- 1Department of Biological Sciences, Dartmouth College, Hanover, NH, USA
- 2Department of Agricultural Biotechnology, National Academy of Agricultural Science, Rural Development Administration, Jeonju-si, South Korea
Plant development is exquisitely sensitive to the environment. Light quantity, quality, and duration (photoperiod) have profound effects on vegetative morphology and flowering time. Recent studies have demonstrated that ambient temperature is a similarly potent stimulus influencing morphology and flowering. In Arabidopsis, ambient temperatures that are high, but not so high as to induce a heat stress response, confer morphological changes that resemble the shade avoidance syndrome. Similarly, these high but not stressful temperatures can accelerate flowering under short day conditions as effectively as exposure to long days. Photoperiodic flowering entails a series of external coincidences, in which environmental cycles of light and dark must coincide with an internal cycle in gene expression established by the endogenous circadian clock. It is evident that a similar model of external coincidence applies to the effects of elevated ambient temperature on both vegetative morphology and the vegetative to reproductive transition. Further study is imperative, because global warming is predicted to have major effects on the performance and distribution of wild species and strong adverse effects on crop yields. It is critical to understand temperature perception and response at a mechanistic level and to integrate this knowledge with our understanding of other environmental responses, including biotic and abiotic stresses, in order to improve crop production sufficiently to sustainably feed an expanding world population.
Introduction
Plant development is highly sensitive to the environment. For example, light dramatically affects plant morphology (Arsovski et al., 2012). When grown in the dark, dicot seedlings become etiolated, develop elongated hypocotyls, and are pale because chloroplast formation and chlorophyll biosynthesis requires exposure to light. In contrast, seedlings grown in the light undergo photomorphogenesis, exhibiting short embryonic stems and expanded green cotyledons. Light quality also has a profound influence on plant morphology. Shading, in which the ratio of red to far-red light is decreased, induces a suite of morphological changes that includes the elongation of hypocotyls and petioles and upward (hyponastic) growth of the petioles and leaves to yield an open rosette (Casal, 2012). In addition, the relative duration of light and dark during the day, photoperiod, has a major influence on the transition to flowering (Song et al., 2015).
Plant morphology and reproductive development are also strongly influenced by temperature (Wigge, 2013; Quint et al., 2016). Ambient temperatures that are high, yet insufficient to cause heat stress, induce a suite of morphological changes that are collectively termed thermomorphogenesis. In Arabidopsis, growth at 27°C results in elongated hypocotyls and petioles and other morphological changes that are reminiscent of the response to shade. In addition, elevated temperature accelerates flowering, especially in short days that are non-inductive in Arabidopsis grown at lower ambient temperatures (e.g., 15–20°C).
In this mini review, we will consider the similarities and differences in the thermoresponsiveness of growth and flowering, with an emphasis on Arabidopsis, where our mechanistic understanding is greatest.
Thermomorphogenesis
One of the first manifestations of thermomorphogenesis, the suite of responses in growth to elevated temperature, is increased elongation of the hypocotyl. The similarity of thermomorphogenesis to responses to shading suggested common underlying mechanisms. PHYTOCHROME INTERACTING FACTOR4 (PIF4) and PIF5, basic helix-loop-helix (bHLH) transcription factors that are key components of phytochrome signaling with central roles in photomorphogenesis (Leivar and Monte, 2014), also play pivotal roles in thermomorphogenesis (Wigge, 2013; Quint et al., 2016). Loss of PIF4 function attenuates hypocotyl elongation at elevated temperature (Koini et al., 2009). Similarly, mutants that disrupt auxin signaling block thermoresponsive hypocotyl elongation (Gray et al., 1998). PIF4 interacts with BRASSINAZOLE-RESISTANT1 to regulate many genes associated with growth regulation (Oh et al., 2012), integrating multiple hormone (auxin, brassinolide, gibberellin, and cytokinin) signaling pathways in the growth response.
Expression of PIF4 and PIF5 is tightly regulated at both transcriptional and post-transcriptional levels. PIF4 and PIF5 transcription and mRNA accumulation are regulated by the circadian clock (Nozue et al., 2007; Niwa et al., 2009; Kunihiro et al., 2011; Nusinow et al., 2011). During the light and early evening, PIF4 transcription is repressed by ELONGATED HYPOCOTYL5 (HY5) and the evening complex (EC) as well as by additional transcriptional repressors (Lee et al., 2007; Toledo-Ortiz et al., 2014; Quint et al., 2016). Photoperiod affects PIF4 expression, with transcripts accumulating during the night in short days but only at about dawn in long days. This permits increased PIF4 accumulation and greater hypocotyl elongation in short days due to the greater stability and activity of PIF4 in the dark (Quint et al., 2016). DE-ETIOLATED1 (DET1) plays a role in this stabilization of PIF4 (Dong et al., 2014; Shi et al., 2015). Although DET1 has not been shown to directly contribute to PIF4 accumulation at elevated temperatures, det1 mutants are impaired in thermoresponsive hypocotyl growth (Delker et al., 2014). Thus, PIF4 plays a central role in integrating photoperiodic and circadian clock control of hormone signaling into the growth response through the external coincidence of clock and photoperiod regulated PIF4 expression with environmentally imposed dark (Nomoto et al., 2012b). Similarly, in long days at elevated temperature PIF4 accumulates earlier in the dark, again providing an example of external coincidence of clock-, photoperiod-, and temperature-regulated PIF4 expression with environmentally imposed dark (Nomoto et al., 2012a).
