- 1School of Biological Sciences, The University of Auckland, Auckland, New Zealand
- 2The New Zealand Institute for Plant and Food Research Limited, Auckland, New Zealand
Plant species that bear fruit often utilize expansion of an ovary (carpel) or accessory tissue as a vehicle for seed dispersal. While the seed(s) develop, the tissue(s) of the fruit follow a common progression of cell division and cell expansion, promoting growth of the fruit. Once the seed is fully developed, the fruit matures and the surrounding tissue either dries or ripens promoting the dissemination of the seed. As with many developmental processes in plants, plant hormones play an important role in the synchronization of signals between the developing seed and its surrounding fruit tissue(s), regulating each phase of fruit development. Following pollination, fruit set is achieved through a de-repression of growth and an activation of cell division via the action of auxin and/or cytokinin and/or gibberellin. Following fruit set, growth of the fruit is facilitated through a relatively poorly studied period of cell expansion and endoreduplication that is likely regulated by similar hormones as in fruit set. Once the seeds reach maturity, fruit become ready to undergo ripening and during this period there is a major switch in relative hormone levels of the fruit, involving an overall decrease in auxin, gibberellin, and cytokinin and a simultaneous increase in abscisic acid and ethylene. While the role of hormones in fruit set and ripening is well documented, the knowledge of the roles of other hormones during growth, maturation, and some individual ripening components is sketchy.
Background
The fruiting body of flowering (angiosperm) plants has evolved to best aid seed protection and dispersal. A diverse range of fruit types within angiosperm species exists and these variations are exemplified between fleshy fruits, that have evolved with an enlargement of the tissue surrounding the seed to create attractive flesh for seed dispersing animals, and “dry” fruit, that split open (dehisce) to release the seed via abiotic dispersal mechanisms. Evolutionary studies have revealed that plant species producing fleshy fruit have evolved from ancestral dry fruit producing species, suggesting common mechanisms between dry and fleshy fruit (Knapp, 2002). Pulling upon the literature from across different species, we have revealed common trends in the hormonal regulation of the different stages of fruit development (Figure 1). In all cases, dry or fleshy fruit undergo a progression of specific steps including: fruit set, fruit growth, maturation, and ripening/senescence. The crosstalk between hormones that occurs during most of these steps is scarce, nevertheless with the advent of genomic and high throughput technologies there has been significant progress in characterizing hormones and the expression of associated downstream genes in both model and non-model organisms. In this review, we aim to give an overview of the way plant hormones interact to control these different developmental steps and the switch(es) between them as well as highlight areas that require further research to understand these complex processes.
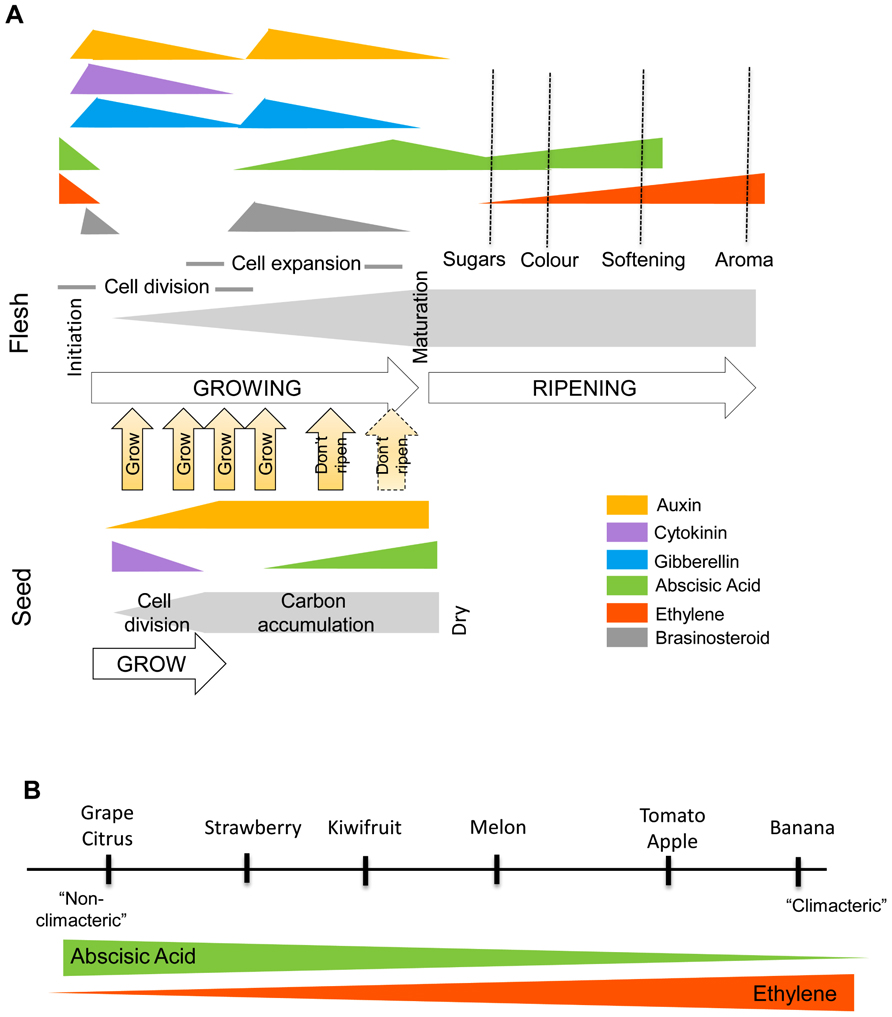
FIGURE 1. (A) Hormonal changes that occur in a generic fruit during development and ripening. Differential hormone concentrations occur in the seed and the surrounding tissue with the developing seed influencing its environment. Multiple studies have shown that increases in auxin, cytokinin, gibberellin, and brassinosteroid at fruit set, and an involvement of auxin, gibberellin, and brassinosteroid at fruit growth. For fruit maturation there is an inhibition of auxin transport from the seed and increase in ABA. This triggers the ripening/senescence program which leads to an increase in ABA and/or ethylene biosynthesis and response in the surrounding tissue. (B) The spectrum of ripening dependencies to ABA and ethylene. All fruit appear to respond to ABA and ethylene. In historically considered “climacteric fruit,” ABA indirectly regulates ripening through ethylene. In “non-climacteric” fruit, the ABA has a more dominant role but the fruit still have ethylene-dependant ripening characters.
