- Department of Horticulture, University of Kentucky, Lexington, KY, USA
Plant cell walls provide physical strength, regulate the passage of bio-molecules, and act as the first barrier of defense against biotic and abiotic stress. In addition to providing structural integrity, plant cell walls serve an important function in connecting cells to their extracellular environment by sensing and transducing signals to activate cellular responses, such as those that occur during pathogen infection. This mini review will summarize current experimental approaches used to study cell wall functions during plant-pathogen interactions. Focus will be paid to cell imaging, spectroscopic analyses, and metabolic profiling techniques.
Introduction
The plant cell wall is a complex network consisting of diverse polysaccharides, lignin, and proteins (Mutwil et al., 2008). It provides physical strength, maintains cell shape, resists internal turgor pressure, regulates cell differentiation and growth, mediates bio-molecule transit, and serves as the first barrier of defense against biotic and abiotic stress (Knox, 2008; Collinge, 2009; Endler and Persson, 2011). Cell walls are highly dynamic and are capable of modifying their structural and chemical compositions to maintain functionality during developmental growth (Brewin, 2004; Somerville et al., 2004; Gorshkova et al., 2013; Bellincampi et al., 2014). In addition to its structural roles, plant cell walls serve an important function in connecting extracellular and intracellular environments by sensing and transducing signals, and activating cellular responses (Pogorelko et al., 2013) to environmental change and pathogen attack (Aziz et al., 2004; Vorwerk et al., 2004; Hématy et al., 2009). At pathogen infection sites plants generally accumulate callose, phenolic compounds, and lignin (Underwood, 2012), and in some cases metabolites and proteins that can directly inhibit the growth of pathogens (Vorwerk et al., 2004; Haas et al., 2009). The importance of plant cell wall integrity and cell wall-mediated resistance during plant-microbial interaction has been demonstrated, but the related components and signaling pathways have not been fully elucidated (Mellersh and Heath, 2001; Collinge, 2009; Hématy et al., 2009).
This mini review will summarize current experimental approaches that may be used as tools to study the cell wall with a focus on techniques that could be applied during the interaction between a plant and an interacting microbe. In particular, focus will be given to techniques for assessing changes in metabolites during plant-microbe interaction as well as techniques for imaging the cell wall. We are particularly interested in how the phytobiome, including mutualistic endophytes, pathogens and symbionts alike interact with the plants central architectural framework, the cell wall, and how this information could be harnessed for isolation of new herbicides (Xia et al., 2014) and/or plant defense systems.
Metabolic Profiling Focused on Interactions between Plant and Microbe
Metabolic profiling is the characterization and quantification of low-molecular weight metabolites and their intermediates in biological systems (Roessner and Bowne, 2009). This profiling aims to capture metabolites involved in the dynamic plant response to genetic modification, growth and developmental manipulation, and biotic/abiotic stresses (Clarke and Haselden, 2008). During plant-pathogen interaction, pathogens attempt to utilize the metabolism of host plants to suppress plant defense and to obtain nutrients (Dangl and Jones, 2001; Chisholm et al., 2006; Collinge, 2009). Metabolites that are synthesized by a host plant during a plant-microbe interaction can serve as signals, sedatives, or toxins to either aid the association with the microbe, or to attempt to limit the proliferation of the microbe (Thomma et al., 2002; Krishnan et al., 2005; Allwood et al., 2010, 2011; Schwessinger et al., 2012). Ultimately, most metabolic profiling will aim to capture in situ changes in cellular output in a spatially or temporal discrete region (Sumner, 2006; Timischl et al., 2008; Sumner et al., 2011; Khakimov et al., 2012). In the case of plant-pathogen interactions, profiling generally focuses on the plants metabolic response. Assaying microbial metabolites that are involved in plant-microbe interaction remains challenging. Assigning signals produced by a microbe requires separating them from those of the host plant and when grown in isolation, their metabolic output may not reflect a pathogenic state. When considering the plant cell wall, the relative predictability of metabolites in specific tissues provides an excellent starting point for looking at metabolic shifts associated with microbial ingress.
Analysis of Plant Cell Wall Polysaccharides through Metabolic Profiling
At a broad scale, measurement of cell wall metabolites has been well-defined for decades. Neutral and acidic polysaccharides (Blakeney et al., 1983), acid insoluble and soluble glucose (Updegraff, 1969), soluble and insoluble lignin fractions (NREL, 2000) and the linkages between glycosyl units (Tong and Gross, 1988) can be examined with spectrophotometric, high performance liquid chromatography (HPLC), or gas chromatography (GC) coupled to mass spectroscopy (GC MS) to obtain a snapshot of the cell wall composition (Kopka, 2006). Similarly, at a much higher resolution, the structure of cell wall polysaccharides can be examined by uniformly feeding the plant with a isotope trace (13C-glucose and 15N-ammonia) and then employing 13C-magic angle spinning solid state nuclear magnetic resonance spectroscopy (SS-NMR) (Dick-Pérez et al., 2011; Fernandes et al., 2011; Harris et al., 2012). However, the capacity to look at spatially discrete regions of cell wall composition, which are linked to microbial association, can be difficult due to the relatively large amount of material needed for many of these techniques. To get around this limitation, combining systematic metabolite profiling with immunological approaches can be effective. For example, immunological approaches have been used to investigate the glycome profiling of wide array of plant cell wall polysaccharides (Pattathil et al., 2010, 2012; DeMartini et al., 2011; Fangel et al., 2012). Currently, around 150 antibodies that can recognize diverse epitopes present on each of the major classes of plant polysaccharides exist and are continuing to be developed. These antibodies have been used for in situ localization of epitopes to further our understanding of cell wall composition (Pattathil et al., 2012). Carbohydrate Microarray Polymer Profiling (CoMPP) has been streamlined as a screening platform to analyze cell wall polysaccharides by combining the specificity of monoclonal antibodies with a high-throughput microarray system (Alonso-Simón et al., 2009; Moller et al., 2012). In the context of microbial ingress, antibody based polysaccharide visualization has been utilized to observe altered xyloglucan arising from infection by the fungal pathogen Botrytis cinerea (Nguema-Ona et al., 2012, 2013). While difficulties arise in assigning quantitative data for localized metabolite profiles via immunological techniques, the capacity to gain unparalleled qualitative data is emerging. Additionally, a versatile high-resolution oligosaccharide microarray has been developed for cell wall analysis, which aids in the validation and characterization of target oligosaccharides produced via hydrolysis of polysaccharides or de novo synthesis (Pedersen et al., 2012). This library of cell wall oligosaccharides has been created by coupling target oligosaccharides with cognate proteins to form neoglycoconjugates, which in turn can be printed onto a microarray format (Pedersen et al., 2012). One can imagine the importance of such techniques to identify and characterize oligosaccharides identified during metabolic profiling.
