- 1State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, Chinese Academy of Sciences, Beijing, China
- 2Tourism and Air Service College, Guizhou Minzu University, Guizhou, China
- 3University of Chinese Academy of Sciences, Beijing, China
Elevated atmospheric CO2 typically enhances photosynthesis of C3 plants and alters primary and secondary metabolites in plant tissue. By modifying the defensive signaling pathways in host plants, elevated CO2 could potentially affect the interactions between plants, viruses, and insects that vector viruses. R gene-mediated resistance in plants represents an efficient and highly specific defense against pathogens and herbivorous insects. The current study determined the effect of elevated CO2 on tomato plants with and without the nematode resistance gene Mi-1.2, which also confers resistance to some sap-sucking insects including whitefly, Bemisia tabaci. Furthermore, the subsequent effects of elevated CO2 on the performance of the vector whiteflies and the severity of Tomato yellow leaf curl virus (TYLCV) were also determined. The results showed that elevated CO2 increased the biomass, plant height, and photosynthetic rate of both the Moneymaker and the Mi-1.2 genotype. Elevated CO2 decreased TYLCV disease incidence and severity for Moneymaker plants but had the opposite effect on Mi-1.2 plants whether the plants were agroinoculated or inoculated via B. tabaci feeding. Elevated CO2 increased the salicylic acid (SA)-dependent signaling pathway on Moneymaker plants but decreased the SA-signaling pathway on Mi-1.2 plants when infected by TYLCV. Elevated CO2 did not significantly affect B. tabaci fitness or the ability of viruliferous B. tabaci to transmit virus regardless of plant genotype. The results indicate that elevated CO2 increases the resistance of Moneymaker plants but decreases the resistance of Mi-1.2 plants against TYLCV, whether the plants are agroinoculated or inoculated by the vector. Our results suggest that plant genotypes containing the R gene Mi-1.2 will be more vulnerable to TYLCV and perhaps to other plant viruses under elevated CO2 conditions.
Introduction
The atmospheric CO2 concentration, which has risen from 280 to 400 ppm since the industrial revolution, now exceeds any level in the past 65,000 years and is predicted to reach 540–900 ppm by the end of this century (IPCC, 2013). Increases in atmospheric CO2 alter photosynthetic rates, carbohydrate accumulation, transpiration, and other aspects of plant physiology (Ainsworth and Long, 2005; Ainsworth et al., 2008). These effects can lead to changes in the primary and secondary metabolites in plant tissue, and may therefore affect interactions between plants and pathogens, between plants and insects, and between plants, viruses, and virus vectors (Chakraborty and Datta, 2003).
The effect of elevated CO2 on the incidence and severity of diseases caused by plant pathogens differs among pathogens. Free-air CO2 enrichment (FACE) studies have indicated that elevated CO2 increases plant susceptibility to certain fungal species (Kobayashi et al., 2006; Melloy et al., 2010) but reduces susceptibility to certain bacterial pathogens and some fungal species (Jwa and Walling, 2001; Zhang et al., 2015). These results were largely explained by the cross-talk between jasmonic acid (JA)- and salicylic acid (SA)-signaling pathways, which are vital for plant resistance against different types of pathogens (Eastburn et al., 2011; Zhang et al., 2015). Elevated CO2 increased plant resistance against Potato virus Y in tobacco and Tomato yellow leaf curl virus (TYLCV) in tomato (Matros et al., 2006; Huang et al., 2012). In the field, these plant viruses are transmitted by insect vectors, most of which are sap-sucking insects (i.e., aphids and whiteflies) whose performance could be affected by elevated CO2 (Sun et al., 2013; Wang et al., 2014). Some aphid species exhibit increased fecundity, abundance, and survival under elevated CO2 (Pritchard et al., 2007; Robinson et al., 2012). In contrast, elevated CO2 reduced whitefly abundance at 1000 ppm but had no effect at 700 ppm (Butler et al., 1986; Tripp et al., 1992; Wang et al., 2014). It is unclear whether the effects of elevated CO2 on the performance of insect vectors could in turn alter virus transmission to plants.
