- 1School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, China
- 2State Key Laboratory of Grassland Agro-ecosystems, College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou, China
- 3Lanzhou Institute of Husbandry and Pharmaceutical Science, Chinese Academy of Agricultural Sciences, Lanzhou, China
Salinity is one of the major abiotic stresses that limit the growth and productivity of sugar beet (Beta vulgaris L.). To improve sugar beet’s salinity tolerance, the ZxNHX and ZxVP1-1 genes encoding tonoplast Na+/H+ antiporter and H+-PPase from xerophyte Zygophyllum xanthoxylum were co-expressed by Agrobacterium tumefaciens-mediated transformation. It is showed here that co-expression of ZxNHX and ZxVP1-1 confers enhanced salinity tolerance to the transformed sugar beet plants compared with the wild-type (WT) plants. The chimeric plants grew well in the presence of high salinity (400 mM NaCl), whereas WT plants displayed chlorosis and died within 8 days. Compared to WT plants, the chimeric plants co-expressing ZxNHX and ZxVP1-1 accumulated more proline, Na+ and K+ in their leaves and petioles when exposed to high salinity, which caused lower solute potential, retained more water and thus subjected to lesser cell membrane damage. Interestingly, the chimeric plants accumulated higher sucrose, glucose and fructose contents in their storage roots than WT plants in the absence or presence of high salinity. Our results suggested that co-expression of ZxNHX and ZxVP1-1 improved the osmoregulatory capacity in chimeric sugar beet through increased compartmentalization of ions into the vacuoles by enhancing the activity of proton pumps and thus mitigated Na+-toxicity for plants.
Introduction
Salinity is a major abiotic stress limiting growth and productivity of plants in many areas of the world due to increasing use of poor quality of water for irrigation and soil salinization (Munns and Tester, 2008; Rozema and Flowers, 2008; Zhang and Shi, 2013). Saline stress to most plant species mainly accounts for the enhancement in cytoplasmic osmotic stress and Na+-specific toxicity (Munns and Tester, 2008; Maathuis et al., 2014). Excessive accumulation of Na+ in the cytosol leads to inhibitions of protein synthesis, many enzymatic reactions, and photosynthetic processes (Kronzucker et al., 2013). Plants have evolved various adaptive strategies to cope with saline stress (Shabala, 2013; Zhang and Shi, 2013; Roy et al., 2014). One of those that plant cells employ for the alleviation of excess cytosolic Na+ is to compartmentalize Na+ into the vacuoles (Yamaguchi et al., 2013). Compartmentalization of Na+ into vacuoles not only protects the cytoplasm from Na+-toxicity, but also allows plant to use Na+ as a benefit osmiticum for lowering its cellular osmotic potential and as such prevents water loss (Kronzucker and Britto, 2011; Shabala, 2013; Maathuis et al., 2014). It was well known that Na+ compartmentalization is thought to be mediated through tonoplast Na+/H+ antiporter (NHX), which are driven by proton motive forces across tonoplast generated by H+ transporting pumps, such as H+-ATPase and H+-PPase (VP) (Bassil and Blumwald, 2014). Theoretically, overexpression of the tonoplast Na+/H+ antiporter and H+-PPase genes should enhance the sequestration of Na+ into the vacuole, thus alleviating the toxicity of Na+ in the cytosol and increasing vacuolar osmoregulatory capacity, which could confer salinity tolerance to plants (Apse et al., 1999; Bhaskaran and Savithramma, 2011; Bao et al., 2014).
Indeed, over the last 16 years, numerous studies have demonstrated that overexpression of the genes encoding tonoplast Na+/H+ antiporters conferred enhanced salinity tolerance in various transgenic plants, such as Arabidopsis thaliana (Apse et al., 1999; Bayat et al., 2011), rice (Oryza sativa) (Ohta et al., 2002; Chen et al., 2007a), maize (Zea mays) (Chen et al., 2007b), wheat (Triticum aestivum) (Xue et al., 2004), tomato (Solanum lycopersicum) (Zhang and Blumwald, 2001; Yarra et al., 2012), rapeseed (Brassica napus) (Zhang et al., 2001; Rajagopal et al., 2007), soybean (Glycine max) (Sun et al., 2006; Li et al., 2010a), apple (Malus × domestica) (Li et al., 2010b), kiwifruit (Actinidia deliciosa) (Tian et al., 2011), peanut (Arachis hypogaea) (Banjara et al., 2012), cowpea (Vigna unguiculata) (Mishra et al., 2014), alfalfa (Medicago sativa) (Li et al., 2011; Zhang et al., 2014), and sweet potato (Ipomoea batatas) (Fan et al., 2014). Genetic engineering of stronger vacuolar proton pumping for improved plant salinity and/or drought resistance has also been achieved by expression of genes encoding tonoplast H+-PPases in transgenic plants of Arabidopsis (Gaxiola et al., 2001; Yao et al., 2012; Gamboa et al., 2013), alfalfa (Bao et al., 2009), creeping bentgrass (Agrostis stolonifera) (Li et al., 2010c), cotton (Gossypium hirsutum) (Pasapula et al., 2011), and peanut (Qin et al., 2013). It has been demonstrated that co-expression of Pennisetum glaucum tonoplast Na+/H+ antiporter PgNHX1 and Arabidopsis H+-PPase AVP1 genes confers higher salinity tolerance than expression of single PgNHX1 or AVP1 in transgenic tomato (Bhaskaran and Savithramma, 2011). Similar findings were observed in transgenic tobacco co-expressing wheat tonoplast Na+/H+ antiporter TNHXS1 and H+-PPase TVP1 genes (Gouiaa et al., 2012), and cotton co-expressing Arabidopsis AtNHX1 and AVP1 (Shen et al., 2015).
Zygophyllum xanthoxylum is a salt-accumulating xerophyte that colonizes arid areas in northwestern of China (Ma et al., 2012; Yue et al., 2012). It has been shown that Z. xanthoxylum can accumulate larger quantities of Na+ than K+ in shoot tissues for osmotic adjustment even at low salt soils (Wang et al., 2004). Further studies showed that low concentrations of Na+ significantly increased growth and alleviated water stress in this species (Ma et al., 2012; Yue et al., 2012). The ZxNHX and ZxVP1-1 genes, encoding tonoplast Na+/H+ antiporter and H+-PPase, were cloned from Z. xanthoxylum (Wu et al., 2011). A positive correlation was observed between up-regulation of ZxNHX and accumulation of Na+ in leaf of Z. xanthoxylum in the presence of salt, suggesting that ZxNHX is involved in compartmentalization of Na+ in shoots (Wu et al., 2011). Our recent studies showed that ZxNHX controls Na+ and K+ homeostasis at the whole-plant level in Z. xanthoxylum by feedback regulation of the expression of genes involved in their transport (Yuan et al., 2015). Bao et al. (2014) reported that coordinating expression of ZxNHX and ZxVP1-1 enhanced salt- and drought-tolerance in transgenic Lotus corniculatus by increasing Na+, K+, and Ca2+ accumulation. These results suggested that coordinating expression of these two genes is more valuable way for obtaining transgenic plants with enhanced stress tolerance. However, there are no reports regarding improving salinity tolerance of sugar crops by co-expressing tonoplast Na+/H+ antiporter and H+-PPase.
Sugar beet (Beta vulgaris L.), a species of Chenopodiaceae family, is an important sugar crop that supplies approximately 35% of the world’s sugar (Liu et al., 2008). Sugar beet is regarded as one of the relatively more salinity-tolerant crops (Wu et al., 2013, 2015a); therefore, it is a good choice for the reclamation of saline land. However, sugar beet’s growth and development, especially its yield and sugar quality, are negatively affected by high salinity. Therefore, breeding sugar beet varieties with higher salinity tolerance is necessary for this important crop to adapt to high salinity. A consider amount of time is required to select salinity tolerance plants through traditional breeding procedure. The genetic engineering approach, however, provides an efficient way to improve salinity tolerance in sugar beet. Here we investigated the possibility of co-expression of Z. xanthoxylum ZxNHX and ZxVP1-1 to enhance salinity tolerance in sugar beet.
