- Department of Plant Pathology, University of California, Davis, Davis, CA, USA
Branched β-1,3-glucans and the eicosapolyenoic acids (EP) are among the best characterized oomycete elicitors that trigger innate immune responses in plants. These elicitors were identified over three decades ago, and they were useful in the study of the sequence of physiological, biochemical and molecular events that induce resistance in plants. However, in spite of the cross-kingdom parallels where these molecules are well-characterized as immune system modulators in animals, their perception and modes of action in plants remains obscure. Oomycetes are among the most important plant pathogens, responsible for diseases that devastate crops, ornamentals, and tree species worldwide. With the recent interest and advances in our understanding of innate immunity in plants, and the redefining of many of the classical elicitors as microbe-associated molecular patterns (MAMPs), it seems timely and important to reexamine β-glucans and EP using contemporary approaches. In this review, we highlight early studies of β-glucans and EP, discuss their roles as evolutionarily conserved signals, and consider their action in relation to current models of MAMP-triggered immunity.
Introduction
Over 30 years ago branched β-1→3-glucans and the EP – AA and EPA – were characterized as potent oomycete elicitors of innate immune responses in plants. These and the Phytophthora elicitin proteins with activities in a somewhat narrower host range (Tyler, 2002) figured prominently in the literature in subsequent years, and were used to examine physiological, biochemical and molecular events associated with the HR and induced resistance. Intriguing is that β-glucans and EP are important in modulating innate immunity and inflammation in animals, although these cross-kingdom parallels are likely not fully appreciated by the plant and animal research communities.
Oomycetes are among the most important plant pathogens, responsible for devastating plant diseases worldwide. New Phytophthora species, in particular, are continually being discovered, with the number of species identified nearly double that of only a decade ago (Hansen et al., 2012; Kroon et al., 2012). Downy mildew pathogens and the diseases they cause are also current threats to U.S. and world agriculture, with two listed as Select Agents as serious threats to U.S. agriculture (http://www.selectagents.gov). The Phytophthora research community is attuned to the need and urgency to develop novel control strategies that are broadly applicable yet sustainable, with vigorous research programs studying population genetics, genomics, effector biology, host resistance, and disease epidemiology and management. Within this research portfolio, determining how β-glucans and EP are perceived and act in plants could be useful for enhancing disease resistance against oomycetes and possibly other attackers. In this review, we highlight early studies of β-1→3-glucans and EP, discuss their roles as evolutionarily conserved signals, and consider their action in relation to current models of MAMP1-triggered immunity.
Eicosapolyenoic Acids
Arachidonic acid (AA; 20:4 Δ5,8,11,14) and eicosapentaenoic acid (EPA; 20:5 Δ5,8,11,14,17) are 20-carbon, all-cis PUFAs containing four and five double bonds, respectively (Figure 1). In mammals, AA and EPA undergo enzymatic oxidation to oxylipins, referred to as eicosanoids, which serve crucial signaling functions in stress responses (Blee, 2002; Bostock et al., 2011). Examples of these eicosanoids include prostaglandins and thromboxanes, formed via the action of cyclooxygenases, and leukotrienes, formed via the action of LOXs. Eicosanoid-mediated stress responses include pain, inflammation and fever (prostaglandins), platelet aggregation and vasoconstriction (thromboxanes), and allergic responses and asthma (leukotrienes; De Caterina and Basta, 2001; Murakami, 2011). Although higher plants do not contain AA and EPA, AA and EPA are found in oomycete pathogens and plants are exposed to these fatty acids during infection (Walley et al., 2013).
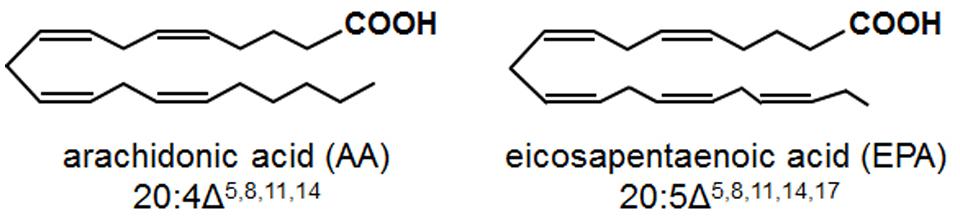
FIGURE 1. Chemical structures of the eicosapolyenoic acids, arachidonic acid, and eicosapentaenoic acid.
Many molecules of microbial pathogens identified as elicitors in earlier studies have been reclassified as MAMPS to conform to terminology used in animal immunity. MAMPs are motifs in essential molecules such as proteins, lipids, and polysaccharides that are present in entire classes of microbes (pathogenic or non-pathogenic). These molecular motifs are generally absent from hosts and can be recognized by plants and animals, such as in response to attempted infection or colonization. Defense responses induced by MAMPS in plants are referred to as PTI (Nurnberger et al., 2004; Boller and Felix, 2009; Zipfel and Robatzek, 2010). Studies of PTI have focused on the bacterial peptides flagellin and EF-Tu and their action in Arabidopsis. These peptides are perceived by PRRs, receptor-like kinases that are crucial for perception of flagellin/EF-Tu and activation of PTI. However, unlike flagellin and EF-Tu, many of the historical elicitors that stimulate well-characterized defense responses in plants have not been sufficiently investigated to resolve their modes of action (Nurnberger et al., 2004; Boller and Felix, 2009; Nguyen et al., 2010; Zipfel and Robatzek, 2010). The elicitors AA and EPA conform to the definition of MAMPs: they are not present in higher plants, are essential components in oomycete cells, are largely absent from other classes of microbes, and elicit similar defense responses in plant species where they have been studied (Tyler, 2002; Bostock et al., 2011; Walley et al., 2013).
Eicosapolyenoic acid elicitor activity in plants was first discovered in the interaction between Phytophthora infestans and potato. Mycelial extracts of P. infestans induced sesquiterpenoid phytoalexins, lignin deposition and cell death in potato tissue in a reaction similar to a HR to incompatible races of the pathogen. Purification and analysis of all active fractions in these extracts identified AA and EPA, without exception, either free or esterified to other molecules (Bostock et al., 1981, 1982). Elicitation was specific to AA and EPA. Treatment with 15 other fatty acids, including LA (18:2Δ9,12) and ALA (18:3Δ9,12,15), the primary unsaturated fatty acids found in higher plants (Kachroo and Kachroo, 2009), as well as structurally similar eicosatrienoic acid (20:3Δ11,14,17) and arachidonyl alcohol, did not elicit defense responses. Treatment of tuber disks with AA also protected them from subsequent P. infestans infection (Bostock et al., 1981, 1982).
EP-Induced Resistance Against Pathogens and Phytohormone Defense Signaling
Eicosapolyenoic acids induce systemic resistance in potato as well as in other plant species to various pathogens. Although the mechanisms remain unresolved, EP have been shown to elicit SA, JA, and ET in different experimental systems. Colonization of avocado seedling roots by P. cinnamomi was reduced in roots treated with AA prior to inoculation (Romero-Correa et al., 2014). Pearl millet seedlings were protected to a greater degree against infection by the downy mildew pathogen, Sclerospora graminicola, following seed treatment with AA or EPA, in contrast to seedlings emerging from seeds treated with LA, ALA, DHA or water (Amruthesh et al., 2005).
EP elicit SAR or SAR-like responses in tobacco, potato, and tomato. Treatment of lower leaves of tobacco plants with AA induced local and SAR to TMV (Rozhnova et al., 2003). EP treatment of the lower leaves of potato plants protected the upper leaves from infection by P. infestans, a systemic resistance that developed within 5 days of the inducing treatment (Cohen et al., 1991). Plants treated with LA, ALA, or oleic acid displayed partial protection but not to the level of EP-treated plants. AA also induced resistance in potato leaves to the early blight pathogen, Alternaria solani, with levels of SA and a PR1-like protein elevated in the AA-treated leaves (Coquoz et al., 1995). AA-treatment of tomato leaves induced localized accumulation of transcripts for P4 (Fidantsef et al., 1999), a PR-1 family member and SAR marker in tomato (Van Kan et al., 1992), but did not induce expression of the proteinase inhibitor gene PI-2. The latter is strongly induced by wounding and JA treatment and serves as a marker for JA-mediated resistance in tomato (Fidantsef and Bostock, 1998; Fidantsef et al., 1999).
Although the studies in tobacco, potato, and tomato indicate that EP-induced resistance may operate through SA, recent research suggests EP action is more complex (Savchenko et al., 2010). Treatment of tomato and Arabidopsis leaves with AA increased JA levels, reduced SA levels, and increased resistance to Botrytis cinerea. Arabidopsis plants transformed to produce small amounts of EPs (named EP plants) were less susceptible to P. capsici, B. cinerea, and feeding by aphids. However, these plants were more susceptible to Pseudomonas syringae pv. tomato (DC3000). The EP plants had constitutively elevated levels of JA and JA-marker gene expression and reduced levels of SA and SA-marker gene expression relative to wild-type plants. The differential effect of EP on disease and pest outcomes corresponds to EP’s impact on SA and JA defense signaling, and this effect is dependent upon JA as demonstrated with a JA-deficient aos mutant line (Savchenko et al., 2010).