The interactions between insect vectors and plant viruses are often assumed to be mediated by plant defenses (Belliure et al., 2005; Colvin et al., 2006; Stout et al., 2006). A growing number of studies have reported that virus infection can decrease the resistance of host plants against insect vectors. Infection of tobacco plants by Tomato Yellow Leaf Curl China Virus (TYLCCNV) suppresses JA-dependent defenses and terpenoid synthesis, thereby favoring the performance of the whitefly vector, Bemisia tabaci, on virus-infected plants (Zhang et al., 2012; Luan et al., 2013). Viruliferous B. tabaci fed more than non-viruliferous B. tabaci and spent more time salivating into sieve tube elements, thereby enhancing virus infection and spread (Liu et al., 2013).
Tomato yellow leaf curl virus, which severely damages tomato crops in many tropical and subtropical regions worldwide (Czosnek and Laterrot, 1997; Zhang et al., 2009), is mainly transmitted by the whitefly B. tabaci in a persistent-circulative manner (Hogenhout et al., 2008). B. tabaci and TYLCV have a mutualistic relationship involving their shared host plants (McKenzie, 2002; Colvin et al., 2006; Jiu et al., 2007). Thus, the interaction between B. tabaci and the host plant is a key determinant of TYLCV transmission and infection.
In tomato, a well-studied R gene, Mi-1.2, encodes a protein with a nucleotide-binding domain and a leucine-rich repeat region (Milligan et al., 1998). Tomato plants with Mi-1.2 are resistant to three species of root-knot nematodes (Meloidogyne arenaria, M. incognita, and M. javanica) and sap-sucking insects such as whiteflies, aphids and pysllids. This gene reduces nematode or insect reproduction and abundance (Kaloshian et al., 1995; Milligan et al., 1998 ; Vos et al., 1998; Nombela et al., 2003; Casteel et al., 2006). Given that the Mi-1.2 gene confers a moderate level of resistance to whiteflies, we suspect that the Mi-1.2 gene might also affect TYLCV acquisition and transmission by its vectors.
In host plants not infected with virus, SA-signaling defenses reduce the feeding efficiency of viruliferous B. tabaci, which may subsequently affect TYLCV transmission and infection of plants (Shi et al., 2013). TYLCV infection alone can induce SA-dependent defenses, which increases the defense against subsequent feeding by B. tabaci (Huang et al., 2012; Shi et al., 2013). Moreover, the SA-signaling pathway is involved in R gene Mi-1.2-mediated resistance (Li et al., 2006). The transcript levels of PR1 in the resistant Mi-1.2 plants accumulated faster and at higher amounts than in the susceptible mi-1.2 plants after aphid infestation (Martinez de Ilarduya et al., 2003). Thus, the regulation of the SA-signaling pathway appears to be crucial for plant resistance against both virus and vector. In tomato and other crops, the SA-signaling pathway can be modified by the environment (Huang et al., 2012; Sun et al., 2013), suggesting that environmental change could affect phytohormone SA-induced defenses in Mi-1.2 contained plants, which may affect the severity of TYLCV and the fitness of vector B. tabaci.
In the current study, we assessed the effects of elevated CO2 on the tritrophic interactions among tomato, B. tabaci, and TYLCV. Two tomato cultivars were used: whitefly resistant cultivar Motelle (Mi-1.2) plants and its near-isogenic susceptible cultivar Moneymaker. We tested two hypotheses: (1) the Mi-1.2 genotype of tomato would reduce TYLCV transmission and severity due to the higher resistance ability, which may indirectly suppress B. tabaci fitness; and (2) elevated CO2 would enhance plant resistance against TYLCV and B. tabaci by up-regulating the SA- signaling pathway.
