- 1Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, Yokohama, Japan
- 2Technical Department, Biomaterial Analysis Center, Tokyo Institute of Technology, Yokohama, Japan
- 3Biological Science Laboratories, Kao Corporation, Tochigi, Japan
- 4Core Research for Evolutional Science and Technology, Japan Science and Technology Agency, Tokyo, Japan
- 5Earth-Life Science Institute, Tokyo Institute of Technology, Tokyo, Japan
Inorganic phosphate (Pi) depletion is a serious problem for plant growth. Membrane lipid remodeling is a defense mechanism that plants use to survive Pi-depleted conditions. During Pi starvation, phospholipids are degraded to supply Pi for other essential biological processes, whereas galactolipid synthesis in plastids is up-regulated via the transcriptional activation of monogalactosyldiacylglycerol synthase 3 (MGD3). Thus, the produced galactolipids are transferred to extraplastidial membranes to substitute for phospholipids. We found that, Pi starvation induced oil accumulation in the vegetative tissues of various seed plants without activating the transcription of enzymes involved in the later steps of triacylglycerol (TAG) biosynthesis. Moreover, the Arabidopsis starchless phosphoglucomutase mutant, pgm-1, accumulated higher TAG levels than did wild-type plants under Pi-depleted conditions. We generated transgenic plants that expressed a key gene involved in TAG synthesis using the Pi deficiency–responsive MGD3 promoter in wild-type and pgm-1 backgrounds. During Pi starvation, the transgenic plants accumulated higher TAG amounts compared with the non-transgenic plants, suggesting that the Pi deficiency–responsive promoter of galactolipid synthase in plastids may be useful for producing transgenic plants that accumulate more oil under Pi-depleted conditions.
Introduction
Plants possess various mechanisms, including membrane lipid remodeling (Essigmann et al., 1998; Härtel et al., 2000; Andersson et al., 2003, 2005; Jouhet et al., 2004; Nakamura, 2013; Shimojima et al., 2013), to adapt to inorganic phosphate (Pi)-limited conditions, which are distinct from those related to nitrogen (N)-limited conditions. During Pi deficiency, phospholipids in the biological membranes are degraded and the phosphorus generated via phospholipid breakdown is used for other essential biological processes in the cell.
To compensate for the lack of phospholipids in the membranes under Pi-limited conditions, galactolipid synthesis in the outer envelope membranes of plastids is up-regulated (Awai et al., 2001; Kobayashi et al., 2004). Plants have two species of galactolipids: monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG). Under normal growth conditions, both MGDG and DGDG are produced and remain in plastids as components of the thylakoid membrane (Benning and Ohta, 2005; Shimojima and Ohta, 2011). However, under Pi-depleted conditions the amount of DGDG doubles and is exported to the extraplastidial membranes by a yet unknown mechanism to substitute for the major phospholipid, phosphatidylcholine. In plastids, DGDG is mainly produced by an additional transfer of a galactose moiety to MGDG by DGDG synthases (DGD1 and DGD2) on the outer envelope membrane (Härtel et al., 2000; Kelly and Dörmann, 2002; Kelly et al., 2003). A previous analysis of an Arabidopsis mutant clearly showed that MGDG synthesis on the outer envelope membrane performed by type B MGDG synthases MGD2 and MGD3 has an important role in increasing the DGDG content during Pi depletion (Kobayashi et al., 2009). An Arabidopsis mgd3 knock-out mutant showed a severe growth defect during Pi depletion, whereas an mgd2 knock-out did not show a significant growth difference compared with wild type (WT; Kobayashi et al., 2009). Thus, between the two isoforms of type B MGDG synthase, MGD3 is predominantly involved in lipid remodeling during Pi starvation.
Type B MGDG synthase genes are widely conserved in seed plant genomes, suggesting that the enhancement of galactolipid synthesis under Pi starvation have been widely conserved in higher plants for adaptation to Pi-poor environments (Russo et al., 2007; Tjellström et al., 2008; Lambers et al., 2012; Yuzawa et al., 2012). Based on these previous findings, we hypothesized that a MGD3 promoter might be useful for efficiently expressing introduced genes in the shoots and roots of plants in response to Pi starvation.
Plant storage lipids, triacylglycerols (TAGs), can be used as feedstock for the production of biodiesel or highly valuable fatty acids (Durrett et al., 2008; Dyer and Mullen, 2008; Riediger et al., 2009; Lu et al., 2011). However, most plant TAGs are synthesized and stored in seeds, which constitute a small portion of the total plant biomass. Although, TAGs are also synthesized in non-seed and vegetative tissues, such as leaves, the amount of TAG in vegetative tissues is usually very low (Chapman and Ohlrogge, 2012; Chapman et al., 2013). Many techniques have been used to increase TAG levels in vegetative tissues (Chapman and Ohlrogge, 2012; Chapman et al., 2013), and those approaches were based on knowledge obtained from TAG synthesis and breakdown in seeds. Most experiments involved ectopically overexpressing genes involved in TAG synthesis and knocking down/out genes involved in TAG breakdown. The transcription factors LEAFY COTYLEDON1 and 2 (LEC1 and 2) are involved in seed maturation and TAG biosynthesis, respectively (Santos Mendoza et al., 2005; Mu et al., 2008). In Arabidopsis, the overexpression of LEC1 or LEC2 in WT plants and of LEC2 in the fatty acid–breakdown mutant COMATOSE leads to TAG accumulation in vegetative tissues (Santos Mendoza et al., 2005; Mu et al., 2008; Slocombe et al., 2009; Kim et al., 2015). Transgenic tobacco that overexpresses DIACYLGLYCEROL ACYLTRANSFERASE 1 (DGAT1) and LEC2 also accumulates TAG in its leaves (Andrianov et al., 2010). Sanjaya et al. (2011) elevated TAG levels in vegetative tissues by (i) suppressing APS1, which encodes the small subunit of ADP-glucose pyrophosphorylase, which catalyzes the first step of starch biosynthesis, producing a starchless mutant and by (ii) overexpressing the transcription factor WRINKLED1 (WRI1), which regulates TAG synthesis in Arabidopsis seeds and shoots. The ectopic overexpression of Chlamydomonas reinhardtii DGAT in Arabidopsis also elevates TAG levels in leaves (Sanjaya et al., 2013). The Arabidopsis COMPARATIVE GENE IDENTIFICATION-58 (CGI-58) homolog controls TAG breakdown exclusively in vegetative tissues by interacting with PEROXISOMAL ABC-TRANSPORTER 1 (PXA1), and the knockout mutant accumulates higher TAG levels in vegetative tissues compared with WT plants (James et al., 2010; Park et al., 2013). TAG levels in Arabidopsis vegetative tissues are also increased when DGAT1 and WRI1 are constitutively overexpressed in sdp1 knockout mutant plants, which are defective in TAG breakdown under nutrient-sufficient conditions (Kelly et al., 2013). The overexpression of PHOSPHOLIPID:DIACYLGLYCEROL ACYLTRANSFERASE 1 (PDAT1) with oleosin in Arabidopsis is also effective for enhancing TAG levels in leaves (Fan et al., 2013). Recently, Vanhercke et al. (2014) succeeded in producing transgenic Nicotiana tabacum (tobacco) in which TAG comprised >15% of the leaf dry weight by co-expressing three genes, oleosin, DGAT1, and WRI1, without severely affecting plant development. Thus, the previous reports clearly showed that genetically engineered vegetative tissues have the potential to store relatively high levels of TAGs.
Here, as an application of the lipid remodeling system during Pi starvation, we produced transgenic Arabidopsis plants that express high levels of TAG synthesis genes under the control of the MGD3 promoter. We analyzed the effects of this approach on plant growth and the TAG content in vegetative tissues to evaluate its efficiency in producing more oil in plant vegetative tissues.