Salicylic acid and JA can be mutually antagonistic (Bostock, 2005), making it difficult to reconcile these different findings. AA treatment elicits ET production in both pepper and potato (Bostock et al., 1986; Garcia-Pineda and Lozoya-Gloria, 1999), and ET can modulate SA- and JA-defense networks (Pieterse et al., 2012). The different experimental outcomes may result in part from differences in EP concentrations used in the various studies. Higher concentrations of EP can induce an intense, localized necrosis at the site of application, particularly in solanaceous plants. This strong phenotype could trigger or result from phytohormone changes different from those induced by low concentrations. Also, it is possible that all three phytohormones (SA, JA, and ET) are important in establishing EP-induced resistance through a process of transitional signaling (Truman et al., 2010). A study in potato indicates that both SA and JA are important in PTI responses (Halim et al., 2009), and a study of PTI in Arabidopsis using signal allocation analysis of mutants deficient in ET, SA, and JA signaling indicated that PTI depends on synergy among ET, SA, and JA (Tsuda et al., 2009). Further research is needed to fully elucidate the interactions among SA, JA, and ET in their involvement in EP-induced resistance and defense responses.
Phytoalexin Induction
Eicosapolyenoic acids have been useful in dissecting aspects of secondary metabolism in plants, with a focus on sesquiterpenoid phytoalexins in solanaceous plants. However, EP elicits production of defense metabolites in other plant families as well. The isoflavanoid phytoalexins phaseollin and coumestrol accumulate in leaves of French bean following infiltration with AA (Longland et al., 1987). Phenol-2,4-bis (1,1-dimethylethyl), a defense compound in avocado, is induced in roots treated with AA as well as with SA (Romero-Correa et al., 2014). Among solanaceous plants EP elicit sesquiterpenoid phytoalexin synthesis in thorn-apple, eggplant, chili pepper, green pepper, potato, and tomato (Bloch et al., 1984; Whitehead et al., 1990; Hoshino et al., 1994; Castoria et al., 1995; Garcia-Pineda and Lozoya-Gloria, 1999). In potato tuber, AA elicits sesquiterpenoid phytoalexin biosynthesis with strong expression of sesquiterpene cyclase, a committed step in the pathway. Concurrent with this is a complete suppression of wound-induced squalene synthase and steroid glycoalkaloid accumulation (Tjamos and Kuć, 1982; Zook and Kuć, 1991). HMGR catalyzes the first step in the synthesis of stress-induced isoprenoids from mevalonate in potato. Three isoforms of HMGR are differentially induced by wounding and AA treatment (Choi et al., 1992), and a similar expression pattern of the corresponding HMGR isoforms occurs in tomato (Rodriguez-Concepcion and Gruissem, 1999).
Generation of Reactive Oxygen Species/Programmed Cell Death
In addition to potato, EP have been shown to elicit PCD, characteristic of the HR, in other plant species. Pearl millet seedlings treated with AA displayed a HR similar to that induced by the oomycete, S. graminicola, the causal agent of downy mildew. Following treatment with AA, the HR developed more quickly in pearl millet seedlings with genotypes rated as resistant versus susceptible to S. graminicola (ratings were based on field studies; Geetha et al., 1996). Tomato protoplasts treated with AA underwent PCD with characteristic DNA fragmentation and laddering, while LA and ALA treatment had no PCD-inducing effects (Knight et al., 2001).
In both potato and pepper, AA was found to induce ROS in a similar manner. AA treatment of potato tuber disks elicited a biphasic oxidative burst (generation of ROS) peaking at 1 and 6–9 h after treatment and increased expression of StRBOHB, a homolog of gp91(phox), which encodes a subunit within the neutrophil NADPH oxidase complex (Yoshioka et al., 2001). As in potato, treatment of pepper fruit with AA elicited an immediate, rapid ROS burst. When DPI, an inhibitor of NADPH-dependent oxidases, was applied to the fruit prior to application of AA, ROS generation decreased as the concentration of DPI was increased (Araceli et al., 2007).
How Do AA and EPA Elicit Defense Responses?
The mode of action of EP in PTI is unresolved, although the structural requirements of EP as elicitors are well characterized. These include at least a 20 carbon backbone with all cis-1,4-pentadiene unsaturation beginning at the Δ5 position and at least four double bonds in the chain (Bostock et al., 1981, 1982; Preisig and Kuć, 1985; Savchenko et al., 2010). While this specificity could provide evidence for involvement of a receptor that recognizes these structural features, previous studies of EP in potato indicate that initial perception by plant cells may be quite different than other MAMPs. Initial recognition of AA and EPA may occur by specific disruption of host membrane integrity and/or perturbation of oxylipin metabolism, with the possibility that plant cells produce novel oxylipins from EP (Bostock et al., 1992; Ricker and Bostock, 1992, 1994; Fidantsef and Bostock, 1998). Studies in potato showed that U-14C radiolabeled AA was quickly incorporated into neutral lipids (mono-, di-, and tri-glycerides) and polar lipids (glycolipids and phospholipids). A small fraction, ∼2–5% of the AA, was oxidized (Preisig and Kuć, 1988; Ricker and Bostock, 1992). Also, sporangia of P. infestans readily incorporated exogenous 14C-AA into phospholipids (primarily), diglycerides and TGs. By 12–14 h after inoculation, microautoradiographic studies revealed that the radioactivity from sporangia was released into the epidermal and palisade mesophyll cells adaxial to the inoculated leaf surface and distant from fungal structures (Ricker and Bostock, 1992). Plant phospholipases are activated following attack by pathogens or treatment of plants with elicitors (Bostock, 1989; Canonne et al., 2011). This could create an opportunity for any EP incorporated into plant lipids during infection to be released and accessible to plant oxylipin enzymes.
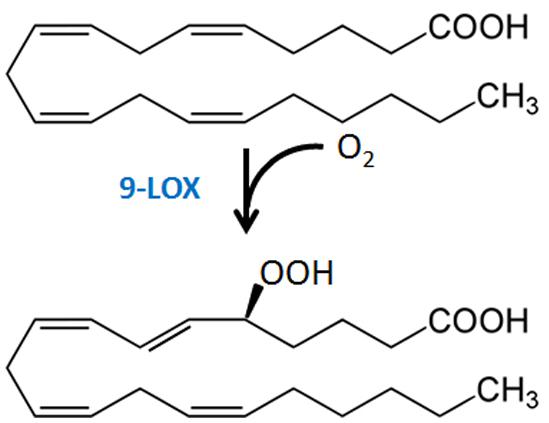
Research in potato and tomato indicates that the 9-LOX pathway may play an important role in EP action. The first step in the enzymatic formation of phyto-oxylipins involves the action of LOX (Figure 2). Plant LOXs act on PUFA containing a cis-(1,4)-pentadiene system, inserting an oxygen molecule (O2) to form hydroperoxy fatty acids. These are further metabolized to various oxylipin families by members of CYP74 cytochrome P450s: AOSs, HPLs, and DESs, or by less well-characterized POX or PXG and EASs (Blee, 2002; Feussner and Wasternack, 2002; La Camera et al., 2004; Kachroo and Kachroo, 2009; Mosblech et al., 2009).
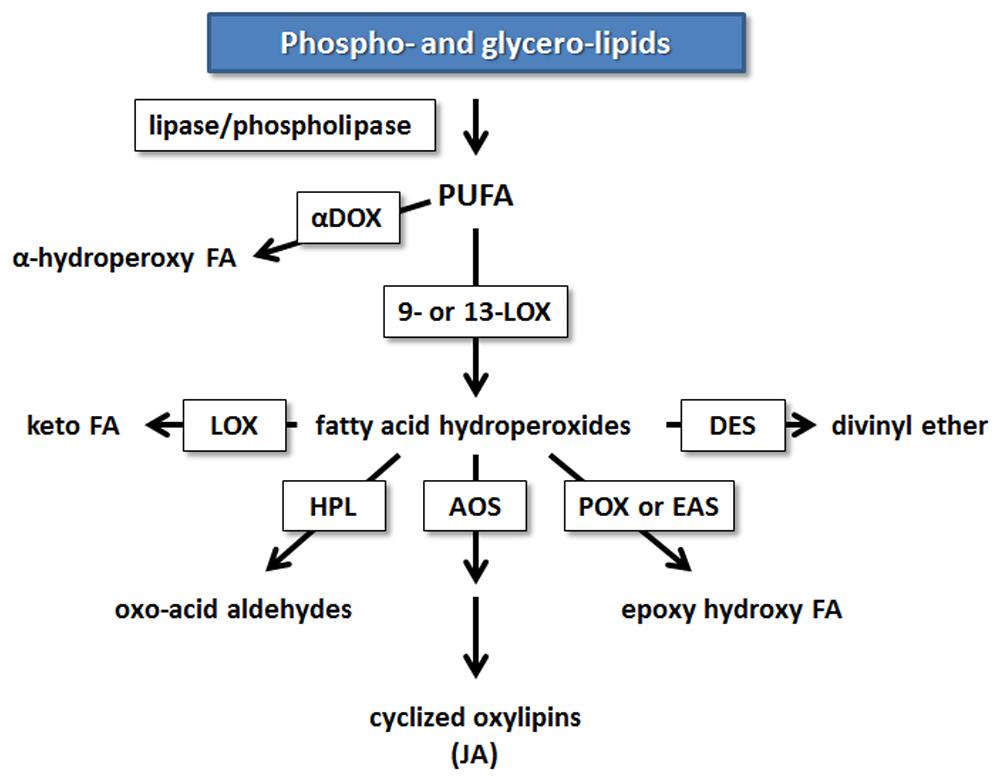
FIGURE 2. Enzymatic mechanisms leading to the synthesis of oxylipins in plants from PUFA. Enzymes involved in oxylipin metabolism are boxed. AOS, allene oxide synthase; αDOX, α-dioxygenase; DES, divinyl ether synthase; EAS, epoxy alcohol synthase; FA, fatty acid; HPL, hydroperoxide lyase; HPR, hydroperoxide reductase; JA, jasmonic acid; LOX, lipoxygenase; POX, peroxygenase [modified from (Shah, 2005)].
