Arabidopsis seeds is seen enveloping the inner layer when seeds are stained without agitation. It is diffuse, with no obvious structure, and poorly adherent such that it is easily extracted by shaking seeds in water or a mild chelator (Western et al., 2000; Macquet et al., 2007a). Tests of water absorption over time demonstrate that it is capable of rapid and significant water absorption (Arsovski et al., 2009a). Linkage analysis and antibody staining of extracted outer mucilage suggests that it is primarily unbranched RG I (>80–90% of sugars in mucilage are Rha and GalA, with an approximately equal molar ratio as would be expected for the RG I backbone) with only a small proportion of branched RG I and arabinoxylan (Dean et al., 2007; Macquet et al., 2007a; Naran et al., 2008; Arsovski et al., 2009a). HG of differing degrees of methylation has also been detected using dot blots with JIM5 and JIM7 (Willats et al., 2001).
Inner Mucilage
Unlike the outer mucilage, the inner mucilage layer is strongly adherent to the seed and cannot be removed only by agitation: treatment with harsh chemicals (strong acid and base) or enzymatic digestion is required, suggesting bonding with structures of the seed coat (Western et al., 2000; Macquet et al., 2007a; Huang et al., 2011; Walker et al., 2011). Ruthenium red staining of the inner layer is darker than the outer layer, suggesting that the pectin network is denser (Figure 1A). This was confirmed by the inability of large molecules (e.g., the colloidal molecules that make up India ink) to penetrate the inner mucilage layer (Windsor et al., 2000; Western et al., 2001). Willats et al. (2001) demonstrated that, while FITC-labeled 40 kDa dextrans can enter the inner mucilage layer, 150 kDa dextrans cannot. Both Usadel et al. (2004) and Macquet et al. (2007a) showed that there are at least two distinct molecular size fractions in Arabidopsis mucilage, one of ∼600 kDa (outer mucilage) and another of ∼50,000 kDa (inner mucilage – molecular sizes from Macquet et al., 2007a). Mucilage extracted with mild chelator followed by increasing concentrations of alkali allowed comparison of the water holding capacity of inner versus outer mucilage, and showed that both the rate and degree of water absorption is reduced for inner mucilage compared with outer mucilage (Arsovski et al., 2009a).
The increased density and altered swelling properties of the inner mucilage layer is explained by its increased chemical complexity as identified through chemical, histochemical, and immunological analyses. While the majority of the inner mucilage is still comprised of unbranched RG I (most of inner layer can be removed by digestion with RG I hydrolase), linkage analysis has suggested that branched RG I, arabinans, galactans, arabinogalactans, and arabinoxylans are present in increased amounts compared with the outer mucilage. Further, when inner mucilage is extracted with sequentially harsher treatments, the proportion of complex/branched pectins increases (Dean et al., 2007; Macquet et al., 2007a; Arsovski et al., 2009a; Huang et al., 2011; Walker et al., 2011). Whole seed immunofluorescence supports the inner mucilage localization of RG I (CCRC-M36; INRA-RU2), arabinans (LM6), galactans (LM5), and arabinogalactans (JIM13), but also suggests the presence of both low and highly methylesterified HG (JIM5 and JIM7), and XG (α-XG; Willats et al., 2001; Macquet et al., 2007a; Young et al., 2008; Arsovski et al., 2009a; Sullivan et al., 2011; HE McFarlane and TL Western, unpublished data). In addition to pectins and hemicellulose, the adherent mucilage contains cellulose arranged in dense “rays” that radiate out from the top of each columella separated by a less dense network of cellulose spanning the inner mucilage layer between the rays (Figure 1B; Willats et al., 2001; Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011). It has been calculated that ∼12–19% of the adherent mucilage layer is comprised of cellulose (Macquet et al., 2007a). Finally, Macquet et al. (2007a) suggest that the adherent mucilage could be divided into two layers based on chemical composition: a region that contains RG I, HG, cellulose, and galactans that is immediately adjacent to the seed surface, and a zone beyond that that is comprised of RG I, HG, galactans, and arabinans. While there is certainly structure within the inner adherent layer, it is unclear if it can be simply defined into layers within itself – there is some contradictory data as to the exact localization of arabinans and highly methylesterified versus more de-esterified HG within the inner adherent mucilage (Willats et al., 2001; Macquet et al., 2007a; Arsovski et al., 2009a). Further, changes in mucilage staining density with ruthenium red surrounding the cellulosic rays shows structural complexity in multiple dimensions within the adherent layer (Western et al., 2000).
