- Rubiales Laboratory, Department of Plant Breeding, Institute for Sustainable Agriculture, Consejo Superior de Investigaciones Científicas, Córdoba, Spain
Orobanche crenata is a holoparasitic plant that is potentially devastating to crop yield of legume species. Soil temperature and humidity are known to affect seed germination, however, the extent of their influence on germination and radicle growth of those of O. crenata is largely unknown. In this work, we studied the effects of temperature, water potential (Ψt) and the type of water stress (matric or osmotic) on O. crenata seeds during conditioning and incubation periods. We found that seeds germinated between 5 and 30°C during both periods, with a maximum around 20°C. Germination increased with increasing Ψt from −1.2 to 0 MPa during conditioning and incubation periods. Likewise, seed germination increased logarithmically with length of conditioning period until 40 days. The impact of the type of water stress on seed germination was similar, although the radicle growth of seeds under osmotic stress was lower than under matric stress, what could explain the lowest infestation of Orobanche sp. in regions characterized by saline soil. The data in this study will be useful to forecast infection of host roots by O. crenata.
Introduction
The holoparasitic weed Orobanche crenata Forsk. is responsible for important crop losses across the Mediterranean and West Asia where it parasitizes mainly Fabaceae species such as faba bean (Vicia faba), grasspea (Lathyrus sativus), lentil (Lens culinaris), pea (Pisum sativum), and vetches (Vicia sp.), but also Umbelliferae such as carrot (Daucus carota) (Grenz and Sauerborn, 2007; Parker, 2009). Because each Orobanche plant produces 1000s of minute seeds that persist viable in the soil for many years increasing the parasite seedbank in the soil and because infection and pathogenic process takes place underground (Joel et al., 2007) the effective control of this weedy species is extremely difficult (Rubiales et al., 2009).
Orobanche seeds germinate under favorable environmental conditions and the presence of chemical stimulants in the root exudates of proper plant species (Fernández-Aparicio et al., 2009). Before this, the Orobanche seeds are in an inactive state. Only after a conditioning period of several days that follows seed imbibition, O. crenata seeds can respond to germination stimulants (Van Hezewijk et al., 1993). However, this is not the case for other Orobanche species such as O. cumana and O. aegyptiaca (syn. Phelipanche aegyptiaca) in which this conditioning helps, but is not essential for stimulant receptivity (Plakhine et al., 2009). During the conditioning period, temperature (T), water potential (Ψt), oxygen availability and growth regulators are known to affect the seed viability of several species of Orobanche and their germination response (Van Hezewijk et al., 1993; Kebreab and Murdoch, 1999; Gibot-Leclerc et al., 2004; Song et al., 2005).
Environmental factors, especially T and Ψt, affect the germination of conditioned seeds during incubation after exposure to germination stimulants (Kebreab and Murdoch, 2000). Once the parasitic seed germination is induced, an infective radicle arises from the seed coat and grows, following a positive gradient of germination stimulants until the host root, to which it can adhere and penetrate (Fernández-Aparicio et al., 2010). Therefore, the seeds, which show a large radicle, reach far roots what increases the infection efficiency. Temperature and Ψt can also affect the seed radicle elongation (Dodd and Donovan, 1999). Nevertheless, the impact of both T and Ψt on radicle elongation of Orobanche seeds are little understood. This information is essential for the development of germination and infection submodels, critical components to forecast effects of O. crenata on legume hosts.
Water potential quantifies the tendency of water to move from one point to another. In the soil, Ψt is mainly the sum of: (i) the gravitational potential (Ψg); (ii) osmotic potential (Ψo) as a consequence of the presence of ionic changes due to salts and non-ionically due to water binding by components on plant parts or other solutes; and (iii) matric potential (Ψm) caused by water adsorption and surface tension phenomena in soil (Papendick and Campbell, 1980). Whereas Ψm and Ψo can change substantially and therefore affect seed germination, gravitational potential is determined solely by elevation of a point to some arbitrary reference point being negligible in near points (i.e., seed and adjacent water). In non-saline soils, Ψm is the dominant component (Papendick and Campbell, 1980; Chowdhury et al., 2011).
Furthermore, seed germination is differently affected by comparable Ψm and Ψo, although their free energy measurements are equal (Hillel, 1972; Schmidhalter and Oertli, 1991). Thus, the seeds of different plants (Meiri, 1984; Schmidhalter and Oertli, 1991) and several microorganisms (Ramirez et al., 2004; Chowdhury et al., 2011) have been demonstrated to be more sensitive to low Ψm than low Ψo. However, very little attention has been given to study the impact of Ψm and Ψo on seed germination of Orobanche species.
In this study, our objectives were to determinate the influence of temperature (T), water stress (Ψt), and type of water stress (Ψm and Ψo) on seed germination and radicle length of O. crenata during both the conditioning and the incubation periods. These results will be of value for development of predictive infection models.
Materials and Methods
Orobanche Seeds
Seeds were collected from O. crenata plants infecting faba beans during 2010 in Córdoba (37.51°N, 4.80°W, elevation of 110 m), southern Spain. Dry seeds were stored in glass containers in the dark at room temperature until use. Before use, seeds were disinfected with formaldehyde as described by González-Verdejo et al. (2005). To ensure that all germination requirements other than T and Ψt were satisfied, we included exogenous application of 1.2 mL of water or solution per 5-cm Petri dish, with 10 ppm GR24, a synthetic germination stimulant (Johnson et al., 1976) that was applied after conditioning period in the three experiments. In addition, we included seeds which were not exposed to GR24, which were incubated at 20°C at water potential value of 0 MPa, for assuring that this stimulant, is needed to seed germination in O. crenata. For each treatment, three replicate Petri dishes were used and the experiments were carried out twice. In all experiments, control seeds were conditioned with sterile distilled water.
Water Potential Treatments
Because polyethylene glycol (PEG) solutions are relatively non-toxic to seeds (Song et al., 2005), aqueous solutions of PEG 8000 (Sigma 25322-68-3), or Milli-Q water were used for producing a range of matric potentials (0, −0.3, −0.6, −0.9, −1.2, and −3 MPa). The amount of PEG required for each combination T – Ψt was calculated using the polynomial equation of Michel and Kaufmann (1973) and revised by Michel (1983).
Likewise, sterile milli-Q water was modified osmotically by the addition of non-ionic glycerol (Panreac 56-81-5) to 0, −0.3, −0.6, −0.9, −1.2, and −3 MPa (Dallyn and Fox, 1980). The quantity of glycerol used to adjust the water activity (aw) of each solution was calculated using the Norrish’s equation (Harris, 1980) modified by Baeza et al. (2010). Finally, for a sample at given T, the Ψt was uniquely related to the aw through the Kelvin equation:
where R is the universal gas constant, Tk is Kelvin temperature and Mw is the molecular mass of water (Papendick and Campbell, 1980).
The Ψt of all solutions was then confirmed by measurement in a dewpoint potentiameter (WP4 Aqua Lab Water Meter, Decagon Devices, Pullman, WA, USA) and subjected to a slight adjustment when necessary.
Effect of Temperature and Water Potential During the Conditioning Period
Experiment 1
Around 150 seeds of O. crenata were sown per 10-mm disks of glass fiber filter paper (WHATMAN 3645, 175 g m−2). Three disks (pseudoreplicates) were placed in a sterile 5-cm Petri dish lined with two layers of 50-mm diameter glass filter paper wetted with 1.5 mL of sterile milli-Q water or different conditioning media as described by Song et al. (2005). These media were PEG or glycerol solutions at −0.3, −0.6, −0.9, −1.2, and −3 MPa. The Petri dishes were sealed with Parafilm and wrapped with aluminum foil to provide absolute darkness. The Petri dishes were then placed in growth chambers at different temperatures (5, 10, 15, 20, 25, 30, and 35°C). After 5 days, other 0.6 mL of sterile water or conditioning medium was added to each Petri dish to maintain the Ψt and the dishes were placed back in the chambers for two more days.
