- 1School of Agriculture, Yunnan University, Kunming, China
- 2Flower Research Institute of Yunnan Academy of Agricultural Sciences, Kunming, China
- 3National Engineering Research Center for Ornamental Horticulture, Kunming, China
Rhododendron delavayi Franch is an evergreen shrub or small tree with large scarlet flowers that makes it highly attractive as an ornamental species. The species is native to southwest China and southeast Asia, especially the Himalayan region, showing good adaptability, and tolerance to drought. To understand the water stress coping mechanisms of R. delavayi, we analyzed the plant's photosynthetic performance during water stress and recovery. In particular, we looked at the regulation of stomatal (gs) and mesophyll conductance (gm), and maximum rate of carboxylation (Vcmax). After 4 days of water stress treatment, the net CO2 assimilation rate (AN) declined slightly while gs and gm were not affected and stomatal limitation (SL) was therefore negligible. At this stage mesophyll conductance limitation (MCL) and biochemical limitation (BL) constituted the main limitation factors. After 8 days of water stress treatment, AN, gs, and gm had decreased notably. At this stage SL increased markedly and MCL even more so, while BL remained relatively constant. After re-watering, the recovery of AN, gs, and gm was rapid, although remaining below the levels of the control plants, while Vcmax fully regained control levels after 3 days of re-watering. MCL remained the main limitation factor irrespective of the degree of photosynthetic recovery. In conclusion, in our experiment MCL was the main photosynthetic limitation factor of R. delavayi under water stress and during the recovery phase, with the regulation of gm probably being the result of interactions between the environment and leaf anatomical features.
Introduction
Low water availability is considered as one of the main environmental factors limiting plant growth and productivity worldwide (Chaves et al., 2009). The majority of climate change scenarios predict an increase in drought incidents throughout the world (Lemke et al., 2007). Thus, the strategies of tolerance, adaption, and survival will be of major importance for plants growing under water stress. It has been shown that water stress primarily affects photosynthetic CO2 assimilation, and therefore limits plant productivity and growth (Flexas et al., 2006). The response of photosynthesis to water stress has received considerable attention in recent decades, especially the key factors limiting photosynthesis under water stress conditions (Flexas et al., 2002; Lawlor and Cornic, 2002). However, there is an on-going debate about whether the determinant for photosynthesis under water stress conditions is stomatal closure, diffusive resistance, or metabolic uncoupling (Lawlor and Cornic, 2002; Flexas et al., 2009, 2014; Pinheiro and Chaves, 2011; Campos et al., 2014; Chen et al., 2015).
Stomatal closure is generally considered as the initial and main cause of the decrease in photosynthesis under water stress conditions, as diffusion of CO2 from the atmosphere to the sites of carboxylation in the chloroplast is impaired (Flexas et al., 2009; Rho et al., 2013). However, reduced leaf CO2 diffusion conductance is not only due to stomatal closure, but also due to the decreased internal conductance of CO2 diffusion (mesophyll conductance, gm; Galmés et al., 2007; Zhou et al., 2014; Chen et al., 2015). As a result of the recognition of the importance of gm, the studies pertaining to gm have increased exponentially in recent years (Zhou et al., 2007; Flexas et al., 2009; Galle et al., 2009; Niinemets et al., 2009; Rancourt et al., 2015). Some studies suggested that gm was consistently delayed by a few days compared with gs (Rancourt et al., 2015), while other studies suggested that gm can vary at least as fast as gs, and gm contributes greatly to the limitation of photosynthesis during water stress and recovery after water stress (Gomes et al., 2008). Recently, the work by Carriquí et al. (2015) suggested that gs, and gm were co-responsible for the lower photosynthesis observed in ferns as compared with angiosperms, and that gm was the most constraining factor for photosynthesis in ferns. These findings support the idea of an important role for gm in the photosynthetic responses of plants to climatic constraints.
Additionally, photosynthesis also decreases due to metabolic impairments and/or cell damage, especially under severe water stress combined with intensive light and high temperature (Flexas et al., 2006; Zhou et al., 2007). Under these circumstances, down-regulation of photosynthesis increases the production of reactive oxygen species (Takahashi and Murata, 2005) and even leads to photoinhibition (Massacci et al., 2008; Wang et al., 2012; Chastain et al., 2014). Furthermore, the carbon balance of a plant enduring a water stress period depends as much on the rate and degree of photosynthetic recovery (Flexas et al., 2006). The capability for photosynthetic recovery after exposure to water stress conditions is essential to understand the plant response to water stress, and to determine appropriate irrigation in agricultural practices. Many studies have addressed the response of photosynthesis to water stress, but the underlying process of photosynthetic recovery from water stress is poorly understood. In particular, knowledge about the factors limiting photosynthesis under these conditions, and their possible interactions with other environmental conditions are scarce (Flexas et al., 2009; Campos et al., 2014).
A previous study proposed a method which divides the total limitation into stomatal limitation (SL), mesophyll conductance limitation (MCL), and biochemical limitation (BL; Grassi and Magnani, 2005). Galmés et al. (2007) found that MCL was the main limiting factor for photosynthesis recovery after re-watering in 10 Mediterranean species. The results of Flexas et al. (2009) suggested that stomatal closure recovered much more slowly than gm, thus photosynthesis recovery after re-watering was mostly limited by SL. These findings underline the importance of gm during water stress conditions, and further suggest an important contribution to the overall adaptation of plants to drought stress conditions. However, current knowledge about the physiological limitations to photosynthesis during short-term water stress and recovery after re-watering is scarce (Flexas et al., 2009, 2014). It would be necessary to apply this method to analyze photosynthetic limitations in plants subjected to water stress, especially in circumstances pertaining to a shorter period of water stress and recovery after re-watering.
