- 1State Key Laboratory of Hybrid Rice, College of Life Sciences, Wuhan University, Wuhan, China
- 2Key Laboratory of Plant Germplasm Enhancement and Specialty Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
Cytokinins in plants are crucial for numerous biological processes, including seed germination, cell division and differentiation, floral initiation and adaptation to abiotic stresses. The salt stress can promote reactive oxygen species (ROS) production in plants which are highly toxic and ultimately results in oxidative stress. However, the correlation between endogenous cytokinin production and ROS homeostasis in responding to salt stress is poorly understood. In this study, we analyzed the correlation of overexpressing the cytokinin biosynthetic gene AtIPT8 (adenosine phosphate-isopentenyl transferase 8) and the response of salt stress in Arabidopsis. Overproduction of cytokinins, which was resulted by the inducible overexpression of AtIPT8, significantly inhibited the primary root growth and true leaf emergence, especially under the conditions of exogenous salt, glucose and mannitol treatments. Upon cytokinin overproduction, the salt stress resistance was declined, and resulted in less survival rates and chlorophyll content. Interestingly, ROS production was obviously increased with the salt treatment, accompanied by endogenously overproduced cytokinins. The activities of catalase (CAT) and superoxide dismutase (SOD), which are responsible for scavenging ROS, were also affected. Transcription profiling revealed that the differential expressions of ROS-producing and scavenging related genes, the photosynthesis-related genes and stress responsive genes were existed in transgenic plants of overproducing cytokinins. Our results suggested that broken in the homeostasis of cytokinins in plant cells could modulate the salt stress responses through a ROS-mediated regulation in Arabidopsis.
Introduction
Cytokinins play important and complex roles in plant growth and abiotic stress responses (Wang et al., 2011; Ha et al., 2012; Hwang et al., 2012; Zwack and Rashotte, 2015). Numerous evidences indicate that cytokinins have both positive and negative effects on stress tolerance. Many studies have reported that, in response to extended stress, the concentrations of cytokinins were decreased in plants (Kudoyarova et al., 2007; Ghanem et al., 2008; Merewitz et al., 2011; Nishiyama et al., 2011). Contrarily, both short-term and sustained increase in cytokinin levels may also occur in plants while encountering severe stress conditions (Pospisilova et al., 2005; Alvarez et al., 2008; Dobra et al., 2010). Cytokinin biosynthesis genes IPTs (adenosine phosphate-isopentenyl transferases) can be up-regulated by NaCl treatment, and the deficiency in cytokinin biosynthesis may result in a strong salt-tolerant phenotype (Nishiyama et al., 2011). Many studies have examined the effects of exogenous cytokinin applications in abiotic stress responses. Exogenously supplied cytokinins not only can improve salt tolerance in young wheat seedlings, but also can result in more susceptible phenotype to the salt treatment in beans (Kirkham et al., 1974; Abdullah and Ahmad, 1990). After cytokinin application, the Arabidopsis plants are of higher survival ability when they are exposed to freezing or dehydrated conditions (Jones et al., 2010; Kang et al., 2012). The effects of changed endogenous cytokinin levels in transgenic plants overexpressing cytokinin biosynthesis genes (IPTs), or cytokinin degraded genes (CKXs), are demonstrated. Overproduction of endogenous cytokinins enhances drought stress tolerance. However, decrease in cytokinin levels produce a positive consequence in drought tolerance (Rivero et al., 2007; Werner et al., 2010; Qin et al., 2011; Macková et al., 2013).
The components of cytokinin signaling also play complex roles in responses to abiotic stresses. For instance, Arabidopsis AHK1, the histidine kinase 1 of cytokinin signaling, plays as a positive regulator in the responses of drought and salt stresses. The loss-of-function mutations, such as ahk2, ahk3, and ahk2 ahk3 are of strong tolerance to drought and salt stresses (Tran et al., 2007; Wohlbach et al., 2008; Kumar et al., 2013). AHPs (histidine phosphotransfer proteins) are involved in regulating the responses to drought stress in a negative and redundant manner (Hutchison et al., 2006; Hwang et al., 2012; Nishiyama et al., 2013). The resistant to salt stress phenotype is reported in studying the quadruple loss-of-function mutant arr3arr4arr5arr6 (Mason et al., 2010). Collectively, all these studies suggest the impact of cytokinin metabolism and signaling in the stress responses in intricate manners.
The reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide radical (O2-), and hydroxyl radical (OH-), all can be induced by drought, salt, and low temperature conditions (Sharma et al., 2012; Choudhury et al., 2013; Petrov et al., 2015). To detoxify, plants have evolved ROS scavenging systems that involve in enzymic and non-enzymic antioxidants. The major antioxidant enzymes include superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT) and glutathione peroxidase (GPX). SOD converts superoxide into H2O2, while APX, GPX, and CAT detoxify H2O2 (Mittler, 2002; Apel and Hirt, 2004; Das and Roychoudhury, 2014). The cross-talk between the cytokinin signaling and ROS production and scavenging systems is demonstrated in Arabidopsis. In cytokinin-deficient mutant ipt1,3,5,7, the genes involving in ROS breakdown are greatly affected (Nishiyama et al., 2012). The treatment of N(6)-benzyladenine (6-BA) induces massive production of ROS, eventually, results in a loss of cell viability in tobacco BY-2 cells (Mlejnek et al., 2003). Exogenous applications of cytokinins lead to increasing in APX and CAT activities during dark-induced senescence (Zavaleta-Mancera et al., 2007), as well as raise of SOD and CAT activities after heat stress (Liu and Huang, 2002). In overexpressing CKX transgenic Arabidopsis lines, declined cytokinin levels may cause alterations in activities of antioxidants, while responding to abiotic stresses (Mýtinová et al., 2010; Lubovská et al., 2014). Hence, the impact of cytokinins on ROS homeostasis in plants responding to environmental stresses is imperative.
To in-depth study the correlation between endogenous cytokinin levels and ROS homeostasis in plants responding to abiotic stresses especially to the salt stress, we analyzed the inducible transgenic line overexpressing AtIPT8, a cytokinin biosynthesis gene. The results indicated that endogenous cytokinin overproduction, which was promoted by AtIPT8 overexpression, resulted in enhanced-sensitive phenotype to the salt treatment. Dependent on salt treatment, the ROS contents were strongly increased in plants of overproducing cytokinin; and, the activities of antioxidants and the contents of total chlorophyll were significantly declined with comparing to those in the wild-type (Col). Moreover, many genes involving in photosynthesis and abiotic stress responses were differentially expressed in plants of overexpressing AtIPT8. In this study, we provided evidences in that overproduction of endogenous cytokinin could decrease salt resistance, through modulating endogenous ROS homeostasis in Arabidopsis.
Materials and Methods
Plant Materials and Growth Conditions
Arabidopsis thaliana ecotype Columbia (Col) was used in this study as wild-type control. Seeds were surface sterilized and sown on Murashige and Skoog (MS) agar plates containing full-strength MS salts, 0.8% (w/v) agar, and 1% (w/v) sucrose. The 17-β-estradiol (Sigma-Aldrich, E8875) was dissolved in DMSO (Dimethyl Sulfoxide) and used in this study. The seeds were stratified in darkness at 4°C for 4 days and then transferred to growth chamber with 16 h/8 h light/dark cycle at 23°C, or were directly sown in soil after stratification under the same conditions. Overexpressing AtIPT8 transgenic plants (OE) were generated in Col-0 background as described by Wang et al. (2011). The homozygous T4 transgenic lines were used in this study.
Cytokinins Extraction and Quantification
Cytokinins were extracted and purified from 2 g of 2-week-old seedlings which were induced with 17-β-estradiol (10 μM) for 24 h (Wang et al., 2011). The extraction procedure was performed according to methods described in previous reports (Åstot et al., 2000; Dobrev and Kamínek, 2002; Hoyerová et al., 2006). The internal standards of Deuterium-labeled cytokinin (Olchemim, Czech) were added to the extraction buffer (100 ng per sample). Detection and quantification of cytokinins were performed with HPLC-MS system (Agilent 1200 series HPLC, Agilent Technologies, Palo Alto, CA, USA; AB 3200 Q trap MS/MS, Applied Biosystems, USA).
Comparisons of Root Growth, Survival Rates and Chlorophyll Contents
To compare the primary root growth under various stress treatments, seeds were respectively sown on MS agar plates supplied with NaCl (100 mM), glucose (300 mM) and D-mannitol (300 mM). 17-β-estradiol (10 μM) or DMSO (mock) was added to the plates. After stratified at 4°C for 4 days, the plates were transferred to growth chamber and placed vertically. The primary root length was measured at the 10 days after transferring. For salt resistance treatment, 5-day-old seedlings grown on MS plates were transferred to fresh salt-containing MS plates, and then calculated the survival rates after 10 days treatment. The seedlings after survival rates calculation were collected and used for chlorophyll contents determination. Total chlorophyll was extracted in 85% acetone as described by Porra et al. (1989). The contents of chlorophyll were determined at settings of 639 nm and 645 nm, respectively with spectrophotometer. All experiments were performed three times independently.
Determination of ROS Production and Antioxidant Enzymes Activities
Reactive oxygen species production was detected in roots and cotyledons using dichlorofluorescein (DCF; Foreman et al., 2003). The 5-day-old seedlings were treated with 100 mM NaCl plus or minus 17-β-estradiol in plates. After treatment, the seedlings were incubated with 20 μM DCF. To detect the DCF fluorescent signals, images were acquired with confocal laser scanning microscopy (TCS SP8, Leica, Germany) under 488 nm excitation and 525 nm emission. Fluorescence intensity was quantified using LAS AF software. Quantification of H2O2 content was determined using the method described by Hu et al. (2012). Ten-day-old seedlings were pre-treated with 100 mM NaCl plus or minus 17-β-estradiol in plates. H2O2 content and activity of antioxidant enzymes were measured after salt treatments. The detailed procedure has been described by Wang et al. (2013).
