- 1International Center for Ecology, Meteorology and Environment, School of Applied Meteorology, Nanjing University of Information Science and Technology, Nanjing, China
- 2Department of Environmental Sciences, University of Toledo, Toledo, OH, USA
- 3Department of Biology, University of Maryland, College Park, MD, USA
- 4Department of Biological Sciences, Rutgers University, Newark, NJ, USA
Heat-waves with higher intensity and frequency and longer durations are expected in the future due to global warming, which could have dramatic impacts in agriculture, economy and ecology. This field study examined how plant responded to heat-stress (HS) treatment at different timing in naturally occurring vegetation. HS treatment (5 days at 40.5∘C) were applied to 12 1 m2 plots in restored prairie vegetation dominated by a warm-season C4 grass, Andropogon gerardii, and a warm-season C3 forb, Solidago canadensis, at different growing stages. During and after each heat stress (HS) treatment, temperature were monitored for air, canopy, and soil; net CO2 assimilation (Anet), quantum yield of photosystem II (ΦPSII), stomatal conductance (gs), and internal CO2 level (Ci), specific leaf area (SLA), and chlorophyll content of the dominant species were measured. One week after the last HS treatment, all plots were harvested and the biomass of above-ground tissue and flower weight of the two dominant species were determined. HS decreased physiological performance and growth for both species, with S. canadensis being affected more than A. gerardii, indicated by negative HS effect on both physiological and growth responses for S. canadensis. There were significant timing effect of HS on the two species, with greater reductions in the net photosynthetic rate and productivity occurred when HS was applied at later-growing season. The reduction in aboveground productivity in S. canadensis but not A. gerardii could have important implications for plant community structure by increasing the competitive advantage of A. gerardii in this grassland. The present experiment showed that HS, though ephemeral, may promote long-term effects on plant community structure, vegetation dynamics, biodiversity, and ecosystem functioning of terrestrial biomes when more frequent and severe HS occur in the future.
Introduction
The increased concentration of CO2 and other greenhouse gasses in atmosphere is causing a future climate with higher temperatures and dramatic changes in rainfall patterns (IPCC, 2013). In addition to rising mean annual temperatures, the frequency, duration, and severity of periods with exceptionally high temperatures are also increasing (Easterling et al., 2000; Tripathi et al., 2016). HS events with a trend of high frequency and extremity have already been reported in different parts of the world (Henderson and Muller, 1997; Gaffen and Ross, 1998; Yan, 2002). Thus, plants in the future will be exposed to both higher mean temperatures, and likely more extreme HS. The World Meteorological Organization (WMO) defines HS events as episodes of 5 or more continuous days with air temperatures over 5°C above daily maximum temperatures (Frich et al., 2002). During extreme climate events the acclimatory capacities of an organism are substantially exceeded (Gutschick and BassiriRad, 2003) and the impact of extreme climate events can be significantly greater than those associated with mean temperature increases (Karl et al., 1997). Combining the climatological and biological definitions, Smith (2011) stated that an extreme climate event is an episode in which a statistically rare climatic period could cause community responses, with loss of key species, invasion by novel species, and alteration ecosystem structure and/or function outside the bounds of normal variability. Therefore, extreme climate events, in spite of their ephemeral nature, can potentially cause shifts in the structure of plant communities (Smith, 2011) and greatly impact ecosystem productivity (Ciais et al., 2005) and biodiversity (Thomas et al., 2004). It is, however, difficult to determine whether the ecological response is explicitly attributable to an extreme climate event, since it may not be extreme enough to cause ecological consequences (Niu et al., 2014).
Accordingly, research has started to not only focus on the impact of the trend of gradual increases in mean temperatures but also on the effects of increasing extreme HS events (Brown et al., 2004; Jentsch et al., 2011; Sentis et al., 2013). However, due to the difficulties in conducting experiments and simulating extreme heat events under field conditions, they are most frequently conducted under controlled conditions in the laboratory (Wang et al., 2008a, 2012). Therefore, the effects of extreme heat events on the vegetation structure and dynamics remained less well understood than effects of climate warming and atmospheric CO2 enrichment, especially on crops photosynthesis, respiration, and growth (Long et al., 2004; Ainsworth et al., 2008). To date, only a few experiments with HS treatment have been conducted in plant communities, and these studies focused on recolonization, competition, invasion, and the role of species richness during extreme events in community processes. HS manipulations were conducted mostly on grassland (White et al., 2001; Van Peer et al., 2004) or arctic species (Marchand et al., 2005, 2006). In this study, we will apply short-term HS treatment in a restored prairie and concentrate on the ecophysiological and growth responses of two dominant warm-season tall-grass prairie species with contrasting photosynthetic pathways (a C4 grass and a C3 forb) to HS.
The negative effects of HS on plants growth and crop yield mainly was caused through its negative effects on photosynthetic process, which is among the most thermosensitive aspects of plant functions (Wang et al., 2008a). Due to inter-annual variations in the timing and duration of hot days, HS events may affect plant physiological processes and community structure differently. Hence, the response of plants photosynthetic activity to HS will depend on the season and growing stage when HS events occur (Xu and Baldocchi, 2003; Yu et al., 2003; Richardson et al., 2010). Although the research of the effects of the timing of the extreme events is urgently needed, there is still a lack of studies in this regard (Jentsch et al., 2011; Smith, 2011). We urgently need to advance research on the effect of the timing of extreme events and their consequences by collecting evidence from experimental studies in natural field conditions. Therefore, this study will simulate HS events in the key phenological stages of the dominant species in an old prairie to investigate how variation in the timing of HS events during the growing season influences physiological processes and growth of individual species and community dynamics.
The optimal temperature for photosynthesis is typically higher for C4 species than for C3 species because C4 species usually have higher water use efficiency and lower photorespiration due to its CO2-accumulating mechanisms in the leaf (Sage and Monson, 1999). This may contribute to greater tolerance to HS for C4 species than co-occurring C3 species (Coleman and Bazzaz, 1992; Ehleringer et al., 1997; Wang et al., 2008b). Differential sensitivities to HS among different species may lead to divergent responses in these dominant species, particularly if the stress exceeds species-specific physiological thresholds (Gutschick and BassiriRad, 2003). In natural systems, the significance of climate warming for C4 vegetation can depend less on the mean increase in global temperature and more on the spatial and temporal variation of the temperature increase (Sage and Kubien, 2003). In New Zealand, for example, episodic heat events inhibit C3 plants more than C4 grasses, and as a result, facilitate C4 grass invasion of C3-dominated grasslands (White et al., 2000, 2001). However, whether the timing of HS events impacts differently on C3 vs. C4 species remains to be determined and the differences in the responses to HS applied at different growing stages will have a bearing on the relative impact of global environmental change on the abundance, productivity and distribution of C3 and C4 species and therefore community structure.
To examine the influence of HS on plants ecophysiological and growth response in naturally occurring mixed C3–C4 vegetation, we conducted a field study and aimed with the following two major objectives: (1) to determine how HS affects the ecophysiological and morphological characteristics of a C4 and C3 species which co-dominate a restored prairie community; (2) to determine the effect of timing of HS on each species growth and physiology. Our specific hypotheses were as follows: (1) HS will have a less pronounced negative effect on the C4 than the C3 species; (2) differences in the responses to HS applied at different growing stage (HS timing effect) will lead to differences in plants ecophysiological responses and growth.
Materials and Methods
Field Site and Experimental Treatments
The experiment site was located within a restored prairie vegetation at the University of Toledo’s Stranahan Arboretum (Toledo, OH, USA), within the oak-savannah glacial-sand ecosystem referred to as “Oak Openings” region1. Andropogon gerardii (big bluestem), a warm-season C4 perennial grass, and Solidago canadensis (goldenrod), a warm-season C3 perennial herbaceous dicot, together account for almost 95% plant canopy cover and the majority of total aboveground productivity in this ecosystem. Top-vented 1 m3-chambers made with transparent plastic attached to a wooden frame was used to simulate HS treatment. Heat treatment was applied in situ from June 21 to June 25, July 22 to July 26, and August 28 to September 1 in 2007 (as in Wang et al., 2008b). There was no obvious drought situation before each heat treatment. For each heat treatment, eight 1 m × 1 m plots were selected randomly for use; four were untreated controls and four were heated to 39–41°C daytime temperature. A portable electric heater (Heat Runner model 33551, 1500 W), suspended near a corner of the chamber, was used to increase and regulate chamber temperature, and a fan was used to distribute warm air inside the chamber. Spatial variation in temperature within chambers was found to be minimal. The temperature in the central chamber was 0.5 ± 0.3 (standard deviation)°C higher than the edge of the chamber. Plants were not watered during the heat treatment. This experimental design did not allow for determination of chamber effects on plants (increased humidity, decreased wind, and slightly decreased light levels), but such effects would only serve to minimize the negative effects of HS, and make detection of heat effects more difficult. Air temperature was monitored continuously, with either a temperature probe and data logger (HOBO8, Onset Computer Corp, Bourne, MA, USA) or a fine-wire thermocouple and data logger (LI-1000, LiCOR, Lincoln, NE, USA). Leaf temperature was measured with an IR thermometer (cross-checked against the probes above). Soil temperature (10 cm) during midday and at the end of the HS treatment was monitored with a temperature probe and a thermometer (Wang et al., 2008b; Mainali et al., 2014). Heat-treatments in this study were chosen to represent those HS events encountered by vegetation in the Toledo region (northwest Ohio, USA) during summer months. On average, there is about 10 days of HS in July and August during which day time maximal temperatures are higher than 32°C in Toledo. The recorded daytime maximal temperatures for June, July, and August were 40, 41, and 39°C, respectively, and the mean daytime maximal temperatures for June, July, and August were 28, 30, and 29°C, respectively2. Therefore, the target HS treatment temperature was set at 40, 41, and 39°C for June, July, and August, respectively, in this experiment.