Fruit Set
Fruit set is the first step in fruit development; it is established during and soon after fertilization. Seed bearing plants have a unique double fertilization event with two pollen nuclei fertilizing the embryo and the endosperm (Dumas et al., 1998; Raghavan, 2003; Hamamura et al., 2012). The role of hormones during embryo development and seed maturation has been well reviewed (for example: Gutierrez et al., 2007; Sun et al., 2010). The fertilization event leads to the development of the seed that de-represses cell division and fruit growth in a synchronized manner (review: Fuentes and Vivian-Smith, 2009). Fruit set has traditionally been attributed to the action of three hormones, auxin, and/or gibberellin, and/or cytokinin (Mariotti et al., 2011). Application of these hormones alone can trigger fruit development to a certain extent and, in many plant species, application in combination will induce normal fruit growth even in the absence of fertilization (parthenocarpy; Nitsch, 1952; Crane, 1964; Gillaspy et al., 1993; Vivian-Smith and Koltunow, 1999), indicating that an interplay between these hormones is necessary for fruit set and fruit growth. In many species, auxin and cytokinin levels in the seed increase during seed development until maturity (Nitsch, 1950; Blumenfeld and Gazit, 1970; Varga and Bruinsma, 1976; Yang et al., 2002; Devoghalaere et al., 2012) and in pea, removal of the seed leads to reduced gibberellin biosynthesis in the pericarp (García-Martínez and Carbonell, 1980; Ozga et al., 1992). These observations led to the “seed control” hypothesis where the seeds communicate through hormones to the surrounding tissue(s) to promote fruit growth through firstly cell division and later on cell expansion (Ozga et al., 2002).
At the molecular level, the main advances have been on how gibberellin and auxin pathways interact to promote fruit set in both dry fruit, such as Arabidopsis thaliana (Arabidopsis), and fleshy fruit, such as tomato (de Jong et al., 2009a; Carrera et al., 2012; Ruan et al., 2012). Early studies showed that elevated levels of gibberellins and auxin are present in fruits from plants that exhibit parthenocarpy (Talon et al., 1990) and auxin levels increase during seed development while gibberellin levels increase in the ovaries following fertilization (Olimpieri et al., 2007; Hu et al., 2008). In Arabidopsis, fruit development induced by auxin occurs solely through activation of gibberellin signaling and the current, simplified model, of auxin and gibberellin action is the following: auxin, synthesized in the ovules on fertilization is transported to the pericarp where it induces gibberellin biosynthesis (Zhao, 2010). In turn, the newly synthesized gibberellin will lead to the release of growth repression (Fuentes et al., 2012). There are additional layers of regulation, for example, it has been shown that a threshold level of gibberellins in the gynoecium is required to initiate auxin biosynthesis, providing a feedback loop (Vivian-Smith and Koltunow, 1999). Tomato fruit set can be achieved by application of auxin or gibberellin. Auxin appears to act partly through gibberellin, as it can induce gibberellin biosynthesis early during fruit development (Serrani et al., 2008), but each hormone seems to also play a specific role on its own. Auxin-induced fruit contain many more cells compared to gibberellin-induced fruits, which contain fewer larger cells (Bungerkibler and Bangerth, 1983). One of the key players in gibberellin–auxin crosstalk is an auxin response factor (ARF), SlARF7, which when mutated causes parthenocarpic fruit development. The mutated fruit display a thick pericarp with large cells having a similar appearance to gibberellin-induced fruit. Molecular analysis has showed that SlARF7 was partly controlling both auxin and gibberellin signaling (de Jong et al., 2009b, 2011). This pathway was further characterized through the analysis of the tomato procera parthenocarpic mutant, with a constitutive gibberellin response, and indicate that activation of the gibberellin signaling pathway after fertilization also controls SlARF7 expression (Carrera et al., 2012).