The importance of LOX, in particular a 9-LOX2, in EP elicitor activity is supported by fatty acid structure-activity requirements and studies of LOX expression. The carboxyl function of EP is critical, a feature consistent with the substrate requirement of plant LOXs (Preisig and Kuć, 1985; Feussner and Wasternack, 2002). A Δ5 double bond at the beginning of a methylene-interrupted series with at least four double bonds provides the highest elicitor activity (Bostock et al., 1981, 1982; Preisig and Kuć, 1985; Feussner and Wasternack, 2002). AA stimulates LOX expression in potato and tomato (Bostock et al., 1992; Robinson et al., 2014), with 5-HPETE (Figure 3) a principal LOX product formed after treatment of tissue with AA (Ricker and Bostock, 1994; Robinson et al., 2014). Expression of pLOX1, a potato LOX gene now identified as a 9-LOX type 1 (Andreou et al., 2009), was strongly induced in AA-treated and P. infestans-inoculated potato tuber disks and leaves (Fidantsef and Bostock, 1998), as was a tomato LOX in AA-treated tomato leaves (Fidantsef et al., 1999). LA-treatment did not induce pLOX1 expression or LOX activity. Heat treatment of tuber disks inactivates enzyme activity and abolishes HPETE formation following AA treatment (Ricker and Bostock, 1994), and EP-induced responses are strongly diminished when LOX activity is inhibited or absent (Preisig and Kuć, 1987; Vaughn and Lulai, 1992). Nonetheless, definitive experiments with LOX knock-out/knock-down or overexpression lines to critically test specific LOX isoforms in EP action have not been reported. While it has been proposed and is quite likely that the 9-oxylipin pathway metabolites of AA may directly act as signal molecules to activate defense responses (Regdel et al., 1994), AA and/or its metabolites may also induce expression and activity of oxylipin pathway enzymes to form biologically active metabolites from the plant LA and ALA pools.
Studies during the past 15 years in solanaceous plants point to the importance of 9-LOX and the 9-oxylipin pathway in defense, and have demonstrated that the 9-LOXs from potato, tobacco, and pepper can utilize AA as a substrate. Many of these studies have investigated defense responses against oomycete pathogens or used elicitor preparations from oomycetes likely containing EP (Fournier et al., 1993; Veronesi et al., 1996; Gobel et al., 2001, 2002; Andreou et al., 2009; Hwang and Hwang, 2010). 9-hydroperoxy fatty acids can be utilized by downstream oxylipin pathway enzymes to form other compounds that have been found to function in defense. In particular, DESs are induced in response to elicitors and pathogen attack in several solanaceous species including potato, tobacco, and pepper (Weber et al., 1999; Stumpe et al., 2001; Fammartino et al., 2007; Gullner et al., 2010). DESs are CYP74D P450s that produce the divinyl ethers CA from 9-HPOD and CnA from 9-HPOT.
Recent experiments indicate that treatment of tomato roots with EP induces resistance against P. capsici. Hydroponically grown tomato plants whose roots were treated with EP and subsequently inoculated with P. capsici experience significantly less rot and collapse at the crowns than plants whose roots were treated with H2O, LA, or ALA, indicating that exposure of tomato roots to EP prior to inoculation with P. capsici reduces susceptibility of the plants to P. capsici (Roberts et al., 2013). Further experiments demonstrate that roots and crowns display significantly increased lignification responses following root treatment with AA and EPA and subsequent inoculation with P. capsici compared to roots treated with H2O, LA, and ALA. AA-treatment of tomato roots elicits increased expression of 9-LOX and 9-DES genes in tomato roots compared to control treatments (LA and H2O). Expression of 9-DES is also increased following inoculation of roots with P. capsici (Robinson et al., 2014).
In conclusion, although EP action in plants is complicated, evidence supports an important role for LOX and likely a 9-oxylipin pathway in the initiation of plant responses. Furthermore, in Arabidopsis an intact JA pathway is required for AA activity, implicating a 13-LOX. Whether DES and divinyl ethers participate in the plant response to EP observed in solanaceous plants is unresolved, although ongoing research in our laboratory will address this issue. The search for a traditional PRR for EP in plant cells analogous to those for other MAMPs, although intriguing, may not be productive given other mechanisms for rapid uptake of PUFA by plant cells and their entry into oxylipin metabolism.
β-Glucans and Related Oligosaccharins in Plant Immunity
β-linked glucose polysaccharides are the most abundant component of Phytophthora cell walls, comprising more than 80% of the wall dry weight (Bartnicki-Garcia and Wang, 1983). These include insoluble β-1→4-linked (cellulosic) and β-1→3, β-1→6-linked glucans, with the latter by far the more abundant of these polymers. In addition to the abundance of glucose, compositional analyses of cell walls also reveal minor amounts of mannose and glucosamine, as well as protein and lipid similar to levels found in cell walls of fungi. In addition to the insoluble glucans, soluble β-1→3-linked glucans are present at various developmental stages in the oomycete life cycle. For example these can be found in the germination fluids of cystospores as well as other stages, and during synthesis and remodeling of the wall during growth, thus making them potentially available at the host–pathogen interface during infection (Doke et al., 1980; Waldmuller et al., 1992). Laminarans are linear β-1→3-linked glucans that provide the dominant storage carbohydrate in Phytophthora and other oomycetes, as well as other stramenopiles (Bartnicki-Garcia and Wang, 1983).
The β-1→3, β-1→6-linked glucans present a very complex array of possible structures, some with well-established activity in modulating plant innate immunity. The most prominent example is the elicitor activity associated with glucans isolated from cultures and cell walls of the soybean pathogen P. sojae (formerly P. megasperma f. sp. glycinea). Albersheim et al. (1983) showed that these were potent inducers of the flavonoid phytoalexin, glyceollin, and related defense reactions in soybean cotyledons. β-glucan oligosaccharide fractions of varying complexity had elicitor activity suggesting a model whereby cell wall fragments released during infection provide the physiological triggers of the plant defense response. The smallest active fragment following partial acid hydrolysis of P. sojae cell walls was purified and shown to be a hexa (β-D-glucopyranosyl)-D-glucitol. This oligosaccharide and its corresponding unreduced hepta-β-glucoside elicited at concentrations between 10-7 to 10-9 M (Sharp et al., 1984; Figure 4). Subsequent work by Michael Hahn and coworkers further defined the branched β-1→3, β-1→6 structural motif essential to maximally induce phytoalexin accumulation (Cheong et al., 1991) and found that the hepta-β-glucoside specifically bound to soybean membranes with high affinity (Cheong and Hahn, 1991). These investigators provided strong evidence that the binding activity was associated with a membrane protein or glycoprotein. Subsequent efforts by other laboratories identified hydrophobic membrane proteins that bind β-glucans with high affinity from soybean (Cosio et al., 1992; Umemoto et al., 1997; Mithofer and Ebel, 1999) and other legumes (Mithofer et al., 1999). Reconstitution of the soybean homolog in lipid vesicles strongly bound the hepta-β-glucoside (Kd = 6–7 nM, with even higher affinities reported in other studies), which could be displaced by glucans with different degrees of polymerization in competitive binding assays.