Rhododendron is one of the most well-known alpine flowers. Of the approximately 571 Rhododendron species in China, 320 species are found within the Yunnan Province of southwestern China (Fang et al., 2005). Most of the above Rhododendron species are distributed in alpine areas and commonly are less constrained by water shortage. However, the five consecutive years of spring drought experienced in Southwest China since 2009 have had great adverse effects on the growth and flowering of Rhododendron. Furthermore, droughts frequently occur in winter and spring, water supply limitation is gradually becoming one of the dominant limitations for the growth and application of Rhododendron. Currently, little is known about the physiological responses during the process of water stress and the recovery after re-watering.
Rhododendron delavayi Franch is an evergreen shrub or small tree with large scarlet flowers that makes it highly attractive as an ornamental species. The species is native to southwest China and southeast Asia, especially the Himalayan region. The leaf of R. delavayi is leathery, and the abaxial surface with 1-layered, spongy or somewhat agglutinated, whitish to fawn indumentums (Fang et al., 2005). In our previous study, we found that the stomata of R. delavayi exist only on the abaxial surface. When compared with R. irroratum and R. yunnanense which grow under the same conditions, R. delavayi exhibited perfect adaptability and tolerance to dry and high radiation environments, including traits such as with smaller stomata, larger stomatal density, and higher ratio of palisade and spongy tissue (Cai et al., 2014). The aim of the present work was to evaluate the responses of photosynthesis to water stress and recovery, and analyze the main limiting factors of photosynthesis during water stress and recovery, emphasizing the leaf internal diffusive components. Our hypotheses were that: (1) excitation pressure imposed by water stress will cause a general decline of the photosynthetic performance; (2) down-regulation of mesophyll conductance to CO2 may impose a similar or even greater limitation to photosynthesis than that imposed by stomatal closure during water stress treatment; (3) photosynthetic recovery after re-watering may be mostly limited by mesophyll conductance limitations.
Materials and Methods
Plant Materials and Treatments
The experiment was carried out in a greenhouse in Kunming, China (a1t1926 m, E 102°46′, N 25°07′). Five-year-old plants of R. delavayi were grown in 9-L plastic pots (one plant per pot) filled with a mixture of red soil and humus (V/V, 1/3). The plants were housed in the experimental greenhouse under natural light and temperature conditions. From budbreak (20 March) to the beginning of the experimental period (25 June), the plants were irrigated three times per week to maintain sufficient water supply. Then, 30 plants were placed in the same greenhouse, and subdivided randomly into two groups: the control and the stressed plants. The control plants were irrigated daily to field capacity, while the treatment plants were not irrigated. Ten days after stopping the irrigation, the leaf stomatal conductance (gs) decreased to 0.02 mol H2O m−2s−1 (severe water stress), at which point all of the plants were re-watered to field capacity for recovery.
Relative Water Content (RWC)
Plant water status was assessed by relative water content (RWC) on the first whorl of the leaves. In order to determine RWC, four fresh leaves per replication were collected and their fresh weights (FW) were obtained. Next, these leaves were placed in water to float for 24 h at 4°C in the dark to obtain their turgid weights (TW). The leaves were then oven dried at 72°C for 48 h to obtain their dry weights (DW). RWC was determined by the formula: RWC(%) = (FW − DW)/(TW − DW) × 100 (de Souza et al., 2013).
Soil Moisture Content (SMC)
In order to determine soil moisture content (SMC), the soil of four pots per replication were collected in an aluminum box and their fresh weights (FW) determined. The soil sample was then oven dried at 105°C for 48 h to obtain their dry weights (DW). SMC was determined by the formula: SMC(%) = (FW−DW)/DW×100.
Instantaneous Gas Exchange
Instantaneous gas exchange measurements were tested daily, between 12:00 and 13:00 h local time, using an open gas-exchange system (Li-6400XT; Li-Cor, Inc., Nebraska, USA) equipped with a light source (Li-6400-02B). No measurements were taken on day 6 due to technical problems with the Li-6400. All measurements were made on the young, fully expanded leaves at a saturating photosynthetic photo flux density (PPFD) of 1000 μmol m−2s−1, with a CO2 concentration of 400 μmol mol−1 in the leaf cuvette. During the instantaneous measurements, net CO2 assimilation rate (AN), stomatal conductance (gs), intercellular CO2 concentration (Ci), transpiration rate (Tr), air temperature (Tair), and leaf-to-air vapor pressure deficit (VPD) were recorded automatically by the Li-6400XT.
CO2 Response Curve and Chlorophyll Fluorescence
The CO2 response curve (AN–Ci curves) and chlorophyll fluorescence were measured simultaneously with a combined open gas exchange system and chlorophyll fluorescence system (Li-6400-40; Li-Cor Inc., Nebraska, USA) on four specific sampling days for each treatment: day 4 and day 8 after stopping the irrigation, and then day 1 and day 3 after re-watering.
AN–Ci curves were constructed as a function of the ambient CO2 concentration (Ca) ranging from 50 to 2000 μmol mol−1. Light intensity was maintained at 1000 μmol m−2 s−1. The flow rate within the chamber was controlled at 500 mmol air min−1, and the block temperature at 25°C. VPD was kept within a variation of 0.5 kPa during the performance of a single curve. At first, Ca in the leaf chamber decreased stepwise from 400 to 50 μmol mol−1. After that Ca was returned to 400 μmol mol−1 to restore the original AN. Then, Ca was increased stepwise until 2000 μmol mol−1 to complete the curve. The number of different Ca values used for the response curve was 13, and the time lag between two consecutive measurements at each Ca was restricted to 2–4 min. The AN-Ci curves were used to estimate the maximum carboxylation rate of Rubisco (Vcmax), the maximum electron transport rate (Jmax) used a utility developed by Sharkey et al. (2007), and was based on an alternative AN-Ci curve fitting method through a non-rectangular hyperbola version of the model provided by Farquhar et al. (1980).