Gene Expression Analysis by Microarray and Quantitative Real-time RT-PCR (qRT-PCR)
For microarray analysis, 10-day-old plants of Col and AtIPT8-OE were pre-treated with 17-β-estradiol (10 μM) or DMSO for 24 h, respectively. Afterward, the seedlings were collected for total RNA extraction and transcriptomic analysis. The detailed procedure has been described by Wang et al. (2011). To confirm the expression patterns of differentially expressed genes obtained from microarray analysis, qRT-PCR was employed after the seedlings pretreated with or without 17-β-estradiol. Total RNA was extracted using a plant RNA purification kit (Tiangen, catalog number #DP4321). Equal amounts of RNA were used for reverse transcription with ReverTra Ace-α-TM (TOYOBO, catalog number FSK-1002) according to the manufacturer’s instructions. The primers used in real-time quantitative RT-PCR were designed by web tool3. The primers used for qRT-PCR experiment are listed in Supplementary Table S1.
Results
Induced-overexpression of AtIPT8 Resulted in Endogenous Cytokinin Overproduction
Due to the lethality caused by constitutively overexpressed AtIPT8 in plants, we generated transgenic plants with estradiol-inducible overexpression of AtIPT8, and the line AtIPT8-OE was selected (Wang et al., 2011) for further analysis in this study. First, we examined the relative expression levels of AtIPT8 in transgenic plants using methods of semi-quantitative RT-PCR and qRT-PCR (Figures 1A,B). The results showed that expression level of AtIPT8 gene was induced more than 40-fold higher upon estradiol induction (Figure 1B). To examine the effect of AtIPT8 on the production of endogenous cytokinins, the total cytokinin contents were quantified in plants of Col and AtIPT8-OE plants. Upon estradiol induction, the contents of iP and iP9G (iP-type) were increased, more than 100-fold in AtIPT8-OE plants than that in Col plants. Moreover, the concentrations of tZ, ZR and ZRMP (Z-type) cytokinins were also elevated more than 10-fold in AtIPT8-OE plants (Figure 1C). Thus, the quantitative analysis on cytokinin contents indicated that inducer-dependent activation of AtIPT8 could lead to elevation of cytokinin contents in Arabidopsis.
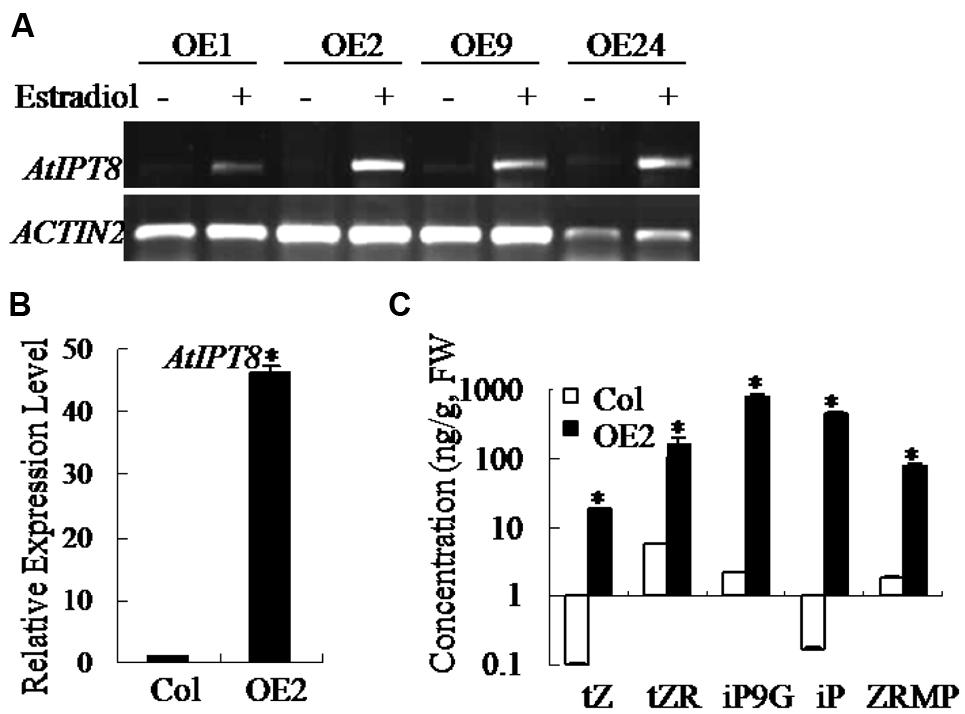
FIGURE 1. Overexpression of AtIPT8 promoted overproduction of endogenous cytokinins in Arabidopsis. Asterisk symbols (∗) indicate p < 0.05 (Student t-test). (A) Semi-quantitative RT-PCR to examine the expression levels of AtIPT8 in independent 17-β-estradiol-inducible transgenic lines. Overexpressing AtIPT8 transgenic lines (OE1, OE2, OE9, and OE24) showed higher expression levels of AtIPT8 in an estradiol-dependent manner. ACTIN2 was used as the internal control. Estradiol: 17-β-estradiol (10 μM). (B) The AtIPT8 expression level was more than 40-fold higher in OE2 plants after 10 μM 17-β-estradiol treatment for 24 h. The values of Y-axis indicate means ± SE (standard errors) of three independent experimental repeats. (C) The cytokinin varieties in the 2-week-old seedlings of Col and OE2 were quantified using GC-MS. Seedlings of OE2 were pre-treated with 10 μM 17-β-estradiol for 24 h. The data represent the means ± SE of two reproducible experiments (fresh weight, FW).
Endogenous Cytokinin Overproduction Modulated Salt Stress Responses
To determine whether the endogenous cytokinin levels could affect the abiotic stress responses, we compared the stress resistant phenotypes between Col and AtIPT8-OE plants in treatments of NaCl, glucose and mannitol. Seeds were germinated on the freshly prepared MS plates containing NaCl, glucose and mannitol, and then, the fresh weight and primary root length were analyzed after 10 days of treatments. The results showed that cytokinin overproduction limited plant growth (Figure 2A, upper panel). Significantly, combining with salt and osmotic stress conditions, the growth of roots and true leaves was inhibited (Figure 2A). The fresh weight of plants was obviously decreased in the same stress treatments (Figure 2B). Furthermore, we examined the primary root length. The severe effect on root growth was observed with AtIPT8-OE plants that were treated by estradiol and NaCl or glucose or mannitol (Figure 2C). Interestingly, the most obvious inhibitory effect in the growth of primary roots was showed in the treatment of glucose (300 mM) (Figure 2C). The inhibitory effect in primary root growth by cytokinin overproduction was rescued by exogenously addition of auxin (IAA). Application of 2,4-D could trigger more callus generation in AtIPT8-OE plants (Supplementary Figure S1). Together, these results suggested that cytokinin overproduction may reduce plant’s tolerance in salt and osmotic stress conditions.
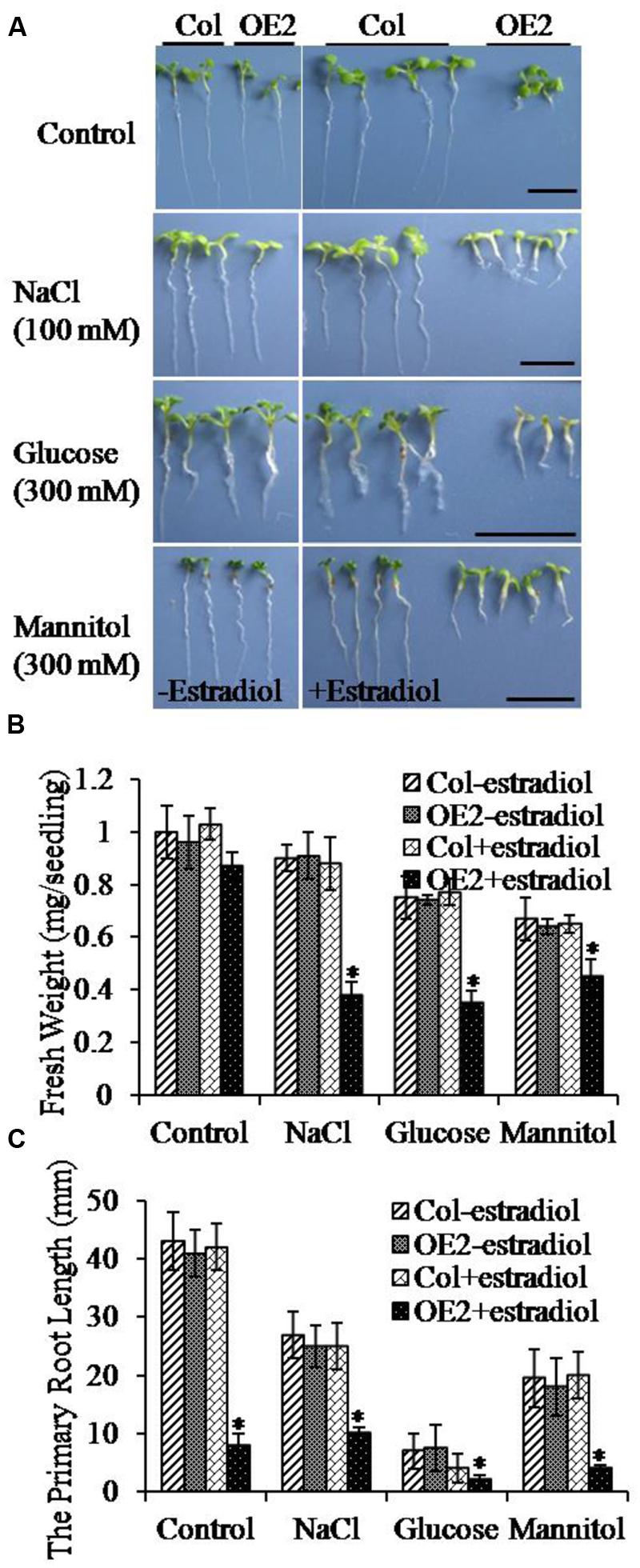
FIGURE 2. The growth of cotyledons and primary roots of OE2 seedlings was altered under various stress conditions. Asterisk symbols (∗) indicate p < 0.05 (Student t-test). (A) The OE2 seedlings showed hypersensitivity to treatments of NaCl, glucose and mannitol. Seeds were germinated on the indicated plates, and photos were taken after 10 days of seedlings grown on the indicated plates. Bar = 10 mm. Estradiol: 17-β-estradiol (10 μM). (B) The fresh weights of Col and OE2 seedlings were quantified. The values were determined by using the data from three independent experiments (seedling numbers, n > 20 per experiment). (C) The primary roots lengths of Col and OE2 seedlings were measured. The values were determined by using the data from three independent experiments (seedling numbers, n > 20 per experiment).
