- 1Integrated Molecular Plant Physiology Research, Department of Biology, University of Antwerp, Antwerp, Belgium
- 2Faculty of Science, Department of Botany, University of Beni-Suef, Beni-Suef, Egypt
- 3Centre of Excellence Plant and Vegetation Ecology, Department of Biology, University of Antwerp, Antwerp, Belgium
Elevated atmospheric CO2 can stimulate plant growth by providing additional C (fertilization effect), and is observed to mitigate abiotic stress impact. Although, the mechanisms underlying the stress mitigating effect are not yet clear, increased antioxidant defenses, have been held primarily responsible (antioxidant hypothesis). A systematic literature analysis, including “all” papers [Web of Science (WoS)-cited], addressing elevated CO2 effects on abiotic stress responses and antioxidants (105 papers), confirms the frequent occurrence of the stress mitigation effect. However, it also demonstrates that, in stress conditions, elevated CO2 is reported to increase antioxidants, only in about 22% of the observations (e.g., for polyphenols, peroxidases, superoxide dismutase, monodehydroascorbate reductase). In most observations, under stress and elevated CO2 the levels of key antioxidants and antioxidant enzymes are reported to remain unchanged (50%, e.g., ascorbate peroxidase, catalase, ascorbate), or even decreased (28%, e.g., glutathione peroxidase). Moreover, increases in antioxidants are not specific for a species group, growth facility, or stress type. It seems therefore unlikely that increased antioxidant defense is the major mechanism underlying CO2-mediated stress impact mitigation. Alternative processes, probably decreasing the oxidative challenge by reducing ROS production (e.g., photorespiration), are therefore likely to play important roles in elevated CO2 (relaxation hypothesis). Such parameters are however rarely investigated in connection with abiotic stress relief. Understanding the effect of elevated CO2 on plant growth and stress responses is imperative to understand the impact of climate changes on plant productivity.
Introduction
The changing earth's atmosphere includes a gradual increase in CO2 to possibly double the current concentration (IPCC, 2012). Such increase in primary carbon (C) source, will affect plant metabolism, growth, and development (fertilizing effect), especially under favorable water and nutrient conditions. Such effect may be transient, and differ among plant groups, particularly between C3- and C4-type photosynthesis.
This subject is extensively reviewed (e.g., Long et al., 2006; Ainsworth et al., 2008; Albert et al., 2011; Dieleman et al., 2012; Xu et al., 2013, 2015; Huang and Xu, 2015; Pandey et al., 2015; Kimball, 2016), and is commonly covered in plant physiology text books.
Less documented are the interactions of elevated CO2 with plant responses to the environment, such as in stress conditions. Nevertheless, the importance of understanding such interactions, given the high-CO2 future climate scenario's, is increasingly recognized, (e.g., Mittler and Blumwald, 2010; Feng et al., 2014; Xu et al., 2015), also in text books (e.g., see “stress matrix” in Taiz et al., 2015). One effect of elevated CO2 on plant responses, is the reduction of stress impact. This is demonstrated at the plant growth level, but also at the level of cellular oxidative damage (e.g., lipid peroxidation, protein oxidation), and at the level of stress-generated reactive oxygen species (ROS; Geissler et al., 2010; Mishra et al., 2013; Zinta et al., 2014; AbdElgawad et al., 2015).
A reasonable number of papers has reported the stress-reducing effect of elevated CO2. Nevertheless, until recently, this topic was rarely covered in reviews. Now, recent overviews of CO2 effects in plants, start dedicating attention to this effect (Feng et al., 2014; Misra and Chen, 2015; Xu et al., 2015). It is generally recognized that CO2 effects on abiotic stress impact vary considerably, and, that the underlying mechanisms remain elusive. It is clear that, in addition to providing extra C, elevated CO2 induces stomatal closing. This improves water use, protecting against drought stress, and helps to explain reduced impact of ozone stress (reduced uptake). However, reduced oxidative damage and ROS levels under elevated CO2, probably involves so called non-stomatal factors (Ghannoum, 2009), including metabolic changes. More specifically, increased C availability, possibly resulting in increased supply of defense (antioxidant) molecules, is often held primarily responsible for improved protection against oxidative damage in elevated CO2 (antioxidant hypothesis).