After conditioning, the seeds were blotted to remove excessive water or conditioning media. Each disk from every replicate Petri dish were then transferred to a separate new 5-cm Petri dish containing two layers of filter paper wetted with 1.2 mL of sterile milli-Q water with 10 ppm GR24. Petri dishes were incubated at 20°C in the dark as describe above. Germination was examined under a compound microscope (Nikon Eclipse 80i; Nikon Corp., Tokyo) at 7 days after GR24 addition counting around 200 seeds per Petri dish. In addition, we randomly selected 30–40 germinated seeds per treatment and the length of their emerging radicle was also measured at this time. In total, 84 treatments [seven temperatures × six Ψt × two types of water stress (Ψm and Ψo)] were evaluated. In all experiments, for each treatment, three replicate Petri dishes were used and the experiment was carried out twice.
Experiment 2
This experiment was nearly similar to our previous experiment but seed germination was assessed periodically at 2, 7, 10, 20, and 40 days after GR24 addition allowing calculation of seed germination percentage. Conditioning temperature was fixed to 20°C, maintaining the PEG and glycerol solutions at 0, −0.3, −0.6, −0.9, −1.2, and −3 MPa. In this case, between 0.1 and 0.3 mL of sterile water or conditioning medium was periodically added to each Petri dish to maintain the Ψt.
Because the germinated seeds were removed to measure the size of the radicles at 7, 20, and 40 days, this evaluation corresponds to seeds germinated between 0–7, 8–20, and 21–40 conditioning days (see Evaluation). Therefore, the radicle of seeds that germinated between the 10th to 20th days had 10 days to develop, and those that germinated between the 20th and 40th day had 20 days to develop. In total, the seeds were subjected to 12 treatments [six Ψt × two types of water stress (Ψm and Ψo)] that were evaluated after five conditioning periods.
Effect of Temperature and Water Potential During the Incubation Period
Experiment 3
This experiment was similar to Experiment 1 except that O. crenata seeds were conditioned at 20°C in the dark for 10 days on the paper disks with sterile distilled water (160 μL per disk). Then the disks were blotted and transferred to new Petri dishes containing filter paper wetted with 1.2 mL of PEG or glycerol solution (0, −0.3, −0.6, −0.9, −1.2, and −3 MPa). Petri dishes were then incubated at 5, 10, 15, 20, 25, 30, and 35°C in the dark. The seed germination was evaluated at 7 and 10 days and the length of the emerging radicle of 30–40 seeds at 7 days as described above. In this case, 84 treatments [seven T × six Ψt × two types of water stress (Ψm and Ψo)] were evaluated and the experiment was conducted twice.
Evaluation
In all cases, the germination of O. crenata seeds were directly quantified on the Petri dishes using a magnification of 40× with the aid of a compound microscope (Nikon Eclipse 80i; Nikon Corp., Tokyo). For that, we counted the total of seeds of several fields of view that was taken at random. Seeds were considered germinated when the length of the emerging radicle was equal to or longer than its width. Length of the emerging radicle of seed was measured at a magnification of 200× with the aid of a compound microscope using the NIS-Element software (Nikon Corp., Tokyo, Japan).
Statistical Analysis
Analysis of variance (ANOVA) was performed on germination percentage or radicle length depending on the design of each experiment. Both germination percentage and radicle length were log or arcsin-transformed when necessary for normality or homogeneity of variances. All experiments were repeated at once, and data from repetitions of each experiment were combined after checking for homogeneity of the experimental error variances by the F test (two variances). Because there were several interactions among independent variables (Experiments 1 and 3), to clarify the effects of Ψt (water vs. negative potentials) or type of water stress (osmotic vs. matric) on the dependent variables, we compared among them using orthogonal contrasts. When the type of water stress did not affect to type of water stress, ANOVA or regression analysis was performed on the whole of data using this variable as repetitions.
Linear regression analysis was used to evaluate the relationship between duration of conditioning period (days) and the cumulative germination percentage (Experiment 2). The duration of the conditioning period (days) was log-transformed. Various linear and non-linear regression models were evaluated for describing the effect of T and Ψt on seed germination and radicle length during the conditioning and the incubation periods (Experiments 1 and 3). The models tested were the generalized Analysis β model (Hau and Kranz, 1990), the Schödter angular model (Hau and Kranz, 1990), the Yin’s model (Yin et al., 1995) and several second- or third-order polynomial equations based on results of ANOVA analysis. We included the Ψt on the models as (Ψt - Ψtmin)c when necessary. The used models were developed with an empirical approach according to the collected data. These models have been used to describe the influence of temperature and water stress on germination of broomrape seeds for the first time.
The Analysis β model (Hau and Kranz, 1990) was selected because it provided a good fit for all experiments and because each parameter has biological meaning. The Analysis β model uses the following equation:
in which Y = standardized germination percentage or radicle length that varied from 0 to 1 (Y = G/Gmax or Y = RS/RSmax); t = standardized temperature [(t = (T -Tmin)/(Tmax -Tmin)]; Ψt = water potential (0 ≥ Ψt ≥ Ψtmin); and k, a, b, and c are unknown parameters. Tmin, Tmax, and Ψtmin were the minimum temperature, maximum temperature, and minimum Ψt for seed germination, respectively. Maximum Y is reached when standardized t =a/(a + b). Thus, for a given Ψt, if the parameter a < b or a > b, the optimum temperature is shifted to the left of right, respectively. In this study, Tmin and Tmax were selected according to the data of our study, and the Ψtmin used on the models (−2 MPa) was selected according previous experiments. A linear regression was applied to test the relationship between data estimated by non-linear regression and observed data. In all cases, the best regression model was chosen from many combinations of terms based on the significance of the estimated parameters (P ≤ 0.05), Mallow’s Cp statistic, Akaike’s information criterion modified for small data sets, the coefficient of determination (R2), R2 adjusted for degrees of freedom (Ra2), and the pattern of residuals over predicted and independent variables.
Results
Both T and Ψt affected the germination of O. crenata seeds during conditioning and incubation periods. Overall, the seeds germinated in a range of T between 10 and 25°C during conditioning and incubation periods, being germination strongly reduced at 25°C. On the contrary, no germination occurred at 5 or 35°C or under −3 MPa at any temperature and it was very limited at 30°C. For this reasons, equations of models were developed in all cases considering 5 and 35°C as Tmin and Tmax, respectively (Table 1). Moreover, there were several double and triple interactions among the independent variables depending on the experiments. No O. crenata seeds untreated with GR24 germinated.
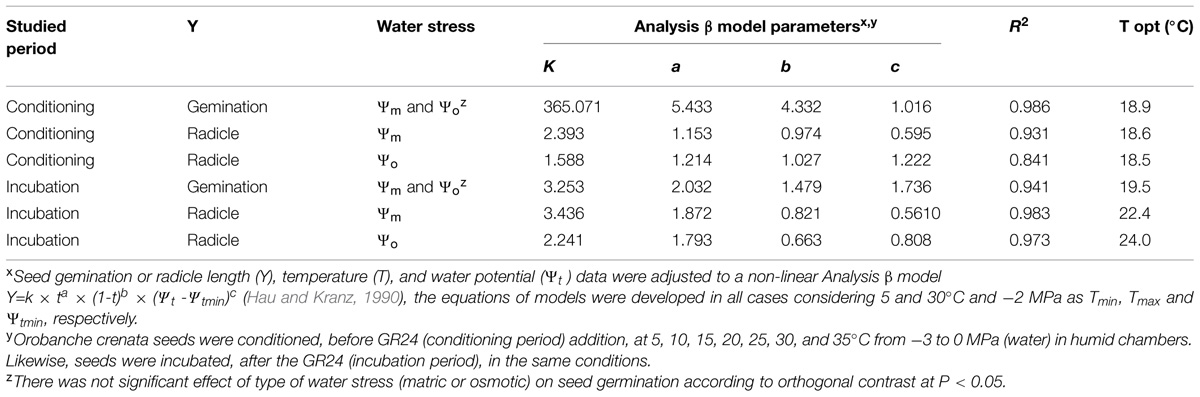
TABLE 1. Effect of temperature (T), water potential (Ψt), and type of water stress [matric (Ψm) or osmotic (Ψo)] during conditioning (Experiment 1) and incubation (Experiment 3) periods on seed germination and radicle length (0–1) of Orobanche crenata seeds according to the fitted Analysis β models.