Cytokinin levels also increase after pollination (Matsuo et al., 2012). Although cytokinins are generally considered to play a critical role in the stimulation of cell division during fruit development (Wismer et al., 1995; Srivastava and Handa, 2005), very few experimental data support the involvement of this hormone in the initial cell division phase of fruit growth (Mariotti et al., 2011). It is well known that cytokinin promotes cell proliferation at shoot apical meristems and interact closely with auxin (Murray et al., 2012) and are likely to function in a similar manner in the developing gynoecia (Lindsay et al., 2006; Bartrina et al., 2011). A recent study in Arabidopsis showed that cytokinin plays at least two roles during fruit development: an early proliferation-inducing role at the medial region of the developing gynoecia and a later role during formation of fruit valve margins (Marsch-Martinez et al., 2012). Finally brassinosteroids might also have a role in fruit set (Fu et al., 2008), however, the interaction with other hormones has not been investigated.
While auxin, gibberellins, and cytokinin levels are increasing at fruit set, abscisic acid (ABA) levels decrease (Hein et al., 1984; Kojima et al., 1993). Consistent with these observations, a transcriptomic analysis showed that mRNA levels of several ABA biosynthesis genes decrease after pollination, while expression of ABA degradation genes increases (Vriezen et al., 2008). ABA has also been shown to counteract the effect of gibberellin on fruit set in pea (García-Martínez and Carbonell, 1980). Expression of ethylene biosynthesis and signaling genes also decrease after pollination while in unpollinated tomato ovaries ethylene biosynthesis and signaling genes are highly expressed.
Overall, these data demonstrate that fruit set relies on a fine balance between plant hormones; the concerted action of auxin and/or gibberellin and/or cytokinin (dependency toward a specific hormone will likely depend on the plant species) will ultimately lead to activation of core cell cycle genes. We can also speculate that ABA and ethylene could have an antagonistic effect on fruit set but this will require further investigation (Figure 1A).
Fruit Growth
The developing seed continually sends signals to the surrounding tissue to expand and there is usually a positive correlation between seed number and fruit size (Nitsch, 1970). The developing fruit must also signals back to the rest of the plant so that it is provided with enough nutrients and does not abort. The extent of growth of the fruit from anthesis to maturity is extremely variable; in some species the fruit enlarge relatively little while in others they may increase in volume many thousand times. Unique to fleshy fruit, concomitant with cell expansion, there is an accumulation of storage products and an increase in sugar accumulation (Coombe, 1976). While fruit expansion is a key event, there is little literature covering the role of hormones in the transition for the division to the expansion phases and to the sustained growth of the fruit. Drawing on literature outside the fruit environment it is clear that cell expansion is regulated by auxin, gibberellin, and brassinosteroid (Davies, 2010; Pattison and Catala, 2012).
Cell enlargement depends on both cell wall loosening and increases in turgor pressure (Cosgrove, 2005). While auxin mostly controls cell division during fruit set, it is thought to play an important role during the growth phase by influencing cell enlargement together with gibberellins (Csukasi et al., 2011). In tomato, the maintenance of auxin gradients, through the precise localization of auxin transporters, such as the PIN transporters, will be essential for fruit growth (Pattison and Catala, 2012). A transcriptomic approach focusing on the cell expansion phase revealed that in the growing exocarp and locular tissues, a range of cell wall-related proteins are up-regulated during the expansion stage of the fruit, as well as sugar transport proteins and various glycolytic enzymes. Some genes belonging to the expansins, endo-xyloglucan transferase and pectate lyases families have been shown to be regulated by either auxin, gibberellin, or both in tomato (de Jong et al., 2011; Carrera et al., 2012). A genome-wide approach in apple, focusing on the role of auxin during cell expansion, showed that auxin action potentially involves an ARF gene, which is linked to quantitative trait loci (QTLs) for fruit size (Devoghalaere et al., 2012). ABA has also been associated with the expansion phase in tomato (Gillaspy et al., 1993) and ABA-deficient mutants have a reduced fruit size (Nitsch et al., 2012). The source of these hormones originates mostly from the seed and has to be transported to the surrounding tissue and/or is synthesized directly in the expanding tissue but, expect for auxin, our current knowledge is, however, limited in this area.
Fruit Maturation
Fruit maturity is a developmental point where the fruit has reached the competence to ripen, but has yet to start the ripening process. Auxin and maybe cytokinin appear to be key regulators of fruit maturation. Genetic studies have shown that the tomato ripening inhibitor (rin) mutant that displays a non-ripening phenotype, have higher levels of auxin and cytokinin at breaker stage compared to wild-type fruit (Davey and Van Staden, 1978; Rolle and Chism, 1989). The suppression of a rin-like MADS-box gene in apple (Ireland et al., 2013), resulted in a maintenance of high auxin concentration during fruit maturation and fruit that did not ripen (Ireland et al., 2013; Schaffer et al., 2013). In Arabidopsis and Brassica napus, a low auxin is required for seed dehiscence (pod shatter) to occur (Chauvaux et al., 1997; Sorefan et al., 2009). A mutation in INDEHISCENT (IND) results in high levels of auxin within the valve margins of the dehiscence zone compared to wild-type controls and it has been postulated that this high intracellular auxin at least partially inhibits dehiscence (Sorefan et al., 2009). In tomato, reduction of auxin by the over-expression of a Capsicum chinense auxin-conjugating enzyme (GH3) leads to decreased auxin and an increased sensitivity to ethylene at an earlier stage of development (Liu et al., 2005). In strawberry, when achene’s are removed from immature fruit, precocious ripening of the receptacle occurs (Given et al., 1988), this ripening can be stopped by the application of exogenous auxin. During fruit growth, auxin levels in the seed are higher than in the surrounding fruit tissue (Devoghalaere et al., 2012) and this suggests as the seeds become dormant, auxin biosynthesis or transport to the rest of the fruit is inhibited, allowing the mature fruit to ripen. This appears to be supported across fruit species as addition of auxin to mature fruit invariably delays ripening (Vendrell, 1985; Manning, 1994; Davies et al., 1997; Aharoni et al., 2002). It should also be noted that although seeds have a strong influence on maturity, parthenocarpic fruit still ripen suggesting a developmental regulation may also be involved.
