- Key Laboratory of Molecular Epigenetics of Ministry of Education, Northeast Normal University, Changchun, China
Aux/IAA proteins are transcriptional repressors that control auxin signaling by interacting with auxin response factors (ARFs). So far all of the identified Aux/IAA mutants with auxin-related phenotypes in Arabidopsis and rice (Oryza sativa) are dominant gain-of-function mutants, with mutations in Domain II that affected stability of the corresponding Aux/IAA proteins. On the other hand, morphological changes were observed in knock-down mutants of Aux/IAA genes in tomato (Solanum lycopersicum), suggesting that functions of Aux/IAA proteins may be specific for certain plant species. We report here the characterization of PtrIAA14.1, a poplar (Populus trichocarpa) homolog of IAA7. Bioinformatics analysis showed that PtrIAA14.1 is a classic Aux/IAA protein. It contains four conserved domains with the repressor motif in Domain I, the degron in Domain II, and the conserved amino acid signatures for protein–protein interactions in Domain III and Domain IV. Protoplast transfection assays showed that PtrIAA14.1 is localized in nucleus. It is unable in the presence of auxin, and it represses auxin response reporter gene expression. Expression of wild-type PtrIAA14.1 in Arabidopsis resulted in auxin-related phenotypes including down-curling leaves, semi-draft with increased number of branches, and greatly reduced fertility, but expression of the Arabidopsis Aux/IAA genes tested remain largely unchanged in the transgenic plants. Protein–protein interaction assays in yeast and protoplasts showed that PtrIAA14.1 interacted with ARF5, but not other ARFs. Consistent with this observation, vascular patterning was altered in the transgenic plants, and the expression of AtHB8 (Arabidopsis thaliana homeobox gene 8) was reduced in transgenic plants.
Introduction
Auxin regulates most, if not all, aspects of plant growth and development, such as organ formation, lateral root initiation, stem and root elongation, vascular tissue differentiation, and apical dominance (Davies, 1995). Auxin-regulated plant growth and development is likely initiated by the rapid response of specific genes to local changes in auxin concentration. These genes are referred to as early auxin response genes. Aux/IAA genes are one of the several different types of early auxin response genes in Arabidopsis (Guilfoyle, 1999; Hagen and Guilfoyle, 2002).
There are 29 Aux/IAA genes in the Arabidopsis genome (Reed, 2001; Liscum and Reed, 2002). Aux/IAA proteins are transcription repressors that involve in the regulation of auxin signaling. Based on their amino acid sequence similarity, Aux/IAA proteins generally contain four conserved domains, Domain I, II, III, and IV. Domain I contains a conserved LxLxL motif that functions as an active repression domain. Domain II contains a conserved degron that regulates the stability of Aux/IAA proteins in an auxin-dependent manner. Domains III and IV are related to the conserved C-terminal dimerization domains of auxin response factors (ARFs), and contain conserved amino acids that are required for homo and hetero interactions among Aux/IAA proteins and ARFs (Ulmasov et al., 1997, 1999; Ramos et al., 2001; Tiwari et al., 2001, 2003, 2004; Dreher et al., 2006; Nanao et al., 2014).
Based on the results obtained largely from Arabidopsis protoplast transient transfection and yeast two-hybrid assays, it was proposed that when auxin concentrations are low, Aux/IAA repressors are dimerized with ARF activators that bound on the TGTCTC auxin response elements (AuxREs) in the promoter regions of auxin response genes, resulting in repression of auxin response genes. When auxin concentrations are elevated, Aux/IAA repressors are degraded by the ubiquitin–proteasome pathway, allowing the ARF activators to activate auxin response genes (Ulmasov et al., 1999; Tiwari et al., 2001, 2004; Guilfoyle and Hagen, 2007).
Functions of Aux/IAA genes in regulating plant growth and development are largely revealed by characterization of dominant gain-of-function mutations in Domain II of Aux/IAA proteins. Several different types of phenotypes were observed in the dominant iaa mutants (Nagpal et al., 2000; Rogg et al., 2001; Fukaki et al., 2002; Uehara et al., 2008; Ploense et al., 2009; Rinaldi et al., 2012), and some of the dominant gain-of-function iaa mutants even have opposite phenotypes (Tian and Reed, 1999; Tian et al., 2002; Knox et al., 2003). Promoter-exchange experiments further confirmed that Aux/IAA genes have both specific and similar functions (Muto et al., 2007). On the other hand, all single T-DNA insertion mutants identified in Arabidopsis so far, and double or triple mutants of closely related Aux/IAA genes in Arabidopsis showed no visible developmental defects (Overvoorde et al., 2005).
Consistent with the phenotypes observed in the T-DNA insertion and dominant gain-of-function Aux/IAA mutants, Arabidopsis transgenic plants expressing glucocorticoid hormone-binding domain (GR) fused protein of wild-type IAA1 (IAA1-GR) did not exhibit any obvious phenotypic differences upon DEX treatment (Park et al., 2002). Transgenic Arabidopsis plants expressing wild-type VvIAA19, a grape (Vitis vinifera) Aux/IAA gene, are also morphological similar to wild-type plants (Fujita et al., 2012; Kohno et al., 2012). However, auxin-related phenotypes were observed in DEX treated transgenic plants expressing GR fused IAA1 with a mutation in Domain II (iaa1-GR; Park et al., 2002), and transgenic plants expressing mIAA14-GR under the control of the stele-specific SHORT-ROOT promoter (Fukaki et al., 2005). Transgenic Arabidopsis plants expressing IAA20 and IAA30, two Aux/IAA proteins lacking Domain II, and IAA31, an Aux/IAA with a partial, dominant mutation-type domain II, also showed auxin-related phenotypes (Sato and Yamamoto, 2008).
Similar to the observations in Arabidopsis, the Aux/IAA mutants with auxin-related phenotypes isolated in rice (Oryza sativa), including osiaa11, osiaa13, and osiaa23 were all caused by dominant gain-of-function mutations in the Domain II (Jun et al., 2011; Kitomi et al., 2012; Zhu et al., 2012), and auxin-related phenotypes were observed in transgenic rice plants expressing OsIAA4, an Aux/IAA with a dominant mutation-type domain II (Song and Xu, 2013).
In tomato (Solanum lycopersicum), however, phenotypic changes were observed in knock-down mutants of Aux/IAA genes (Wang et al., 2005a; Bassa et al., 2012; Deng et al., 2012; Su et al., 2014), suggesting that functions of Aux/IAA proteins may be specific for particular plant species.
There are a total of 35 genes in poplar (Populus trichocarpa) encoding Aux/IAA proteins (Kalluri et al., 2007), but their functions remain largely unknown. Because IAA7 is a classic Auxin/IAA protein and its roles in auxin signaling and auxin-regulated plant growth and development have been well characterized in Arabidopsis (Nagpal et al., 2000; Muto et al., 2007), we decided to use IAA7 homolog in poplar as an example to study the roles of poplar Aux/IAA proteins in auxin signaling and plant growth and development.
Using the full length amino acid sequence of IAA to BLAST search poplar protein database (www.phytozome.net), we identified PtrIAA14.1 as a poplar homolog of Arabidopsis IAA7. We showed that PtrIAA14.1 regulates auxin response reporter gene expression in protoplasts, and that expressing wild-type PtrIAA14.1 in Arabidopsis resulted in auxin-related phenotypes.
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis thaliana (Arabidopsis) ecotype Columbia (Col-0) was used for protoplasts isolation and plant transformation. DR5:GUS transgenic plants were used for crossing and protoplasts isolation, and leaves from field-grown poplar (P. trichocarpa) tree were used for PtrIAA14.1 gene cloning and expression analysis.