Gas Exchange and Leaf Trait Measurements
Photosynthetic measurements were conducted during and after each HS treatment in order to determine the timing effect of HS on foliar gas exchange. During and after each heat treatment, one fully expanded leaves were chosen randomly from each plot and net photosynthetic rate, stomatal conductance to water vapor, and internal CO2 level were measured daily with a portable infrared gas analyzer (LI-COR 6400LCF; LI-COR, Lincoln, NE, USA). During measurements, CO2 concentration of 380 μmol mol-1, leaf temperature of 25°C, photosynthetic photon flux (PPFD) of 1500 μmol m-2 s-1 and airflow through the chamber of 250 μmol s-1 were set in the leaf chamber. Net photosynthetic rate (Anet) was taken as the rate of photosynthesis at a PPFD of 1500 μmol m-2 s-1. The parameters including stomatal conductance (gs) and intercellular CO2 concentration (Ci) were recorded during the photosynthetic measurement. Intrinsic water use efficiency (iWUE) was calculated as the ratio of net photosynthetic rate to stomatal conductance. Quantum yield of PSII electron transport (ΦPSII) was measured with a pulse-amplitude-modulated (PAM) fluorometer with a saturating pulse of 3000 μmol photons m-2 s-1 (Model PAM 101/103, Walz, Germany) on ambient light-adapted (∼800 μmol photons m-2 s-1) plants, as in Wang et al. (2008b). Leaf area index (LAI) was measured once per week, using LAI-2000 (LI-COR Biosciences, Lincoln, NE, USA). After gas-exchange measurements of last heat-stress treatment, ten 0.5 cm2 leaf punches from each leaf were taken and oven-dried at 65°C for 2 weeks for measurement of SLA (m2 kg-1) and LWC (%). An index of the total leaf chlorophyll content was measured using a chlorophyll meter (SPAD-502, Konica Minolta, Japan). Readings were taken along the middle section of the four leaves of one plant and the mean value was used for analysis. The measurements were made on five plants from each treatment before, during and after the HS.
Biomass and C, N Measurements
Four-week after the last HS treatment, 40 cm × 50 cm of each plot was harvested. The clipped plants were sorted into different categories (species, green and senescent leaves, stems and flowers), oven-dried at 65°C for 1 week and weighed.
Statistical Analysis
All statistics were tested in the R statistical language3. The normality of the residuals of all the variables was tested using the Shapiro–Wilk test. Fixed effects of species, heating stress at different time and their interactions on the morphological, biochemical, and physiological parameters were tested by a linear mixed-effects model, using the lme4 package4. The measuring time were specified as a random factor to control for their associated intra-class correlation. Linear mixed-effects models also tolerate the necessarily unequal number of responses and unbalanced sample sizes for each treatment. We obtained p-values for regression coefficients using the nlme package. For the sake of brevity, we present only the F tests from the LMER results here (type III Wald F tests with Kenward–Roger degrees of freedom approximation). A Post hoc Tukey HSD tests were made on specific contrasts to examine significant treatment effects among groups (step function in the nlme package, R). End of season measurements of aboveground primary production, flower weight and leaf morphological parameters were analyzed via t-tests to account for heat-stress timing effect. All statistical tests were considered significant at P ≤ 0.05. Mean values of each variable were expressed with their standard error (SE).
Results
Air temperature in the heated plots increased on average to 40.5 ± 2.8°C during HS treatment (data not shown, as in Wang et al., 2008b; Mainali et al., 2014). During the 5-days HS treatment, leaf temperature of A. gerardii and S. canadensis in heated plots was higher than that in control plots, but returned to control levels right after the end of HS (Figure 1; Table 1). For HS applied during early-, peak-, and reproductive- growing season, leaf temperature reached 34.9, 34.3, and 33.2 for A. gerardii and 34.1, 34.1, and 33.8°C for S. canadensis in the heated plots, respectively (Figure 1).
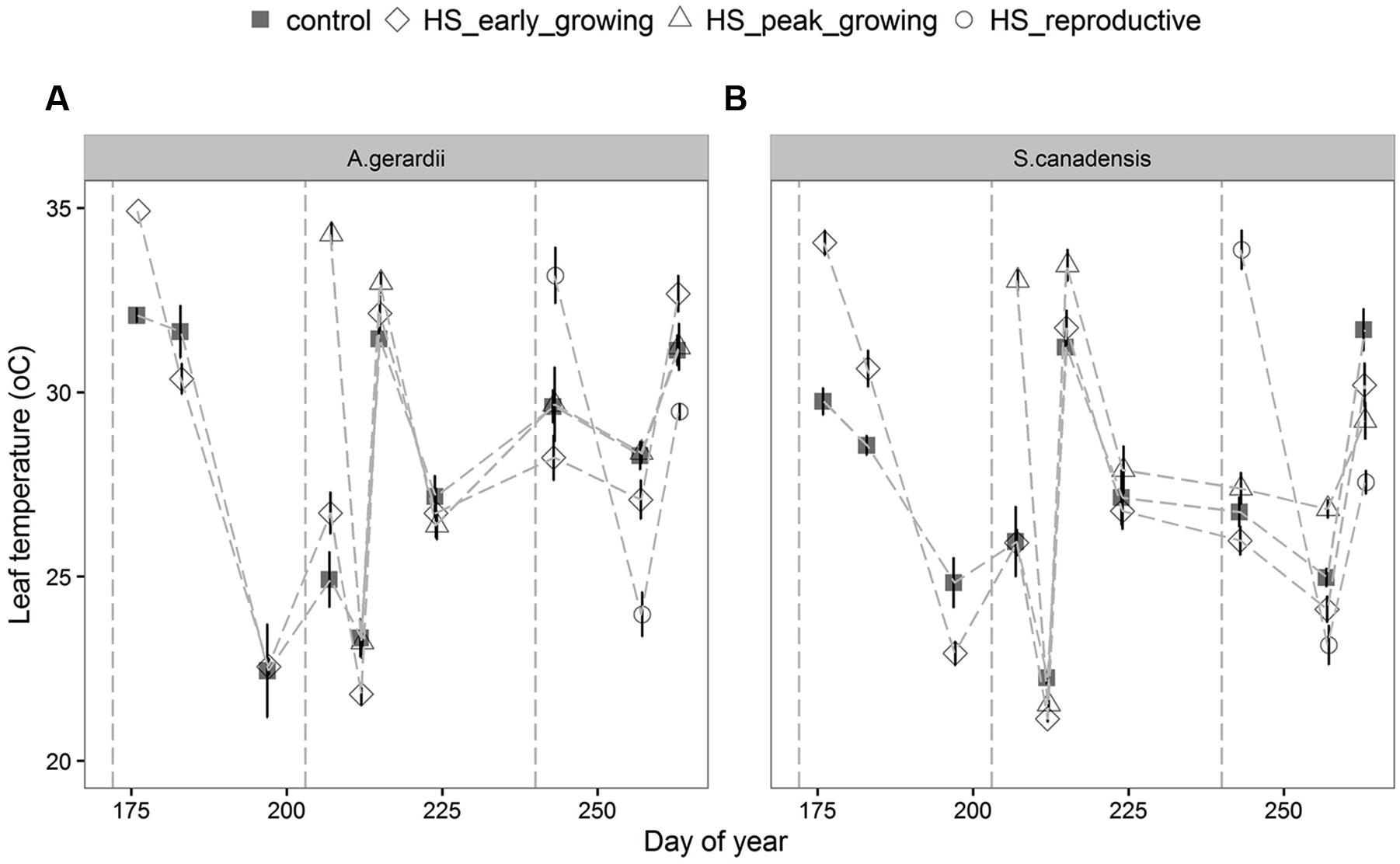
FIGURE 1. Effects of HS applied at different growing stage on leaf temperature of (A) Andropogon Gerardii and (B) Solidago Canadensis. Measurements were taken during and after each heat-stress treatment. Values are means ± 1 SD; n = 4.