Materials and Methods
Open-Top Field Chambers and CO2 Levels and Plants
The experiment was carried out in eight open-top field chambers (OTCs). Four of the OTCs were continuously maintained at the current ambient level of CO2 (about 400 ppm), and four were continuously maintained at an elevated level of CO2 (about 750 ppm, the predicted level by the end of this century) (IPCC, 2013). Details of the automatic control system for CO2 concentrations and OTCs are provided in Chen et al. (2005). Air temperature was measured three times daily (8:00, 14:00, and 18:00) throughout the experiment and did not differ significantly between the two sets of OTCs during the experiment.
Two near-isogenic tomato (Solanum lycopersicum) lines, the susceptible cultivar Moneymaker and the resistant cultivar Motelle (Mi-1.2), were used in our experiments. Motelle carries a 650-kb segment of S. peruvianum DNA that harbors the Mi-1.2 gene, which makes it genetically distinct from Moneymaker (Milligan et al., 1998). These lines were selected for study due to whitefly resistance (Nombela et al., 2000). Seeds of Moneymaker and Mi-1.2 (Motelle) plants were obtained from the National Engineer and Research Center for Vegetable, Academy of Agricultural and Forestry Sciences, Beijing, China. One week after germination, when the cotyledons were beginning to expand, the seedlings were transplanted singly into plastic pots (25 cm × 21 cm × 22 cm) containing sterilized loamy field soil (organic carbon 75 g/kg; available N 500 mg/kg; available P 200 mg/kg; available K 300 mg/kg). The pots were placed in ventilated insect-proof cages in octagonal OTCs until they grew to the 3- to 4-leaf stage. Pot placement was re-randomized within each OTC once each week. No chemical fertilizers and insecticides were used. Water was added to each pot every 2 days. Five groups of plants were used for the experiments described in the following sections (Supplementary Figure S1).
Plant Growth Traits and Photosynthesis as Affected by Plant Genotype and CO2 Level (Group 1)
Six undamaged 8-week-old plants of each genotype in each OTC (=24 plants per treatment and 96 plants in total) were selected for measurement of photosynthetic rate and plant growth traits. The net photosynthetic rate was determined according to Guo et al. (2012) with some modification. The net photosynthetic rate of each plant was measured with a Li-Cor 6400 gas exchange system (Li-Cor Inc., Lincoln, NE, USA). The fourth mature leaf from the base of the stem was selected for measurement. All measurements were done between 9:00 and 12:00 am. The CO2 concentration of the incoming air was adjusted to 400 μmol mol-1 CO2 or 750 μmol mol-1. Relative humidity corresponded to ambient conditions. Before gas exchange was measured, photosynthetic active radiation for the leaf in the measuring cuvette was increased in steps to 1200 μmol m-2 s-1. When the CO2 assimilation rate was stable for at least 2 min, measurements were recorded. After that, the plants were harvested for measurement of biomass, stem diameter, and height.
TYLCV Incidence and Disease Index as Affected by Plant Genotype, CO2 Level, and Agroinoculation vs. Whitefly Virus Inoculation (Group 2)
The plant–virus interactions could be affected by both plant physiology and vector transmission ability, thus, in current study, we determined the effects of elevated CO2 on the disease incidence and index of TYLCV by either agroinoculation or transmitted by whitefly. For agroinoculation of TYLCV, 25 8-week-old plants of each genotype in each OTC (25 plants × 4 OTC × 2 genotypes × 2 CO2 levels and 400 plants in total) were selected and agroinoculated as described previously (Huang et al., 2012). The TYLCV infection of tomato plants was achieved using Agrobacterium tumefaciens-mediated infectious inoculation (Zhang et al., 2009; Al Abdallat et al., 2010), and the infectious 2 clone (pBINPLUS-SH2-1.4A) of TYLCV- Israel [China: Shangai2] was constructed into A. tumefaciens strain EHA105 as described previously (Zhang et al., 2009). The infectious clone of TYLCV was provided by Professor Xueping Zhou (State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, China). The culture of TYLCV clone was grown in LB culture medium with kanamycin (50 μg/ml) and rifampicin (50 μg/ml) at 28°C (250 rpm) for 24 h (OD600 = 1.5). The bacteria culture was centrifuged for 10 min at 2500 g and resuspended with 50 ml buffer (10 mM MgC12, 10 mM 2-(N-morpholino) ethanesulfonic acid, 200 μM acetosyringone) after which 0.2 ml of the culture was injected three times into the phloem (about 1 mm in depth) of the tomato stem at the three to four leaf stage to achieve inoculation; a sterile syringe (1 ml) with a beveled needle (0.5 mm × 20 mm) was used for injection. Inoculated plants were grown in ventilated cages in the OTCs. The incidence of TYLCV infection (percentage of plants with disease symptoms) and the disease index were determined 6 weeks after agroinoculation. Disease index values were determined as follows (Curvers et al., 2010):
where Ni represents the number of plants in disease symptom ranking i, Ri represents the disease symptom rank (i = 0–4), N represents the total number of plants investigated, and Rh represents the highest disease symptom rank. Disease symptoms were ranked mainly according to Friedmann et al. (1998): 0 = no visible symptoms: inoculated plants show the same growth and development as non-inoculated plants; 1 = very slight yellowing of apical leaf margins; 2 = some yellowing and minor curling of leaf ends; 3 = widespread leaf yellowing, curling, and cupping, with some reduction in size, but plants continue to develop; 4 = severe plant stunting and yellowing, and pronounced cupping and curling of leaves; plants stop growing.
Bemisia tabaci of the B biotype (Middle East Asia Minor 1, aka MEAM 1), which were kindly provided by Professor Youjun Zhang of the Institute of Vegetable and Flower, Chinese Academy of Agricultural Sciences, were reared on cabbage (non-host of TYLCV) grown in insect-proof wooden cages as previously described (Jiu et al., 2007). Viruliferous whiteflies were caged on the TYLCV-infected tomato plants in a separate greenhouse. Whiteflies from the viruliferous colony were confirmed to be infected with TYLCV prior to infestation by PCR analysis (Zhang et al., 2009). For transmission of TYLCV to tomato plants by B. tabaci, 60 8-week-old plants of each genotype in each OTC were randomly selected, and each of 20 plants was infested by 5, 15, or 25 viruliferous B. tabaci for 48 h (20 plants × 4 OTC × 2 genotypes × 2 CO2 levels × 3 whiteflies densities and 960 plants in total). The virus incidence and disease index of the tomato plants were determined 6 weeks after B. tabaci infestation.
The Abundance and Fecundity of B. tabaci as Affected by Plant Genotype, CO2 Level, and TYLCV Infection (Group 3)
To determine the effect of TYLCV infection on B. tabaci numbers and fecundity on tomato, 16 5-week-old plants of each genotype in each OTC were randomly selected. Eight plants were agroinoculated with TYLCV, and the other eight were not. Three weeks later, we checked the TYLCV copy numbers of the new emerged leaf by qPCR and confirmed that they are all successfully infected by TYLCV. Then, 4 8-week plants from each tomato genotype and TYLCV treatment per OTC (4 plants × 4 OTC × 2 genotypes × 2 CO2 levels × 2 TYLCV treatment and 128 plants in total) were selected. Five newly emerged females and five newly emerged males were released onto each plant; each plant was kept in a separate whitefly proof, ventilated cage (120 mesh). After 28 days, the numbers of each developmental stage of B. tabaci were determined for each of the four replicates in each OTC.
To determine the effect of TYLCV infection of tomato on B. tabaci fecundity, 4 8-week plants from each tomato genotype and TYLCV treatment per OTC (4 plants × 4 OTC × 2 genotypes × 2 CO2 levels × 2 TYLCV treatment and 128 plants in total) were randomly selected, one mated females were introduced into each plant with a whitefly proof, ventilated cage. The females were then transferred daily to fresh leaves until they died, and the number of eggs deposited by each female was determined.