Quantitative trait locus (QTL) mapping with Arabidopsis natural accessions revealed variation in thermoresponsive hypocotyl growth and implicated the EC components, EARLY FLOWERING3 (ELF3) and LUX ARRHYTHMO (LUX), as well as PHYB. The elf3-1 loss of function mutant exhibits enhanced growth under control temperatures and does not increase growth at high temperature, coinciding with elevated PIF4 levels under both conditions. These mutants also lose the high temperature induction of LUX expression suggesting that ELF3 is required for this rapid thermoresponsiveness (Box et al., 2015). Natural allelic variation in ELF3 also alters the hypocotyl elongation response to shading; QTL mapping in an Arabidopsis Bay-0 x Sha recombinant inbred line (RIL) population revealed that the Bay-0 ELF3 allele confers longer period and greater response to shade than the Sha allele (Jiménez-Gómez et al., 2010; Coluccio et al., 2011). Loss of ELF3 also disrupts rhythmic root growth rates under diurnal and free running conditions (Yazdanbakhsh et al., 2011).
Flowering Time in Arabidopsis
Arabidopsis has at least four flowering pathways: autonomous, vernalization, gibberellic acid (GA), and photoperiodic (Simpson and Dean, 2002; Amasino and Michaels, 2010). Recent evidence strongly supports a fifth, thermoresponsive, pathway (Capovilla et al., 2015). The autonomous pathway induces flowering in an environmentally (temperature and photoperiod) insensitive fashion. However, flowering is sensitive to environmental conditions, particularly to photoperiod and temperature. In Arabidopsis, a facultative long day plant, flowering is accelerated both in long days and at elevated temperatures. In addition, many accessions require vernalization, an extended period of cold temperature that mimics winter, in order to flower.
Vernalization
Much is known about vernalization in Arabidopsis (Kim et al., 2009; Sheldon et al., 2009; Song et al., 2012; Berry and Dean, 2015; Hepworth and Dean, 2015). Two critical components include FRIGIDA (FRI), an inducer of the flowering repressor, FLOWERING LOCUS C (FLC). FLC complexes with SHORT VEGETATIVE PHASE (SVP) to form a potent transcriptional repressor of floral inducers, including FLOWERING LOCUS T (FT), FD, and SUPPRESSOR OF CONSTANS 1 (SOC1) (Figure 1) (Amasino, 2010). The expression of FLC, which encodes a MADS domain transcriptional repressor, is progressively downregulated in response to chromatin changes resulting from prolonged (weeks to months) cold. Loss of function of either FRI or FLC eliminates the vernalization requirement, permitting accelerated flowering and a summer annual lifecycle, whereas accessions with functional FRI and FLC genes have a vernalization requirement and a winter annual habit (Gazzani et al., 2003; Song et al., 2012). A second transcriptional repressor closely related to FLC, MADS AFFECTING FLOWERING 2 (MAF2), is more slowly downregulated in response to vernalization than is FLC, and prevents premature vernalization in response to brief cold spells, although loss of MAF2 function does not eliminate the vernalization requirement (Ratcliffe et al., 2003). Like FLC, MAF2 also interacts with SVP; multiple tetrameric complexes, such as FLC-SVP-MAF3-MAF4 and SVP-FLM (FLOWERING LOCUS M)-MAF2-MAF4 have been postulated (Gu et al., 2013b; Airoldi et al., 2015).
