- 1Australia–China Research Centre for Crop Improvement and School of Environmental and Life Sciences, The University of Newcastle, Callaghan, NSW, Australia
- 2Institute of Vegetables, Zhejiang Academy of Agricultural Sciences, Hangzhou, China
Hydrogen peroxide (H2O2) is a major reactive oxygen species (ROS) and plays diverse roles in plant development and stress responses. However, its localization in large and thick plant organs (e.g., stem, roots, and fruits), other than leaves, has proven to be challenging due to the difficulties for the commonly used H2O2-specific chemicals, such as 3,3′-diaminobenzidine (DAB), cerium chloride (CeCl3), and 2′,7′-dichlorofluorescin diacetate (H2DCF-DA), to penetrate those organs. Theoretically, the reaction of endogenous H2O2 with these chemicals could be facilitated by using thin organ sections. However, the rapid production of wound-induced H2O2 associated with this procedure inevitably disturbs the original distribution of H2O2 in vivo. Here, by employing tomato seedling stems and fruits as testing materials, we report a novel, simple, and rapid protocol to localize H2O2 in those organs using DAB-mediated tissue printing. The rapidity of the protocol (within 15 s) completely avoided the interference of wound-induced H2O2 during experimentation. Moreover, the H2O2 signal on the printing was stable for at least 1 h with no or little background produced. We conclude that DAB-mediated tissue printing developed here provide a new feasible and reliable method to localize H2O2 in large plant organs, hence should have broad applications in studying ROS biology.
Introduction
Reactive oxygen species (ROS)accumulate when plants are under various biotic (pathogen attack) and abiotic (e.g., high light, drought, heat, salt, and heavy metal) stresses (Apel and Hirt, 2004; Suzuki et al., 2012; Choudhury et al., 2013). On one hand, excessive ROS cause oxidative damage to proteins, DNA, and lipids. On the other hand, ROS also act as signaling molecules to regulate development and stress responses (Apel and Hirt, 2004). There are different kinds of ROS in plants, including singlet oxygen(1O2), superoxide (), H2O2, and hydroxyl radical (OH-). Among them, H2O2 is thought to be relatively stable (Bienert et al., 2007) and the most likely signaling ROS to regulate developmental and stress responses (Van Breusegem et al., 2008), and thus one of the most studied ROS species.
Hydrogen peroxide could be detected quantitatively and qualitatively. Accurate quantification of H2O2 in plant organs, however, is difficult to achieve owing to the unique properties of H2O2 being highly metabolically active with a half-life of only 1 ms in plants (Reth, 2002; Veljovic-Jovanovic et al., 2002; Petrov and Van Breusegem, 2012). Even storage of plant materials at -80°C may result in the loss of H2O2 by as much as 60% within 7 days (Cheeseman, 2006). Moreover, H2O2 may react with many reduced compounds released during homogenization of plant materials, such as ascorbic acid which leads to underestimation of H2O2 levels. The level of H2O2 could also be overestimated because of endogenous phenolics in plant tissues (Veljovic-Jovanovic et al., 2002). In fact, it has been reported that H2O2 content can span more than several orders of magnitude even for leaves from the same species (from nM to mM; Cheeseman, 2006; Razem, 2008), indicating major challenges in H2O2 quantification.
Hydrogen peroxide can also be qualitatively localized at a tissue or cellular level. Compared to the measurement of H2O2 extracted from whole plant organs, this approach has the advantage of localizing H2O2 in particular cellular sites in a multicellular tissue or organ, thereby potentially providing deep insights into the cellular origin and function of the H2O2. Localization of H2O2 relies on histochemical staining of plant organs. The most commonly used chemicals to localize H2O2 in planta are 3,3′-diaminobenzidine (DAB), cerium chloride (CeCl3), and 2′,7′-dichlorofluorescin diacetate (H2DCF-DA). However, detection of H2O2 with DAB requires a long incubation time with DAB solution. For example, H2O2 localization in detached leaves and tender seedling roots usually needs more than 8 h incubation in DAB solution (Thordal-Christensen et al., 1997; Salzer et al., 1999). To gain higher resolution of the cellular localization of H2O2, a TEM method has also been employed. In this method, endogenous H2O2 reacts with exogenously supplied CeCl3 to form cerium perhydroxide, which gives dark deposits under TEM. However, before samples are fixed for TEM, leaves must be incubated in CeCl3 solution for at least 1 h to allow the penetration of CeCl3 into the tissue and the formation of cerium perhydroxide (Bestwick et al., 1997; Romero-Puertas et al., 2004). Such a long period of incubation of detached plant organs in DAB and CeCl3 solution would inevitably change the distribution pattern of H2O2 in vivo, because of both the rapid degradation of original H2O2 due to its short half-life and the de novo production of wound-induced H2O2. Queval et al. (2008) has suggested that DAB staining only reflect the production of H2O2 rather than its original concentration or distribution. Indeed, it has been reported that H2O2 accumulates rapidly at the cutting site of Arabidopsis stem, within 1 min after cutting, and furthermore the wound-induced H2O2 signal can travel at a speed of 8.4 cm min-1 and induce dramatic increase of H2O2 in distal cotyledons within 2 min (Miller et al., 2009).