Materials and Methods
Plasmid and Bacteria Strains
The plasmid pCAMBIA1302-ZxNHX-ZxVP1-1, which contains the cauliflower mosaic virus 35S (CaMV 35S) promoter driving ZxNHX (GenBank accession number: EU103624, encoding tonoplast Na+/H+ antiporter) and ZxVP1-1 (GenBank accession number: EU103625, encoding tonoplast H+-PPase) from Z. xanthoxylum, has been previously constructed in our laboratory (Bao et al., 2014). The constructs were mobilized into Agrobacterium tumefaciens strain GV3101 by electroporation for subsequent plant transformation (Bao et al., 2014). Agrobacterium suspension was obtained after incubation in 100 mL Luria-bertani (LB) liquid medium (pH 7.0) containing 50 μg mL–1 kanamycin, 50 μg mL–1 gentamicin, and 25 μg mL–1 rifampicin overnight at 28°C under constant rotation at 200 rpm and resuspension in the appropriate volume of free-antibiotics LB liquid medium to an OD600 of 0.6–0.8.
Plant Materials and Transformation
Seeds of sugar beet (B. vulgaris L. cv. “Gantang7”) were kindly provided by Wuwei Sannong Seed Technology Co., Ltd., Gansu province, China, in mid March 2013. Seeds were surface sterilized for 1 min in 75% ethanol (v/v) and rinsed 3 times with distilled water, soaked in distilled water for 1 day and then germinated at 25 °C in the dark for 3 days. Uniform seedlings were carefully transferred to plastic containers (5 cm × 5 cm × 5 cm; two seedlings/container) filled with vermiculite and irrigated with the modified Hoagland nutrient solution containing 2.5 mM KNO3, 1 mM NH4H2PO4, 0.5 mM Ca(NO3)2, 0.5 mM MgSO4, 60 μM Fe-Citrate, 92 μM H3BO3, 18 μM MnCl2·4H2O, 1.6 μM ZnSO4·7H2O, 0.6 μM CuSO4·5H2O, and 0.7 μM (NH4)6Mo7O24·4H2O. Solutions were renewed every 3 days. The seedlings were grown in a greenhouse, where the temperature was regulated 20°C at night and 25°C in the day and the relative humidity averaged 65 and 75% for day and night, respectively. The daily photoperiod was 16/8 h (day/night) and the light flux density during the light period was between 550 and 600 μmol m–2 s–1. 2-week-old seedlings were used for genetic transformation.
The transformation was performed using the procedure as described by Weeks et al. (2008) with a slight modification. Briefly, after the cotyledons of 2-week-old seedlings expanded and the apical node emerged, the apical node was excised and the wound covered with cotton wool wetted with Agrobacterium suspension as described above. For wild-type (WT) control, the Agrobacterium suspension was replaced by distilled water. The seedlings were put in the dark for 3 h and then cotton was removed. Thereafter, the seedlings were transferred into and grown in the greenhouse and irrigated with modified Hoagland nutrient solution as described above. 5 days later, a new apical node emerged from the wound region and formed new shoots 25 days later. All the T0 transformed plants were used for molecular characterization.
Molecular Characterization of Chimeric Sugar Beet
For PCR analysis, plant genomic DNA was extracted from fresh leaves of putative chimeric and WT plants using the Ezup Column Genome DNA Isolation Kit (Sangon, Shanghai, China). The ZxNHX and ZxVP1-1 genes in chimeric and WT plants were amplified from same DNA templates using the following sets of specific primers: (i) 5′-CATCGGTGGTGCTTTTCAAT-3′ and 5′-GCAGCTCTACCAGCCATCAC-3′ for ZxNHX, (ii) 5′-GCTGGAATCGAATTTGTGGA-3′ and 5′-TGCAGCCTTATGTGCATCTG-3′ for ZxVP1-1. The PCR amplification conditions for the ZxNHX and ZxVP1-1 fragments were carried out with initial denaturation at 94 °C for 2 min, followed by 30 cycles of 94°C for 30 s, 54°C for 45 s, 72°C for 30 s, and final extension at 72°C for 10 min. PCR products were electrophoresed on a 1.2% agarose gel, respectively.
For RT-PCR analysis, total RNA was extracted from 50 mg of young leaves of PCR-positive plants and WT plants with Trizol reagent (Sangon, Shanghai, China) according to manufacturer’s instructions. DNase treated total RNA samples were used for the synthesis of first strand cDNA using M-MLV Reverse Transcriptase Kit (Sangon, Shanghai, China). PCR amplification of the ZxNHX and ZxVP1-1 gene was carried out according to the conditions described above and ACTIN was used as a internal control in the semi-quantitative RT-PCR. The primer sequences of ACTIN are 5′-GTGGTCGTAC AAC(A/T)GGTATTGTG-3′ and 5′-GA(A/G/T)CCTCCAATCCAGACACTG-3′, which give a 598 bp fragment with cDNA. The all amplified products were electrophoresed on 1.2 % agarose gel, and visualized by AlphaImager (ver. 4.0.1; Alpha Innotech Co., San Leandro, CA, USA) for subsequent analysis, respectively. Experiments were repeated at least three times.
Treatments of Chimeric and Wild-type Plants
Uniform T0 chimeric (uniform expression levels of genes) and WT plants were exposed to the modified Hoagland nutrient solution as described above supplemented without or with 400 mM NaCl for 8 days, respectively. Treatment solution was renewed every 2 days. All the plants were grown in the greenhouse as described above.
Determination of Plant Growth and Water Content
At the end of treatments, the plants were divided into separate leaf, petiole, storage root, and lateral root fractions. Fresh weights (FW) of the tissues were weighed immediately. The samples were then dried in oven at 80°C for 72 h to determine dry weights (DW). Water content (WC) was calculated according to the following equation as described by Wu et al. (2013): WC = (FW – DW)/DW.
Determination of Malondialdehyde (MDA) Concentration
Malondialdehyde (MDA) was determined using the thiobarbituric acid (TBA) protocol as described by Peever and Higgins (1989) with slight modifications. The absorbances at 450, 532, and 600 nm (A450, A532, and A600, respectively) were measured using an UV-VIS spectrophotometer (UV-300 OPC, Mapada Co., Shanghai, China). The concentration of MDA in nmol/g FW was calculated according to the equation of Bao et al. (2009): MDA concentration (nmol/g FW) = C (μmol/L) × V (L)/FW (g) × 1000, where C (μmol/L) = 6.45 × (A532 – A600) – 0.56 A450, and V represents the volume (L) of extracting solution.
Determination of Solute Potential (ΨS)
Plant tissues (leaf, petiole, storage root, and lateral root) were frozen in liquid nitrogen and then stored at –80°C until the analysis of solute potential (Ψs). Ψs was detected with the sap squeezed out from the thawed shoot at 25°C with a cryoscopic osmometer (Osmomat-030, Gonotec GmbH, Berlin, Germany). The reading (mmol kg–1) was used to calculate the Ψs in MPa with the formula Ψs = -moles of solute × R × T, where R = 0.008314 and T = 298.8 (Ma et al., 2012).
Determination of Proline and Sugar Contents
Proline was measured according to the method of Bates et al. (1973) using the ninhydrin reagent. Proline content was estimated using an UV-VIS spectrophotometer at 520 nm and determined from calibration curve using L-Proline (Sangon, Shanghai, China) as standard. Sugars contents were determined according to the method as described by Liu et al. (2008). Briefly, sugars were extracted from dried plant tissues in 80% ethanol at 80°C for 40 min. Sucrose, glucose, and fructose contents were read at 480, 630, and 480 nm and determined from calibration curve using Sucrose, Glucose and D-Fructose (Sangon, Shanghai, China) as standard, respectively.