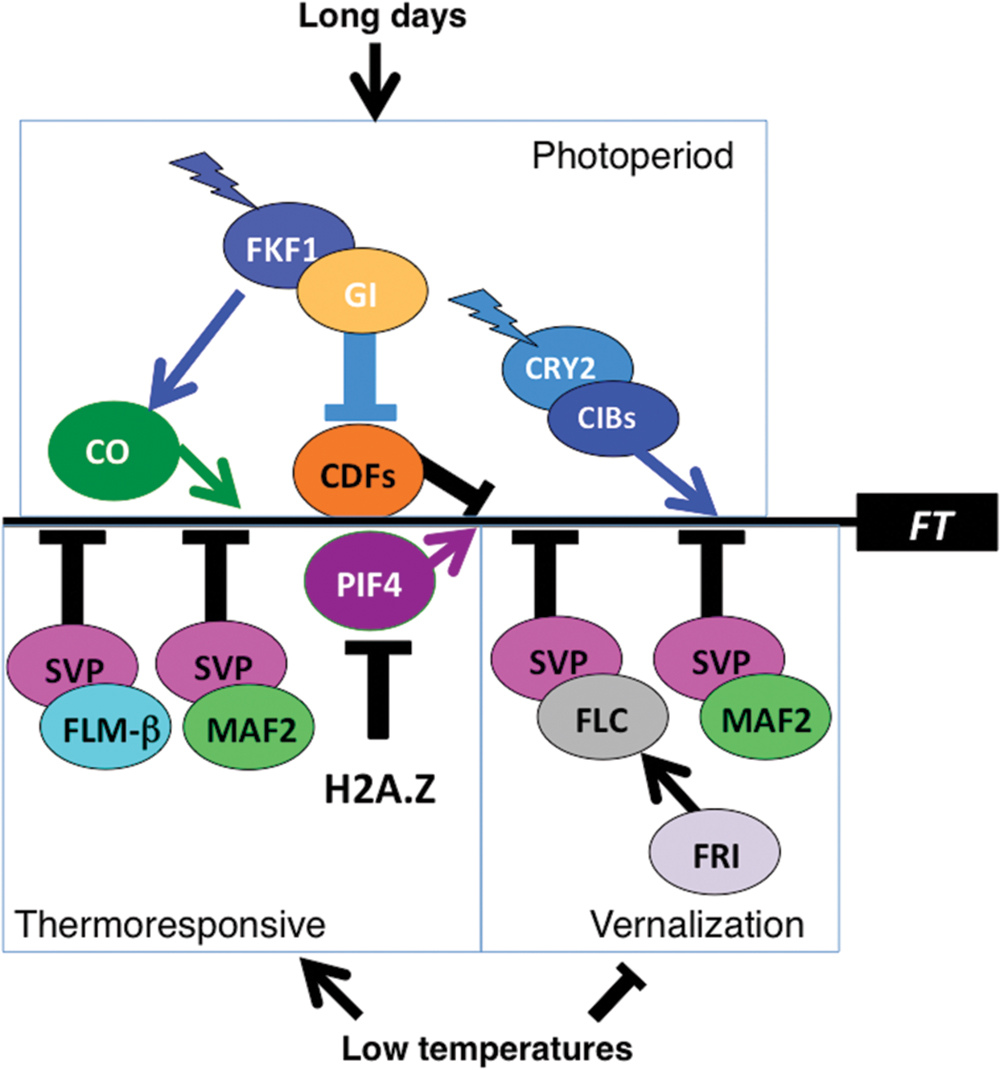
FIGURE 1. Photoperiod and thermoresponsive pathways regulate expression of FLOWERING LOCUS T (FT). Positive regulators of FT expression are indicated by white lettering and negative regulators by black lettering. The thin black horizontal line represents the FT promoter but only to indicate that all these regulatory inputs converge on the FT promoter, without depicting the number or spatial arrangement of binding sites within the promoter. Jagged arrows indicate blue light input in the late afternoon of long days, conferring photoperiod sensitivity in examples of external coincidence of light with a photosensitive phase defined by the circadian clock. Abundance of SVP (SHORT VEGETATIVE PHASE)/MAF2 (MADS AFFECTING FLOWERING 2) and SVP/FLM (FLOWERING LOCUS M)-β complexes declines with increasing temperatures. Additional complexes of SVP with other MAFs are formed but have been omitted for simplicity. Incorporation of the H2A.Z variant, which reduces access of transcriptional activators to the FT promoter, declines with increasing temperature. Similarly, after exposure to low temperature, FLC (FLOWERING LOCUS C) and MAF2 expression is reduced allowing plants requiring vernalization to flower.
Thermoresponsive Flowering
The transition to flowering is influenced by moderate changes in ambient temperature. Genome-wide association (GWAS) and QTL studies in Arabidopsis indicate a complex architecture of natural variation in thermal responses (Sanchez-Bermejo et al., 2015). A growth promoting temperature change from 23 to 27°C is as effective at inducing flowering as the transfer from non-inductive (8-h) short days to inductive (16-h) long days (Balasubramanian et al., 2006). Although, thermoresponsive flowering is not as well understood as vernalization, evidence supports a number of independent thermoresponsive pathways (Capovilla et al., 2015).
The histone variant H2A.Z acts as a thermosensor for flowering time. H2A.Z is incorporated into nucleosomes by a chromatin-remodeling complex that includes ACTIN-RELATED PROTEIN6 (ARP6) and PHOTOPERIOD-INSENSITIVE EARLY FLOWERING1 (PIE1) (Talbert and Henikoff, 2014). H2A.Z incorporation into nucleosomes makes DNA less accessible for transcription factors and slows RNA polymerase II. This limits gene expression at lower temperatures because, with increasing temperature, H2A.Z nucleosomes are depleted (Talbert and Henikoff, 2014). Of relevance to flowering, H2A.Z occupancy at the FT promoter is decreased at higher temperatures, permitting promoter binding by PIF4 (Kumar and Wigge, 2010; Kumar et al., 2012). PIF4, initially identified as important in the shade avoidance response, was implicated in thermoresponsive flowering because the pif4 mutant failed to accelerate flowering at elevated temperatures (Kumar et al., 2012). Similarly, pif5 loss of function delays flowering at high temperature and the pif4 pif5 double mutant flowered later than either single mutant, showing that both PIF4 and PIF5 accelerate flowering at elevated temperature (Fernández et al., 2016).