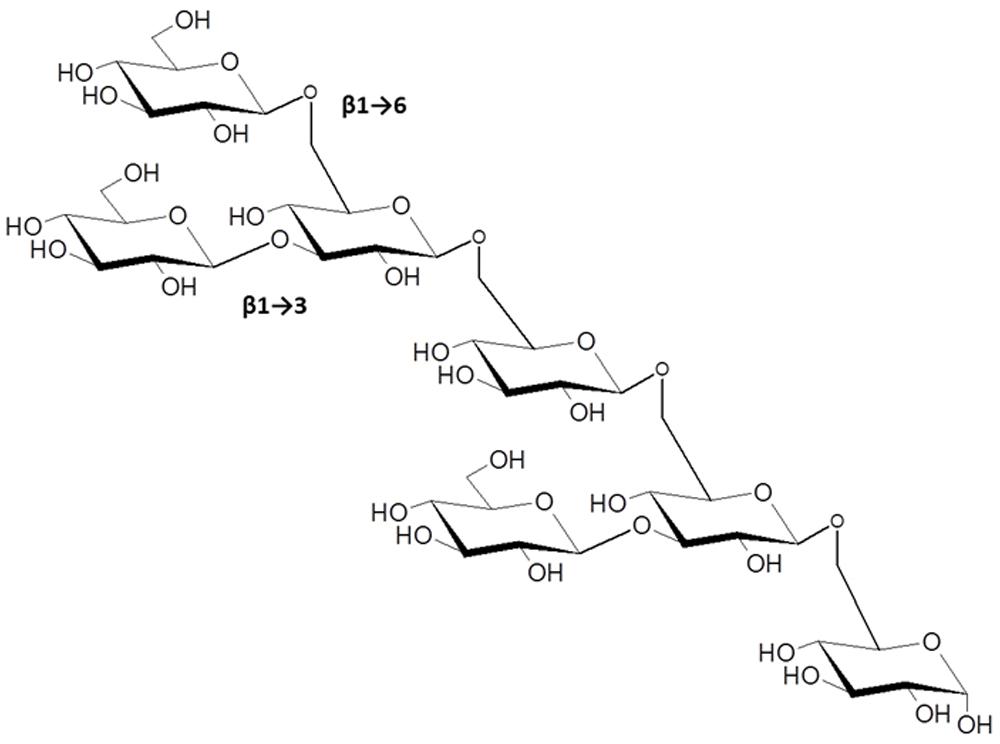
FIGURE 4. The β-1→3, 1→6-linked hepta-β-glucoside from Phytophthora sojae, with potent elicitor activity in members of the Fabaceae (from Cheong et al., 1991). Other β-1→3, 1→6-glucans with higher degrees of polymerization have immunomodulatory functions in plant-microbe interactions as discussed in the text.
The elicitor activity and high affinity binding of the hepta-β-glucoside and related β-glucans are limited to members of the Fabaceae (Ebel, 1998; Fliegmann et al., 2004). Biochemical purification and additional studies indicate the binding proteins from legumes constitute a family of proteins of different sizes (75–150 kDa; Ebel, 1998), with different carbohydrate active domains, one that binds β-glucans and another with glucanase activity capable of releasing elicitor-active fragments from Phytophthora cell walls (Fliegmann et al., 2004). What would further strengthen the case for these as physiological receptors for β-glucan-triggered immune responses in soybean is evidence that the binding specificity for diverse oligoglucosides matches their bioactivity as elicitors. To our knowledge corresponding knock-out or knock-down genetic experiments within legumes to corroborate receptor function have not been reported, although the soybean protein expressed in tomato confers binding of the hepta-β-glucoside (Mithofer et al., 2000).
β-Glucans in Immune Suppression and Activation in the Solanaceae
β-1→3-glucans also figure prominently as immune modulators in the potato – P. infestans interaction, although the story here is complicated by their reported action as both enhancers and suppressors of elicitor activity. However, this differential activity has not been reconciled with the degree of biochemical resolution as was done with P. sojae glucans to unambiguously assign enhancer or suppressor activity to the various oligoglucosides within the active fractions. Doke and Tomiyama (1980) using a potato protoplast assay showed that water soluble, anionic and non-anionic β-glucans suppressed the elicitor activity of a crude hyphal wall fraction from P. infestans. They suggested a degree of race-specificity in that glucans from compatible races of the pathogen were more active than those of incompatible races in suppressing the HR and ROS induced by the hyphal wall elicitor. The suppressive glucans were partially characterized and shown to have a DP of 17–23 glucose units with β-1→3 and β-1→6 linkages, and were present in the fluids of germinating cystospores (Doke et al., 1979). The purified hepta-β-glucoside from P. sojae was neither active as an elicitor nor as a suppressor in potato. A subsequent study showed that water soluble glucans from spore germination fluids of P. capsici have similar effect in suppressing elicitor-induced cell death in pepper and tomato cell suspensions (Sanchez et al., 1994). Race specificity attributed to the glucans in the context of HR suppression is difficult to reconcile with the contemporary paradigm of effector-triggered immunity and resistance (R)-gene action (Chisholm et al., 2006).
The model for β-glucans as suppressors is further complicated by their enhancement of EP elicitor activity. β-glucans, although lacking inherent elicitor activity in potato, can strongly enhance the activity of EP. Several lines of evidence suggest the combined action of eliciting (EP) and non-eliciting (β-glucans) components provide a maximal defense response. Initial evidence came from reconstitution experiments whereby highly elicitor-active, solubilized cell wall fractions were hydrolyzed in base-borohydride, leaving polysaccharides intact but hydrolyzing any esterified fatty acids, which were then removed by solvent extraction. This resulted in complete loss of elicitor activity, which was restored by addition of AA and EPA to the base-hydrolyzed wall fractions at their levels initially present (Bostock et al., 1982). Subsequent fractionation, partial purification and analysis showed that the enhancers were indeed β-1→3-glucans (Maniara et al., 1984). Preisig and Kuć (1985) further demonstrated that the glucans provide a 10–100 fold enhancement of the activity of AA concentrations that alone are below the threshold for induction of phytoalexins and related responses. The glucans also revealed elicitor activity of other EPs, particularly Δ5-eicosatrienoic acids. The most active β-glucan fractions had similar DP as the suppressor glucans, and were then found to suppress the HR induced by incompatible races of P. infestans, suggesting that the enhancers and suppressors could be the same.
These classic experiments indicate that members of the Solanaceae have an intriguing system for perceiving specific β-glucans and EP to coordinate a strong resistance response. The activity of these glucans in modulating immunity in potato, in particular, suggests a receptor-mediated process subject to attenuation by competing ligands as observed in legumes. For example, the suppressive action of the β-glucans against the HR induced by pathogen inoculum or the crude hyphal wall elicitor may have resulted from similarities in oligosaccharin motifs that compete for a putative MAMP receptor. Algal polysaccharides, such as the storage β-glucans laminarin and carrageenan, activate defense responses in some plants, although sulfated carrageenans appear to be far more active than laminarins as elicitors (Klarzynski et al., 2000; Mercier et al., 2001). However, in potato, laminarin neither elicits nor suppresses, providing a negative control treatment in the studies of the more complex β-1→3-linked glucans (Bostock et al., 1982; Maniara et al., 1984; Preisig and Kuć, 1985). Although considerably less active than the β-glucans, N, N’-diacetyl-D-chitobiose, the hapten for the potato lectin, inhibited the HR induced by incompatible races of P. infestans in potato (Nozue et al., 1980) and modestly enhanced the elicitor activity of AA (Maniara et al., 1984). Although other carbohydrates may modulate the plant immune response to some degree, the exceptionally strong biological activity of the oomycete oligosaccharins indicates considerable structural specificity in their action.
β-Glucan Recognition in Antifungal Immunity in Vertebrates
Protection against fungi in vertebrates involves both innate and adaptive immunity (Brown, 2011). Innate antifungal immunity is primarily mediated by diverse pattern-recognition receptors associated with phagocytes, which upon activation ingest and kill or degrade the invading microbe. Carbohydrates associated with the fungal cell wall, in particular, are well positioned to be recognized by these receptors. The adaptive and highly specific immune response to the invader is then engaged following generation of cytokines and chemokines along with the presentation of microbial antigens to lymphocytes.
There are multiple pattern-recognition receptors for β-glucans in phagocytes and the molecular details for some of these interactions have been characterized (Brown and Gordon, 2005). These include the transmembrane dectin-1, a natural killer-cell-receptor-like C-type lectin (calcium dependent) found on macrophages, neutrophils and dendritic cells, which specifically recognizes β-1→3- and β-1→6-linked glucans as well as intact yeast cells (Brown, 2006; Schorey and Lawrence, 2008). Zymosan, a complex cell wall preparation from Saccharomyces cerevisiea used to promote inflammation in experimental models, also stimulates dectin-1 and macrophage activation. Of particular interest in relation to the topic of this review is that zymosan induces cytosolic phospholipase A2 in macrophages that releases AA for conversion into pro-inflammatory prostaglandins and leukotrienes (Suram et al., 2006; Olsson and Sundler, 2007). Intriguing here is the apparent cross-kingdom conservation whereby β-glucans operate in concert with AA metabolites and other signals to orchestrate an innate immune response. The extent to which this analogy and underlying mechanisms translate to plant–oomycete interactions remains to be determined. Arabidopsis and Solanum species have proteins with C-type lectin motifs with some homology to dectin-1. However, they appear to be rare in plants and their functions are unresolved (Singh and Zimmerli, 2013).