Measurement of Na+, K+, and Ca2+ Concentrations
Na+, K+, and Ca2+ concentrations were detected according to the method described by Wu et al. (2013, 2015a). Briefly, Na+, K+, and Ca2+ were extracted from dried plant tissues (leaf, petiole, storage root, and lateral root) in 100 mM acetic acid at 90°C for 2 h and ions analysis was performed by a flame spectrophotometer (2655-00, Cole-Parmer Instrument Co., Vernon Hills, USA) (Wu et al., 2013, 2015a).
Statistical Analysis
Data were performed by one-way analysis of variance (ANOVA) using statistical software (SPSS 19.0, Chicago, USA). Duncan’s multiple range tests were used to detect significant difference between means at a significant level of P < 0.05.
Results
Production and Molecular Characterization of Chimeric Sugar Beet Plants Co-expressing ZxNHX and ZxVP1-1
The 35S-ZxNHX1-ZxVP1-1 construct described by Bao et al. (2014) was introduced into sugar beet via Agrobacterium-mediated transformation (Weeks et al., 2008). To validate whether the ZxNHX and ZxVP1-1 genes from xerophyte Z. xanthoxylum integrated into sugar beet genome, PCR reactions were performed with specific primers to amplify the fragments of ZxNHX and ZxVP1-1, respectively. We totally obtained 40 T0 chimeric plants that showed the fragments of 504 bp for ZxNHX and 471 bp for ZxVP1-1 by PCR amplification, respectively. A stable transformation efficiency of 9.95% was observed using a total of 402 plants in three different experiments generating 40 T0 chimeric sugar beet plants. The expression levels of ZxNHX and ZxVP1-1 were monitored by RT-PCR performed on young leaves of the all T0 chimeric plants and WT plants, respectively. As expected, co-expression of ZxNHX and ZxVP1-1 in all PCR-positive T0 chimeric plants were showed and their expression levels almost were consistent among all different T0 chimeric plants, while the WT plants did not show amplification fragments (Figure 1). Sugar beet plants were difficultly propagated from their leaves and petioles, therefore, 36 of these T0 chimeric plants were directly chose to further analyze physiological parameters.
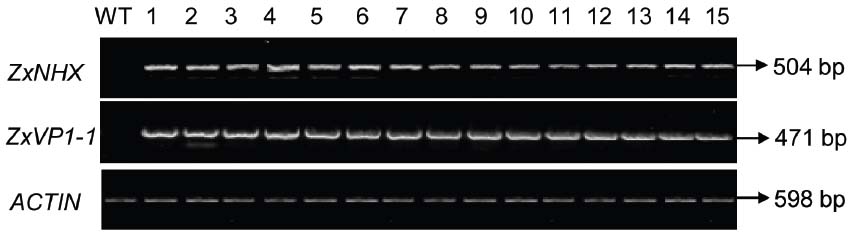
Figure 1. RT-PCR analysis of ZxNHX and ZxVP1-1 genes in T0 chimeric sugar beet plants. Specific PCR products of ZxNHX for 504 bp and ZxVP1-1 for 471 bp were detected in the new leaves of chimeric sugar beet plants, respectively. WT, wild type plant; 1–15, the T0 chimeric plants of co-expressing ZxNHX and ZxVP1-1. A 598 bp ACTIN fragment was amplified by RT-PCR as an internal control.
Co-expression of ZxNHX and ZxVP1-1 Enhances Salinity Tolerance in Chimeric Sugar Beet
Both T0 chimeric and WT plants grew well vigorously under control condition, while chimeric plants developed faster to larger size than WT plants. When exposed to high salinity (400 mM NaCl), WT plants wilted and died, but the chimeric plants grew well (Figure 2A). These results indicated that co-expression of ZxNHX and ZxVP1-1 enhanced salinity tolerance in chimeric sugar beet plants.
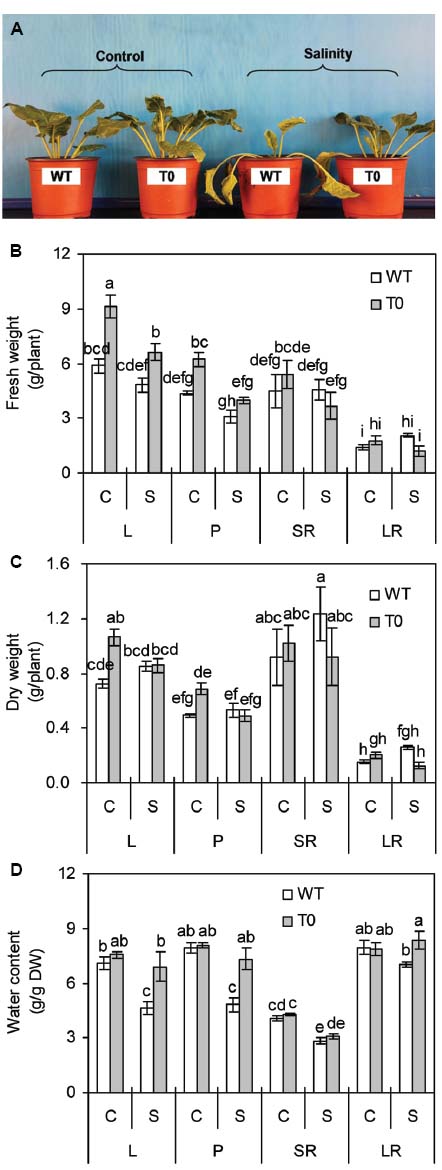
Figure 2. Morphology (A), fresh weight (B), dry weight (C), and water content (D) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). L: leaf; P: petiole; SR: storage root; LR: lateral root. Data in (B–D) are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
It was observed that FW and dry weight (DW) in leaf and petiole of chimeric plants were significantly higher than that of WT plants under control condition (P < 0.05) (Figures 2B,C). When subjected with salinity, FW in leaf of chimeric plants was 38% higher than that of WT plants. However, no significant differences in tissues DW were found between WT and chimeric plants (Figure 2C). Salinity caused a significant reduction of WC in storage root of both WT and chimeric plants (P < 0.05). WC of leaf and petiole in WT plants significantly reduced by 35 and 64% under saline stress, respectively, whilst remained unchanged in chimeric plants (Figure 2D). WC in leaf, petiole, and lateral root of chimeric plants was 49, 51, and 19% higher than that in WT plants under saline stress, respectively (P < 0.05) (Figure 2D). These results suggested that chimeric sugar beet plants co-expressing ZxNHX and ZxVP1-1 displayed greater water retention capacity compared with WT plants.
Co-expression of ZxNHX and ZxVP1-1 Maintains the Stability of Cell Membrane in Chimeric Sugar Beet Under Saline Stress
To investigate the stability of membrane in WT and chimeric plants under saline stress, MDA concentration that represents the degree of cell membrane damage was determined (Yazici et al., 2007; Han et al., 2014). Under control condition, no significant differences were seen in all the tissues from WT and chimeric plants. When subjected to saline stress, MDA concentration in leaf was 69% lower in chimeric plants than in WT plants (P < 0.05) (Figure 3). These results suggested that the cell membrane of chimeric sugar beet was healthier or subjected to less damage than WT plants under saline stress.