PHYTOCHROME INTERACTING FACTOR3, PIF4, and PIF5, but not PIF1 and PIF6, promote flowering when overexpressed in the phloem companion cells (Galvão et al., 2015). The PIFs promote flowering through induction of FT and its paralog TWIN SISTER OF FT (TSF) in response to warm nights and independently of FT during warm days (Thines et al., 2014; Galvão et al., 2015; Fernández et al., 2016). The increased expression of FT at high temperatures requires CO in addition to PIF4 and PIF5; co pif4 double, and co pif4 pif5 triple mutants flower later than pif single or double mutants. PIF4 and CO physically interact and this complex contributes to the induction of FT and TSF expression (Fernández et al., 2016). However, the co pif4 pif5 triple mutant still flowers earlier at 27 than at 21°C (Fernández et al., 2016). Similarly, quadruple pif1 pif3 pif4 pif5 (also called pifQ) loss of function mutants only partially suppress the early flowering and elevated FT expression at high temperature persists in the arp6 mutant (Galvão et al., 2015). This indicates that the mechanism by which H2A.Z delays flowering must be more complex than simply through PIF interaction with the FT promoter and that there is additional complexity in the acceleration of flowering in response to elevated temperature.
SHORT VEGETATIVE PHASE plays a central role in thermoresponsive flowering as well as in vernalization. SVP encodes a flowering repressor and thermoresponsive flowering likely includes a reduction of SVP expression at higher temperatures, because SVP overexpression delays flowering at 27°C (Fernández et al., 2016) and svp loss of function mutants flower early and fail to modify their flowering time in response to temperature (Capovilla et al., 2015) (Figure 1). SVP forms repressor complexes with MADS box transcription factors related to FLC: FLM and MAF (Ratcliffe et al., 2003; Lee et al., 2013; Posé et al., 2013; Gu et al., 2013a). These complexes repress FT and SOC1 transcription at low temperatures but decline in abundance at higher temperatures, relieving repression (Figure 1). The circadian clock imposes a circadian oscillation on SVP expression, linking thermosensitivity to circadian cycling (Fujiwara et al., 2008).
Gibberellic acid stimulates flowering. GA signaling entails the degradation of the DELLA transcriptional repressors; low GA levels allow the accumulation of the DELLAs, which delays flowering (Galvão et al., 2012; Yu et al., 2012). Inhibition of GA biosynthesis suppresses the acceleration of flowering at high temperature (Balasubramanian et al., 2006) and blocks the acceleration of flowering and increase of FT expression seen in the arp6 mutant (Galvão et al., 2015). However, both ft and ft tsf mutants still accelerate flowering in response to exogenous active GA, indicating that GA can act independently of FT and TSF. Similarly, GA can accelerate flowering in the arp6 mutant and the pif3 pif4 pif5 triple mutant indicating that GA can act independently of H2A.Z incorporation and the PIF genes. Expression of a constitutively active DELLA protein at the shoot apical meristem (SAM) but not in the phloem companion cells prevented GA-induced flowering, indicating that the GA acts at the SAM, consistent with its independence from the PIFs, which induce FT and TSF in phloem companion cells. The action of GA at the SAM, at least in part, involves the induction of the floral inducers SPL3 and SPL5 (Galvão et al., 2012, 2015; Porri et al., 2012; Yu et al., 2012).
There is considerable natural variation in thermoresponsive flowering and FLM is a major-effect QTL (Balasubramanian et al., 2006). Consistent with FLM as a flowering repressor, the loss of function flm-3 allele confers early flowering (Lee et al., 2013; Posé et al., 2013). The FLM primary transcript undergoes temperature dependent alternative splicing to yield two main isoforms that differ in terms of use of exon 2 (FLM-β) or exon 3 (FLM-δ); FLM-β binds DNA but FLM-δ does not (Lee et al., 2013; Posé et al., 2013). At lower temperatures the SVP-FLM-β complex is abundant and represses the floral integrators, FT and SOC1, but at higher temperatures the abundance of both FLM-β and SVP decreases, relieving repression (Lee et al., 2013; Posé et al., 2013). At higher (27°C) temperatures additional longer transcripts arise due to intron retention and the use of novel splice sites (Sureshkumar et al., 2016). Most of these longer transcripts include premature termination codons and are subjected to non-sense-mediated decay. The net result is a decreased abundance of the FLM-β transcript, the FLM-β isoform, and the SVP-FLM-β repressor complex (Sureshkumar et al., 2016).
A natural allele of FLM, in which a LINE retrotransposon has inserted into the first intron, confers early flowering both at 15 and at 21°C, although the effect was more pronounced at 15°C (Lutz et al., 2015). This insertion reduces abundance of both the major FLM transcripts, although temperature-dependent alternative splicing is preserved. Similar alleles were found in additional accessions, suggesting that this class of insertion confers early flowering at 15°C in summer annual accessions through reduced expression of the FLM-β isoform and the SVP-FLM-β repressor complex (Lutz et al., 2015).