For auxin treatment and RNA isolation from seedlings, seeds were sterilized and sown on plates containing 0.6% (w/v) phytoagar solidified 1/2 Murashige & Skoog (MS) medium with vitamins (PlantMedia) and 1% (w/v) sucrose. After kept at 4°C in darkness for 2 days, the plates were moved into a growth room. For plant transformation and phenotypic analysis for adult plants, Arabidopsis seeds were sown directly into soil and kept in a growth room. Arabidopsis plants were grown at 20°C, with a 16 h light/8 h darkness photoperiod at approximately 120 μmol m–2 s–1.
Constructs
The LexA-Gal4-GUS reporter, and GD, LD-VP, and CAT constructs used for protoplast transfection were described previously (Tiwari et al., 2003; Wang et al., 2007).
To generate HA or GD tagged constructs for PtrIAA14.1 for protoplast transfection assays, the full-length open-reading frame (ORF) of PtrIAA14.1 was amplified by RT-PCR using RNA isolated from the poplar leaves, and the PCR products were cloned in frame with an N-terminal HA or GD tag into the pUC19 vector under the control of the double 35S enhancer promoter of CaMV (Tiwari et al., 2003; Wang et al., 2005b). For plant transformation, the pUC19HA-PtrIAA14.1 construct was digested with proper enzymes, and subcloned into the binary vector pPZP211 (Hajdukiewicz et al., 1994) to generate binary vector pPZP211HA-PtrIAA14.1.
To generate GD tagged PtrIAA14.1CTD for protoplast transfection, ORF sequences corresponding to the amino acid residues 131–248 of PtrIAA14.1 were amplified by PCR using pUC19HA-PtrIAA14.1 plasmids as template, and cloned in frame with an N-terminal GD tag into the pUC19 vector.
To generate GFP tagged PtrIAA14.1 for protoplast transfection, ORF of PtrIAA14.1 without stop codon were amplified by PCR using pUC19HA-PtrIAA14.1 plasmids as template, and cloned in frame with an C-terminal GFP tag into the pUC19 vector.
To generate pDHB1ARF5CTD construct for yeast-two-hybrid assays, ORF sequences corresponding to the amino acid residues 767–901 of ARF5 were amplified by PCR using pUC19HA-ARF5 (Wang et al., 2005b) plasmids as template, and cloned into pDHB1 vector. pPR3-NPtrIAA14.1 were generated by cloning PCR amplified PtrIAA14.1 full length ORF into pPR3-N vector.
All primers used for gene cloning and gene expression analysis are listed in Table 1.
Plant Transformation and Transgenic Plants Selection
To generate transgenic plants, about 5-week-old plants with several mature flowers on the main inflorescence were transformated via Agrobacterium tumefaciens (GV3101) using the floral dip method (Clough and Bent, 1998). To select transgenic plants, T1 seeds were sterilized and sown on 1/2 MS plates containing 50 μg ml–1 kanamycin. Phenotypes of transgenic plants were examined in the T1 generation, and confirmed in following generations. More than 10 transgenic lines with similar phenotypes were obtained. Homozygous T3 or T4 plants were used for the experiments.
Auxin Treatment and Root Elongation Assay
For PtrIAA14.1 expression analysis, leaves were collected from a 15-year-old field-grown poplar tree (Liu et al., 2013; Wang et al., 2014; Zhou et al., 2014), and leaf discs were prepared and treated with 10 μM IAA for 4 h in darkness on a shaker at 40 rpm. Samples were frozen in liquid N2, and kept in –80°C for RNA isolation.
For Aux/IAA gene expression analysis, 10-day-old wild-type and transgenic Arabidopsis seedlings grown on 1/2 MS plates were treated with 10 μM IAA for 4 h in darkness on a shaker at 40 rpm.
For root elongation and lateral root formation assays, 4-day-old wild-type and transgenic Arabidopsis seedlings grown on vertical plates were transferred onto new plates at the presence or absent of 0.1 μM IAA. Pictures were taken after growing vertically for additional 4 days, and root length was measured using ImageJ software.
RNA Isolation and RT-PCR
Total RNA from poplar leaf discs was isolated as described previously (Geraldes et al., 2011; Liu et al., 2013; Wang et al., 2014). cDNA was synthesized using the Omniscript RT Kit (Qiagen) according to the manufacturer’s instructions.
Total RNA from Arabidopsis seedlings was isolated using Easy-Pure Plant RNA Kit (TransGen Biotech), and cDNA was synthesized using the EazyScript First-Strand DNA Synthesis Super Mix (TransGen Biotech) according to the manufacturer’s instructions.
All RT-PCR analyses were repeated at least three times and the representative images were presented. Arabidopsis gene ACTIN2 (ACT2) and poplar gene UBQ were used as controls for RT-PCR.
Yeast Two Hybridization
Split-ubiquitin system was used to detect the interaction between PtrIAA14.1 and ARF5. ARF5CTD was cloned into bait vector pDHB1, and PtrIAA14.1 was cloned into prey vector pPR3-N. Bait and prey constructs were cotransformed into yeast strain NMY51. Cotransformation of empty bait and prey construct, and empty prey with bait construct were used as controls. The yeast transformants containing both prey and bait were able to grow on minimum synthetic dextrose dropout medium lacking both Trp and Leu (SD-trp-leu). A positive interaction between two proteins is indicated by the growth of yeast colony on the minimum synthetic dextrose medium lacking Leu, Trp, and His (SD-trp-leu-his), and a white color due to the activation of Ade synthesis.
Protoplasts Isolation, Transfection, GUS Activity Assays, and Florescence Examination
Reporter and effector plasmids were prepared using the GoldHi EndoFree Plasmid Maxi Kit (CW Biotech) according to the manufacturer’s instructions. Protoplasts isolation, transfection, and GUS activity assays were performed by following the procedures described previously (Wang et al., 2005b; Tiwari et al., 2006). Florescence was examined under an Olympus BX-UCB/BX61 microscope.
Vascular Patterning Observation
Cotyledons for vascular patterning observation were prepared as described by Krogan et al. (2012). Briefly, cotyledons from 10-day-old seedlings were fixed with ethanol: acetic acid (3:1, v/v) for 1 h, washed with 95% ethanol for 2 h, stored in 70% ethanol overnight, and then cleared in chloral hydrate: glycerol: water (8:3:1, w/v/v) overnight. Pictures were taken under an Olympus BX-UCB/BX61 microscope.
Results
PtrIAA14.1 is a Poplar Homolog of Arabidopsis IAA7
PoptrIAA7.1 and PoptrIAA7.2 have been shown to be paralogs and are closely related to IAA7 and IAA14 (Kalluri et al., 2007). Because the poplar genome is still under fine annotation, we BLAST searched the updated poplar protein database (www.phytozome.net) using entire amino acid of IAA7 to confirm/identify its poplar homologs. We found that PoptrIAA7.1 and PoptrIAA7.2 are indeed homologs of IAA7 but they have been renamed PtrIAA14.1 (Potri.008G161200) and PtrIAA14.2 (Potri.010G078300), respectively. As shown in Figure 1A, the amino acid identity and similarity between IAA7 and PtrIAA14.1 are 66.92 and 72.62%, respectively, between IAA7 and PtrIAA14.2 are 67.3 and 73.38%, respectively, and between PtrIAA14.1 and PtrIAA14.2 are 93.15 and 96.19%, respectively.