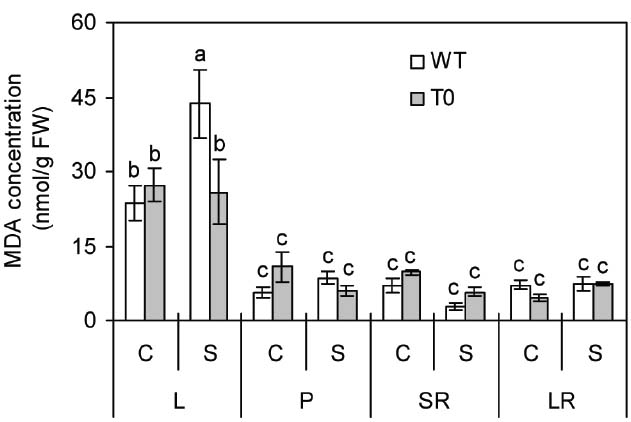
Figure 3. Malondialdehyde (MDA) concentration in leaf (L), petiole (P), storage root (SR), and lateral root (LR) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). Data are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
Chimeric Sugar Beet Plants Retain More Solute in Shoot
To assay the amount of solute in the tissues, the tissues solute potential (Ψs) of WT and chimeric plants was determined. Under control condition, the Ψs of storage root was significantly lower than that of other tissues in either WT or chimeric plants (P < 0.05). When exposed to saline stress, all the tissues Ψs of both chimeric and WT plants dropped significantly (P < 0.05). However, the Ψs of both leaf and petiole was more negative in chimeric plants than that in WT plants (P < 0.05) (Figure 4). These results suggested that chimeric plants had higher osmoregulatory capacity compared to WT plants.
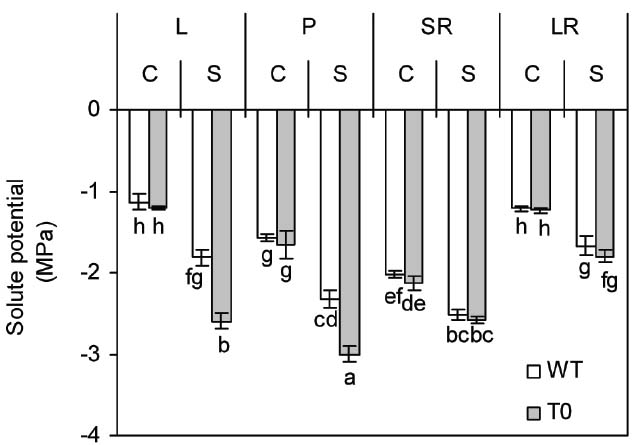
Figure 4. Solute potential in leaf (L), petiole (P), storage root (SR), and lateral root (LR) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). Data are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
In the absence of salinity, proline content in petiole of chimeric plants was 38% higher than that of WT plants, however, in other tissues, no clear differences were seen between WT and chimeric plants (Figure 5). In the presence of salinity, proline content in shoots (leaf and petiole) of both chimeric plants and WT plants remarkably increased, whereas to a lesser degree in WT than in chimeric plants. It was also observed that chimeric plants accumulated more proline in their leaf and petiole by 70% and 55% compared with WT plants, respectively (P < 0.05) (Figure 5).
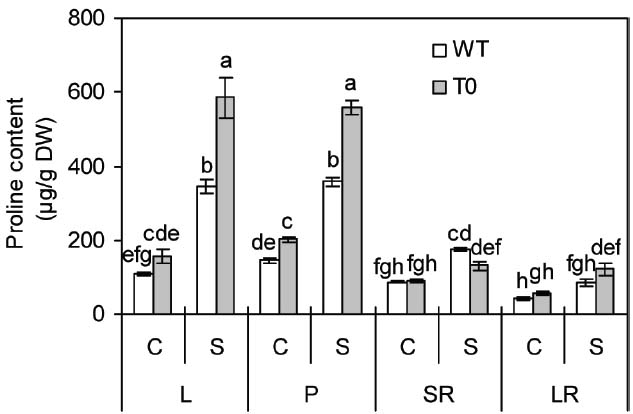
Figure 5. Proline content in leaf (L), petiole (P), storage root (SR), and lateral root (LR) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). Data are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
Chimeric Sugar Beet Plants Accumulate More Na+, K+ in Shoots and More Ca2+ in Lateral Root
To investigate whether chimeric plants co-expressing ZxNHX and ZxVP1-1 could accumulate more ions, the concentrations of Na+, K+, and Ca2+ in chimeric and WT plants were measured. In the absence of salinity, no remarkable differences were found in leaf, petiole, storage root and lateral root Na+ concentration from WT and chimeric plants. When exposed to saline stress, Na+ concentration of leaf and petiole increased obviously in all plants (P < 0.05) (Figure 6A). However, leaf and petiole Na+ concentrations in chimeric plants were 28 and 15% higher than those in WT plants, respectively (P < 0.05) (Figure 6A). Compared to control, saline stress significantly increased Na+ concentrations of storage root and lateral root by 3.9- and 1.7-fold in WT plants (P < 0.05), respectively, but not in chimeric plants. These results suggested that chimeric sugar beet accumulated more Na+ in shoots but less in roots.
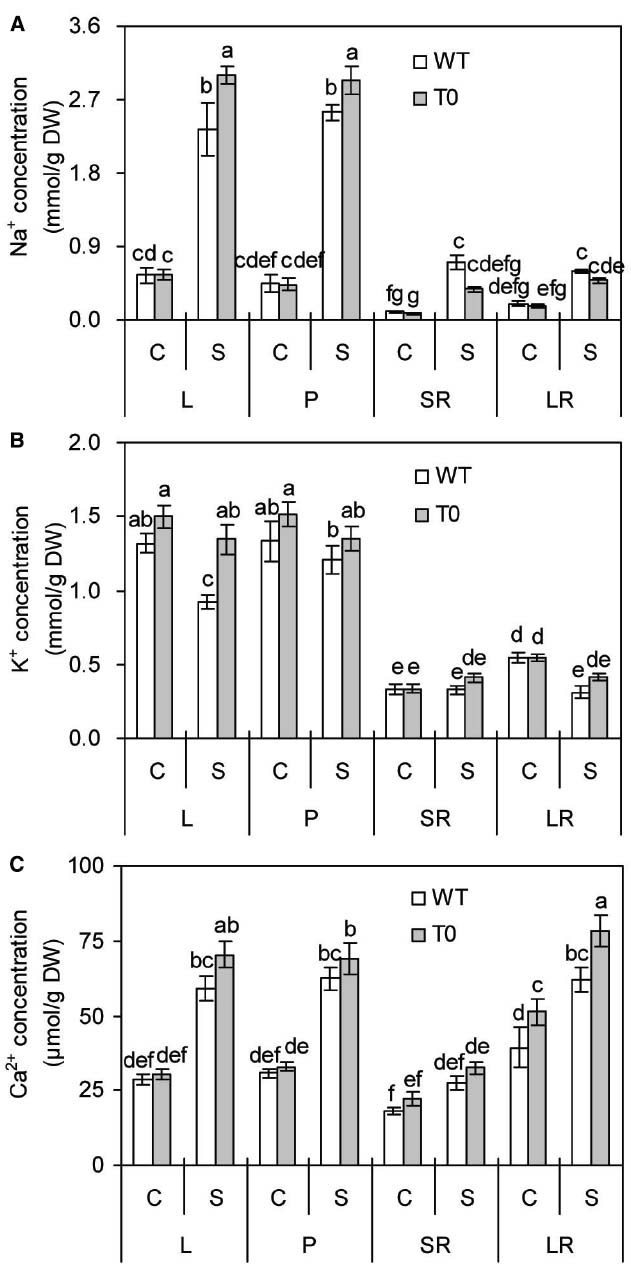
Figure 6. Na+ (A), K+ (B), and Ca2+ (C) concentrations in leaf (L), petiole (P), storage root (SR), and lateral root (LR) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). Data are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
It is clear that salinity caused a significant reduction of leaf and lateral K+ concentrations by 30 and 43% in WT plants compared to control (P < 0.05), respectively. By contrast, chimeric plants maintained stable K+ concentration in all the tissues under saline stress (Figure 6B). It was observed that there are no significant differences between WT and chimeric plants under control condition. However, K+ concentration in leaf of chimeric plants was 45% higher than that of WT plants under saline condition (P < 0.05). It was found that saline stress remarkably increased leaf, petiole, lateral root Ca2+ concentrations in both WT and chimeric plants (P < 0.05), whereas to a greater degree in chimeric plants than in WT plants (Figure 6C). Especially, lateral root Ca2+ concentration of chimeric plants was 30 or 26% higher than that of WT plants in the absence or presence of salinity.