Effect of Temperature and Water Potential During the Conditioning Period
Experiment 1
Seeds of O. crenata conditioned in water potentials ≥−1.2 MPa germinated over the glass fiber filter papers at temperatures between 10 and 25°C. However, when conditioned at Ψt = −1.2 MPa the seeds did not germinate at 10°C. Maximum seed germination approached 41% in water at 20°C. The germination percentage of seeds conditioned in water was significantly (orthogonal contrasts, P = 0.035) higher than that of the seeds conditioned in negative water potentials. Conversely, the both matric and osmotic stress have similar (orthogonal contrasts, P = 0.6318) impact on the germination percentage of the seeds. For example, the mean of seed germination for the seeds conditioned at 20°C among −0.3 and −1.2 MPa under osmotic or matric stress were 25.9 and 23.7%, respectively. For that, the data from each type of water stress were used as independent repetitions to fit the models (Table 1). The fitted Analysis β model of Ψt-T affecting the germination percentage is illustrated in Figure 1. The fitted model was highly significant (P < 0.001; R2 and Ra2 were >0.90) and the standardized residuals were randomly distributed over predicted values. The optimum T for maximum seed germination, obtained with fitted model, was 18.9°C. Thus, germination higher than 40% is only obtained with a conditioning at 17–20°C at water potential value of 0 MPa (Figure 1).
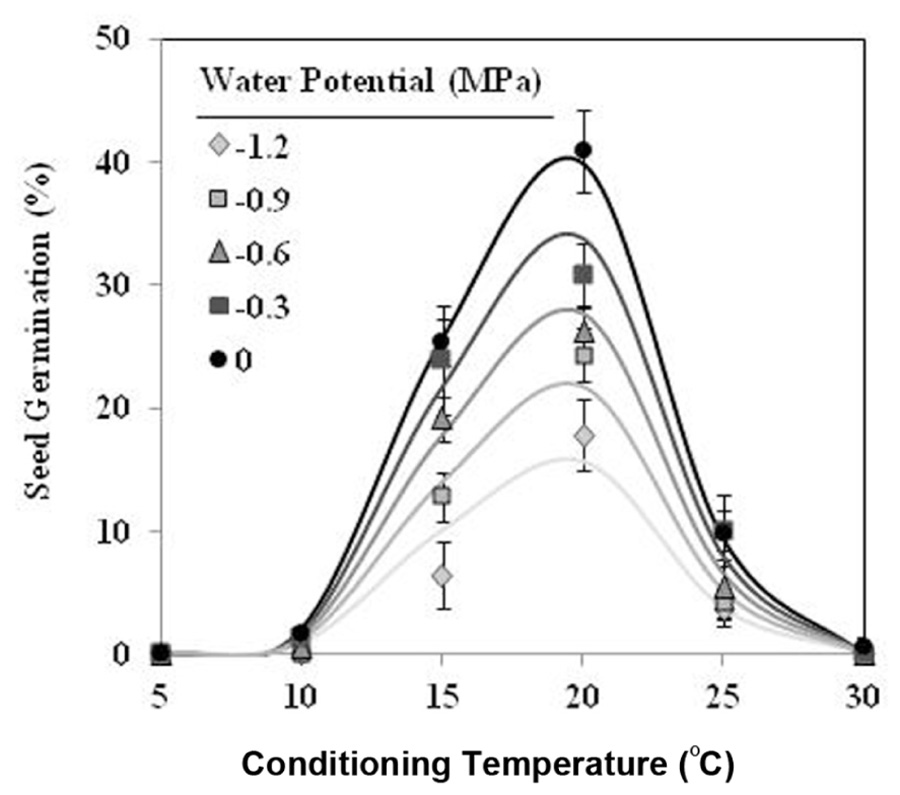
FIGURE 1. Effects of temperature (°C) and water potential (MPa) on seed germination of Orobanche crenata during conditioning period. The lines were fitted according to Analysis β equation (Hau and Kranz, 1990; Table 1). Points represent the average of 12 repetitions. Bars represent the SD of the mean.
The effect of T on radicle length followed a similar trend as percentage of seed germination, although showing an optimum temperature lower. The largest radicle lengths were observed at 15 and 20°C for seed conditioned at water potential value of 0 MPa, which showed mean radicle lengths 1170 ± 75 μm and 826 ± 31 μm, respectively. For lower or higher temperatures the radicle lengths were shorter than previous one. Water potential (water vs. negative potentials) and the types (matric vs. osmotic stress) of water stress had significant (orthogonal contrast, P < 0.001) effect on radicle length of germinated seeds of O. crenata. Overall, the radicle length increased with increasing of Ψt from −1.2 to 0 MPa. Even so, the radicle length of seeds that were conditioned under osmotic stress (467 ± 11 μm) was significantly shorter (orthogonal contrast, P < 0.001) than those conditioned under matric stress (652 ± 12 μm). For this reason, two regression models were independently fitted for the data of each type of water stress. The Analysis β model showed an excellent fit for the radicle length data of both type of water stress (Table 1). The fitted models were highly significant (P < 0.001; R2 and Ra2 were >0.84) and the standardized residuals were randomly distributed over predicted values. The predicted optimum temperatures for maximum radicle length were around 18.5°C under both types of water stress. According to both fitted models, only the seed that were conditioned in water showed a radicle length over 900 μm (Figures 2A,B; Table 1).
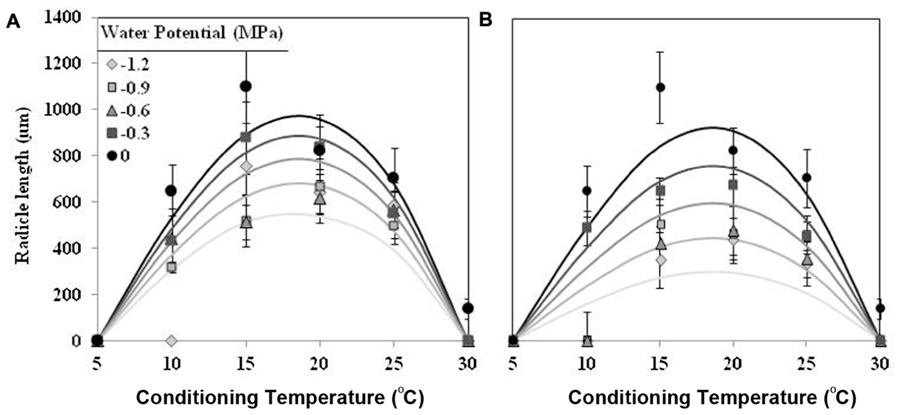
FIGURE 2. Effects of temperature (°C) and matric (A) or osmotic (B) potentials (MPa) on radicle length of O. crenata seeds during conditioning period. The lines were fitted according to Analysis β equation (Hau and Kranz, 1990; Table 1). Points represent the average of six repetitions. Bars represent the SD of the mean.
Experiment 2
Orobanche crenata seeds conditioned at 20°C and Ψt ≥−1.2 MPa during 40 days, germinated under both types of water stress. No seeds geminated when they were conditioned at −3 MPa. Maximum germination (57.60 ± 4.5%) was obtained for seeds conditioned in water at the end of experiment (Figure 3). The germination percentage was significantly (orthogonal contrasts, P = 0.022) lower at negative potentials than water, but there was not significant differences between both types of waster stress (orthogonal contrasts, P = 0.411). For this reason, we used the data from each type of water stress as repetitions to fit regression lines (Figure 3). The cumulative percentage germination increased log-linearly (R2 = 0.706; P < 0.001) with increasing of length of the conditioning period from 0 to 40 days (Figure 3). The short conditioning period that resulted in seed germination was the 7 days, with a germination percentage of ≈18% for the seeds conditioned in water. The comparison among the linear regression lines of each Ψt (between −1.2 and 0 MPa) showed equality of variances (P = 0.715), with significant differences between elevations (P < 0.001) but not (P = 0.475) among slopes (b = the apparent rate of germination increase). Thus, significant differences were found in the line slopes of water potentials 0 and −1.2 MPa (P < 0.05), which were significantly different (P < 0.05) to the remaining water potentials that formed a homogeneous group (P > 0.05; Figure 3).