Results
Pi Depletion Increases TAG Levels in Arabidopsis WT Plants
We first compared the phenotypes and TAG levels of WT plants grown under N-depleted and Pi-depleted conditions (Figure 1). The seedlings grown under N-depleted conditions were relatively more chlorotic than seedlings grown under Pi-depleted conditions (Figure 1A), and the TAG levels in N-depleted plants after 7 d were 1.5-fold higher than in the Pi-depleted plants after 10 d (Figure 1B), suggesting that the higher accumulation of TAG during N depletion was a consequence of the rapid breakdown of photosynthetic membranes. Consistent with the phenotypes shown in Figure 1A, WT seedlings grown under N-depleted conditions for 7 d were very small, and their shoot fresh weight was half that of plants grown under N-sufficient or Pi-depleted conditions (Figure 1C). The TAG content per seedling in WT shoots under Pi-depleted conditions was ~1.5-fold higher than that of WT shoots under N-depleted conditions (Figure 1D).
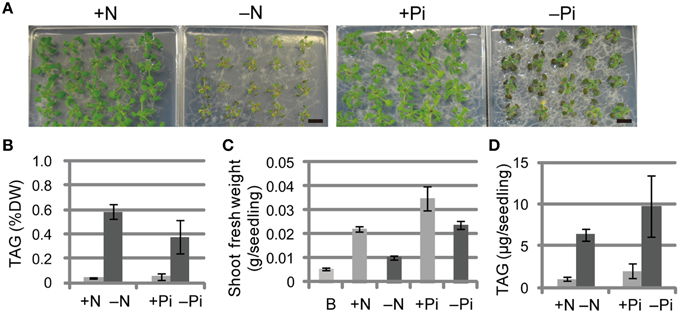
Figure 1. Growth phenotypes and TAG accumulation in shoots of WT Arabidopsis plants grown under Pi- or N-depleted conditions. WT plants (10 d old) were transferred to MS agar containing 1% (w/v) sucrose with (+Pi; 1 mM) or without (−Pi; 0 mM) Pi for 10 d, or with (+N; 3.5 mM) or without (−N; 0 mM) N for 7 d. (A) Growth under Pi-depleted or N-depleted conditions. Bars = 1 cm. (B) TAG levels in shoots under Pi-depleted or N-depleted conditions. DW, dry weight. (C) Shoot fresh weight under Pi-depleted or N-depleted conditions. B, before transfer to Pi- or N-depleted conditions. (D) TAG content in shoots per seedling under N- or Pi-depleted conditions. Data are the means ± SD from three independent experiments.
Pi Limitation Induces TAG Accumulation in Vegetative Tissues of Various Seed Plants
We also measured TAG levels in the roots of WT Arabidopsis plants grown under Pi-sufficient and Pi-depleted conditions. TAG levels in WT plants grown under Pi-depleted conditions were 5- to 6-fold higher in shoots and 1.5- to 2-fold higher in roots compared with those of plants grown under Pi-sufficient conditions (Figure 2A). It should be noted that we have also presented additional data here for shoot TAG levels for comparison with root TAG levels.
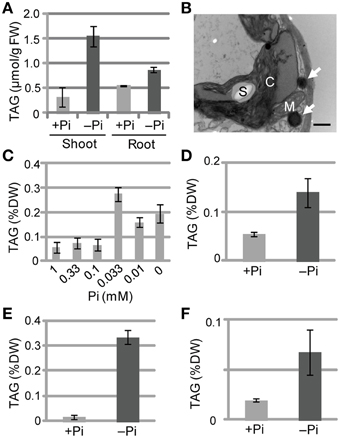
Figure 2. TAG accumulation in WT plants under Pi-depleted growth conditions. (A,B) WT Arabidopsis seedlings (10 d old) were transferred to MS agar containing 1% (w/v) sucrose and 0 mM (–Pi) or 1.0 mM (+Pi) Pi and were grown for 10 d. (A) TAG content in shoots and roots of WT Arabidopsis plants. FW, fresh weight. (B) Electron microscopy of WT Arabidopsis leaves. White arrows, oil droplets; C, chloroplast; M, mitochondrion; S, starch. Bar = 0.5 μm. (C) Effect of Pi concentration on shoot TAG levels in WT Arabidopsis plants. WT Arabidopsis seedlings (10 d old) were transferred to MS agar containing 1% (w/v) sucrose and various concentrations of Pi and were grown for 10 d. DW, dry weight. (D) TAG levels in shoots of tomato (Solanum lycopersicum L.) plants grown for 8 d with 1 mM Pi followed by 28 d with (+) or without (–) Pi. (E) Tobacco (Nicotiana tabacum) plants grown for 10 d with Pi followed by 21 d with (+) or without (–) Pi. (F) Barnyard grass (Echinochloa crus-galli) plants grown for 8 d with Pi followed by 28 d with (+) or without (–) Pi. Data are the mean ± SD from three independent experiments.
We used electron microscopy to assess the impact of low Pi availability on the accumulation of oil droplets in leaf mesophyll cells. Pi starvation resulted in many large starch granules within chloroplasts and oil droplets outside of chloroplasts, whereas no oil droplets were observed inside chloroplasts under these experimental conditions (Figure 2B; Supplementary Figure 1). At Pi levels between 0.1 and 1 mM, TAG levels were similar in plant leaves; however, when the Pi level was < 0.1 mM the TAG levels increased, with 0.033 mM Pi producing the highest TAG level (Figure 2C). Thus, a Pi concentration of ≤0.033 mM was required to observe the low Pi–dependent TAG accumulation in leaves. We also analyzed the TAG levels of other plant species under Pi-depleted conditions. Increased TAG levels upon Pi starvation also occurred in tomato (Solanum lycopersicum L.; Figure 2D; Supplementary Figure 2A), tobacco (N. tabacum; Figure 2E), and barnyard grass (Echinochloa crus-galli; Figure 2F; Supplementary Figure 2B), suggesting that the phenomenon is widely conserved among seed plants, including monocots.
The Vegetative Tissues of the Starchless Phosphoglucomutase Mutant pgm-1 Accumulate Higher Levels of TAGs Under Pi-depleted Conditions
Under Pi-depleted growth conditions, starch accumulates in leaf chloroplasts (Nielsen et al., 1998). Under nutrient-sufficient growth conditions, mutant Arabidopsis plants with low starch levels accumulate more TAGs in their vegetative tissues than WT plants (Sanjaya et al., 2011). To test whether the same pool of carbon sources was used for starch and oil synthesis in leaves under Pi-depleted conditions, we examined Arabidopsis pgm-1 mutants, which lack almost all of the transitory starch in leaves because of a point mutation in the plastidic phosphoglucomutase gene (Caspar et al., 1985; Periappuram et al., 2000). Although, the shoots of pgm-1 plants accumulated more anthocyanin than did WT plants under both Pi-sufficient and Pi-depleted conditions, their fresh weights were similar under both conditions (Figures 3A,B).