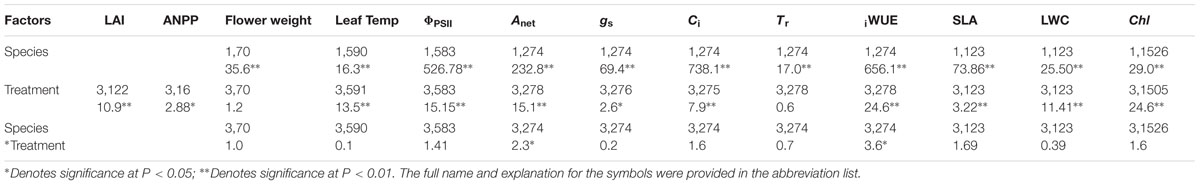
TABLE 1. Degrees of freedom (numerator, denominator) and F-statistics from the linear mixed effect model on the fixed effect of treatment on the morphological, biochemical, and physiological parameters.
Aboveground net primary production at the end of growing season differed significantly among different treatments (Figure 2A). ANPP of the plots heat-stressed at reproductive-growing season was significantly lower than that of the control plots. The productivity of S. canadensis, but not A. gerardii, was significantly reduced by HS. The flower weight of S. canadensis was higher than that of A. gerardii, but neither was affected by HS (Figure 2B). LAI was significantly lower at the plots heat-stressed at reproductive growing stage than the control plots (Figure 2C). LAI was highest at the plots heat-stressed at early-growing season and lowest at the plots heat-stressed at reproductive-growing stage (Figure 2C).
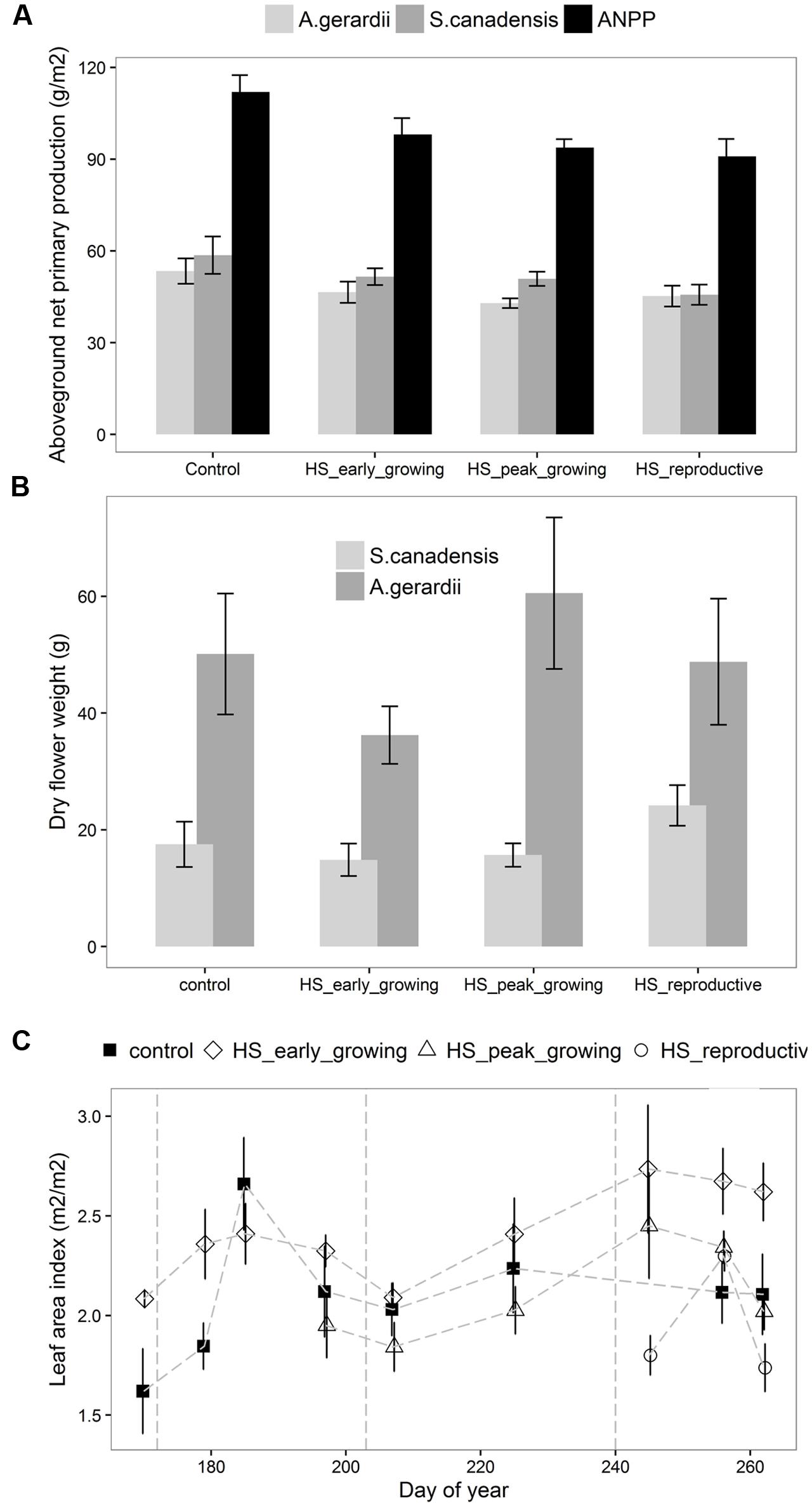
FIGURE 2. Effects of HS applied at different growing stage on (A) the aboveground net primary productivity, productivity of A. gerardii and S. Canadensis; (B) the flower weight of A. gerardii and S. Canadensis; (C) leaf area index (LAI). LAI was measured once during and twice after each heat-stress treatment. Values are means ± 1 SD; n = 4.
Specific leaf area of S. canadensis was higher than that of A. gerardii. Compared with control plots, HS at peak-growing stage significantly increased SLA for both A. gerardii and S. canadensis (Figure 3A). SLA of plants heat-stressed at peak- and reproductive- stages was significantly higher than that heat-stressed at early-growing stages. LWC of S. canadensis was higher than that of A. gerardii. Compared with control plots, HS at reproductive-growing stages significantly decreased LWC for S. canadensis. And for A. gerardii, LWC of plants at the plots heat-stressed at peak-growing stage was significantly lower than that at control plots and plots heat-stressed at early-growing stages (Figure 3B).
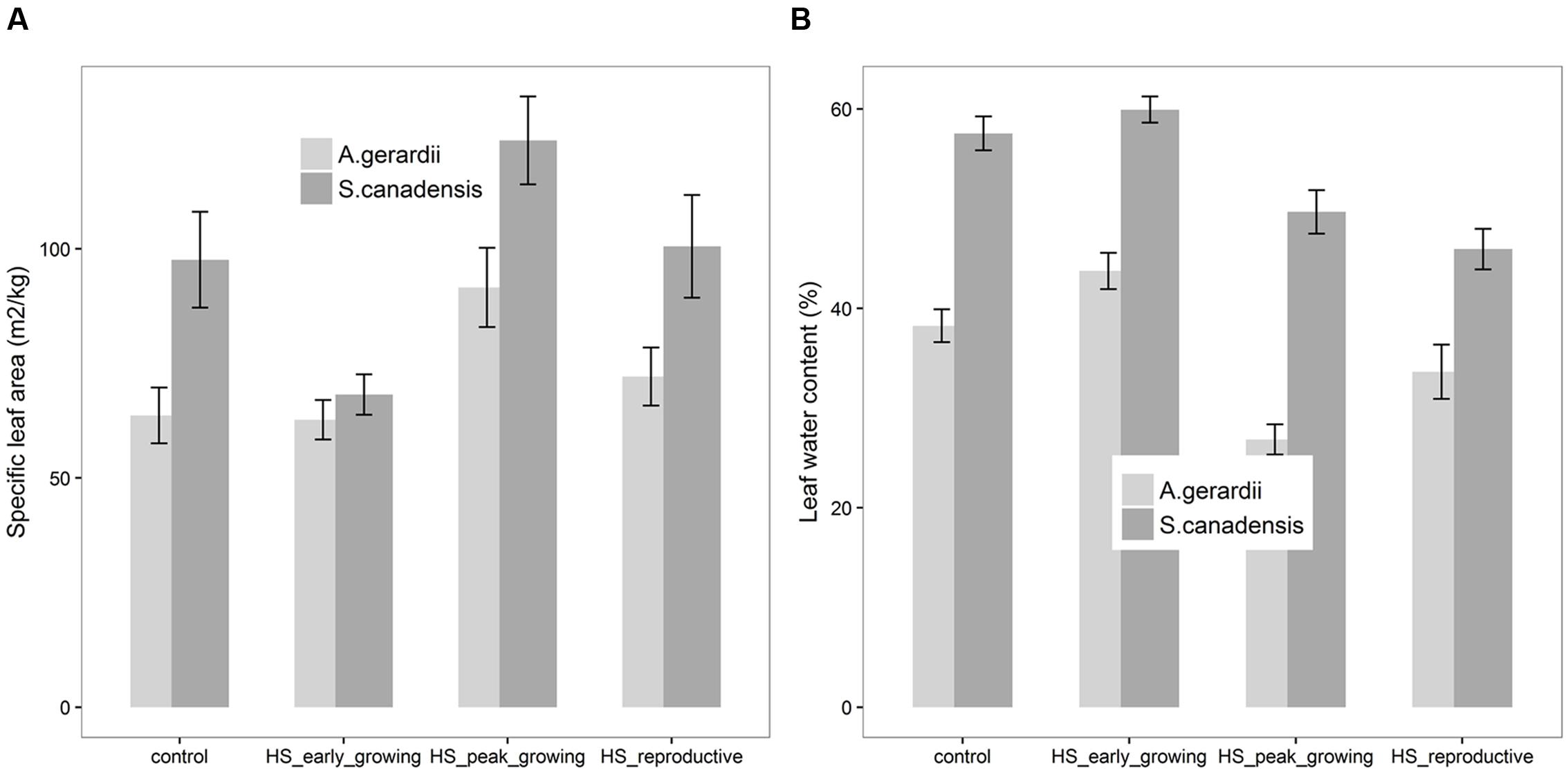
FIGURE 3. Effects of HS applied at different growing stage on (A) specific leaf area (SLA) and (B) relative leaf water content of A. gerardii and S. canadensis. Values are means ± 1 SD; n = 4.