For the chlorophyll fluorescence measurements, the leaf was irradiated by an actinic radiation of 1000 μmol m−2 s−1 (90–10% red-blue light) for 15–20 min until stable photosynthesis occurred. Then the steady state fluorescence (Fs) was recorded and subsequently another saturating light pulse around 6000 μmol m−2s−1 was applied to determine the maximum fluorescence (F). Actinic light was removed and the leaves were irradiated with far-red light to obtain Fo adapted to light (F). From these values, the photochemical quenching (qP) was calculated as: , the actual photochemical efficiency of photosystem II was calculated as: (; Genty et al., 1989), and the electron transport rate was calculated as: (Jflu) = ΦPSII × PPFD × 0.5 × 0.85 (Valentini et al., 1995).
After an adaptation period of 30 min in the dark, the minimum fluorescence (Fo) was measured with the light which was sufficiently low to avoid a photochemical reaction. The maximum fluorescence (Fm) was obtained by applying a saturating light pulse of 6000 μmol m−2 s−1 for 0.8 s. The maximum quantum efficiency of photosystem II (Fv/Fm) was calculated as: FV/Fm = (Fm−Fo)/Fm(Genty et al., 1989).
Determination of Mesophyll Conductance
Mesophyll conductance (gm) was calculated using the “variable” method as described by Harley et al. (1992):
where, AN and Ci are taken from AN–Ci curve, and Jflu was estimated from chlorophyll fluorescence on the same leaf, and Γ* was 37.43 μmol mol−1 at 25°C according to Bernacchi et al. (2002). Rd was respiration in the light and determined according to the method of Laisk (1977). gm values were calculated for measurements of the net assimilation rate at a Ci level of 100–300 μmol mol−1, and the average value of gm was determined for each leaf. The calculated values for gm were used to estimate the chloroplast CO2 concentration (Cc) as: Cc = Ci−(AN/gm)
Photosynthetic Limitation Analysis
To compare photosynthetic limitations during water stress and recovery, the approach proposed by Grassi and Magnani (2005) was used to partition photosynthetic limitation into three components related to stomatal conductance (SL), mesophyll conductance (MCL), and leaf biochemical characteristics (BL). This requires assuming a reference which had a maximum assimilation rate. In the current study, the maximum assimilation rate, concomitant with gs, gm, and Vcmax, was generally found under well-watered conditions. Therefore, the control treatment was used as a reference. According to this method, non-stomatal limitations were defined as the sum of the contributions of mesophyll conductance and leaf biochemistry (NSL = MCL + BL), while the diffusive limitations were the sum of the contributions of the stomatal and mesophyll conductance (DL = SL+MCL).
Results
Experimental Conditions and Plant Water Stress
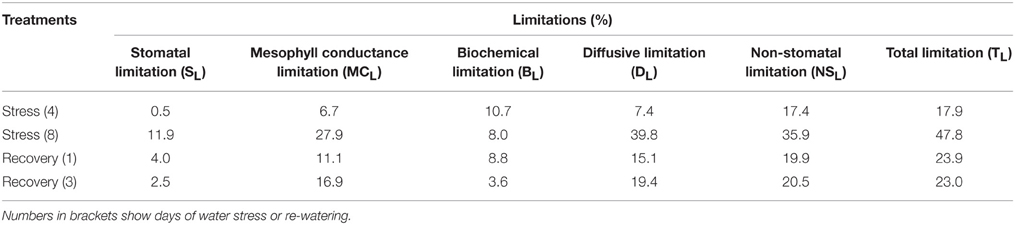
During the experiment VPD of the control and stressed plants remained mostly above 1.5 kPa. Air temperature (Tair) was between 23.9 and 37.9°C (Figures 1A,B). The highest VPD and Tair corresponded to the most severely stressed plants without irrigation for 10 days. For the three days (3, 7, and 11 days after the onset of the experiment) with clouds or rainfall, Tair values of all of the plants were close to 25°C, VPD also decreased regardless of the treatments.
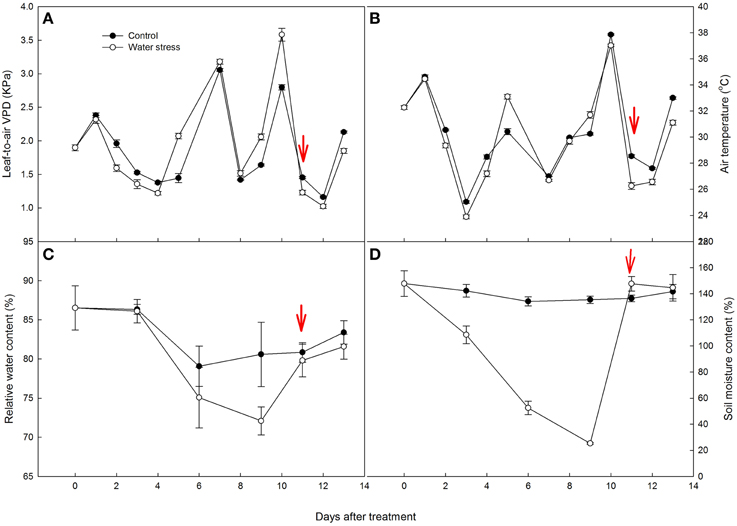
Figure 1. Variations of (A) leaf-to-air vapor pressure deficit (VPD), (B) air temperature (Tair), (C) leaf relative water content (RWC), and (D) soil moisture content (SMC) during the experiment. The values represent means ± SE (n = 10 for A,B, n = 4 for C,D). Day 0 corresponds to the first day of water stress and the arrow indicates the start of re-watering.
During the experiment period, RWC in the control plants had some fluctuation, with an average of 82.8%. After 6 days of water stress, RWC in the stressed plants decreased slightly. After 9 days of water stress, RWC in the stressed plants decreased to 72.1%, and there was a significant difference between the control and stressed plants. After re-watering, RWC in the water stressed plants increased to values similar to those in the control plants (Figure 1C).
During the experiment period, SMC of the control plants was between 134.1 and 147.8%, while SMC of the stressed plants decreased significantly, from 147.8% at the beginning of the treatment to 25.3% after 9 days of water stress (Figure 1D).