Figure 1. PtrIAA14.1 is a homolog of Arabidopsis Aux/IAA protein IAA7. (A) Amino acid sequence identities and similarities of poplar Aux/IAA proteins PtrIAA14.1 and PtrIAA14.2 and the Arabidopsis Aux/IAA proteins IAA7 and IAA14. Green stands for identities, and blue refers to similarities. (B) Amino acid sequence alignment of PtrIAA14.1, PtrIAA14.2, IAA7, and IAA14. The four conserved domains are underlined. Triangles indicate conserved KR residues between Domain I and Domain II that are crucial for protein degradation, closed circles indicate putative nucleus localization signals, asterisks indicate conserved residues crucial for protein–protein interaction, and open circle indicates conserved W residue in OsIAA23 that is crucial for protein-protein interaction. (C) Expression of PtrIAA14.1 and PtrIAA14.2 in poplar leaves in response to IAA treatment. RNA was isolated from IAA treated poplar leaf discs and RT-PCR was used to examine the expression of PtrIAA14.1. Expression of UBQ was used as a control.
PtrIAA14.1 and PtrIAA14.2 contain the four conserved domains found in most of the Arabidopsis Aux/IAA proteins including IAA7 and IAA14 (Figure 1B). In addition to the conserved LxLxL motif in Domain I, and the conserved QVVGWPPVRSYRK degron in Domain II (Tiwari et al., 2004; Dreher et al., 2006), amino acid sequence alignment showed that the predicted nucleus localization signal in Arabidopsis Aux/IAA proteins, the KR residues between Domain I and II that are crucial for 26 proteasome degradation of Arabidopsis Aux/IAA proteins (Dreher et al., 2006), the conserved amino acid residues in Domain III and IV that are require for homo and hetero interactions among Aux/IAA proteins and ARFs (Nanao et al., 2014), and the conserved W residue in OsIAAs and OsARFs that is crucial for protein–protein interaction (Ni et al., 2014) are all conserved in PtrIAA14.1 and PtrIAA14.2 (Figure 1B). Thus only PtrIAA14.1 was chosen for further functional characterization.
Expression of most Arabidopsis Aux/IAA genes including IAA7 were auxin inducible. To test if PtrIAA14.1 is also auxin inducible, leaf discs from poplar were treated with exogenously IAA, and RT-PCR was used to examine the expression levels of PtrIAA14.1. As shown in Figure 1C, the transcription level of both PtrIAA14.1 and PtrIAA14.2 was slight increased in response to auxin treatment.
PtrIAA14.1 is a Transcription Repressor
Because PtrIAA14.1 is highly similar to IAA7 at amino acid sequence level, and it contains all the conserved motifs and amino acid signatures presented in IAA7 (Figure 1B), we examined if PtrIAA14.1 functions as transcriptional repressor as well with protoplast transfection assays.
We first tested if PtrIAA14.1 is a nuclear protein by expressing PtrIAA14.1-GFP fusion proteins in protoplasts. As shown in Figure 2A, florescence was observed predominately in nucleus. We then co-transfected N-terminal GAL4 DNA binding domain (GD) fused with PtrPtrIAA14.1 (GD-PtrIAA14.1) together with transcriptional activator LD-VP, and the LexA-GAL4-GUS reporter gene into protoplasts. Co-transfection of GD was used as a control. As shown in Figure 2B, in the absence of IAA, co-transfection of LD-VP and GD activated the reporter gene, while co-transfection of LD-VP and GD-PtrIAA14.1 resulted in repression of the reporter gene, suggesting that PtrIAA14.1 functions as a transcriptional repressor. Application of auxin partially released the repression on the expression of the reporter gene by PtrIAA14.1 (Figure 2B), indicating that PtrIAA14.1, similar to IAA7, is unstable in the presence of auxin. We also examined if PrIAA14.1 represses auxin response gene expression by expressing PtrIAA14.1 in protoplasts containing an integrated auxin response gene reporter DR5:GUS. As shown in Figure 2C, PtrIAA14.1 repressed DR5:GUS expression.
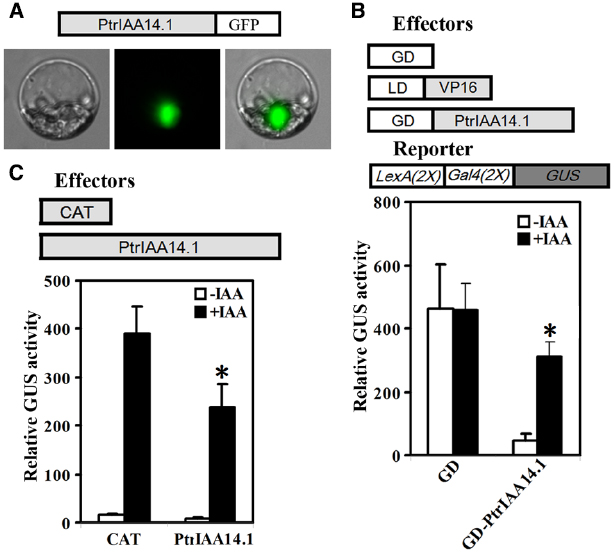
Figure 2. PtrIAA14.1 is a transcriptional repressor. (A) Nuclear localization of PtrIAA14.1. PtrIAA14.1-GFP (diagrammed on the top of the figure). Plasmids were transfected into protoplasts isolated from Arabidopsis leaves and incubated in darkness for 20–22 h before florescence was examined under a florescence microscope. Repression of LexA-Gal4-GUS reporter gene (B) and the integrated DR5:GUS reporter gene (C) by PtrIAA14.1. Effectors and reporters (diagrammed on the top of the figures) or effectors plasmids alone were transfected into protoplasts and incubated for 20–22 h before GUS activity was assayed. Data represent the mean ± SD of three replicates. *Significantly different from absence of IAA (B), or CAT control (C) (P < 0.05).
Expression of PtrIAA14.1 Resulted in Morphological Changes in Arabidopsis
To further explore the function of PtrIAA14.1 in auxin signaling and plant growth and development, we generated transgenic plant expressing PtrIAA14.1 under the control of the 35S promoter. Because PtrIAA14.1 contains a wild-type Domain II, we were not expecting to see any morphological alternations in the transgenic plants if PtrIAA14.1 acts similarly as Arabidopsis Aux/IAA proteins. However, more than 10 lines of transgenic plants with similar phenotypic changes were obtained. Because fertility in strongest transgenic lines was abolished, two moderate transgenic lines were selected for further phenotypic analysis.
As shown in Figure 3, adult transgenic plants expressing PtrIAA14.1 showed several auxin-related phenotypes, including down-curling leaves, semi-draft with increased number of branches (Figure 3A), and greatly reduced fertility (Figure 3B). Quantitative analysis showed that the length of main inflorescence stems in transgenic plants was greatly reduced, whereas length of branch stems on the main inflorescences was largely unaffected (Figure 3C). Our analysis also showed that when compared with that in wild-type plants, the number of primary rosette-leaf branch (RI) was increased dramatically, while the number of primary cauline-leaf branch (CI) remained unchanged in the transgenic plants (Figure 3D). On the other hand, under our growth conditions, wild-type plants did not, but transgenic plants produced secondary rosette-leaf branch (RII) and secondary branch cauline-leaf (CII; Figure 3D).