This conclusion is indeed supported by studies, showing increased antioxidant levels and/or antioxidant enzyme activities (Lin and Wang, 2002; Geissler et al., 2010; Pintó-Marijuan et al., 2013; Zinta et al., 2014). However, there is a considerable number of reports in which elevated CO2 had little or no effect on antioxidants, or even decreased their levels (Erice et al., 2007; Pérez-López et al., 2010; Farfan-Vignolo and Asard, 2012; Mishra et al., 2013). This indicates that the stress-mitigating effect of elevated CO2 cannot be universally attributed to increased antioxidant defenses. A key alternative process probably involved in the effect of elevated CO2 on oxidative stress, is photorespiration. Elevated CO2 promotes carboxylation over oxygenation at rubisco, reducing reactive oxygen species (ROS) formation (relaxation hypothesis; Long and Drake, 1991; Booker et al., 1997; Ainsworth et al., 2008; Zinta et al., 2014; AbdElgawad et al., 2015). Moreover, when measured simultaneously, reduced photorespiration correlates well with the decrease in H2O2 and lower oxidative damage levels under high CO2 in some studies (Aranjuelo et al., 2008; Mishra et al., 2013). Therefore, whether elevated CO2 reduces stress impact through “increased defense or decreased challenge,” remains unaddressed (e.g., Tausz-Posch et al., 2013; Xu et al., 2015).
A Systematic Literature Analysis
To gain insight in this issue, we performed a systematic literature analysis, using an “as complete as possible” collection of studies (Web of Science-indexed), meeting the following criteria; (1) analyzing oxidative stress markers (electrolyte leakage, protein oxidation, lipid peroxidation) and antioxidants (molecules and enzymes), (2) in plant shoots grown under ambient and elevated CO2, in presence and absence of abiotic stress. The selection of papers was based on a search using the keyword combination “elevated CO2” AND antioxidants OR oxidative stress AND plants, resulting in 238 hits (December 2015). From the combined lists, double references were removed; manuscripts were removed because measurements were not performed on shoots; because they were reviews; or because one of the keywords (elevated CO2, antioxidants, oxidative stress) was in fact not studied in detail but occurred only in the extended keyword list. Eventually, 105 papers were analyzed (details in Supplementary Table 1 and Datasheet 1).
To perform the analysis in a quantitative manner, we scored the number of occurrences (observations) in which a given parameter (e.g., lipid peroxidation, ascorbate level, APX activity,…), significantly increased (+), remained unchanged (=), or decreased (−). This number of observations (+ or = or −), is expressed relative, as a fraction (%) of the total number (sum = n) of observations on that parameter. As not all 105 studies report on all parameters, the number of observations is often lower than 105. On the other hand, often papers report on changes in one particular parameter, in multiple measurements and/or conditions, or development stages, with and without elevated CO2. Therefore, the number of observations can be considerably higher than 105 (see figures).
Changes (+/ = /−) were recorded for three plant treatments: effect of: (1) elevated CO2 relative to ambient CO2 (labeled “C” in heat maps); (2) stress exposure in ambient CO2 (relative to non-stressed, “S”); (3) stress exposure in elevated CO2 (relative to stress exposure in ambient CO2, “CS”). These nine sets of observations (+/ = /− for each C/S/CS), are presented in 3 × 3 heat-map format (vertically treatments: C, S, CS; horizontally: +, =, −). Statistical significance of the observations was taken as reported by the authors of the original papers.
The following, commonly quantified parameters were included; (1) cell damage: lipid peroxidation (malondialdehyde, MDA), protein oxidation (carbonylation), electrolyte leakage; (2) molecular and enzymatic antioxidants: phenolics (PHEN), ascorbate (ASC), glutathione (GSH), tocopherol (TOH), superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione peroxidase (GPX), glutathione reductase (GR); (3) reactive oxygen species (ROS): hydrogen peroxide (H2O2), and superoxide ().
To approximate changes in “overall (total) oxidative damage,” and “overall (total) antioxidants,” we calculated the mathematical sum of the number of observations (either +, =, or −) of all oxidative damage parameters (lipid peroxidation + protein oxidation + electrolyte leakage), and the sum of the number of changes, in all antioxidant parameters (PHEN + ASC + GSH + TOH + SOD + CAT + POX + APX + MDHAR + DHAR + GPX + GR), respectively. To evaluate if elevated CO2 effects on antioxidants were specific for certain species groups, growth facility or stress type, we categorized the observations in, (a) C3/C4 metabolism, (b) species groups [grasses (Poaceae), legumes (Fabaceae), trees], (c) growth facility [growth chamber, green house, open top chambers (OTC), Free Air Concentration Enrichment (FACE)]; and (d) stress type (heavy metals, drought, high temperature, ozone, salinity).