The role of cytokinin during fruit maturation is less well documented but cytokinin-deficient Arabidopsis fruit show non-synchronous ripening with fewer viable seeds compared to controls suggesting cytokinin also has a role in the regulation of silique maturation and ripening (Werner et al., 2003). Finally decreases in free cytokinin and auxin levels are also observed before ripening in orange and grape (Minana et al., 1989; Bottcher et al., 2011).
One of the challenges in future work will be to better understand the molecular mechanisms underlying fruit maturation and interaction between these hormones.
Fruit Ripening/Senescence
The progression of fruit ripening or senescence is a complex process involving changes to the metabolic and physiological traits of a fruit. In all fruit, in the tissue surrounding the seed, there is a color change and a change in cell wall composition causing either a dehiscence or a softening (Klee and Giovannoni, 2011). Unique to fleshy fruit there is often a breakdown of stored carbohydrates to sugars and a decrease in acidity along with an increase in flavor and aroma volatiles (Klee and Giovannoni, 2011). The control of ripening appears to be achieved predominantly through the ripening hormones ABA and ethylene (reviews: Fedoroff, 2002; Giovannoni, 2004; Setha, 2012), ethylene being the most studied. Fruit types that have a strong requirement for ethylene to ripen such as tomatoes, peaches, bananas, apples, and melon have previously been labeled climacteric and the role of ethylene in both these fruit types has been extensively reviewed (for example, Bapat et al., 2010; Paul et al., 2012). In peaches and tomato, indole-3-acetic acid (IAA) has also been reported to have some crosstalk with ethylene during ripening as (i) production of ethylene can be concomitant with an increase of IAA and (ii) auxin-signaling components can be up-regulated by ethylene and vice versa (Jones et al., 2002; Trainotti et al., 2007). In fruit that have a lower requirement of ethylene to ripen (referred as non-climacteric fruit such as grape and citrus), ABA appears to have a stronger role (Setha, 2012). It has been shown that in the climacteric fruits tomato and banana, there is an increase in ABA preceding an increase in ethylene. Exogenous application of ABA induces ethylene through the biosynthesis genes (Jiang et al., 2000; Zhang et al., 2009), while a suppression of ABA leads to a delay in fruit ripening (Figure 1B; Sun et al., 2012a). In the dry dehiscent fruit Arabidopsis, again ABA increases with silique maturation (Kanno et al., 2010) and has been linked with the promotion of dehiscence, an ethylene mediated event (Child et al., 1998; Kou et al., 2012).
While there is a considerable amount of literature on fruit ripening, researchers have often only focused on a small number of physiological changes to document the ripening process. For example color change and/or fruit firmness are often used as a surrogate for ripening, with other ripening characters completely overlooked. It is becoming clear that some ripening traits are independently controlled from each other (Johnston et al., 2009; Ireland et al., 2013). The use of single physiological marker(s) may hence lead to a misrepresentation of this complex process. Here we have summarized the literature based on how different traits respond to hormones rather than considering ripening as one single process.
Sugar Accumulation
There is little literature on the hormonal control of starch hydrolysis and the resulting sugar accumulation. There have been a number of studies that have documented the metabolic changes that occur during maturation and ripening (Fait et al., 2008; Osorio et al., 2011, 2012), though the link between hormonal control and metabolite accumulation is limited; however, Johnston et al. (2009) observed in apple that, while this could progress independently of ethylene, it was highly sensitive to ethylene. In melon, the application of exogenous ABA was shown to promote starch hydrolysis (Sun et al., 2012b), different from growth section, however, this was confounded by the fact that the ABA also increased the ethylene levels.
Color Change
Much of the literature documents the control of color change during fruit ripening. This is achieved by a combination chlorophyll loss (degreening) and production of secondary color metabolites such as carotenoids and anthocyanins. Color change in many fruit species is associated with an increase of ABA and/or ethylene. In apple, the degreening occurs independently of ethylene but ethylene can accelerate the process (Johnston et al., 2009). Citrus and melon also both require ethylene for the degreening of the skin. The production of secondary color metabolites is strongly ethylene regulated in tomato, though some intermediates can be produced in the absence of ethylene. Application of ABA to tomato fruit results in an enhanced onset of breaker stage compared to controls, further implicating ABA as being positive regulator of ripening in tomato (Buta and Spaulding, 1994). In grape and strawberry, the color change is strongly regulated by ABA (Deytieux et al., 2005; Jia et al., 2011), though application of 1-methylcyclopropene (1-MCP; an inhibitor of ethylene response) can delay this process, suggesting that ethylene may play a role (Chervin et al., 2004). There are also reports of color change being inhibited by brassinosteroids in grape and strawberry (Symons et al., 2006; Chai et al., 2013).