The cohesion of mucilage, and the adhesion to the seed of the inner adherent mucilage layer suggests specific structural organization. A key determinant of this organization is the cellulose that spans the adherent layer. The structuring of the inner adherent layer by cellulose is clearly demonstrated by several mutants affecting cellulose production within mucilage, including the cellulose synthase subunit cesa5, the fasciclin-like AGP sos5, and the leucine rich receptor-like kinase fei2. All three mutants lack or have significantly reduced cellulose, leading to an increase in the amount of diffuse, non-adherent mucilage, and decreased inner adherent layer (Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011). Suggested mechanisms for cellulose anchoring of the pectins comprising the majority of the mucilage include non-covalent bonding between cellulose and pectins, and molecular entanglement, whereby the large, branched pectins are captured through tangling with each other and with the cellulose microfibrils projecting at right angles to the seed surface (Macquet et al., 2007a; Harpaz-Saad et al., 2011; Mendu et al., 2011; Sullivan et al., 2011). Interactions between HGs through Ca2+ bridges are also implicated in maintaining the density of the inner mucilage layer. Treatment of seeds with Ca2+-specific or general heavy metal chelators leads to the apparent expansion of the inner, adherent layer (Willats et al., 2001; Rautengarten et al., 2008).
Roles and Relationship between Inner and Outer Mucilage
The exact relationship between the outer, non-adherent mucilage layer and inner adherent mucilage is uncertain. It is unclear whether the structural layers exist in mucilage as it is deposited or whether they resolve once the mucilage is released. Due to differences in swelling, molecular size and likelihood of molecular entanglement, it is possible that the unbranched RG I could simply diffuse away from the seed, while the more complex, branched pectins are trapped within the cellulose-based scaffolding. Whether the polysaccharides comprising mucilage are deposited or organized within the mucilage pocket in a stratified fashion is unknown. However, results from studies of mutants in genes affecting the β-galactosidase MUM2 and the β-xylosidase/α-arabinofuranosidase BXL1 demonstrate that post-deposition trimming of pectin side-chains in mucilage does occur and affects mucilage hydration (Dean et al., 2007; Macquet et al., 2007b; Arsovski et al., 2009a). It has been proposed that a population of RG I molecules that is accessible to these and other enzymes in the mucilage pocket becomes the outer soluble layer as it is both divested of its side-chains and digested into smaller molecules (Walker et al., 2011).
Clearly, there are structural and chemical differences between the two mucilage layers, but whether they are separate functional entities still needs to be elucidated. Differences in their speed and extent of swelling could provide for separate roles. The significant and rapid expansion of the primarily unbranched RG I in the outer mucilage layer could be responsible for breakage of the outer primary cell wall of the mucilage cells to allow mucilage release. This is consistent with results from mum2 and bxl1 mutants that have impaired mucilage release due to reduced mucilage swelling when hydrated (Dean et al., 2007; Macquet et al., 2007b; Arsovski et al., 2009a). It also has been suggested that the inner mucilage, being strongly adherent to the seed, is involved in seed hydration and/or adhesion to soil or animals (Macquet et al., 2007a). A role for outer non-adherent mucilage in increasing adhesion to soil substrates also has been proposed for myxospermous seeds in general (Grubert, 1974).
Advantages of Using Seed Coat Epidermal Cells to Study Cell Wall Structure and Function
As described above, Arabidopsis seed coat mucilage is composed of pectin, cellulose, hemicellulose, and protein and therefore could be described as a specialized cell wall. Since it is synthesized following cessation of cell growth, mucilage is most accurately described as a specialized, pectin-rich, secondary cell wall even though its composition is distinct from the more typical cellulose-rich secondary cell walls. Despite the fact that its structure and function are unique, differing in many ways from other cell walls, it has potential as a model for investigating the chemical and physical properties of different cell wall components and their interactions in vivo, especially with respect to its major component RGI. The value of Arabidopsis seed coat mucilage lies in its unique features. First, mucilage is copious and easily accessible, simplifying its extraction. Second, typical cell wall preparations are derived from tissues with multiple cell types, each of which could have a distinct cell wall. Mucilage, on the other hand, is derived from a single cell type and its structure is relatively simple with unbranched RGI and no RGII. Third, the ability of mucilage to expand and adhere to the seed represent important cell wall properties that are easy to observe