Heat stress treatment reduced net CO2 assimilation rate and stomatal conductance in heat-stressed plants. Anet (net photosynthetic rate) was higher for A. gerardii than that of S. canadensis. Anet was significantly lower in heated plots than in control plots during HS for A. gerardii and S. canadensis (statistical results not shown). Anet remained depressed for at least 1 week after HS in heated plants, relative to unheated plants. Throughout the experimental duration, Anet was significantly decreased by heat-stress at peak and reproductive growing stages, compared with control plots. Anet was lowest for the plots heat-stressed at reproductive stage, followed by the plots heat-stressed at peak-growing and early-growing stage (Figure 4). Stomatal conductance to water vapor (gs) varied among different species and treatment. For A. gerardii and S. canadensis, gs was lower in heated plots. There was also a similar significant timing effect of HS on gs as on Anet, with lowest gs achieved at plots heat-stressed at reproductive stage (Figure 4; Table 1). Variation in internal CO2 (Ci) was also a function of species and treatment. For A. gerardii and S. canadensis, Ci was higher in heated plots. There was also a significant timing effect of HS on Ci, with highest Ci achieved at plots heat-stressed at reproductive stage than that of control plots and plots heat-stressed at early- and peak- growing stages (Figure 4; Table 1). The intrinsic water use efficiency (iWUE) was lower for the plants heat-stressed at the reproductive stages than control plants. There was no significant difference between control plots and plots heat-stressed at early- and peak- growing season (Figure 4).
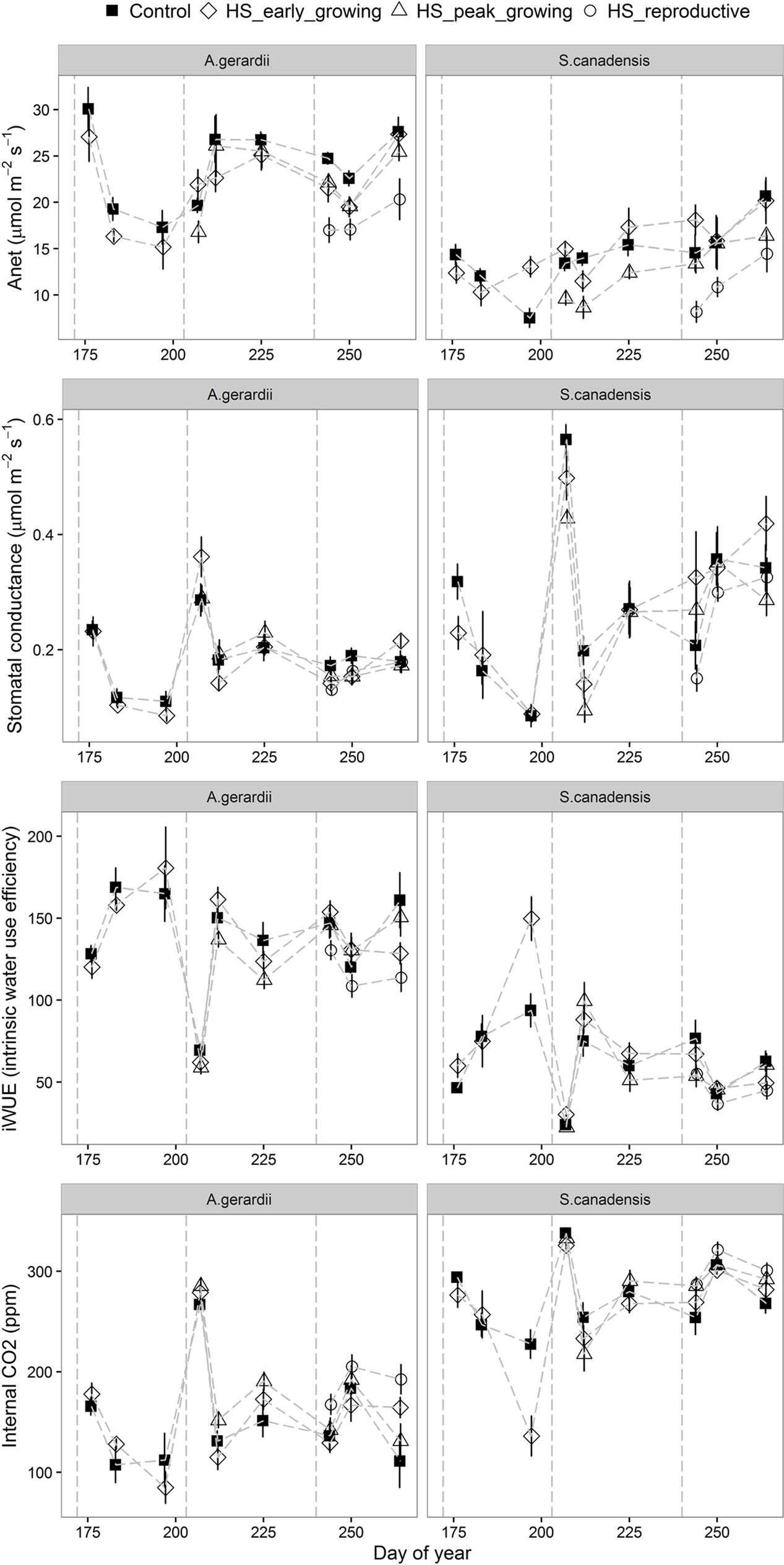
FIGURE 4. Effects of HS applied at different growing stage on net photosynthesis (Anet), stomatal conductance (gs), leaf internal CO2 (Ci) and intrinsic water use efficiency of A. gerardii and S. canadensis. Measurements were taken once during and twice after each heat-stress treatment. Values are means ± 1 SD; n = 4.
Quantum yield of PSII electron transport (ΦPSII) was higher for S. canadensis than for A. gerardii. HS at different growing stages played a significant role in affecting ΦPSII. HS decreased ΦPSII significantly when it was applied at the peak-growing season, compared with control plots (Figure 5). The content of chlorophyll (chlorophyll a + chlorophyll b) was significantly different among different treatments. For both species, chlorophyll content in newly developed leaves was significantly lower than that in the fully developed and senescent leaves (statistical not shown). The chlorophyll content was affected by heat-stress at different growing stages significantly. HS applied at different growing stages all lowered chlorophyll content significantly compared with control plots for both species. Chlorophyll content of the two species heat stressed at peak and reproductive growing stage was lower than that heat-stressed at early growing stages (Figure 6).