Chimeric Sugar Beet Plants Accumulate More Soluble Sugars in Storage Root
To understand whether co-expression of ZxNHX1 and ZxVP1-1 would affect sugar metabolism in chimeric plants, we determined the sucrose, fructose and glucose contents of WT and chimeric plants grown under saline condition. It was showed that the sucrose, fructose, and glucose contents in storage root of chimeric plants were significantly higher than those of WT plants under either control or saline condition, respectively (P < 0.05) (Figures 7A–C). However, in other tissues, no significant differences were found between WT and chimeric plants (Figures 7A–C). It was also found that storage root displayed the highest sugars contents among all the tissues in either WT or chimeric plants (P < 0.05) (Figures 7A–C). These results suggested that chimeric plants can accumulate more soluble sugars in storage root.
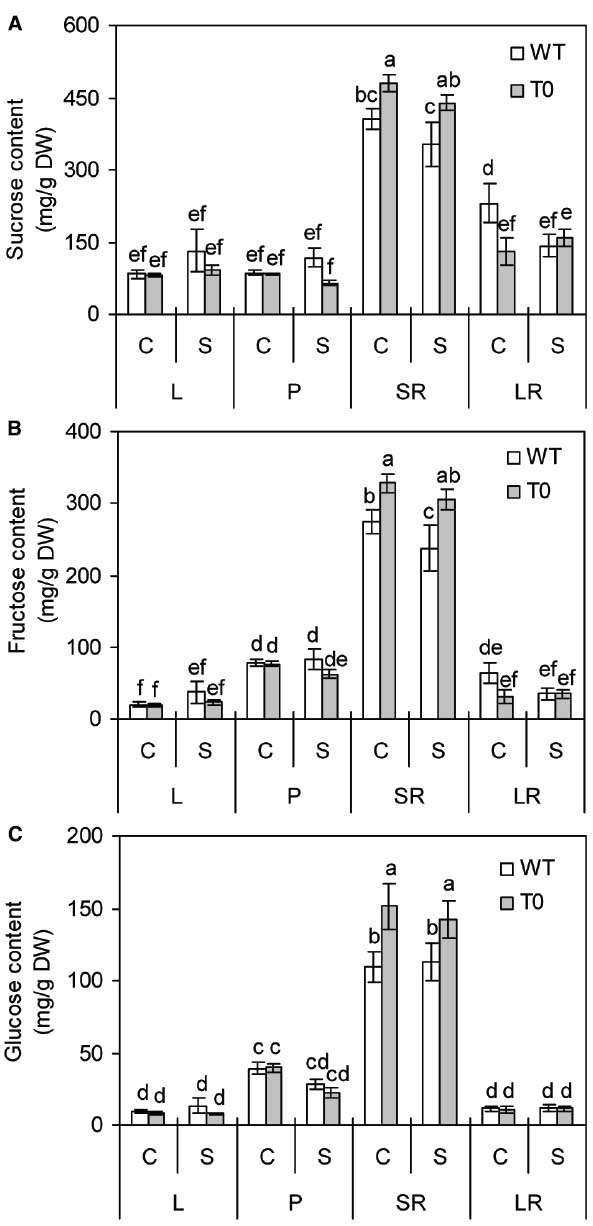
Figure 7. Sucrose (A), fructose (B), and glucose (C) contents in leaf (L), petiole (P), storage root (SR), and lateral root (LR) of 2-month-old wild-type (WT) and chimeric plants (T0) co-expressing ZxNHX and ZxVP1-1 grown under control (C) and salinity (S) condition (treated with 400 mM NaCl for 8 days). Data are mean ± SE (n = 6) and bars indicate the SE. For T0 chimeric plants, values are derived from a combination of single plants each from one of six lines. Columns with different letters indicate significant difference at P < 0.05 (Duncan’s test).
Discussion
In the present study, the ZxNHX and ZxVP1-1 genes from xerophyte Z. xanthoxylum were introduced into the important sugar crop sugar beet. We obtained T0 chimeric sugar beet plants co-expressing ZxNHX and ZxVP1-1 (Figure 1). However, the transgenes are present and detectable in the new upper leaves but would not be present in the non-transformed root tissues—i.e., the storage root and the lateral root would not be transgenic in the T0 plants. Thus, it is clear that these T0 plants are likely to be “chimeric” transgenic plants.
Compared with the WT plants, the T0 chimeric sugar beet plants displayed improved salinity tolerance. After stress with high salinity (400 mM NaCl), the WT plants displayed growth inhibition, chlorosis, and even death, whereas the T0 chimeric plants exhibited normal growth and survival (Figure 2A). This can be attributed to the enhanced compartmentalization of Na+ into vacuoles resulting from co-expression of tonoplast Na+/H+ antiporter and H+-PPase (Gaxiola et al., 2001, 2007; Bao et al., 2009; Yamaguchi et al., 2013; Fan et al., 2014). This mechanism could provide better protection for plant cells via mitigating the toxic effects of Na+ in the cytosol, maintaining ions homeostasis, and increasing vacuolar osmoregulatory capacity (Gaxiola et al., 2007; Bao et al., 2014). This notion is further supported in the present study by the measurements of ions accumulation; the chimeric plants accumulated more Na+ and K+ in their leaf and petiole as well as more Ca2+ in lateral root compared with the WT plants (P < 0.05) (Figures 6A–C). These results corroborate earlier findings of salinity tolerance by co-expressing ZxNHX and ZxVP1-1 in L. corniculatus (Bao et al., 2014).
It was also observed that chimeric plants grew better than WT plants even under control conditions (Figures 2A–C). Similar phenotypes were observed in transgenic cotton overexpressing AVP1 (Pasapula et al., 2011) and L. corniculatus co-expressing ZxNHX and ZxVP1-1 (Bao et al., 2014). It was well demonstrated that tonoplast Na+/H+ antiporter and H+-PPase played essential roles in plant growth and development (Apse et al., 2003; Li et al., 2005; Gaxiola et al., 2007; Bassil et al., 2011). Apse et al. (2003) found that tonoplast Na+/H+ antiporters control cell expansion and leaf development by regulating vacuolar pH and ions homeostasis. These findings ware further confirmed by Bassil et al. (2011), who reported that Arabidopsis double mutant nhx1/nhx2 exhibited remarkably reduced growth, smaller cells, shorter hypocotyls in etiolated seedlings and abnormal stamens in mature flowers compared with WT plants. Tonoplast H+-PPase has been shown to play an important role in facilitating auxin transport and distribution, and thus regulating auxin-related developmental processes (Li et al., 2005). The over-expression of AVP1 resulted in increased cell division at the onset of organ formation and hyperplasia by enhancing auxin transport in transgenic Arabidopsis, while avp1-1 null mutants showed severely disrupted root and shoot development by reducing auxin transport (Li et al., 2005; Gaxiola et al., 2007).