Elevated CO2 Mitigates Oxidative Stress in Plants
First we addressed the question whether changes in “overall oxidative damage,” induced by C, S, and CS, correlate with observations of “overall changes in antioxidants.” Such correlation could point to a causal link, e.g., increased antioxidant defenses being responsible for reduced stress impact in elevated CO2 (antioxidant hypothesis). Obviously, estimating overall levels is a rather crude approach, and does not eliminate the possibility of elevated CO2 specifically affecting one or more antioxidant components (analyzed below).
Changes in overall oxidative damage (Figure 1A), largely confirm previous knowledge. First, elevated CO2 alone is not often reported to cause cell damage (17% “+,”lane “C”), it rather leaves damage levels unchanged (60% “ = ”), or, in some instances, decreased (23% “−”). Second, abiotic stress increases cell damage indicators in most observations (55% “+,”lane “S”). Third, elevated CO2 is frequently reported to reduce stress impact (47% “−,”lane “CS,” also compare “S”and “CS”in “+”row). This pattern of changes in C, S and CS, correlates very well with the changes in ROS levels (mostly H2O2; Figure 1A, panel “ROS”). Elevated CO2alone (C) was not often observed to increase ROS levels (24% “+”), but rather did not affect (55% “ = ”), or decrease ROS (21% “−”). Stress increased ROS levels at ambient CO2 (67% “+”). The correlation between changes in oxidative damage and ROS levels, is perhaps not very surprising, and probably indicates that cell damage under stress is primarily caused by oxidative effects.
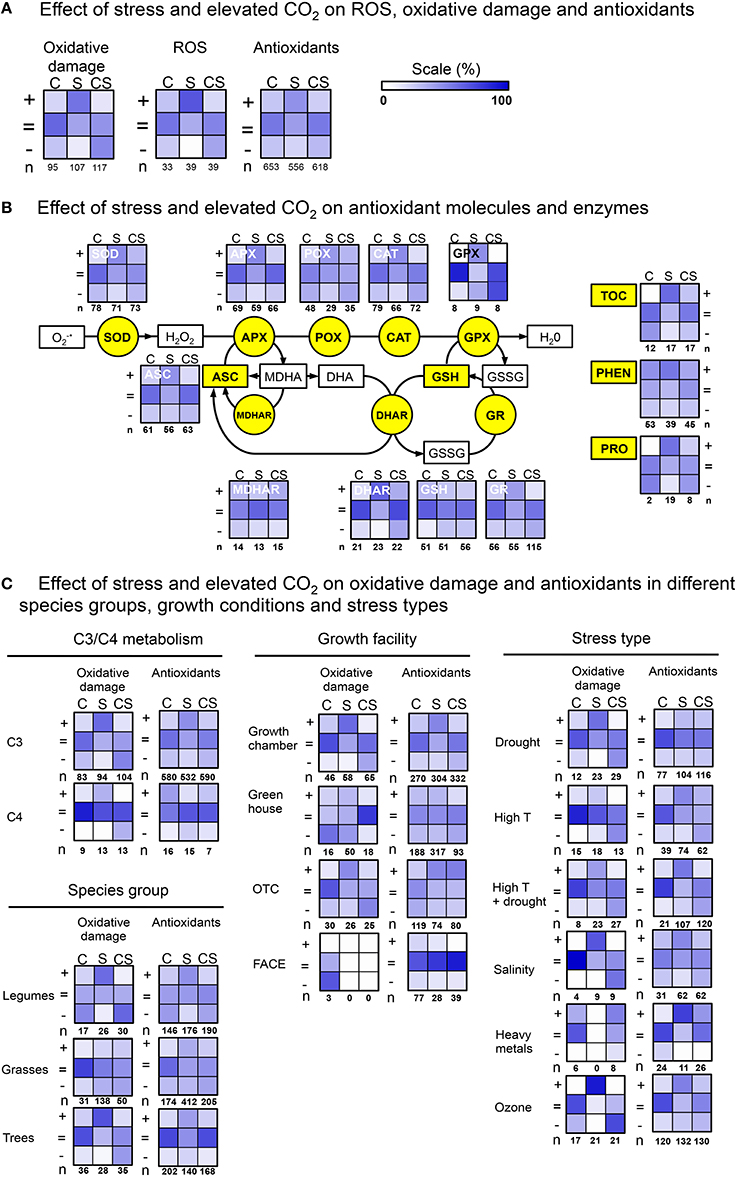
Figure 1. Heat maps showing the relative numbers of observations (%), demonstrating the effect (increase “+, ”no change “ =, ” decrease “−”) of elevated CO2 (C), stress (S), and their combination (CS), on oxidative damage and antioxidants. (A) Comparing overall effects on oxidative damage, ROS and antioxidants. (B) Effects individual antioxidant components. (C) Effects categorized by metabolism type, species group, growth conditions and stress type.