Other techniques for assessing metabolites on a screening scale include Oligosaccharide Mass Profiling (OLIMP) coupled with Matrix-Assisted Laser Desorption/Ionization Time Of Flight (MALDI-TOF)-MS (Obel et al., 2009), or using of a suite of 74 polysaccharide degrading enzymes (Bauer et al., 2006). Both techniques were developed for the small-scale assessment of plant cell wall polysaccharides and to examine the oligosaccharides formed from polysaccharides that are digested by specific degrading enzymes (Bauer et al., 2006; Obel et al., 2009). OLIMP has particularly high sensitivity, thus making it ideal for small samples. It needs short preparation time and is suitable for in situ wall analysis at the cellular level. Importantly, OLIMP enables the comparative analysis of the wall polymers in a Golgi-enriched fraction vs. the apoplast fraction based on matrix polysaccharides, which may extend information about cellular functions during plant-pathogen interaction. OLIMP has been used to examine microbial alterations of the cell wall (Lionetti et al., 2007; Manabe et al., 2011), and allowed researchers to pinpoint that the alteration in esterification of pectin and xylan influenced the outcome of B. cinerea infection.
Cell Imaging and Spetroscopic Techniques
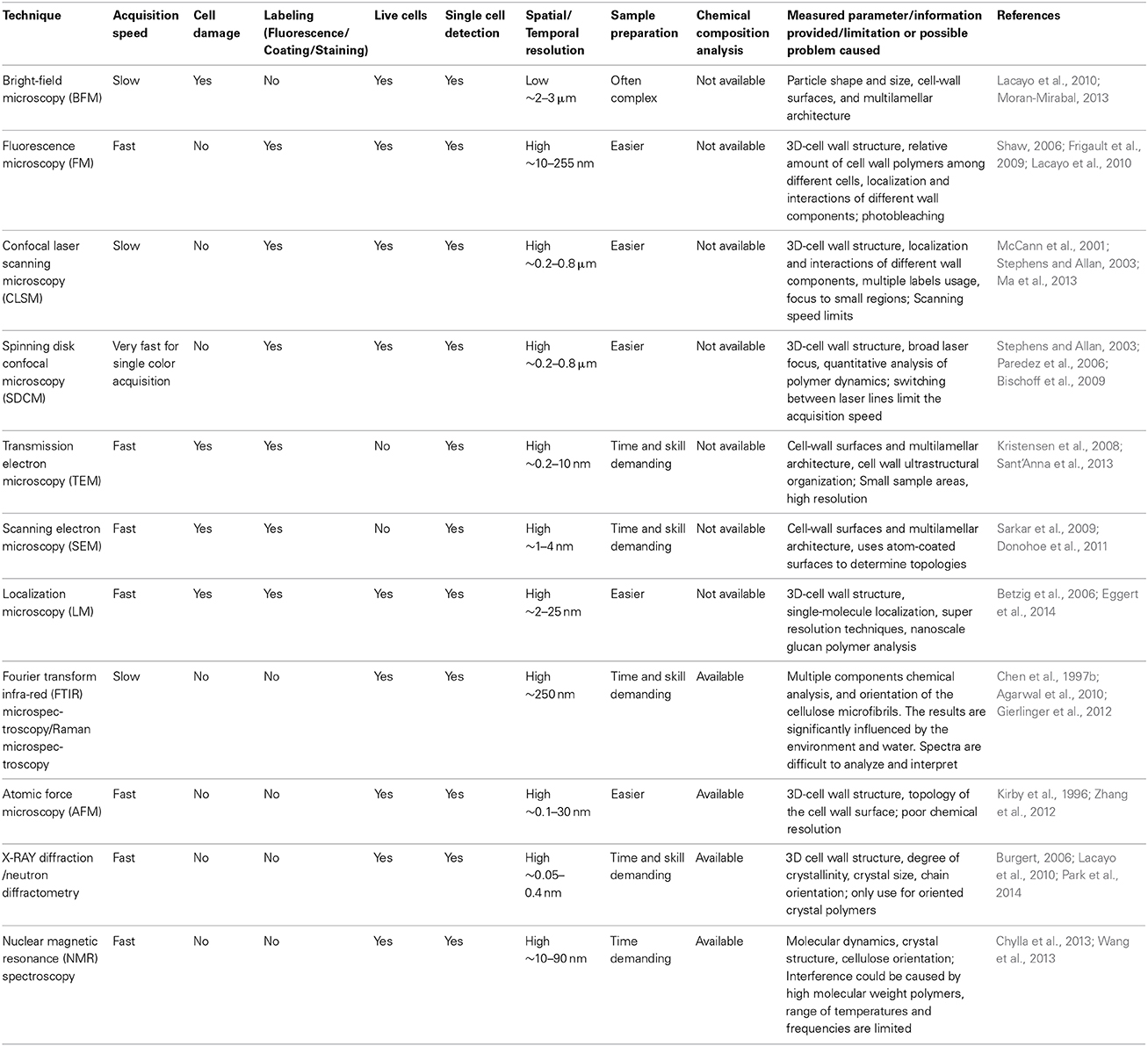
Advanced cellular imaging can be useful to investigate phenotypes linked to plant-microbe associations. Cellular imaging can be particularly important when applying a quantitative methodology to imaging techniques. Many microscopic techniques are available, including light (Wilt et al., 2009), fluorescence (Lichtman and Conchello, 2005), and confocal microscopy (Nwaneshiudu et al., 2012). However, the outcomes of cell imaging can be influenced by many factors, such as microscope resolution, the rate at which images can be acquired, cell type being examined and the abundance/size of the tagged protein or structure being observed (Stephens and Allan, 2003; Shaw, 2006; Table 1 for more details). Live cell imaging techniques (Table 1) have facilitated our understanding of plant cell wall dynamics in several different applications (Lee et al., 2011; Sappl and Heisler, 2013), and have been broadly applied when studying specific aspects of cell wall alteration during the interaction between a host plant and microbe.