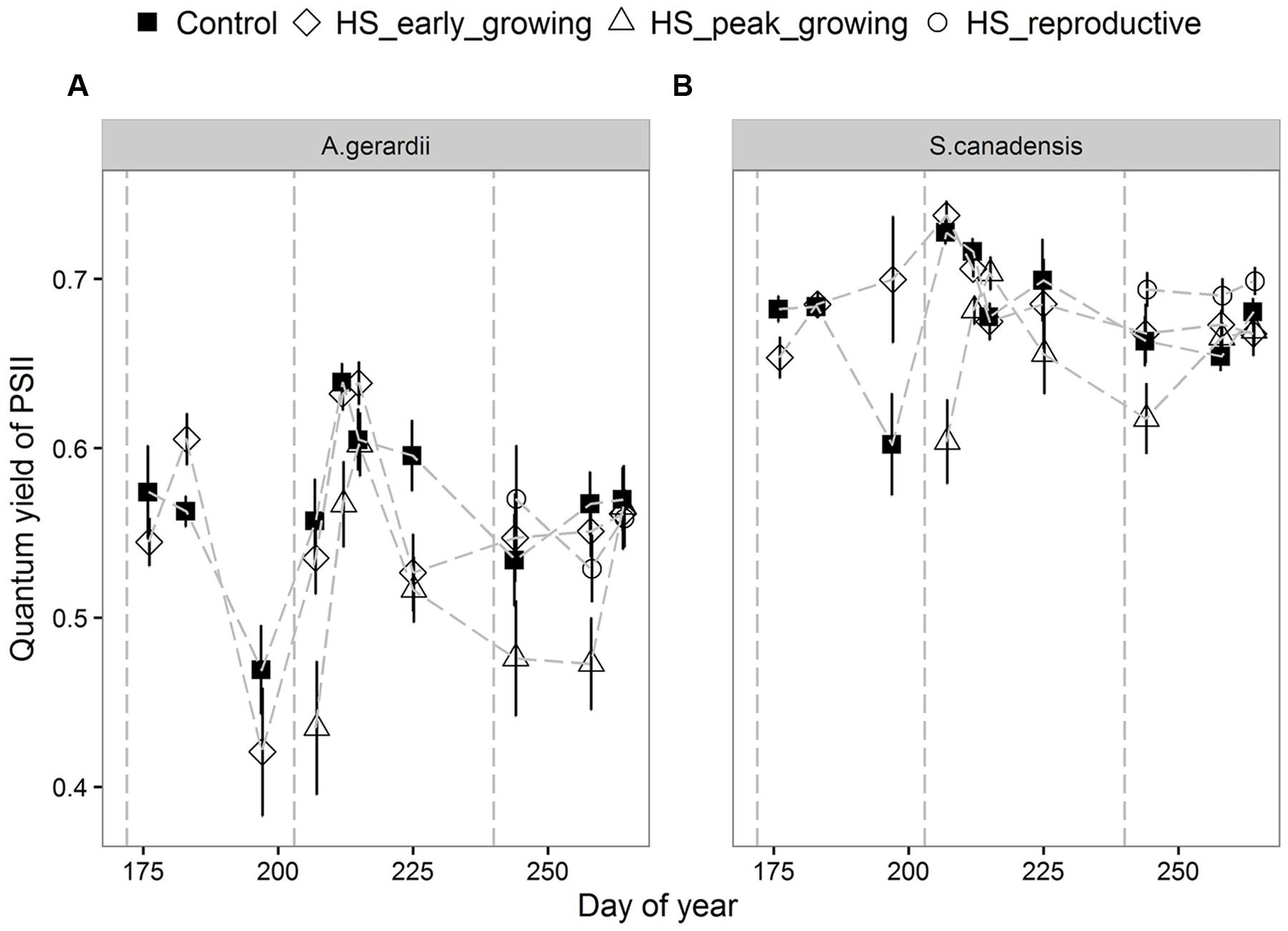
FIGURE 5. Effects of HS applied at different growing stage on quantum yield of photosystem II (ΦPSII) of (A) A. gerardii and (B) S. canadensis. Measurements were taken during and after each heat-stress treatment. Values are means ± 1 SD; n = 4.
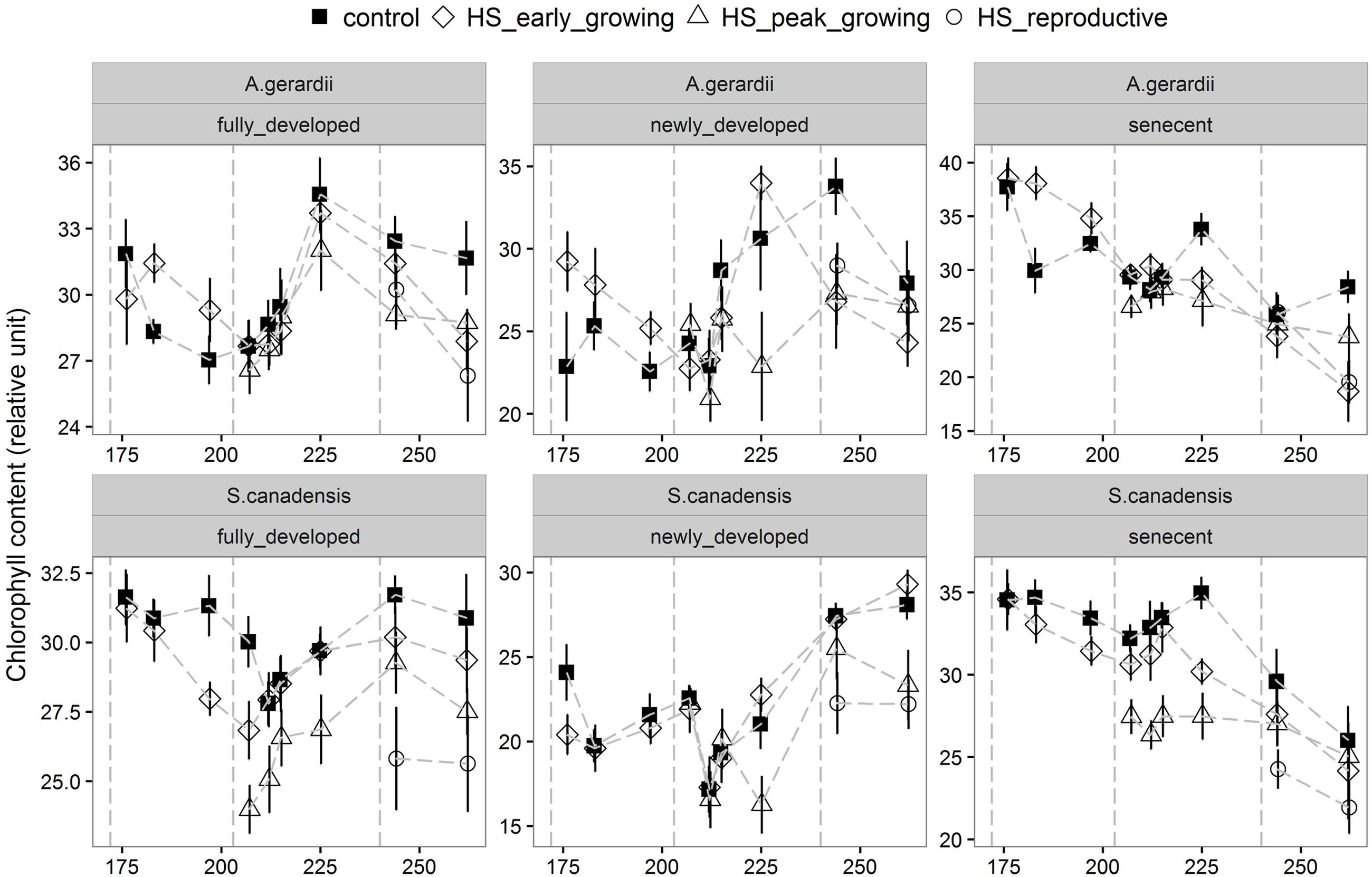
FIGURE 6. Effects of HS applied at different growing stage on relative chlorophyll content of the newly develop, fully developed and senescent leaves of A. gerardii and S. canadensis. Measurements were taken during and after each heat-stress treatment. Values are means ± 1 SD; n = 4.
Discussion
Extreme climate events have long been acknowledged as a universal phenomenon in recent years and caused great agricultural, economic and ecological consequences (IPCC, 2013). However, in natural field conditions, comprehensive investigations of the effect of HS occurring at different growing stage on plants ecophysiology and growth are still scarce. In this study, we simulated HS events in a tallgrass prairie and collected plant ecophysiological and growth data throughout a growing season. These in situ physiological and whole-plant responses of the two dominant species showed different sensitivity to temperature manipulations exposed at different growing stages. Overall, we found that (1) the physiology of both species and growth of S. canadensis were affected by HS treatment; (2) the degree of HS effect varied when it applied at different growing stages, with greater negative effect associated with HS applied at later-growing season; (3) the physiology and growth of the two dominant species showed differential sensitivity to HS, with S. canadensis being affected more than A. gerardii.