Although H2DCF-DA staining takes a shorter incubation time (10 min) than DAB and CeCl3 staining, it is mostly used for the visualization of H2O2 on the surface of plant organs such as, epidermis of citrus fruit (Macarisin et al., 2007) and tobacco leaves (Essmann et al., 2008). To the best of our knowledge, there have been no reports on using the above-mentioned three chemicals to localize H2O2 in the inner parts of large plant organs (e.g., stem and fruit). The most likely reason for this scenario is the difficulties for these chemicals to infiltrate into the large plant organs. This was indeed the case in our preliminary studies on tomato ovaries using DAB and H2DCF-DA staining. Only recently, H2O2 localization in seed has been reported in a study on rice using H2DCF-DA (Nagasawa et al., 2013), in which seeds were sectioned in half before staining. Although sectioning facilitated the reaction of H2DCF-DA with H2O2, the rapid production of wound-induced H2O2 could dramatically alter the original distribution of H2O2 and yield an artifact of overestimate of H2O2 level, just as discussed above. Thus, how to avoid or minimize the interference of wound-induced H2O2 is a prerequisite for the accurate localization of H2O2 in organ sections.
Some studies have endeavored to tackle the problem of wound-induced production of H2O2. For example, starch/KI-mediated tissue printing has been used to localize H2O2 in the stem of seedlings from different plant species including soybean, pea, common bean, sunflower, and cucumber (Schopfer, 1994). In this procedure, the sections of seedling stem were pressed for 60 s, immediately after cutting, on nitrocellulose paper impregnated with starch/KI solution. The oxidation of KI to I2 by H2O2 can produce the blue–black I2-starch complex (Olson and Varner, 1993), which can be photographed under the microscope. The whole procedure can be completed in just 70 s, and thus wound-induced H2O2 can be avoided to a large extent (Schopfer, 1994). However, the intensity of color increases with time after printing, and therefore the signal must be observed and recorded immediately (Schopfer, 1994). In addition, there is a background color due to continuous autoxidation of KI, which interferes and blurs the results (Neves et al., 1998; Przymusiński et al., 2007).
After being absorbed into plant cells, DAB reacts with H2O2 to form a reddish-brown polymer in the presence of peroxidase (Thordal-Christensen et al., 1997). DAB-mediated tissue printing has been employed to localize peroxidase in plants (Spruce et al., 1987). However, there has been no report on DAB-mediated tissue printing to directly localize H2O2 in plants. Here, we described such a novel procedure. The new protocol can rapidly and reliably localize H2O2 in sections of large tomato organs, namely stems and fruits. The whole procedure was completed within 15 s which avoided the interference of wound-induced H2O2. At the same time, the signal was very stable for at least 1 h with little background produced.
Materials and Methods
H2O2 Localization by DAB-Mediated Tissue Printing
Nitrocellulose membrane (0.45 μm in pore size, HybondTM-C Extra, Amersham) was soaked in 5 mg mL-1 DAB–HCl solution (pH 3.8) and then air-dried at room temperature for 30 min in the dark. The soaked nitrocellulose membrane was placed on a layer of un-soaked nitrocellulose membrane, which can absorb excessive plant exudate from cutting site during tissue printing. Tissue printing was performed at ∼20°C. Free-hand sections in 1.0 mm thickness were prepared with a razor blade. The sections were cut from the top, middle, and bottom positions of stems of 50-days old seedlings, or transversely from the middle of fruits at 5 and 10 DAF. The sections were gently pressed onto the impregnated nitrocellulose membrane with forefinger for 10 s to ensure that H2O2 in sections is successfully transferred to membrane and at the same time the sections are not crushed by the press. Then, the sections were carefully removed with forceps. This, together with the 5 s required for the cutting of the section, renders the total time for tissue printing being only 15 s. The membrane was then washed in 100% ethanol to remove the possible interfering substance (e.g., chlorophyll) and photographed under a dissection microscope after 5 min at room temperature to allow completion of the reaction between the H2O2 derived from plant cells and DAB pre-soaked in the membrane.
To verify the specificity of reaction, tissue printings were also done as above on membranes pre-soaked in 5 mg mL-1 DAB–HCl solution (pH 3.8) containing 100 mM ascorbic acid. To test whether there is a production of wound-induced H2O2 under our experimental conditions, tissue printing was performed at 0, 1, and 2 min after sectioning. To test if endogenous peroxidase was sufficient to support the reaction, H2O2 was introduced exogenously by soaking nitrocellulose membrane in 5 mg mL-1 DAB–HCl solution (pH 3.8) containing 20 mM H2O2 and tissue printing was done as described above. If the endogenous peroxidase is sufficient, it can be expected that the tissue printing with exogenously supplied H2O2 would produce stronger signals.
H2O2 Quantification in Tomato Stems
Hydrogen peroxide extraction was carried out according to Veljovic-Jovanovic et al. (2002). Briefly, 100 mg of stem from the top, middle, and bottom part of tomato seedlings was harvested, snap-frozen in liquid nitrogen and analyzed immediately. Samples were homogenized in 1.5 mL 1 M HClO4 with 100 mg of insoluble polyvinylpyrrolidone, which can remove phenolic compounds. Homogenates were centrifuged at 13000 × g for 10 min at 4°C. The H2O2 content in the supernatant was then determined as described by Cheeseman (2006). Briefly, 60 μL extract was mixed with 600 μL eFOX reagents (containing 250 μM ferrous ammonium sulfate, 100 μM sorbitol, 100 μM xylenol orange, and 1% ethanol in 25 mM H2SO4). Then, the difference in absorbance between 550 and 800 nm was recorded at least 30 min after mixing the supernatant with the eFOX reagents. The content of H2O2 was calculated using a standard curve of H2O2.
Statistical Analysis
One-way ANOVA was done using IBM SPSS Statistics 20.