Endogenous Cytokinin Overproduction Decreased Salt Stress Resistance
To further analyze the salt stress response with overproduced cytokinins in plants, we transferred 5-day-old seedlings to the MS plates containing NaCl, and treated for 10 days. We observed that, after estradiol induction, AtIPT8-OE plants appeared more sensitive to the treatments of NaCl (Figure 3A). Then, we measured the relative survival rates under the conditions of the NaCl treatment. Results showed that, lessen survival rates were scored with AtIPT8-OE plants which were induced by estradiol and treated with NaCl; without estradiol induction, no obvious differences, in terms of survival rates, were obtained in plants of Col and AtIPT8-OE (Figure 3B). The chlorophyll contents are usually used to evaluate the tolerance of plants after stress treatments (Tanaka et al., 2011). Therefore, we measured the total chlorophyll contents in the seedlings. We obtained that decrease in chlorophyll contents caused by NaCl treatments were showing in plants of both Col and AtIPT8-OE, more than two-fold decrease in chlorophyll contents was scored with AtIPT8-OE plants followed by estradiol induction (Figure 3C). Collectively, these results suggested that overproduction of endogenous cytokinins might play a negative effect on surviving in the salinity condition.
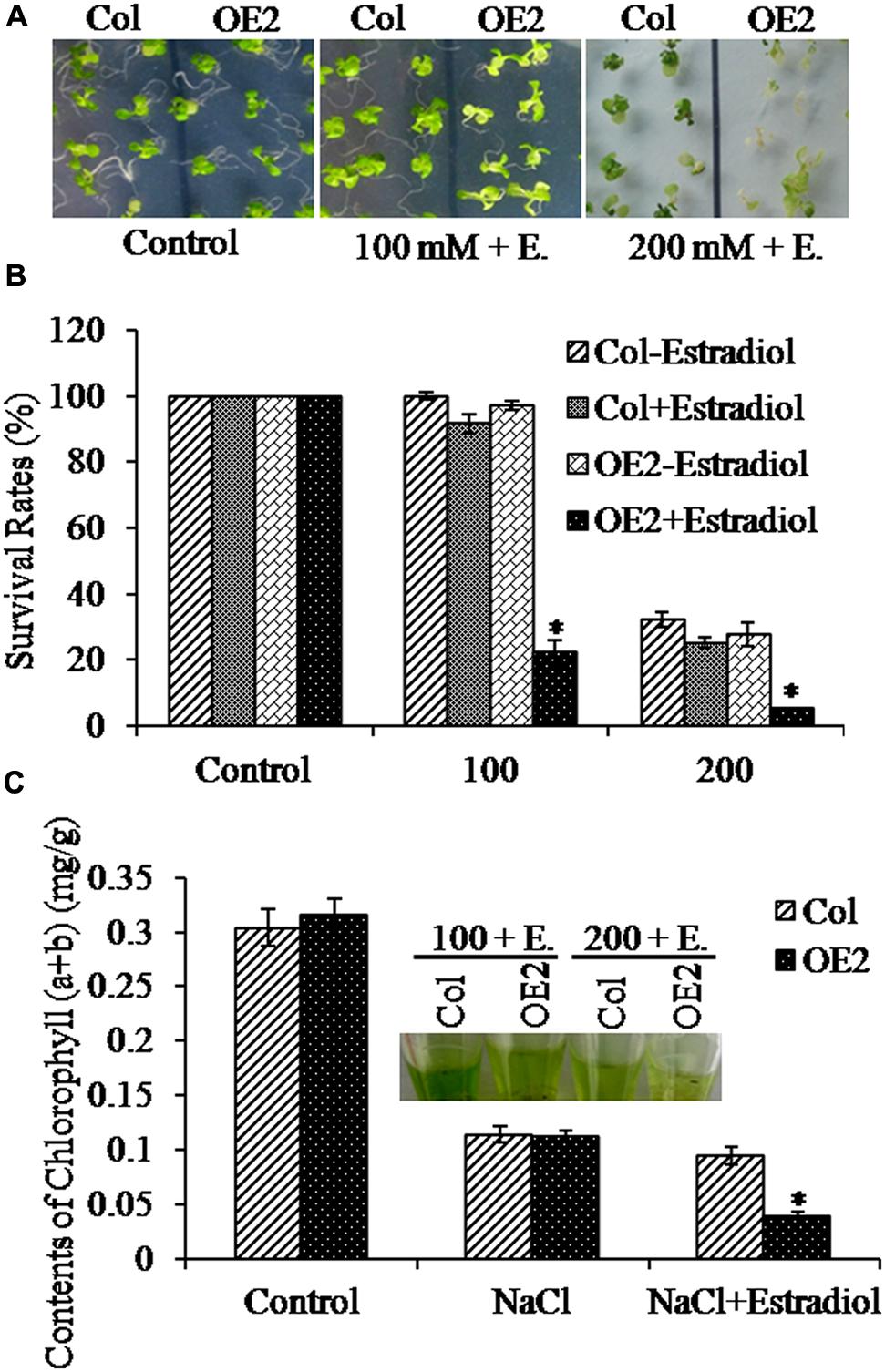
FIGURE 3. Analyses of salt stress responses in OE2 plants. Asterisk symbols (∗) indicate p < 0.05 (Student t-test). (A) Phenotypes of 15-day-old plants of Col and OE2 on MS plates containing NaCl (100 and 200 mM, respectively). The 5-day-old seedlings grown on MS plates were transferred into the corresponding NaCl-containing plates. Photos were taken after 10 days of treatments. Estradiol: 17-β-estradiol (10 μM). (B) The survival rates of Col and OE2 plants were measured after treated with NaCl (100 and 200 mM, respectively). The data are means ± SE of three independent experiments (n > 15 for each repeat). (C) The chlorophyll contents of Col and OE2 plants were measured after treated with NaCl (100 mM) with or without estradiol induction. The data are means ± SE of three independent experiments (n > 15 for each repeat).
Endogenous Cytokinin Overproduction Increased ROS Accumulation
Salt stress triggers the accumulation of intracellular ROS (Das and Roychoudhury, 2014). To investigate the correlation of cytokinin overproduction and ROS homeostasis in plant cells, we pretreated the seedlings with NaCl and then analyzed the ROS production by quantifying DCF fluorescent intensity. The ROS levels were compared in roots and cotyledons between Col and AtIPT8-OE plants. As shown in the results, the NaCl-treatment could promote ROS generation in roots and cotyledons of Col and AtIPT8-OE plants (Figure 4A). Moreover, the relative salt-induced ROS levels were significantly increased after estradiol-dependent cytokinin overproduction in all of the detected roots and cotyledons (Figure 4A). The ROS levels increased more than 10-fold without estradiol induction after salt treatment, however, after estradiol induction, the relative ROS contents were extensively increased about 18-fold in AtIPT8-OE plants; whereas there was only 11-fold increased in Col plants when compared with the control treatment (Figure 4B). We also examined the effect of exogenous application of 6-BA on ROS production in Col plants. The results indicated that exogenous cytokinin could promote ROS generation under the condition of salt treatment (Supplementary Figure S2). To further determine the characteristic of ROS, we examined the contents of hydrogen peroxide H2O2. As shown in the results, H2O2 contents were obviously increased in AtIPT8-OE plants under the conditions of estradiol-induction and salt treatment (Figure 5A). To assess the effect of cytokinin overproduction on ROS-scavenging capacity, the major antioxidant enzymes activities of CAT and SOD were compared between Col and AtIPT8-OE plants. As the results, the activities of CAT and SOD increased about 1.8-fold and 2.5-fold, respectively, after salt treatment in Col and AtIPT8-OE plant without estradiol induction. However, after estradiol application the activities of CAT and SOD showed only 1.2-fold and 1.7-fold increase in AtIPT8-OE plants (Figures 5B,C). These results suggested that the weakened performance of AtIPT8-OE plants against salt stress was due to elevated ROS production and declined SOD and CAT activities.