However, changes induced by C, S, and CS in “overall antioxidants” (Figure 1A) are different from changes in oxidative damage and ROS. In particular, stress induces increases in antioxidants less frequent (43%, “+”) than increases in oxidative damage (55% “+”) or ROS (67% “+”). And, antioxidants were more often reported to not change (50% “ = ”), then this is the case for oxidative damage (36% “ = ”) and ROS (33% “ = ”) changes. Therefore, it appears questionable whether increases in antioxidants can be considered a likely general cause of lowered ROS and oxidative damage in elevated CO2.
Effect of Elevated CO2 on Individual Antioxidant Components
It is conceivable that elevated CO2 specifically increases particular antioxidants. Such information might be lost in the somewhat “crude” summation of all observations (as done above). We therefore zoomed-in on individual molecules and enzymes from various antioxidant defense pathways (Figure 1B). This analysis confirms reported increases of the activities of many antioxidant components under abiotic stress (lanes S). Increases were particularly frequently observed for PHEN (54% “+”), DHAR (61% “+”), and TOC (59% “+”). However, for all antioxidant components there is a considerable number of observations indicating no changes (from 22 to 62% “ = ”).
In elevated atmospheric CO2 during stress exposure, the levels and activities of nearly all molecular and enzymatic antioxidants (except GPX) have been reported to increase, but with variable and often low (10–40%) frequencies. Increases were reported particularly frequently for PHEN (38%), POX (26%), SOD (29%), and MDHAR (40%). None of all antioxidant components accounts solely, throughout all studies, for increased antioxidant defenses in high CO2. The frequently observed increases of DHAR and MDHAR, together with ASC and GSH levels reported to remain largely unchanged (56 and 54% “ = ”), may indicate that the ASC/GSH-cycle is often involved in the antioxidant response in elevated CO2. However, MDHAR/DHAR activities are reported in only 12 of the 105 papers, and further verification is therefore necessary.
Are Antioxidants Specifically Responsive to Elevated CO2 in Particular Species or Growth Conditions?
We next addressed the question whether increases in antioxidant capacity in elevated CO2 were possibly specific for metabolism type (C3 vs. C4), species-group, stress type or growth facilities (details in Supplementary Table 1). For example, one could hypothesize that in C4 plants, extra CO2 is more likely to alleviate stress impact through increased antioxidant capacity, then through suppression of photorespiration. Our dataset contains 54 species with C3, and 8 with C4 metabolism. Heat maps summarizing the observations on oxidative damage and antioxidant changes (Figure 1C), show considerable reduction of stress impact by elevated CO2 and increases in antioxidants for C3 as well as C4 plants.
A large number (95%) of all species in which abiotic stress, elevated CO2 and antioxidant changes are studied, are either legumes (10), grasses (18), or trees (22) (Supplementary Table 1). Oxidative damage as a result of abiotic stress, was considerably more frequently observed in the legumes (58%) and trees (68%), compared to grasses (17%) (Figure 1C). Also the reduction in oxidative damage by elevated CO2, was more frequently reported in legumes (47%), than in grasses (16%) and trees (11%). It is not immediately clear what the basis is for this difference in responsiveness. Increases in antioxidants are reported equally frequent in each species group.
Another factor that could explain some inconsistency in reports on antioxidant increases in elevated CO2, is the variation in growth facility. Elevated CO2 effects on growth seem less pronounced in FACE experiments, than in growth cabinet-experiments (Ainsworth et al., 2008). On the other hand, in field conditions (e.g., OTC, FACE) stress is often more severe and prolonged (Mittler and Blumwald, 2010). The literature analysis shows that reduction of stress impact on cell damage, and increases in antioxidants, are reported in all facilities (Figure 1C, insufficient data for FACE).
Finally, we also sorted the observations by stress type (Figure 1C). It is apparent that the stress mitigating effect on oxidative damage occurs for all abiotic stresses, with the notable exception of heavy metal stress. However, the effect of elevated CO2 on heavy metal stress is only reported in 4/105 papers, and needs further confirmation.