Analysis of Cell Wall Structure and Function with Cell Imaging Techniques
There are several techniques that may be used to investigate structural and functional changes of plant cell walls during plant-microbe interactions. Aside from examining the phenotype, actually pinpointing defects in the cell wall often requires the merger to two or more techniques, including profiling cell wall structure as described above. Electron microscopy, both scanning (SEM) and transmission (TEM), along with fluorescence microscopy (FM) in the form of laser scanning or spinning disk confocal microscopy are of particularly interest. These techniques have been used together to examine plant-microbe interaction through alterations in the cell wall. Here, FM and TEM (Table 1) revealed that multi-vesicular bodies participated in cell wall-associated defense to powdery mildew in barley (An et al., 2006). As individual techniques, neither could ascertain mechanistic association, but together these techniques allowed a snapshot of inter and intracellular occurrences. Further, the relevance of plasma membrane–cell wall adhesion for cowpea resistance to rust fungi penetration was pinpointed by an integrated use of light and confocal microscopy (Mellersh and Heath, 2001). Laser scanning confocal microscopy can be used to track both plant and microbial proteins in live tissue. For example, confocal microscopy was used to track the dynamics of a Xanthomonas outer protein J (XopJ) in tobacco plants (Table 1), and revealed its interference (alteration of intracellular vesicle trafficking and polarized protein secretion) with cell wall-associated defense responses (Bartetzko et al., 2009). Similarly, but for a plant protein, the application of spinning disk confocal microscopy allowed the visualization of the CELLULOSE SYNTHASE (CeSA) complexes after exposure to the dinitrite-peptide Thaxtomin-A, which is a phytotoxin produced by Streptomyces scabies and S. eubacteria (Bischoff et al., 2009). Confocal microscopy allows the user to observe the microbial effector while it influences the target plant protein or cellular process. We recently utilized a screen of microbial endophytes (Xia et al., 2013) to identify microbial factors that induce cellulose inhibition and identified the compound acetobixan from a Bacillus sp. (Xia et al., 2014). Confocal microscopy allowed us to validate that the target process that the microbe was altering in the host plant was cellulose biosynthesis, which revealed a specific mechanism for this association.
The mechanisms of plant cell wall organization and dynamics have been extensively studied, and the use of suitable chemical probes to examine cell wall polysaccharide organization is expanding (Vorwerk et al., 2004; Lee et al., 2011). Recently, small molecule probes that bind to polysaccharides with high resolution and sensitivity have been developed (Knox, 2008; Pattathil et al., 2010; Lee et al., 2011), particularly in the form of click chemistry (Wallace and Anderson, 2012). An example of this approach was the utilization of an alkynylated fucose analog (FucAl) incorporated into the cell wall pectin fraction to elucidate pectin delivery, architecture, and dynamics in Arabidopsis (Anderson et al., 2012). Ultimately, the development of small molecule probes compatible with live-cell imaging can further enhance the understanding of fundamental biological questions pertinent to the cell wall during plant-microbe interaction, and can even be targeted to specific events.
Additional Imaging Techniques of Note
Atomic force microscopy (AFM) is a technique with expanding use and potential. The extremely high resolution of AFM can allow the examination of events occurring within the nm scale. AFM has recently been used to detect the interaction of a synthetic carbohydrate-binding module with plant cellulose, and the structural changes of crystalline cellulose at a cell-wall surface (Zhang et al., 2012, 2013). In terms of plant microbe interactions, AFM recently provided nanoscale imaging of cell surfaces in their native state and revealed cell wall dynamics and modification during Arabidopsis and Fusarium oxysporum interaction (Adams et al., 2012). These selected studies underline the necessity to utilize the ever-expanding technological advances in imaging systems, often in concert with metabolic profiling, to maximize the detail of the investigation.