K+ is the major ionic osmoticum in plant cells and occurs in two major pools, in the vacuole and in the cytosol (Barragán et al., 2012). In the present study, the chimeric plants co-expressing ZxNHX and ZxVP1-1 accumulated a markedly higher level of K+ in their leaves under saline conditions as compared with WT plants (Figure 6B). Significant enhancement in K+ during salinity stress has also been reported in transgenic tomato overexpressing AtNHX1 (Leidi et al., 2010), L. corniculatus co-expressing ZxNHX and ZxVP1-1 (Bao et al., 2014), and alfalfa overexpressing TaNHX2 from wheat (Zhang et al., 2015). The increase in K+ accumulation of transgenic plants is likely to be due to increased NHX activity as these transporters are also known to use K+ as a substrate (Shabala and Pottosin, 2014). It was showed that the overexpression of tonoplast Na+/H+ antiporters led to more vacuolar K+ accumulation and greater K+ uptake in transgenic plants compared to WT plants (Leidi et al., 2010; Zhang et al., 2015), whereas Arabidopsis double mutant nhx1/nhx2 displayed lower K+ contents than WT plants (Bassil et al., 2011; Barragán et al., 2012). These results implied that tonoplast Na+/H+ antiporters are crucial for the accrual of K+ in shoot tissues of plants.
There were evidences that accumulating more ions such as Na+ and K+ resulting from the overexpression of the genes encoding tonoplast Na+/H+ antiporter and H+-PPase can improve the osmoregulation capacity, which serves as a force to drive water uptake and thus, maintain the turgor of transgenic plants under salinity condition (Bao et al., 2009, 2014; Gouiaa et al., 2012). Our results supported this point again. Owing to lower leaf and petiole solute potential in chimeric sugar beet (Figure 4), leaves and petioles of chimeric plants retained more water compared with WT plants (Figure 2D). Similarly, lower solute potential was also observed in transgenic L. corniculatus co-expressing ZxNHX and ZxVP1-1 that is in agreement with the findings of the present study and may be due to superior osmotic adjustment of transgenic plants (Bao et al., 2014).
Salinity induces oxidative stress in cells, leading to damage to cellular machinery including free-radical-mediated lipid peroxidation (Mishra et al., 2014). MDA acts as an effective indicator for measuring the degree of lipid peroxidation (Yazici et al., 2007). Under salinity condition, chimeric sugar beet plants exhibited a lower concentration of MDA in their leaf than the WT plants (Figure 3), indicative of the occurrence of less oxidative damage with co-expression of ZxNHX and ZxVP1-1. Tian et al. (2011) also reported that transgenic kiwifruit plants overexpressing AtNHX1 showed a significantly lower MDA content than WT plants when exposed to salinity conditions. These were in accordance with results for transgenic cowpea overexpressing VrNHX1 from mungbean (Vigna radiata) (Mishra et al., 2014) and sweet potato overexpressing AtNHX1 (Fan et al., 2014). These findings perhaps ascribed to the enhanced sequestration of Na+ into the vacuoles, which may protect the cells from the toxicity of excess Na+. Therefore, it can be assumed that co-expression of ZxNHX and ZxVP1-1 might protect the cell membrane from injuries induced by saline stress.
The accumulation of proline in response to high salinity has been well documented (Juan et al., 2005; Wu et al., 2015b). Proline, an important osmoprotectants, contributes to osmotic adjustment, stabilization of proteins and protein complexes, protection of sub-cellular structures like membranes and macromolecules as well as photosynthetic apparatus under salinity stress, and acts as a reactive-oxygen-species (ROS) scavenger (Mishra et al., 2014; Wu et al., 2015a). Evidence supporting the role of proline during saline stress was obtained based on salinity tolerance in transgenic tobacco plants with increased levels of proline biosynthesis (Kishor et al., 1995) and salinity tolerance of Arabidopsis with suppressed levels of proline degradation (Nanjo et al., 1999). In the present study, the accumulation of proline under saline stress was dramatically increased in leaf and petiole of both chimeric and WT plants. However, the accumulation was more pronounced in chimeric plants than in WT plants (Figure 5). An increase of proline content during saline stress has also been observed in AtNHX1-expressing transgenic sweet potato (Fan et al., 2014) and VrNHX1-expressing transgenic cowpea (Mishra et al., 2014). These results implied that the sequestration of excessive Na+ into the vacuoles caused by increasing tonoplast Na+/H+ transport activity may activate the expression of salinity tolerance-related genes, which contribute to the enhancement of proline accumulation.
It is well known that the sugar content of storage root is the main qualitative determinant of the crop, which is one of the most important raw material sources for the production of manufactured sugar throughout the world (Liu et al., 2008). Therefore, the sugar content is also an important objective in sugar beet breeding. Under either control or salinity conditions, contents of sucrose, glucose and fructose in storage root of chimeric plants were significantly higher than those in WT plants (Figures 7A–C). Similar results were observed in transgenic sugar beet overexpressing AtNHX3 as described by Liu et al. (2008), who reported that the maintenance of ions homeostasis diminished the toxic effects of Na+ on the expression of enzymes associated with sucrose synthesis, allowing increased sucrose production in transgenic plants overexpressing AtNHX3 (Liu et al., 2008). However, when grown under control or salinity conditions, no significant difference was seen on the sugar contents of shoots in WT and transgenic plants (Figures 7A–C). Recently, Pizzio et al. (2015) showed that AVP1 has essential functions independent of phloem sucrose loading in Arabidopsis. These are probably related to AVP1 in the vacuolar and prevacuolar compartments mediating PPi hydrolysis and lumen acidification (Pizzio et al., 2015). In addition, although the accumulations of soluble sugars in many species have been widely reported as a response to salinity stress (Ashraf and Harris, 2004; Juan et al., 2005; Nemati et al., 2011), other studies do not support this response (Ashraf, 1997). In the present study, contents of sugar in sugar beet plants were not remarkably affected by salinity stress (Figures 7A–C). Similar results were reported in this species (Wu et al., 2013, 2015a) and other plant species (Morgan, 1992). This could be attributed to the excessive accumulation of Na+ in shoot tissues plants that inhibits enzymes involved in carbohydrate metabolism under high salinity conditions (Munns and Tester, 2008).
In conclusion, our results demonstrated that co-expression of ZxNHX and ZxVP1-1 can enhance salinity tolerance in chimeric sugar beet. It was noteworthy that chimeric plants accumulated more sucrose, glucose and fructose in their storage roots.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31460101 and 31260294) and the Elitist Program of Lanzhou University of Technology (Grant No. J201404). We are also grateful to the anonymous reviewers for their constructive comments on the manuscript.
References
Apse, M. P., Aharon, G. S., Snedden, W. A., and Blumwald, E. (1999). Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiporter in Arabidopsis. Science 285, 1256–1258. doi: 10.1126/science.285.5431.1256
Apse, M. P., Sottosanto, J. B., and Blumwald, E. (2003). Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. Plant J. 36, 229–239. doi: 10.1046/j.1365-313X.2003.01871.x
Ashraf, M. (1997). Changes in soluble carbohydrates and soluble proteins in three arid-zone grass species under salt stress. Trop. Agric. 74, 234–237.
Ashraf, M., and Harris, P. J. C. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166, 3–16. doi: 10.1016/j.plantsci.2003.10.024
Banjara, M., Zhu, L., Shen, G., Payton, P., and Zhang, H. (2012). Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol. Rep. 6, 59–67. doi: 10.1007/s11816-011-0200-5
Bao, A. K., Wang, S. M., Wu, G. Q., Xi, J. J., Zhang, J. L., and Wang, C. M. (2009). Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 176, 232–240. doi: 10.1016/j.plantsci.2008.10.009
Bao, A. K., Wang, Y. W., Xi, J. J., Liu, C., Zhang, J. L., and Wang, S. M. (2014). Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Funct. Plant Biol. 41, 203–214. doi: 10.1071/FP13106
Barragán, V., Leidi, E. O., Andres, Z., Rubio, L., De Luca, A., Fernández, J. A., et al. (2012). Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24, 1127–1142. doi: 10.1105/tpc.111.095273
Bassil, E., and Blumwald, E. (2014). The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr. Opin. Plant Biol. 22, 1–6. doi: 10.1016/j.pbi.2014.08.002
Bassil, E., Tajima, H., Liang, Y. C., Ohto, M., Ushijim, K., Nakano, R., et al. (2011). The Arabidopsis Na+/H+ antiporters nhx1 and nhx2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23, 3482–3497. doi: 10.1105/tpc.111.089581
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant Soil. 39, 205–207. doi: 10.1007/BF00018060
Bayat, F., Shiran, B., and Belyaev, D. V. (2011). Overexpression of HvNHX2, a vacuolar Na+/H+ antiporter gene from barley, improves salt tolerance in Arabidopsis thaliana. Aust. J. Crop Sci. 5, 428–432.