Photosynthetic Responses to Water Stress and Recovery
AN of the control plants oscillated during the experiment, from 15.2 μmol CO2 m−2s−1 by day 4 to 10.5 μmol CO2 m−2s−1 by day 7. Water stress caused a slight reduction in AN from day 1 to day 7, and AN of the stressed plants was more than 80% of the control plants. However, water stress resulted in a significant reduction in AN by day 8, and the value of AN rapidly decreased to 1.68 μmol CO2 m−2s−1 by day 9, only 14% of the value of the control plants. After 1 d of re-watering, AN was restored to 80% of the control value, but no further recovery of AN was observed afterwards (Figure 2A).
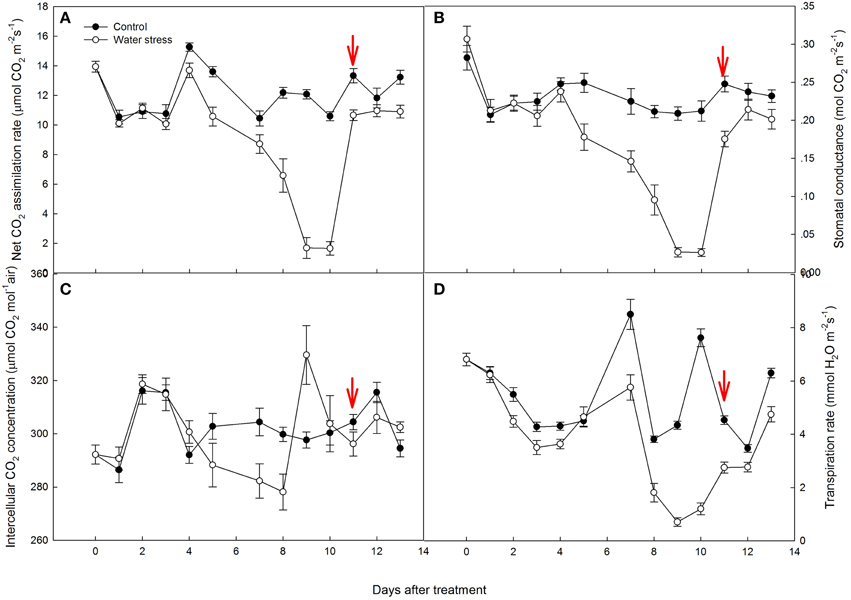
Figure 2. Variations of (A) net CO2 assimilation rate (AN), (B) stomatal conductance (gs), (C) intercellular CO2 concentration (Ci), and (D) transpiration rate (Tr) during the experiment. Values represent means ± SE (n =10). Day 0 corresponds to the first day of water stress and the arrow indicates the start of re-watering.
Stomatal conductance (gs) showed a similar variation to AN. In the control plants, gs were maintained above 0.20 mol CO2 m−2s−1, with an average value of 0.22 mol CO2 m−2s−1. The stressed plants had similar gs values to the control plants from day 1 to day 4. Water stress caused a gradual decline in gs from day 5 to day 8, and gs values of the stressed plants were approximately 0.10 mol CO2 m−2s−1 by day 8. However, gs quickly declined to 0.02 mol CO2 m−2s−1 by day 9. Like AN, the recovery of gs after re-watering was very quick, restored to 70% of the control value in the first day, and restored to 86% of the control plants after 3 days of re-watering (Figure 2B).
As a consequence of the decreased gs during water stress treatment, Ci and Tr were also depressed (Figures 2C,D). It is worth noting that the change in Ci did not simply follow that in gs. During the last 2 days of water stress treatment, Ci increased quickly and exceeded the control plants. After re-watering, Ci decreased and maintained lower values than that observed in the control plants.
When compared with the control plants, Vcmax and Jmax in the stressed plants were reduced by 22 and 14% by day 4, respectively. However, Vcmax and Jmax did not decrease further after 8 days of water stress treatment. After re-watering, Vcmax and Jmax increased to the levels equivalent to that in the control plants, and even higher than the control plants after 3 days of re-watering (Table 1).
Table 1. Net CO2 assimilation rate (AN), stomatal conductance (gs), Rubisco maximum carboxylation rate (Vcmax), maximum electron transport rate (Jmax), mesophyll conductance (gm) and chloroplast CO2 concentration (Cc) under water stress and re-watering.
Mesophyll conductance (gm) did not decline by day 4, but suddenly decreased to 53% of the control plants by day 8. However, gm totally recovered during the first day of re-watering. As a consequence of the decreased gm during water stress treatment, Cc was also depressed. The depression was more remarkable by day 8. After re-watering, due to the rapid recovery of gm, Cc increased and was higher than the control plants during the first day after re-watering (Table 1).
Change in Chlorophyll Fluorescence During Water Stress and Recovery
The chlorophyll fluorescence parameters are shown in Figure 3. The values for Fv/Fm were kept above 0.82 throughout the experimental period, and were not significantly different between the control plants and stressed plants (Figure 3A). By day 4, the values for ΦPSII, qP, and Jflu of the stressed plants significantly declined, and further declined by day 8. After re-watering, the recovery of ΦPSII, qP, and Jflu was slight on the first day, and these recovered progressively to the levels seen in the control plants within 3 days (Figures 3B–D).
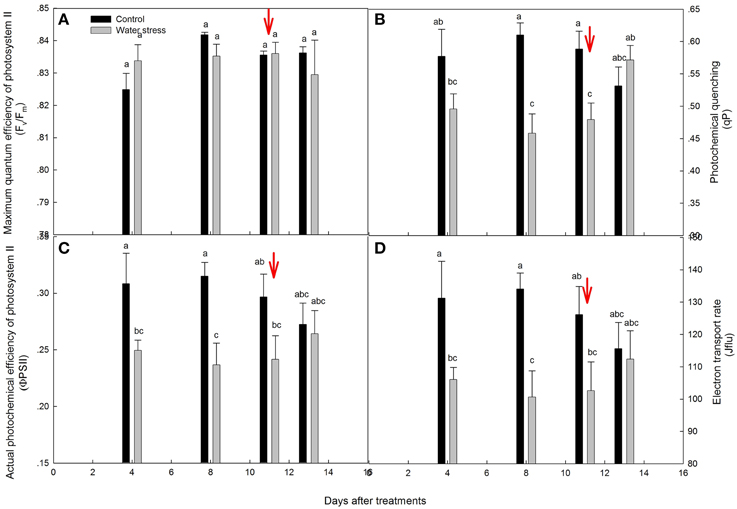
Figure 3. Variations of (A) maximum quantum efficiency of photosystem II (Fv/Fm), (B) photochemical quenching (qP), (C) actual photochemical efficiency of photosystem II (ΦPSII), and (D) electron transport rate (Jflu) during the experiment. Values represent means ± SE (n = 4). Day 0 corresponds to the first day of water stress and the arrow indicates the start of re-watering.