Localization microscopy, which is a form of super-resolution microscopy, focuses on the localization of single fluorescent molecule. Such super-resolution microscopy has been used to analyze the infection site of the fungal pathogen powdery mildew on Arabidopsis plants at a nanoscale level (Eggert et al., 2014). The technique was sensitive enough to show that the microbial pathogen induced the synthesis of the (1,3)-β-glucan cell wall polymer callose, which interacted with the (1,4)-β-glucan cellulose to form a three-dimensional network for preventing pathogen infection. The formation of callose associated with pathogen ingress has been well-studied, but such inter-polymer associations could not have been proven without technical breakthroughs. It will be interesting to see whether such techniques are combined with click-chemistry to observe an increasing number of interactions simultaneously.
The combined approaches of microscopy with spectroscopy can also facilitate the investigation of wall associated ultra-structure modifications, and the chemical compositions of the plant cell wall during plant-pathogen interaction (Table 1 for more details). Fourier Transform Infrared (FT-IR) spectromicroscopy has been used to determine the presence and orientation of functional groups of cellulose and pectin in plant cell walls for well over a decade (Chen et al., 1997a,b; Kaèuráková et al., 2000; Wilson et al., 2000). This technique was used to show that a mutation in Arabidopsis PMR6, which encodes a pectate lyase-like protein and is required for the growth and reproduction of plant fungal powdery mildew pathogen-Erysiphe cichoracearum, altered plant cell wall composition by increasing pectin accumulation. Both absorbance peaks attributed to cellulose and xyloglucan shifted down in energy and broadened in the spectra of pmr6-1 cell walls, which indicated that either the –CH2OH group or the hydrogen bond of cellulose in pmr6-1 had been changed (Vogel et al., 2002).
Raman microscopy (Inelastic scattering with a photon from a laser light source) combined with FT-IR spectromicroscopy (Photon absorption) can facilitate the observation of ultra-structure, such as cellulosic crystals on the micro-scale (<0.5 μm) level (Agarwal et al., 2010), as well as the alignment and orientation of cellulose microfibrils with respect to the fiber axis between different cell wall layers (Gierlinger et al., 2012). This combined approach also improves our ability to visualize and analyze the chemical composition of plant cell walls. For instance, the spectra of the two wall-matrix polymers: lignin and pectin display discernable marker bands, which do not overlap with the cellulose signature, so their distribution in the plant cell wall can be easily visualized, imaged, and analyzed using these techniques (Gierlinger and Schwanninger, 2006; Richter et al., 2011; Gierlinger et al., 2012).
Prospects
Recent technical breakthroughs in combining higher resolution imaging and metabolic profiling techniques have yielded numerous discoveries in how plant cell wall function is modulated during microbial interaction. Although much effort was spent to be inclusive in this mini-review, due to space constraints we apologize for excluding numerous developing techniques not limited to but including those associated with biochemical pull downs, protein-protein interaction arrays and more. Although advanced cell imaging and spectroscopic techniques have facilitated such studies, the recent identification of the enormously complex phytobiome (Bulgarelli et al., 2012; Lundberg et al., 2012, 2013) reveals an outstanding question of the function of the phytobiome in plant-microbe associations. The use of next generation sequencing has revealed that many more microbes are present within plant tissue than those previously identified as obligate endophytes. It remains unclear how these microbial mutualists are associating with (or avoiding) the plant cell wall and associated defense pathways, and whether under pathogen interaction, they matter?
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
Funding support was provided by NSF-IOS and USDA (Hatch/FAPRU) to Seth DeBolt.
References
Adams, E., Emerson, D., Croker, S., Kim, H. S., Modla, S., Kang, S., et al. (2012). “Atomic force microscopy: a tool for studying biophysical surface properties underpinning fungal interactions with plants and substrates,” in Plant Fungal Pathogens, Vol. 835, eds M. D. Bolton and B. P. H. J. Thomma (New York, NY: Humana Press), 151–164.
Agarwal, U., Reiner, R., and Ralph, S. (2010). Cellulose I crystallinity determination using FT-Raman spectroscopy: univariate and multivariate methods. Cellulose 17, 721–733. doi: 10.1007/s10570-010-9420-z
Allwood, J. W., Clarke, A., Goodacre, R., and Mur, L. A. J. (2010). Dual metabolomics: a novel approach to understanding plant–pathogen interactions. Phytochemistry 71, 590–597. doi: 10.1016/j.phytochem.2010.01.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Allwood, J. W., De, V., Ric, C. H., Moing, A., Deborde, C., Erban, A., et al. (2011). “Chapter sixteen–plant metabolomics and its potential for systems biology research: background concepts, technology, and methodology,” in Methods in Enzymology, Vol. 500, eds M. V. Daniel Jameson and V. W. Hans (Waltham, MA: Academic Press), 299–336.