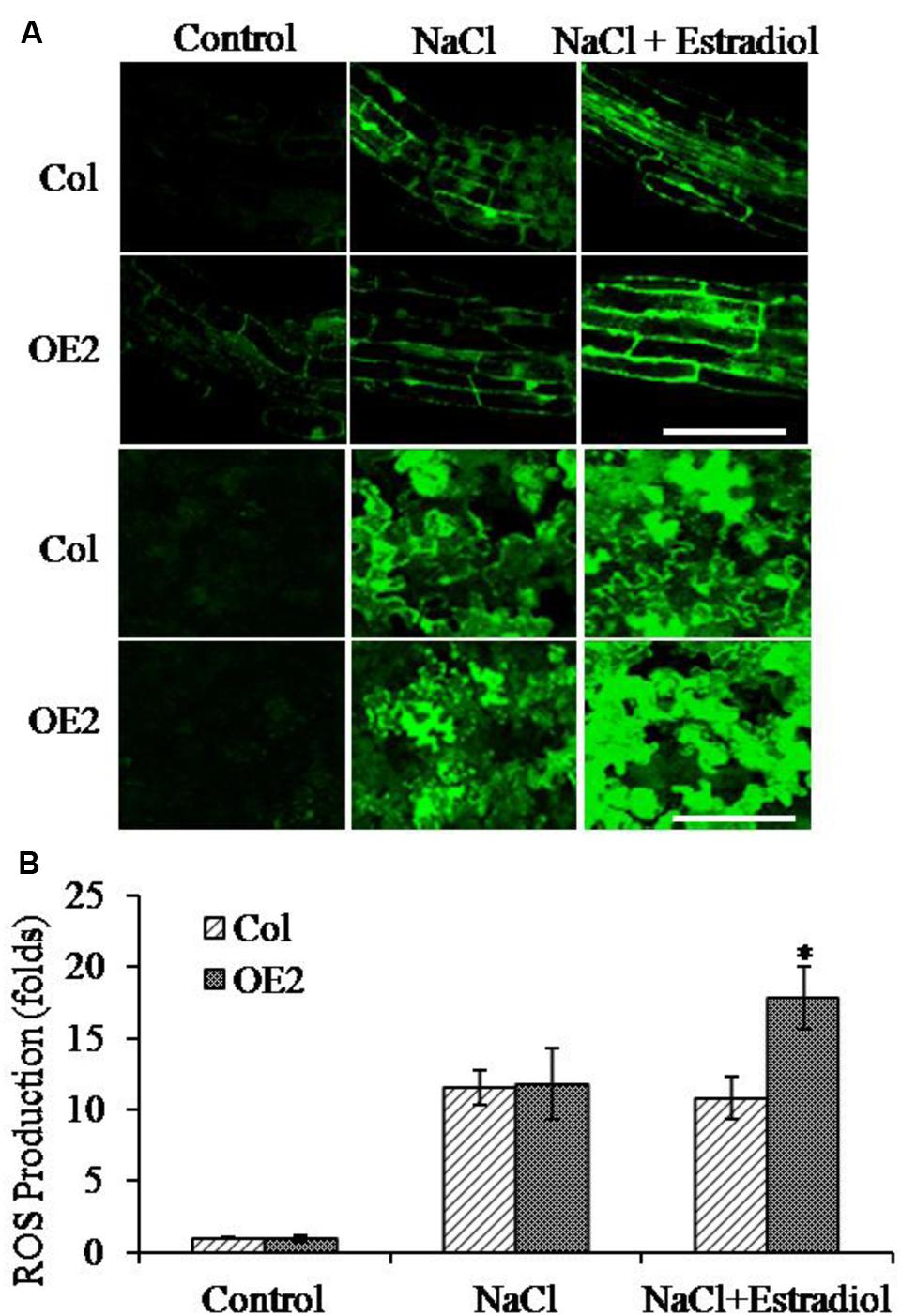
FIGURE 4. Reactive oxygen species (ROS) production in roots and cotyledons was analyzed under NaCl treatment. The salt concentration was 100 mM in this assay. Estradiol, 10 μM. Asterisk symbols (∗) indicate p < 0.05 (Student t-test). (A) The fluorescent intensity of DCF was observed with the confocal microscropy. Bar, 50 μm. (B) The ROS contents in Col and OE2 plants were quantified. The data are means ± SE of three independent experiments (n = 10). The fluorescent intensity of cotyledons in Col without salt treatment was taken as “1.”
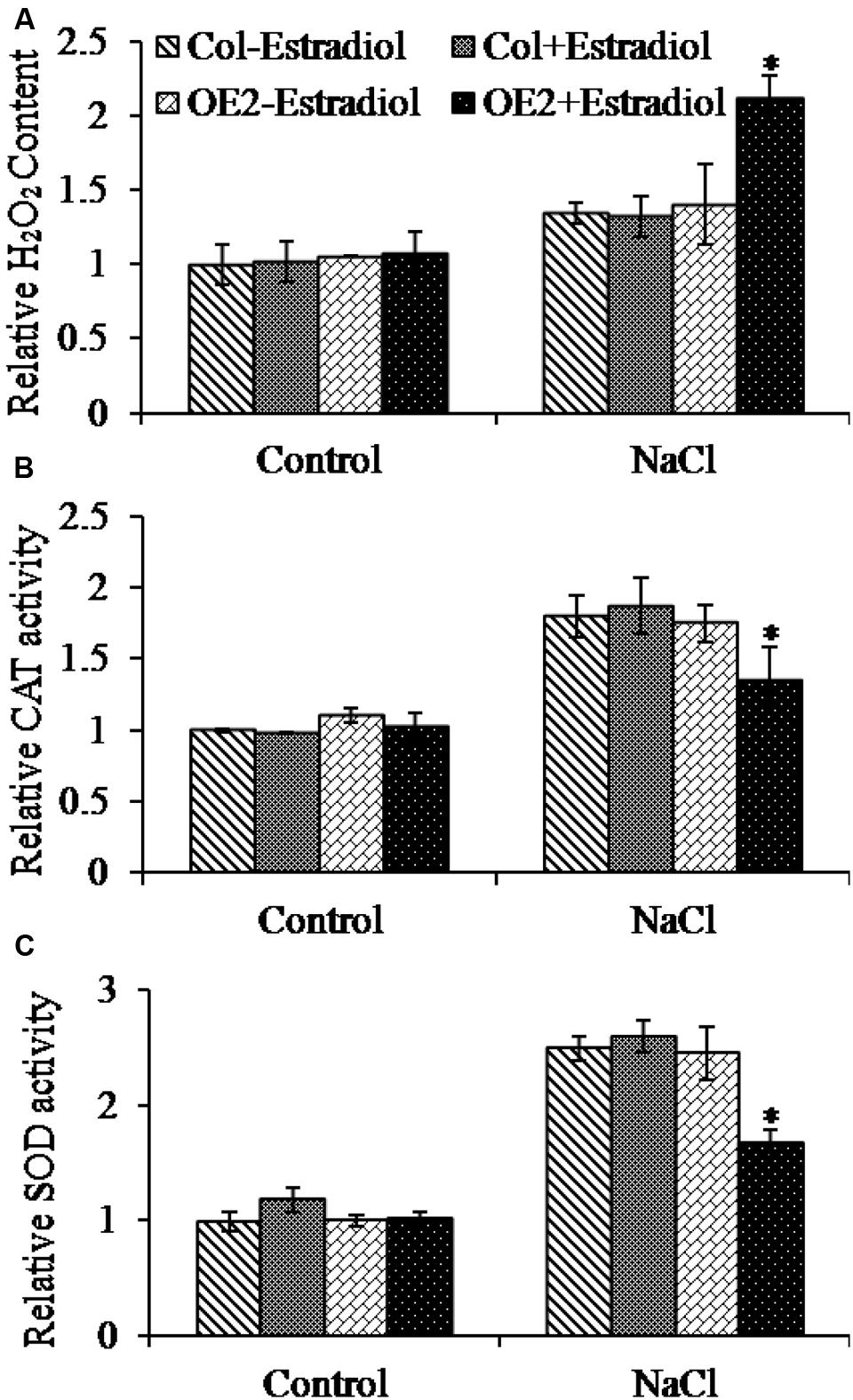
FIGURE 5. Determination of relative H2O2 content and antioxidant enzyme activities in Col and OE2 plants. Asterisk symbols (∗) indicate p < 0.05 (Student t-test). The 5-day-old seedlings grown on MS plated were transferred into the indicated treatments for 7 days, and then the seedlings were collected for analyzing H2O2 and antioxidant enzyme activities (NaCl, 100 mM; Estradiol, 10 μM). Data are means ± SE of three independent measurements. (A) Comparison of H2O2 contents in Col and OE2 plants after NaCl treatment. (B) and (C) Changes in CAT and SOD activities of Col and OE2 were compared.
Transcriptomic Analysis on the Effect of Endogenous Cytokinin Overproduction
To assess the transcriptomic changes which might have been affected by endogenous cytokinin overproduction in AtIPT8-OE plants, we conducted the microarray analysis to analyze the potential genes with differential expression levels in Col and AtIPT8-OE plants. Ten-day-old seedlings were pre-treated with or without estradiol for 24 h, and then total RNA were extracted for microarray analysis. A Two-Way Arabidopsis Genome Array (CapitalBio Corp.4) was used in this study (Patterson et al., 2006; Wang et al., 2011). Upon estradiol-induction, 425 genes exhibited more than twofold changes in the transcription levels between Col and AtIPT8-OE plants (Figure 6A; Supplementary Table S2) (Wang et al., 2011). Functional categorization of the differentially expressed genes revealed that cytokinin overproduction affected the expression of many genes involving in biological process, carbohydrate metabolisms, photosynthesis, transcription regulations and abiotic stress responses (Figure 6A). Detailed functional categorization indicated that many differentially expressed genes were the members which could be involved in responding to various stresses, such as the defense responses, oxidation reductions, cold, salt and water deprivation responses. Hierarchical cluster analysis on genes regulated by cytokinin overproduction in Arabidopsis indicated that 197 genes were up-regulated and 228 genes were down-regulated in AtIPT8-OE plant, when compared with Col control after estradiol-induction (Figure 6B). Among them, many ABA- and abiotic stress-related genes might be affected by overproducing cytokinin (Wang et al., 2011). To rule out the effect of cytokinin overeproduction in ROS generation/signaling and salt stress response, we compared the differentially expressed genes by cytokinin with salt- and oxidative-regulated genes, which were downloaded from the public microarray data5. Interestingly, among 425 differentially expressed genes in AtIPT8-OE plants dependent upon estradiol induction (Figure 6A; Supplementary Table S2), only 406 genes could be found in the data from the transcriptomic database (Supplementary Figure S3A; Supplementary Table S2). There were 104 genes with significant changes (folds ≥ 2.0) after treated by cytokinin and salt, respectively; and among of them, 40 genes were up-regulated and 64 genes were down-regulated. Forty-two genes were co-regulated by both of cytokinin and oxidative stress, and among of them 32 genes were up-regulated (Supplementary Figures S3B,C). Only 25 genes have been co-regulated by all of the cytokinin overproduction, salt and oxidative stresses treatment (Supplementary Figure S3B).