Acquisition and Transmission of TYLCV by B. tabaci as Affected by Plant Genotype and CO2 Level (Group 4)
Forty-eight 4-week-old tomato plants were agroinoculated with the virus. Once the plants exhibited obvious symptoms 4 weeks later and were confirmed as TYLCV infected by detecting the TYLCV copies with RT-PCR in the systemic leaves according to Zhang et al. (2009), we started to inoculate whiteflies. To determine the effects of plant genotype and CO2 level on transmission of TYLCV by B. tabaci, 100 adult whiteflies were caged on the second true leaf (numbered from the apex down) of each TYLCV-infected tomato plants to obtain enough viruliferous whiteflies. After a 48-h acquisition access period, 20 viruliferous whiteflies were then caged on the second true leaf of each of four 5-week-old tomato plants (at the four-leaf stage) at three time points of each genotype in each OTC (4 plants × 4 OTC × 2 genotypes × 2 CO2 levels × 3 three time points and 192 plants in total) (Rubinstein and Czosnek, 1997). The whiteflies were removed after 8, 24, and 48 h inoculation access period. Infection was assessed 4 weeks later based on the appearance of TYLCV symptoms and on the number of copies of TYLCV in the leaf tissue, which was determined according to Zhang et al. (2009).
To determine the effects of plant genotype and CO2 level on the acquisition of TYLCV by B. tabaci, four six-leaf stage virus-infected tomato plants (9-week-old) of each genotype at each time point in each OTC were selected (4 plants × 4 OTC × 2 genotypes × 2 CO2 levels × 3 three time points and 192 plants in total); the plants had been agroinoculated about 4 weeks earlier. Before releasing whiteflies, we confirmed as TYLCV infected by detecting the TYLCV copies with PCR in the systemic leaves according to Zhang et al. (2009). Fifty adult B. tabaci were caged on the second true leaf (numbered from the apex down). After acquisition access periods of 2, 8, and 24 h, ten B. tabaci were removed from each cage, and the TYLCV copy number in each group of ten B. tabaci was determined.
Quantification of Phytohormone Content, Defensive Enzyme Activity, and Defensive Gene Expression (Group 5)
For measurement of the contents of the phytohormones JA and SA and the activities of the defensive enzymes phenylalanine ammonia lyase (PAL) and lipoxygenase (LOX) in tomato plants as affected by TYLCV and CO2 level, four 5-week-old plants of each genotype in each OTC were agroinoculated with TYLCV; another four plants of each genotype in each OTC were not inoculated and served as controls. Four weeks later, 500 mg of leaves were collected from each plant. The leaf samples were immediately stored in liquid N until analyzed.
For measurement of expression of JA- and SA-dependent defense genes, 16 5-week-old plants of each genotype in each OTC were agroinoculated with TYLCV, and another 16 plants of each genotype in each OTC were not inoculated and served as controls. After 0, 2, 8, and 24 h, the leaves of four plants (±inoculation) of each genotype in each OTC were harvested. The leaf samples were immediately stored in liquid N until analyzed.
Measurement of Phytohormone Content and Defensive Enzyme Activity
The contents of endogenous JA and SA in the plant leaves were measured as described by Sun et al. (2013). The activities of PAL and LOX were measured according to Guo et al. (2012).