Increases in antioxidants are reported for all stresses, and are therefore not stress-type specific. Interestingly, reported increases in antioxidants for drought and ozone stress, suggest that in addition to stomatal closure by elevated CO2, other processes contribute to the reduction of drought and ozone impact. The data sorted by stress type, also illustrate that in the majority of the reports, that whereas antioxidant activities generally increase in response to the stress (S), they do not increase further or even decrease when the stress is combined with elevated CO2 (CS).
Conclusions
The question whether mitigation of stress impact by elevated CO2 occurs through “increased defense or decreased challenge” is probably best answered by “both.” Clearly increased defenses have been demonstrated, but only in a minority of the reports, and, this effect is not specific for any particular antioxidant, C3, or C4 metabolism, for a particular species group, growth facility-type or stress type. This suggests that decreased challenge also plays an important role in stress mitigation in elevated CO2. The primary candidate process for this, is reduced hydrogen peroxide production by elevated CO2 in photorespiration. Effects of elevated CO2 on photorespiration in stress conditions, have also been reported, and point to effects on photosynthesis (Booker et al., 1997; Aranjuelo et al., 2008; Pérez-López et al., 2012; AbdElgawad et al., 2015). However, despite the possibly important role of photorespiration in stress responses under future climate conditions, these aspects are rarely investigated simultaneously (only 4 of 105 papers investigated photorespiration changes). It is therefore of great interest to further unravel the role of antioxidants and photorespiration in elevated CO2 effects. Moreover, the causal role of antioxidant increases to reduce oxidative damage and ROS under elevated CO2, is almost exclusively inferred from “correlative changes.” This conclusion is not, yet, supported by studies performing plant manipulations, e.g., use of mutants, overexpressor lines, or pharmacological treatments. Engineered plants with elevated antioxidant enzymes, sometimes show increased stress resistance (Eltayeb et al., 2007; Lee et al., 2007; Avramova et al., 2015). However, these lines have not been tested under elevated CO2. Recently, a mutant screening assay was developed employing the effect of elevated CO2 on hydrogen peroxide production (Queval et al., 2012), which underlines the relevance of understanding photorespiration in altered CO2 conditions.
However, also in C4 plants, in which photorespiration is not very active, elevated CO2 reduces ROS levels and oxidative damage, without changes in antioxidants. This suggests that other non-stomatal processes, apart from antioxidant defenses and photorespiration, contribute to stress mitigation. Stress induces ROS production at various cellular sites (e.g., Foyer et al., 1994; Schwanz et al., 1996; Gill and Tuteja, 2010; Miller et al., 2010; Das and Roychoudhury, 2014), which each can be affected by elevated CO2 (Figure 2). Apart from changes in antioxidants and photorespiration (described above), some evidence indicates that elevated CO2 reduces mitochondrial and chloroplast ROS formation, and NADPH oxidase activity, but, little is known at the level of β-oxidation or other cellular oxidases (Gonzalez-Meler et al., 1996; Booker et al., 1997; Gonzàlez-Meler and Siedow, 1999; Lin and Wang, 2002). Understanding the effect of elevated CO2 in plant stress responses is further complicated by the fact that additional C affects multiple metabolic processes, as demonstrated by non-targeted (omics) approaches (e.g., Sicher and Barnaby, 2012; Xu et al., 2014; Zinta et al., 2014; AbdElgawad et al., 2015; Misra and Chen, 2015).
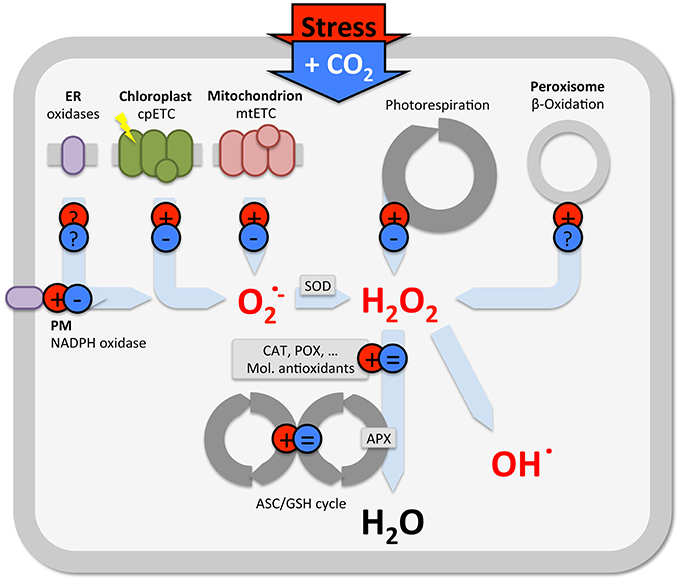
Figure 2. Schematic view summarizing the predominant effect of stress (red) and elevated CO2 (blue) on ROS production in various subcellular locations. Note, there is considerable variety in the outcome of studies on antioxidants (see text), only the predominant effects are indicated in this generalization.