Perspectives
The “renaissance of elicitors” heralded in the excellent review by Boller and Felix (2009) reflects a raised awareness and renewed interest in some of the classic elicitors. Recasting these as MAMPs has provided a framework that can inform and guide research into their perception and action in plant cells. The extent that different MAMPs collaborate in vivo during infection to synergize a strong defense response is unclear, although the cellular machinery seems to be present to do so. The oligomerization of receptors upon MAMP stimulation – the ligand-induced FLS2-BAK1 interaction and coordination with brassinosteroid signaling being a canonical example (Wang, 2012) – should encourage research in other systems for similar examples. It appears that the well-studied receptor-like kinases provide one of several strategies plants use to perceive elicitors to trigger innate immunity (Boller and Felix, 2009; Greeff et al., 2012). A challenge with different MAMPs apparently operating within the same infection interface is that mixed and potentially conflicting messages emanate from phytohormone-regulated response networks, leading to unwanted tradeoffs in the resistance phenotype (Bostock, 2005). How the plant negotiates these trade-offs will be an important consideration.
An implicit feature of innate immunity is that MAMPs be presented in their most biologically active form. The β-1→3-glucanase activity of the soybean binding proteins seems to be ideally positioned to release active β-glucan oligomers from invading hyphal walls (Fliegmann et al., 2004), and immunomodulatory glucans from Phytophthora spp. can be found in spore germination fluids (Doke et al., 1979; Waldmuller et al., 1992; Sanchez et al., 1994). The overwhelming evidence indicates that EP must be released from esterified forms for them to be perceived to trigger cellular responses (Bostock, 1989; Ricker and Bostock, 1992). A better understanding of how, when and where EP and β-glucans are deployed during the infection and whether they converge to coordinate immune responses will help to fully realize the potential of MAMP-triggered immunity in plant–oomycete interactions (Figure 5). With sequenced genomes, technical advances in transcriptomics, proteomics and metabolic profiling, and high-throughput functional assays, now is an opportune time to re-examine these elicitors in crop models.
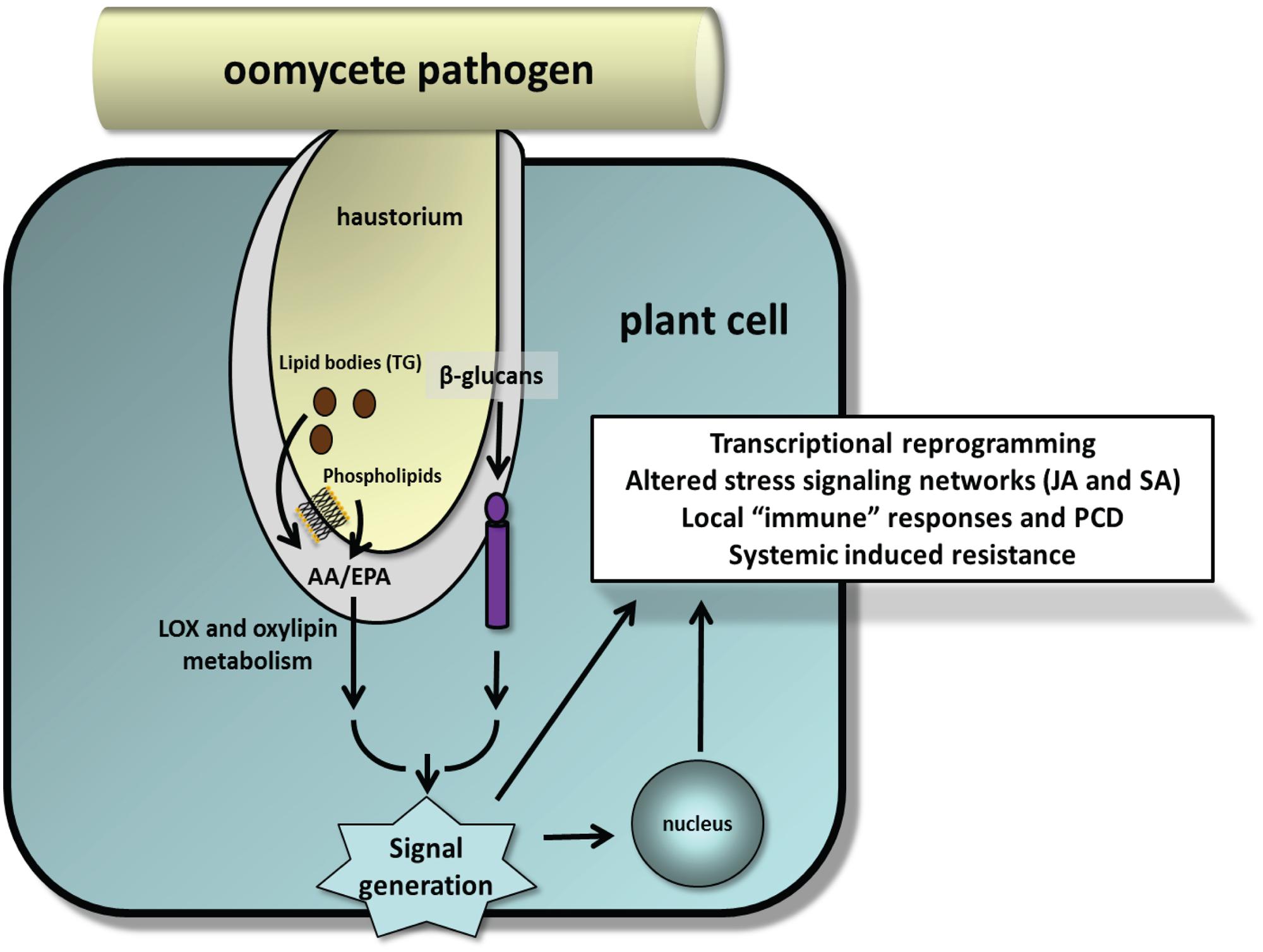
FIGURE 5. Model illustrating release of EP from phospholipids and TG-rich lipid bodies and β-glucans during early stages of plant–oomycete interactions as suggested by experimental studies. Implicit in this model are the fusion of lipid bodies with the haustorial membrane and activation of appropriate lipases and glucanases at the host–parasite interface to release these MAMPs. Minor amounts of EP also can be detected in cell wall fractions. How pathogenic oomycetes suppress these processes for successful infection and colonization is unresolved.
Conflict of Interest Statement
The Guest Associate Editor Gitta Coaker declares that, despite being affiliated to the same institution as the authors, the review process was handled objectively and no conflict of interest exists. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Sara M. Robinson is supported by a National Science Foundation Graduate Research Fellowship under Grant No. DGE-1148897, a Jastro-Shields award, and funds from the University of California Agricultural Experiment Station. We thank M. Hahn for his insights on the hepta-glucoside elicitor.
Abbreviations
AA, arachidonic acid (20:4 Δ5,8,11,14); ALA, α-Linolenic acid (18:3Δ 9,12,15); AOS, allene oxide synthase; CA, colneleic acid; a divinyl ether from peroxidized LA; CnA, colnelenic acid; a divinyl ether from peroxidized ALA; DES, divinyl ether synthase; DHA, docosahexaenoic acid (22:6 Δ4,7,10,13,16,19); DP, degree of polymerization; DPI, diphenyleneiodonium; EAS, epoxy alcohol synthase; EP, eicosapolyenoic acids (arachidonic acid and/or eicosapentaenoic acid); EPA, eicosapentaenoic acid (20:5 Δ5,8,11,14,17); ET, ethylene; HMGR, 3-Hydroxy-3-methylglutaryl coenzyme A reductase; HPETE, Hydroperoxyeicosatetraenoic acid; HPL, Hydroperoxide lyase; HPOD, Hydroperoxyoctadienoic acid (from LA); HPOT, Hydroperoxyoctatrienoic acid (from ALA); HR, hypersensitive response; JA, jasmonic acid; LA, linoleic acid (18:2Δ9,12); LOX, lipoxygenase; MAMP, microbe associated molecular pattern; PCD, programmed cell death; POX or PXG, peroxygenase; PR, pathogenesis-related (proteins); PRR, pattern recognition receptor; PTI, pattern triggered immunity; PUFA, polyunsaturated fatty acid; ROS, reactive oxygen species; SA, salicylic acid; SAR, systemic acquired resistance; TG, triglyceride; TMV, tobacco mosaic virus.
Footnotes
- ^ Microbe-associated molecular pattern.
- ^ In plants, 9-LOXs insert oxygen at the 9-carbon of LA and ALA, which is carbon 1 in the (1Z,4Z)-pentadiene system closest to the carboxyl end of the molecule. The Δ5-carbon of AA is in the carbon 1 position of the (1Z,4Z)-pentadiene system closest to the carboxyl group.