Cell Wall Hydrolysis
There is a considerable set of literature covering ripening related changes in the cell wall (review: Brummell, 2006). Depending on the fruit type these can manifest as a formation of a dehiscence zone, or through the softening of the flesh tissue. In each case there is a suite of cell wall-related genes that are up-regulated, and in many instances each is differentially regulated. In the case of fruit softening, loss of a single gene can be compensated by other gene action (Powell et al., 2003). In apple and melon, there are both ethylene-independent and ethylene-dependent softening which can be observed in the differential regulation of cell wall-related genes. In banana, it has been shown that ABA can act synergistically with ethylene to promote softening (Lohani et al., 2004) and in grape ABA has been shown to cause fruit softening (Cantin et al., 2007).
Studies of Arabidopsis silique dehiscence indicate that ethylene, jasmonic acid, and ABA work in conjunction with each other to promote normal floral organ abscission via the up-regulation of genes like POLYGALACTURONASE (ADPG1; Ogawa et al., 2009). In Arabidopsis, a delayed dehiscent phenotype is associated with reduction in the ability of Arabidopsis fruit to produce ethylene and that a wild-type time to dehiscence can be restored with treatment of exogenous ethylene (Child et al., 1998; Patterson, 2001). Finally salicylic acid has been shown to delay softening in banana (Srivastava and Dwivedi, 2000).
Flavor and Aroma Production
In apple, aroma volatiles are the least ethylene sensitive, and most ethylene-dependant of the ripening traits. Consistent with this, there are a significant number of publications linking the production of aroma with ethylene (Flores et al., 2002; Botondi et al., 2003; Defilippi et al., 2005; Schaffer et al., 2007). There is, however, remarkably little literature examining if other hormones contribute to the regulation of volatile production in fruit.
Summary
It is clear that there is still considerable work needed to better understand the way that hormones interact during fruit development. While there are areas that have been quite extensively covered such as fruit set and the role of ethylene in fruit ripening, there are considerable gaps in our understanding of the hormonal control and crosstalk of other areas, such as fruit expansion, endoreduplication, starch hydrolysis, and flavor development. While much of the physiology is now documented there are considerable opportunities to further our molecular understanding of these complex processes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aharoni, A., Keizer, L. C. P., Van den Broeck, H. C., Blanco-Portales, R., Munoz-Blanco, J., Bois, G., et al. (2002). Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiol. 129, 1019–1031.
Bapat, V. A., Trivedi, P. K., Ghosh, A., Sane, V. A., Ganapathi, T. R., and Nath, P. (2010). Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol. Adv. 28, 94–107.
Bartrina, I., Otto, E., Strnad, M., Werner, T., and Schmulling, T. (2011). Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23, 69–80.
Blumenfeld, A., and Gazit, S. (1970). Cytokinin activity in avocado seeds during fruit development. Plant Physiol. 46, 331–333.
Botondi, R., DeSantis, D., Bellincontro, A., Vizovitis, K., and Mencarelli, F. (2003). Influence of ethylene inhibition by 1-methylcyclopropene on apricot quality, volatile production, and glycosidase activity of low- and high-aroma varieties of apricots. J. Agric. Food Chem. 51, 1189–1200.
Bottcher, C., Harvey, K., Forde, C. G., Boss, P. K., and Davies, C. (2011). Auxin treatment of pre-veraison grape (Vitis vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Aust. J. Grape Wine Res. 17, 1–8.
Bungerkibler, S., and Bangerth, F. (1983). Relationship between cell number, cell size and fruit size of seeded fruits of tomato (Lycopersicon esculentum Mill.), and those induced parthenocarpically by the application of plant growth regulators. Plant Growth Regul. 1, 143–154.
Buta, J. G., and Spaulding, D. W. (1994). Changes in indole-3-acetic acid and abscisic acid levels during tomato (Lycopersicon esculentum Mill.) fruit development and ripening. J. Plant Growth Regul. 13, 163–166.
Cantin, C. M., Fidelibus, M. W., and Crisostoc, C. H. (2007). Application of abscisic acid (ABA) at veraison advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol. Technol. 46, 237–241.
Carrera, E., Ruiz-Rivero, O., Peres, L. E., Atares, A., and Garcia-Martinez, J. L. (2012). Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 160, 1581–1596.
Chai, Y.-M., Zhang, Q., Tian, L., Li, C. L., Xing, Y., Qin, L., et al. (2013). Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul. 69, 63–69.
Chauvaux, N., Child, R., John, K., Ulvskov, P., Borkhardt, B., Prinsen, E., et al. (1997). The role of auxin in cell separation in the dehiscence zone of oilseed rape pods. J. Exp. Bot. 48, 1423–1429.