SHORT VEGETATIVE PHASE also forms floral repressor complexes with MAF2, MAF3 and MAF4 (Ratcliffe et al., 2003; Gu et al., 2013a). MAF2 has evolved a temperature dependent alternative splicing pattern independently from FLM. The abundant MAF2 splice form at low temperatures encodes a functional MAF2 isoform that complexes with SVP to generate a floral repressor, but at elevated temperature an alternatively spliced intron-retaining variant encodes a prematurely truncated and non-functional MAF2 isoform that fails to repress flowering (Airoldi et al., 2015).
Temperature influences flowering, but the magnitude and direction of the temperature response depends on ecological details of the species under consideration (Capovilla et al., 2015). In Boechera stricta, a perennial relative of Arabidopsis, elevated temperature delays flowering (Anderson et al., 2011).
Photoperiodic Flowering
In the photoperiodic pathway, the circadian clock regulates the induction of critical flowering inducers, CONSTANS (CO) and FT, via an external coincidence mechanism in which the external stimulus, light, must coincide with an inductive window that is restricted (gated) by the circadian clock (Romera-Branchat et al., 2014; Greenham and McClung, 2015; Song et al., 2015). The following simplification emphasizes several examples of external coincidence.
The circadian clock drives morning-specific expression of several CYCLING DOF FACTOR (CDF) genes whose protein products repress CO transcription. The CDF proteins are targeted for degradation by a SCF complex containing FLAVIN BINDING, KELCH REPEAT, F-BOX1 (FKF1), and GIGANTEA (GI) (Figure 1). Both FKF1 and GI exhibit circadian cycling in protein abundance. In short days, GI protein abundance peaks at dusk while FKF1 protein peaks after dark. This leads to the formation of the FKF1-GI complex in the dark. Thus, CO transcription is repressed until about dusk and CO mRNA accumulates after dusk. CO protein is unstable in the dark so, in short days, CO protein fails to accumulate and FT transcription is not induced.
In long days the phase of peak GI expression is delayed and coincides with that of FKF1 in late afternoon. FKF1 is a blue-light photoreceptor, and the interaction of FKF1 with GI is enhanced by blue light. This is a second example of external coincidence, when the peaks of FKF1 and GI proteins coincide in the light to allow the formation of the FKF1-GI complex to degrade the CDFs in the late afternoon. As a consequence, transcriptional repression of CO is relieved in the afternoon of long days and CO mRNA accumulates in the light, which permits the stabilization of nascent CO protein and activation of FT transcription.
FLOWERING LOCUS T transcription is also induced independently of CO. Several CRY2-INTERACTING bHLH (CIB) transcription factors accumulate in long days to stimulate FT transcription. The CIBs are activated in the afternoon by blue-light dependent interaction with CRY2 (Liu et al., 2008). In addition, CIB protein stability is enhanced via a blue light dependent interaction with the FKF1 relatives, ZEITLUPE (ZTL) and LOV KELCH PROTEIN 2 (LKP2), although not with FKF1 (Liu et al., 2013). Thus, this CO-independent induction of FT is mediated in the afternoon/evening of long days via two classes of blue light photoreceptors, CRY2 for CIB activation and ZTL/LKP2 for CIB stabilization, a third example of external coincidence.
Conclusion
Studies of growth and flowering have emphasized the effects light intensity, quality, and duration (photoperiod), each of which has dramatic effects on vegetative morphology and the developmental transition from vegetative to reproductive growth (Arsovski et al., 2012; Casal, 2012; Song et al., 2015). Temperature also affects plant growth and reproduction, but only in recent years has it been realized that the effects of elevated but non-stressful temperatures on growth and reproduction can be of similar magnitude to those of light quantity and quality (Wigge, 2013; Quint et al., 2016). Temperature and light share some regulatory networks, but also employ specific regulatory pathways. One particularly prominent theme is the strong intersection of light and temperature signaling with time of day imposed by the circadian clock. In thermomorphogenesis as well as in thermosensitive and photoperiodic flowering, internal rhythms established by the circadian clock must coincide with the externally imposed environmental cycle of light and dark, an intersection termed “external coincidence.” Against a backdrop of global warming predicted to have major effects on the performance and distribution of wild species and strong adverse effects on crop yields (Willis et al., 2008; Wolkovich et al., 2012; McClung, 2014), it is crucial to integrate our understanding of temperature perception and response with other environmental responses, including biotic and abiotic stresses. Circadian clock function is intricately intertwined with each of these environmental response pathways (Greenham and McClung, 2015).
It is well-established that there is heterogeneity among plant species in terms of clock function and its relationship to flowering time (Song et al., 2015), so extrapolation to crops will require dedicated study in each species under consideration, using models established in Arabidopsis as guides.