An explanation for the relatively large variety in antioxidant responses to elevated CO2, is that these responses possibly occur relatively far downstream from the CO2 primary targets. As a result, changes in antioxidants may not directly correlate to the stress and CO2 treatment only, but are an integrated response of changes in various metabolic processes. The primary targets for CO2, i.e., where cellular “perception” of altered CO2 levels first occur, are probably changes in stomatal opening, suppression of photorespiration and increased levels of carbohydrates through increased C fixation. Changes in antioxidants are probably an integrated downstream overall result from changes in these processes. It therefore appears that an important topic, as the response of plants to adverse growth conditions, in future climate-levels CO2, is underexplored, and leaves many unanswered questions (also Xu et al., 2015).
Finally, with regard to textbook knowledge, our analysis indicates that positive interactions of CO2 have been shown for salinity and heat stress. We therefore suggest to update the very instructive Mittler and Blumwald “stress matrix” (Mittler and Blumwald, 2010; Taiz et al., 2015) accordingly.
Author Contributions
HAb: Literature analysis and review writing. GZ: Literature analysis and review writing. GB: Review writing. IJ: Review writing. HA: Literature analysis and review writing.
Funding
This work was supported by the Research Council of the University of Antwerp as concerted research project (GOA-BOF-UA-2007), and Flemish Science Foundation as concerted research project (FWO, G0D0514N). GZ acknowledges support from Methusalem Funding to the Centre of Excellence “PLECO,” University of Antwerp.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.00556
Supplementary Table 1 and Datasheet 1. Overview of the papers used in this study.
References
AbdElgawad, H., Farfan-Vignolo, E. R., De Vos, D., and Asard, H. (2015). Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 231, 1–10. doi: 10.1016/j.plantsci.2014.11.001
Ainsworth, E. A., Beier, C., Calfapietra, C., Ceulemans, R., Durand-Tardif, M., Farquhar, G. D., et al. (2008). Next generation of elevated CO2 experiments with crops: a critical investment for feeding the future world. Plant Cell Environ. 31, 1317–1324. doi: 10.1111/j.1365-3040.2008.01841.x
Albert, K. R., Mikkelsen, T. N., Michelsen, A., Ro-Poulsen, H., and Van Der Linden, L. (2011). Interactive effects of drought, elevated CO2 and warming on photosynthetic capacity and photosystem performance in temperate heath plants. J. Plant Physiol. 168, 1550–1561. doi: 10.1016/j.jplph.2011.02.011
Aranjuelo, I., Erice, G., Nogués, S., Morales, F., Irigoyen, J. J., and Sánchez-Díaz, M. (2008). The mechanism (s) involved in the photoprotection of PSII at elevated CO2 in nodulated alfalfa plants. Environ. Exp. Bot. 64, 295–306. doi: 10.1016/j.envexpbot.2008.01.002
Avramova, V., Abdelgawad, H., Zhang, Z., Fotschki, B., Casadevall, R., Vergauwen, L., et al. (2015). Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiol. 169, 1382–1396. doi: 10.1104/pp.15.00276
Booker, F. L., Reid, C. D., Brunschön-Harti, S., Fiscus, E. L., and Miller, J. E. (1997). Photosynthesis and photorespiration in soybean [Glycine max (L.) Merr.] chronically exposed to elevated carbon dioxide and ozone. J. Exp. Bot. 48, 1843–1852. doi: 10.1093/jxb/48.10.1843
Das, K., and Roychoudhury, A. (2014). Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2:53. doi: 10.3389/fenvs.2014.00053