Results and Discussion
The DAB-Mediated Tissue Printing for Localizing H2O2 is Simple, Rapid, and Reliable
Unless otherwise specified, tissue printing of tomato stem was always conducted on sections from the middle part of the seedling. We found that tissue printing of stem sections on nitrocellulose membrane for 10 s produced significant reddish-brown color at the cortex and vascular bundle regions (Figures 1A,B). To verify if the color is H2O2-specific, the reaction was done in the presence of ascorbic acid, a specific H2O2 scavenger (Thordal-Christensen et al., 1997). Since the nitrocellulose membrane needed to be air-dried for 30 min before it being used for tissue printing, it is possible that the soaked ascorbic acid might be oxidized. Therefore, to ensure there was sufficient reduced form ascorbic acid in membrane for the specific reaction with H2O2, we employed higher concentration of ascorbic acid (100 mM) than the commonly used concentration (10 mM; Thordal-Christensen et al., 1997; Salzer et al., 1999). It was found that no color was produced under ascorbic acid treatment (Figure 1C), indicating that the reaction was H2O2-specific. The same printing in Figure 1A was photographed again 1 h later with no significant changes in the H2O2 signal strength (Figure 1D). Furthermore, no obvious background color was developed following 1 h at room temperature (Figure 1D). Therefore, it can be concluded that our protocol to localize H2O2 in stem with DAB-mediated tissue printing is simple, rapid, and reliable.
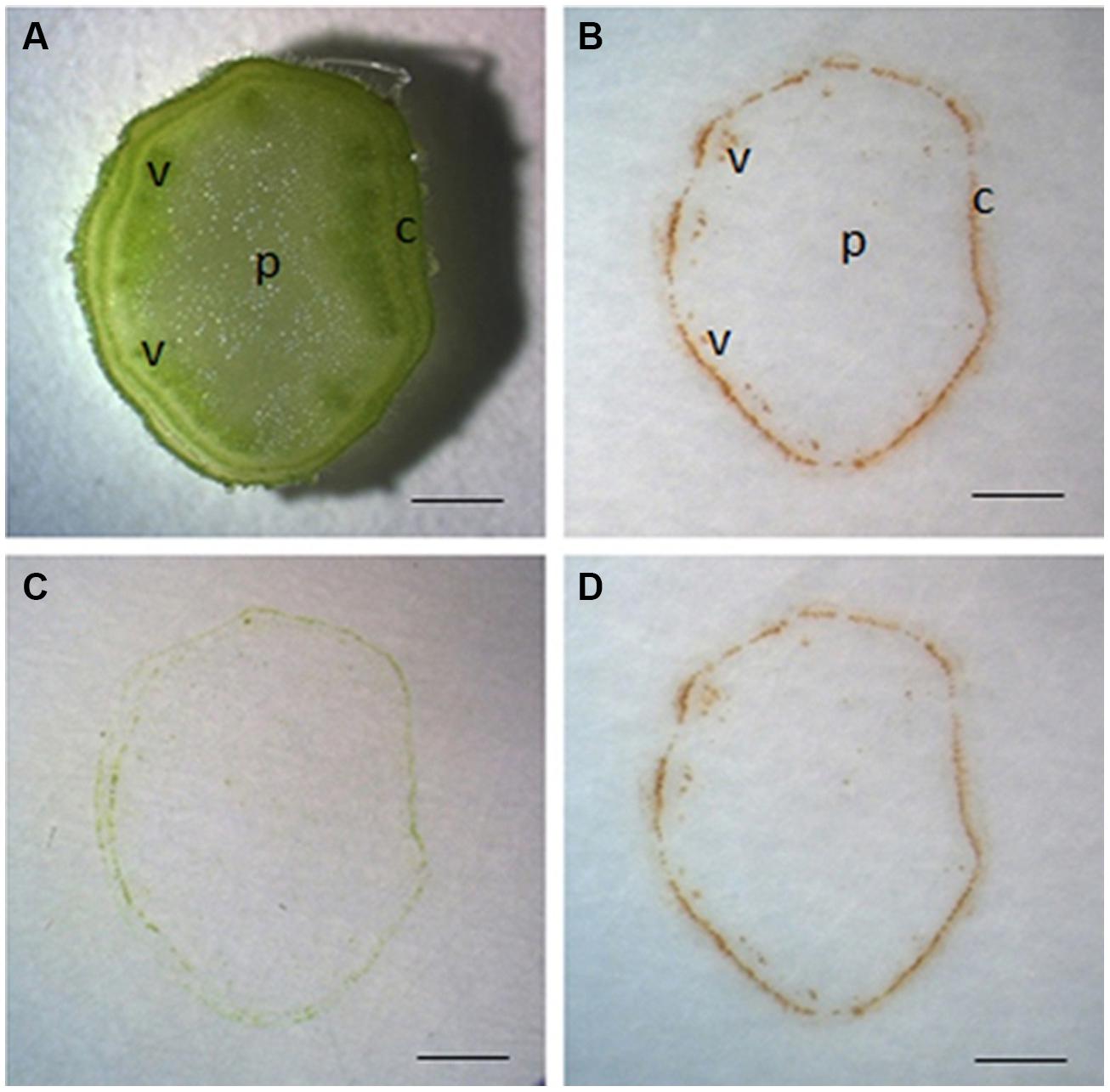
FIGURE 1. Localization of H2O2 by DAB-mediated tissue printing in stems of tomato seedlings. Free-hand sections (1 mm thick) were cut from the middle of tomato stem for tissue printing (A). The sections were pressed for 10 s on nitrocellulose membrane impregnated in 5 mg mL-1 DAB–HCl solution (pH3.8) and photographed immediately (B) or 1 h later (D). To verify the specificity of reaction, the sections were also pressed to membrane impregnated in 5 mg mL-1 DAB–HCl solution (pH3.8) plus 100 mM ascorbic acid (C). Sections used in this figure were consecutive sections from the same stem. c, cortex; p, pith; v, vascular bundle. Scale bar = 1 mm in (A–D).