Author Contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by grants from the Research Program for Agricultural Science & Technology Development (Project No. PJ012091) to JK, and the National Academy of Agricultural Science and the Rural Development Administration, Republic of Korea (BioGreen 21 Program Project No. PJ011069 and PJ009615) to JK and CM, and the National Science Foundation (IOS-1025965 and IOS-1257722) to CM.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Airoldi, C. A., McKay, M., and Davies, B. (2015). MAF2 is regulated by temperature-dependent splicing and represses flowering at low temperatures in parallel with FLM. PLoS ONE 10:e0126516. doi: 10.1371/journal.pone.0126516
Amasino, R. (2010). Seasonal and developmental timing of flowering. Plant J. 61, 1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x
Amasino, R. M., and Michaels, S. D. (2010). The timing of flowering. Plant Physiol. 154, 516–520. doi: 10.1104/pp.110.161653
Anderson, J. T., Lee, C. R., and Mitchell-Olds, T. (2011). Life-history QTLs and natural selection on flowering time in Boechera stricta, a perennial relative of Arabidopsis. Evolution 65, 771–787. doi: 10.1111/j.1558-5646.2010.01175.x
Arsovski, A. A., Galstyan, A., Guseman, J. M., and Nemhauser, J. L. (2012). Photomorphogenesis. Arabidopsis Book. 10:e0147. doi: 10.1199/tab.0147
Balasubramanian, S., Sureshkumar, S., Lempe, J., and Weigel, D. (2006). Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2:e106. doi: 10.1371/journal.pgen.0020106
Berry, S., and Dean, C. (2015). Environmental perception and epigenetic memory: mechanistic insight through FLC. Plant J. 83, 133–148. doi: 10.1111/tpj.12869
Box, M. S., Huang, B. E., Domijan, M., Jaeger, K. E., Khattak, A. K., Yoo, S. J., et al. (2015). ELF3 controls thermoresponsive growth in Arabidopsis. Curr. Biol. 25, 194–199. doi: 10.1016/j.cub.2014.10.076
Capovilla, G., Schmid, M., and Pose, D. (2015). Control of flowering by ambient temperature. J. Exp. Bot. 66, 59–69. doi: 10.1093/jxb/eru416
Coluccio, M. P., Sanchez, S. E., Kasulin, L., Yanovsky, M. J., and Botto, J. F. (2011). Genetic mapping of natural variation in a shade avoidance response: ELF3 is the candidate gene for a QTL in hypocotyl growth regulation. J. Exp. Bot. 62, 167–176. doi: 10.1093/jxb/erq253
Delker, C., Sonntag, L., James, G. V., Janitza, P., Ibañez, C., Ziermann, H., et al. (2014). The DET1-COP1-HY5 pathway constitutes a multipurpose signaling module regulating plant photomorphogenesis and thermomorphogenesis. Cell Rep. 9, 1983–1989. doi: 10.1016/j.celrep.2014.11.043
Dong, J., Tang, D., Gao, Z., Yu, R., Li, K., He, H., et al. (2014). Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating Phytochrome-Interacting Factors in the dark. Plant Cell 26, 3630–3645. doi: 10.1105/tpc.114.130666
Fernández, V., Takahashi, Y., Le Gourrierec, J., and Coupland, G. (2016). Photoperiodic and thermosensory pathways interact through CONSTANS to promote flowering at high temperature under short days. Plant J. 86, 426–440. doi: 10.1111/tpj.13183
Fujiwara, S., Oda, A., Yoshida, R., Niinuma, K., Miyata, K., Tomozoe, Y., et al. (2008). Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell 20, 2960–2971. doi: 10.1105/tpc.108.061531
Galvão, V. C., Collani, S., Horrer, D., and Schmid, M. (2015). Gibberellic acid signaling is required for ambient temperature-mediated induction of flowering in Arabidopsis thaliana. Plant J. 84, 949–962. doi: 10.1111/tpj.13051
Galvão, V. C., Horrer, D., Kuttner, F., and Schmid, M. (2012). Spatial control of flowering by DELLA proteins in Arabidopsis thaliana. Development 139, 4072–4082. doi: 10.1242/dev.080879
Gazzani, S., Gendall, A. R., Lister, C., and Dean, C. (2003). Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 132, 1107–1114. doi: 10.1104/pp.103.021212
Gray, W. M., Östin, A., Sandberg, G., Romano, C. P., and Estelle, M. (1998). High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 95, 7197–7202. doi: 10.1073/pnas.95.12.7197
Greenham, K., and McClung, C. R. (2015). Integrating circadian dynamics with physiological processes in plants. Nat. Rev. Genet. 16, 598–610. doi: 10.1038/nrg3976
Gu, X., Le, C., Wang, Y., Li, Z., Jiang, D., Wang, Y., et al. (2013a). Arabidopsis FLC clade members form flowering-repressor complexes coordinating responses to endogenous and environmental cues. Nat. Commun. 4, 1947. doi: 10.1038/ncomms2947