References
Albersheim, P., Darvill, A. G., Mcneil, M., Valent, B. S., Sharp, J. K., Nothnagel, E. A.,et al. (1983). “Oligosaccharins: naturally occurring carbohydrates with biological regulatory functions,” in Structure and Function of Plant Genomes, eds O. Ciferri and L. Dure (New York, NY: Plenum Press), 293–312.
Amruthesh, K. N., Geetha, N. P., Jorgensen, H. J. L., De Neergaard, E., and Shetty, H. S. (2005). Unsaturated fatty acids from zoospores of Sclerospora graminicola induce resistance in pearl millet. Eur. J. Plant Pathol. 111, 125–137. doi: 10.1007/s10658-004-1590-9
Andreou, A. Z., Hornung, E., Kunze, S., Rosahl, S., and Feussner, I. (2009). On the substrate binding of linoleate 9-lipoxygenases. Lipids 44, 207–215. doi: 10.1007/s11745-008-3264-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Araceli, A. C., Elda, C. M., Edmundo, L. G., and Ernesto, G. P. (2007). Capsidiol production in pepper fruits (Capsicum annuum L.) induced by arachidonic acid is dependent of an oxidative burst. Physiol. Mol. Plant Pathol. 70, 69–76. doi: 10.1016/j.pmpp.2007.07.002
Bartnicki-Garcia, S., and Wang, M. C. (1983). “Biochemical aspects of morphogenesis in Phytophthora,” in Phytophthora: Its Biology, Taxonomy, Ecology, and Pathology, eds D. C. Erwin, S. Bartnicki-Garcia, and P. H. Tsao (Saint Paul, MN: American Phytopathological Society), 121–137.
Blee, E. (2002). Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 7, 315–321. doi: 10.1016/s1360-1385(02)02290-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bloch, C. B., Dewit, P., and Kuć, J. (1984). Elicitation of phytoalexins by arachidonic and eicosapentaenoic acids – a host survey. Physiol. Plant Pathol. 25, 199–208. doi: 10.1016/0048-4059(84)90058-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Boller, T., and Felix, G. (2009). A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. doi: 10.1146/annurev.arplant.57.032905.105346
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bostock, R. M. (1989). Metabolism of lipids containing arachidonic and eicosapentaenoic acids in race-specific interactions between Phytophthora infestans and potato. Phytopathology 79, 898–902. doi: 10.1094/Phyto-79-898
Bostock, R. M. (2005). Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu. Rev. Phytopathol. 43, 545–580. doi: 10.1146/annurev.phyto.41.052002.095505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bostock, R. M., Kuć, J. A., and Laine, R. A. (1981). Eicosapentaenoic and arachidonic acids from Phytophthora infestans elicit fungitoxic sesquiterpenes in the potato. Science 212, 67–69. doi: 10.1126/science.212.4490.67
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bostock, R. M., Laine, R. A., and Kuć, J. A. (1982). Factors affecting the elicitation of sesquiterpenoid phytoalexin accumulation by eicosapentaenoic and arachidonic acids in potato. Plant Physiol. 70, 1417–1424. doi: 10.1104/pp.70.5.1417
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bostock, R. M., Schaeffer, D. A., and Hammerschmidt, R. (1986). Comparison of elicitor activities of arachidonic-acid, fatty-acids and glucans from Phytophthora infestans in hypersensitivity expression in potato tuber. Physiol. Mol. Plant Pathol. 29, 349–360. doi: 10.1016/S0048-4059(86)80051-0
Bostock, R., Tatyana, S., Lazarus, C., and Dehesh, K. (2011). Eicosapolyenoic acids: novel MAMPs with reciprocal effect on oomycete-plant defense signaling networks. Plant Signal. Behav. 6, 531–533. doi: 10.4161/psb.6.4.14782
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bostock, R. M., Yamamoto, H., Choi, D., Ricker, K. E., and Ward, B. L. (1992). Rapid stimulation of 5-lipoxygenase activity in potato by the fungal elicitor arachidonic acid. Plant Physiol. 100, 1448–1456. doi: 10.1104/pp.100.3.1448
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brown, G. D. (2006). Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6, 33–43. doi: 10.1038/nri1745
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brown, G. D. (2011). Innate antifungal immunity: the key role of phagocytes. Annu. Rev. Immunol. 29, 1–21. doi: 10.1146/annurev-immunol-030409-101229
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brown, G. D., and Gordon, S. (2005). Immune recognition of fungal beta-glucans. Cell. Microbiol. 7, 471–479. doi: 10.1111/j.1462-5822.2005.00505.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Canonne, J., Froidure-Nicolas, S., and Rivas, S. (2011). Phospholipases in action during plant defense signaling. Plant Signal. Behav. 6, 13–18. doi: 10.4161/psb.6.1.14037
Castoria, R., Fanelli, C., Zoina, A., and Scala, F. (1995). Analysis of fatty acids in lipids of Verticillium dahliae and induction of lubimin accumulation in eggplant. Plant Pathol. 44, 791–795. doi: 10.1111/j.1365-3059.1995.tb02737.x
Cheong, J. J., Birberg, W., Fugedi, P., Pilotti, A., Garegg, P. J., Hong, N.,et al. (1991). Structure-activity-relationships of oligo-beta-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell 3, 127–136. doi: 10.1105/tpc.3.2.127
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cheong, J. J., and Hahn, M. G. (1991). A specific, high-affinity binding-site for the hepta-beta-glucoside elicitor exists in soybean membranes. Plant Cell 3, 137–147. doi: 10.1105/tpc.3.2.137
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chisholm, S. T., Coaker, G., Day, B., and Staskawicz, B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. doi: 10.1016/j.cell.2006.02.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Choi, D., Ward, B. L., and Bostock, R. M. (1992). Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme-A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell 4, 1333–1344. doi: 10.1105/tpc.4.10.1333
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cohen, Y., Gisi, U., and Mosinger, E. (1991). Systemic resistance of potato plants against Phytophthora infestans induced by unsaturated fatty acids. Physiol. Mol. Plant Pathol. 38, 255–263. doi: 10.1016/S0885-5765(05)80117-1
Coquoz, J. L., Buchala, A. J., Meuwly, P., and Metraux, J. P. (1995). Arachidonic acid induces local but not systemic synthesis of salicylic acid and confers systemic resistance in potato plants to Phytophthora infestans and Alternaria solani. Phytopathology 85, 1219–1224. doi: 10.1094/Phyto-85-1219
Cosio, E. G., Frey, T., and Ebel, J. (1992). Identification of a high-affinity binding-protein for a hepta-β-glucoside phytoalexin elicitor in soybean. Eur. J. Biochem. 204, 1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
De Caterina, R., and Basta, G. (2001). n-3 fatty acids and the inflammatory response – biological background. Eur. Heart J. Suppl. 3, D42–D49. doi: 10.1016/S1520-765X(01)90118-X
Doke, N., Garas, N. A., and Kuć, J. (1979). Partial characterization and aspects of the mode of action of a hypersensitivity-inhibiting factor (HIF) isolated from Phytophthora infestans. Physiol. Plant Pathol. 15, 127–140. doi: 10.1016/0048-4059(79)90061-4
Doke, N., Garas, N. A., and Kuć, J. (1980). Effect on host hypersensitivity of suppressors released during the germination of Phytophthora infestans cystospores. Phytopathology 70, 35–39. doi: 10.1094/Phyto-70-35
Doke, N., and Tomiyama, K. (1980). Effect of hyphal wall components from Phytophthora infestans on protoplasts of potato tuber tissues. Physiol. Plant Pathol. 16, 169–172. doi: 10.1016/0048-4059(80)90031-4
Ebel, J. (1998). Oligoglucoside elicitor-mediated activation of plant defense. Bioessays 20, 569–576. doi: 10.1002/(SICI)1521-1878(199807)20:7<569::AID-BIES8>3.0.CO;2-F
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fammartino, A., Cardinale, F., Gobel, C., Mene-Saffrane, L., Fournier, J., Feussner, I.,et al. (2007). Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol. 143, 378–388. doi: 10.1104/pp.106.087304
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Feussner, I., and Wasternack, C. (2002). The lipoxygenase pathway. Annu. Rev. Plant Biol. 53, 275–297. doi: 10.1146/annurev.arplant.53.100301.135248
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fidantsef, A. L., and Bostock, R. M. (1998). Characterization of potato tuber lipoxygenase cDNAs and lipoxygenase expression in potato tubers and leaves. Physiol. Plant. 102, 257–271. doi: 10.1034/j.1399-3054.1998.1020214.x
Fidantsef, A. L., Stout, M. J., Thaler, J. S., Duffey, S. S., and Bostock, R. M. (1999). Signal interactions in pathogen and insect attack: expression of lipoxygenase, proteinase inhibitor II, and pathogenesis-related protein P4 in the tomato, Lycopersicon esculentum. Physiol. Mol. Plant Pathol. 54, 97–114. doi: 10.1006/pmpp.1998.0192