Real-Time Quantitative PCR of Defensive Gene Expression
For real-time quantitative PCR, each treatment sample had four technical replicates for each of the biological replications. The RNeasy Mini Kit (Qiagen, Valencia, CA, USA) was used to isolate total RNAs from tomato leaves (0.05 g from samples stored at -70°C), and about 2 μg quantities of the RNAs were used to generate the cDNAs with the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA, USA). The mRNA amounts of four target genes were quantified by real-time quantitative PCR: proteinase inhibitor (PI-1), lipoxygenase (LOX2), phenylalanine ammonia lyase (PAL5), and pathogenesis-related protein (PR1a). Specific primers for each gene were designed from the tomato EST sequences using PRIMER5 software (Supplementary Table S1). The PCR reactions were performed in a 20-μL total reaction volume including 10 μL of 2x SYBRs Premix EX TaqTM (Qiagen) master mix, 5 mM of each gene-specific primer, and 1 μL of cDNA template. PCR reactions were carried out on an Mx 3000P detection system (Stratagene, USA) as follows: 5 min at 95°C; then 40 cycles of 10 s at 95°C and 20 s at 62°C; and finally one cycle of 30 s at 95°C, 30 s at 55°C, and 30 s at 95°C. A standard curve was derived from the serial dilutions to quantify the copy numbers of target mRNAs. The relative level of each target gene was standardized by comparing the copy numbers of target mRNAs with the copy number of β-actin (Actin7) (the housekeeping gene; Zhai et al., 2013), which remains constant under different treatment conditions. The β-actin mRNAs of the control were examined in every plate of PCR to eliminate systematic error.
Statistical Analyses
All data were checked for normality and equality of residual error variances and were appropriately transformed (log or square-root) if needed to satisfy the assumptions of analysis of variance. A split-split plot design was used to analyze the univariate responses of the phytohormone contents, enzyme activities, and gene expression in plants (ANOVA, PASW Statistics 18.0, SPSS Inc., Chicago, IL, USA). In the following ANOVA model, CO2 and block (a pair of OTCs with ambient and elevated CO2) were the main effects, tomato genotype was the subplot effect, and TYLCV infection level was the sub-subplot effect:
where C is the CO2 treatment (i = 2), B is the block (j = 4), G is the tomato genotype (k = 2), and H is the virus infection treatment (l = 2). em(ijkl) represents the error because of the smaller scale differences between samples and variability within blocks (ANOVA, SAS Institute). Effects were considered significant if P < 0.05. Because the effect of block and the interactive effects of block and other factors were not significant (P > 0.45), the effect of block and its interaction with other factors are not presented to simplify the presentation. Tukey’s multiple range tests were used to separate means when ANOVAs were significant. For analysis of the plant growth traits (biomass, stem diameter, plant height, and photosynthetic rate), TYLCV incidence and index, and the ability of B. tabaci to acquire and transmit TYLCV under two CO2 levels, a split-plot design was also applied, with CO2 and block as the main effects and tomato genotype as the subplot effect.
Results
Plant Growth Traits and Photosynthesis as Affected by Plant Genotype and CO2 Level (Group 1)
Under ambient CO2, growth and photosynthesis did not significantly differ between Moneymaker and Mi-1.2 plants except for the height (Figure 1; Supplementary Table S2). Elevated CO2 increased biomass by 38.2%, height by 28.6%, and photosynthetic rate by 75.1% for Moneymaker plants, and increased biomass by 15.5%, height by 33.3%, and photosynthetic rate by 62.3 % for Mi-1.2 plants. Mi-1.2 plants had a lower biomass, a lower photosynthetic rate, and a greater height than Moneymaker plants under elevated CO2 (Figures 1A,C,D).