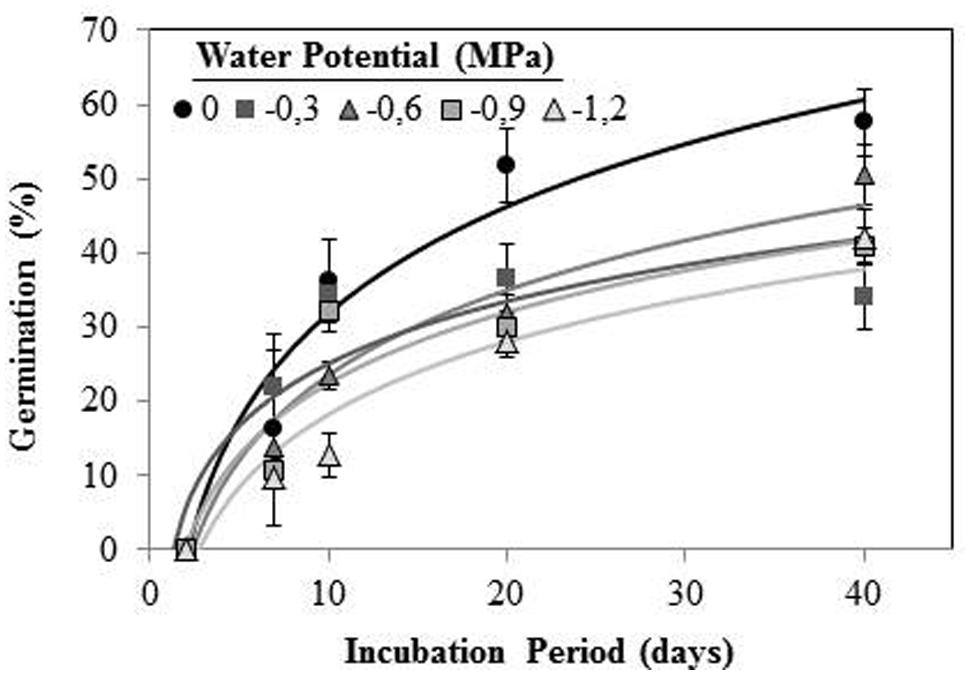
FIGURE 3. Cumulative germination percentage of O. crenata conditioned at 20°C under different water potentials (MPa) during 40 days. The lines were fitted according to logarithmic equation [G (%) =a +log (days)] for each water potential. Points represent the average of 12 repetitions. Bars represent the SD of the mean.
The mean radicle length of O. crenata seeds germinated in water during the first week ranged 1165 ± 68 μm (Figure 4). The effects of type of water stress, germination period and different interactions among independent variable (type of water stress-germination period and type of water stress-germination period-Ψt) on the radicle length were significant (P < 0.05). The radicle length of the seeds conditioned in water (863 ± 28 μm) was higher (orthogonal contrast, P < 0.001) than radicle length of seeds (605.2 ± 9.5 μm) conditioned in negative water potentials. Moreover, the type of water stress also had significant (P < 0.001) effect on the radicle length being 725 ± 13 and 484 ± 11 μm under matric and osmotic stress, respectively. Overall, seeds germinated during the first days of the conditioning period showed a larger radicle than the later germinated ones. In the case of seeds conditioned in osmotic solutions, however, the seeds conditioned at −0.9 and −1.2 MPa showed a radicle length roughly constant (Figures 4A,B).
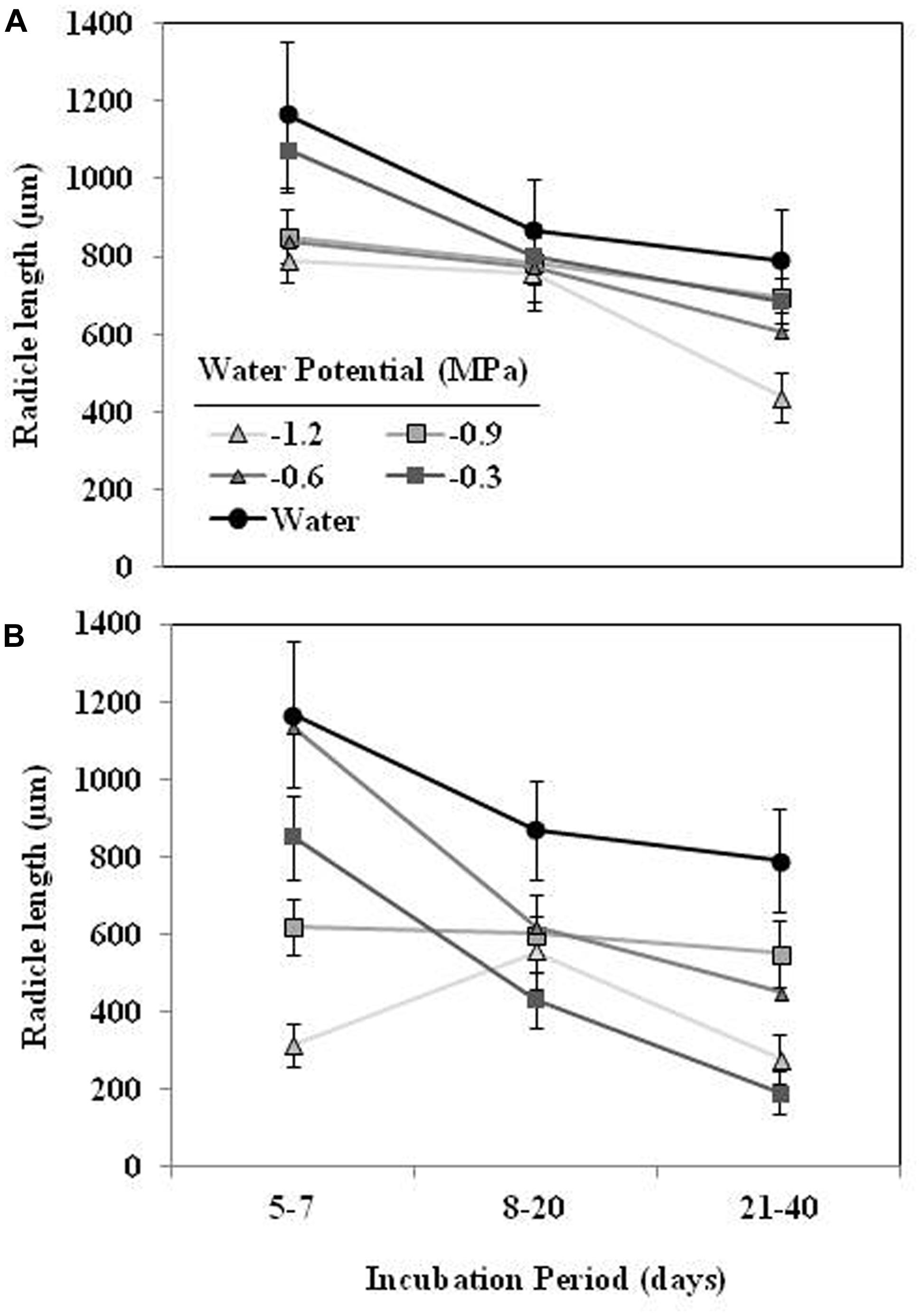
FIGURE 4. Effect of the incubation period, during which the O. crenata seeds germinated (days after GR24 addition), on the radicle length seeds (μm) under matric (A) or osmotic potentials (B). Points represent the average of six repetitions. Bars represent the SE of each mean.