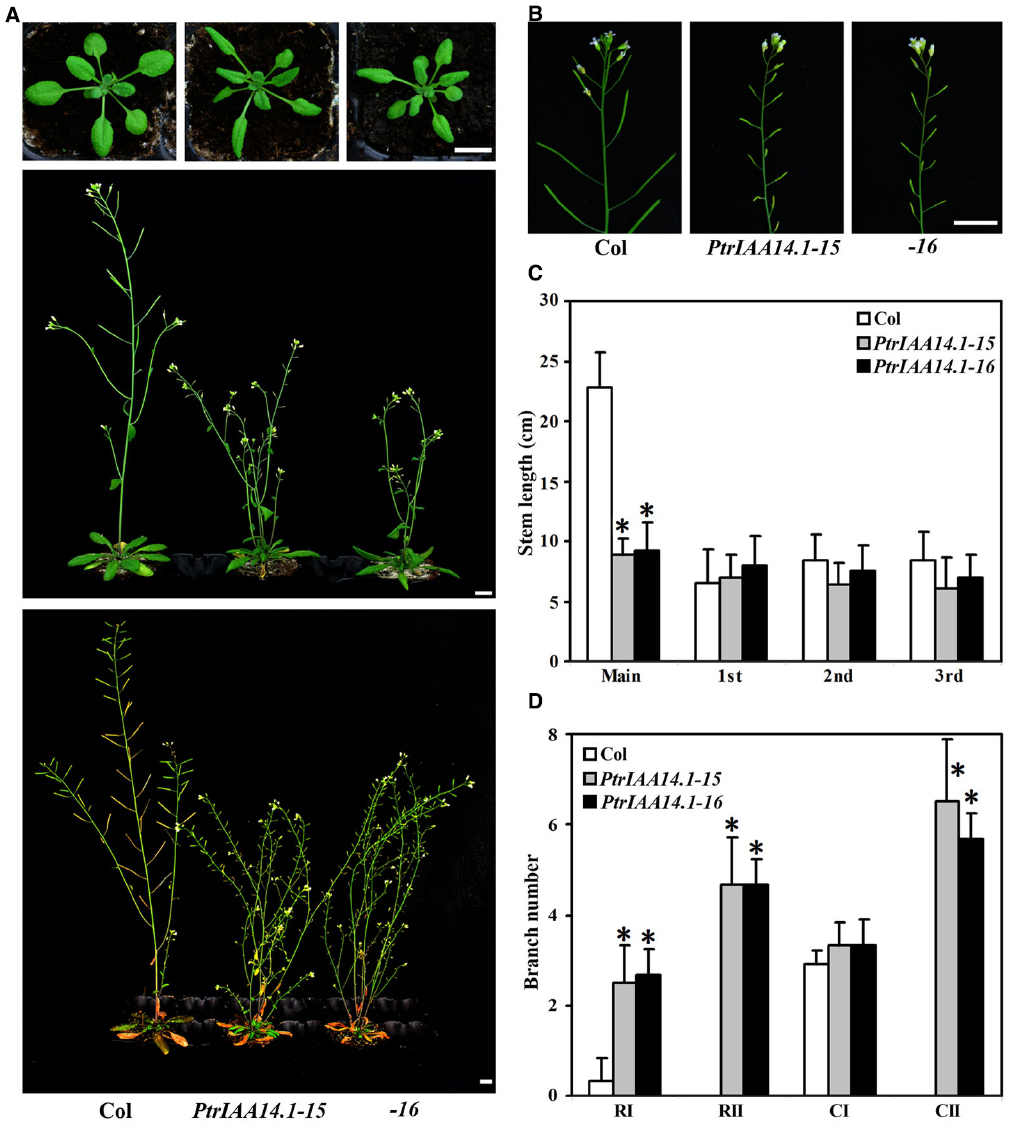
Figure 3. Morphological comparison of wild-type plants and transgenic Arabidopsis plants expressing wild-type PtrIAA14.1. (A) Three-week-old (top panel), 5-week-old (middle panel), and 7-week-old (bottom panel) wild-type and transgenic plants. (B) Inflorescence stems from 5-week-old wild-type and transgenic plants. (C) Stem length of wild-type and transgenic plants. Length of main and branch stems from 5-week-old plants was measured. (D) Branch number of wild-type and transgenic plants. Branches on 7-week-old were counted. The names used for different branches were as described by Aguilar-Martínez et al. (2007). Scale bars: (A,B), 1 cm. Data in (C,D) represent the mean ± SD of 5–11 plants. *Significantly different from wild-type (P < 0.01).
Root Elongation in Arabidopsis Seedlings Expressing PtrIAA14.1 is Less Sensitive to Auxin Treatment
Though pleiotropic phenotypes were observed in adult transgenic plants expressing PtrIAA14.1 (Figure 3), transgenic plant seedlings were largely indistinguishable from wild-type (Figure 4A). To examine if auxin responsiveness in transgenic plants is altered, we analyzed the effects of auxin on root elongation and lateral root formation in transgenic plant seedlings.
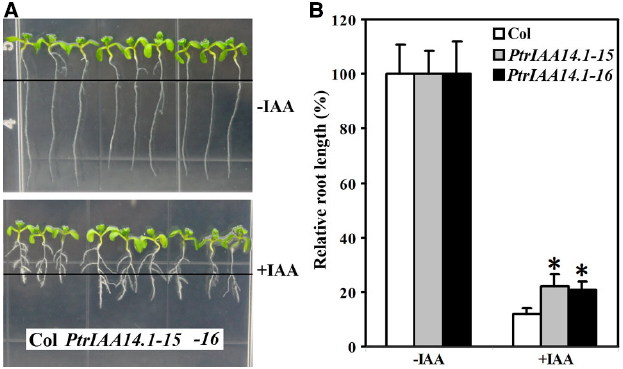
Figure 4. Effects of auxin on root elongation and lateral root formation in wild-type and transgenic plants. (A) Eight-day-old seedlings on vertical plates at the presence (bottom panel) and absent (top panel) of 0.1 μM IAA. Four-day-old seedlings grown on vertical plates were transferred into new plates with or without auxin added, and grown vertically for additional 4 days. Lines indicate the root tip positions immediately after transfer. (B) Inhibition of root elongation by auxin. Length of new elongated roots was measured and relative root length was calculated. Data represent the mean ± SD of at least 10 seedlings. *Significantly different from wild-type (P < 0.0001).
Four-day-old seedlings of wild-type and transgenic plants grown on vertical 1/2 MS plates were transferred to new 1/2 MS plates with or without 0.1 μM IAA and grown vertically for additional 4 days. The root length was measured and relative root growth was calculated. As shown in Figure 4, Arabidopsis seedlings expressing PtrIAA14.1 was less sensitive to auxin treatments. The root length of wild-type seedlings with auxin treatments averaged about 10% of the root length (i.e., reduction by 90%) as compared to seedlings grown in normal conditions. In comparison, PtrIAA14.1 seedlings with auxin treatments grew to about 20% of the normal root length (i.e., reduction by 80%; Figure 4B). Lateral root formation in transgenic plant seedlings in response to auxin, however, remains unchanged when compared with that in wild type (Figure 4A).
Expression of the Arabidopsis Aux/IAA genes in Transgenic Plants is Largely Unaffected
PtrIAA14.1 repressed auxin response reporter gene expression in protoplasts (Figure 2C). Adult transgenic plants expressing PtrIAA14.1 have pleiotropic phenotypes (Figure 3), and was less sensitive to auxin treatments as measured by the root growth inhibition assays (Figure 4). We wanted to further examine if expression of auxin responsive genes in the transgenic plants was affected. We first crossed DR5:GUS reporter line to the PtrIAA14.1 transgenic line, and examined reporter gene expression by detecting GUS activity in the seedlings. As shown in Figure 5A, in both wild-type and transgenic plant background, GUS activity was mainly observed in root and cotyledon tips in the absent of auxin treatment, and GUS activity was detected in nearly all parts of the seedlings when treated with 10 μM IAA, however, no dramatically difference was observed between wild-type and transgenic plants. On the other hand, slightly increased GUS activity was observed near the edges of full expanded rosette leaves from 3-week-old transgenic plants, and in the veins of the leaves upon auxin treatment (Figure 5B).