Alonso-Simón, A., Kristensen, J. B., Øbro, J., Felby, C., Willats, W. G. T., and Jørgensen, H. (2009). High-throughput microarray profiling of cell wall polymers during hydrothermal pre-treatment of wheat straw. Biotechnol. Bioeng. 105, 509–514. doi: 10.1002/bit.22546
An, Q., Hückelhoven, R., Kogel, K. H., Van, B., and Aart, J. E. (2006). Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol. 8, 1009–1019. doi: 10.1111/j.1462-5822.2006.00683.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Anderson, C. T., Wallace, I. S., and Somerville, C. (2012). Metabolic click-labeling with a fucose analog reveals pectin delivery, architecture, and dynamics in Arabidopsis cell walls. Proc. Natl. Acad. Sci. U.S.A. 109, 1329–1334. doi: 10.1073/pnas.1120429109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aziz, A., Heyraud, A., and Lambert, B. (2004). Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 218, 767–774. doi: 10.1007/s00425-003-1153-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bartetzko, V., Sonnewald, S., Vogel, F., Hartner, K., Stadler, R., Hammes, U. Z., et al. (2009). The Xanthomonas campestris pv. vesicatoria type III effector protein XopJ inhibits protein secretion: evidence for interference with cell wall-associated defense responses. Mol. Plant Microbe Interact. 22, 655–664. doi: 10.1094/MPMI-22-6-0655
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bauer, S., Vasu, P., Persson, S., Mort, A. J., and Somerville, C. R. (2006). Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc. Natl. Acad. Sci. U.S.A. 103, 11417–11422. doi: 10.1073/pnas.0604632103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bellincampi, D., Cervone, F., and Lionetti, V. (2014). Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 5:228. doi: 10.3389/fpls.2014.00228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Betzig, E., Patterson, G. H., Sougrat, R., Lindwasser, O. W., Olenych, S., Bonifacino, J. S., et al. (2006). Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645. doi: 10.1126/science.1127344
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bischoff, V., Cookson, S. J., Wu, S., and Scheible, W. R. (2009). Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J. Exp. Bot. 60, 955–965. doi: 10.1093/jxb/ern344
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Blakeney, A. B., Harris, P. J., Henry, R. J., and Stone, B. A. (1983). A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 113, 291–299. doi: 10.1016/0008-6215(83)88244-5
Brewin, N. J. (2004). Plant cell wall remodeling in the Rhizobium–legume symbiosis. Crit. Rev. Plant Sci. 23, 293–316. doi: 10.1080/07352680490480734
Bulgarelli, D., Rott, M., Schlaeppi, K., Ver Loren van, T. E., Ahmadinejad, N., Assenza, F., et al. (2012). Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488, 91–95. doi: 10.1038/nature11336
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Burgert, I. (2006). Exploring the micromechanical design of plant cell walls. Am. J. Bot. 93, 1391–1401. doi: 10.3732/ajb.93.10.1391
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, L. M., Wilson, R. H., and McCann, M. C. (1997a). Infra-red microspectroscopy of hydrated biological systems: design and construction of a new cell with atmospheric control for the study of plant cell walls. J. Microsc. 188, 62–71. doi: 10.1046/j.1365-2818.1997.2470805.x
Chen, L. M., Wilson, R. H., and McCann, M. C. (1997b). Investigation of macromolecule orientation in dry and hydrated walls of single onion epidermal cells by FT-IR microspectroscopy. J. Mol. Struct. 408, 257–260. doi: 10.1016/S0022-2860(96)09539-7
Chisholm, S., Coaker, G., Day, B., and Staskawicz, B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. doi: 10.1016/j.cell.2006.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chylla, R., Van Acker, R., Kim, H., Azapira, A., Mukerjee, P., Markley, J. L., et al. (2013). Plant cell wall profiling by fast maximum likelihood reconstruction (FMLR) and region-of-interest (ROI) segmentation of solution-state 2D 1H-13C NMR spectra. Biotechnol. Biofuels 6:45. doi: 10.1186/1754-6834-6-45
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Clarke, C. J., and Haselden, J. N. (2008). Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol. Pathol. 36, 140–147. doi: 10.1177/0192623307310947
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Collinge, D. B. (2009). Cell wall appositions: the first line of defense. J. Exp. Bot. 60, 351–352. doi: 10.1093/jxb/erp001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dangl, J. L., and Jones, J. D. G. (2001). Plant pathogens and integrated defense responses to infection. Nature 411, 826–833. doi: 10.1038/35081161
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
DeMartini, J. D., Pattathil, S., Avci, U., Szekalski, K., Mazumder, K., Hahn, M. G., et al. (2011). Application of monoclonal antibodies to investigate plant cell wall deconstruction for biofuels production. Energy Environ. Sci. 4, 4332–4339. doi: 10.1038/nature11336
Dick-Pérez, M., Zhang, Y., Hayes, J., Salazar, A., Zabotina, O. A., and Hong, M. (2011). Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50, 989–1000. doi: 10.1021/bi101795q
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Donohoe, B. S., Vinzant, T. B., Elander, R. T., Pallapolu, V. R., Lee, Y. Y., Garlock, R. J., et al. (2011). Surface and ultrastructural characterization of raw and pretreated switchgrass. Bioresour. Technol. 102, 11097–11104. doi: 10.1016/j.biortech.2011.03.092
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eggert, D., Naumann, M., Reimer, R., and Voigt, C. A. (2014). Nanoscale glucan polymer network causes pathogen resistance. Sci. Rep. 4, 1–6. doi: 10.1038/srep04159
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Endler, A., and Persson, S. (2011). Cellulose synthases and synthesis in Arabidopsis. Mol. Plant 4, 199–211. doi: 10.1093/mp/ssq079
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fangel, J. H., Pedersen, S., Vidal-Melgosa, L., Ahl, A., Salmean, J., Egelund, M., et al. (2012). “Carbohydrate microarrays in plant science,” in High-Throughput Phenotyping in Plants, Vol. 918, ed J. Normanly (New York, NY: Humana Press), 351–362.