Photosynthetic Limitations During Water Stress and Recovery
After 4 days of water stress treatment, SL was negligible, while MCL and BL imposed some limitations to photosynthesis. With the further imposition of water stress treatment, SL and MCL increased rapidly, while BL did not increase by day 8. It is worth noting that MCL was more than two times the level observed for SL. After 1 d of re-watering, SL and MCL decreased rapidly, while BL did not change. After 3 days of re-watering, SL and BL further declined, while MCL remained stable, and thus MCL was the main limitation to photosynthesis during recovery (Table 2).
Discussion
Photosynthetic Responses to Water Stress and Recovery
The water stress-induced decline of AN, gs, and gm was always slower than the recovery after re-watering. After 4 days of water stress treatment, AN declined slightly, and there was almost no change in the values for gs and gm. As the duration of the water stress treatment increased, AN, gs, and gm decreased markedly by day 8. After 9 days of water stress, the values of AN was close to zero, gs reached 0.02 mol CO2 m−2s−1. There was no further reduction in the next continued 2 days drought. After re-watering for 1 day, no plant died and all of them showed some recovery. Results suggested that gs can use as a reference parameter to justify the levels of water stress of R. delavayi, i.e., severe water stress (gs near 0.02 mol CO2 m−2s−1). Flexas et al. (2009) also use gs to define the levels of water stress of Vitis hybrid Richter-110, i.e., moderate water stress (gs near 0.15 mol CO2 m−2s−1) and severe water stress (gs near 0.05 mol CO2 m−2s−1).
Once water stress was established and maintained, gs was more stable than gm. An intriguing behavior of gm was a total recovery after irrigation for 1 day, while gs and AN were restored to above 70% of the control values. Throughout the periods of water stress and recovery, AN and gs of followed the same course, indicating a strong co-regulation of these parameters, as shown in many studies (Chaves et al., 2002; Lawlor and Cornic, 2002). However, with the further recovery of gs and AN, the values for gm were not restored further, and even slightly decreased after re-watering for 3 days, indicating an independent regulation for gs and gm. Previous studies suggested that gs appears to be more independent of environmental conditions except for VPD (Pou et al., 2008; Hanson et al., 2013), and the opening and closing of stomata is regulated by the integration of environmental signals and endogenous hormonal stimuli (Daszkowska-Golec and Szarejko, 2013). However, the response of gm to water stress strongly depends on the water channels aquaporins (Daszkowska-Golec and Szarejko, 2013), and the impact of additional environmental factors, especially light condition (Zhou et al., 2007). Regulatory mechanisms such as the phosphorylation of aquaporins can be light dependent (Tournaire-Roux et al., 2003). The results of Galle et al. (2009) further showed that gm declines considerably and recovers slightly under high light conditions; while under low light conditions it does not decrease under water stress. In the present study, the first day of re-watering was cloudy with rain, and Tair was close to 25°C with a decreasing VPD. Considered together with the present results, it seems that the adaptation of gm to water stress and its rapid recovery after rewatering is related to additional factors, such as light intensity and temperature, and suggests the necessity to better understand the regulation of mesophyll conductance, which conceivably depends on the environmental conditions.
In plants under water stress treatment, stomatal closure results in rapid decrease of gs and AN (Campos et al., 2014). However, non-stomatal factors were also important for the regulation of photosynthetic capacity, as reflected by both the reduction of Vcmax and Jmax. The decrease in Vcmax is mostly due to the reduced activity of fructose-1,6-biphosphate phosphatase, as well as the decreased activity of Rubisco (Maroco et al., 2002; Bota et al., 2004). However, recent transcriptomic analysis in plants subjected to water stress showed that some genes related to Rubisco activase, Calvin cycle enzymes, and PSI and PSII, are conversely up-regulated during the acclimation to water stress (Cramer et al., 2007; Song et al., 2014). Proteomic analysis also confirmed that some photosynthetic proteins such as notably Rubisco and sedoheptulose 1,5-bisphosphatase, and mitochondrial glycine decarboxylase complex (GDC) protein were up-regulated (Vincent et al., 2007; Zhang et al., 2010). It has been verified that irrigated and water-stressed plants often show similar values for Vcmax and Jmax (de Souza et al., 2005), and Vcmax remained almost unchanged both under water stress and recovery (Galle et al., 2009). In the present study, these two effects were observed. Firstly, the reduction of AN, gs, and gm were accompanied by reductions in Vcmax and Jmax under water stress conditions (Table 1), but the magnitudes of the reduction of Vcmax and Jmax were much smaller than for AN, gs, and gm. Secondly, AN, gs, and gm remained below the levels of the control plants after 3 days of re-watering, but Vcmax and Jmax were totally restored and even surpassed the control value. In accordance with the reports by Tang et al. (2013) and Galle et al. (2011), the increased Vcmax in response to the decreased Cc and gm improved the photosynthetic capacity, and contributed to the maintenance of photosynthesis under water stress treatment, most notably, during recovery.