Effect of Temperature and Water Potential During Incubation Period
Experiment 3
The germination percentage of conditioned seeds increased with increasing incubation period from 5 to 10 days. The maximum germination percentage increased with the T between 10 and 20°C and then decreased between 20 and 30°C. Likewise, the final germination percentage increased with increasing Ψt from −1.2 to 0 MPa, approaching around 43% in water at 15°C (Figure 5). The germination percentage of conditioned seeds incubated in water was significantly higher (orthogonal contrasts, P < 0.001) than other incubated at negative potentials. On the contrary, there was no significant (orthogonal contrasts, P = 0.107) differences on the maximum germination percentage of conditioned seeds that incubated under matric or osmotic stress. The Analysis β equations (Table 1; Figure 5) fitted satisfactorily to the data of final germination percentage at each T-Ψt combination (P < 0.001; R2 and Ra2 > 0.93). The standardized residuals were randomly distributed over predicted again. The obtained-optimum T for seed germination was 19.5°C (Table 1; Figure 5).
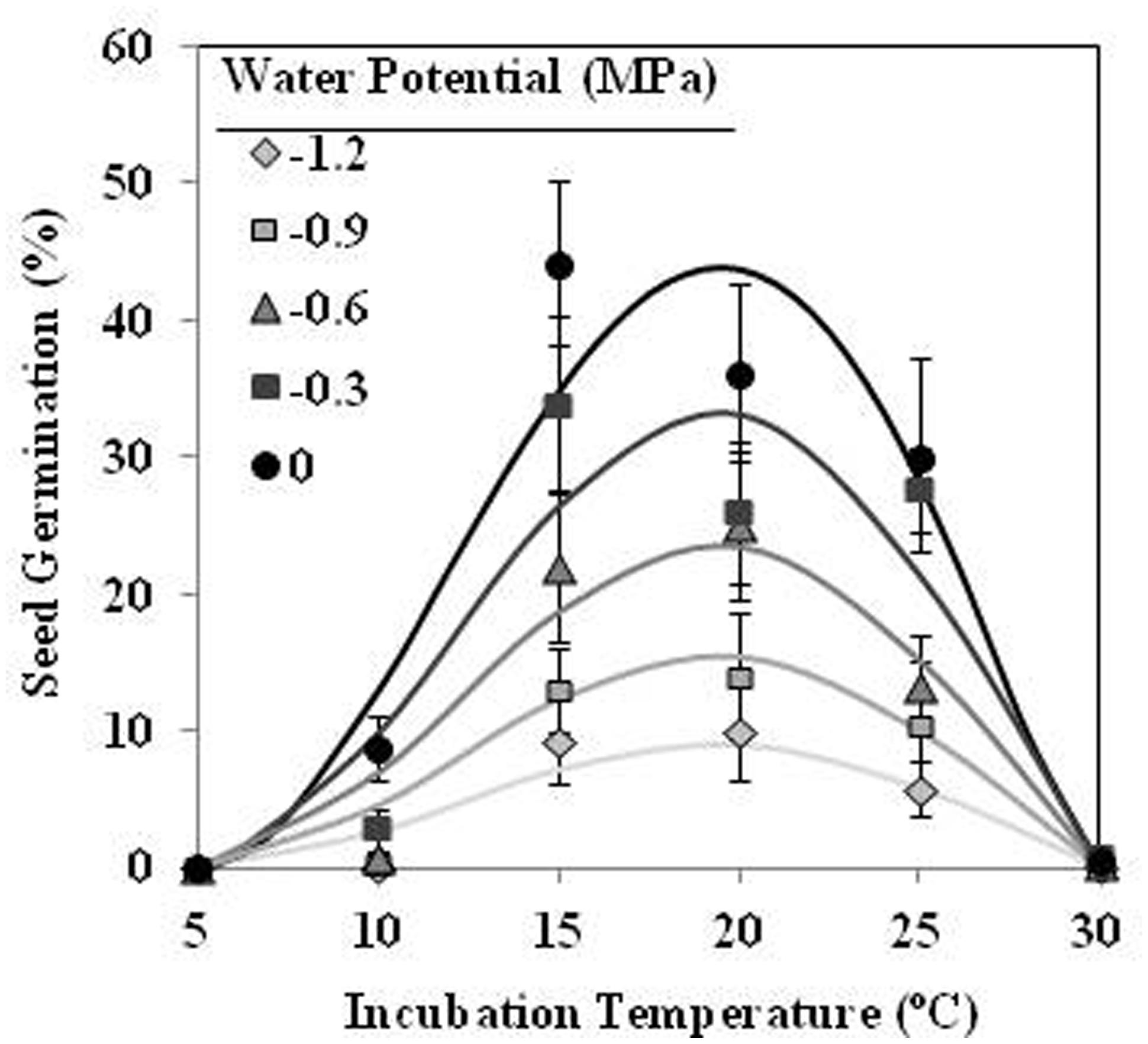
FIGURE 5. Effects of temperature (°C) and water potential (MPa) on seed germination of O. crenata during incubation period. The lines were fitted according to Analysis β equation (Hau and Kranz, 1990; Table 1). Points represent the average of 12 repetitions. Bars represent the SD of the mean.
Radicle length of seeds of O. crenata was highly affected by Ψt and T during the incubation period. For example, in the case of seeds incubated in water, average radicle length increased gradually from 304 μm at 10°C until a maximum of 1991 μm at 25°C (Figure 6). In addition, the conditioned seeds, which were incubated in water, showed higher (orthogonal contrasts, P < 0.001) radicle length than other incubated at negative water potentials. The type of water stress had too significant (orthogonal contrasts, P < 0.001) effect on the radicle length of seeds, being higher (1150 ± 588 μm) in the seeds incubated in PEG solutions than other (975 ± 498 μm) incubated in glycerol solutions. The curves describing the effect of Ψt and T on the radicle length of O. crenata seeds fitted satisfactorily (P < 0.001; R2 and Ra2 > 0.95) for each type of water stress (Figures 6A,B). The obtained optimum temperatures for radicle growth were 22.3 and 24.5°C under matric or osmotic stress, respectively (Figures 6A,B; Table 1). The same results were obtained when we studied the germination percentage at seven incubation days.
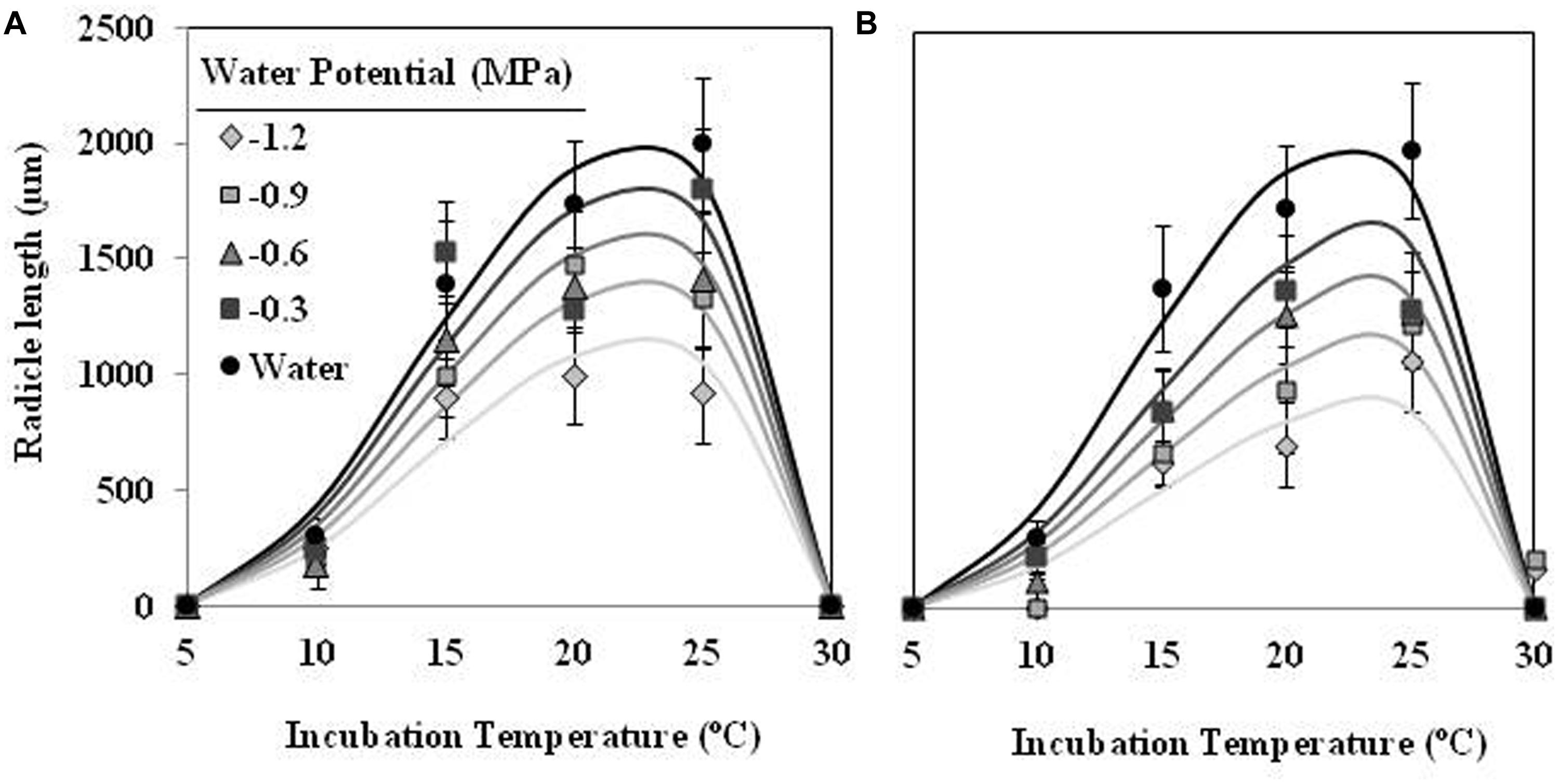
FIGURE 6. Effects of temperature (°C) and matric (A) or osmotic (B) potentials (MPa) on radicle length of O. crenata seeds during incubation period. The lines were fitted according to Analysis β equation (Hau and Kranz, 1990; Table 1). Points represent the average of six repetitions. Bars represent the SD of the mean.