Wound-Induced H2O2 is Avoided in the Reaction
Previous studies have shown that H2O2 can be rapidly induced by wounding (Olson and Varner, 1993; Miller et al., 2009). To test the possible involvement of wound-induced H2O2 in the current protocol, tissue printing was performed at 0, 1, and 2 min after sectioning using consecutive sections. It was found there was no difference in H2O2 distribution between 0 and 1 min after sectioning, and H2O2 was mainly confined in the cortex and vascular bundles with no signal found in the pith (Figures 2A,B). However, obvious H2O2 signal was produced in the pith when tissue printing was conducted 2 min after sectioning (Figure 2C), indicating the production of wound-induced H2O2. These results indicate that although wound-induced H2O2 accumulated very quickly (within 2 min after cutting), the rapidity of our protocol (within 15 s, see Materials and Methods for details) can completely avoid the interference of wound-induced H2O2.
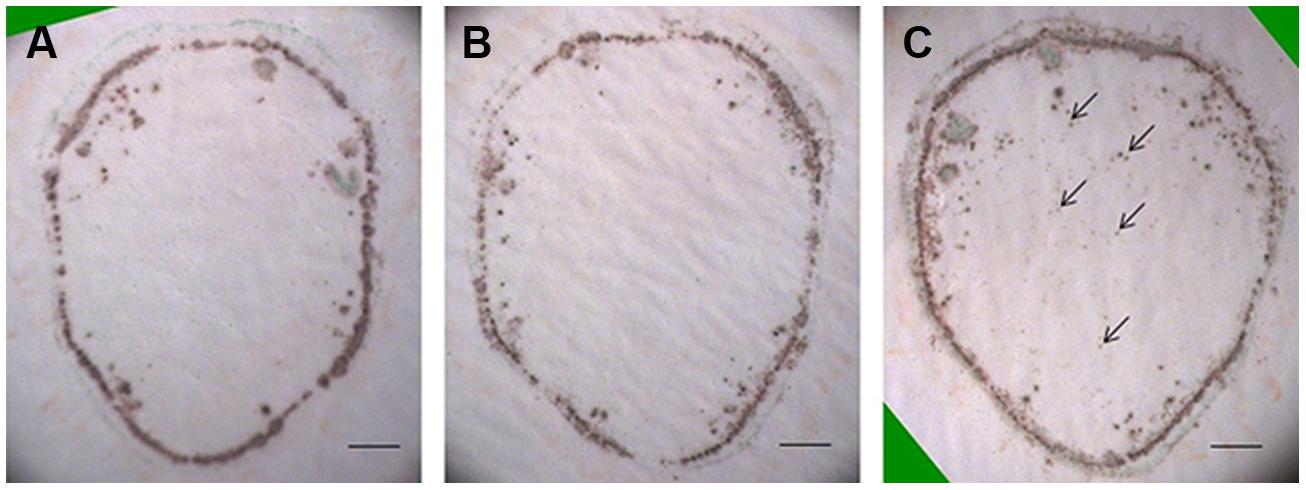
FIGURE 2. Accumulation of wound-induced H2O2 in tomato stem after sectioning. Free-hand sections (1 mm thick) were cut from the middle of tomato stem for tissue printing at 0 (A), 1 (B), and 2 (C) min after cutting. In (A,B), no difference was found in H2O2 distribution pattern. In (C), however, H2O2 was found in pith area (arrows) where no H2O2 was detected in (A,B), indicating the accumulation of wound-induced H2O2 at 2 min after cutting. Sections used in this figure were consecutive sections from the same stem. Scale bar = 1 mm in (A–C).
Endogenous Peroxidase is Sufficient to Support the Reaction
The reaction between DAB and H2O2 relies on the activity of peroxidase (Thordal-Christensen et al., 1997). In our protocol, H2O2 was localized using exogenous DAB and endogenous peroxidase. Undoubtedly, the exogenously supplied DAB used in our protocol was sufficient to support the reaction (Spruce et al., 1987; Thordal-Christensen et al., 1997). However, it is unknown whether endogenous peroxidase activity is enough for the reaction. To address this issue, exogenous H2O2 was introduced by soaking nitrocellulose in DAB solution containing 20 mM H2O2. If the activity of endogenous peroxidase from the stem section is more than enough for the reaction, the color intensity for H2O2 on tissue printing should become stronger in the presence of exogenously supplied H2O2. As expected, H2O2 was found in the cortex but not in the pith in the absence of exogenously supplied H2O2 (Figure 3A). However, H2O2 signal strength increased not only in the cortex but also appeared in the pith when the nitrocellulose membrane was pre-soaked with exogenous H2O2 (Figure 3B). These observations indicate that the activity of endogenous peroxidase was sufficient to support the reaction between endogenous H2O2 and exogenously supplied DAB.