Chervin, C., El-Kereamy, A., Roustan, J. P., Latche, A., Lamon, J., and Bouzayen, M. (2004). Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 167, 1301–1305.
Child, R. D., Chauvaux, N., John, K., Ulvskov, P., and Van Onckelen, H. A. (1998). Ethylene biosynthesis in oilseed rape pods in relation to pod shatter. J. Exp. Bot. 49, 829–838.
Crane, J. C. (1964). Growth substances in fruit setting and development. Annu. Rev. Plant Physiol. 15, 303–326.
Csukasi, F., Osorio, S., Gutierrez, J. R., Kitamura, J., Giavalisco, P., Nakajima, M., et al. (2011). Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol. 191, 376–390.
Davey, J., and Van Staden, J. (1978). Endogenous cytokinins in the fruits of ripening and non-ripening tomatoes. Plant Sci. Lett. 11, 359–364.
Davies, C., Boss, P. K., and Robinson, S. P. (1997). Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 115, 1155–1161.
Davies, P. J. (2010). Plant Hormones: Biosynthesis, Signal Transduction, Action! Dordrecht: Springer.
Defilippi, B. G., Dandekar, A. M., and Kader, A. A. (2005). Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 53, 3133–3141.
de Jong, M., Mariani, C., and Vriezen, W. H. (2009a). The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 60, 1523–1532.
de Jong, M., Wolters-Arts, M., Feron, R., Mariani, C., and Vriezen, W. H. (2009b). The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 57, 160–170.
de Jong, M., Wolters-Arts, M., Garcia-Martinez, J. L., Mariani, C., and Vriezen, W. H. (2011). The Solanum lycopersicum AUXIN RESPONSE FACTOR 7 (SlARF7) mediates cross-talk between auxin and gibberellin signalling during tomato fruit set and development. J. Exp. Bot. 62, 617–626.
Devoghalaere, F., Doucen, T., Guitton, B., Keeling, J., Payne, W., Ling, T. J., et al. (2012). A genomics approach to understanding the role of auxin in apple (Malus × domestica) fruit size control. BMC Plant Biol. 12:7. doi: 10.1186/1471-2229-12-7
Deytieux, C., Geny, L., and Doneche, B. (2005) Relation between hormonal balance and polygalacturonase activity in grape berry. Acta Hortic. 682, 163–170.
Dumas, C., Berger, F., Faure, J. E., and Matthys-Rochon, E. (1998). Gametes, fertilization and early embryogenesis in flowering plants. Adv. Bot. Res. 28, 231–261.
Fait, A., Hanhineva, K., Beleggia, R., Dai, N., Rogachev, I., Nikiforova, V. J., et al. (2008). Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 148, 730–750.
Flores, F., El Yahyaoui, F., de Billerbeck, G., Romojaro, F., Latche, A., Bouzayen, M., et al. (2002). Role of ethylene in the biosynthetic pathway of aliphatic ester aroma volatiles in Charentais Cantaloupe melons. J. Exp. Bot. 53, 201–206.
Fu, F. Q., Mao, W. H., Shi, K., Zhou, Y. H., Asami, T., and Yu, J. Q. (2008). A role of brassinosteroids in early fruit development in cucumber. J. Exp. Bot. 59, 2299–2308.
Fuentes, S., Ljung, K., Sorefan, K., Alvey, E., Harberd, N. P., and Ostergaard, L. (2012). Fruit growth in Arabidopsis occurs via DELLA-dependent and DELLA-independent gibberellin responses. Plant Cell 24, 3982–3996.
Fuentes, S., and Vivian-Smith, A. (2009). “Fertilization and fruit initiation,” in Fruit Development and Seed Dispersal, ed. L. østergaard, Vol. 38 (Oxford: Wiley Blackwell Publishing), 107–171.
García-Martínez, J. L., and Carbonell, J. (1980). Fruit-set of unpollinated ovaries of Pisum sativum L. – influence of plant-growth regulators. Planta 147, 451–456.
Gillaspy, G., Ben-David, H., and Gruissem, W. (1993). Fruits: a developmental perspective. Plant Cell 5, 1439–1451.
Giovannoni, J. J. (2004). Genetic regulation of fruit development and ripening. Plant Cell 16, 170–180.
Given, N. K., Venis, M. A., and Grierson, D. (1988). Hormonal-regulation of ripening in the strawberry, a non-climacteric fruit. Planta 174, 402–406.
Gutierrez, L., Van Wuytswinkel, O., Castelain, M., and Bellini, C. (2007). Combined networks regulating seed maturation. Trends Plant Sci. 12, 294–300.
Hamamura, Y., Nagahara, S., and Higashiyama, T. (2012). Double fertilization on the move. Curr. Opin. Plant Biol. 15, 70–77.
Hein, M. B., Brenner, M. L., and Brun, W. A. (1984). Concentrations of abscisic acid and indole-3-acetic acid in soybean seeds during development. Plant Physiol. 76, 951–954.
Hu, J., Mitchum, M. G., Barnaby, N., Ayele, B. T., Ogawa, M., Nam, E., et al. (2008). Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20, 320–336.