Dieleman, W. I., Vicca, S., Dijkstra, F. A., Hagedorn, F., Hovenden, M. J., Larsen, K. S., et al. (2012). Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Glob. Chang. Biol. 18, 2681–2693. doi: 10.1111/j.1365-2486.2012.02745.x
Eltayeb, A. E., Kawano, N., Badawi, G. H., Kaminaka, H., Sanekata, T., Shibahara, T., et al. (2007). Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225, 1255–1264. doi: 10.1007/s00425-006-0417-7
Erice, G., Irigoyen, J. J., Sánchez-Díaz, M., Avice, J.-C., and Ourry, A. (2007). Effect of drought, elevated CO2 and temperature on accumulation of N and vegetative storage proteins (VSP) in taproot of nodulated alfalfa before and after cutting. Plant Sci. 172, 903–912. doi: 10.1016/j.plantsci.2006.12.013
Farfan-Vignolo, E. R., and Asard, H. (2012). Effect of elevated CO2 and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 59, 55–62. doi: 10.1016/j.plaphy.2012.06.014
Feng, G.-Q., Li, Y., and Cheng, Z.-M. (2014). Plant molecular and genomic responses to stresses in projected future CO2 environment. CRC Crit. Rev. Plant Sci. 33, 238–249. doi: 10.1080/07352689.2014.870421
Foyer, C. H., Lelandais, M., and Kunert, K. J. (1994). Photooxidative stress in plants. Physiol. Plant. 92, 696–717. doi: 10.1111/j.1399-3054.1994.tb03042.x
Geissler, N., Hussin, S., and Koyro, H.-W. (2010). Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta 231, 583–594. doi: 10.1007/s00425-009-1064-6
Ghannoum, O. (2009). C4 photosynthesis and water stress. Ann. Bot. 103, 635–644. doi: 10.1093/aob/mcn093
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Gonzalez-Meler, M. A., Ribas-Carbó, M., Siedow, J. N., and Drake, B. G. (1996). Direct inhibition of plant mitochondrial respiration by elevated CO2. Plant Physiol. 112, 1349–1355.
Gonzàlez-Meler, M. A., and Siedow, J. N. (1999). Direct inhibition of mitochondrial respiratory enzymes by elevated CO2: does it matter at the tissue or whole-plant level? Tree Physiol. 19, 253–259. doi: 10.1093/treephys/19.4-5.253
Huang, B., and Xu, Y. (2015). Cellular and molecular mechanisms for elevated CO2 regulation of plant growth and stress adaptation. Crop Sci. 55, 1405. doi: 10.2135/cropsci2014.07.0508
IPCC (2012). Summary for policymakers, in Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation A Special Report of Working Groups I and II of the Intergovernmental Panel On Climate Change, eds C. B. Field, V. Barros, T. F. Stocker, D. Qin, D. J. Dokken, K. L. Ebi, M. D. Mastrandrea, K. J. Mach, G.-K. Plattner, S. K. Allen, M. Tignor, and P. M. Midgley (Cambridge, UK; New York, NY: Cambridge University Press), 1–19.
Kimball, B. A. (2016). Crop responses to elevated CO2 and interactions with H2O, N, and temperature. Curr. Opin. Plant Biol. 31, 36–43. doi: 10.1016/j.pbi.2016.03.006
Lee, Y.-P., Kim, S.-H., Bang, J.-W., Lee, H.-S., Kwak, S.-S., and Kwon, S.-Y. (2007). Enhanced tolerance to oxidative stress in transgenic tobacco plants expressing three antioxidant enzymes in chloroplasts. Plant Cell Rep. 26, 591–598. doi: 10.1007/s00299-006-0253-z
Lin, J.-S., and Wang, G.-X. (2002). Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci. 163, 627–637. doi: 10.1016/S0168-9452(02)00173-5
Long, S. P., Ainsworth, E. A., Leakey, A. D., Nösberger, J., and Ort, D. R. (2006). Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312, 1918–1921. doi: 10.1126/science.1114722
Long, S. P., and Drake, B. G. (1991). Effect of the long-term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scirpus olneyi. Plant Physiol. 96, 221–226. doi: 10.1104/pp.96.1.221
Miller, G., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
Mishra, A. K., Rai, R., and Agrawal, S. (2013). Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J. Biochem. Biophys. 50, 139–149.