Discussion
It has widely acknowledged that germination of Orobanche sp. seeds is influenced by environmental and microbiological factors, including T, Ψt (Kebreab and Murdoch, 1999, 2000; Song et al., 2005) as well as by microbe interactions in the rhizosphere (Mabrouk et al., 2007; Fernández-Aparicio et al., 2010). Traditionally, physiology-based models have been used to describe the effect of the environmental parameters on the germination of Orobanche seeds, although they have not been used to radicle length and have additional limitations. For example, the hydrothermal time model (Gummerson, 1986) requires daily evaluations for a good calculation of its rate of germination and it assumes that there is no interaction between Ψt and T. Kebreab and Murdoch (1999) proposed an alternative model to explain the interaction of Ψt and T, although it predicts that the seed population will eventually achieve 100% germination, which is not the case. To overcome this limitation, they later refined the model (Kebreab and Murdoch, 2000). Even so, hydrothermal time is currently the most used model to study seed germination of different weeds (Finch-Savage and Leubner-Metzger, 2006; Guillemin et al., 2013). Here we studied the effects of T, type of water stress (matric or osmotic) and Ψt on seed germination and radicle length of O. crenata seeds before and after exposure to GR24 as necessary exogenous stimulus for O. crenata germination in the absence of an appropriate host. For this purpose, we used the Analysis β model (Hau and Kranz, 1990) that has a series of advantages: (i) it provided an excellent fit for germination of O. crenata seeds after conditioning period and during the incubation period; (ii) it showed a good fit for radicle length of the seeds; and (iii) its parameters Tmin, Tmax, and Ψtmin have biological significance, although it is mainly empirical model. Even so, empirical approach may be satisfactory for ecological modeling of seed germination (Forcella et al., 2000). Furthermore, mechanistic risk models can be easily developed considering the normalized rates of seed germination and radicle growth during conditioning and incubation periods using the Analysis β model. To develop mechanistic models, the main steps of the parasite life cycle Orobanche sp. (i.e., germination, radicle elongation to the host root, penetration, establishment, and plant develop) can be organized in a relational diagram according to the principles of the “systems analysis” and considering the normalized rates of the these steps (Leffelaar and Ferrari, 1989).
As a new feature, we studied separately the impact of both matric and osmotic stresses on seed germination of this parasitic plant due to little attention that has been paid to the differences between both stresses. For example, different authors have considered that the water stress caused by PEG solutions is of osmotic type (Kebreab and Murdoch, 2000; Song et al., 2005); although it has been previously shown that the water potential generated by PEG is predominantly (99%) due to matric forces (Steuter et al., 1981).
During the incubation period, the maximum germination of O. crenata seeds was obtained at 18–21°C. These values are mainly within the optimal range of 15–20°C for germination of O. crenata seeds (Van Hezewijk et al., 1993; Kebreab and Murdoch, 1999, 2000; Song et al., 2005). Small differences in optimum temperature for seed germination could be due to genetic variation within and among populations of O. crenata that attack legumes in different geographic regions (Van Hezewijk et al., 1993). This is in agreement with the substantial diversity among O. crenata populations revealed by molecular analyses (Román et al., 2002). At the optimal temperature for conditioning (20°C), the percentage of germinated seed decreased at both type of water stress from 0 to −1.2 MPa, the latter which is near to permanent wilting point of soil that is reached at −1.5 MPa (Cassel and Nielsen, 1986). Conversely, the seed germination was totally prevented at Ψt of −2 MPa in previous experiments. Our results are in agreement with Kebreab and Murdoch (2000) who showed a reduction in O. aegyptiaca seed germination when the water potential decreases from 0 to −1.33 MPa. Conversely, Song et al. (2005) did not observe significant decrease from 0 to −1 MPa in O. aegyptiaca and O. ramosa seed germination; although both species showed a marked reduction in seed germination at −2 MPa. It is interesting to remark that for some species as O. ramosa, the duration of the conditioning period influences the Ψt effect. E.g., the percentage of seed germination of this species is close to zero when the seeds are conditioned at −2 MPa during 4 days, and it is around 77% for the seed conditioned during 20 days (Gibot-Leclerc et al., 2004).
Even though the radicle elongation of seeds is an essential step in the parasite life cycle of Orobanche species, it has been scarcely studied when compared with seed germination. These few studies have focused on the effect of environmental and microbiological factors on radicle elongation. E.g., Westwood and Foy (1999) observed that radicle of Orobanche seeds are more sensible to nitrogen in ammonium form than nitrate. Barghouthi and Salman (2010) identified potential Biological Control Agents (BCAs), mainly Pseudomonas and Bacillus species, which adversely affected radicle elongation of O. aegyptiaca and O. cernua. The inhibition of radicle elongation of Orobanche seeds have also been identified as a resistance mechanism of red clover (Trifolium pratense) that is activated on plants treated with salicylate (Kusumoto et al., 2007). Orobanche radicle growth inhibition has also been reported by a number of plants and fungal metabolites (Fernández-Aparicio et al., 2013; Cimmino et al., 2014, 2015). In our study we found differences in the radicle length of O. crenata seeds in response to T, Ψt and type of water stress during conditioning period. Radicle length was maximum in the treatment of 15°C/0 MPa. In addition, at a given Ψt, radicle length was more sensitive to changes in osmotic than in matric potential. The effect of low osmotic potentials on seed germination and radicle elongation of O. minor during conditioning and incubation periods has been observed when using NaCl solutions; although the reduced radicle elongation could also be due to the toxic effect of ions on seeds (Hassan et al., 2010). In previous reports, matric stress exerts a more negative effect than osmotic stress on germination and seedling growth of different plants such as carrot (Schmidhalter and Oertli, 1991), bean (Meiri, 1984), pepper, and cotton (Shalhevet and Hsiao, 1986). These results may be explained by the fact that O. crenata seeds make up for the water stress in different ways and depending on the type of stress. For example, the plants can easily adjust their Ψt using the solutes under a saline medium, while they are less effective reducing the Ψt under matric stress due to a high metabolic energy requirement (Schmidhalter and Oertli, 1991). Likewise, fungi are able to reduce their Ψt by increasing the concentration of total sugar alcohols, although the patterns of accumulation of sugar alcohols change depending on the type of water stress (Ramirez et al., 2004). In addition, at low Ψo, fungi are able to take up solutes to reduce their internal osmotic potential, an unavailable option when the Ψt is mainly matric (Jones et al., 2011).