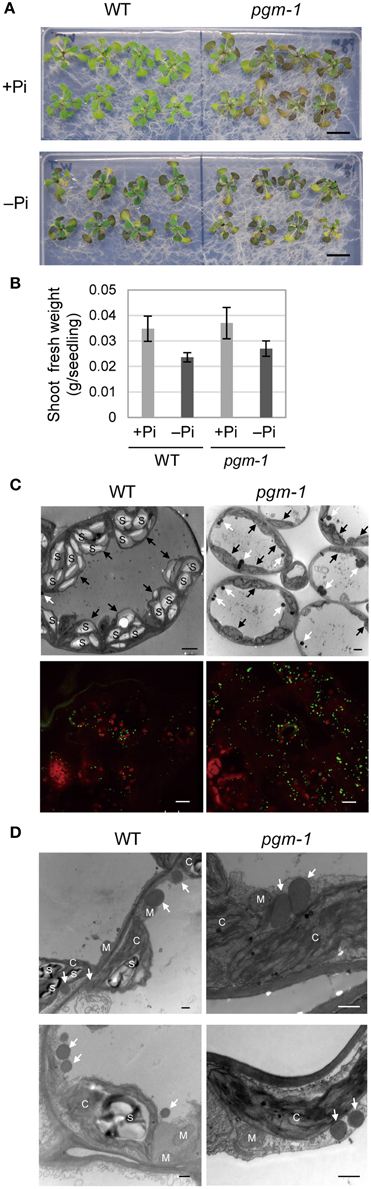
Figure 3. Growth phenotypes and TAG accumulation in leaves of WT and starchless mutant pgm-1 Arabidopsis plants. WT and pgm-1 plants (10 d old) were transferred to MS agar containing 1% (w/v) sucrose and 0 mM (−Pi) or 1 mM (+Pi) Pi and were grown for 10 d. (A) Growth under Pi-sufficient (+Pi) or Pi-depleted (–Pi) conditions. Bars = 1.0 cm. (B) Shoot fresh weight of seedlings grown under Pi-sufficient and Pi-depleted conditions. (C) Oil droplets in leaf mesophyll cells under Pi-depleted conditions. Electron microscopy of leaf mesophyll cells (upper panels; white arrows, oil droplets; black arrows, chloroplasts; S, starch) and representative confocal fluorescence micrographs of leaves (lower panels) showing chloroplasts (red) and oil droplets stained with Nile red (green). Bars = 2 μm in upper images and 10 μm in lower images. (D) Electron microscopy of leaf mesophyll cells in WT and pgm-1 plants grown under Pi-depleted conditions. White arrows indicate oil droplets. S, starch; M, mitochondrion; C, chloroplast. Bars = 0.5 μm.
Lipid droplets in leaf mesophyll cells of WT and pgm-1 plants grown under Pi-depleted conditions were visualized using electron microscopy (Figure 3C, upper panels) and also with a neutral lipid–selective fluorescent dye, Nile red (Figure 3C, lower panels, green). The number of lipid droplets was higher in pgm-1 than in WT plants, and, based on electron microscopy observations, these lipid droplets were likely to be located outside of the chloroplasts (Figure 3D).
Pi Starvation–induced TAG Accumulation Occurs without the Transcriptional Activation of the Key Steps in TAG Biosynthesis
TAG accumulation during senescence is related to the transcriptional up-regulation of DGAT1 (Kaup et al., 2002). TAG accumulation during N starvation occurs with the concomitant induction of the genes involved in TAG synthesis and accumulation, such as DGAT1 and OLEOSIN1 (Yang et al., 2011). Thus, we analyzed the expression of the key TAG biosynthetic genes DGAT1, DGAT2, and PDAT1 in WT and pgm-1 plants under Pi-sufficient and Pi-depleted conditions (Figures 4A–C). Distinct from TAG accumulation during senescence or N starvation, the high TAG accumulation in WT and pgm-1 plants under Pi-depleted conditions (Figure 3C) did not correlate with the transcriptional up-regulation of these genes (Figures 4A–C). The expression levels of these genes in WT roots under Pi-sufficient and Pi-depleted conditions were also analyzed and were clearly shown to be unchanged or decreased during Pi starvation (Figure 4D). These results suggested that the overexpression of these genes under Pi-depleted conditions might further increase TAG levels in the leaves and roots of WT and pgm-1 mutants.
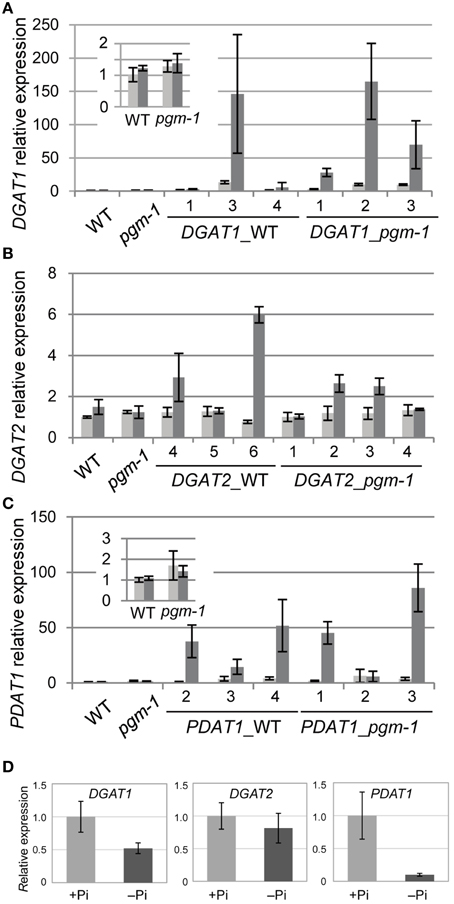
Figure 4. Quantitative reverse transcription-PCR analysis of DGAT1, DGAT2, and PDAT1 expression in WT, pgm-1, and transgenic Arabidopsis plants under Pi-sufficient or Pi-depleted conditions. Seedlings (10 d old) of transgenic lines were transferred to MS medium containing 1% (w/v) sucrose, and 0 mM Pi (dark gray bars) or 1 mM Pi (light gray bars) and were grown for 10 d. (A) DGAT1, (B) DGAT2, and (C) PDAT1 mRNA levels in shoots of WT, pgm-1, and transgenic lines in the WT and pgm-1 backgrounds are shown. Line numbers are indicated for each transgenic strain. The expression level of each gene is relative to that in WT under Pi-sufficient conditions. Data are the mean ± SD from three independent experiments. (D) mRNA levels of DGAT1, DGAT2, and PDAT1 in roots of WT plants under Pi-sufficient (+Pi) and Pi-depleted (–Pi) conditions. The expression level of each gene is relative to that in WT under Pi-sufficient conditions. Data are the mean ± SD from three independent experiments.
Pi Starvation–induced Overexpression of DGAT1, DGAT2, or PDAT1 Does Not Affect Plant Biomass
Previously, our group analyzed the promoter region of Arabidopsis MGD3 (ProMGD3), which encodes a key enzyme in membrane lipid remodeling under Pi-depleted conditions. We showed a significant increase in MGD3 expression levels in shoots and roots of WT plants upon Pi starvation (Awai et al., 2001; Kobayashi et al., 2004). To express TAG synthesis genes under Pi starvation, we produced transgenic Arabidopsis plants in WT and pgm-1 backgrounds harboring the construct ProMGD3:DGAT1 (designated as DGAT1_WT and DGAT1_pgm-1, respectively), ProMGD3:DGAT2 (designated as DGAT2_WT and DGAT2_pgm-1, respectively), or ProMGD3:PDAT1 (designated as PDAT1_WT and PDAT1_pgm-1, respectively). In shoots, compared with WT or pgm-1 plants, DGAT1 expression in DGAT1_WT line 3 and DGAT1_pgm-1 line 2 were markedly higher under Pi-sufficient (~10- to 14-fold higher) and Pi-depleted (~140- to 170-fold higher) conditions (Figure 4A). In transgenic plants harboring ProMGD3:DGAT2, DGAT2 expression in DGAT2_WT line 6 was ~6-fold higher than that in WT and pgm-1 plants under Pi-depleted conditions, whereas expression under Pi-sufficient conditions was similar to that of WT and pgm-1 plants (Figure 4B). Among three lines of DGAT2_pgm-1 transgenic plants, DGAT2 expression levels in lines 2 and 3 were only slightly higher than that in WT and pgm-1 plants under Pi-depleted conditions (~2.5-fold higher; Figure 4B). PDAT1 expression levels in PDAT1_WT line 4 and PDAT1_pgm-1 line 3 were ~2.5-fold higher under Pi-sufficient conditions and markedly higher under Pi-depleted conditions (~50− and ~80-fold higher, respectively) compared with WT and pgm-1 plants (Figure 4C).