Gu, X., Wang, Y., and He, Y. (2013b). Photoperiodic regulation of flowering time through periodic histone deacetylation of the florigen gene FT. PLoS Biol. 11:e1001649. doi: 10.1371/journal.pbio.1001649
Hepworth, J., and Dean, C. (2015). Flowering Locus C’s lessons: conserved chromatin switches underpinning developmental timing and adaptation. Plant Physiol. 168, 1237–1245. doi: 10.1104/pp.15.00496
Jiménez-Gómez, J. M., Wallace, A. D., and Maloof, J. N. (2010). Network analysis identifies ELF3 as a QTL for the shade avoidance response in Arabidopsis. PLoS Genet. 6:e1001100. doi: 10.1371/journal.pgen.1001100
Kim, D. H., Doyle, M. R., Sung, S., and Amasino, R. M. (2009). Vernalization: winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol. 25, 277–299. doi: 10.1146/annurev.cellbio.042308.113411
Koini, M. A., Alvey, L., Allen, T., Tilley, C. A., Harberd, N. P., Whitelam, G. C., et al. (2009). High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr. Biol. 19, 408–413. doi: 10.1016/j.cub.2009.01.046
Kumar, S. V., Lucyshyn, D., Jaeger, K. E., Alós, E., Alvey, E., Harberd, N. P., et al. (2012). Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245. doi: 10.1038/nature10928
Kumar, S. V., and Wigge, P. A. (2010). H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147. doi: 10.1016/j.cell.2009.11.006
Kunihiro, A., Yamashino, T., Nakamichi, N., Niwa, Y., Nakanishi, H., and Mizuno, T. (2011). PHYTOCHROME-INTERACTING FACTOR 4 and 5 (PIF4 and PIF5) activate the homeobox ATHB2 and auxin-inducible IAA29 genes in the coincidence mechanism underlying photoperiodic control of plant growth of Arabidopsis thaliana. Plant Cell Physiol. 52, 1315–1329. doi: 10.1093/pcp/pcr076
Lee, J., He, K., Stolc, V., Lee, H., Figueroa, P., Gao, Y., et al. (2007). Analysis of transcription factor HY5 genomic binding sites revealed Its hierarchical role in light regulation of development. Plant Cell 19, 731–749. doi: 10.1105/tpc.106.047688
Lee, J. H., Ryu, H.-S., Chung, K. S., Posé, D., Kim, S., Schmid, M., et al. (2013). Regulation of temperature-responsive flowering by MADS-Box transcription factor repressors. Science 342, 628–632. doi: 10.1126/science.1241097
Leivar, P., and Monte, E. (2014). PIFs: systems integrators in plant development. Plant Cell 26, 56–78. doi: 10.1105/tpc.113.120857
Liu, H., Wang, Q., Liu, Y., Zhao, X., Imaizumi, T., Somers, D. E., et al. (2013). Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 110, 17582–17587. doi: 10.1073/pnas.1308987110
Liu, H., Yu, X., Li, K., Klejnot, J., Yang, H., Lisiero, D., et al. (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1538. doi: 10.1126/science.1163927
Lutz, U., Poseì, D., Pfeifer, M., Gundlach, H., Hagmann, J., Wang, C., et al. (2015). Modulation of ambient temperature-dependent flowering in Arabidopsis thaliana by natural variation of FLOWERING LOCUS M. PLoS Genet. 11:e1005588. doi: 10.1371/journal.pgen.1005588
Niwa, Y., Yamashino, T., and Mizuno, T. (2009). The circadian clock regulates the photoperiodic response of hypocotyl elongation through a coincidence mechanism in Arabidopsis thaliana. Plant Cell Physiol. 50, 838–854. doi: 10.1093/pcp/pcp028
Nomoto, Y., Kubozono, S., Miyachi, M., Yamashino, T., Nakamichi, N., and Mizuno, T. (2012a). A circadian clock- and PIF4-mediated double coincidence mechanism is implicated in the thermosensitive photoperiodic control of plant architectures in Arabidopsis thaliana. Plant Cell Physiol. 53, 1965–1973. doi: 10.1093/pcp/pcs141
Nomoto, Y., Kubozono, S., Yamashino, T., Nakamichi, N., and Mizuno, T. (2012b). Circadian clock- and PIF4-controlled plant growth: a coincidence mechanism directly integrates a hormone signaling network into the photoperiodic control of plant architectures in Arabidopsis thaliana. Plant Cell Physiol. 53, 1950–1964. doi: 10.1093/pcp/pcs137
Nozue, K., Covington, M. F., Duek, P. D., Lorrain, S., Fankhauser, C., Harmer, S. L., et al. (2007). Rhythmic growth explained by coincidence between internal and external cues. Nature 448, 358–361. doi: 10.1038/nature05946
Nusinow, D. A., Helfer, A., Hamilton, E. E., King, J. J., Imaizumi, T., Schultz, T. F., et al. (2011). The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475, 398–402. doi: 10.1038/nature10182
Oh, E., Zhu, J. Y., and Wang, Z. Y. (2012). Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 14, 802–809. doi: 10.1038/ncb2545