and measure and may provide insight into common aspects of cell wall biology such as the role of the middle lamella in cell–cell adhesion. Fourth, mucilage synthesis occurs over a short (4 days) period of seed development facilitating the analysis of cellular changes that accompany cell wall synthesis. Finally, seed coat mucilage is amenable to genetic analysis. Genetic manipulation is a powerful approach that can be used to establish causal links between the biochemical function of a gene product, cell wall structure, and its role in vivo. However, changing the cell wall composition of many tissues can have severe consequences on plant viability thus limiting genetic analysis. Seed coat epidermal cells are dispensable (Western et al., 2001), thus even genetic manipulations that severely impact the cell can be isolated or engineered. Forward genetic screens for mutants with altered mucilage have already identified a number of genes involved in cell wall biology (reviewed in Arsovski et al., 2010; Western, 2012) and more will be found as screens become more sophisticated (e.g., Arsovski et al., 2009b). Unfortunately, because the seed coat differentiates late in the life cycle of the plant (seed coat is part of the maternal generation) many proteins required for its cell walls will also be important for cell types earlier in development. Mutations in genes encoding such proteins may result in plant lethality or sterility and therefore be missed in forward genetic screens for seed coat defects due to the failure of the mutant plant to produce seeds. In addition, seed coat mutant screens will be subject to the same problems of genetic redundancy that have plagued previous attempts to identify mutant phenotypes for genes encoding carbohydrate active enzymes. An alternate way that mucilage/seed coat epidermal cells could be used to study cell walls is as a bioassay to evaluate the function of carbohydrate active enzymes on the structure and properties of carbohydrates. Genes encoding such enzymes can be expressed in the seed coat epidermis with tissue specific promoters, their protein products targeted to the apoplast or Golgi, and their effect on the structure and properties of mucilage or columella cell wall carbohydrates evaluated without fear of lethal effects. It is conceivable that, with enough knowledge, specific types of cell wall carbohydrates could be engineered and properties tested by introducing sets of enzymes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to express our thanks to Jonathan Griffiths for supplying images for Figure 1, Jonathan Griffiths and Bronwen Forward for comments on the manuscript, and our apologies to those researchers whose studies we were unable to include due to space considerations. This work was supported by Natural Science and Engineering Research Council of Canada grants to George W. Haughn and Tamara L. Western.
References
Arsovski, A. A., Haughn, G. W., and Western, T. L. (2010). Seed coat mucilage cells of Arabidopsis thaliana as a model for plant cell wall research. Plant Signal. Behav. 5, 1–6.
Arsovski, A. A., Popma, T. M., Haughn, G. W., Carpita, N. C., McCann, M. C., and Western, T. L. (2009a). AtBXL1 encodes a bifunctional beta-D-xylosidase/alpha-L-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol. 150, 1219–1234.
Arsovski, A. A., Villota, M. M., Rowland, O., Subramaniam, R., and Western, T. L. (2009b). MUM ENHANCERS are important for seed coat mucilage production and mucilage secretory cell differentiation in Arabidopsis thaliana. J. Exp. Bot. 60, 2601–2612.
Blake, A. W., McCartney, L., Flint, J. E., Bolam, D. N., Boraston, A. B., Gilbert, H. J., and Knox, J. P. (2006). Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in Prokaryotic enzymes. J. Biol. Chem. 281, 29321–29329.
Dean, G. H., Zheng, H., Tewari, J., Huang, J., Young, D. S., Hwang, Y. T., Western, T. L., Carpita, N. C., Mccann, M. C., Mansfield, S. D., and Haughn, G. W. (2007). The Arabidopsis MUM2 gene encodes a beta-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19, 4007–4021.
Goto, N. (1985). A mucilage polysaccharide secreted from testa of Arabidopsis thaliana. Arabidopsis Inf. Serv. 22, 143–145.
Grubert, M. (1974). Studies on the distribution of myxospermy among seeds and fruits of Angiospermae and its ecological importance. Acta Biol. Venez. 8, 315–551.
Harholt, J., Suttangkakul, A., and Vibe Scheller, H. V. (2010). Biosynthesis of pectin. Plant Physiol. 153, 384–395.
Harpaz-Saad, S., Mcfarlane, H. E., Xu, S., Divi, U. K., Forward, B., Western, T. L., and Kieber, J. J. (2011). Cellulose synthesis via the FEI2 RLK/SOS5 pathway and CELLULOSE SYNTHASE 5 is required for the structure of seed coat mucilage in Arabidopsis. Plant J. 68, 941–953.
Huang, J., Bowles, D., Esfandiari, E., Dean, G., Carpita, N. C., and Haughn, G. W. (2011). The Arabidopsis transcription factor LUH/MUM1 is required for extrusion of seed coat mucilage. Plant Physiol. 156, 491–502.
Macquet, A., Ralet, M. C., Kronenberger, J., Marion-Poll, A., and North, H. M. (2007a). In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol. 48, 984–999.