Under both Pi conditions, growth phenotypes and shoot fresh weights of DGAT1_WT line 3 and DGAT1_pgm-1 line 2 were similar to those of WT and pgm-1 plants (Figures 3A, 5A,D). DGAT2_WT line 6 and DGAT2_pgm-1 line 2 accumulated slightly less anthocyanin than did WT and pgm-1 plants under both Pi conditions (Figures 3A, 5B,D). The shoot fresh weight of DGAT2_WT line 6 grown under Pi-sufficient conditions was greater than those of WT and pgm-1 plants, although under Pi-depleted conditions the fresh weight was similar to those of WT and pgm-1 plants (Figure 5D). The growth phenotype of PDAT1_WT line 4 was similar to that of PDAT1_pgm-1 line 3 under both Pi conditions but differed from those of WT and pgm-1 plants (Figures 3A, 5C). Under Pi-depleted conditions, seedlings of both lines were yellowish and accumulated markedly less anthocyanin than did WT and pgm-1 plants (Figures 3A, 5C). Moreover, the fresh weight of PDAT1_pgm-1 line 3 was significantly greater than those of WT and pgm-1 plants under both Pi conditions (Figure 5D). Taken together, the shoot fresh weights of all of the transgenic plants was similar to, or higher than, those of WT and pgm-1 plants under both Pi conditions (Figure 5D).
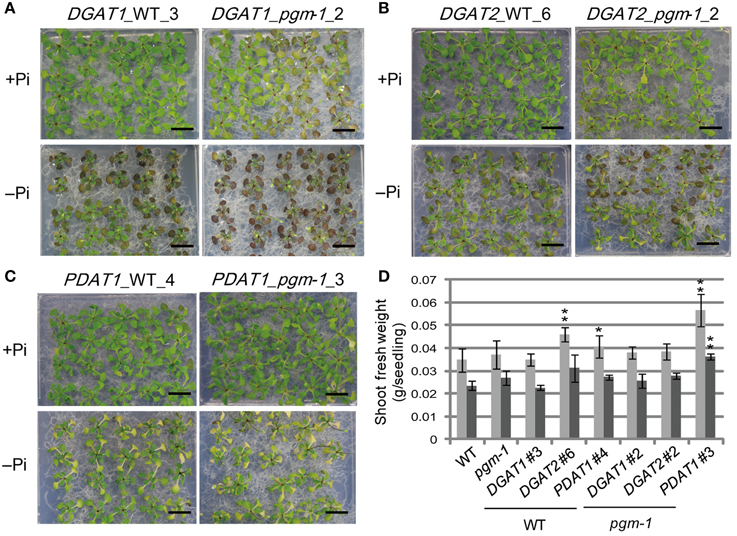
Figure 5. Growth phenotypes and fresh weight of transgenic lines under Pi-sufficient and Pi-depleted conditions. Seedlings (10 d old) of transgenic lines were transferred to MS agar containing 1% (w/v) sucrose and 0 mM (–Pi) or 1 mM (+Pi) Pi and were grown for 10 d. Growth phenotypes of (A) DGAT1_WT_3 and DGAT1_pgm-1_2, (B) DGAT2_WT_6 and DGAT2_pgm-1_2, and (C) PDAT1_WT_4 and PDAT1_pgm-1_3. Bars = 1 cm. (D) Shoot fresh weights of seedlings grown under Pi-sufficient (light gray) and Pi-depleted (dark gray) conditions. Data are the mean ± SD from three independent experinments; *P < 0.05 or **P < 0.01 for t-test vs. WT under Pi-sufficient conditions.
Pi Starvation–induced Overexpression of DGAT1, DGAT2, and PDAT1 Enhances TAG Accumulation in Vegetative Tissues
We analyzed TAG levels in vegetative tissues of WT, pgm-1, and transgenic plants (Figure 6). Compared with WT plants under Pi-sufficient conditions, TAG levels in pgm-1 under Pi-sufficient and Pi-depleted conditions were 1.8-fold and 13-fold higher, respectively (Figure 6A). Moreover, TAG levels in DGAT1_pgm-1 line 2, DGAT2_pgm-1 line 2, and PDAT1_pgm-1 line 3 transgenic lines under Pi-depleted conditions were 26-,16-, and 23-fold higher, respectively, than WT under Pi-sufficient conditions (Figure 6A). Moreover, the TAG content in shoots per seedling of pgm-1, DGAT1_pgm-1 line 2, DGAT2_pgm-1 line 2, and PDAT1_pgm-1 line 3 transgenic lines under Pi-depleted conditions was 9.5-, 19-, 12-, and 23-fold higher, respectively, than that of WT seedlings under Pi-sufficient conditions, and the TAG content was 1.8-, 3.5-, 2.3-, and 4.3-fold higher, respectively, than that of WT seedlings under Pi-depleted conditions (Figure 6B).
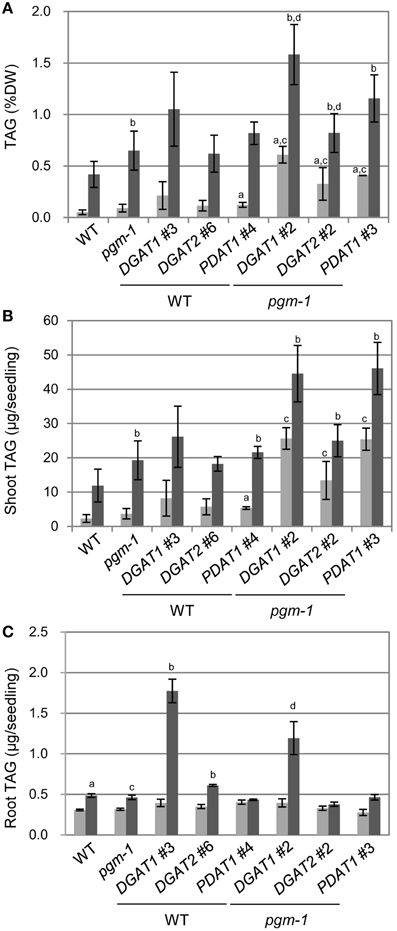
Figure 6. TAG accumulation in Arabidopsis transgenic lines under Pi-sufficient and Pi-depleted conditions. Plants were grown on MS agar with 1% (w/v) sucrose for 10 d and then were transferred to MS agar containing 1% (w/v) sucrose with 1 mM Pi or without Pi for 10 d. (A) TAG levels in shoots per dry weight (DW) in WT, pgm-1, and transgenic plant lines under Pi-sufficient (light gray) and Pi-depleted (dark gray) conditions. (B,C) TAG content in shoots (B) and roots (C) per each seedling of WT, pgm-1, and transgenic plant lines, as in (A). Data are the mean ± SD from three independent experiments; a−dt-test significant at P < 0.05 vs. aWT under Pi-sufficient conditions, bWT under Pi-depleted conditions, cpgm-1 under Pi-sufficient conditions, or dpgm-1 under Pi-depleted conditions.