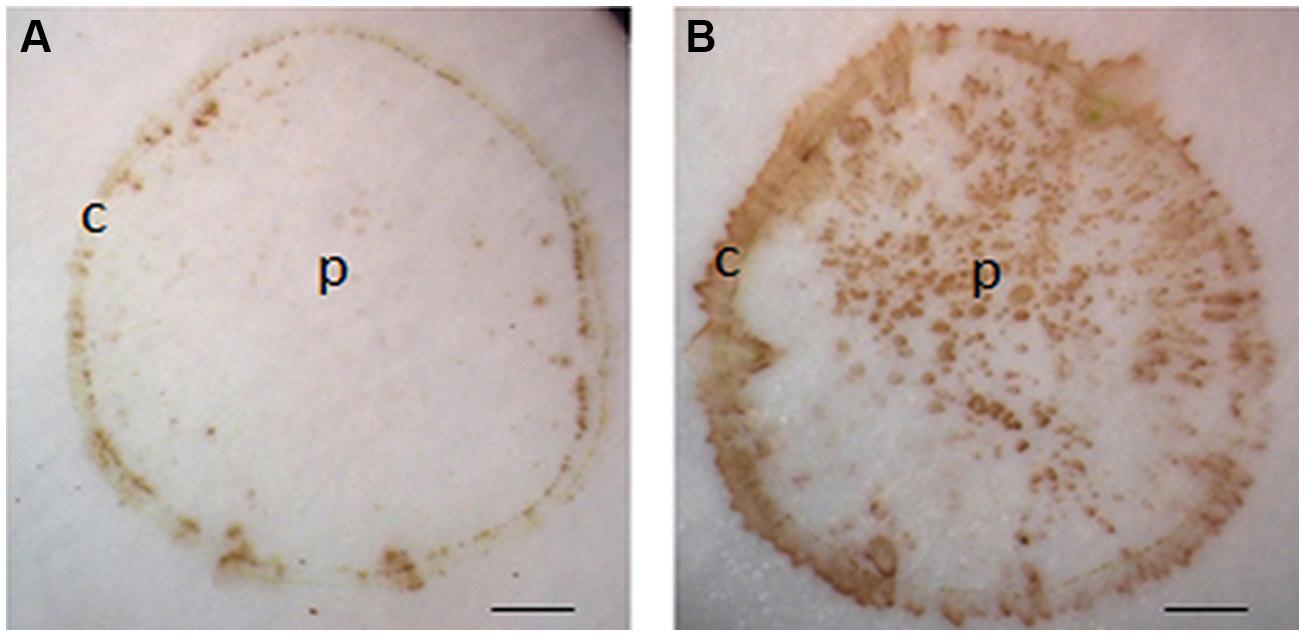
FIGURE 3. Effects of exogenously applied H2O2 on H2O2 signal strength in tomato seedling stems. Tissue printing was conducted on nitrocellulose membranes without (A) and with (B) exogenously supplied 20 mM H2O2. Note, application of exogenously supplied H2O2 increased the intensity of signals in the cortex, and with additional signals apparently detected in the pith region of tomato stem in (B) as compared to that in (A). c, cortex; p, pith; Scale bar = 1 mm in (A,B).
H2O2 Distribution Changes Developmentally in Tomato Stems and Fruits
The production of H2O2 is tightly regulated during plant development. For example, using starch/KI-mediated tissue printing, Schopfer (1994) showed that H2O2 level dramatically increases from the hook region toward the root in 5 days old soybean seedlings. Using the current protocol, we studied H2O2 distribution along the stem of tomato seedlings (Figure 4A). The analyzes revealed that the distribution patterns of H2O2 were strikingly different among the top, middle, and bottom regions of tomato seedling. H2O2 was distributed throughout the whole section at the top of seedlings (Figure 4B). However, the signal strength of H2O2 in pith decreased at the middle and bottom regions of the stem (Figures 4C,D), with H2O2 detected only in the cortex at the bottom area of the stem (Figure 4D). Accompanying the changed distribution pattern, H2O2 content appeared to decrease along stems from the top to the bottom. This observation on H2O2 gradient along tomato stems is contrary to that reported in soybean (Schopfer, 1994). The reason for the discrepancy may lie in different cultivation conditions. The soybean seedlings were grown in darkness (Schopfer, 1994), whereas our tomato seedlings were grown under normal conditions (10/14 h, day/night). To verify the reliability of our observation, H2O2 concentration was measured in tissue extracts at the top, middle, and bottom of tomato seedling stems. It was found that H2O2 content indeed decreased down the stem. H2O2 content at the top of seedling stem was one and two times higher than that at the middle and bottom part, respectively (Figure 5). The consistency between the quantified value (Figure 5) and the localized signal strength in sections (Figure 4) provides further evidence that our DAB-mediated tissue printing is reliable and can semi-quantitatively reflect H2O2 distribution in plant organs.
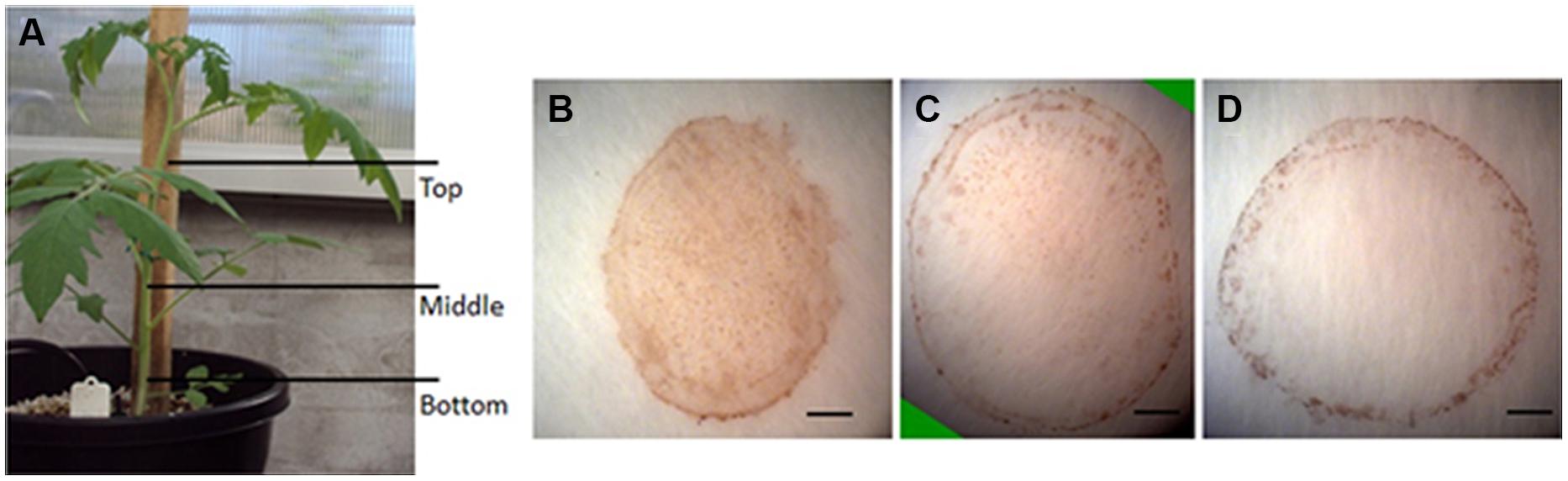
FIGURE 4. Localization of H2O2 in different parts of tomato seedling stems. Stem sections were cut from different positions as indicated in (A) for H2O2 localization at the top (B), middle (C), and bottom (D) of tomato stems using DAB-mediated tissue printing. Scale bars in (B–D) represent 1, 1.2, and 1 mm, respectively.