Both A. gerardii (C4) and S. canadensis (C3) experienced decreased Anet and intrinsic water use efficiency during HS. The decreases in Anet were still evident 1 week after heat treatment ended and the recovery to the control level took at least 1 week, which indicates that under moderate HS conditions (most commonly reported at temperatures between 35 and 40°C), photosynthesis can be reversibly reduced (Sharkey and Zhang, 2010; Huve et al., 2011). The direct effects of HS could have led to thermal damage to the photosynthetic machinery. We detected differences in Anet among the HS treatments persisting after the treatments ended, as well as a negative response in end of season aboveground productivity for S. canadensis, so there could have been significant thermal damage to the photosynthetic capacity of the two species. Photosynthesis can be reduced directly through non-stomatal limitations or indirectly through stomatal limitations under HS conditions (Sage et al., 2008; Bussotti et al., 2014). In this study, the decrease in Anet was associated with either reduced stomatal conductance for S. canadensis or down-regulation of quantum yield photosystem II (PSII) for both species (Rennenberg et al., 2006; Sage et al., 2008). Preventing excessive water loss and hydraulic failure through stomatal closure can also limit evaporative cooling and restrict CO2 input into the leaf, which is a strategy to save water before further damages happen due to increase in the temperature and/or drought stress (Bauweraerts et al., 2014; Teskey et al., 2014). The restrictions on CO2 input to the leaf due to stomatal closure resulted in reduced carbon assimilation (McDowell et al., 2008). In contrast to heat-induced stomatal closure, A. geraidii kept stomata relatively open under HS conditions which could enable effective transpirational cooling and prevent leaf from overheating (McDowell et al., 2008). Also, reduced intercellular CO2 concentration suggested that CO2 concentration also had negative effects on carbon assimilation, as observed previously in other species (Wang et al., 2008a,b, 2012).
When absorbed light are not dissipated efficiently as heat or used in the photosynthetic process, stomatal closure and reduced CO2 uptake can lead to the photo-oxidative stress (Demmig-Adams et al., 2012; Foyer et al., 2012). The quantum yield of PSII (ΦPSII) measures the proportion of light absorbed by chlorophyll associated with PSII system that is used in photochemistry (Baker and Rosenqvist, 2004). In this study, S. canadensis exhibited higher ΦPSII than A. gerardii during and after HS, but the decrease of ΦPSII compared to control samples during HS for A. gerardii was not significantly different from S. canadensis (Figure 4; Table 1). The significantly decreased ΦPSII suggested that both species engaged flexible heat dissipation in response to HS, presumably because the activation of Rubisco was inhibited at higher temperatures (Feller et al., 1998). The observed reduction of ΦPSII was indicative of acclimation responses or repair processes rather than sustained damages to PSII, because ΦPSII recovered after HS treatment ended. The relative chlorophyll content of the leaves in the two species decreased significantly after HS and most of them did not recover completely, which is more evident in S. canadensis and when HS was applied during the later-growing season. HS have also been found to decrease total chlorophyll content significantly in eight Australian wheat varieties when the temperature increased from 28 to 36°C during 6 days (Balouchi, 2010). Efeoglu and Terzioglu (2009) reported that the total chlorophyll content in two wheat cultivars did not change during an 8 h HS treatment of 37°C, but significantly decreased during an 8 h HS treatment of 45°C. The high chlorophyll contents have been associated with heat tolerance in some wheat varieties (Reynolds et al., 1997).
Specific leaf area of both species in this study increased due to HS treatment applied in the peak-growing season. Alterations in leaf structure are an important mode of acclimation in many species (Wright et al., 2005). Higher SLA is beneficial for obtaining higher potential evaporative demand and a more extensive foliar display that captures more light for constant biomass investment (Schuepp, 1993; Niinemets, 1999; Wright et al., 2004). SLA was reported to be higher in higher growth temperatures (Williams and Black, 1993; Loveys et al., 2002), while others reported no systematic temperature-induced change in SLA of five deciduous and evergreen tree species grown at five temperatures (Tjoelker et al., 1999). The impact of temperature on SLA therefore depends on which species is being investigated and the temperature regimes at which the plants are grown and treated.
The optimal temperature for photosynthesis ranges between 20 and 35°C for most plant species (Rennenberg et al., 2006; Sage et al., 2008). However, thermotolerance of photosynthesis to HS differs in different species (Berry and Björkman, 1980) and foliage types (Dreyer et al., 2001; Duan et al., 2014). The responses of photosynthesis to HS depends on adaptation strategies to habitat conditions (Knight and Ackerly, 2002; Cunningham and Read, 2006; Weston and Bauerle, 2007; Gunderson et al., 2010) and climate change scenarios such as CO2 elevation (Wang et al., 2008a, 2012). However, the species differences were not always found (Ghannoum et al., 2010) or the thermotolerance of species was reported to be unrelated to the temperature at their site of origin (Lin et al., 2013). In contrast with our hypothesis, both species showed reduced Anet during HS and the sensitivity of Anet of the two species responding to the HS did not vary significantly (Figure 5; Table 1). However, the C3 species, S. canadensis, tended to close stomata in response to HS, leading to reduced transpiration (and therefore reduced transpirational cooling upon HS). In contrast, the C4 species, A. gerardii, tended to keep stomata relatively open and maintained high transpiration rates which would limit negative temperature effects on the foliage.
The response of plants to HS was variable depending on the season or life stage during which the HS event happened. Plants were reported to be more susceptible to HS during later reproductive developmental stages (Cross et al., 2003). Early-growing or peak-growing season HS had neutral effects on plants growth for both species. In contrast, later-growing season HS significantly decreased the productivity of S. canadensis. Mid- or late- summer heat event was associated with strong physiological stress (De Boeck et al., 2011). In this study, ANPP and LAI of the plots heat-stressed at reproductive season was significantly lower than that of the control plots and the reduction was mostly caused by the negative HS effect on S. canadensis. The reduction in ANPP was mostly due to the experimental treatment, as the ratio between the two species in each treatment was not significantly different (Figure 2A). Consistently, the physiological performance of Anet, gs, and ΦPSII were all decreased more during HS applied at peak- or reproductive- growing stages.
Notably, in this study, the flower weight of the two species was not affected by HS (Figure 2B), which is contrary to what have reported that heat-stressed plants decreased flower production and produced later flowers on existing inflorescences (Sato et al., 2000; Cross et al., 2003). HS applied at different growing stage had no effect on mortality for the two species (data not shown). S. canadensis had similar mortality rate, while A. gerardii showed no mortality among different treatments. Andrello et al. (2012) reported increased mortality of juvenile plants in the endangered Eryngium alpinum L. during HS, while adult plants were less affected. Similarly, mortality of the Tenerife endemic Helianthemum juliae seedling reached nearly 100% in years of extreme drought (Marrero-Gómez et al., 2007).
Conclusion
Understanding the responses of dominant species to climate extremes is essential to predict future ecosystem dynamics and is particularly important when these species experience similar growing phenology but differ in their sensitivities to the climate factors. In this study, we examined the timing effects of HS on two dominant species in a tallgrass prairie ecosystem. There were two main conclusions drawn from this research. First, the photosynthetic and growth responses of these two species to HS were different, with S. canadensis being affected more than A. gerardii, indicated by the negative HS effect on both physiological and growth responses for S. canadensis; secondly, there were significant timing effect of HS on the two species, with greater reductions in photosynthesis and productivity occurred when HS was applied at later-growing season. The reduction in aboveground productivity in S. canadensis but not A. gerardii can increase the competitive advantage of A. gerardii, which therefore could have dramatic implications for species abundance, distribution and community structure. The present experiment showed that ephemeral HS may promote stochastic successions at the community level (Kreyling et al., 2011) or promote long-term effects on deterministic trajectories at the ecosystem scale (Allen and Breshears, 1998). It is worth pointing out that the negative HS effect in this study may be smaller than likely to occur, as HS treatment applied in this experiment was a single HS event and plants in Northwest Ohio experience multiple HS events per summer. Thus, the negative HS effect could be underestimated in this study and special caution should be paid when to predict long-term heat-stress consequences and differences between C3 and C4 plants. Furthermore, this study focused on plants ecophysiological processes and only examined short-term plant responses to HS within one generation of perennial plants. The results suggest that long-term effect of HS on plant communities and ecosystems dynamics should be studied more extensively and with longer experimental durations, particularly in combination with other potentially interactive aspects of global environmental change including increases in atmospheric CO2 and O3 concentration and altered precipitation pattern (Wang et al., 2014a,b).
Author Contributions
DW and SH came up with the research idea. DW led the experiment and writing. KM and RT assisted conducting the experiment and revising the manuscript.
Funding
Funding for this research was provided by grants from the Jiangsu Distinguished Professorship Award, National Science Foundation to SH and Nanjing University of Information Science and Technology, Jiangsu Natural Science Foundation (BK20150894), national natural science foundation of China (31500503) and the International S&T Cooperation Program of China (2012DFA60830) to DW.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Daryl Moorhead, Sandra Stutzenstein, and Walter Schulisch for providing access to experimental field sites, as well as for logistical support and assistance conducting the experiment. We thank Dr. Jiquan Chen for providing us vehicles and experimental equipments. We appreciate the reviewers for giving their advice for the manuscript.
Abbreviations
Anet, net photosynthetic rate (μmol m-2 s-1); ANPP, Aboveground net primary production (g); gs, stomatal conductance to water vapor (mol m-2 s-1); HS, heat stress; LAI, leaf area index (m2 m-2); LWC, (leaf water content, %); SLA, specific leaf area (m2 kg-1); Wa, aboveground biomass (g); Wf, biomass of flowers (g); iWUE, intrinsic water use efficiency (the ratio of Anet to gs); ΦPSII, quantum yield of electron transport of photoystem II.