We also measured the TAG content in roots per seedling in six transgenic lines under Pi-sufficient and Pi-depleted conditions (Figure 6C). The overexpression of these three genes had different effects in roots than in shoots. First, the TAG content in roots per seedling of WT and pgm-1 was comparable under both Pi conditions (Figure 6C). Second, DGAT1 overexpression in WT and pgm-1 plants was the most effective way to produce more TAGs under Pi-depleted conditions compared with DGAT2- or PDAT1-containing transgenic plants in the same background (Figure 6C). Under Pi-depleted conditions, the TAG content in DGAT1_WT line 3 and DGAT1_pgm-1 line 2 increased to 5.8-fold and 4-fold of the levels in WT and pgm-1, respectively (Figure 6C). However, in the other transgenic plants, the TAG content was comparable with that in the non-transformed backgrounds, WT and pgm-1 (Figure 6C). Thus, using the starchless mutant background during Pi starvation in combination with the overexpression of DGAT1, DGAT2, or PDAT1 was the most efficient way to increase TAG levels in shoots (Figures 6A,B), but for roots, the use of the pgm-1 background for transgenic plants, relative to WT, had a negative effect on increasing TAG levels. Although, the level of TAG in DGAT1_pgm-1 roots increased by 4-fold under Pi-depleted conditions compared with that in pgm-1 under Pi-sufficient conditions, the TAG level in the roots of pgm-1 and the other pgm-1 background transgenic plants was similar to, or smaller than, that in WT and the other WT background transgenic plants (Figure 6C). Thus, our results clearly showed that the Pi starvation–induced overexpression of DGAT1 or PDAT1 in the pgm-1 mutant background is the most efficient way to increase TAG levels in shoots, but DGAT1 overexpression in WT is effective for enhancing TAG accumulation in roots.
Discussion
Plants increase TAG levels in vegetative tissues during senescence and under several stresses such as freezing, drought stress, or oxidative stress (Sakaki et al., 1990a,b,c; Kaup et al., 2002; Gaude et al., 2007; Moellering et al., 2010; Lippold et al., 2012; Troncoso-Ponce et al., 2013). Among the essential macronutrients for plants, N starvation is a well-known trigger for TAG synthesis in leaves (Gaude et al., 2007; Yang et al., 2011; Lippold et al., 2012). However, N starvation promotes senescence and degradation of chlorophyll, causing a severe reduction in photosynthetic activity, and thus results in smaller seedlings as compared with well-fertilized plants (Yang et al., 2011). We showed that Pi starvation alters TAG levels in seedlings and compared the effect with N starvation (Figures 1, 2). Although, N starvation also results in TAG accumulation, the biomass of plants grown under N-depleted conditions was markedly smaller than that under Pi-depleted conditions (Figure 1C). Moreover, chloroplast ultrastructure was not severely affected, during Pi deficiency except for an increase in the number of starch granules in the stroma (Supplementary Figure 1). Taken together, these results suggest that either N or Pi starvation results in TAG accumulation in leaves, but the damage to plants is relatively more severe during N starvation. Indeed, Arabidopsis WT plants under Pi-depleted conditions maintain ~85% of their photosynthetic activity as compared with plants grown under Pi-sufficient conditions (Kobayashi et al., 2009). These results also suggest that both types of nutrient deficiency result in TAG accumulation in leaves, but the pathways and the mechanisms of TAG synthesis might differ slightly.
Because, TAG levels were increased in both shoots and roots, we first thought that the TAG synthesis genes were up-regulated under Pi-depleted conditions. Unexpectedly, the expression levels of three major TAG synthesis genes, DGAT1, DGAT2, and PDAT1, were not significantly increased in WT shoots under Pi-depleted conditions (Figures 4A–C). Moreover, in WT roots, although expression levels of DGAT2 remained unchanged under both Pi conditions, those of DGAT1 and PDAT1 under Pi-depleted conditions were significantly decreased compared with those under Pi-sufficient conditions (Figure 4D). These results suggested that enhanced TAG accumulation under Pi-depleted conditions was not correlated with the transcriptional up-regulation of TAG synthesis genes, as was also suggested by Pant et al. (2015), but could be due to the down-regulation of genes involved in TAG breakdown or homeostasis during Pi depletion (James et al., 2010; Kelly et al., 2013; Park et al., 2013). Currently, the molecular mechanisms behind Pi starvation–induced TAG accumulation are unclear but are under investigation.
We clearly showed that the Pi starvation–inducible promoter ProMGD3 is a useful engineering tool for producing transgenic plants that accumulate TAG in shoots in response to Pi starvation; however, it was only partially successful in roots. As the roots of DGAT1_WT accumulated significant levels of TAG in response to Pi starvation (Figure 6C), the reason for unchanged TAG levels in DGAT2- or PDAT1-containing transgenic plants might be due to the decreased availability of the preferred substrates in roots compared with shoots under Pi-depleted conditions. Indeed, the substrate preferences of Arabidopsis DGAT1 and DGAT2 were reported to be different (Zhou et al., 2013; Ayme et al., 2014). As for the fatty acid composition of TAGs in roots of WT, pgm-1, and transgenic plants under Pi-depleted conditions, both DGAT1_WT and DGAT1_pgm-1 showed notable increases in the 18:1 ratio and decreases in the 18:3 ratio compared with those in the other plants (Supplementary Figure 3). However, for the fatty acid composition of TAGs in shoots, an increase in the 18:1 ratio and a decrease in the 18:3 ratio were observed only in DGAT1-containing transgenic plants under Pi-depleted conditions (Supplementary Figure 4). The fatty acid composition of TAGs in DGAT1-containing transgenic plants was in agreement with the substrate preference, 18:1 over 18:3, of DGAT1 (Zhou et al., 2013). Thus, these transgenic plants might also be useful for further analyzing the differences in TAG accumulation mechanisms and the availability of substrates in shoots and roots under Pi starvation.
In this study, we enhanced TAG accumulation in vegetative tissues using the promoter of a plastid-localized galactolipid synthase gene in combination with the lipid remodeling system under Pi starvation, but the amount of TAG was still low compared with the levels seen in previous studies. The highest TAG levels in shoot of our transgenic plants was ~1.5 % D.W. as shown in Figure 6A. Fan et al. (2013) showed that coexpression of PDAT1 with oleosin in wild-type background and tgd1-1 mutant background boost leaf TAG content by up to 6.4–8.6% of the dry weight, respectively. Kelly et al. (2013) showed that in transgenic plants constitutively coexpressing WRINKLED1 and DGAT1 in sdp1 mutant background, the accumulation of TAG in roots, stems, and leaves was elevated to levels ranging from 5 to 8% of dry weight. Thus, all the other works were performed by engineering multiple genes involved in TAG accumulation or degradation such as co-overexpression of oleosin or knock-out of SDP1. Given that we only introduced single gene into the transgenic plants, we still have chance to elevate TAG levels in leaves by engineering multiple genes using our system. Moreover, we are currently investigating whether this system can be applied to crop plants, and we are determining the best soil growth conditions for enhanced TAG accumulation without extreme plant growth retardation. Although, we showed its ability to produce TAG only in vegetative tissues, this system can be applied to producing other useful industrial compounds by introducing the corresponding synthesis genes in place of the TAG synthesis genes. Thus, we hope that the system will be used in many industrial applications in the near future.
Materials and Methods
Plant Material and Growth Conditions
Seeds of pgm-1 were obtained from the Arabidopsis Biological Resource Center. Surface-sterilized seeds of WT A. thaliana (Columbia-0), the starchless mutant pgm-1, and transgenic mutant lines were incubated at 4°C in darkness for 3 d prior to plating on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) containing 0.8% (w/v) agar (MS agar) supplemented with 1% (w/v) sucrose and were then incubated at 23°C under continuous white light (40–50 μmol m−2 s−1) for all growth conditions. Arabidopsis, tomato (S. lycopersicum L.), barnyard grass (E. crus-galli), and tobacco (N. tabacum) seeds were grown on solidified MS agar supplemented with 1% (w/v) sucrose for 8 d (tomato, barnyard grass) or 10 d (Arabidopsis and tobacco) and then were grown for another 10 d (Arabidopsis), 21 d (tobacco), or 28 d (tomato and barnyard grass) on solidified Pi-sufficient (1 mM Pi) or Pi-depleted (0 mM Pi) medium (Härtel et al., 2000) supplemented with 1% (w/v) sucrose, or for 7 d on solidified N-sufficient (3.5 mM N) or N-depleted (0 mM N) medium supplemented with 1% (w/v) sucrose, with KNO3 and Ca(NO3)2 4H2O replaced with KCl2 and CaCl2, respectively.