Fernandes, A. N., Thomas, L. H., Altaner, C. M., Callow, P., Forsyth, V. T., Apperley, D. C., et al. (2011). Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. U.S.A. 108, 1195–1203. doi: 10.1073/pnas.1108942108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Frigault, M. M., Lacoste, J., Swift, J. L., and Brown, C. M. (2009). Live-cell microscopy–tips and tools. J. Cell Sci. 122, 753–767. doi: 10.1242/jcs.033837
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gierlinger, N., Keplinger, T., and Harrington, M. (2012). Imaging of plant cell walls by confocal Raman microscopy. Nat. Protoc. 7, 1694–1708. doi: 10.1038/nprot.2012.092
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gierlinger, N., and Schwanninger, M. (2006). Chemical imaging of poplar wood cell walls by confocal Raman microscopy. Plant Physiol. 140, 1246–1254. doi: 10.1104/pp.105.066993
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gorshkova, T. A., Kozlova, L. V., and Mikshina, P. V. (2013). Spatial structure of plant cell wall polysaccharides and its functional significance. Biochemistry (Moscow) 78, 836–853. doi: 10.1134/S0006297913070146
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haas, B., Kamoun, S., Zody, M. C., Jiang, R. H. Y., Handsaker, R. E., Cano, L. M., et al. (2009). Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461, 393–398. doi: 10.1038/nature08358
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Harris, D. M., Corbin, K., Wang, T., Gutierrez, R., Bertolo, A. L., Petti, C., et al. (2012). Cellulose microfibril crystallinity is reduced by mutating C-terminal transmembrane region residues CESA1A903V and CESA3T942I of cellulose synthase. Proc. Natl. Acad. Sci. U.S.A. 109, 4098–4103. doi: 10.1073/pnas.1200352109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hématy, K., Cherk, C., and Somerville, S. (2009). Host–pathogen warfare at the plant cell wall. Curr. Opin. Plant Biol. 12, 406–413. doi: 10.1016/j.pbi.2009.06.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kaèuráková, M., Capek, P., Sasinková, V., Wellner, N., and Ebringerová, A. (2000). FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 43, 195–203. doi: 10.1016/S0144-8617(00)00151-X
Khakimov, B., Amigo, J. M., Bak, S., and Engelsen, S. B. (2012). Plant metabolomics: resolution and quantification of elusive peaks in liquid chromatography-mass spectrometry profiles of complex plant extracts using multi-way decomposition methods. J. Chromatogr. 1266, 84–94. doi: 10.1016/j.chroma.2012.10.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kirby, A., Gunning, A. P., Waldron, K. W., Morris, V. J., and Ng, A. (1996). Visualization of plant cell walls by atomic force microscopy. Biophys. J. 70, 1138–1143. doi: 10.1016/S0006-3495(96)79708-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Knox, J. P. (2008). Revealing the structural and functional diversity of plant cell walls. Curr. Opin. Plant Biol. 11, 308–313. doi: 10.1016/j.pbi.2008.03.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kopka, J. (2006). “Gas chromatography mass spectrometry,” in Plant Metabolomics, eds K. Saito, R. Dixon, and L. Willmitzer (Berlin: Springer-Verlag Press), 3–20.
Krishnan, P., Kruger, N. J., and Ratcliffe, R. G. (2005). Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 56, 255–265. doi: 10.1093/jxb/eri010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kristensen, J., Thygesen, L., Felby, C., Jorgensen, H., and Elder, T. (2008). Cell-wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 1, 5. doi: 10.1186/1754-6834-1-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lacayo, C. I., Malkin, A. J., Holman, H. Y. N., Chen, L., Ding, S. Y., Hwang, M. S., et al. (2010). Imaging cell wall architecture in single Zinnia elegans tracheary elements. Plant Physiol. 154, 121–133. doi: 10.1104/pp.110.155242
Lee, K. J. D., Marcus, S. E., and Knox, J. P. (2011). Cell wall biology: perspectives from cell wall imaging. Mol. Plant 4, 212–219. doi: 10.1093/mp/ssq075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lichtman, J. W., and Conchello, J. A. (2005). Fluorescence microscopy. Nat. Methods 2, 910–919. doi: 10.1038/nmeth817
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lionetti, V., Raiola, A., Camardella, L., Giovane, A., Obel, N., Pauly, M., et al. (2007). Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol. 143, 1871–1880. doi: 10.1104/pp.106.090803
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lundberg, D. S., Lebeis, S. L., Paredes, S. H., Yourstone, S., Gehring, J., Malfatti, S., et al. (2012). Defining the core Arabidopsis thaliana root microbiome. Nature 488, 86–90. doi: 10.1038/nature11237
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lundberg, D. S., Yourstone, S., Mieczkowski, P., Jones, C. D., and Dangl, J. (2013). Practical innovations for high-throughput amplicon sequencing. Nat. Methods 10, 999–1002. doi: 10.1038/nmeth.2634
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ma, J., Ji, Z., Zhou, X., Zhang, Z. H., and Xu, F. (2013). Transmission electron microscopy, fluorescence microscopy, and confocal raman microscopic analysis of ultrastructural and compositional heterogeneity of Cornus alba L. Wood cell wall. Microsc. Microanal. 19, 243–253. doi: 10.1017/S1431927612013906
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Manabe, Y., Nafisi, M., Verhertbruggen, Y., Orfila, C., Gille, S., Rautengarten, C., et al. (2011). Loss-of-function mutation of REDUCED WALL ACETYLATION2 in Arabidopsis leads to reduced cell wall acetylation and increased resistance to Botrytis cinerea. Plant Physiol. 155, 1068–1078. doi: 10.1104/pp.110.168989
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McCann, M. C., Bush, M., Milioni, D., Sado, P., Stacey, N., Catchpole, G., et al. (2001). Approaches to understanding the functional architecture of the plant cell wall. Phytochemistry 57, 811–821. doi: 10.1016/S0031-9422(01)00144-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mellersh, D. G., and Heath, M. C. (2001). Plasma membrane–cell wall adhesion is required for expression of plant defense responses during fungal penetration. Plant Cell 13, 413–424. doi: 10.1105/tpc.13.2.413
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moller, I. E., Pettolino, F. A., Hart, C., Lampugnani, E. R., Willats, W. G., and Bacic, A. (2012). Glycan profiling of plant cell wall polymers using microarrays. J. Vis. Exp. 17:e4238. doi: 10.3791/4238
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moran-Mirabal, J. M. (2013). “Advanced-microscopy techniques for the characterization of cellulose structure and cellulose-cellulase interactions,” in Cellulose–Fundamental Aspects, eds V. T. van de and L. Godbout (Rijeka: InTech Press), 1–44.