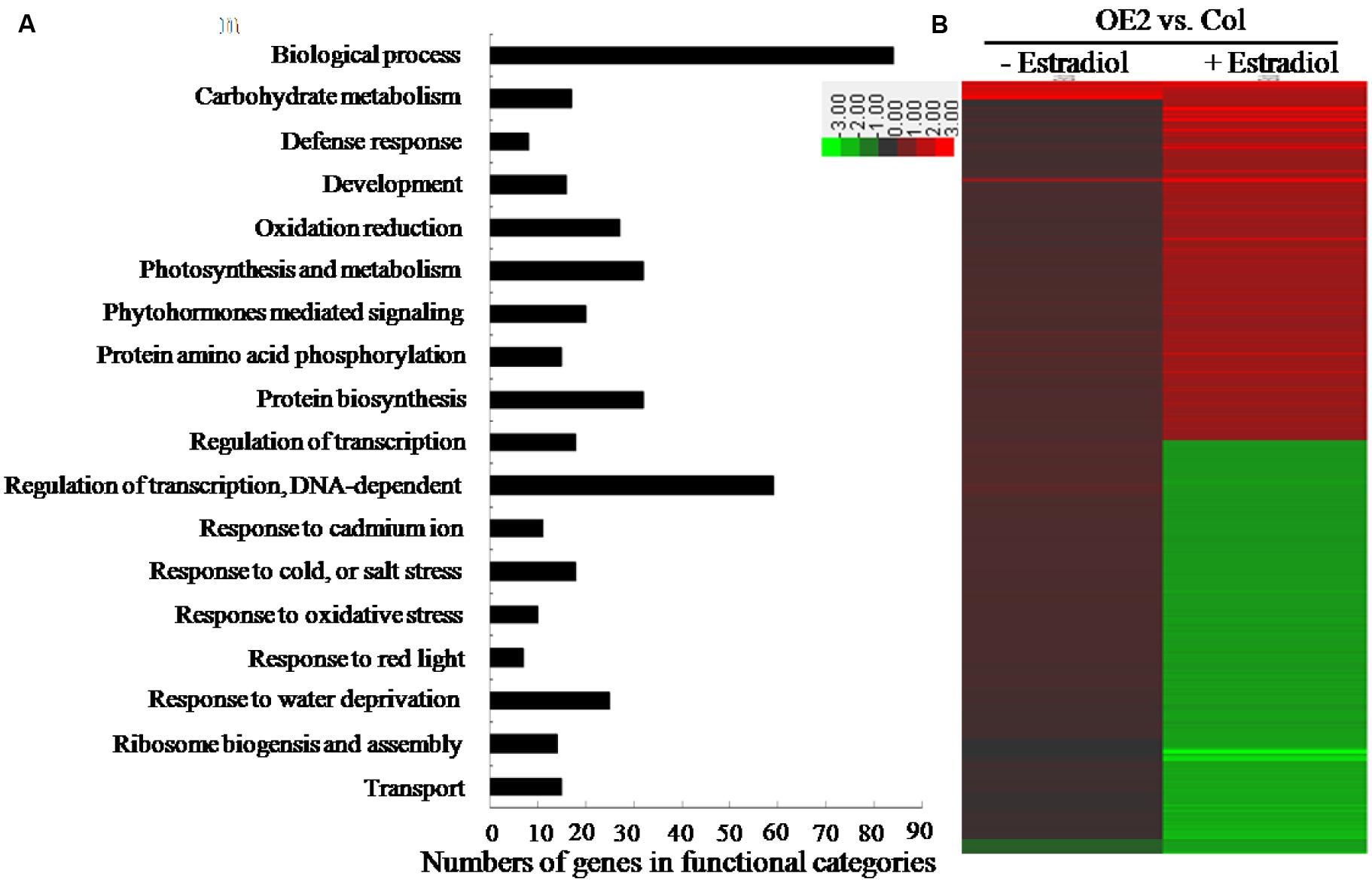
FIGURE 6. Differentially expressed genes were identified by microarray analysis between Col and OE2 plants. The 10-day-old seedlings were pretreated with 17-β-estradiol (10 μM) or DMSO for 24 h, respectively, and then processed for microarray analysis. (A) Profiling for the differentially expressed genes using the CapitalBio® Molecular Annotation System V4.0 (CB-MAS) functional catalog. Categories of the differentially expressed genes in OE2 and Col plants after 17-β-estradiol (10 μM) treatment for 24 h. (B) Hierarchical cluster analysis of genes regulated by AtIPT8 overexpression in Arabidopsis. The differentially expressed genes in OE2 plants after 17-β-estradiol (10 μM) induction were imported for cluster analysis by using Cluster 3.0, and the resulting tree figure was displayed using the Java Treeview software. The detailed information of genes was listed in Supplementary Table S2
Transcriptional Alterations of ROS-scavenging and -production Related-genes by Endogenous Cytokinin Overproduction and Salt Stress
Next, we selected some genes which were responsible for ROS-production and -scavenging for follow-up qRT-PCR analyses. Ten-day-old seedlings were pretreated with or without estradiol for 24 h, and then treated with NaCl for 3 h. Because RbohD, RbohF, and RbohJ are responsible for fine tuning the control of ROS production, we attested their expression levels. As shown in the results, three examined Rboh genes could be up-regulated by NaCl-treatment either in Col or in AtIPT8-OE plants; the significantly enhanced expression levels of these three Rboh genes were scored with AtIPT8-OE plants upon estradiol-induction and salt treatment (Figure 7).
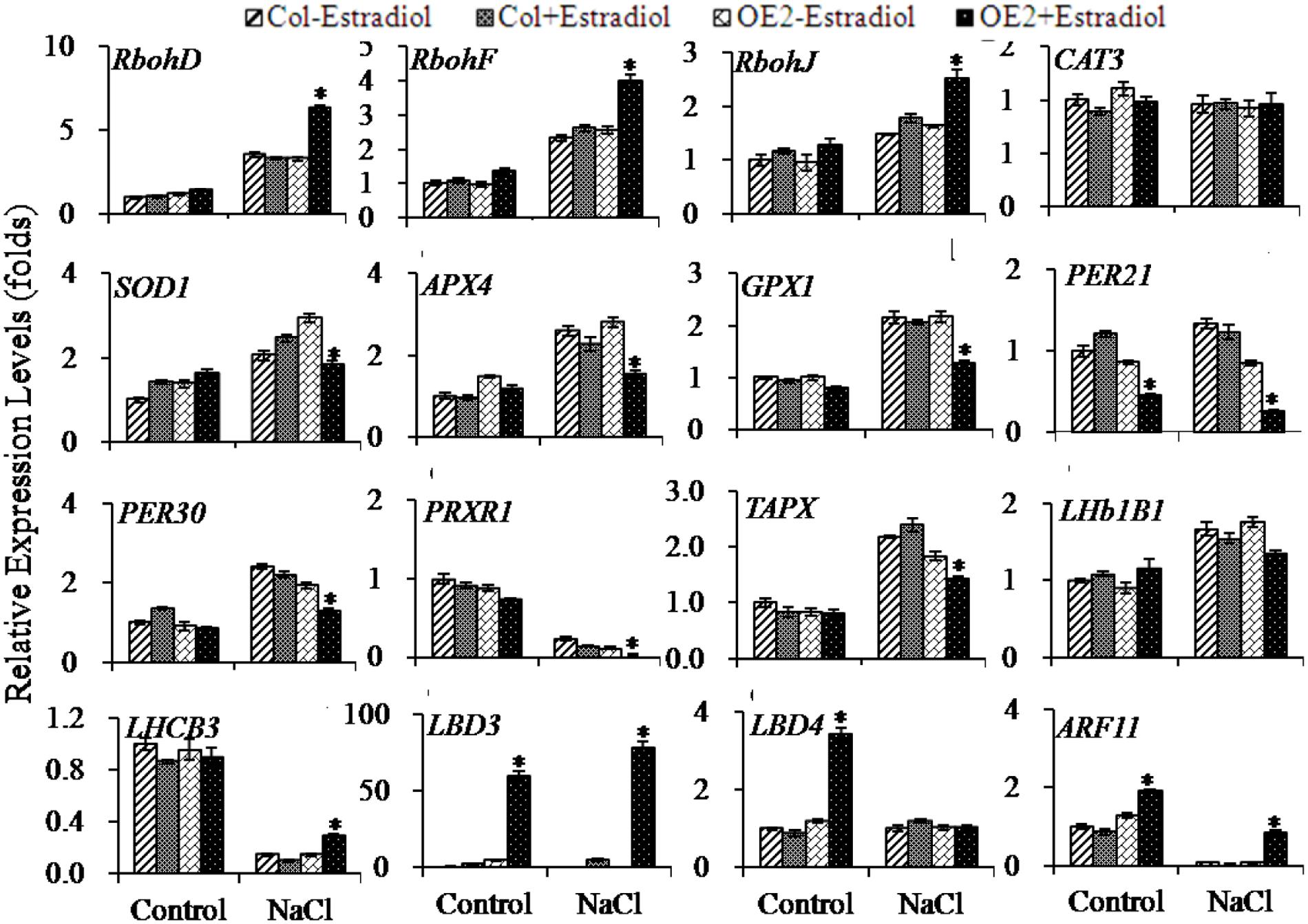
FIGURE 7. Quantitative analysis on the expression levels of ROS-related and photosynthesis-related genes. 10-day-old plants grown on MS plates were pretreated with DMSO or 17-β-estradiol (10 μM) for 24 h, and then subjected to treatments (NaCl, 100 mM; with or without estadiol) in liquid MS medium for 3 h. The seedlings were then collected for total RNA extraction and qRT-PCR analysis. Gene expression levels were normalized to their expression levels in Col plants, which were taken as 1. The data are means ± SE of three independent repeats. Asterisk symbols (∗) indicate p < 0.05 (Student t-test).