Misra, B. B., and Chen, S. (2015). Advances in understanding CO2 responsive plant metabolomes in the era of climate change. Metabolomics 11, 1478–1491. doi: 10.1007/s11306-015-0825-4
Mittler, R., and Blumwald, E. (2010). Genetic engineering for modern agriculture: challenges and perspectives. Annu. Rev. Plant Biol. 61, 443–462. doi: 10.1146/annurev-arplant-042809-112116
Pandey, P., Zinta, G., AbdElgawad, H., Ahmad, A., Jain, V., and Janssens, I. A (2015). Physiological and molecular alterations in plants exposed to high CO2 under phosphorus stress. Biotechnol. Adv. 33, 303–316. doi: 10.1016/j.biotechadv.2015.03.011
Pérez-López, U., Robredo, A., Lacuesta, M., Sgherri, C., Mena-Petite, A., Navari-Izzo, F., et al. (2010). Lipoic acid and redox status in barley plants subjected to salinity and elevated CO2. Physiol. Plant. 139, 256–268. doi: 10.1111/j.1399-3054.2010.01361.x
Pérez-López, U., Robredo, A., Lacuesta, M., Mena-Petite, A., and Muñoz-Rueda, A. (2012). Elevated CO2 reduces stomatal and metabolic limitations on photosynthesis caused by salinity in hordeum vulgare. Photosyn. Res. 111, 269–283. doi: 10.1007/s11120-012-9721-1
Pintó-Marijuan, M., Joffre, R., Casals, I., De Agazio, M., Zacchini, M., García-Plazaola, J. I., et al. (2013). Antioxidant and photoprotective responses to elevated CO2 and heat stress during holm oak regeneration by resprouting, evaluated with NIRS (near-infrared reflectance spectroscopy). Plant Biol. 15, 5–17. doi: 10.1111/j.1438-8677.2011.00538.x
Queval, G., Neukermans, J., Vanderauwera, S., Van Breusegem, F., and Noctor, G. (2012). Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ. 35, 374–387. doi: 10.1111/j.1365-3040.2011.02368.x
Schwanz, P., Picon, C., Vivin, P., Dreyer, E., Guehl, J.-M., and Polle, A. (1996). Responses of antioxidative systems to drought stress in pendunculate oak and maritime pine as modulated by elevated CO2. Plant Physiol. 110, 393–402.
Sicher, R. C., and Barnaby, J. Y. (2012). Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiol. Plant 144, 238–253. doi: 10.1111/j.1399-3054.2011.01555.x
Taiz, L., Zeiger, E., Møller, I. M., and Murphy, A. (2015). Plant Physiology and Development, 6th Edn. Sunderland, MA: Sinauer Associates.
Tausz-Posch, S., Borowiak, K., Dempsey, R. W., Norton, R. M., Seneweera, S., Fitzgerald, G. J., et al. (2013). The effect of elevated CO2 on photochemistry and antioxidative defence capacity in wheat depends on environmental growing conditions–A FACE study. Environ. Exp. Bot. 88, 81–92. doi: 10.1016/j.envexpbot.2011.12.002
Xu, Z., Jiang, Y., and Zhou, G. (2015). Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 6:701. doi: 10.3389/fpls.2015.00701
Xu, Z., Shimizu, H., Ito, S., Yagasaki, Y., Zou, C., Zhou, G., et al. (2014). Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239, 421–435. doi: 10.1007/s00425-013-1987-9
Xu, Z., Shimizu, H., Yagasaki, Y., Ito, S., Zheng, Y., and Zhou, G. (2013). Interactive effects of elevated CO2, drought, and warming on plants. J. Plant Growth Regul. 32, 692–707. doi: 10.1007/s00344-013-9337-5
Zinta, G., Abdelgawad, H., Domagalska, M. A., Vergauwen, L., Knapen, D., Nijs, I., et al. (2014). Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 20, 3670–3685. doi: 10.1111/gcb.12626
Keywords: abiotic stress, reactive oxygen species, oxidative damage, antioxidants, future climate, elevated CO2, stress mitigation, photorespiration
Citation: AbdElgawad H, Zinta G, Beemster GTS, Janssens IA and Asard H (2016) Future Climate CO2 Levels Mitigate Stress Impact on Plants: Increased Defense or Decreased Challenge? Front. Plant Sci. 7:556. doi: 10.3389/fpls.2016.00556
Received: 15 February 2016; Accepted: 11 April 2016;
Published: 02 May 2016.
Edited by:
Mohammad Anwar Hossain, Bangladesh Agricultural University, BangladeshReviewed by:
Devesh Shukla, Western Kentucky University, USAUsue Pérez-López, University of the Basque Country, Spain
Copyright © 2016 AbdElgawad, Zinta, Beemster, Janssens and Asard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamada AbdElgawad, Hamada.AbdElgawad@uantwerpen.be
†Present Address: Gaurav Zinta, Shanghai Center for Plant Stress Biology, Shanghai Institute for Biological Sciences, Chinese Academy of Sciences, Shanghai, China
‡These authors have contributed equally to this work.
 Hamada AbdElgawad
Hamada AbdElgawad Gaurav Zinta
Gaurav Zinta Gerrit T. S. Beemster
Gerrit T. S. Beemster Ivan A. Janssens
Ivan A. Janssens Han Asard
Han Asard