In our experiments, percentage of seed germination increased logarithmically with the length of conditioning period during 40 days. This is concordant with the observation that O. crenata seeds reach maximum germination after a period of conditioning of 18–21 days (Van Hezewijk et al., 1993; Kebreab and Murdoch, 2000). Nevertheless, we did not distinguish clearly the secondary dormancy (wet dormancy) of the O. crenata seeds, i.e., a decreased in germination percentage after 21 or 49 days of conditioning at 20 and 10–15°C, respectively, as it has been observed for this species by Van Hezewijk et al. (1993). According to Kebreab and Murdoch (2000), O. crenata seeds, however, showed similar germination percentages when they were conditioned at 20°C during 20–40 days, and they needed more than 70 conditioning days to enter in a state of secondary dormancy. The induction of secondary dormancy at low temperatures during winter, might explain the decline in Orobanche infection observed by farmers in the case of late sowing (Parker and Riches, 1993). In addition, we have observed that seeds, that need more conditioning time to germinate, show the smallest radicles. The latter might also lead to a decline in infection rate of the crop.
During incubation period, O. crenata seeds germinated in a similar range of temperatures (10–25°C) than that which was required during conditioning, and the thermal optimum was the same (about 20°C). Similar optimum temperatures have been described for this species (Van Hezewijk et al., 1993), O. aegyptiaca (Jain and Foy, 1992; Kebreab and Murdoch, 2000), and O. ramosa (Gibot-Leclerc et al., 2004). Likewise, the germination percentage decreased with decreasing Ψt from 0 to −1.2 MPa, with no apparent differences between the types of water stress. According to our data, at given temperature (20°C), the percentage of germination of O. crenata seeds during conditioning period decreased at 16.4% per MPa, whereas the germination declined a 22.4% per MPa during the incubation. This fact suggests that O. crenata seeds appear more sensitive to low levels of Ψt during conditioning period than during the subsequent incubation phase. Water stress may be more limiting in the conditioning period, during which it is necessary that water enters into the seeds, than during the incubation period, when the seeds are already hydrated (Finch-Savage and Leubner-Metzger, 2006). The radicle length of O. crenata seeds was shorter of the seed incubated in osmotic than matric potentials, which has been previously discussed. This fact, the high sensibility of radicle elongation to osmotic stress could be related with the lowest infestation of Orobanche sp. in regions characterized by saline soil, as the region south to the Dead Sea in Jordan (Abu Irmaileh, 1998).
In summary, the results of this study clearly show that low matric and low osmotic potential had negative impacts on seed germination and radicle length of O. crenata seeds. At given Ψt, the reduced percentage of seed germination was similar under matric and osmotic stress during the conditioning or incubation period. In contrast, our results show that low Ψo had a stronger negative effect on radicle length of O. crenata seeds than low Ψm during both periods.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by Spanish Ministry of Education and Science (project AGL AGL2014-558724-R) and by the FP7-ARIMNet (Medileg project). JM is a contract holder under the Juan de la Cierva Programme of the Spanish Ministry of Education and Science, co-financed by FEDER. We thank the JA Gómez-Calero, who was in charge of the Aqua Lab Water Meter; and Antonio Trapero and Themis J. Michailides for critical reviews of the manuscript.
References
Abu Irmaileh, B. E. (1998). “Effect of salinity on Orobanche germination and establishment,” in Proceeding of the Fourth International Workshop on Orobanche Current Problem of Orobanche Researches, eds K. Wegmann, L. Musselman, and D. Joel, Albena.
Baeza, R., Pérez, A., Sánchez, V., Zamora, M. C., and Chirife, J. (2010). Evaluation of Norrish’s equation for correlating the water activity of high concentrated solutions of sugars, polyols, and polyethylene glycols. Food Bioprocess Technol. 3, 87–92. doi: 10.1007/s11947-007-0052-8
Barghouthi, S., and Salman, M. (2010). Bacterial inhibition of Orobanche aegyptiaca and Orobanche cernua radical elongation. Biocontrol Sci. Technol. 20, 423–435. doi: 10.1080/09583150903340544
Cassel, D. K., and Nielsen, D. R. (1986). “Field capacity and available water capacity,” in Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods, ed. A. Klute (Madison, WI: Soil Science Society of America), 901–926.
Cimmino, A., Fernández-Aparicio, M., Andolfi, A., Basso, S., Rubiales, D., and Evidente, A. (2014). Effect of fungal and plant metabolites on broomrape (Orobanche and Phelipanche spp.) seed germination and radicle growth. J. Agricul. Food Chem. 62, 10485–10492. doi: 10.1021/jf504609w
Cimmino, A., Fernández-Aparicio, M., Avolio, F., Andolfi, A., Yoneyama, K., Rubiales, D., et al. (2015). Ryecyanatines A and B and ryecarbonitrilines A and B, substituted cyanatophenol, cyanatobenzo[1,3]diole, and benzo[1,3]dioxolecarbonitriles from rye (Secale cereale L.) root exudates: novel metabolites with allelophatic activity on Orobanche seed germination and radicle growth. Phytochemistry 109, 57–65. doi: 10.1016/j.phytochem.2014.10.034
Chowdhury, N., Marschener, P., and Burns, R. (2011). Response of microbial activity and community structure to decreasing soil osmotic and matric potential. Plant Soil 344, 241–254. doi: 10.1007/s11104-011-0743-9
Dallyn, H., and Fox, A. (1980). Spoilage of material of reduced water activity byxerophilic fungi. Soc. Appl. Bacteriol. Tech. Ser. 15, 129–139.
Dodd, G., and Donovan, L. A. (1999). Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am. J. Bot. 86, 1146–1153. doi: 10.2307/2656978
Fernández-Aparicio, M., Cimmino, A., Evidente, A., and Rubiales, D. (2013). Inhibition of Orobanche crenata seed germination and radicle growth by allelochemicals identified in cereals. J. Agric. Food Chem. 61, 9797–9803. doi: 10.1021/jf403738p
Fernández-Aparicio, M., García-Garrido, J. M., Ocampo, J. A., and Rubiales, D. (2010). Colonization of field pea roots by arbuscular mycorrhizal fungi reduces Orobanche and Phelipanche species seed germination. Weed Res. 50, 262–268. doi: 10.1111/j.1365-3180.2010.00771.x
Fernández-Aparicio, M., Flores, F., and Rubiales, D. (2009). Recognition of root exudates by seeds of broomrape (Orobanche and Phelipanche) species. Ann. Bot. 103, 423–431. doi: 10.1093/aob/mcn236
Finch-Savage, W. E., and Leubner-Metzger, G. (2006). Seed dormancy and the control of germination. New Phytol. 171, 501–523. doi: 10.1111/j.1469-8137.2006.01787.x
Forcella, F., Benech-Arnold, R. L., Sánchez, R., and Ghersa, C. M. (2000). Modeling seedling emergence. Field Crops Res. 67, 123–139. doi: 10.1016/S0378-4290(00)00088-5
Gibot-Leclerc, S., Corbineau, F., Sallé, G., and Côme, D. (2004). Responsiveness of Orobanche ramosa L. seeds to GR 24 as related to temperature, oxygen availability and water potential during preconditioning and subsequent germination. J. Plant Growth Regul. 43, 63–71. doi: 10.1023/B:GROW.0000038242.77309.73
González-Verdejo, C. I., Barandiaran, X., Moreno, M. T., Cubero, J. I., and Di-Pietro, A. (2005). An improved axenic system for studying pre-infection development of the parasitic plant Orobanche ramosa. Ann. Bot. 96, 1121–1127. doi: 10.1093/aob/mci263
Grenz, J. H., and Sauerborn, J. (2007). Mechanisms limiting the geographical range of the parasitic weed Orobanche crenata. Agric. Ecosys. Environ. 122, 275–281. doi: 10.1016/j.agee.2007.01.014
Guillemin, J. P., Gardarin, A., Granger, S., Reibel, C., Munier-Jolain, N., and Colbach, N. (2013). Assessing potential germination period of weeds with base temperatures and base water potentials. Weed Res. 53, 76–87. doi: 10.1111/wre.12000
Gummerson, R. J. (1986). The effect of constant temperatures and osmotic potential on the germination of sugar beet. J. Exp. Bot. 41, 1431–1439. doi: 10.1093/jxb/37.6.729
Harris, R. (1980). “Effect of water potential on microbial growth and activity,” in Water Potential Relations in Soil Microbiology, eds J. Parr and W. Gardner (Madison, WI: Soil Science Society of America), 23–95.