Fliegmann, J., Mithofer, A., Wanner, G., and Ebel, J. (2004). An ancient enzyme domain hidden in the putative β-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 279, 1132–1140. doi: 10.1074/jbc.M308552200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fournier, J., Pouenat, M. L., Rickauer, M., Rabinovitch-Chable, H., Rigaud, M., and Esquerre-Tugaye, M. T. (1993). Purification and characterization of elicitor induced lipoxygenase in tobacco cells. Plant J. 3, 63–70. doi: 10.1111/j.1365-313X.1993.tb00011.x
Garcia-Pineda, E., and Lozoya-Gloria, E. (1999). Induced gene expression of 1-aminocyclopropane-1-carboxylic acid (ACC oxidase) in pepper (Capsicum annuum L.) by arachidonic acid. Plant Sci. 145, 11–21. doi: 10.1016/S0168-9452(99)00065-5
Geetha, S., Shetty, S. A., Shetty, H. S., and Prakash, H. S. (1996). Arachidonic acid-induced hypersensitive cell death as an assay of downy mildew resistance in pearl millet. Ann. Appl. Biol. 129, 91–96. doi: 10.1111/j.1744-7348.1996.tb05734.x
Gobel, C., Feussner, I., Hamberg, M., and Rosahl, S. (2002). Oxylipin profiling in pathogen-infected potato leaves. Biochim. Biophys. Acta 1584, 55–64. doi: 10.1016/S1388-1981(02)00268-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gobel, C., Feussner, I., Schmidt, A., Scheel, D., Sanchez-Serrano, J., Hamberg, M.,et al. (2001). Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. J. Biol. Chem. 276, 6267–6273. doi: 10.1074/jbc.M008606200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Greeff, C., Roux, M., Mundy, J., and Petersen, M. (2012). Receptor-like kinase complexes in plant innate immunity. Front. Plant Sci. 3:209. doi: 10.3389/fpls.2012.00209
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gullner, G., Kuenstler, A., Kiraly, L., Pogany, M., and Tobias, I. (2010). Up-regulated expression of lipoxygenase and divinyl ether synthase genes in pepper leaves inoculated with Tobamoviruses. Physiol. Mol. Plant Pathol. 74, 387–393. doi: 10.1016/j.pmpp.2010.06.006
Halim, V. A., Altmann, S., Ellinger, D., Eschen-Lippold, L., Miersch, O., Scheel, D.,et al. (2009). PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J. 57, 230–242. doi: 10.1111/j.1365-313X.2008.03688.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hansen, E. M., Reeser, P. W., and Sutton, W. (2012). Phytophthora beyond agriculture. Annu. Rev. Phytopathol. 50, 359–378. doi: 10.1146/annurev-phyto-081211-172946
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hoshino, T., Chida, M., Yamaura, T., Yoshizawa, Y., and Mizutani, J. (1994). Phytoalexin induction in green pepper cell cultures treated with arachidonic acid. Phytochemistry 36, 1417–1419. doi: 10.1016/S0031-9422(00)89733-2
Hwang, I. S., and Hwang, B. K. (2010). The pepper 9-lipoxygenase gene CaLOX1 functions in defense and cell death responses to microbial pathogens. Plant Physiol. 152, 948–967. doi: 10.1104/pp.109.147827
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kachroo, A., and Kachroo, P. (2009). Fatty acid-derived signals in plant defense. Annu. Rev. Phytopathol. 47, 153–176. doi: 10.1146/annurev-phyto-080508-081820
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Klarzynski, O., Plesse, B., Joubert, J. M., Yvin, J. C., Kopp, M., Kloareg, B.,et al. (2000). Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124, 1027–1037. doi: 10.1104/pp.124.3.1027
Knight, V. I., Wang, H., Lincoln, J. E., Lulai, E. C., Gilchrist, D. G., and Bostock, R. M. (2001). Hydroperoxides of fatty acids induce programmed cell death in tomato protoplasts. Physiol. Mol. Plant Pathol. 59, 277–286. doi: 10.1006/pmpp.2001.0366
Kroon, L., Brouwer, H., De Cock, A., and Govers, F. (2012). The genus Phytophthora anno 2012. Phytopathology 102, 348–364. doi: 10.1094/PHYTO-01-11-0025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
La Camera, S., Gouzerh, G., Dhondt, S., Hoffmann, L., Fritig, B., Legrand, M.,et al. (2004). Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 198, 267–284. doi: 10.1111/j.0105-2896.2004.0129.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Longland, A. C., Slusarenko, A. J., and Friend, J. (1987). Arachidonic and linoleic acids elicit isoflavonoid phytoalexin accumulation in Phaseolus vulgaris (French bean). J. Phytopathol. 120, 289–297. doi: 10.1111/j.1439-0434.1987.tb00492.x
Maniara, G., Laine, R., and Kuć, J. (1984). Oligosaccharides from Phytophthora infestans enhance the elicitation of sesquiterpenoid stress metabolites by arachidonic acid in potato. Physiol. Plant Pathol. 24, 177–186. doi: 10.1016/0048-4059(84)90026-2
Mercier, L., Lafitte, C., Borderies, G., Briand, X., Esquerre-Tugaye, M. T., and Fournier, J. (2001). The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 149, 43–51. doi: 10.1046/j.1469-8137.2001.00011.x
Mithofer, A., and Ebel, J. (1999). Functional reconstitution of β-glucan elicitor-binding activity upon incorporation into lipid vesicles. FEBS Lett. 458, 129–132. doi: 10.1016/S0014-5793(99)01126-6
Mithofer, A., Fliegmann, J., and Ebel, J. (1999). Isolation of a French bean (Phaseolus vulgaris L.) homolog to the β-glucan elicitor-binding protein of soybean (Glycine max L.). Biochim. Biophys. Acta 1418, 127–132. doi: 10.1016/S0005-2736(99)00010-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mithofer, A., Fliegmann, J., Neuhaus-Url, G., Schwarz, H., and Ebel, J. (2000). The hepta-β-glucoside elicitor-binding proteins from legumes represent a putative receptor family. Biol. Chem. 381, 705–713. doi: 10.1515/bc.2000.091
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mosblech, A., Feussner, I., and Heilmann, I. (2009). Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 47, 511–517. doi: 10.1016/j.plaphy.2008.12.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Murakami, M. (2011). Lipid mediators in life science. Exp. Anim. 60, 7–20. doi: 10.1538/expanim.60.7
Nguyen, H. P., Chakravarthy, S., Velasquez, A. C., Mclane, H. L., Zeng, L. R., Nakayashiki, H.,et al. (2010). Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Mol. Plant Microbe Interact. 23, 991–999. doi: 10.1094/MPMI-23-8-0991
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nozue, M., Tomiyama, K., and Doke, N. (1980). Effect of N, N’-diacetyl-D-chitobiose, the potato lectin hapten and other sugars on hypersensitive reaction of potato tuber cells infected by incompatible and compatible races of Phytophthora infestans. Physiol. Plant Pathol. 17, 221–224. doi: 10.1016/0048-4059(80)90055-7
Nurnberger, T., Brunner, F., Kemmerling, B., and Piater, L. (2004). Innate immunity in plants and animals: striking similarities and obvious differences. Immunol. Rev. 198, 249–266. doi: 10.1111/j.0105-2896.2004.0119.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Olsson, S., and Sundler, R. (2007). The macrophage β-glucan receptor mediates arachidonate release induced by zymosan: essential role for Src family kinases. Mol. Immunol. 44, 1509–1515. doi: 10.1016/j.molimm.2006.09.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pieterse, C. M., Van Der Does, D., Zamioudis, C., Leon-Reyes, A., and Van Wees, S. C. (2012). Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 28, 489–521. doi: 10.1146/annurev-cellbio-092910-154055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Preisig, C. L., and Kuć, J. A. (1985). Arachidonic acid-related elicitors of the hypersensitive response in potato and enhancement of their activities by glucans from Phytophthora infestans (Mont) Debary. Arch. Biochem. Biophys. 236, 379–389. doi: 10.1016/0003-9861(85)90638-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Preisig, C. L., and Kuć, J. A. (1987). Inhibition by salicylhydroxamic acid, BW755C, eicosatetraynoic acid, and disulfiram of hypersensitive resistance elicited by arachidonic acid or poly-L-lysine in potato tuber. Plant Physiol. 84, 891–894. doi: 10.1104/pp.84.3.891
Preisig, C. L., and Kuć, J. A. (1988). Metabolism by potato tuber of arachidonic acid, an elicitor of hypersensitive resistance. Physiol. Mol. Plant Pathol. 32, 77–88. doi: 10.1016/S0885-5765(88)80007-9
Regdel, D., Kuhn, H., and Schewe, T. (1994). On the reaction specificity of the lipoxygenase from tomato fruits. Biochim. Biophys. Acta 1210, 297–302. doi: 10.1016/0005-2760(94)90232-1
Ricker, K. E., and Bostock, R. M. (1992). Evidence for release of the elicitor arachidonic acid and its metabolites from sporangia of Phytophthora infestans during infection of potato. Physiol. Mol. Plant Pathol. 41, 61–72. doi: 10.1016/0885-5765(92)90049-2
Ricker, K. E., and Bostock, R. M. (1994). Eicosanoids in the Phytophthora infestans potato interaction - lipoxygenase metabolism of arachidonic acid and biological activities of selected lipoxygenase products. Physiol. Mol. Plant Pathol. 44, 65–80. doi: 10.1016/S0885-5765(05)80095-5
Roberts, S. M., Pye, M. F., Dehesh, K., and Bostock, R. M. (2013). Eicosapolyenoic fatty acids induce resistance in tomato to root and crown infection by Phytophthora capsici. Phytopathology 103, 122–122.