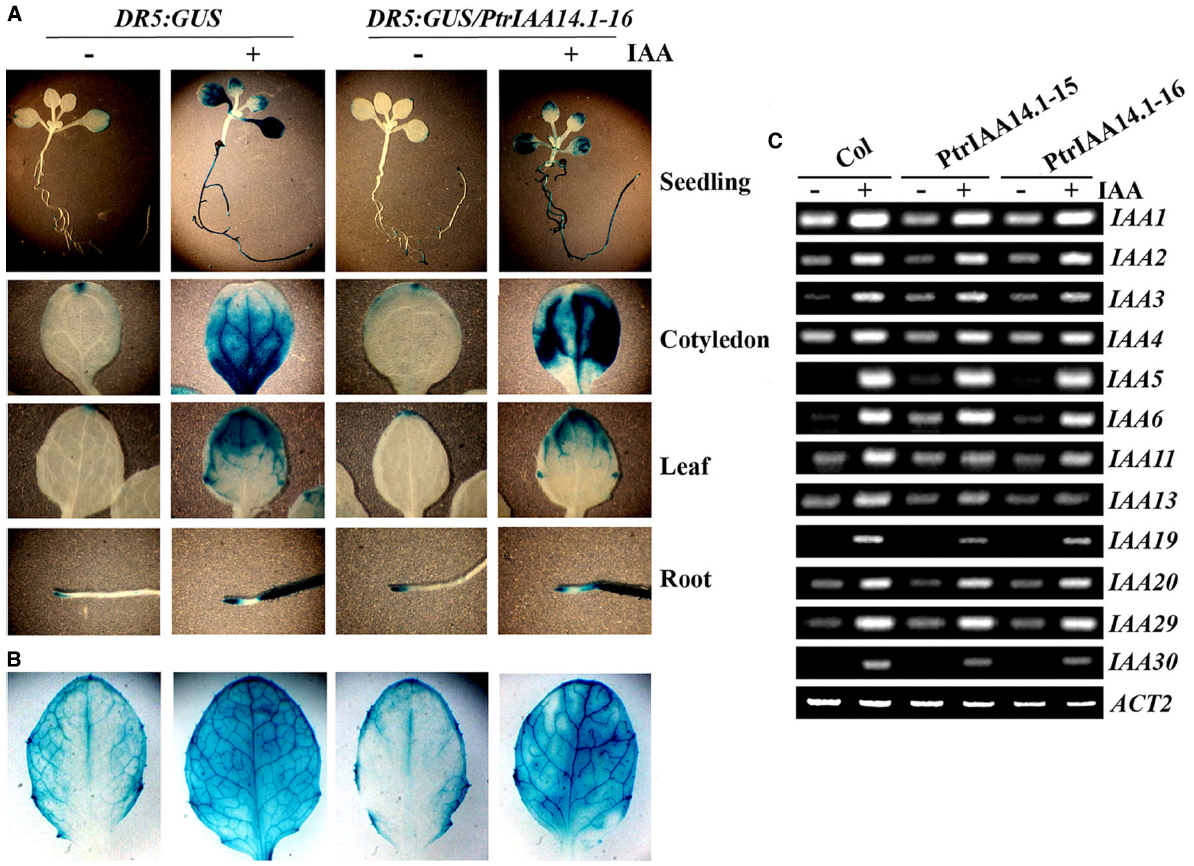
Figure 5. Expression of auxin response genes in transgenic plants expressing PtrIAA14.1. Expression of DR5:GUS reporter gene in wild-type and transgenic seedlings (A) and mature rosette leaves from 3-week-old plants (B). X-Gluc (5-bromo-4-chloro-3indolyl-β-d-glucuronide) was used for histochemical staining of GUS activity. (C) Expression of Aux/IAA genes in wild-type and transgenic plants. Ten-day-old seedlings were treated with 10 μM IAA for 4 h, and then RNA was isolated. RT-PCR was used to examine the expression of Aux/IAA genes. Expression of ACT2 was used as a control.
By using RT-PCR, we also examined the expression of some endogenous Aux/IAA genes in the transgenic plants, including IAA1 to 6, IAA11, IAA13, IAA19, IAA20, IAA29, and IAA30. Expression of all the Arabidopsis Aux/IAA genes tested was elevated in response to auxin treatment, and in general, their expression in transgenic plants remains largely unchanged (Figure 5C). Yet some slight changes in the expression of some Aux/IAA genes were observed in the transgenic plants. For example, the expression of IAA6 was slightly increased in the transgenic plants in the absent of auxin treatment, and auxin responsiveness of IAA11 was slightly decreased in the transgenic plants (Figure 5C).
PtrIAA14.1 Interacts With ARF5 in Yeast and in Plant Cells
Interplay of ARFs and Aux/IAA proteins controls auxin signaling in Arabidopsis. There are only five ARFs including ARF5, ARF6, ARF7, ARF8, and ARF19, that are transcription activators. Because that PtrIAA14.1 repressed auxin response reporter gene expression in protoplasts (Figure 2C), and transgenic plants expressing PtrIAA14.1 have auxin-related phenotypes (Figure 3), we further examined if PtrIAA14.1 regulates auxin signaling and plant growth and development via interacting with ARF activators by using yeast two hybridization and protoplast transfection assays.
Empty bait vector pDHB1 or bait vector containing ARF activator CTD coding sequences and prey vector containing full-length PtrIAA14.1 coding sequence or empty prey vector pPR3-N were cotransformed into yeast strain NMY51, and grown in SD-trp-leu and SD-trp-leu-his plates. The results showed that only yeast transformed with bait vector containing ARF5CTD and prey vector containing full-length PtrIAA14.1 grew on SD-trp-leu-his plates (Figure 6A), indicating that PtrIAA14.1 interacts with ARF5 in yeast.
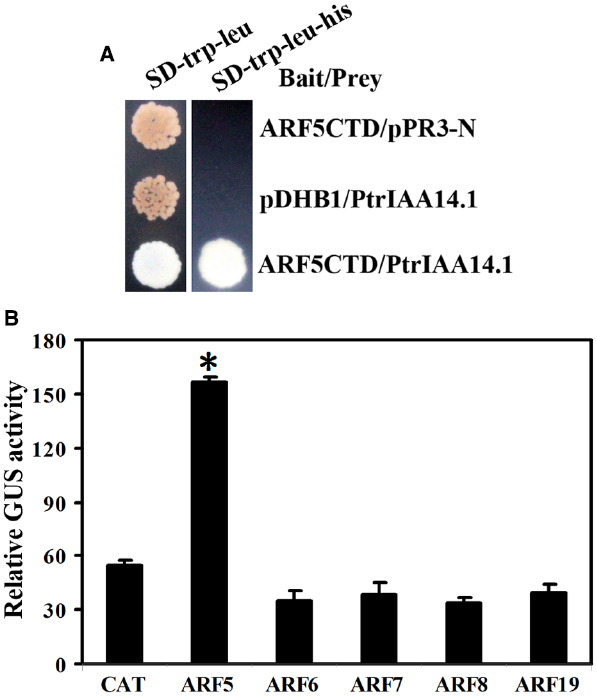
Figure 6. Interaction between PtrIAA14.1 and ARF5 in yeast and plant cells. (A) PtrIAA14.1 interacts with ARF5 in yeast. Bait and prey plasmids were cotransformed into NMY51 yeast cells and grown in SD-trp-Leu and SD-trp-leu-his plates for 2–4 days before picture were taken. (B) PtrIAA14.1 interacts with ARF5 in protoplasts. PtrIAA14.1CTD and ARF5 plasmids were co-transfected into protoplasts and incubated for 20–22 h before GUS activity was assayed. Data represent the mean ± SD of three replicates. *Significantly different from CAT control (P < 0.0001).