The ROS-scavenging related-genes were also compared between Col and AtIPT8-OE plants. Under the condition of salt treatment, the promoted expression levels of SOD1, APX4 and GPX1 were measured in Col and AtIPT8-OE plants while estradiol was present or absent. The expression levels of SOD1, APX4 and GPX1 were obviously lower in AtIPT8-OE plants when compared with those in Col under salt treatment (Figure 7). Interestingly, the CAT3 expression in both of Col and AtIPT8-OE plants was not affected in all tested conditions. We also compared the expression levels of some genes encoding peroxidases. As shown in Figure 7, upon estradiol-induction the expression levels of PER21 and PER30 were inhibited despite of the salt treatment. Moreover, PRXR1 and TAPX, which are involved in the hydrogen peroxide catabolic and oxidation-reduction processes, were analyzed. The down-regulated PRXR1 transcripts showed in the salt treatment, and significant decrease in expression levels of PRXR1 were scored in the AtIPT8-OE plants that were induced by estradiol. In contrast, up-regulated TAPX expression was detected after salt treatment, but estradiol-induction weakened the TAPX expression (Figure 7).
In addition, we examined expressions of those genes which are functional in the photosystem (Supplementary Table S2). Thus, expressions of LHb1B1 and LHCB3 were compared under the condition with or without salt treatment. We observed that increased LHb1B1 expression level could be triggered by the salt treatment. Notably, the elevated LHb1B1 expression level could be retracted in AtIPT8-OE plants upon estradiol-induction. As for the expression of LHCB3, it was obviously inhibited by the salt treatment in all the examined plants; recovered level of LHCB3 expression was detected with the estradiol-induction in AtIPT8-OE plants (Figure 7). Furthermore, we analyzed the expressions of LBD3 and LBD4, known as LOB domain-containing proteins and playing roles in the determination of bilateral symmetry (Shuai et al., 2002). The LBD3 (ASL9, ASYMMETRIC LEAVES 2 LIKE 9) can be exclusively regulated by the plant hormone cytokinins in a manner of depending on His-Asp phosphorelay signal transduction (Naito et al., 2007). In this study, we found that LBD3 expression was significantly enhanced in AtIPT8-OE plants in the estradiol-dependent manner. When treated with NaCl, the higher level of LBD3 expression was sustained (Figure 7; Supplementary Table S2). LBD4 showed less than four fold increase in AtIPT8-OE plants that were induced by estradiol, but the salt treatment revoked the effect of cytokinin overproduction on LBD4 expression. We also evaluated the auxin responsive factor ARF11 that could be down-regulated by cytokinin overproduction (Supplementary Table S2). As shown in Figure 7, expression level of ARF11 was decreased by the salt treatment in Col and AtIPT8-OE plants; however, slight rebound of ARF11 expression was produced with estradiol-induction.
Discussion
Maintaining cytokinin homeostasis is essential for plant growth and development, as well as plant adaptation to environmental stresses. Numerous studies demonstrate that abiotic stresses have both positive and negative effects on the metabolism of endogenous cytokinins (Hansen and Dörffling, 2003; Kudoyarova et al., 2007; Alvarez et al., 2008). It is usually difficult to define the working concentrations in plant cells for exogenous application of cytokinins. In this study, through analyzing inducible-AtIPT8 overexpression transgenic plants we investigated the effects of modulating endogenous cytokinin production in salt treatments. Results in this study demonstrated that inducible AtIPT8 overexpression could significantly promote endogenous cytokinin overproduction, and affect the responses of Arabidopsis plants to salt stresses. The balance of endogenous cytokinin and auxin contents is critical for maintaining primary root growth (Dello Ioio et al., 2007; Müller and Sheen, 2008; Moubayidin et al., 2010; De Rybel et al., 2014; Schaller et al., 2015). In AtIPT8-OE plants, the growth of primary roots was significantly inhibited by overproduction of endogenous cytokinin upon estradiol-induction (Figures 1 and 2). We further assessed the effect of cytokinin overproduction on osmotic and salt stress responses. Our results indicated that both of salt and osmotic stress treatments inhibited plant leaf and root growth (Figure 2). Notably, the most significance in inhibitory effect was observed after glucose treatment, which resulted in an extremely shorten roots and etiolated cotyledons (Figure 2). Salt and osmotic stresses have similar effects on water potential, but salinity has additional cytotoxic effects within the cell (Zhu, 2002). When exposed to high salt concentrations, the AtIPT8-OE plants showed less survival rates and the chlorophyll contents were significantly decreased after estradiol application (Figure 3). It has been shown that, under normal conditions, exogenous cytokinin (6-benzyladenine) application is able to promote chlorophyll biosynthesis in roots, but, mutations in cytokinins receptors (ahk2-2ahk3-3 and cre1-12ahk3-3) result in lower chlorophyll contents (Kobayashi et al., 2012). In this study, we observed that, if only overproduced endogenous cytokinin in AtIPT8-OE plants it had no obvious effects on chlorophyll contents (Figure 3). It is likely, the regulations of cytokinins and chlorophyll biosynthesis is much more complicated than we would have expected. Future studies on this point will expand our understanding on the complications of cytokinins and chlorophyll biosynthesis in Arabidopsis.
Chlorophyll accumulation is important in abiotic stress responses, because plant cells must strictly regulate their metabolisms to coincide with the machinery of photosynthesis (Tanaka et al., 2011). Interestingly, in our results, we have noticed that many genes, which are involved in the photosynthesis and metabolism, were differentially expressed in the AtIPT8-OE plants that were overproducing endogenous cytokinins (Figures 6 and 7, Supplementary Table S2). For instance, genes encoding the components of light harvesting protein complexes, such as LHb1B1, LHCB2.2, LHCB3, and LHCB4 were differentially regulated by overproduced endogenous cytokinins and the salt treatment (Figure 7; Supplementary Table S2). Expression levels of the photosystem II subunits including PSAK, PSAN, PSBP, and PSBQ, which are involved in oxygen evolution, were down-regulated by cytokinin overproduction (Supplementary Table S2). Nowadays, fewer evidences in the involvement of photosystem II subunits in abiotic stress responses are reported. With altered functions of chlorophyll-binding proteins, the sensitivity of ABA and dehydration conditions may be influenced in plants (Xu et al., 2012). Our results in analyzing the chlorophyll contents and in profiling the photosystem related genes suggested an indispensable mechanism that may involve in modulating endogenous cytokinin levels and responding to abiotic stress conditions.
The expression levels of stress-responsive genes that can be altered at various degrees after cytokinin treatment were revealed by genome-wide transcriptome analyses (Brenner et al., 2012; Bhargava et al., 2013; Brenner and Schmülling, 2015). The effects of salt stress and cytokinin-deficiency on gene expression have been demonstrated, in which a subset of stress-responsive genes are significantly modified in the cytokinin-deficient mutant ipt1,3,5,7, under normal and salinity conditions (Nishiyama et al., 2012). Under salinity conditions, cytokinin-deficiency may up-regulate many stress-responsible genes, including DREB-type transcriptional factors, ABA-responsive components, as well as salt-inducible NAC and ZFHD genes (Nishiyama et al., 2012). In agreement with this trend, we demonstrated that cytokinin-overproduction inhibited ABA-signaling downstream targets such as ABF3, RAB18, RD29B, RD26, DREB2A, as well as homeobox proteins ATHB5, ATHB7, and ATHB12 (Supplementary Table S2). Thus, cytokinin and ABA are functionally antagonized in the regulation of plant growth and the adaption of abiotic stresses (Shkolnik-Inbar and Bar-Zvi, 2010; Nishiyama et al., 2011; Wang et al., 2011; Liu et al., 2013; Guan et al., 2014; Yang et al., 2014).
In general, abiotic stress triggers oxidative responses and then stimulates ROS production. In this study, many of the differentially expressed genes, which were triggered by the overproduction of endogenous cytokinins, could be categorized into oxidation reduction and oxidative stress responses (Figure 6; Supplementary Table S2). Endogenous cytokinin overproduction enhanced ROS generation and decreased the activities of ROS-scavenging enzymes (Figures 4 and 5). In plant cells, ROS production occurs mainly in membrane-enclosed compartments such as chloroplasts, mitochondria and peroxisomes. In chloroplasts, photosystem I and II (PSI and PSII) are the major sites for ROS generation. Emerging evidences have implicated that cytokinin signaling in abiotic stresses lead to photosynthetic dysfunction and ROS production, by affecting genes expression of PSII subunits (Yi et al., 2008; Kobayashi et al., 2012). The enhancement of expressions of RbohD, RbohF, and RbohJ genes, which was triggered by the salt treatment and cytokinin overproduction, suggested the complex network of cytokinin, salt stress and ROS generation in plant cells (Figure 7). Overexpression of ROS-scavenging enzymes, such as isoforms of SOD, CAT and APX, can stimulate abiotic stress tolerance in various crop plants (Apel and Hirt, 2004). In this study, expressions of SOD1, APX4, GPX1, PER21, PER30, PXRX1, and TAPX1 were significantly down-regulated by endogenous cytokinin-overproduction and salt-treatment (Figure 7; Supplementary Table S2). In contrast to the complex effects of cytokinin homeostasis to drought stress tolerance, cytokinin deficient mutant ipt1,3,5,7 resists to salt stress (Nishiyama et al., 2011). Notably, in agreement with this study, we showed that overproduction cytokinin could enhance salt sensitivity in Arabidopsis. Thus, under the conditions of endogenous cytokinin overproduction and salt treatment, it is likely that, the lower expression levels of ROS-scavenging related-genes and the promotion of ROS-production were attributed to the decrease in antioxidant enzyme activities and the increase in ROS contents in AtIPT8-OE plants.