Robinson, S. M., Dehesh, K., and Bostock, R. M. (2014). Eicosapolyenoic fatty acids induce expression of 9-oxylipin pathway genes and resistance in tomato to Phytophthora capsici. Phytopathology 104(Suppl. 3), 99.
Rodriguez-Concepcion, M., and Gruissem, W. (1999). Arachidonic acid alters tomato HMG expression and fruit growth and induces 3-hydroxy-3-methylglutaryl coenzyme A reductase-independent lycopene accumulation. Plant Physiol. 119, 41–48. doi: 10.1104/pp.119.1.41
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Romero-Correa, M. T., Villa-Gomez, R., Castro-Mercado, E., and Garcia-Pineda, E. (2014). The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 87, 32–41. doi: 10.1016/j.pmpp.2014.05.003
Rozhnova, N. A., Gerashchenkov, G. A., and Babosha, A. V. (2003). The effect of arachidonic acid and viral infection on the phytohemagglutinin activity during the development of tobacco acquired resistance. Russ. J. Plant Physiol. 50, 661–665. doi: 10.1023/A:1025696325679
Sanchez, L. M., Doke, N., Ban, Y., and Kawakita, K. (1994). Involvement of suppressor glucans and plant epidermal cells in host-selective pathogenesis of Phytophthora capsici. J. Phytopathol. 140, 153–164. doi: 10.1111/j.1439-0434.1994.tb00187.x
Savchenko, T., Walley, J. W., Chehab, E. W., Xiao, Y. M., Kaspi, R., Pye, M. F.,et al. (2010). Arachidonic acid: an evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 22, 3193–3205. doi: 10.1105/tpc.110.073858
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schorey, J. S., and Lawrence, C. (2008). The pattern recognition receptor Dectin-1: from fungi to mycobacteria. Curr. Drug Targets 9, 123–129. doi: 10.2174/138945008783502430
Shah, J. (2005). Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu. Rev. Phytopathol. 43, 229–260. doi: 10.1146/annurev.phyto.43.040204.135951
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sharp, J. K., Valent, B., and Albersheim, P. (1984). Host-pathogen interactions 26. Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J. Biol. Chem. 259, 1312–1320.
Singh, P., and Zimmerli, L. (2013). Lectin receptor kinases in plant innate immunity. Front. Plant Sci. 4:124. doi: 10.3389/fpls.2013.00124
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stumpe, M., Kandzia, R., Gobel, C., Rosahl, S., and Feussner, I. (2001). A pathogen-inducible divinyl ether synthase (CYP74D) from elicitor-treated potato suspension cells. FEBS Lett. 507, 371–376. doi: 10.1016/S0014-5793(01)03019-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Suram, S., Brown, G. D., Ghosh, M., Gordon, S., Loper, R., Taylor, P. R.,et al. (2006). Regulation of cytosolic phospholipase A2 activation and cyclooxygenase 2 expression in macrophages by the β-glucan receptor. J. Biol. Chem. 281, 5506–5514. doi: 10.1074/jbc.M509824200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tjamos, E. C., and Kuć, J. A. (1982). Inhibition of steroid glycoalkaloid accumulation by arachidonic and eicosapentaenoic acids in potato. Science 217, 543–544. doi: 10.1126/science.217.4559.542
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Truman, W. M., Bennett, M. H., Turnbull, C. G. N., and Grant, M. R. (2010). Arabidopsis auxin mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds. Plant Physiol. 152, 1562–1573. doi: 10.1104/pp.109.152173
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tsuda, K., Sato, M., Stoddard, T., Glazebrook, J., and Katagiri, F. (2009). Network properties of robust immunity in plants. PLoS Genet. 5:e1000772. doi: 10.1371/journal.pgen.1000772
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tyler, B. M. (2002). Molecular basis of recognition between Phytophthora pathogens and their hosts. Annu. Rev. Phytopathol. 40, 137–167. doi: 10.1146/annurev.phyto.40.120601.125310
Umemoto, N., Kakitani, M., Iwamatsu, A., Yoshikawa, M., Yamaoka, N., and Ishida, I. (1997). The structure and function of a soybean b-glucan-elicitor-binding protein. Proc. Natl. Acad. Sci. U.S.A. 94, 1029–1034. doi: 10.1073/pnas.94.3.1029
Van Kan, J. A. L., Joosten, M. H. A. J., Wagemakers, C. A. M., Vandenbergvelthuis, G. C. M., and Dewit, P. J. G. M. (1992). Differential accumulation of messenger RNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirulent races of Cladosporium fulvum. Plant Mol. Biol. 20, 513–527. doi: 10.1007/BF00040610
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vaughn, S. F., and Lulai, E. C. (1992). Further evidence that lipoxygenase activity is required for arachidonic acid-elicited hypersensitivity in potato callus cultures. Plant Sci. 84, 91–98. doi: 10.1016/0168-9452(92)90212-5
Veronesi, C., Rickauer, M., Fournier, J., Pouenat, M. L., and Esquerre-Tugaye, M. T. (1996). Lipoxygenase gene expression in the tobacco – Phytophthora parasitica nicotianae interaction. Plant Physiol. 112, 997–1004. doi: 10.1104/pp.112.3.997
Waldmuller, T., Cosio, E. G., Grisebach, H., and Ebel, J. (1992). Release of highly elicitor-active glucans by germinating zoospores of Phytophthora megasperma f. sp. glycinea Planta 188, 498–505. doi: 10.1007/BF00197041
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Walley, J. W., Kliebenstein, D. J., Bostock, R. M., and Dehesh, K. (2013). Fatty acids and early detection of pathogens. Curr. Opin. Plant Biol. 16, 520–526. doi: 10.1016/j.pbi.2013.06.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, Z.-Y. (2012). Brassinosteroids modulate plant immunity at multiple levels. Proc. Natl. Acad. Sci. U.S.A. 109, 7–8. doi: 10.1073/pnas.1118600109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Weber, H., Chetelat, A., Caldelari, D., and Farmer, E. E. (1999). Divinyl ether fatty acid synthesis in late blight-diseased potato leaves. Plant Cell 11, 485–493. doi: 10.2307/3870875
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Whitehead, I. M., Atkinson, A. L., and Threlfall, D. R. (1990). Studies on the biosynthesis and metabolism of the phytoalexin lubimin and related compounds in Datura stramonium L. Planta 182, 81–88. doi: 10.1007/BF00239988
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yoshioka, H., Sugie, K., Park, H. J., Maeda, H., Tsuda, N., Kawakita, K.,et al. (2001). Induction of plant gp91 phox homolog by fungal cell wall, arachidonic acid, and salicylic acid in potato. Mol. Plant Microbe Interact. 14, 725–736. doi: 10.1094/MPMI.2001.14.6.725
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zipfel, C., and Robatzek, S. (2010). Pathogen-associated molecular pattern-triggered immunity: veni, vidi...? Plant Physiol. 154, 551–554. doi: 10.1104/pp.110.161547
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zook, M. N., and Kuć, J. A. (1991). Induction of sesquiterpene cyclase and suppression of squalene synthetase activity in elicitor-treated or fungal-infected potato tuber tissue. Physiol. Mol. Plant Pathol. 39, 377–390. doi: 10.1016/0885-5765(91)90018-D
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: arachidonic acid, eicosapolyenoic acid, innate immunity, oligosaccharins, oxylipins, Phytophthora
Citation: Robinson SM and Bostock RM (2015) β-glucans and eicosapolyenoic acids as MAMPs in plant–oomycete interactions: past and present. Front. Plant Sci. 5:797. doi: 10.3389/fpls.2014.00797
Received: 12 September 2014; Accepted: 22 December 2014;
Published online: 13 January 2015.
Edited by:
Gitta Coaker, University of California, Davis, USAReviewed by:
Choong-Min Ryu, Korea Research Institute of Bioscience and Biotechnology, South KoreaJulio Vega-Arreguin, National Autonomous University of Mexico, Mexico
Copyright © 2015 Robinson and Bostock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard M. Bostock, Department of Plant Pathology, University of California, Davis, One Shields Avenue, Davis, CA 95616, USA e-mail: rmbostock@ucdavis.edu
 Sara M. Robinson
Sara M. Robinson Richard M. Bostock
Richard M. Bostock