Electron Microscopy
Leaf segments were fixed with 2% (w/v) paraformaldehyde and 2.5% (w/v) glutaraldehyde in 0.067 M sodium phosphate buffer (pH 7.4) for 2 h at room temperature and then for 16 h at 4°C. Samples were then washed six times in the sodium phosphate buffer for 10 min each at room temperature. They were post-fixed with 2% (w/v) osmium tetroxide in 0.067 M sodium phosphate (pH 7.4) for 2 h at room temperature. The fixed samples were dehydrated in a graded ethanol series and embedded in epoxy resin mixture (Quetol 651 mixture; Nissin EM). Ultrathin 70-nm sections were cut with a diamond knife on a Leica Ultracut UCT ultramicrotome and were transferred onto copper grids. The sections were stained with 2% (w/v) uranyl acetate for 15 min followed by 0.4% (w/v) lead citrate for 5 min at room temperature. The specimens were observed on a Hitachi H-7500 transmission electron microscope at an accelerating voltage of 80 kV.
Lipid Analysis
Total lipid was extracted from tissues as described by Bligh and Dyer (1959). The polar membrane lipids were separated by two-dimensional thin-layer chromatography (Kobayashi et al., 2007). TAGs were separated by one-dimensional thin-layer chromatography using the solvent system of hexane/diethyl ether/acetic acid (160:40:4, v/v/v). Lipids on silica gel plates were visualized with 0.01% (w/v) primuline in 80% (v/v) acetone under UV light. Lipids isolated from silica gel plates were methylated, and fatty acid methyl esters were quantified by gas chromatography using pentadecanoic acid as an internal standard (Kobayashi et al., 2006).
Imaging Lipid Droplets In situ
Leaves were vacuum-fixed in 4% (w/v) paraformaldehyde in 50 mM PIPES buffer (pH 7.0) and stained with 50 μg ml−1 Nile red (Sigma) to selectively visualize lipid droplets in situ (Greenspan et al., 1985). The Nile Red signal and chlorophyll autofluorescence were observed using a confocal laser-scanning microscope (TCS SE; Leica) with an argon laser for excitation at 488 nm, a 556- to 580-nm filter for detection of the Nile Red signal, and a 718- to 749-nm filter for detection of chlorophyll fluorescence. Images were merged and pseudocolored using Leica confocal software.
Generation and Selection of Transgenic Plants
To obtain transgenic plants, the complete coding sequences of DGAT1, DGAT2, and PDAT1 from A. thaliana were amplified from the reverse transcript of WT leaf total RNA. The primers used were DGAT1_FW (5′ CGCCCGGGTATGGCGATTTTGGATTCTGCTGGC 3′), DGAT1_RV (5′ GCGAGCTCTCATGACATCGATCCTTTTCGGTTC 3′); DGAT2_FW (5′ GCCCCGGGTATGGGTGGTTCCAGAGAGTTCCGAG 3′), DGAT2_RV (5′ GCGAGCTCTCAAAGAATTTTCAGCTCAAGATC 3′); and PDAT1_FW (5′ CGCCCGGGTATGCCCCTTATCATCGGAAAAAG 3′), PDAT1_RV (5′ GCGAGCTCTCACAGCTTCAGGTCAATACGCTC 3′). Each amplified fragment was cloned into the pZErO cloning vector (Life Technologies). To remove the Sac I site in the DGAT1 coding sequence, the obtained vector was subjected to a Quikchange Lightning reaction (Qiagen) using the following primers: DGAT1_c845t_fw (5′ GTCTCCTACTACGTTAGCTTGAAGAGCTTGGCATATTTC 3′) and DGAT1_c845t_rv (5′ GAAATATGCCAAGCTCTTCAAGCTAACGTAG TAGGAGAC3′). Vectors were subjected to restriction analysis and DNA sequencing to confirm the presence of the expected sequences. Each DGAT1, DGAT2, and PDAT1 fragment was digested with Sma I and Sac I and independently ligated into the Sma I and Sac I sites of plasmid atMGD3::GUS/pBI101 (Kobayashi et al., 2004). All of the Arabidopsis transformants described here were produced using a modified version of the floral dip method (Clough and Bent, 1998) and were selected on MS agar containing 50 μg ml−1 kanamycin.
Quantitative Reverse Transcription–PCR
Total RNA was isolated from three independent plant samples using the SV Total RNA Isolation System (Promega). Reverse transcription was performed using the PrimeScript RT reagent kit (TaKaRa Bio). cDNA amplification was carried out using SYBR PreMix Ex Taq (TaKaRa Bio). Signal detection and quantification were performed in duplicate using the Thermal Cycler Dice Real Time System (TaKaRa Bio). Quantitative PCR determination of DGAT1, DGAT2, and PDAT1 transcripts was carried out using the Arabidopsis UBQ10 transcript levels for normalization (Narise et al., 2010). Expression levels were obtained from at least three replicates. The gene-specific primers used were as follows: DGAT1_fw (5′ GAGAGAGAGTCCA CTTAGCTC 3′), DGAT1_rv (5′ CGTTCTGATCAAC CAACCATAC 3′); DGAT2_fw (5′ TCCAGCCTAATCG TGCCTATG 3′), DGAT2_rv (5′ GGGAGTGTAGAAT ATAGCACTAC 3′); PDAT1_fw (5′ AGGCAAACAATGCGCT GATGG 3′), PDAT1_rv (5′ TGTCAAGTGACAT GTGTTCCAC 3′); UBQ10_fw (5′ GGCCTTGTATAATCCC TGATGAATAAG 3′), UBQ10_rv (5′ AAAGAGATAACAGGA ACGGAAACATAGT 3′).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (No. 23119506, No. 25119708, and No. 24580091) and JST CREST from the Ministry of Education, Sports, Science, and Culture in Japan.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00664
Supplementary Figure 1. Electron microscopy of leaf mesophyll cells in WT Arabidopsis plants. Plants were grown on MS agar with 1% (w/v) sucrose for 10 d and then were transferred to MS agar containing 1% (w/v) sucrose without (0 mM) Pi for 10 d. White arrows indicate oil droplets. S, starch; M, mitochondrion. Bars = (A) 10 μm and (B) 0.5 μm.
Supplementary Figure 2. TAG levels in various plants under Pi-depleted conditions. (A,B) TAG levels in tomato (S. lycopersicum L.) shoots (A) and barnyard grass (E. crus-galli) (B) grown for 13 d with Pi and then for 31 d with (+) or without (–) Pi. For all conditions: +Pi, 1 mM; –Pi, 0 mM. (C,D) Fatty acid profiles of TAGs in shoots of tomato (C) and barnyard grass (D). Data are the mean ± SD from three independent experiments.
Supplementary Figure 3. Fatty acid composition of TAG in roots of WT, pgm-1, and transgenic plant lines grown under Pi-sufficient and Pi-depleted conditions. Plants were grown on MS agar with 1% (w/v) sucrose for 10 d and then were transferred to MS agar containing 1% (w/v) sucrose with (A) or without (B) Pi for 10 d. Data are the mean ± SD from three independent experiments.
Supplementary Figure 4. Fatty acid composition of TAG in shoots of WT, pgm-1, and transgenic plant lines. Plants were grown under Pi-sufficient (gray) and Pi-depleted (black) conditions in all of the figures, except the WT (N) figure, in which plants were grown under N-sufficient (gray) and N-depleted (black) conditions. Data are the mean ± SD from three independent experiments.