Ireland, H. S., Yao, J. L., Tomes, S., Sutherland, P. W., Nieuwenhuizen, N., Gunaseelan, K., et al. (2013). Apple SEPALLATA1/2-like genes control fruit flesh development and ripening. Plant J. 73, 1044–1056.
Jia, H. F., Chai, Y. M., Li, C. L., Lu, D., Luo, J. J., Qin, L., et al. (2011). Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 157, 188–199.
Jiang, Y., Joyce, D. C., and Macnish, A. J. (2000). Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J. Plant Growth Regul. 19, 106–111.
Johnston, J. W., Gunaseelan, K., Pidakala, P., Wang, M., and Schaffer, R. J. (2009). Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J. Exp. Bot. 60, 2689–2699.
Jones, B., Frasse, P., Olmos, E., Zegzouti, H., Li, Z. G., Latche, A., et al. (2002). Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 32, 603–613.
Kanno, Y., Jikumaru, Y., Hanada, A., Nambara, E., Abrams, S. R., Kamiya, Y., et al. (2010). Comprehensive hormone profiling in developing Arabidopsis seeds: examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 51, 1988–2001.
Klee, H. J., and Giovannoni, J. J. (2011). Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 45, 41–59.
Knapp, S. (2002). Tobacco to tomatoes: a phylogenetic perspective on fruit diversity in the Solanaceae. J. Exp. Bot. 53, 2001–2022.
Kojima, K., Kuraishi, S., Sakurai, N., and Fusao, K. (1993). Distribution of abscisic-acid in different parts of the reproductive-organs of tomato. Sci. Hortic. 56, 23–30.
Kou, X. H., Watkins, C. B., and Gan, S. S. (2012). Arabidopsis AtNAP regulates fruit senescence. J. Exp. Bot. 63, 6139–6147.
Lindsay, D. L., Sawhney, V. K., and Bonham-Smith, P. C. (2006). Cytokinin-induced changes in CLAVATA1 and WUSCHEL expression temporally coincide with altered floral development in Arabidopsis. Plant Sci. 170, 1111–1117.
Liu, K. D., Kang, B. C., Jiang, H., Moore, S. L., Li, H. X., Watkins, C. B., et al. (2005). A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol. Biol. 58, 447–464.
Lohani, S., Trivedi, P. K., and Nath, P. (2004). Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 31, 119–126.
Manning, K. (1994). Changes in gene-expression during strawberry fruit ripening and their regulation by auxin. Planta 194, 62–68.
Mariotti, L., Picciarelli, P., Lombardi, L., and Ceccarelli, N. (2011). Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J. Plant Growth Regul. 30, 405–415.
Marsch-Martinez, N., Ramos-Cruz, D., Irepan Reyes-Olalde, J., Lozano-Sotomayor, P., Zuniga-Mayo, V. M., and de Folter, S. (2012). The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning. Plant J. 72, 222–234.
Matsuo, S., Kikuchi, K., Fukuda, M., Honda, I., and Imanishi, S. (2012). Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 63, 5569–5579.
Minana, F. M. H., Primomillo, E., and Primomillo, J. (1989). Isolation and identification of cytokinins from developing citrus-fruits. Citriculture 1–4, 367–379.
Murray, J. A., Jones, A., Godin, C., and Traas, J. (2012). Systems analysis of shoot apical meristem growth and development: integrating hormonal and mechanical signaling. Plant Cell 24, 3907–3919.
Nitsch, J. (1950). Growth and morphogenesis of the strawberry as related to auxin. Am. J. Bot. 37, 211–215.
Nitsch, J. P. (1970). “Hormonal factors in growth and development,” in The Biochemistry of Fruits and Their Products, ed. A. Hulme (London: Academic Press), 427–472.
Nitsch, L., Kohen, W., Oplaat, C., Charnikhova, T., Cristescu, S., Michieli, P., et al. (2012). ABA-deficiency results in reduced plant and fruit size in tomato. J. Plant Physiol. 169, 878–883.
Ogawa, M., Kay, P., Wilson, S., and Swain, S. M. (2009). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21, 216–233.
Olimpieri, I., Siligato, F., Caccia, R., Mariotti, L., Ceccarelli, N., Soressi, G. P., et al. (2007). Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis. Planta 226, 877–888.
Osorio, S., Alba, R., Damasceno, C. M. B., Lopez-Casado, G., Lohse, M., Zanor, M. I., et al. (2011). Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiol. 157, 405–425.
Osorio, S., Alba, R., Nikoloski, Z., Kochevenko, A., Fernie, A. R., and Giovannoni, J. J. (2012). Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiol. 159, 1713–1729.
Ozga, J. A., Brenner, M. L., and Reinecke, D. M. (1992). Seed effects on gibberellin metabolism in pea pericarp. Plant Physiol. 100, 88–94.
Ozga, J. A., van Huizen, R., and Reinecke, D. M. (2002). Hormone and seed-specific regulation of pea fruit growth. Plant Physiol. 128, 1379–1389.
Patterson, S. E. (2001). Cutting loose. Abscission and dehiscence in Arabidopsis. Plant Physiol. 126, 494–500.
Pattison, R. J., and Catala, C. (2012). Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 70, 585–598.
Paul, V., Pandey, R., and Srivastava, G. C. (2012). The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene – an overview. J. Food Sci. Technol. 49, 1–21.