Mutwil, M., Debolt, S., and Persson, S. (2008). Cellulose synthesis: a complex complex. Curr. Opin. Plant Biol. 11, 252–257. doi: 10.1016/j.pbi.2008.03.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nguema-Ona, E., Moore, J., Fagerstrom, A., Fangel, J., Willats, W. G. T., Hugo, A., et al. (2013). Overexpression of the grapevine PGIP1 in tobacco results in compositional changes in the leaf arabinoxyloglucan network in the absence of fungal infection. BMC Plant Biol. 13:46. doi: 10.1186/1471-2229-13-46
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nguema-Ona, E., Moore, J. P., Fagerström, A., Fangel, J. U., Willats, W. G. T., Hugo, A., et al. (2012). Profiling the main cell wall polysaccharides of tobacco leaves using high-throughput and fractionation techniques. Carbohydr. Polym. 88, 939–949. doi: 10.1016/j.carbpol.2012.01.044
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
NREL National Renewable Energy Laboratories. (2000). Technical Report: Determining the Cost of Producing Ethanol from Corn Starch and Lignocellulosic Feedstocks. NREL/TP-580–28893. Golden, CO: NREL Press.
Nwaneshiudu, A., Kuschal, C., Sakamoto, F. H., Anderson, R. R., Schwarzenberger, K., and Young, R. C. (2012). Introduction to confocal microscopy. J. Invest. Dermatol. 132, e3. doi: 10.1038/jid.2012.429
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Obel, N., Erben, V., Schwarz, T., Kühnel, S., Fodor, A., and Pauly, M. (2009). Microanalysis of plant cell wall polysaccharides. Mol. Plant 2, 922–932. doi: 10.1093/mp/ssp046
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Paredez, A. R., Somerville, C., and Ehrhardt, D. W. (2006). Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495. doi: 10.1126/science.1126551
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Park, Y. B., Lee, C. M., Kafle, K., Park, S., Cosgrove, D. J., and Kim, S. H. (2014). Effects of plant cell wall matrix polysaccharides on bacterial cellulose structure studied with vibrational sum frequency generation spectroscopy and x-ray diffraction. Biomacromolecules 15, 2718–2724. doi: 10.1021/bm500567v
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pattathil, S., Avci, U., Baldwin, D., Swennes, A. G., McGill, J. A., Popper, Z., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153, 514–552. doi: 10.1104/pp.109.151985
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pattathil, S., Avci, U., Miller, J., and Hahn, M. G. (2012). “Immunological approaches to plant cell wall and biomass characterization: glycome profiling,” in Biomass Conversion, Vol. 908, ed M. E. Himmel (New York, NY: Humana Press), 61–72.
Pedersen, H. L., Fangel, J. U., McCleary, B., Ruzanski, C., Rydahl, M. G., Ralet, M. C., et al. (2012). Versatile high resolution oligosaccharide microarrays for plant glycobiology and cell wall research. J. Biol. Chem. 287, 39429–39438. doi: 10.1074/jbc.M112.396598
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pogorelko, G., Lionetti, V., Bellincampi, D., and Zabotina, O. (2013). Cell wall integrity: targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signal. Behav. 8:e25435. doi: 10.4161/psb.25435
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Richter, S., Müssig, J., and Gierlinger, N. (2011). Functional plant cell wall design revealed by the Raman imaging approach. Planta 233, 763–772. doi: 10.1007/s00425-010-1338-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Roessner, U., and Bowne, J. (2009). What is metabolomics all about? Biotechniques 46, 363–365. doi: 10.2144/000113133
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sant'Anna, C., Costa, L. T., Abud, Y., Biancatto, L., Miguens, F. C., and de Souza, W. (2013). Sugarcane cell wall structure and lignin distribution investigated by confocal and electron microscopy. Microsc. Res. Tech. 76, 829–834. doi: 10.1002/jemt.22235
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sappl, P. G., and Heisler, M. G. (2013). Live-imaging of plant development: latest approaches. Curr. Opin. Plant Biol. 16, 33–40. doi: 10.1016/j.pbi.2012.10.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sarkar, P., Bosneaga, E., and Auer, M. (2009). Plant cell walls throughout evolution: towards a molecular understanding of their design principles. J. Exp. Bot. 60, 3615–3635. doi: 10.1093/jxb/erp245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schwessinger, B., Ronald, P. C., Schwessinger, B., and Ronald, P. C. (2012). Plant innate immunity: perception of conserved microbial signatures. Annu. Rev. Plant Biol. 63, 451–482. doi: 10.1146/annurev-arplant-042811-105518
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shaw, S. L. (2006). Imaging the live plant cell. Plant J. 45, 573–598. doi: 10.1111/j.1365-313X.2006.02653.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Somerville, C., Bauer, S., Brininstool, G., Facette, M., Hamann, T., Milne, J., et al. (2004). Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211. doi: 10.1126/science.1102765
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stephens, D. J., and Allan, V. J. (2003). Light microscopy techniques for live cell imaging. Science 300, 82–86. doi: 10.1126/science.1082160
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sumner, L. W. (2006). “Current status and forward looking thoughts on LC/MS metabolomics,” in Plant Metabolomics, Vol. 57, eds K. Saito, R. Dixon, and L. Willmitzer (Berlin: Springer Press), 21–32.