Indeed, chlorophyll fluorescence analysis also supported this conclusion. After 4 days of water stress treatment, decreases in Jflu and ΦPSII were observed, and indicated a decline in the quantum yield of the electron transport in PSII (de Souza et al., 2013), and limited the synthesis of ATP and the regeneration of RuBP (Lin et al., 2009), which caused the low activation of Rubisco (Vcmax). However, Fv/Fm was maintained between 0.82 and 0.85 throughout the experiment, indicating that PSII was quite resistant to water stress treatment. In addition, qP, Jflu, and ΦPSII were fully recovered after 3 days of re-watering. The above results suggest that water stress inevitably damaged the light reactions, with possible damage to PSII functionality, but the occurrence of damage did not seem to be irreversible. The same trend has already been shown in some species, particularly in those showing increased paraheliotropism in response to water stress (Pastenes et al., 2005; Wang et al., 2012).
Photosynthetic Limitations During Water Stress and Recovery
The photosynthetic limitation analysis showed that SL was negligible, and that MCL and BL were the main limitation factors after 4 days of water stress treatment (Table 2). This indicates that 4 days of water stress treatment did not affect the opening of the stomata, the internal transfer of CO2, or the photosynthetic rate. With the continuing water stress treatment, AN, gs and gm declined rapidly and significantly by day 8. At the same time, SL and MCL increased markedly, and the increase of MCL was far greater than that of SL, and thus MCL became the main important limitation factor under water stress conditions. It is worth noting that gm of R. delavayi was remarkably smaller than gs, even in the control plants, although the differences became smaller as the water stress intensified. This phenomenon has been widely described in woody plants, especially for sclerophyllous species (Hanba et al., 2002; Warren and Dreyer, 2006; Galmés et al., 2007), although not in all cases. Previous studies indicate that leaf anatomical characteristics have an important role in driving the photosynthesis and the potential gm (Tosens et al., 2012; Tomás et al., 2013). In particular, CO2 diffusion through the mesophyll tissues is significantly limited by the cell wall thickness and the chloroplast envelope. Besides, the chloroplast surface area exposed to intercellular air spaces per leaf area have been proposed as major determinants of differences in gm between species (Terashima et al., 2011; Tomás et al., 2013). Our previous study found that leaf anatomical characteristics of R. delavayi may have effects on gm and CO2 transfer, including traits such as smaller stomata, higher stomatal density, and a higher ratio of palisade to spongy mesophyll tissues (Cai et al., 2014). Also, other leaf anatomical characteristics might have contributed to the regulation of gm, emphasizing the need for further investigations.
After re-watering for 1 day, gm was almost complete recovery. gs showed some recovery and not fully restored to the control level after 3 days of re-watering. The recovery extent largely depended on the species, from almost null (e.g., Pistacia lentiscus) to almost complete (e.g., Limonium magallufianum) after re-watering for 1 day (Galmés et al., 2007). The limitation analysis performed for recovery data revealed that the recovery of photosynthesis was most affected by MCL rather than by SL and BL (Table 2). This result contrasts with the results of Ennahli and Earl (2005), who showed in cotton that, after severe water stress, recovery 24 h after re-watering was mostly caused by biochemical limitations, while stomatal and mesophyll limitations were almost totally absent. However, our results are in agreement with the results of Galmés et al. (2007), who showed that gm was the most important factor limiting photosynthesis recovery after severe water stress treatment, regardless of the plant growth form and leaf anatomy. To our knowledge, there are few reports showing that limited recovery of gm is the most important factor limiting photosynthesis recovery after a severe water stress. Our findings strongly reinforced the important role of gm in response of photosynthesis in R. delavayi plants under water stress and recovery, and indicate the necessity of better understanding the regulation of gm, which likely depend on the metabolism related to environmental conditions and leaf anatomy.
In conclusion, 4 days of water stress had little effect on AN, gs, and gm of R. delavayi. After 8 days of water stress treatment, a marked stomatal closure and a decrease in AN and gm were observed. After re-watering, gm recovered faster than AN and gs did. Photosynthetic limitation revealed that down-regulation of gm was the main limitation factor both under water stress and recovery.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (31460217) and by the Innovation Talents Promotion Project, Ministry of Science and Technology of China (2014HE002). The authors are grateful to Ph.D. Christina Baker Starrman for revising the language.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.01089
Table S1. Leaf relative water content (RWC) of R. delavayi.
Figure S1. The leaf adaxial surface of R. delavayi.
Figure S2. The leaf abaxial surface of R. delavayi.
Figure S3. Recovery of the R. delavayi during the experiment was repeated in 2015, the red circles indicate survival plants after re-watering. Re-watering was done when the stomatal conductance reached 0.02 mol CO2 m−2s−1 and the drought continued for 4 days.