Footnotes
- ^ http://oakopen.org/
- ^ http://www.ncdc.noaa.gov
- ^ http://www.r-project.org/
- ^ http://cran.r-project.org/web/packages/lme4/index.html
References
Ainsworth, E. A., Rogers, A., and Leakey, A. D. B. (2008). Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 147, 13–19. doi: 10.1104/pp.108.117101
Allen, C. D., and Breshears, D. D. (1998). Drought-induced shift of a forest-woodland ecotone: rapid landscape response to climate variation. Proc. Natl. Acad. Sci. U.S.A. 95, 14839–14842. doi: 10.1073/pnas.95.25.14839
Andrello, M., Bizoux, J. P., Barbet-Massin, M., Gaudeul, M., Nicole, F., and Till-Bottraud, I. (2012). Effects of management regimes and extreme climatic events on plant population viability in Eryngium alpinum. Biol. Conserv. 147, 99–106. doi: 10.1016/j.biocon.2011.12.012
Baker, N. R., and Rosenqvist, E. (2004). Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 55, 1607–1621. doi: 10.1093/jxb/erh196
Balouchi, H. (2010). Screening wheat parents of mapping population for heat and drought tolerance, detection of wheat genetic variation. Int. J. Biol. Life Sci. 6, 56–66.
Bauweraerts, I., Ameye, M., Wertin, T. M., McGuire, M. A., Teskey, R. O., and Steppe, K. (2014). Acclimation effects of heat waves and elevated [CO2] on gas exchange and chlorophyll fluorescence of northern red oak (Quercus rubra L.) seedlings. Plant Ecol. 215, 733–746. doi: 10.1007/s11258-014-0352-9
Berry, J. A., and Björkman, O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M., and West, G. B. (2004). Toward a metabolic theory of ecology. Ecology 85, 1771–1789. doi: 10.1890/03-0800
Bussotti, F., Ferrini, F., Pollastrini, M., and Fini, A. (2014). The challenge of Mediterranean sclerophyllous vegetation under climate change: from acclimation to adaptation. Environ. Exp. Bot. 103, 80–98. doi: 10.1016/j.envexpbot.2013.09.013
Ciais, P. H., Reichstein, M., Viovy, N., Granier, A., Ogée, J., Allard, V., et al. (2005). Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437, 529–533. doi: 10.1038/nature03972
Coleman, J. S., and Bazzaz, F. A. (1992). Effects of CO2 and temperature on growth and resource use of cooccurring C3 and C4 annuals. Ecology 73, 1244–1259. doi: 10.2307/1940673
Cross, R. H., Mckay, S. A. B., Mchughen, A. G., and Bonham-Smith, P. C. (2003). Heat-stress effects on reproduction and seed set in Linum usitatissimum L. (flax). Plant Cell Environ. 26, 1013–1020. doi: 10.1046/j.1365-3040.2003.01006.x
Cunningham, S. C., and Read, J. (2006). Foliar temperature tolerance of temperate and tropical evergreen rain forest trees of Australia. Tree Physiol. 26, 1435–1443. doi: 10.1093/treephys/26.11.1435
De Boeck, H. J., Dreesen, F. E., Janssens, I. A., and Nijs, I. (2011). Whole-system responses of experimental plant communities to climate extremes imposed in different seasons. New Phytol. 189, 806–817. doi: 10.1111/j.1469-8137.2010.03515.x
Demmig-Adams, B., Cohu, C. M., Muller, O., and Adams, W. W. (2012). Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth. Res. 113, 75–88. doi: 10.1007/s11120-012-9761-6
Dreyer, E., Roux, X. L., Montpied, P., Daudet, F. A., and Masson, F. (2001). Temperature response of leaf photosynthetic capacity in seedlings from seven temperate tree species. Tree Physiol. 21, 223–232. doi: 10.1093/treephys/21.4.223
Duan, B., Dong, T., Zhang, X., Zhang, Y., and Chen, J. (2014). Ecophysiological responses of two dominant subalpine tree species Betula albosinensis and Abies faxoniana to intra- and interspecific competition under elevated temperature. For. Ecol. Manag. 323, 20–27. doi: 10.1016/j.foreco.2014.03.036
Easterling, D. R., Meehl, G. A., and Parmesan, C. (2000). Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074. doi: 10.1126/science.289.5487.2068
Efeoglu, B., and Terzioglu, S. (2009). Photosynthetic response of two wheat varieties to high temperature. EurAsian J. Biosci. 3, 97–106. doi: 10.5053/ejobios.2009.3.0.13
Ehleringer, J. R., Cerling, T. E., and Helliker, B. R. (1997). C-4 photosynthesis, atmospheric CO2 and climate. Oecologia 112, 285–299. doi: 10.1007/s004420050311
Feller, U., Crafts-Brandner, S. J., and Salvucci, M. E. (1998). Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol. 116, 539–546. doi: 10.1104/pp.116.2.539
Foyer, C. H., Neukermans, J., Queval, G., Noctor, G., and Harbinson, J. (2012). Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 63, 1637–1661. doi: 10.1093/jxb/ers013
Frich, P., Alexander, L. V., Della-Marta, P., Gleason, B., Haylock, M., Tank, A. M. G. K., et al. (2002). Observed coherent changes in climatic extremes during the second half of the twentieth century. Clim. Res. 19, 193–212. doi: 10.3354/cr019193
Gaffen, D. J., and Ross, R. J. (1998). Increased summertime heat stress in the US. Nature 396, 529–530. doi: 10.1007/s00484-013-0634-2
Ghannoum, O., Phillips, N. G., Sears, M. A., Logan, B. A., Lewis, J. D., Conroy, J. P., et al. (2010). Photosynthetic responses of two eucalypts to industrial-age changes in atmospheric [CO2] and temperature. Plant Cell Environ. 33, 1671–1681. doi: 10.1111/j.1365-3040.2010.02172.x
Gunderson, C. A., O’Hara, K. H., Campion, C. M., Walker, A. V., and Edwards, N. T. (2010). Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob. Change Biol. 16, 2272–2286. doi: 10.1111/j.1365-2486.2009.02090.x
Gutschick, V. P., and BassiriRad, H. (2003). Extreme events as shaping physiology, ecology, and evolution of plants: toward a unified definition and evaluation of their consequences. New Phytol. 160, 21–42. doi: 10.1046/j.1469-8137.2003.00866.x
Henderson, K. G., and Muller, R. A. (1997). Extreme temperature days in the south-central United States. Clim. Res. 8, 151–162. doi: 10.1371/journal.pone.0042737
Huve, K., Bichele, I., Rasulov, B., and Niinemets, U. (2011). When it is too hot for photosynthesis: heat-induced instability of photosynthesis in relation to respiratory burst, cell permeability changes and H2O2 formation. Plant Cell Environ. 34, 113–126. doi: 10.1111/j.1365-3040.2010.02229.x
IPCC (2013). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jentsch, A., Kreyling, J., Elmer, M., Gellesch, E., Glaser, B., Grant, K., et al. (2011). Climate extremes initiate ecosystem-regulating functions while maintaining productivity. J. Ecol. 99, 689–702. doi: 10.1111/j.1365-2745.2011.01817.x
Karl, T. R., Nicholls, N., and Gregory, J. (1997). The coming climate. Sci. Am. 276, 78–83. doi: 10.1038/scientificamerican0597-78
Knight, C., and Ackerly, D. (2002). An ecological and evolutionary analysis of photosynthetic thermotolerance using the temperature-dependent increase in fluorescence. Oecologia 130, 505–514. doi: 10.1007/s00442-001-0841-0
Kreyling, J., Jentsch, A., and Beierkuhnlein, C. (2011). Stochastic trajectories of succession initiated by extreme climatic events. Ecol. Lett. 14, 758–764. doi: 10.1111/j.1461-0248.2011.01637.x
Lin, Y. S., Medlyn, B. E., De Kauwe, M. G., and Ellsworth, D. S. (2013). Biochemical photosynthetic responses to temperature: how do interspecific differences compare with seasonal shifts? Tree Physiol. 33, 793–806. doi: 10.1093/treephys/tpt047
Long, S. P., Ainsworth, E. A., Rogers, A., and Ort, D. R. (2004). Rising atmospheric carbon dioxide: plants face the future. Annu. Rev. Plant Biol. 55, 591–628. doi: 10.1146/annurev.arplant.55.031903.141610
Loveys, B. R., Scheurwater, I., Pons, T. L., Fitter, A. H., and Atkin, O. K. (2002). Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast- and slow-growing plant species. Plant Cell Environ. 25, 975–987. doi: 10.1046/j.1365-3040.2002.00879.x
Mainali, K. P., Heckathorn, S. A., Wang, D., Weintraub, M. N., Frantz, J. M., Hamilton, E. W. III, et al. (2014). Impact of a short-term heat event on C and N relations in shoots vs. Roots of the stress-tolerant C-4 grass, Andropogon gerardii. J. Plant Physiol. 171, 977–985. doi: 10.1016/j.jplph.2014.04.006
Marchand, F. L., Kockelbergh, F., Van De Vijver, B., Beyens, L., and Nijs, I. (2006). Are heat and cold resistance of arctic species affected by successive extreme temperature events? New Phytol. 170, 291–300. doi: 10.1111/j.1469-8137.2006.01659.x
Marchand, F. L., Mertens, S., Kockelbergh, F., Beyens, L., and Nijs, I. (2005). Performance of High Arctic tundra plants improved during but deteriorated after exposure to a simulated extreme temperature event. Glob. Change Biol. 11, 2078–2089. doi: 10.1111/j.1365-2486.2005.01046.x
Marrero-Gómez, M. V., Oostermeijer, J. G. B., and Carquelamo, E. (2007). Population viability of the narrow endemic Helianthemum juliae (Cistaceae) in relation to climate variability. Biol. Conserv. 136, 552–562. doi: 10.1016/j.biocon.2007.01.010
McDowell, N., Pockman, W. T., Allen, C. D., Breshears, D. D., Cobb, N., Kolb, T., et al. (2008). Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178, 719–739. doi: 10.1111/j.1469-8137.2008.02436.x
Niinemets, U. (1999). Components of leaf dry mass per area – thickness and density – alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 144, 35–47. doi: 10.1046/j.1469-8137.1999.00466.x
Niu, S., Luo, Y., Li, D., Cao, S., Xia, J., Li, J., et al. (2014). Plant growth and mortality under climatic extremes: an over-view. Environ. Exp. Bot. 98, 13–19. doi: 10.1016/j.envexpbot.2013.10.004
Rennenberg, H., Loreto, F., Polle, A., Brilli, F., Fares, S., Beniwal, R. S., et al. (2006). Physiological responses of forest trees to heat and drought. Plant Biol. 8, 556–571. doi: 10.1055/s-2006-924084
Richardson, A. D., Black, T. A., Ciais, P., Delbart, N., Friedl, M. A., Gobron, N., et al. (2010). Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R Soc. Lond. B Biol. Sci. 365, 3227–3246. doi: 10.1098/rstb.2010.0102
Reynolds, M., Nagarajan, S., Razzaque, M., and Ageeb, O. E. (1997). Using Canopy Temperature Depression to Select for Yield Potential of Wheat in Heat-Stressed Environments, Wheat Special Report No. 42. Mexico, DF: CIMMYT.