LBDs mainly expressed at the base of lateral organs of shoots and roots. Ectopically overexpressing LBD results in smaller organs through limiting the cell division (Shuai et al., 2002). Previous studies indicate that cytokinin is crucial for determining root-meristem size and root stem-cell specification (Dello Ioio et al., 2007; Müller and Sheen, 2008). In this study, significantly up-regulated LBD3 was linked to the overproduction of endogenous cytokinins and the treatment of salt, which was consistent with a previous study (Naito et al., 2007). Not like LBD3, the expression level of LBD4 was slightly increased by overly produced endogenous cytokinins, and the salt treatment antagonized the effect of cytokinin on LBD4 expression (Figure 7; Supplementary Table S2). The pleiotropic defects in the growth of roots and cotyledons, caused by endogenous cytokinin overproduction, might be achieved by enhancing LBDs expression. Collectively, we concluded that endogenous cytokinin overproduction derived by inducible overexpression of AtIPT8 shed a negative effect on plant salt tolerance by modulating stress-responsive gene expression, ROS production and chlorophyll homeostasis.
Author Contributions
In this research, YW was responsible for the experimental design, revising and finalizing the manuscript. YW designed and performed most of the experiments, analyzed the data and drafted the manuscript. WS performed physiological, confocal microscopic imaging and gene expression experiments. ZC provided regents and helpful discussions. All the authors in this research read and approved the final manuscript.
Funding
This work was supported by the grants of the National Natural Science Foundation of China to YW (31300233) and to YW (31270333), and the grant of the Major State Basic Research Program from Ministry of Science and Technology of China (2013CB126900) to YW, and the Chinese 111 Project (B06018). We are grateful to editor and reviewers for their comments and suggestions to this study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Wu lab members for technique help and helpful comments on the manuscripts. We thank Ying Hu (College of Life Sciences, Wuhan University) for the help in the confocal laser scanning microscopy. The expense for using the instruments at College of Life Sciences of Wuhan University was supported by the “Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.”
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.01004
FIGURE S1 | The inhibited elongation of primary roots in OE2 can be rescued by IAA treatment. (A) Exogenously added IAA could rescue estradiol-induced aberrant growth of roots in OE2. Seeds were growing on MS plates containing 17-β-estradiol (10μM) for 2 weeks, then transferred to medium containing IAA (1.0 nM) for 5 days. (Bar = 5cm). (B) The primary root lengths (shown in A) were measured. The results represent the means + SE of three independent experiments (seedling numbers, n > 30 per experiment). (C) 17-β-estradiol (10 μM) treatment could induce more callus generation in OE2 plants than that in Col under the same concentrations of exogenous 2,4-D (5 nM). Photos were taken after 7 days growth on MS plates.
FIGURE S2 | Exogenous application of cytokinin (6-BA) enhanced ROS production under the condition of salt stress. (A) The 5-day-old Col seedlings were pretreated with NaCl (100 mM) plus or minus 6-BA (10 μM) for 3 days. The fluorescent intensity of DCF was observed using the confocal microscope. Bar, 50 μm. (B) The ROS contents in Col plants were quantified. The data are means ± SEs of three independent experiments (n = 10). The fluorescent intensity of cotyledons in Col without salt treatment was taken as “1.”
FIGURE S3 | Comparisons of differentially expressed genes in AtIPT8-OE plants and salt- and/or oxidative-stress regulated genes downloaded from public microarray database (http://bar.utoronto.ca/welcome.htm). (A) Hierarchical cluster analysis of genes affected by AtIPT8 overexpression, salt and oxidative stresses in Arabidopsis. The differentially expressed genes in OE2 plants after 17-β-estradiol (10 μM) induction, and by the salt and oxidative treatments were analyzed by the method of using Cluster 3.0. The resulting tree figure was displayed using the Java Treeview software. The detailed information of genes was listed in Supplementary Table S2. (B) Numbers of overlapping transcripts changed by cytokinin overproduction, salt and oxidative treatments. (C) Numbers of genes with up- or down-regulated expression levels by cytokinin overproduction, salt and oxidative treatments.
TABLE S1 | Primers used for qRT-PCR analysis in this study.
TABLE S2 | The differentially expressed genes in AtIPT8-OE plant and ROS-regulated and/or salt-regulated genes from public microarry data (http://bar.utoronto.ca/welcome.htm).
Footnotes
- ^ http://tiangen.biomart.cn
- ^ http://www.toyobo-global.com
- ^ https://www.genscript.com/ssl-bin/app/primer
- ^ http://www.capitalbio.com
- ^ http://bar.utoronto.ca/welcome.htm
References
Abdullah, Z., and Ahmad, R. (1990). Effect of pre- and post-kinetin treatments on salt tolerance of different potato cultivars growing on saline soils. J. Agron. Crop Sci. 165, 94–102. doi: 10.1111/j.1439-037X.1990.tb00839.x
Alvarez, S., Marsh, E. L., Schroeder, S. G., and Schachtman, D. P. (2008). Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 31, 325–340. doi: 10.1111/j.1365-3040.2007.01770.x
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
Åstot, C., Dolezal, K., Moritz, T., and Sandberg, G. (2000). Deuterium in vivo labelling of cytokinins in Arabidopsis thaliana analysed by capillary liquid chromatography/frit-fast atom bombardment mass spectrometry. J. Mass Spectrom. 35, 13–22.
Bhargava, A., Clabaugh, I., To, J. P., Maxwell, B. B., Chiang, Y. H., Schaller, G. E., et al. (2013). Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-Seq in Arabidopsis. Plant Physiol. 162, 272–294. doi: 10.1104/pp.113.217026
Brenner, W. G., Ramireddy, E., Heyl, A., and Schmülling, T. (2012). Gene regulation by cytokinin. Front. Plant Sci. 3:8. doi: 10.3389/fpls.2012.00008
Brenner, W. G., and Schmülling, T. (2015). Summarizing and exploring data of a decade of cytokinin-related transcriptomics. Front. Plant Sci. 6:29. doi: 10.3389/fpls.2015.00029
Choudhury, S., Panda, P., Sahoo, L., and Panda, S. K. (2013). Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 8:e23681. doi: 10.4161/psb.23681
Das, K., and Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. doi: 10.3389/fenvs.2014.00053
Dello Ioio, R., Linhares, F. S., Scacchi, E., Casamitjana-Martinez, E., Heidstra, R., Costantino, P., et al. (2007). Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol. 17, 678–682. doi: 10.1016/j.cub.2007.02.047
De Rybel, B., Adibi, M., Breda, A. S., Wendrich, J. R., Smit, M. E., Novák, O., et al. (2014). Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 345:1255215. doi: 10.1126/science.1255215
Dobra, J., Motyka, V., Dobrev, P., Malbeck, J., Prasil, I. T., Haisel, D., et al. (2010). Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 167, 1360–1370. doi: 10.1016/j.jplph.2010.05.013
Dobrev, P. I., and Kamínek, M. (2002). Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. A 950, 21–29. doi: 10.1016/S0021-9673(02)00024-9
Foreman, J., Demidchik, V., Bothwell, J. H. F., Mylona, P., Miedema, H., Torres, M. A., et al. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446. doi: 10.1038/nature01485
Ghanem, M. E., Albacete, A., Martínez-Andújar, C., Acosta, M., Romero-Aranda, R., Dodd, I. C., et al. (2008). Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 59, 3039–3050. doi: 10.1093/jxb/ern153
Guan, C., Wang, X., Feng, J., Hong, S., Liang, Y., Ren, B., et al. (2014). Cytokinin antagonizes abscisic acid-mediated inhibition of cotyledon greening by promoting the degradation of ABSCISIC ACID INSENSITIVE5 protein in Arabidopsis. Plant Physiol. 164, 1515–1526. doi: 10.1104/pp.113.234740
Ha, S., Vankova, R., Yamaguchi-Shinozaki, K., Shinozaki, K., and Tran, L. S. (2012). Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci. 17, 172–179. doi: 10.1016/j.tplants.2011.12.005
Hansen, H., and Dörffling, K. (2003). Root-derived trans-zeatin riboside and abscisic acid in drought-stressed and rewatered sunflower plants: interaction in the control of leaf diffusive resistance? Funct. Plant Biol. 30, 365–375. doi: 10.1071/FP02223
Hoyerová, K., Gaudinová, A., Malbeck, J., Dobrev, P. I., Kocábek, T., Solcová, B., et al. (2006). Efficiency of different methods of extraction and purification of cytokinins. Phytochemistry 67, 1151–1159. doi: 10.1016/j.phytochem.2006.03.010
Hu, L., Li, H., Pang, H., and Fu, J. (2012). Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Solanum) differing in salt tolerance. J. Plant Physiol. 169, 146–156. doi: 10.1016/j.jplph.2011.08.020
Hutchison, C. E., Li, J., Argueso, C., Gonzalez, M., Lee, E., Lewis, M. W., et al. (2006). The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18, 3073–3087. doi: 10.1105/tpc.106.045674
Hwang, I., Sheen, J., and Müller, B. (2012). Cytokinin signaling networks. Annu. Rev. Plant Biol. 63, 353–380. doi: 10.1146/annurev-arplant-042811-105503