References
Andersson, M. X., Larsson, K. E., Tjellstrom, H., Liljenberg, C., and Sandelius, A. S. (2005). Phosphate-limited oat. The plasma membrane and the tonoplast as major targets for phospholipid-to-glycolipid replacement and stimulation of phospholipases in the plasma membrane. J. Biol. Chem. 280, 27578–27586. doi: 10.1074/jbc.M503273200
Andersson, M. X., Stridh, M. H., Larsson, K. E., Liljenberg, C., and Sandelius, A. S. (2003). Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett. 537, 128–132. doi: 10.1016/S0014-5793(03)00109-1
Andrianov, V., Borisjuk, N., Pogrebnyak, N., Brinker, A., Dixon, J., Spitsin, S., et al. (2010). Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol. J. 8, 277–287. doi: 10.1111/j.1467-7652.2009.00458.x
Awai, K., Maréchal, E., Block, M. A., Brun, D., Masuda, T., Shimada, H., et al. (2001). Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 10960–10965. doi: 10.1073/pnas.181331498
Ayme, L., Baud, S., Dubreucq, B., Joffre, F., and Chardot, T. (2014). Function and localization of the Arabidopsis thaliana diacylglycerol acyltransferase DGAT2 expressed in yeast. PLoS ONE 9:e92237. doi: 10.1371/journal.pone.0092237
Benning, C., and Ohta, H. (2005). Three enzyme systems for galactoglycerolipid biosynthesis are coordinately regulated in plants. J. Biol. Chem. 280, 2397–2400. doi: 10.1074/jbc.R400032200
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 37, 911–917. doi: 10.1139/o59-099
Caspar, T., Huber, S. C., and Somerville, C. (1985). Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 79, 11–17. doi: 10.1104/pp.79.1.11
Chapman, K. D., Dyer, J. M., and Mullen, R. T. (2013). Commentary: why don't plant leaves get fat? Plant Sci. 207, 128–134. doi: 10.1373/clinchem.2013.202952
Chapman, K. D., and Ohlrogge, J. B. (2012). Compartmentation of triacylglycerol accumulation in plants. J. Biol. Chem. 287, 2288–2294. doi: 10.1074/jbc.R111.290072
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Durrett, T. P., Benning, C., and Ohlrogge, J. (2008). Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 54, 593–607. doi: 10.1111/j.1365-313X.2008.03442.x
Dyer, J. M., and Mullen, R. T. (2008). Engineering plant oils as high-value industrial feedstocks for biorefining: the need for underpinning cell biology research. Physiol. Plant. 132, 11–22. doi: 10.1111/j.1399-3054.2007.01021.x
Essigmann, B., Güler, S., Narang, R. A., Linke, D., and Benning, C. (1998). Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 95, 1950–1955. doi: 10.1073/pnas.95.4.1950
Fan, J., Yan, C., and Xu, C. (2013). Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J. 76, 930–942. doi: 10.1111/tpj.12343
Gaude, N., Bréhélin, C., Tischendorf, G., Kessler, F., and Dörmann, P. (2007). Nitrogen deficiency in Arabidopsis affects galactolipid composition and gene expression and results in accumulation of fatty acid phytyl esters. Plant J. 49, 729–739. doi: 10.1111/j.1365-313X.2006.02992.x
Greenspan, P., Mayer, E. P., and Fowler, S. D. (1985). Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973. doi: 10.1083/jcb.100.3.965
Härtel, H., Dörmann, P., and Benning, C. (2000). DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 97, 10649–10654. doi: 10.1073/pnas.180320497
James, C. N., Horn, P. J., Case, C. R., Gidda, S. K., Zhang, D., Mullen, R. T., et al. (2010). Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc. Natl. Acad. Sci. U.S.A. 107, 17833–17838. doi: 10.1073/pnas.0911359107
Jouhet, J., Maréchal, E., Baldan, B., Bligny, R., Joyard, J., and Block, M. A. (2004). Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria. J. Cell Biol. 167, 863–874. doi: 10.1083/jcb.200407022
Kaup, M. T., Froese, C. D., and Thompson, J. E. (2002). A role for diacylglycerol acyltransferase during leaf senescence. Plant Physiol. 129, 1616–1626. doi: 10.1104/pp.003087
Kelly, A. A., and Dörmann, P. (2002). DGD2, an Arabidopsis gene encoding a UDP-galactose-dependent digalactosyldiacylglycerol synthase is expressed during growth under phosphate-limiting conditions. J. Biol. Chem. 277, 1166–1173. doi: 10.1074/jbc.M110066200
Kelly, A. A., Froehlich, J. E., and Dörmann, P. (2003). Disruption of the two digalactosyldiacylglycerol synthase genes DGD1 and DGD2 in Arabidopsis reveals the existence of an additional enzyme of galactolipid synthesis. Plant Cell 15, 2694–2706. doi: 10.1105/tpc.016675
Kelly, A. A., van Erp, H., Quettier, A. L., Shaw, E., Menard, G., Kurup, S., et al. (2013). The SUGAR-DEPENDENT1 lipase limits triacylglycerol accumulation in vegetative tissues of Arabidopsis. Plant Physiol. 162, 1282–1289. doi: 10.1104/pp.113.219840
Kim, H. U., Lee, K.-R., Jung, S.-J., Shin, H. A., Go, Y. S., Suh, M.-C., et al. (2015). Senescence-inducible LEC2 enhances triacylglycerol accumulation in leaves without negatively affecting plant growth. Plant Biotechnol. J. doi: 10.1111/pbi.12354. [Epub ahead of print].