Powell, A. L. T., Kalamaki, M. S., Kurien, P. A., Gurrieri, S., and Bennett, A. B. (2003). Simultaneous transgenic suppression of LePG and LeExp1 influences fruit texture and juice viscosity in a fresh market tomato variety. J. Agric. Food Chem. 51, 7450–7455.
Raghavan, V. (2003). Some reflections on double fertilization, from its discovery to the present. New Phytol. 159, 565–583.
Rolle, R. S., and Chism, G. W. (1989). Kinetic comparison of cytokinin nucleosidase activity isolated from normally ripening and mutant tomato varieties. Plant Physiol. 91, 148–150.
Ruan, Y. L., Patrick, J. W., Bouzayen, M., Osorio, S., and Fernie, A. R. (2012). Molecular regulation of seed and fruit set. Trends Plant Sci. 17, 656–665.
Schaffer, R. J., Friel, E. N., Souleyre, E. J. F., Bolitho, K., Thodey, K., Ledger, S., et al. (2007). A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 144, 1899–1912.
Schaffer, R. J., Ireland, H. S., Ross, J. J., Ling, T. J., and David, K. M. (2013). SEPALLATA1/2-suppressed mature apples have low ethylene, high auxin and reduced transcription of ripening-related genes. AoB Plants 5, pls047.
Serrani, J. C., Ruiz-Rivero, O., Fos, M., and Garcia-Martinez, J. L. (2008). Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 56, 922–934.
Sorefan, K., Girin, T., Liljegren, S. J., Ljung, K., Robles, P., Galvan-Ampudia, C. S., et al. (2009). A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459, 583–586.
Srivastava, A., and Handa, A. K. (2005). Hormonal regulation of tomato fruit development: a molecular perspective. J. Plant Growth Regul. 24, 67–82.
Srivastava, M. K., and Dwivedi, U. N. (2000). Delayed ripening of banana fruit by salicylic acid. Plant Sci. 158, 87–96.
Sun, L., Sun, Y., Zhang, M., Wang, L., Ren, J., Cui, M., et al. (2012a). Suppression of 9-cis-epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol. 158, 283–298.
Sun, Y., Chen, P., Duan, C., Tao, P., Wang, Y., Ji, K., et al. (2012b). Transcriptional regulation of genes encoding key enzymes of abscisic acid metabolism during melon (Cucumis melo L.) fruit development and ripening. J. Plant Growth Regul. doi: 10.1007/s00344-012-9293-5
Sun, X., Shantharaj, D., Kang, X., and Ni, M. (2010). Transcriptional and hormonal signaling control of Arabidopsis seed development. Curr. Opin. Plant Biol. 13, 611–620.
Symons, G. M., Davies, C., Shavrukov, Y., Dry, I. B., Reid, J. B., and Thomas, M. R. (2006). Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol. 140, 150–158.
Talon, M., Zacarias, L., and Primomillo, E. (1990). Hormonal changes associated with fruit-set and development in mandarins differing in their parthenocarpic ability. Physiol. Plant. 79, 400–406.
Trainotti, L., Tadiello, A., and Casadoro, G. (2007). The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J. Exp. Bot. 58, 3299–3308.
Varga, A., and Bruinsma, J. (1976). Roles of seeds and auxins in tomato fruit growth. Z. Pflanzenphysiol. 80, 95–104.
Vendrell, M. (1985). Dual effect of 2,4-D on ethylene production and ripening of tomato fruit tissue. Physiol. Plant. 64, 559–563.
Vivian-Smith, A., and Koltunow, A. M. (1999). Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol. 121, 437–451.
Vriezen, W. H., Feron, R., Maretto, F., Keijman, J., and Mariani, C. (2008). Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 177, 60–76.
Werner, T., Motyka, V., Laucou, V., Smets, R., Van Onckelen, H., and Schmulling, T. (2003). Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15, 2532–2550.
Wismer, P. T., Proctor, J. T. A., and Elfving, D. C. (1995). Benzyladenine affects cell-division and cell-size during apple fruit thinning. J. Am. Soc. Hortic. Sci. 120, 802–807.
Yang, J., Zhang, J., Huang, Z., Wang, Z., Zhu, Q., and Liu, L. (2002). Correlation of cytokinin levels in the endosperms and roots with cell number and cell division activity during endosperm development in rice. Ann. Bot. 90, 369–377.
Zhang, M., Yuan, B., and Leng, P. (2009). The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J. Exp. Bot. 60, 1579–1588.
Keywords: fruit development, ripening, hormonal regulation
Citation: McAtee P, Karim S, Schaffer R and David K (2013) A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 4:79. doi: 10.3389/fpls.2013.00079
Received: 14 January 2013; Accepted: 19 March 2013;
Published online: 17 April 2013.
Edited by:
Maren Müller, University of Barcelona, SpainReviewed by:
Victoriano Valpuesta, Universidad de Málaga, SpainRuud A. De Maagd, Plant Research International, Netherlands
Copyright: © 2013 McAtee, Karim, Schaffer and David. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Karine David, School of Biological Sciences, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand. e-mail: k.david@auckland.ac.nz