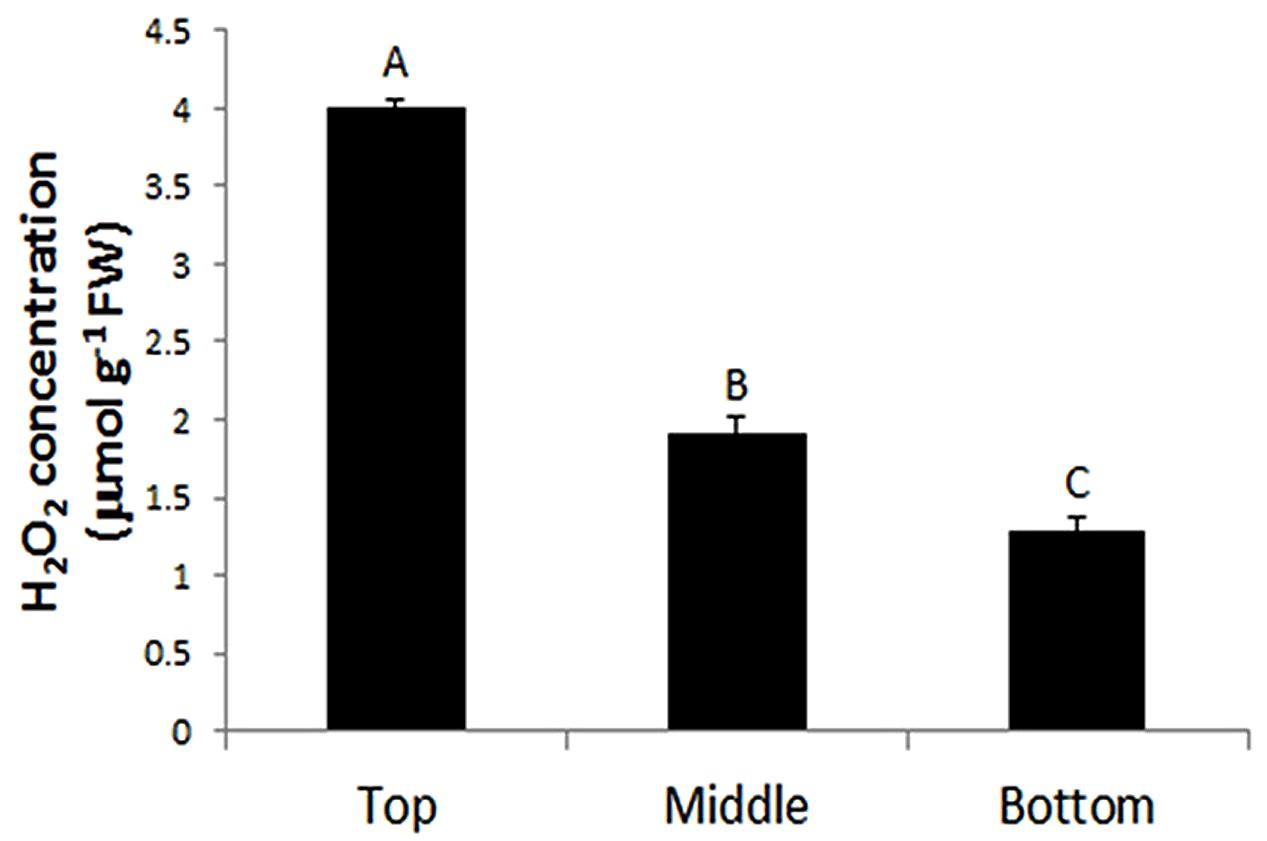
FIGURE 5. Quantification of H2O2 at the top, middle, and bottom regions of tomato seedling stems. Each value is the mean ± SE of four biological replicates (four stem samples from four individual seedlings). Values with different letters indicate significant differences (P ≤ 0.01).
To test if the method is applicable to other large organs, we examined H2O2 localization in tomato fruits at 5 and 10 DAF using DAB-mediated tissue printing. At 5 DAF, H2O2 was detected abundantly and evenly throughout the section (Figure 6A). However, by 10 DAF, H2O2 appeared to be more tissue-specific, in which H2O2 was abundant in pericarp, seed, and septum, and no H2O2 signal was found in columella, locule, and placenta regions (Figures 6B,C). These results suggest that H2O2 distribution is also highly regulated during the development of tomato fruits.