Kobayashi, K., Awai, K., Nakamura, M., Nagatani, A., Masuda, T., and Ohta, H. (2009). Type-B monogalactosyldiacylglycerol synthases are involved in phosphate starvation-induced lipid remodeling, and are crucial for low-phosphate adaptation. Plant J. 57, 322–331. doi: 10.1111/j.1365-313X.2008.03692.x
Kobayashi, K., Awai, K., Takamiya, K., and Ohta, H. (2004). Arabidopsis type B monogalactosyldiacylglycerol synthase genes are expressed during pollen tube growth and induced by phosphate starvation. Plant Physiol. 134, 640–648. doi: 10.1104/pp.103.032656
Kobayashi, K., Kondo, M., Fukuda, H., Nishimura, M., and Ohta, H. (2007). Galactolipid synthesis in chloroplast inner envelope is essential for proper thylakoid biogenesis, photosynthesis, and embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 104, 17216–17221. doi: 10.1073/pnas.0704680104
Kobayashi, K., Masuda, T., Takamiya, K., and Ohta, H. (2006). Membrane lipid alteration during phosphate starvation is regulated by phosphate signaling and auxin/cytokinin cross-talk. Plant J. 47, 238–248. doi: 10.1111/j.1365-313X.2006.02778.x
Lambers, H., Cawthray, G. R., Giavalisco, P., Kuo, J., Laliberté, E., Pearse, S. J., et al. (2012). Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol. 196, 1098–1108. doi: 10.1111/j.1469-8137.2012.04285.x
Lippold, F., vom Dorp, K., Abraham, M., Hölzl, G., Wewer, V., Yilmaz, J. L., et al. (2012). Fatty acid phytyl ester synthesis in chloroplasts of Arabidopsis. Plant Cell 24, 2001–2014. doi: 10.1105/tpc.112.095588
Lu, C., Napier, J. A., Clemente, T. E., and Cahoon, E. B. (2011). New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr. Opin. Biotechnol. 22, 252–259. doi: 10.1016/j.copbio.2010.11.006
Moellering, E. R., Muthan, B., and Benning, C. (2010). Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330, 226–228. doi: 10.1126/science.1191803
Mu, J., Tan, H., Zheng, Q., Fu, F., Liang, Y., Zhang, J., et al. (2008). LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol. 148, 1042–1054. doi: 10.1104/pp.108.126342
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Nakamura, Y. (2013). Phosphate starvation and membrane lipid remodeling in seed plants. Prog. Lipid Res. 52, 43–50. doi: 10.1016/j.plipres.2012.07.002
Narise, T., Kobayashi, K., Baba, S., Shimojima, M., Masuda, S., Fukaki, H., et al. (2010). Involvement of auxin signaling mediated by IAA14 and ARF7/19 in membrane lipid remodeling during phosphate starvation. Plant Mol. Biol. 72, 533–544. doi: 10.1007/s11103-009-9589-4
Nielsen, T. H., Krapp, A., Röper-Schwarz, U., and Stitt, M. (1998). The sugar-mediated regulation of genes encoding the small subunit of Rubisco and the regulatory subunit of ADP glucose pyrophosphorylase is modified by phosphate and nitrogen. Plant Cell Environ. 21, 443–454. doi: 10.1046/j.1365-3040.1998.00295.x
Pant, B. D., Burgos, A., Pant, P., Cuadros-Inostroza, A., Willmitzer, L., and Scheible, W.-R. (2015). The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation. J. Exp. Bot. 66, 1907–1918. doi: 10.1093/jxb/eru535
Park, S., Gidda, S. K., James, C. N., Horn, P. J., Khuu, N., Seay, D. C., et al. (2013). The α/β hydrolase CGI-58 and peroxisomal transport protein PXA1 coregulate lipid homeostasis and signaling in Arabidopsis. Plant Cell 25, 1726–1739. doi: 10.1105/tpc.113.111898
Periappuram, C., Steinhauer, L., Barton, D. L., Taylor, D. C., Chatson, B., and Zou, J. (2000). The plastidic phosphoglucomutase from Arabidopsis. A reversible enzyme reaction with an important role in metabolic control. Plant Physiol. 122, 1193–1199. doi: 10.1104/pp.122.4.1193
Riediger, N. D., Othman, R. A., Suh, M., and Moghadasian, M. H. (2009). A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 109, 668–679. doi: 10.1016/j.jada.2008.12.022
Russo, M. A., Quartacci, M. F., Izzo, R., Belligno, A., and Navari-Izzo, F. (2007). Long- and short-term phosphate deprivation in bean roots: plasma membrane lipid alterations and transient stimulation of phospholipases. Phytochemistry 68, 1564–1571. doi: 10.1016/j.phytochem.2007.03.017
Sakaki, T., Kondo, N., and Yamada, M. (1990a). Pathway for the synthesis of triacylglycerols from monogalactosyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 94, 773–780. doi: 10.1104/pp.94.2.773
Sakaki, T., Kondo, N., and Yamada, M. (1990b). Free Fatty acids regulate two galactosyltransferases in chloroplast envelope membranes isolated from spinach leaves. Plant Physiol. 94, 781–787. doi: 10.1104/pp.94.2.781
Sakaki, T., Saito, K., Kawaguchi, A., Kondo, N., and Yamada, M. (1990c). Conversion of monogalactosyldiacylglycerols to triacylglycerols in ozone-fumigated spinach leaves. Plant Physiol. 94, 766–772. doi: 10.1104/pp.94.2.766
Sanjaya, Miller, R., Durrett, T. P., Kosma, D. K., Lydic, T. A., Muthan, B., et al. (2013). Altered lipid composition and enhanced nutritional value of Arabidopsis leaves following introduction of an algal diacylglycerol acyltransferase 2. Plant Cell 25, 677–693. doi: 10.1105/tpc.112.104752
Sanjaya, Durrett, T. P., Weise, S. E., and Benning, C. (2011). Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol. J. 9, 874–883. doi: 10.1111/j.1467-7652.2011.00599.x
Santos Mendoza, M., Dubreucq, B., Miquel, M., Caboche, M., and Lepiniec, L. (2005). LEAFY COTYLEDON 2 activation is sufficient to trigger the accumulation of oil and seed specific mRNAs in Arabidopsis leaves. FEBS Lett. 579, 4666–4670. doi: 10.1016/j.febslet.2005.07.037
Shimojima, M., and Ohta, H. (2011). Critical regulation of galactolipid synthesis controls membrane differentiation and remodeling in distinct plant organs and following environmental changes. Prog. Lipid Res. 50, 258–266. doi: 10.1016/j.plipres.2011.03.001
Shimojima, M., Watanabe, T., Madoka, Y., Koizumi, R., Yamamoto, M. P., Masuda, K., et al. (2013). Differential regulation of two types of monogalactosyldiacylglycerol synthase in membrane lipid remodeling under phosphate-limited conditions in sesame plants. Front. Plant Sci. 4:469. doi: 10.3389/fpls.2013.00469
Slocombe, S. P., Cornah, J., Pinfield-Wells, H., Soady, K., Zhang, Q., Gilday, A., et al. (2009). Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol. J. 7, 694–703. doi: 10.1111/j.1467-7652.2009.00435.x
Tjellström, H., Andersson, M. X., Larsson, K. E., and Sandelius, A. S. (2008). Membrane phospholipids as a phosphate reserve: the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ. 31, 1388–1398. doi: 10.1111/j.1365-3040.2008.01851.x
Troncoso-Ponce, M. A., Cao, X., Yang, Z., and Ohlrogge, J. B. (2013). Lipid turnover during senescence. Plant Sci. 205–206, 13–19. doi: 10.1016/j.plantsci.2013.01.004
Vanhercke, T., El Tahchy, A., Liu, Q., Zhou, X. R., Shrestha, P., Divi, U. K., et al. (2014). Metabolic engineering of biomass for high energy density: oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 12, 231–239. doi: 10.1111/pbi.12131
Yang, Y., Yu, X., Song, L., and An, C. (2011). ABI4 activates DGAT1 expression in Arabidopsis seedlings during nitrogen deficiency. Plant Physiol. 156, 873–883. doi: 10.1104/pp.111.175950
Yuzawa, Y., Nishihara, H., Haraguchi, T., Masuda, S., Shimojima, M., Shimoyama, A., et al. (2012). Phylogeny of galactolipid synthase homologs together with their enzymatic analyses revealed a possible origin and divergence time for photosynthetic membrane biogenesis. DNA Res. 19, 91–102. doi: 10.1093/dnares/dsr044
Keywords: monogalactosyldiacylglycerol, galactolipid, promoter, triacylglycerol, phosphate starvation, starch
Citation: Shimojima M, Madoka Y, Fujiwara R, Murakawa M, Yoshitake Y, Ikeda K, Koizumi R, Endo K, Ozaki K and Ohta H (2015) An engineered lipid remodeling system using a galactolipid synthase promoter during phosphate starvation enhances oil accumulation in plants. Front. Plant Sci. 6:664. doi: 10.3389/fpls.2015.00664
Received: 17 July 2015; Accepted: 12 August 2015;
Published: 31 August 2015.
Edited by:
Steven James Burgess, University of Cambridge, UKReviewed by:
Vincent Arondel, University of Bordeaux, FrancePhilip David Bates, The University of Southern Mississippi, USA
Copyright © 2015 Shimojima, Madoka, Fujiwara, Murakawa, Yoshitake, Ikeda, Koizumi, Endo, Ozaki and Ohta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mie Shimojima, Graduate School of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259-B-65 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8501, Japan, shimojima.m.aa@m.titech.ac.jp
†These authors have contributed equally to this work.
 Mie Shimojima
Mie Shimojima Yuka Madoka1†
Yuka Madoka1† Masato Murakawa
Masato Murakawa Keiji Endo
Keiji Endo Hiroyuki Ohta
Hiroyuki Ohta