To confirm the interaction of PtrIAA14.1 and ARF5 in planta, plasmids of GD fused PtrIAA14.1CTD (GD-PtrIAA14.1CTD) and ARF activators were cotransfected with Gal4:GUS reporter into protoplasts, and GUS activity was measured after incubation. As shown in Figure 6B, cotransfection of GD-PtrIAA14.1CTD with ARF5, but not ARF6, ARF7, ARF8, and ARF19 activated Gal4-GUS reporter, suggesting that PtrIAA14.1 only interacts with ARF5.
Vascular Patterning is Altered in the PtrIAA14.1 Transgenic Plants
It has been reported that ARF5 regulates vascular development in Arabidopsis (Hardtke and Berleth, 1998; Donner et al., 2009). Our results in yeast and protoplast cells suggest that PtrIAA14.1 interacts with ARF5. We hypothesized that vascular patterning in transgenic plants expressing PtrIAA14.1 may be affected. As shown in Figure 7A, changes of vascular patterning were also observed in full expanded rosette leaves (Figure 5B).
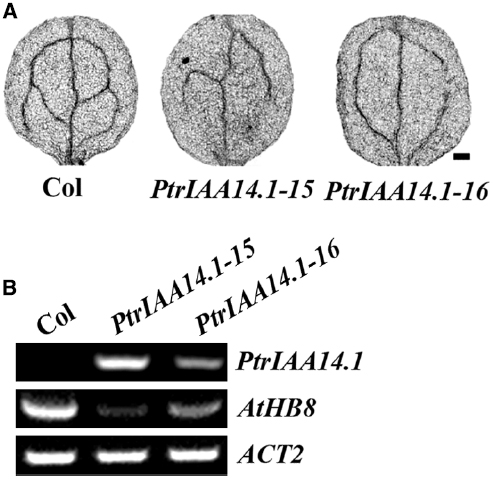
Figure 7. Expression of PtrIAA14.1 in Arabidopsis affected vascular development. (A) Vascular patterning in wild-type and transgenic plant cotyledons. Cotyledons were treated with ethanol: acetic acid (3:1, v/v) solution for 1 h, dehydrated in 95% ethanol for 2 h and in 70% ethanol for overnight, and then cleared in chloral hydrate: glycerol: water (8:3:1, w/v/v) overnight before the pictures were taken. Scale bar: 20 μm. (B) Expression of AtHB8 in transgenic plants. RNA was isolated from 10-day-old seedlings and RT-PCR was used to examine the expression of AtHB8. Expression of ACT2 was used as a control.
Previously studies showed that AtHB8 (Arabidopsis thaliana homeobox gene 8) is an important regulator of vascular development (Baima et al., 2001), and that ARF5 regulates vascular patterning through activating AtHB8 (Donner et al., 2009). Thus we further examined the expression of AtHB8 in PtrIAA14.1 transgenic plants. We found that, unlike the Aux/IAA genes tested, expression of AtHB8 was dramatically decreased in the transgenic plants (Figure 7B), suggesting that PtrIAA14.1 regulates vascular development in Arabidopsis by repressing the expression of AtHB8.
Discussion
Arabidopsis Aux/IAA proteins are transcription repressors that negatively regulate auxin response gene expression by interacting with ARFs (Guilfoyle, 1999; Hagen and Guilfoyle, 2002). Characterization of dominant gain-of-function mutants revealed that Aux/IAA genes regulate many aspects of plant growth and development (Liscum and Reed, 2002). Yet the functions of Aux/IAA proteins in other plants remain largely unknown. Our results in this report showed that poplar Aux/IAA protein PtrIAA14.1 regulates auxin signaling and plant growth and development in Arabidopsis in a way similar but may also be different from that of Arabidopsis Aux/IAA proteins.
PtrIAA14.1 is a Classic Aux/IAA Protein
Most Aux/IAA proteins including IAA7 in Arabidopsis contain four conserved domains, namely Domain I, Domain II, Domain III, and Domain IV. Domain I contains a LxLxL repression motif, Domain II contains a degron, Domain III and IV contain conserved amino acid residues that are required for protein–protein interactions (Ulmasov et al., 1997, 1999; Ramos et al., 2001; Tiwari et al., 2001, 2003, 2004; Dreher et al., 2006; Nanao et al., 2014).
In poplar, there are 35 genes encoding Aux/IAA proteins. PtrIAA14.1 is the most closely related one to Arabidopsis IAA7 (Kalluri et al., 2007). Bioinformatics analysis showed that PtrIAA14.1 shared high sequence identity and similarity to IAA7 on protein sequence level (Figure 1A), and that it contains all of the Aux/IAA features described above (Figure 1B). In addition, PtrIAA14.1 also contains the conserved KR residues that have been shown to be crucial for 26 proteasome degradation of Aux/IAA proteins (Dreher et al., 2006), and the conserved W residue in OsIAAs and OsARFs that has been shown to be crucial for protein–protein interactions (Ni et al., 2014; Figure 1B). Thus we expect PtrIAA14.1 may have similar functions to most Arabidopsis Aux/IAA proteins do. Indeed, protoplast transfection assays showed that PtrIAA14.1 was localized in nucleus. It was unstable in the presence of auxin, and it functioned as a transcription repressor. Moreover, it repressed auxin response reporter gene expression (Figure 2). All these results suggest that PtrIAA14.1 is a classic Aux/IAA protein.
Similarity and Specificity of PtrIAA14.1 and Arabidopsis Aux/IAA Proteins in Regulating Plant Growth and Development
The functions of Arabidopsis Aux/IAA proteins in the regulating of plant growth and development are revealed by characterization of dominant gain-of-function mutants, and all the mutations were occurred within Domain II of corresponding Aux/IAA proteins (Tian and Reed, 1999; Nagpal et al., 2000; Rogg et al., 2001; Fukaki et al., 2002; Tian et al., 2002; Knox et al., 2003; Uehara et al., 2008; Ploense et al., 2009; Rinaldi et al., 2012), indicating that stability of Aux/IAA proteins are crucial for their proper functions in auxin regulated plant growth and development. Consistent with this, transgenic Arabidopsis plants overexpressing wild-type Aux/IAA1 are morphological indistinguishable to wild type (Park et al., 2002). Transgenic Arabidopsis plants expressing a wild-type grape Aux/IAA are also morphological similar to wild-type plants (Fujita et al., 2012; Kohno et al., 2012). Whereas transgenic Arabidopsis plant overexpressing Domain II mutated IAA1, IAA 14, Domain II lacking Aux/IAA, or Aux/IAA with dominant mutation-type Domain II resulted in auxin-related phenotypes (Park et al., 2002; Fukaki et al., 2005; Sato and Yamamoto, 2008).