Jones, B., Gunnerås, S. A., Petersson, S. V., Tarkowski, P., Graham, N., May, S., et al. (2010). Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22, 2956–2969. doi: 10.1105/tpc.110.074856
Kang, N. Y., Cho, C., Kim, N. Y., and Kim, J. (2012). Cytokinin receptor-dependent and receptor-independent pathways in the dehydration response of Arabidopsis thaliana. J. Plant Physiol. 169, 1382–1391. doi: 10.1016/j.jplph.2012.05.007
Kirkham, M. B., Gardner, W. R., and Gerloff, G. C. (1974). Internal water status of kinetin-treated, salt-stressed plants. Plant Physiol. 53, 241–243. doi: 10.1104/pp.53.2.241
Kobayashi, K., Baba, S., Obayashi, T., Sato, M., Toyooka, K., Keränen, M., et al. (2012). Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell 24, 1081–1095. doi: 10.1105/tpc.111.092254
Kudoyarova, G. R., Vysotskaya, L. B., Cherkozyanova, A., and Dodd, I. C. (2007). Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J. Exp. Bot. 58, 161–168. doi: 10.1093/jxb/erl116
Kumar, M. N., Jane, W. N., and Verslues, P. E. (2013). Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 161, 942–953. doi: 10.1104/pp.112.209791
Liu, J., Mehdi, S., Topping, J., Friml, J., and Lindsey, K. (2013). Interaction of PLS and PIN and hormonal crosstalk in Arabidopsis root development. Front. Plant Sci. 4:75. doi: 10.3389/fpls.2013.00075
Liu, X. Z., and Huang, B. (2002). Cytokinin effects on creeping bentgrass response to heat stress. Crop Sci. 42, 466–472. doi: 10.2135/cropsci2002.4660
Lubovská, Z., Dobrá, J., Štorchová, H., Wilhelmová, N., and Vanková, R. (2014). Cytokinin oxidase/dehydrogenase overexpression modifies antioxidant defense against heat, drought and their combination in Nicotiana tabacum plants. J. Plant Physiol. 171, 1625–1633. doi: 10.1016/j.jplph.2014.06.021
Macková, H., Hronková, M., Dobrá, J., Turečková, V., Novák, O., Lubovská, Z., et al. (2013). Enhanced drought and heat stress tolerance of tobacco plants with ectopically enhanced cytokinin oxidase/dehydrogenase gene expression. J. Exp. Bot. 64, 2805–2815. doi: 10.1093/jxb/ert131
Mason, M. G., Jha, D., Salt, D. E., Tester, M., Hill, K., Kieber, J. J., et al. (2010). Type-B response regulators ARR1 and ARR12 regulate expression of AtHKT1;1 and accumulation of sodium in Arabidopsis shoots. Plant J. 64, 753–763. doi: 10.1111/j.1365-313X.2010.04366.x
Merewitz, E. B., Gianfagna, T., and Huang, B. (2011). Photosynthesis, water use, and root viability under water stress as affected by expression of SAG12-ipt controlling cytokinin synthesis in Agrostis stolonifera. J. Exp. Bot. 62, 383–395. doi: 10.1093/jxb/erq285
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
Mlejnek, P., Doležel, P., and Procházka, S. (2003). Intracellular phosphorylation of benzyladenosine is related to apoptosis induction in tobacco BY-2 cells. Plant Cell Environ. 26, 1723–1735. doi: 10.1046/j.1365-3040.2003.01090.x
Moubayidin, L., Perilli, S., Dello Ioio, R., Di Mambro, R., Costantino, P., and Sabatini, S. (2010). The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 20, 1138–1143. doi: 10.1016/j.cub.2010.05.035
Müller, B., and Sheen, J. (2008). Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453, 1094–1097. doi: 10.1038/nature06943
Mýtinová, Z., Motyka, V., Haisel, D., Gaudinová, A., Lubovská, Z., and Wilhelmová, N. (2010). Effect of abiotic stresses on the activity of antioxidative enzymes and contents of phytohormones in wild type and AtCKX2 transgenic tobacco plants. Bio. Plant. 54, 461–470. doi: 10.1007/s10535-010-0082-3
Naito, T., Yamashino, T., Kiba, T., Koizumi, N., Kojima, M., Sakakibara, H., et al. (2007). A link between cytokinin and ASL9 (ASYMMETRIC LEAVES 2 LIKE 9) that belongs to the AS2/LOB (LATERAL ORGAN BOUNDARIES) family genes in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 71, 1269–1278. doi: 10.1271/bbb.60681
Nishiyama, R., Le, D. T., Watanabe, Y., Matsui, A., Tanaka, M., Seki, M., et al. (2012). Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency. PLoS ONE 7:e32124. doi: 10.1371/journal.pone.0032124
Nishiyama, R., Watanabe, Y., Fujita, Y., Le, D. T., Kojima, M., Werner, T., et al. (2011). Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23, 2169–2183. doi: 10.1105/tpc.111.087395
Nishiyama, R., Watanabe, Y., Leyva-Gonzalez, M. A., Ha, C. V., Fujita, Y., Tanaka, M., et al. (2013). Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. U.S.A. 110, 4840–4845. doi: 10.1073/pnas.1302265110
Patterson, T. A., Lobenhofer, E. K., Fulmer-Smentek, S. B., Collins, P. J., Chu, T. M., Bao, W., et al. (2006). Performance comparison of one-color and two-color platforms within the microarray quality control (MAQC) project. Nat. Biotechnol. 24, 1140–1150. doi: 10.1038/nbt1242
Petrov, V., Hille, J., Mueller-Roeber, B., and Gechev, T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6:69. doi: 10.3389/fpls.2015.00069
Porra, R. J., Thompson, W. A., and Kriedemann, P. E. (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 975, 384–394. doi: 10.1016/S0005-2728(89)80347-0
Pospisilova, J., Vagner, M., Malbeck, J., Travnickova, A., and Batkova, P. (2005). Interactions between abscisic acid and cytokinins during water stress and subsequent rehydration. Biol. Plant. 49, 533–540. doi: 10.1007/s10535-005-0047-0
Qin, H., Gu, Q., Zhang, J., Sun, L., Kuppu, S., Zhang, Y., et al. (2011). Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 52, 1904–1914. doi: 10.1093/pcp/pcr125
Rivero, R. M., Kojima, M., Gepstein, A., Sakakibara, H., Mittler, R., Gepstein, S., et al. (2007). Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. U.S.A. 104, 19631–19636. doi: 10.1073/pnas.0709453104
Schaller, G. E., Bishopp, A., and Kieber, J. J. (2015). The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27, 44–63. doi: 10.1105/tpc.114.133595
Sharma, P., Jha, A. B., Dubey, R. S., and Pessarakli, M. (2012). Reactive oxygen species, oxidative damage, and anti-oxidative defense mechanism in plants under stressful conditions. J. Bot. 2012:26. doi: 10.1155/2012/217037
Shkolnik-Inbar, D., and Bar-Zvi, D. (2010). ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22, 3560–3573. doi: 10.1105/tpc.110.074641
Shuai, B., Reynaga-Peña, C. G., and Springer, P. S. (2002). The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 129, 747–761. doi: 10.1104/pp.010926
Tanaka, R., Kobayashi, K., and Masuda, T. (2011). Tetrapyrrole metabolism in Arabidopsis thaliana. Arabidopsis Book 9:e0145. doi: 10.1199/tab.0145
Tran, L. S., Urao, T., Qin, F., Maruyama, K., Kakimoto, T., Shinozaki, K., et al. (2007). Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 104, 20623–20628. doi: 10.1073/pnas.0706547105
Wang, Y., Li, L., Ye, T., Zhao, S., Liu, Z., Feng, Y. Q., et al. (2011). Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. Plant J. 68, 249–261. doi: 10.1111/j.1365-313X.2011.04683.x
Wang, Y., Yang, L., Zheng, Z., Grumet, R., Loescher, W., Zhu, J.-K., et al. (2013). Transcriptomic and physiological variations of three Arabidopsis ecotypes in response to salt stress. PLoS ONE 8:e69036. doi: 10.1371/journal.pone.0069036
Werner, T., Nehnevajova, E., Kollmer, I., Novak, O., Strnad, M., Kramer, U., et al. (2010). Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 22, 3905–3920. doi: 10.1105/tpc.109.072694
Wohlbach, D. J., Quirino, B. F., and Sussman, M. R. (2008). Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell 20, 1101–1117. doi: 10.1105/tpc.107.055871
Xu, Y. H., Liu, R., Yan, L., Liu, Z. Q., Jiang, S. C., Shen, Y. Y., et al. (2012). Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 63, 1095–1106. doi: 10.1093/jxb/err315
Yang, C., Liu, J., Dong, X., Cai, Z., Tian, W., and Wang, X. (2014). Short-term and continuing stresses differentially interplay with multiple hormones to regulate plant survival and growth. Mol. Plant. 7, 841–855. doi: 10.1093/mp/ssu013
Yi, X., Hargett, S. R., Frankel, L. K., and Bricker, T. M. (2008). The effects of simultaneous RNAi suppression of PsbO and PsbP protein expression in photosystem II of Arabidopsis. Photosynth. Res. 98, 439–448. doi: 10.1007/s11120-008-9352-8
Zavaleta-Mancera, H. A., Lopez-Delgado, H., Loza-Tavera, H., Mora-Herrera, M., Trevilla-Garcia, C., Vargas-Suarez, M., et al. (2007). Cytokinin promotes catalase and ascorbate peroxidase activities and preserves the chloroplast integrity during dark-senescence. J. Plant Physiol. 164, 1572–1582. doi: 10.1016/j.jplph.2007.02.003
Zhu, J. K. (2002). Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273. doi: 10.1146/annurev.arplant.53.091401.143329
Keywords: cytokinin overproduction, AtIPT8, ROS homeostasis, salt stress, chlorophyll, transcriptomic analysis
Citation: Wang Y, Shen W, Chan Z and Wu Y (2015) Endogenous Cytokinin Overproduction Modulates ROS Homeostasis and Decreases Salt Stress Resistance in Arabidopsis Thaliana. Front. Plant Sci. 6:1004. doi: 10.3389/fpls.2015.01004
Received: 25 August 2015; Accepted: 30 October 2015;
Published: 19 November 2015.
Edited by:
Ken Yokawa, University of Bonn, GermanyReviewed by:
Rongcheng Lin, Chinese Academy of Sciences, ChinaLizhong Xiong, Huazhong Agricultural University, China
Copyright © 2015 Wang, Shen, Chan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Wu, wuy@whu.edu.cn
†These authors have contributed equally to this work.
 Yanping Wang
Yanping Wang Wenzhong Shen
Wenzhong Shen Zhulong Chan
Zhulong Chan Yan Wu
Yan Wu