Porri, A., Torti, S., Romera-Branchat, M., and Coupland, G. (2012). Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development 139, 2198–2209. doi: 10.1242/dev.077164
Posé, D., Verhage, L., Ott, F., Yant, L., Mathieu, J., Angenent, G. C., et al. (2013). Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature 503, 414–417. doi: 10.1038/nature12633
Quint, M., Delker, C., Franklin, K. A., Wigge, P. A., Halliday, K. J., and van Zanten, M. (2016). Molecular and genetic control of plant thermomorphogenesis. Nat. Plants 2, 15190. doi: 10.1038/nplants.2015.190
Ratcliffe, O. J., Kumimoto, R. W., Wong, B. J., and Riechmann, J. L. (2003). Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15, 1159–1169. doi: 10.1105/tpc.009506
Romera-Branchat, M., Andres, F., and Coupland, G. (2014). Flowering responses to seasonal cues: what’s new? Curr. Opin. Plant Biol. 21, 120–127. doi: 10.1016/j.pbi.2014.07.006
Sanchez-Bermejo, E., Zhu, W., Tasset, C., Eimer, H., Sureshkumar, S., Singh, R., et al. (2015). Genetic architecture of natural variation in thermal responses of Arabidopsis. Plant Physiol. 169, 647–659. doi: 10.1104/pp.15.00942
Sheldon, C. C., Finnegan, E. J., Peacock, W. J., and Dennis, E. S. (2009). Mechanisms of gene repression by vernalization in Arabidopsis. Plant J. 59, 488–498. doi: 10.1111/j.1365-313X.2009.03883.x
Shi, H., Wang, X., Mo, X., Tang, C., Zhong, S., and Deng, X. W. (2015). Arabidopsis DET1 degrades HFR1 but stabilizes PIF1 to precisely regulate seed germination. Proc. Natl. Acad. Sci. U.S.A. 112, 3817–3822. doi: 10.1073/pnas.1502405112
Simpson, G. G., and Dean, C. (2002). Arabidopsis, the rosetta stone of flowering time? Science 296, 285–289. doi: 10.1126/science.296.5566.285
Song, J., Angel, A., Howard, M., and Dean, C. (2012). Vernalization - a cold-induced epigenetic switch. J. Cell Sci. 125, 3723–3731. doi: 10.1242/jcs.084764
Song, Y. H., Shim, J. S., Kinmonth-Schultz, H. A., and Imaizumi, T. (2015). Photoperiodic flowering: time measurement mechanisms in leaves. Annu. Rev. Plant Biol. 66, 441–464. doi: 10.1146/annurev-arplant-043014-115555
Sureshkumar, S., Dent, C., Seleznev, A., Tasset, C., and Balasubramanian, S. (2016). Nonsense-mediated mRNA decay modulates FLM-dependent thermosensory flowering response in Arabidopsis. Nat. Plants 2:16055. doi: 10.1038/nplants.2016.55
Talbert, P. B., and Henikoff, S. (2014). Environmental responses mediated by histone variants. Trends Cell Biol. 24, 642–650. doi: 10.1016/j.tcb.2014.07.006
Thines, B. C., Youn, Y., Duarte, M. I., and Harmon, F. G. (2014). The time of day effects of warm temperature on flowering time involve PIF4 and PIF5. J. Exp. Bot. 65, 1141–1151. doi: 10.1093/jxb/ert487
Toledo-Ortiz, G., Johansson, H., Lee, K. P., Bou-Torrent, J., Stewart, K., Steel, G., et al. (2014). The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 10:e1004416. doi: 10.1371/journal.pgen.1004416
Wigge, P. A. (2013). Ambient temperature signalling in plants. Curr. Opin. Plant Biol. 16, 661–666. doi: 10.1016/j.pbi.2013.08.004
Willis, C. G., Ruhfel, B., Primack, R. B., Miller-Rushing, A. J., and Davis, C. C. (2008). Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc. Natl. Acad. Sci. U.S.A. 105, 17029–17033. doi: 10.1073/pnas.0806446105
Wolkovich, E. M., Cook, B. I., Allen, J. M., Crimmins, T. M., Betancourt, J. L., Travers, S. E., et al. (2012). Warming experiments underpredict plant phenological responses to climate change. Nature 485, 494–497. doi: 10.1038/nature11014
Yazdanbakhsh, N., Sulpice, R., Graf, A., Stitt, M., and Fisahn, J. (2011). Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 34, 877–894. doi: 10.1111/j.1365-3040.2011.02286.x
Keywords: circadian clock, circadian rhythms, photoperiodic flowering, flower induction, thermoresponsive flowering
Citation: McClung CR, Lou P, Hermand V and Kim JA (2016) The Importance of Ambient Temperature to Growth and the Induction of Flowering. Front. Plant Sci. 7:1266. doi: 10.3389/fpls.2016.01266
Received: 15 April 2016; Accepted: 09 August 2016;
Published: 23 August 2016.
Edited by:
Dorothee Staiger, Bielefeld University, GermanyReviewed by:
Sureshkumar Balasubramanian, Monash University, AustraliaRachel Green, Hebrew College, Israel
Copyright © 2016 McClung, Lou, Hermand and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. R. McClung, c.robertson.mcclung@dartmouth.edu Jin A. Kim, jjinbbang8@hanmail.net
 C. R. McClung
C. R. McClung Ping Lou
Ping Lou Victor Hermand
Victor Hermand Jin A. Kim
Jin A. Kim