Bhaskaran, S., and Savithramma, D. L. (2011). Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J. Exp. Bot. 62, 5561–5570. doi: 10.1093/jxb/err237
Chen, H., An, R., Tang, J. H., Cui, X. H., Hao, F. S., Chen, J., et al. (2007a). Overexpression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol. Breed. 19, 215–225. doi: 10.1007/s11032-006-9048-8
Chen, M., Chen, Q. J., Niu, X. G., Zhang, R., Lin, H. Q., Xu, C. Y., et al. (2007b). Expression of OsNHX1 gene in maize confers salt tolerance and promotes plant growth in the field. Plant Soil Environ. 53, 490–498.
Fan, W., Deng, G., Wang, H., Zhang, H., and Zhang, P. (2014). Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol. Plant. doi: 10.1111/ppl.12301 [Epub ahead of print].
Gamboa, M. C., Baltierra, F., Leon, G., and Krauskopf, E. (2013). Drought and salt tolerance enhancement of transgenic Arabidopsis by overexpression of the vacuolar pyrophosphatase 1 (EVP1) gene from Eucalyptus globulus. Plant Physiol. Biochem. 73, 99–105. doi: 10.1016/j.plaphy.2013.09.005
Gaxiola, R. A., Li, J., Undurraga, S., Dang, L. M., Allen, G. J., Alper, S. L., et al. (2001). Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. U.S.A. 98, 11444–11449. doi: 10.1073/pnas.191389398
Gaxiola, R. A, Palmgren, M. G., and Schumacher, K. (2007). Plant proton pumps. FEBS Lett. 581, 2204–2214. doi: 10.1016/j.febslet.2007.03.050
Gouiaa, S., Khoudi, H., Leidi, E. O., Pardo, J. M., and Masmoudi, K. (2012). Expression of wheat Na+/H+ antiporter TNHXS1 and H+-pyrophosphatase TVP1 genes in tobacco from a bicistronic transcriptional unit improves salt tolerance. Plant Mol. Biol. 79, 137–155. doi: 10.1007/s11103-012-9901-6
Han, Q. Q., Lü, X. P., Bai, J. P., Qiao, Y., Paré, P. W., Wang, S. M., et al. (2014). Beneficial soil bacterium Bacillus subtilis (GB03) augments salt tolerance of white clover. Front. Plant Sci. 5:525. doi: 10.3389/fpls.2014.00525
Juan, M., Rivero, R. M., Romero, L., and Ruiz, J. M. (2005). Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ. Exp. Bot. 54, 193–201. doi: 10.1016/j.envexpbot.2004.07.004
Kishor, P. B. K., Hong, Z., Miao, G. H., Hu, C. A. A., and Verma, D. P. S. (1995). Overexpression of D1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 108, 1387–1394.
Kronzucker, H. J., and Britto, D. T. (2011). Sodium transport in plants: a critical review. New Phytol. 189, 54–81. doi: 10.1111/j.1469-8137.2010.03540.x
Kronzucker, H. J., Coskun, D., Schulze, L. M., Wong, J. R., and Britto, D. T. (2013). Sodium as nutrient and toxicant. Plant Soil. 369, 1–23. doi: 10.1007/s11104-013-1801-2
Leidi, E. O., Barragan, V., Rubio, L., El-Hamdaoui, A., Ruiz, M. T., Cubero, B., et al. (2010). The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 61, 495–506. doi: 10.1111/j.1365-313X.2009.04073.x
Li, J., Yang, H., Peer, W. A., Richter, G., Blakeslee, J., Bandyopadhyay, A., et al. (2005). Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science 310, 121–125. doi: 10.1126/science.1115711
Li, T. X., Zhang, Y., Liu, H., Ting, W. Y., Li, W. B., and Zhang, H. X. (2010a). Stable expression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1, and salt tolerance in transgenic soybean for over six generations. Chin. Sci. Bull. 55, 1127–1134. doi: 10.1007/s11434-010-0092-8
Li, Y., Zhang, Y., Feng, F., Liang, D., Cheng, L., Ma, F., et al. (2010b). Overexpression of a Malus vacuolar Na+/H+ antiporter gene (MdNHX1) in apple rootstock M26 and its influence on salt tolerance. Plant Cell Tiss. Organ Cult. 102, 337–345. doi: 10.1007/s11240-010-9738-0
Li, Z., Baldwin, C. M., Hu, Q., Liu, H., and Luo, H. (2010c). Heterologous expression of Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ. 33, 272–289. doi: 10.1111/j.1365-3040.2009.02080.x
Li, W., Wang, D., Jin, T., Chang, Q., Yin, D., Xu, S., et al. (2011). The vacuolar Na+/H+ antiporter gene SsNHX1 from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Mol. Biol. Rep. 29, 278–290. doi: 10.1007/s11105-010-0224-y
Liu, H. L., Wang, Q. Q., Yu, M. M., Zhang, Y. Y., Wu, Y. B., and Zhang, H. X. (2008). Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ. 31, 1325–1334. doi: 10.1111/j.1365-3040.2008.01838.x
Ma, Q., Yue, L. J., Zhang, J. L., Wu, G. Q., Bao, A. K., and Wang, S. M. (2012). Sodium chloride improves the photosynthesis and water status in succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol. 32, 4–13. doi: 10.1093/treephys/tpr098
Maathuis, F. J. M., Ahmad, I., and Patishtan, J. (2014). Regulation of Na+ fluxes in plants. Front. Plant Sci. 4:467. doi: 10.3389/fpls.2014.00467
Mishra, S., Behura, R., Awasthi, J. P., Dey, M., Sahoo, D., Bhowmik, S. S. D., et al. (2014). Ectopic overexpression of a mungbean vacuolar Na+/H+ antiporter gene (VrNHX1) leads to increased salinity stress tolerance in transgenic Vigna unguiculata L. walp. Mol. Breed. 34, 1345–1359. doi: 10.1007/s11032-014-0120-5
Morgan, J. M. (1992). Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Aust. J. Plant Physiol. 19, 67–76. doi: 10.1071/PP9920067
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nanjo, T., Kobayashi, M., Yoshiba, Y., Kakubari, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1999). Antisense suppression of proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461, 205–210. doi: 10.1016/S0014-5793(99)01451-9
Nemati, I., Moradi, F., Gholizadeh, S., Esmaeili, M. A., and Bihamta, M. R. (2011). The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 57, 26–33.