References
Bernacchi, C. J., Portis, A. R., Nakano, H., von Caemmerer, S., and Long, S. P. (2002). Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol. 130, 1992–1998. doi: 10.1104/pp.008250
Bota, J., Medrano, H., and Flexas, J. (2004). Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 162, 671–681. doi: 10.1111/j.1469-8137.2004.01056.x
Cai, Y., Li, S., Li, S., Xie, W., and Song, J. (2014). How do leaf anatomies and photosynthesis of three Rhododendron species relate to their natural environments? Bot. Stud. 55, 36–45. doi: 10.1186/1999-3110-55-36
Campos, H., Trejo, C., Peña-Valdivia, C. B., García-Nava, R., Conde-Martínez, F. V., and Cruz-Ortega, M. R. (2014). Stomatal and non-stomatal limitations of bell pepper (Capsicum annuum L.) plants under water stress and re-watering: delayed restoration of photosynthesis during recovery. Environ. Exp. Bot. 98, 56–64. doi: 10.1016/j.envexpbot.2013.10.015
Carriquí, M., Cabrera, H. M., Conesa, M. À., Coopman, R. E., Douthe, C., Gago, J., et al. (2015). Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 38, 448–460. doi: 10.1111/pce.12402
Chastain, D. R., Snider, J. L., Collins, G. D., Perry, C. D., Whitaker, J., and Byrd, S. A. (2014). Water deficit in field-grown Gossypium hirsutum primarily limits net photosynthesis by decreasing stomatal conductance, increasing photorespiration, and increasing the ratio of dark respiration to gross photosynthesis. J. Plant Physiol. 171, 1576–1585. doi: 10.1016/j.jplph.2014.07.014
Chaves, M. M., Flexas, J., and Pinheiro, C. (2009). Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 103, 551–560. doi: 10.1093/aob/mcn125
Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P. P., Osorio, M. L., et al. (2002). How plants cope with water stress in the field? Photosynthesis and growth. Ann. Bot. 89, 907–916. doi: 10.1093/aob/mcf105
Chen, Y., Yu, J., and Huang, B. (2015). Effects of elevated CO2 concentration on water relations and photosynthetic responses to drought stress and recovery during rewatering in Tall Fescue. J. Am. Soc. Hortic. Sci. 140, 19–26. Available online at: http://journal.ashspublications.org/content/140/1/19.abstract
Cramer, G. C., Ergül, A., Grimplet, J., Tillett, R. L., Tattersall, E. R. A., Bohlman, M. C., et al. (2007). Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct. Integr. Genomic 7, 111–134. doi: 10.1007/s10142-006-0039-y
Daszkowska-Golec, A., and Szarejko, I. (2013). Open or close the gate - stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 4, 138–154. doi: 10.3389/fpls.2013.00138
de Souza, C. R., Maroco, J. P., dos Santos, T. P., Rodrigues, M. L., Lopes, C., Pereira, J. S., et al. (2005). Control of stomatal aperture and carbon uptake by deficit irrigation in two grapevine cultivars. Agric. Ecosyst. Environ. 106, 261–274. doi: 10.1016/j.agee.2004.10.014
de Souza, T. C., Magalhães, P. C., de Castro, E. M., de Albuquerque, P. E. P., and Marabesi, M. A. (2013). The influence of ABA on water relation, photosynthesis parameters, and chlorophyll fluorescence under drought conditions in two maize hybrids with contrasting drought resistance. Acta Physiol. Plant. 35, 515–527. doi: 10.1007/s11738-012-1093-9
Ennahli, S., and Earl, H. J. (2005). Physiological Limitations to Photosynthetic Carbon Assimilation in Cotton under Water Stress. Crop Sci. 45, 2374–2382. doi: 10.2135/cropsci2005.0147
Fang, M. Y., Fang, R. Z., He, M. Y., Hu, L. Z H., and Yang, H. P. (eds.). (2005). Flora of China. Beijing: Science Press.
Farquhar, G. D., von Caemmerer, S., and Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. doi: 10.1007/BF00386231
Flexas, J., Barón, M., Bota, J., Ducruet, J. M., Gallé, A., Galmés, J., et al. (2009). Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandierix V. rupestris). J. Exp. Bot. 60, 2361–2377. doi: 10.1093/jxb/erp069
Flexas, J., Bota, J., Escalona, J. M., Sampol, B., and Medrano, H. (2002). Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471. doi: 10.1071/PP01119
Flexas, J., Bota, J., Galmes, J., Medrano, H., and Ribas−Carbó, M. (2006). Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant. 127, 343–352. doi: 10.1111/j.1399-3054.2006.00621.x
Flexas, J., Carriqui, M., Coopman, R. E., Gago, J., Galmes, J., Martorell, S., et al. (2014). Stomatal and mesophyll conductances to CO2 in different plant groups: underrated factors for predicting leaf photosynthesis responses to climate change? Plant Sci. 226, 41–48. doi: 10.1016/j.plantsci.2014.06.011
Galle, A., Florez-Sarasa, I., Aououad, H. E., and Flexas, J. (2011). The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J. Exp. Bot. 62, 5207–5216. doi: 10.1093/jxb/err233
Galle, A., Florez-Sarasa, I., Tomas, M., Pou, A., Medrano, H., Ribas-Carbo, M., et al. (2009). The role of mesophyll conductance during water stress and recovery in tobacco (Nicotiana sylvestris): acclimation or limitation? J. Exp. Bot. 60, 2379–2390. doi: 10.1093/jxb/erp071
Galmés, J., Medrano, H., and Flexas, J. (2007). Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 175, 81–93. doi: 10.1111/j.1469-8137.2007.02087.x
Genty, B., Briantais, J.-M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA Gen Subjects 990, 87–92. doi: 10.1016/S0304-4165(89)80016-9
Gomes, F. P., Oliva, M. A., Mielke, M. S., de Almeida, A.-A. F., Leite, H. G., and Aquino, L. A. (2008). Photosynthetic limitations in leaves of young Brazilian green dwarf coconut (Cocos nucifera L. ‘nana’) palm under well-watered conditions or recovering from drought stress. Environ. Exp. Bot. 62, 195–204. doi: 10.1016/j.envexpbot.2007.08.006
Grassi, G., and Magnani, F. (2005). Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 28, 834–849. doi: 10.1111/j.1365-3040.2005.01333.x
Hanba, Y. T., Kogami, H., and Terashima, I. (2002). The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ. 25, 1021–1030. doi: 10.1046/j.1365-3040.2002.00881.x
Hanson, D. T., Green, L. E., and Pockman, W. T. (2013). Spatio-temporal decoupling of stomatal and mesophyll conductance induced by vein cutting in leaves of Helianthus annuus. Front Plant Sci. 4:365. doi: 10.3389/fpls.2013.00365
Harley, P. C., Loreto, F., Di Marco, G., and Sharkey, T. D. (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98, 1429–1436. doi: 10.1104/pp.98.4.1429
Laisk, A. (1977). Kinetics of Photosynthesis and Photorespiration of C3 Plants. Moscow: Nauka Press.