Macquet, A., Ralet, M. C., Loudet, O., Kronenberger, J., Mouille, G., Marion-Poll, A., and North, H. M. (2007b). A naturally occurring mutation in an Arabidopsis accession affects a beta-D-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 19, 3990–4006.
Mendu, V., Griffiths, J., Persson, S., Stork, J., Downie, B., Voiniciuc, C., Haughn, G., and Debolt, S. (2011). Subfunctionalization of cellulose synthases in seed coat epidermal cells mediate secondary radial wall synthesis and mucilage attachment. Plant Physiol. 157, 441–453.
Naran, R., Chen, G., and Carpita, N. C. (2008). Novel rhamnogalacturonan I and arabinoxylan polysaccharides of flax seed mucilage. Plant Physiol. 148, 132–141.
Oka, T., Nemoto, T., and Jigami, Y. (2007). Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J. Biol. Chem. 282, 5389–5403.
Pattathil, S., Avci, U., Baldwin, D., Swennes, A. G., Mcgill, J. A., Popper, Z., Bootten, T., Albert, A., Davis, R. H., Chennareddy, C., Dong, R., O’Shea, B., Rossi, R., Leoff, C., Freshour, G., Narra, R., O’Neil, M., York, W. S., and Hahn, M. G. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153, 514–525.
Penfield, S., Meissner, R. C., Shoue, D. A., Carpita, N. C., and Bevan, M. W. (2001). MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13, 2777–2791.
Rautengarten, C., Usadel, B., Neumetzler, L., Hartmann, J., Büssis, D., and Altmann, T. (2008). A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J. 54, 466–480.
Shi, H., Kim, Y., Guo, Y., Stevenson, B., and Zhu, J.-K. (2003). The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell 15, 19–32.
Sullivan, S., Ralet, M. C., Berger, A., Diatloff, E., Bischoff, V., Gonneau, M., Marion-Poll, A., and North, H. M. (2011). CESA5 is required for the synthesis of cellulose with a role in structuring the adherent mucilage of Arabidopsis seeds. Plant Physiol. 156, 1725–1739.
Usadel, B., Kuschinsky, A. M., Rosso, M. G., Eckermann, N., and Pauly, M. (2004). RHM2 is involved in mucilage pectin synthesis and is required for the development of the seed coat in Arabidopsis. Plant Physiol. 134, 286–295.
Vaughan, J. G., and Whitehouse, J. M. (1971). Seed structure and the taxonomy of the Cruciferae. Bot. J. Linn. Soc. 64, 383–409.
Walker, M., Tehseen, M., Doblin, M. S., Pettolino, F. A., Wilson, S. M., Bacic, A., and Golz, J. F. (2011). The transcriptional regulator LEUNIG_HOMOLOG regulates mucilage release from the Arabidopsis testa. Plant Physiol. 156, 46–60.
Western, T. L. (2012). The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci. Res. 22, 1–25.
Western, T. L., Burn, J., Tan, W. L., Skinner, D. J., Martin-Mccaffrey, L., Moffatt, B. A., and Haughn, G. W. (2001). Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol. 127, 998–1011.
Western, T. L., Skinner, D. J., and Haughn, G. W. (2000). Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol. 122, 345–355.
Western, T. L., Young, D. S., Dean, G. H., Tan, W. L., Samuels, A. L., and Haughn, G. W. (2004). MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiol. 134, 296–306.
Willats, W. G. T., Mccartney, L., and Knox, J. P. (2001). In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213, 37–44.
Windsor, J. B., Symonds, V. V., Mendenhall, J., and Lloyd, A. L. (2000). Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J. 22, 483–493.
Keywords: Arabidopsis, mucilage, seed coat, cell wall, pectin, cellulose, xyloglucan, genetic model
Citation: Haughn GW and Western TL (2012) Arabidopsis seed coat mucilage is a specialized cell wall that can be used as a model for genetic analysis of plant cell wall structure and function. Front. Plant Sci. 3:64. doi: 10.3389/fpls.2012.00064
Received: 26 January 2012; Accepted: 16 March 2012;
Published online: 03 April 2012.
Edited by:
Seth DeBolt, University of Kentucky, USAReviewed by:
Seth DeBolt, University of Kentucky, USAHelen M. North, Institut Nationale de Recherche Agronomy, France
Copyright: © 2012 Haughn and Western. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: George W. Haughn, Department of Botany, University of British Columbia, 6270 University Boulevard, Vancouver, BC, Canada V6T 1Z4. e-mail: george.haughn@ubc.ca