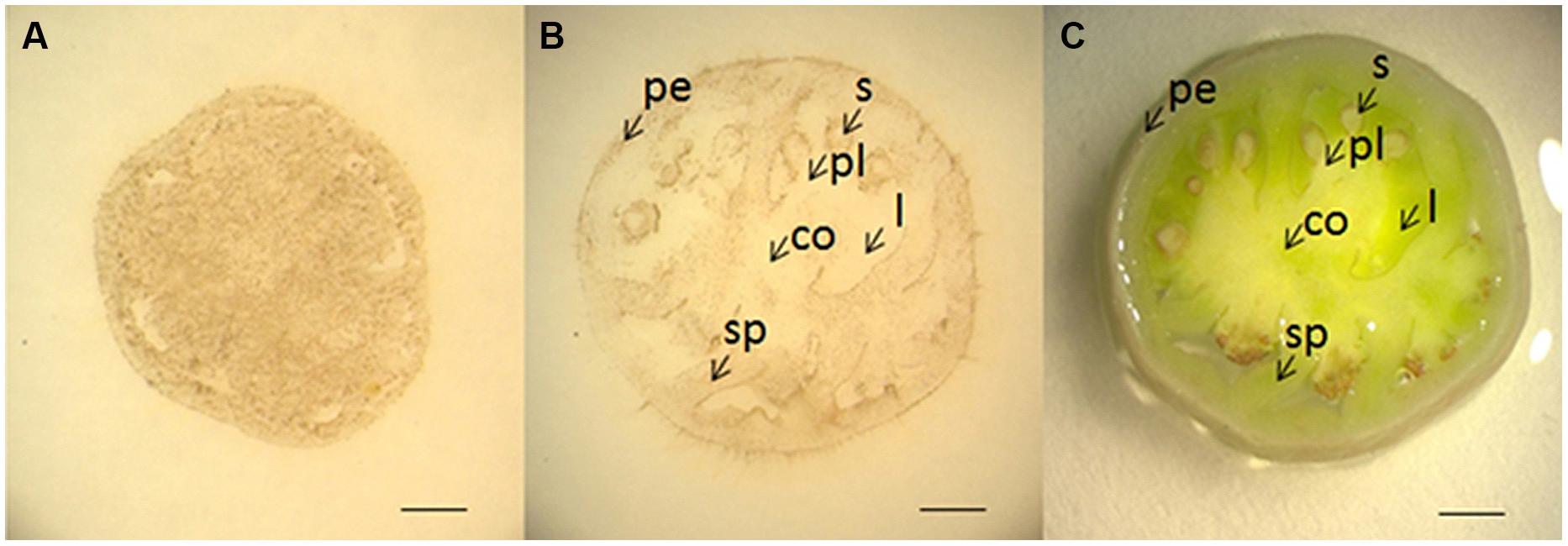
FIGURE 6. Localization of H2O2 in young tomato fruits using DAB-mediated tissue printing. Cross sections (1 mm thick) were cut transversely from the middle of tomato fruits at 5 (A) and 10 (B) DAF. (C) Shows the corresponding picture of fruit section used in (B). co, columella; l, locule; pe, pericarp; pl, placenta; s, seed; sp, septum. Scale bars in (A–C) represent 1, 2, and 2 mm, respectively.
It has been reported that ROS promotes cell division through accelerating auxin-mediated cell cycle entry in alfalfa (Medicago sativa; Fehér et al., 2008). ROS is also involved in the establishment and maintenance of root apical dominance in Arabidopsis (De Tullio et al., 2010) and formation of lateral roots in rice (Chen et al., 2013). Therefore, the higher level of H2O2 in the top of the stem (Figures 4A and 5) and younger fruit (5 DAF; Figure 6A) might be indicative of high activities of cell division in these organs.
In conclusion, localization of H2O2 within large plant organs (e.g., stem and large fruit) other than thin and flat leaves is technically challenging. In this paper, we report the development of a DAB-mediated tissue printing method to localize H2O2 in tomato stem and fruit. The rapidity of our protocol (within 15 s) can effectively avoid the interference of wound-induced H2O2. This represents a major advantage over the protocol reported by Thordal-Christensen et al. (1997) where leaf stripes were incubated in DAB containing solutions for 8 h, inevitably leading to wound-induced H2O2 (Miller et al., 2009; Swanson et al., 2011). Another advantage is that the signal strength of H2O2 from our tissue printing was stably maintained for at least 1 h after tissue printing with no or little background developed. Furthermore, owing to high consistency between the signal intensity of localized H2O2 and its quantified concentration, the protocol can also be used to semi-quantitatively reflect the H2O2 distribution in plant organs. Thus, our protocol is a simple way to specifically, rapidly, and reliably localize H2O2 in large plant organs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was funded, in part, from The University of Newcastle, Australia, as a support to The Australia-China Research Centre for Crop Improvement. Yong-Hua Liu is a recipient of a postgraduate research scholarship from The University of Newcastle. We also thank Joseph Enright for his excellent work in plants cultivation.