Ohta, M., Hayashi, Y., Nakashima, A., Hamada, A., Tanaka, A., Nakamura, T., et al. (2002). Introduction of a Na+/H+ antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett. 532, 279–282. doi: 10.1016/S0014-5793(02)03679-7
Pasapula, V., Shen, G., Kuppu, S., Paez-Valencia, J., Mendoza, M., Hou, P., et al. (2011). Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fibre yield in the field conditions. Plant Biotechnol. J. 9, 88–99. doi: 10.1111/j.1467-7652.2010.00535.x
Peever, T. L., and Higgins, V. J. (1989). Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and non specific elicitors from Cladosporium fulvum. Plant Physiol. 90, 867–875. doi: 10.1104/pp.90.3.867
Pizzio, G. A., Paez-Valencia, J., Khadilkar, A. S., Regmi, K. C., Patron-Soberano, A., Zhang, S., et al. (2015). Arabidopsis type-I proton-pumping pyrophosphatase expresses strongly in phloem, where it is required for pyrophosphate metabolism and photosynthate partitioning. Plant Physiol. 167, 1541–1553. doi: 10.1104/pp.114.254342
Qin, H., Gu, Q., Kuppu, S., Sun, L., Zhu, X., Mishra, N., et al. (2013). Expression of the Arabidopsis vacuolar H+-pyrophosphatase gene AVP1 in peanut to improve drought and salt tolerance. Plant Biotechnol. Rep. 7, 345–355. doi: 10.1007/s11816-012-0269-5
Rajagopal, D., Agarwal, P., Tyagi, W., Singla-Pareek, S. L., Reddy, M. K., and Sopory, S. K. (2007). Pennisetum glaucum Na+/H+ antiporter confers high level of salinity tolerance in transgenic Brassica juncea. Mol. Breed. 19, 137–151. doi: 10.1007/s11032-006-9052-z
Roy, S. J., Negrão, S., and Tester, M. (2014). Salt resistant crop plants. Curr. Opin. Biotechnol. 26, 115–124. doi: 10.1016/j.copbio.2013.12.004
Rozema, J., and Flowers, T. J. (2008). Crops for a salinized world. Science 322, 1478–1480. doi: 10.1126/science.1168572
Shabala, S. (2013). Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 112, 1209–1221. doi: 10.1093/aob/mct205
Shabala, S., and Pottosin, I. (2014). Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiol. Plant. 151, 257–279. doi: 10.1111/ppl.12165
Shen, G., Wei, J., Qiu, X., Hu, R., Kuppu, S., Auld, D., et al. (2015). Co-overexpression of AVP1 and AtNHX1 in cotton further improves drought and salt tolerance in transgenic cotton plants. Plant Mol. Biol. Rep. 33, 167–177. doi: 10.1007/s11105-014-0739-8
Sun, Y., Wang, D., Bai, Y., Wang, N., and Yong, W. (2006). Studies on the overexpression of the soybean GmNHX1 in Lotus corniculatus: the reduced Na+ level is the basis of the increased salt tolerance. Chin. Sci. Bull. 51, 1306–1315. doi: 10.1007/s11434-006-1306-y
Tian, N., Wang, J., and Xu, Z. Q. (2011). Overexpression antiporter gene AtNHX1 from Arabidopsis thaliana improves the salt tolerance of kiwifruit (Actinidia deliciosa). S. Afr. J. Bot. 77, 160–169. doi: 10.1016/j.sajb.2010.07.010
Weeks, J. T., Ye, J. S., and Rommens, C. M. (2008). Development of an in planta method for transformation of alfalfa (Medicago sativa). Transgenic Res. 17, 587–597. doi: 10.1007/s11248-007-9132-9
Wang, S. M., Wan, C. G., Wang, Y. R., Chen, H., Zhou, Z. Y., Fu, H., et al. (2004). The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J. Arid Environ. 56, 525–539. doi: 10.1016/S0140-1963(03)00063-6
Wu, G. Q., Feng, R. J., Liang, N., Yuan, H. J., and Sun, W. B. (2015a). Sodium chloride stimulates growth and alleviates sorbitol-induced osmotic stress in sugar beet seedlings. Plant Growth Regul. 75, 307–316. doi: 10.1007/s10725-014-9954-4
Wu, G. Q., Jiao, Q., and Shui, Q. Z. (2015b). Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower (Helianthus annuus L.). Plant Soil Environ. 61, 220–226. doi: 10.17221/22/2015-PSE
Wu, G. Q., Liang, N., Feng, R. J., and Zhang, J. J. (2013). Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol. Plant. 35, 2665–2674. doi: 10.1007/s11738-013-1298-6
Wu, G. Q., Xi, J. J., Wang, Q., Ma, Q., Bao, A. K., Zhang, J. L., et al. (2011). The ZxNHX gene encoding tonoplast Na+/H+ antiporter in the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. J. Plant Physiol. 168, 758–767. doi: 10.1016/j.jplph.2010.10.015
Xue, Z. Y., Zhi, D. Y., Xue, G. P., Zhang, H., Zhao, Y. X., and Xia, G. M. (2004). Enhanced salt tolerance of transgenic wheat (Triticum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci. 167, 849–859. doi: 10.1016/j.plantsci.2004.05.034
Yamaguchi, T., Hamamoto, S., and Uozumi, N. (2013). Sodium transport system in plant cells. Front. Plant Sci. 4:410. doi: 10.3389/fpls.2013.00410
Yao, M., Zeng, Y., Liu, L., Huang, Y., Zhao, E., and Zhang, F. (2012). Overexpression of the halophyte Kalidium foliatum H+-pyrophosphatase gene confers salt and drought tolerance in Arabidopsis thaliana. Mol. Biol. Rep. 39, 7989–7996. doi: 10.1007/s11033-012-1645-5
Yarra, R., He, S., Abbagani, S., Ma, B., Bulle, M., and Zhang, W. (2012). Overexpression of a wheat Na+/H+ antiporter gene (TaNHX2) enhances tolerance to salt stress in transgenic tomato plants (Solanum lycopersicum L.). Plant Cell Tiss. Organ Cult. 111, 49–57. doi: 10.1007/s11240-012-0169-y
Yazici, I., Tuerkan, I., Sekmen, A. H., and Demiral, T. (2007). Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 61, 49–57. doi: 10.1016/j.envexpbot.2007.02.010
Yuan, H. J., Ma, Q., Wu, G. Q., Wang, P., Hu, J., and Wang, S. M. (2015). ZxNHX controls Na+ and K+ homeostasis at the whole-plant level in Zygophyllum xanthoxylum through feedback regulation of the expression of genes involved in their transport. Ann. Bot. 115, 495–507. doi: 10.1093/aob/mcu177
Yue, L. J., Ma, Q., Li, S. X., Zhou, X. R., Wu, G. Q., Bao, A. K., et al. (2012). NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J. Arid Environ. 87, 153–160. doi: 10.1016/j.jaridenv.2012.06.002
Zhang, H. X., and Blumwald, E. (2001). Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat. Biotechnol. 19, 765–768. doi: 10.1038/90824
Zhang, H. X., Hodson, J. N., Willliams, J. P., and Blumwald, E. (2001). Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc. Natl. Acad. Sci. U.S.A. 96, 1480–1485. doi: 10.1073/pnas.231476498
Zhang, J. L., and Shi, H. Z. (2013). Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 115, 1–22. doi: 10.1007/s11120-013-9813-6
Zhang, L. Q., Niu, Y. D, Huridu, H., Hao, J. F., Qi, Z., and Hasi, A. (2014). Salicornia europaea L. Na+/H+ antiporter gene improves salt tolerance in transgenic alfalfa (Medicago sativa L.). Genet. Mol. Res. 13, 5350–5360. doi: 10.4238/2014.July.24.14
Keywords: salinity tolerance, tonoplast Na+/H+ antiporter, H+-PPase, Na+ compartmentalization, sugar beet
Citation: Wu G-Q, Feng R-J, Wang S-M, Wang C-M, Bao A-K, Wei L and Yuan H-J (2015) Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1-1 confers enhanced salinity tolerance in chimeric sugar beet (Beta vulgaris L.). Front. Plant Sci. 6:581. doi: 10.3389/fpls.2015.00581
Received: 03 June 2015; Accepted: 13 July 2015;
Published: 28 July 2015.
Edited by:
Maki Katsuhara, Okayama University, JapanReviewed by:
Jon Pittman, University of Manchester, UKWoei-Jiun Guo, National Cheng Kung University, Taiwan
Copyright © 2015 Wu, Feng, Wang, Wang, Bao, Wei and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Qiang Wu, School of Life Science and Engineering, Lanzhou University of Technology, 287 Langongping Road, Qilihe District, Lanzhou 730050, China, wugq08@126.com
 Guo-Qiang Wu
Guo-Qiang Wu