Because PtrIAA14.1 is a classic Aux/IAA protein with all the features presented in most Arabidopsis Aux/IAA proteins, including a degron in Domain II, and conserved KR residues between Domain I and Domain II (Figure 1B), and PtrIAA14.1 was unstable in protoplasts in the presence of auxin (Figure 2B), we did not expected that expression of PtrIAA14.1 in Arabidopsis would result in any morphological changes. To our surprise, transgenic Arabidopsis plants expressing PtrIAA14.1 showed several auxin-related phenotypes (Figure 5), and were less sensitive to auxin in term of root elongation (Figure 4). Yet the phenotypes are largely different from that in the dominant gain-of-function mutant iaa7 (Nagpal et al., 2000). On the other hand, the expression of most of the Arabidopsis Aux/IAA genes tested in the transgenic plants remained largely unchanged. These results indicate that unlike most Arabidopsis Aux/IAA proteins, PtrIAA14.1 may use different mechanisms in regulating plant growth and development.
PtrIAA14.1 Regulates Vascular Patterning in Arabidopsis Through Interacting with ARF5
Aux/IAA proteins regulate auxin response gene expression via interacting with ARFs (Guilfoyle and Hagen, 2007). PtrIAA14.1 has all the conserved amino acids required for protein–protein interactions in Domain III and IV (Nanao et al., 2014). It also has the W residue conserved in OsIAAs and OsARFs that is required for protein–protein interactions (Ni et al., 2014). Thus it is reasonable to expect that PtrIAA14.1 interacts with ARFs. Indeed, interaction between PtrIAA14.1 and ARF5 was observed in both yeast and plant cells (Figure 6).
Previously studies have shown that ARF5 regulates vascular development by activating AtHB8 (Donner et al., 2009). Our results also showed that vascular patterning was changed in the transgenic plants expressing PtrIAA14.1, and that the expression of AtHB8 in transgenic plants was also downregulated, suggesting that PtrIAA14.1 regulates vascular development when expressed in Arabidopsis by repressing the expression of AtHB8 via interacting with ARF5. Interaction of PtrIAA14.1 with ARF5, but not other ARF activators (Figure 6B) may also responsible for the auxin-related phenotypes observed in the transgenic plants expressing PtrIAA14.1.
Because there is only one amino acid residue difference found in Domain III and Domain IV, respectively, between PtrIAA14.1 and IAA7, and the total number of amino acid residue difference in the C-terminal of PtrIAA14.1 and IAA7 was less than 10 (Figure 1B), it will be interesting to examine why PtrIAA14.1 interacts specifically with ARF5.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Brian Ellis (University of British Columbia) for providing bait and prey vectors and yeast strain for yeast-two-hybrid analysis. This work was supported by the Department of Human Resources and Social Security of Jilin Province (http://hrss.jl.gov.cn/) and a startup fund from Northeast Normal University (www.nenu.edu.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
Aguilar-Martínez, J. A., Poza-Carrión, C., and Cubas, P. (2007). Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19, 458–472. doi: 10.1105/tpc.106.048934
Baima, S., Possenti, M., Matteucci, A., Wisman, E., Altamura, M. M., Ruberti, I., et al. (2001). The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 126, 643–655. doi: 10.1104/pp.126.2.643
Bassa, C., Mila, I., Bouzayen, M., and Audran-Delalande, C. (2012). Phenotypes associated with down-regulation of Sl-IAA27 support functional diversity among Aux/IAA family members in tomato. Plant Cell Physiol. 53, 1583–1595. doi: 10.1093/pcp/pcs101
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Davies, P. J. (1995). Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht: Kluwer.
Deng, W., Yang, Y., Ren, Z., Audran-Delalande, C., Mila, I., Wang, X., et al. (2012). The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol. 194, 379–390. doi: 10.1111/j.1469-8137.2012.04053.x
Donner, T. J., Sherr, I., and Scarpella, E. (2009). Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 136, 3235–3246. doi: 10.1242/dev.037028
Dreher, K. A., Brown, J., Saw, R. E., and Callis, J. (2006). The Arabidopsis Aux/IAA protein family has diversified in degradation and auxin responsiveness. Plant Cell 18, 699–714. doi: 10.1105/tpc.105.039172
Fukaki, H., Nakao, Y., Okushima, Y., Theologis, A., and Tasaka, M. (2005). Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 44, 382–395. doi: 10.1111/j.1365-313X.2005.02537.x
Fukaki, H., Tameda, S., Masuda, H., and Tasaka, M. (2002). Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J. 29, 153–168. doi: 10.1046/j.0960-7412.2001.01201.x
Fujita, K., Horiuchi, H., Takato, H., Kohno, M., and Suzuki, S. (2012). Auxin-responsive grape Aux/IAA9 regulates transgenic Arabidopsis plant growth. Mol. Biol. Rep. 39, 7823–7829. doi: 10.1007/s11033-012-1625-9
Geraldes, A., Pang, J., Thiessen, N., Cezard, T., Moore, R., Zhao, Y., et al. (2011). SNP discovery in black cottonwood (Populus trichocarpa) by population transcriptome resequencing. Mol. Ecol. Resources Supplemental 1, 81–92. doi: 10.1111/j.1755-0998.2010.02960.x
Guilfoyle, T. J. (1999). “Auxin-regulated genes and promoters,” in Biochemistry and Molecular Biology of Plant Hormones, eds P. J. J. Hooykaas, M. Hall, and K. L. Libbenga (Leiden: Elsevier), 423–459.
Guilfoyle, T. J., and Hagen, G. (2007). Auxin response factors. Curr. Opin. Plant Biol 10, 453–460. doi: 10.1016/j.pbi.2007.08.014
Hagen, G., and Guilfoyle, T. J. (2002). Auxin-responsive gene expression: genes, promoters, and regulatory factors. Plant Mol. Biol. 49, 373–485. doi: 10.1023/A:1015207114117
Hardtke, C. S., and Berleth, T. (1998). The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 17, 1405–1411. doi: 10.1093/emboj/17.5.1405
Hajdukiewicz, P., Svab, Z., and Maliga, P. (1994). The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol. Biol. 25, 989–994. doi: 10.1007/BF00014672
Jun, N., Gaohang, W., Zhenxing, Z., Huanhuan, Z., Yunrong, W., and Ping, W. (2011). OsIAA23-mediated auxin signaling defines postembryonic maintenance of QC in rice. Plant J. 68, 433–642. doi: 10.1111/j.1365-313X.2011.04698.x
Kalluri, U. C., Difazio, S. P., Brunner, A. M., and Tuskan, G. A. (2007). Genome-wide analysis of Aux/IAA and ARF gene families in Populus trichocarpa. BMC Plant Biol. 7:59. doi: 10.1186/1471-2229-7-59
Kitomi, Y., Inahashi, H., Takehisa, H., Sato, Y., and Inukai, Y. (2012). OsIAA13-mediated auxin signaling is involved in lateral root initiation in rice. Plant Sci. 190, 116–122. doi: 10.1016/j.plantsci.2012.04.005
Knox, K., Grierson, C. S., and Leyser, O. (2003). AXR3 and SHY2 interact to regulate root hair development. Development 130, 5769–5777. doi: 10.1242/dev.00659
Kohno, M., Takato, H., Horiuchi, H., Fujita, K., and Suzuki, S. (2012). Auxin-nonresponsive grape Aux/IAA19 is a positive regulator of plant growth. Mol. Biol. Rep. 39, 911–917. doi: 10.1007/s11033-011-0816-0