References
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bestwick, C. S., Brown, I. R., Bennett, M. H., and Mansfield, J. W. (1997). Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9, 209–221. doi: 10.1105/tpc.9.2.209
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bienert, G. P., Møller, A. L. B., Kristiansen, K. A., Schulz, A.,Møller, I. M., Schjoerring, J. K.,et al. (2007). Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192. doi: 10.1074/jbc.M603761200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cheeseman, J. M. (2006). Hydrogen peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 57, 2435–2444. doi: 10.1093/jxb/erl004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, Y. H., Chao, Y. Y., Hsu, Y. Y., and Kao, C. H. (2013). Heme oxygenase is involved in H2O2-induced lateral root formation in apocynin-treated rice. Plant Cell Rep. 32, 219–226. doi: 10.1007/s00299-012-1356-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Choudhury, S., Panda, P., Sahoo, L., and Panda, S. K. (2013). Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 8, e23681. doi: 10.4161/psb.23681
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Tullio, M. C., Jiang, K., and Feldman, L. J. (2010). Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiol. Bioch. 48, 328–336. doi: 10.1016/j.plaphy.2009.11.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Essmann, J., Schmitz-Thom, I., Schön, H., Sonnewald, S., Weis, E., and Scharte, J. (2008). RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 147, 1288–1299. doi: 10.1104/pp.108.121418
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fehér, A., Ötvös, K., Pasternak, T. P., and Szandtner, A. P. (2008). The involvement of reactive oxygen species (ROS) in the cell cycle activation (G0-to-G1 transition) of plant cells. Plant Signal. Behav. 3, 823–826. doi: 10.4161/psb.3.10.5908
Macarisin, D., Cohen, L., Eick, A., Rafael, G., Belausov, E., Wisniewski, M.,et al. (2007). Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 97, 1491–1500. doi: 10.1094/PHYTO-97-11-1491
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miller, G., Schlauch, K., Tam, R., Cortes, D., Torres, M. A., Shulaev, V.,et al. (2009). The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2, ra45. doi: 10.1126/scisignal.2000448
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nagasawa, N., Hibara, K. I., Heppard, E. P., Vander Velden, K. A., Luck, S., Beatty, M.,et al. (2013). GIANT EMBRYO encodes CYP78A13, required for proper size balance between embryo and endosperm in rice. Plant J. 75, 592–605. doi: 10.1111/tpj.12223
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Neves, C., Sá, M. C., and Amâncio, S. (1998). Histochemical detection of H2O2 by tissue printing as a precocious marker of rhizogenesis in grapevine. Plant Physiol. Bioch. 36, 817–824. doi: 10.1016/S0981-9428(99)80019-9
Olson, P., and Varner, J. (1993). Hydrogen peroxide and lignification. Plant J. 4, 887–892. doi: 10.1046/j.1365-313X.1993.04050887.x
Petrov, V. D., and Van Breusegem, F. (2012). Hydrogen peroxide—a central hub for information flow in plant cells. AoB plants pls014. doi: 10.1093/aobpla/pls014
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Przymusiński, R., Rucińska-Sobkowiak, R., Ilska, B., and Gwóźdź, E. A. (2007). Organospecific responses of lupin seedlings to lead localization of hydrogen peroxide and peroxidase activity. Acta Physiol. Plant 29, 411–416. doi: 10.1007/s11738-007-0049-y
Queval, G., Hager, J., Gakière, B., and Noctor, G. (2008). Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J. Exp. Bot. 59, 135–146. doi: 10.1093/jxb/erm193
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Razem, F. A. (2008). An overview of hydrogen peroxide production and cellular determination in plants. Hebron University Res. J. 3, 84–96.
Reth, M. (2002). Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 3, 1129–1134. doi: 10.1038/ni1202-1129
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Romero-Puertas, M. C., Rodríguez-serrano, M., Corpas, F. J., Gómez, M., Del Río, L. A., and Sandalio, L. M. (2004). Cadmium-induced subcellular accumulation of O2middot- and H2O2 in pea leaves. Plant Cell Environ. 27, 1122–1134. doi: 10.1111/j.1365-3040.2004.01217.x
Salzer, P., Corbière, H., and Boller, T. (1999). Hydrogen peroxide accumulation in Medicago truncatula roots colonized by the arbuscular mycorrhiza-forming fungus Glomus intraradices. Planta 208, 319–325. doi: 10.1007/s004250050565
Schopfer, P. (1994). Histochemical demonstration and localization of H2O2 in organs of higher plants by tissue printing on nitrocellulose paper. Plant Physiol. 104, 1269–1275.
Spruce, J., Mayer, A. M., and Osborne, D. J. (1987). A simple histochemical method for locating enzymes in plant tissue using nitrocellulose blotting. Phytochemistry 26, 2901–2903. doi: 10.1016/S0031-9422(00)84559-8
Suzuki, N., Koussevitzky, S., Mittler, R., and Miller, G. (2012). ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 35, 259–270. doi: 10.1111/j.1365-3040.2011.02336.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Swanson, S. J., Choi, W. G., Chanoca, A., and Gilroy, S. (2011). In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant Biol. 62, 273–297. doi: 10.1146/annurev-arplant-042110-103832
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thordal-Christensen, H., Zhang, Z. G., Wei, Y. D., and Collinge, D. B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11, 1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x
Van Breusegem, F., Bailey-Serres, J., and Mittler, R. (2008). Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol. 147, 978–984. doi: 10.1104/pp.108.122325
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: fruits and stems, hydrogen peroxide, localization, tissue printing, reactive oxygen species
Citation: Liu Y-H, Offler CE and Ruan Y-L (2014) A simple, rapid, and reliable protocol to localize hydrogen peroxide in large plant organs by DAB-mediated tissue printing. Front. Plant Sci. 5:745. doi: 10.3389/fpls.2014.00745
Received: 10 October 2014; Accepted: 06 December 2014;
Published online: 22 December 2014.
Edited by:
Jirong Huang, University of Tokyo, JapanReviewed by:
Vasileios Fotopoulos, Cyprus University of Technology, CyprusHan Xiao, Shanghai Institutes for Biological Sciences – Chinese Academy of Sciences, China
Copyright © 2014 Liu, Offler and Ruan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Ling Ruan, Australia–China Research Centre for Crop Improvement and School of Environmental and Life Sciences, The University of Newcastle, Callaghan, NSW 2308, Australia e-mail: yong-ling.ruan@newcastle.edu.au
 Yong-Hua Liu
Yong-Hua Liu Christina E. Offler
Christina E. Offler Yong-Ling Ruan
Yong-Ling Ruan