Sage, R. F., and Kubien, D. S. (2003). Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynth. Res. 77, 209–225. doi: 10.1023/A:1025882003661
Sage, R. F., Way, D. A., and Kubien, D. S. (2008). Rubisco, Rubisco activase, and global climate change. J. Exp. Bot. 59, 1581–1595. doi: 10.1093/jxb/ern053
Sato, S., Peet, M. M., and Thomas, J. F. (2000). Physiological factors limit fruit set of tomato (Lycopersicon esculentum Mill.) under chronic, mild heat stress. Plant Cell Environ. 23, 719–726. doi: 10.1046/j.1365-3040.2000.00589.x
Schuepp, P. H. (1993). Tansley Review No. 59. Leaf boundary layers. New Phytol. 125, 477–507. doi: 10.1111/j.1469-8137.1993.tb03898.x
Sentis, A., Hemptinne, J. L., and Brodeur, J. (2013). Effects of simulated heat waves on an experimental plant–herbivore–predator food chain. Glob. Chang. Biol. 19, 833–842. doi: 10.1111/gcb.12094
Sharkey, T. D., and Zhang, R. (2010). High temperature effects on electron and proton circuits of photosynthesis. J. Integr. Plant Biol. 52, 712–722. doi: 10.1111/j.1744-7909.2010.00975.x
Smith, M. (2011). An ecological perspective on extreme climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663. doi: 10.1111/j.1365-2745.2011.01798.x
Teskey, R., Wertin, T., Bauweraerts, I., Ameye, M., McGuire, M. A., and Steppe, K. (2014). Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 38, 1699–1712. doi: 10.1111/pce.12417
Tjoelker, M. G., Oleksyn, J., and Reich, P. B. (1999). Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob. Chang. Biol. 5, 679–691. doi: 10.1046/j.1365-2486.1999.00257.x
Thomas, C. D., Cameron, A., Green, R. E., Bakkenes, M., Beaumont, L. J., Collingham, Y. C., et al. (2004). Extinction risk from climate change. Nature 427, 145–148. doi: 10.1038/nature02121
Tripathi, A., Tripathi, D. K., Chauhan, D. K., Kumar, N., and Singh, G. S. (2016). Paradigms of climate change impacts on some major food sources of the world: a review on current knowledge and future prospects. Agric. Eecosyst. Environ. 216, 356–373. doi: 10.1016/j.agee.2015.09.034
Van Peer, L., Nijs, I., Reheul, D., and De Cauwer, B. (2004). Species richness and susceptibility to heat and drought extremes in synthesized grassland ecosystems: compositional vs physiological effects. Funct. Ecol. 18, 769–778. doi: 10.1111/j.0269-8463.2004.00901.x
Wang, D., Fan, J., and Heckathorn, S. A. (2014a). Acclimation of photosynthetic tolerance to acute heat stress at elevated CO2 and N. Plant Sci. 226, 162–171. doi: 10.1016/j.plantsci.2014.05.010
Wang, D., Fan, J., and Heckathorn, S. A. (2014b). Effects of CO2 on the tolerance of photosynthesis to heat stress can be affected by photosynthetic pathway and nitrogen. Am. J. Bot. 101, 34–44. doi: 10.3732/ajb.1300267
Wang, D., Heckathorn, S. A., Barua, D., Joshi, P., Hamilton, E. W., and Lacroix, J. J. (2008a). Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C-3, C-4, and CAM species. Am. J. Bot. 95, 165–176. doi: 10.3732/ajb.95.2.165
Wang, D., Heckathorn, S. A., Mainali, K., and Hamilton, E. W. (2008b). Effects of N on plant response to heat-wave: a field study with prairie vegetation. J. Integr. Plant Biol. 50, 1416–1425. doi: 10.1111/j.1744-7909.2008.00748.x
Wang, D., Heckathorn, S. A., Wang, X. Z., and Philpott, S. M. (2012). A meta-analysis of plant physiological and growth responses to temperature and elevated CO2. Oecologia 169, 1–13. doi: 10.1007/s00442-011-2172-0
Weston, D. J., and Bauerle, W. L. (2007). Inhibition and acclimation of C3 photosynthesis to moderate heat: a perspective from thermally contrasting genotypes of Acer rubrum (red maple). Tree Physiol. 27, 1083–1092. doi: 10.1093/treephys/27.8.1083
White, T. A., Campbell, B. D., Kemp, P. D., and Hunt, C. L. (2000). Sensitivity of three grassland communities to simulated extreme temperature and rainfall events. Glob. Change Biol. 6, 671–684. doi: 10.1046/j.1365-2486.2000.00344.x
White, T. A., Campbell, B. D., Kemp, P. D., and Hunt, C. L. (2001). Impacts of extreme climatic events on competition during grassland invasions. Glob. Change Biol. 7, 1–13. doi: 10.1046/j.1365-2486.2001.00381.x
Williams, D. G., and Black, R. A. (1993). Phenotypic variation in contrasting temperature environments: growth and photosynthesis in Pennisetum setaceum from different altitudes on Hawaii. Func. Ecol. 7, 623–633. doi: 10.2307/2390140
Wright, I. J., Reich, P. B., Cornelissen, J. H. C., Falster, D. S., Groom, P. K., Hikosaka, K., et al. (2005). Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14, 411–421. doi: 10.1111/j.1466-822x.2005.00172.x
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Xu, L., and Baldocchi, D. D. (2003). Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol. 23, 865–877. doi: 10.1093/treephys/23.13.865
Yan, Y. Y. (2002). Extreme temperature days in Hong Kong. Phys. Geogr. 23, 476–491. doi: 10.2747/0272-3646.23.6.476
Keywords: global climate change, photosynthesis, aboveground productivity, Solidago canadensis, Andropogon gerardii
Citation: Wang D, Heckathorn SA, Mainali K and Tripathee R (2016) Timing Effects of Heat-Stress on Plant Ecophysiological Characteristics and Growth. Front. Plant Sci. 7:1629. doi: 10.3389/fpls.2016.01629
Received: 25 July 2016; Accepted: 17 October 2016;
Published: 02 November 2016.
Edited by:
Raquel Esteban, University of the Basque Country, SpainReviewed by:
Rubén Retuerto, University of Santiago de Compostela, SpainBartosz Adamczyk, University of Helsinki, Finland
Copyright © 2016 Wang, Heckathorn, Mainali and Tripathee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Wang, wangdan.nuist@outlook.com
 Dan Wang
Dan Wang Scott A. Heckathorn2
Scott A. Heckathorn2 Rajan Tripathee
Rajan Tripathee