Krogan, N. T., Ckurshumova, W., Marcos, D., Caragea, A. E., and Berleth, T. (2012). Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol. 194, 391–401. doi: 10.1111/j.1469-8137.2012.04064.x
Liu, S., Wang, X., Li, E., Douglas, C. J., Chen, J.-G., and Wang, S. (2013). R2R3 MYB transcription factor PtrMYB192 regulates flowering time in Arabidopsis by activating FLOWERING LOCUS C. J. Plant Biol. 56. 243–250. doi: 10.1007/s12374-013-0135-1
Liscum, E., and Reed, J. W. (2002). Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 49, 387–400. doi: 10.1023/A:1015255030047
Muto, H., Watahiki, M. K., Nakamoto, D., Kinjo, M., and Yamamoto, K. T. (2007). Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2 IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol. 144, 187–196. doi: 10.1104/pp.107.096628
Nagpal, P., Walker, L. M., Young, J. C., Sonawala, A., Timpte, C., Estelle, M., et al. (2000). AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol. 123, 563–574. doi: 10.1104/pp.123.2.563
Nanao, M. H., Vinos-Poyo, T., Brunoud, G., Thévenon, E., Mazzoleni, M., Mast, D., et al. (2014). Structural basis for oligomerization of auxin transcriptional regulators. Nat. Commun. 5, 3617. doi: 10.1038/ncomms4617
Ni, J., Zhu, Z., Wang, G., Shen, Y., Zhang, Y., and Wu, P. (2014). Intragenic suppressor of osiaa23 revealed a conserved tryptophan residue crucial for protein-protein interactions. PLoS ONE 9:85358. doi: 10.1371/journal.pone.0085358
Overvoorde, P. J., Okushima, Y., Alonso, J. M., Chan, A., Chang, C., Ecker, J. R., et al. (2005). Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17, 3282–3300. doi: 10.1105/tpc.105.036723
Ploense, S. E., Wu, M. F., Nagpal, P., and Reed, J. W. (2009). A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 136, 1509–1517. doi: 10.1242/dev.025932
Park, J. Y., Kim, H. J., and Kim, J. (2002). Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J. 32, 669–683. doi: 10.1046/j.1365-313X.2002.01459.x
Ramos, J. A., Zenser, N., Leyser, O., and Callis, J. (2001). Rapid degradation of auxin/indoleacetic acid proteins requires conserved amino acids of domain II and is proteasome dependent. Plant Cell 13, 2349–2360. doi: 10.2307/3871512
Reed, J. W. (2001). Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 6, 420–425. doi: 10.1016/S1360-1385(01)02042-8
Rinaldi, M. A., Liu, J., Enders, T. A., Bartel, B., and Strader, L. C. (2012). A gain-of-function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol. Biol. 79, 359–373. doi: 10.1007/s11103-012-9917-y
Rogg, L. E., Lasswell, J., and Bartel, B. (2001). A gain-of-function mutation in IAA28 suppresses lateral root development. Plant Cell 13, 465–480. doi: 10.1105/tpc.13.3.465
Sato, A., and Yamamoto, K. T. (2008). Overexpression of the non-canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol. Plant. 133, 397–405. doi: 10.1111/j.1399-3054.2008.01055.x
Song, Y., and Xu, Z. F. (2013). Ectopic overexpression of an auxin/indole-3-acetic acid (Aux/IAA) gene OsIAA4 in rice induces morphological changes and reduces responsiveness to auxin. Int. J. Mol. Sci. 14, 13645–13656. doi: 10.3390/ijms140713645
Su, L., Bassa, C., Audran, C., Mila, I., Cheniclet, C., Chevalier, C., et al. (2014). The auxin Sl-IAA17 transcriptional repressor controls fruit size via the regulation of endoreduplication-related cell expansion. Plant Cell Physiol. 55, 1969–1976. doi: 10.1093/pcp/pcu124
Tian, Q., and Reed, J. W. (1999). Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126, 711–721.
Tian, Q., Uhlir, N. J., and Reed, J. W. (2002). Arabidopsis SHY2/IAA3 inhibits auxin-regulated gene expression. Plant Cell 14, 301–419. doi: 10.1105/tpc.010283
Tiwari, S., Wang, S., Hagen, G., and Guilfoyle, T. J. (2006). Transfection assays with protoplasts containing integrated reporter genes. Methods Mol. Biol. 323, 237–244. doi: 10.1385/1-59745-003-0:237
Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2003). The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15, 533–543. doi: 10.1105/tpc.008417
Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2004). Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16, 533–543. doi: 10.1105/tpc.017384
Tiwari, S. B., Wang, X.-J., Hagen, G., and Guilfoyle, T. J. (2001). Aux/IAA proteins are active repressors and their stability and activity are modulated by auxin. Plant Cell 13, 2809–2822. doi: 10.2307/3871536
Uehara, T., Okushima, Y., Mimura, T., Tasaka, M., and Fukaki, H. (2008). Domain II mutations in CRANE/IAA18 suppress lateral root formation and affect shoot development in Arabidopsis thaliana. Plant Cell Physiol. 49, 1025–1038. doi: 10.1093/pcp/pcn079
Ulmasov, T., Hagen, G., and Guilfoyle, T. J. (1997). ARF1, a transcription factor that binds auxin response elements. Science 276, 1865–1868.
Ulmasov, T., Hagen, G., and Guilfoyle, T. J. (1999). Activation and repression of transcription by auxin response factors. Proc. Natl. Acad. Sci. U.S.A. 96, 5844–5849.
Wang, H., Jones, B., Li, Z., Frasse, P., Delalande, C., Regad, F., et al. (2005a). The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17, 2676–2692. doi: 10.1105/tpc.105.033415
Wang, S., Tiwari, S. B., Hagen, G., and Guilfoyle, T. J. (2005b). AUXIN RESPONSE FACTOR7 restores the expression of auxin-responsive genes in mutant Arabidopsis leaf mesophyll protoplasts. Plant Cell 17, 1979–1993. doi: 10.1105/tpc.105.031096
Wang, S., Chang, Y., Guo, J., and Chen, J. G. (2007). Arabidopsis ovate family protein 1 is a transcriptional repressor that suppresses cell elongation. Plant J. 50, 858–872. doi: 10.1111/j.1365-313X.2007.03096.x
Wang, S., Li, E., Porth, I., Chen, J. G., Mansfield, S. D., and Douglas, C. J. (2014). Regulation of secondary cell wall biosynthesis by poplar R2R3 MYB transcription factor PtrMYB152 in Arabidopsis. Sci. Rep. 4, 5054. doi: 10.1038/srep05054
Zhu, Z. X., Liu, Y., Liu, S. J., Mao, C. Z., Wu, Y. R., and Wu, P. (2012). A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol. Plant 5, 154–161. doi: 10.1093/mp/ssr074
Keywords: Aux/IAA, auxin response factor, PtrIAA14.1, vascular patterning, Arabidopsis, Populus trichocarpa
Citation: Liu S, Hu Q, Luo S, Li Q, Yang X, Wang X and Wang S (2015) Expression of wild-type PtrIAA14.1, a poplar Aux/IAA gene causes morphological changes in Arabidopsis. Front. Plant Sci. 6:388. doi: 10.3389/fpls.2015.00388
Received: 23 February 2015; Accepted: 15 May 2015;
Published: 02 June 2015.
Edited by:
Yunde Zhao, University of California, San Diego, USAReviewed by:
Zhong-Nan Yang, Shanghai Normal University, ChinaXia Wu, University of Washington, USA
Copyright © 2015 Liu, Hu, Luo, Li, Yang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shucai Wang, School of Life Sciences, Northeast Normal University, 5268 Renmin Street, Changchun 130024, China, wangsc550@nenu.edu.cn
 Shanda Liu
Shanda Liu