Sumner, L. W., Yang, D. S., Bench, B. J., Watson, B. S., Li, C., and Jones, A. D. (2011). “Spatially—resolved metabolomics—challenges for the future,” in The Biology of Plant Metabolomics, Chapter 11, ed R. D. Hall (London: Blackwell-Wiley Press), 343–366.
Thomma, B. P. H. J., Cammue, B. P. A., and Thevissen, K. (2002). Plant defensins. Planta 216, 193–202. doi: 10.1007/s00425-002-0902-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Timischl, B., Dettmer, K., Kaspar, H., Thieme, M., and Oefner, P. J. (2008). Development of a quantitative, validated Capillary electrophoresis-time of flight–mass spectrometry method with integrated high-confidence analyte identification for metabolomics. Electrophoresis 29, 2203–2214. doi: 10.1002/elps.200700517
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tong, C. B. S., and Gross, K. C. (1988). Glycosyl-linkage composition of tomato fruit cell wall hemicellulosic fractions during ripening. Physiol. Plant. 74, 365–370. doi: 10.1111/j.1399-3054.1988.tb00644.x
Underwood, W. (2012). The plant cell wall: a dynamic barrier against pathogen invasion. Front. Plant Sci. 3:85. doi: 10.3389/fpls.2012.00085
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Updegraff, D. M. (1969). Semimicro determination of cellulose inbiological materials. Anal. Biochem. 32, 420–424. doi: 10.1038/nature11336
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vogel, J. P., Raab, T. K., Schiff, C., and Somerville, S. (2002). PMR6, a pectate lyase–like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14, 2095–2106. doi: 10.1105/tpc.003509
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vorwerk, S., Somerville, S., and Somerville, C. (2004). The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci. 9, 203–209. doi: 10.1016/j.tplants.2004.02.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wallace, I. S., and Anderson, C. T. (2012). Small molecule probes for plant cell wall polysaccharide imaging. Front. Plant Sci. 3:89. doi: 10.3389/fpls.2012.00089
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, T., Park, Y. B., Caporini, M. A., Rosay, M., Zhong, L., Cosgrove, D. J., et al. (2013). Sensitivity-enhanced solid-state NMR detection of expansin's target in plant cell walls. Proc. Nat. Acad. Sci. U.S.A. 110, 16444–16449. doi: 10.1073/pnas.1316290110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wilson, R. H., Smith, A. C., Kačuráková, M., Saunders, P. K., Wellner, N., and Waldron, K. W. (2000). The mechanical properties and molecular dynamics of plant cell wall polysaccharides studied by Fourier-transform infrared spectroscopy. Plant Physiol. 124, 397–406. doi: 10.1104/pp.124.1.397
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wilt, B. A., Burns, L. D., Wei Ho, E. T., Ghosh, K. K., Mukamel, E. A., and Schnitzer, M. J. (2009). Advances in light microscopy for neuroscience. Annu. Rev. Neurosci. 32, 435–506. doi: 10.1146/annurev.neuro.051508.135540
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xia, Y., Lei, L., Brabham, C., Stork, J., Strickland, J., Ladak, A., et al. (2013). Characterization of culturable bacterial endophytes of switchgrass (Panicum virgatum L.) and their capacity to influence plant growth. GCB Bioenergy 5, 674–682. doi: 10.1111/j.1757-1707.2012.01208.x
Xia, Y., Lei, L., Brabham, C., Stork, J., Strickland, J., Ladak, A., et al. (2014). Acetobixan, an inhibitor of cellulose synthesis identified by microbial bioprospecting. PLoS ONE 9:e95245. doi: 10.1371/journal.pone.0095245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, M., Chen, G., Kumar, R., and Xu, B. Q. (2013). Mapping out the structural changes of natural and pretreated plant cell wall surfaces by atomic force microscopy single molecular recognition imaging. Biotechnol. Biofuels 6, 147–152. doi: 10.1186/1754-6834-6-147
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, M., Wu, S. C., Zhou, W., and Xu, B. Q. (2012). Imaging and measuring single-molecule interaction between a carbohydrate-binding module and natural plant cell wall cellulose. J. Phys. Chem. 116, 9949–9956. doi: 10.1021/jp304686q
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: cell imaging, metabolic profiling, phytobiome, plant-microbe interaction, cell wall
Citation: Xia Y, Petti C, Williams MA and DeBolt S (2014) Experimental approaches to study plant cell walls during plant-microbe interactions. Front. Plant Sci. 5:540. doi: 10.3389/fpls.2014.00540
Received: 30 April 2014; Accepted: 23 September 2014;
Published online: 14 October 2014.
Edited by:
Vincenzo Lionetti, Sapienza “Università di Roma,” ItalyReviewed by:
Yumiko Sakuragi, University of Copenhagen, DenmarkCharles T. Anderson, The Pennsylvania State University, USA
Copyright © 2014 Xia, Petti, Williams and DeBolt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seth Debolt, Department of Horticulture, University of Kentucky, Plant Science Building, 1405 Veterans Drive, Lexington, KY 40546-0312, USA e-mail: sdebo2@uky.edu
 Ye Xia
Ye Xia Carloalberto Petti
Carloalberto Petti Mark A. Williams
Mark A. Williams Seth DeBolt*
Seth DeBolt*