Lawlor, D. W., and Cornic, G. (2002). Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25, 275–294. doi: 10.1046/j.0016-8025.2001.00814.x
Lemke, P., Ren, R., and Alley, I. (2007). Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Lin, Z. H., Chen, L. S., Chen, R. B., Zhang, F. Z., Jiang, H. X., and Tang, N. (2009). CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 9:43. doi: 10.1186/1471-2229-9-43
Maroco, J. P., Rodrigues, M. L., Lopes, C., and Chaves, M. M. (2002). Limitations to leaf photosynthesis in field-grown grapevine under drought—metabolic and modelling approaches. Funct. Plant Biol. 29, 451–459. doi: 10.1071/PP01040
Massacci, A., Nabiev, S. M., Pietrosanti, L., Nematov, S. K., Chernikova, T., Thor, K., et al. (2008). Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 46, 189–195. doi: 10.1016/j.plaphy.2007.10.006
Niinemets, Ü., Díaz-Espejo, A., Flexas, J., Galmés, J., and Warren, C. R. (2009). Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. J. Exp. Bot. 60, 2271–2282. doi: 10.1093/jxb/erp063
Pastenes, C., Pimentel, P., and Lillo, J. (2005). Leaf movements and photoinhibition in relation to water stress in field-grown beans. J. Exp. Bot. 56, 425–433. doi: 10.1093/jxb/eri061
Pinheiro, C., and Chaves, M. (2011). Photosynthesis and drought: can we make metabolic connections from available data? J. Exp. Bot. 62, 869–882. doi: 10.1093/jxb/erq340
Pou, A., Flexas, J., Alsina, M., del, M., Bota, J., Carambula, C., et al. (2008). Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri x V. rupestris). Physiol. Plant. 134, 313–323. doi: 10.1111/j.1399-3054.2008.01138.x
Rancourt, G. T., Éthier, G., and Pepin, S. (2015). Greater efficiency of water use in poplar clones having a delayed response of mesophyll conductance to drought. Tree Physiol. 35, 172–184. doi: 10.1093/treephys/tpv006
Rho, H., Yu, D. J., Kim, S. J., and Lee, H. J. (2013). Limitation factors for photosynthesis in ‘Bluecrop’ highbush blueberry (Vaccinium corymbosum) leaves in response to moderate water stress. J. Plant Biol. 55, 450–457. doi: 10.1007/s12374-012-0261-1
Sharkey, T. D., Bernacchi, C. J., Farquhar, G. D., and Singsaas, E. L. (2007). Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30, 1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x
Song, Y., Ci, D., Tian, M., and Zhang, D. (2014). Comparison of the physiological effects and transcriptome responses of Populus simonii under different abiotic stresses. Plant Mol. Biol. 86, 139–156. doi: 10.1007/s11103-014-0218-5
Takahashi, S., and Murata, N. (2005). Interruption of the Calvin cycle inhibits the repair of photosystem II from photodamage. Biochim. Biophys. Acta 1708, 352–361. doi: 10.1016/j.bbabio.2005.04.003
Tang, S., Liang, H., Yan, D., Zhao, Y., Han, X., Carlson, J. E., et al. (2013). Populus euphratica: the transcriptomic response to drought stress. Plant Mol. Biol. 83, 539–557. doi: 10.1007/s11103-013-0107-3
Terashima, I., Hanba, Y. T., Tholen, D., and Niinemets, Ü. (2011). Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 155, 108–116. doi: 10.1104/pp.110.165472
Tomás, M., Flexas, J., Copolovici, L., Galmés, J., Hallik, L., Medrano, H., et al. (2013). Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J. Exp. Bot. 64, 2269–2281. doi: 10.1093/jxb/ert086
Tosens, T., Niinemets, U., Vislap, V., Eichelmann, H., and Castro Díez, P. (2012). Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ. 35, 839–856. doi: 10.1111/j.1365-3040.2011.02457.x
Tournaire-Roux, C., Sutka, M., Javot, H., Gout, E., Gerbeau, P., Luu, D. T., et al. (2003). Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425, 393–397. doi: 10.1038/nature01853
Valentini, R., Epron, D., De Angelis, P., Matteucci, G., and Dreyer, E. (1995). In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ. 18, 631–640. doi: 10.1111/j.1365-3040.1995.tb00564.x
Vincent, D., Ergul, A., Bohlman, M. C., Tattersall, E. A. R., Tillett, R. L., Wheatley, M. D., et al. (2007). Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 58, 1873–1892. doi: 10.1093/jxb/erm012
Wang, Z. X., Chen, L., Ai, J., Qin, H. Y., Liu, Y. X., Xu, P. L., et al. (2012). Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 50, 189–196. doi: 10.1007/s11099-012-0023-9
Warren, C. R., and Dreyer, E. (2006). Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. J. Exp. Bot. 57, 3057–3067. doi: 10.1093/jxb/erl067
Zhang, S., Chen, F., Peng, S., Ma, W., Korpelainen, H., and Li, C. (2010). Comparative physiological, ultrastructural and proteomic analyses reveal sexual differences in the responses of Populus cathayana under drought stress. Proteomics 10, 2661–2677. doi: 10.1002/pmic.200900650
Zhou, S., Medlyn, B., Sabaté, S., Sperlich, D., and Prentice, I. C. (2014). Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol. 34, 1035–1046. doi: 10.1093/treephys/tpu072
Keywords: mesophyll conductance, photosynthetic limitation, recovery, Rhododendron delavayi, stomatal conductance, water stress
Citation: Cai Y, Wang J, Li S, Zhang L, Peng L, Xie W and Liu F (2015) Photosynthetic Response of an Alpine Plant, Rhododendron delavayi Franch, to Water Stress and Recovery: The Role of Mesophyll Conductance. Front. Plant Sci. 6:1089. doi: 10.3389/fpls.2015.01089
Received: 20 July 2015; Accepted: 20 November 2015;
Published: 08 December 2015.
Edited by:
Richard Sayre, New Mexico Consortium at Los Alamos National Labs, USAReviewed by:
Jorge E. Mayer, Ag RD&IP Consult P/L, AustraliaHong Hu, Chinese Academy of Sciences, China
Copyright © 2015 Cai, Wang, Li, Zhang, Peng, Xie and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jihua Wang, wjh0505@gmail.com;
Feihu Liu, dmzpynu@126.com
 Yanfei Cai
Yanfei Cai Jihua Wang2,3*
Jihua Wang2,3*