Hassan, M. M., Sugmuto, Y., Babiker, A. G. E., Yamauchi, Y., Osman, M. G. E., and Yagoub, S. O. (2010). Effect of NaCl on Orobanche spp. and Striga hermonthica seeds germination during and after conditioning. Biosci. Res. 7, 26–31.
Hau, B., and Kranz, J. (1990). “Mathematics and statistics for analyses in epidemiology,” in Epidemics of Plant Diseases, ed. J. Kranz (Berlin: Springer-Verlag), 12–46. doi: 10.1007/978-3-642-75398-5_2
Hillel, D. (1972). “Soil moisture and seed germination,” in Water Deficit and Plant Growth, Vol. III, ed. T. T. Koslowski (New York, NY: Academic Press), 65–89.
Jain, R., and Foy, C. L. (1992). Nutrien effects on parasitism and germination of Egyptian Broomrape (Orobanche aegyptiaca). Weed Technol. 6, 269–275.
Joel, D. M., Hershenhorn, J., Eizenberg, H., Aly, R., Ejeta, G., and Rich P. J., et al. (2007). Biology and management of weedy root parasites. Horticul. Rev. 33, 267–349. doi: 10.1002/9780470168011.ch4
Johnson, A. W., Rosebery, G., and Parker, C. (1976). A novel approach to Striga and Orobanche control using synthetic germination stimulants. Weed Res. 16, 223–227. doi: 10.1111/j.1365-3180.1976.tb00406.x
Jones, E. E., Stewart, A., and Whipps, J. M. (2011). Water potential affects Coniothyrium minitans growth, germination and parasitism of Sclerotinia sclerotiorum sclerotia. Fungal Biol. 115, 871–881. doi: 10.1016/j.funbio.2011.06.016
Kebreab, E., and Murdoch, A. J. (1999). Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 50, 655–664. doi: 10.1093/jxb/50.334.655
Kebreab, E., and Murdoch, A. J. (2000). The effect of water stress on the temperature range for germination of Orobanche aegyptiaca seeds. Seed Sci. Res. 10, 127–133. doi: 10.1017/S0960258500000131
Kusumoto, D., Goldwasser, Y., Xie, X., Yoneyama, K., Takeuchi, Y., and Yoneyama, K. (2007). Resistance of red clover (Trifolium pratense) to the root parasitic plant Orobanche minor is activated by salicylate but not by jasmonate. Ann. Bot. 100, 537–544. doi: 10.1093/aob/mcm148
Leffelaar, P. A., and Ferrari, T. J. (1989). “Some elements of dynamic simulation,” in Simulation and Systems Management in Crop Protection, eds R. Rabbinge, S. A. Ward, and H. H. Van Laar (Wageningen: Centre for Agricultural Publishing and Documentation), 19–45.
Mabrouk, Y., Zourgui, L., Sifi, B., Delavault, P., Simier, P., and Belhadj, O. (2007). Some compatible Rhizobium leguminosarum strains in peas decrease infections when parasitized by Orobanche crenata. Weed Res. 47, 44–53. doi: 10.1111/j.1365-3180.2007.00548.x
Meiri, A. (1984). “Plant response to salinity: experimental methodology and application to the field,” in Soil Salinity Under Irrigation, eds I. Shainberg and J. Shalhevet (New York, NY: Springer Verlag), 284–297.
Michel, B. E. (1983). Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 72, 66–70. doi: 10.1104/pp.72.1.66
Michel, B. E., and Kaufmann, M. R. (1973). The osmotic potential of polyethylene glycol 6000. Plant Physiol. 51, 914–916. doi: 10.1104/pp.51.5.914
Papendick, R. I., and Campbell, G. S. (1980). “Theory and measurement of water potential,” in Water Potential Relations in Soil Microbiology, eds J. Parr and W. Gardner (Madison, WI: Soil Science Society of America), 1–22.
Parker, C. (2009). Observations on the current status of Orobanche and Striga problems worldwide. Pest. Manag. Sci. 65, 453–459. doi: 10.1002/ps.1713
Parker, C., and Riches, C. R. (1993). Parasitic Weeds of the World: Biology and Control. Wallingford: CAB International.
Plakhine, D., Hammam. Z., and Joel, D. M. (2009). Is seed conditioning essential for Orobanche germination? Pest. Manag. Sci. 65, 492–496. doi: 10.1002/ps.1716
Ramirez, M. L., Chulze, S. N., and Magan, N. (2004). Impact of osmotic and matric water stress on germination, growth, mycelial water potentials and endogenous accumulation of sugars and sugar alcohols by Fusarium graminearum. Mycologia 96, 470–478. doi: 10.2307/3762167
Román, B., Satovic, Z., Rubiales, D., Torres, A. M., Cubero, J. I., Katzir, N., et al. (2002). Variation among and within populations of the parasitic weed Orobanche crenata from Spain and Israel revealed by inter simple sequence repeat markers. Phytopathology 92, 1262–1266. doi: 10.1094/PHYTO.2002.92.12.1262
Rubiales, D., Fernández-Aparicio, M., Wegmann, K., and Joel, D. (2009). Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res. 49, 23–33. doi: 10.1111/j.1365-3180.2009.00742.x
Schmidhalter, U., and Oertli, J. J. (1991). Germination and seedling growth of carrots under salinity and moisture stress. Plant Soil 132, 243–251.
Shalhevet, J., and Hsiao, T. C. (1986). Salinity and drought. Irrigation Sci. 7, 249–264. doi: 10.1007/BF00270435
Song, W. J., Zhou, W. J., Jin, Z. L., Cao, D. D., Joel, D. M., Takeuchi, Y., et al. (2005). Germination response of Orobanche seeds subjected to conditioning temperature, water potential and growth regulator treatments. Weed Res. 45, 467–476. doi: 10.1111/j.1365-3180.2005.00477.x
Steuter, A. A., Mozafar, A., and Goodin, J. R. (1981). Water potential of aqueous polyethylene glycol. Plant Physiol. 67, 64–67. doi: 10.1104/pp.67.1.64
Van Hezewijk, M. J., Van Beem, A. P., Verkleij, J. A. C., and Pieterse, A. H. (1993). Germination of Orobanche crenata seeds, as influenced by conditioning temperature and period. Can. J. Bot. 71, 786–792. doi: 10.1139/b93-090
Westwood, J. H., and Foy, C. L. (1999). Influence of nitrogen on germination and early development of broomrape (Orobanche spp.). Weed Sci. 47, 2–7.
Keywords: broomrape, water potential, matric and osmotic stress
Citation: Moral J, Lozano-Baena MD and Rubiales D (2015) Temperature and water stress during conditioning and incubation phase affecting Orobanche crenata seed germination and radicle growth. Front. Plant Sci. 6:408. doi: 10.3389/fpls.2015.00408
Received: 09 March 2015; Accepted: 21 May 2015;
Published online: 03 June 2015.
Edited by:
Paul Christiaan Struik, Wageningen University, NetherlandsReviewed by:
Grama Nanjappa Dhanapal, University of Agricultural Sciences, Bangalore, IndiaAad Van Ast, Wageningen University, Netherlands
Copyright © 2015 Moral, Lozano-Baena and Rubiales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Moral, Rubiales Laboratory, Department of Plant Breeding, Institute for Sustainable Agriculture, Consejo Superior de Investigaciones Científicas, Apdo 4084, E-14080 Córdoba, Spain, jmoral@ias.csic.es
 Juan Moral
Juan Moral María Dolores Lozano-Baena
María Dolores Lozano-Baena Diego Rubiales
Diego Rubiales