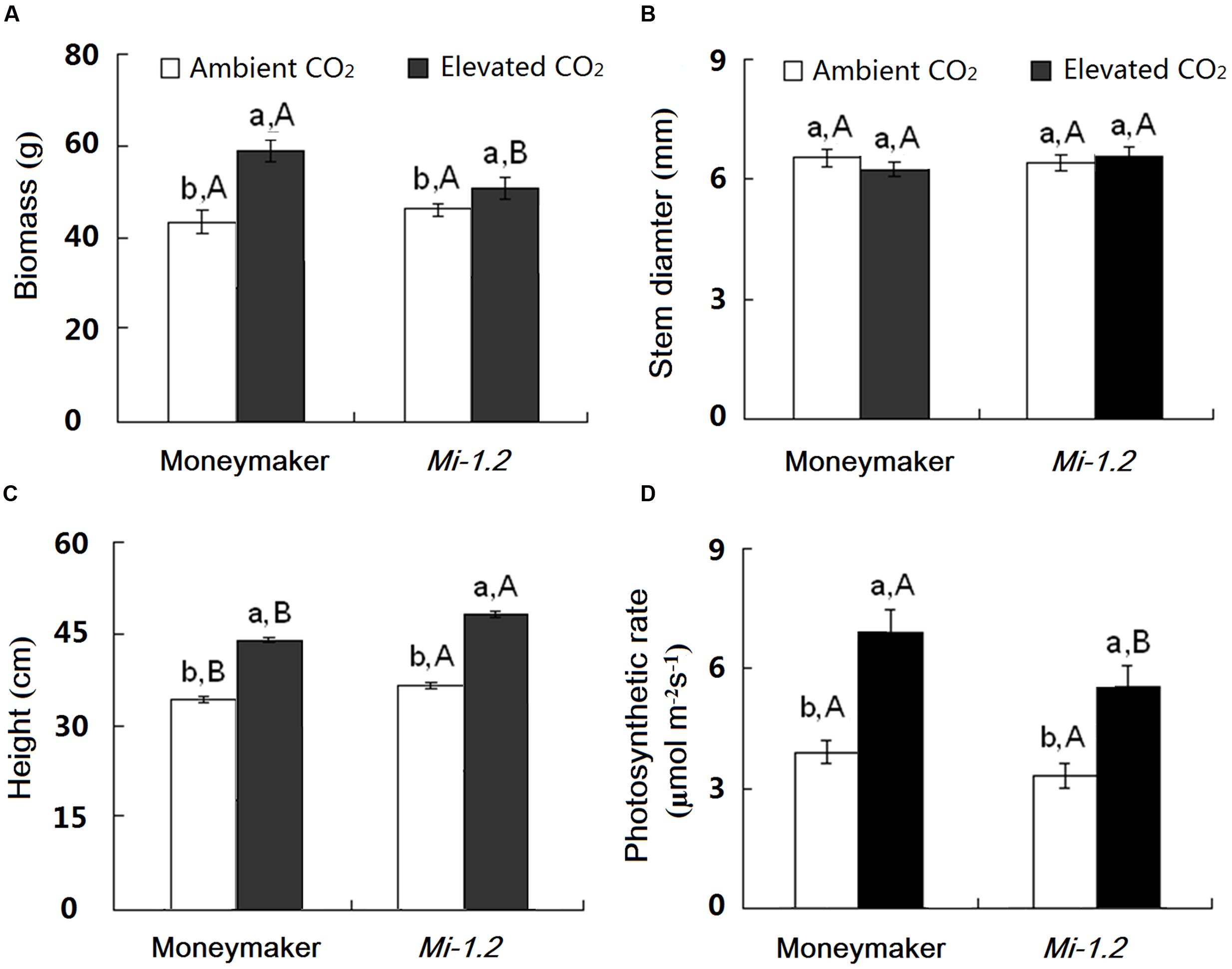
FIGURE 1. Growth traits of two tomato genotypes (Moneymaker and Mi-1.2) grown under ambient CO2 and elevated CO2. (A) Biomass, (B) Stem diameter, (C) Height, and (D) Photosynthetic rate. Different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 within the same genotype. Different uppercase letters indicate significant differences between genotypes within the same CO2 treatment.
TYLCV Incidence and Disease Index as Affected by Plant Genotype, CO2 Level, and Agroinoculation vs. Whitefly Virus Inoculation (Group 2)
For the plants that were agroinoculated with TYLCV, elevated CO2 significantly decreased TYLCV disease incidence and index values for Moneymaker plants but increased those values for Mi-1.2 plants (Figure 2; Supplementary Table S3). For plants that were inoculated with TYLCV by B. tabaci, TYLCV incidence and index values increased as the number of B. tabaci added increased (Figure 3; Supplementary Table S4). Elevated CO2 decreased the TYLCV incidence and disease index values for Moneymaker plants but increased those values for Mi-1.2 plants when infested by the same number of viruliferous B. tabaci (Figure 3).
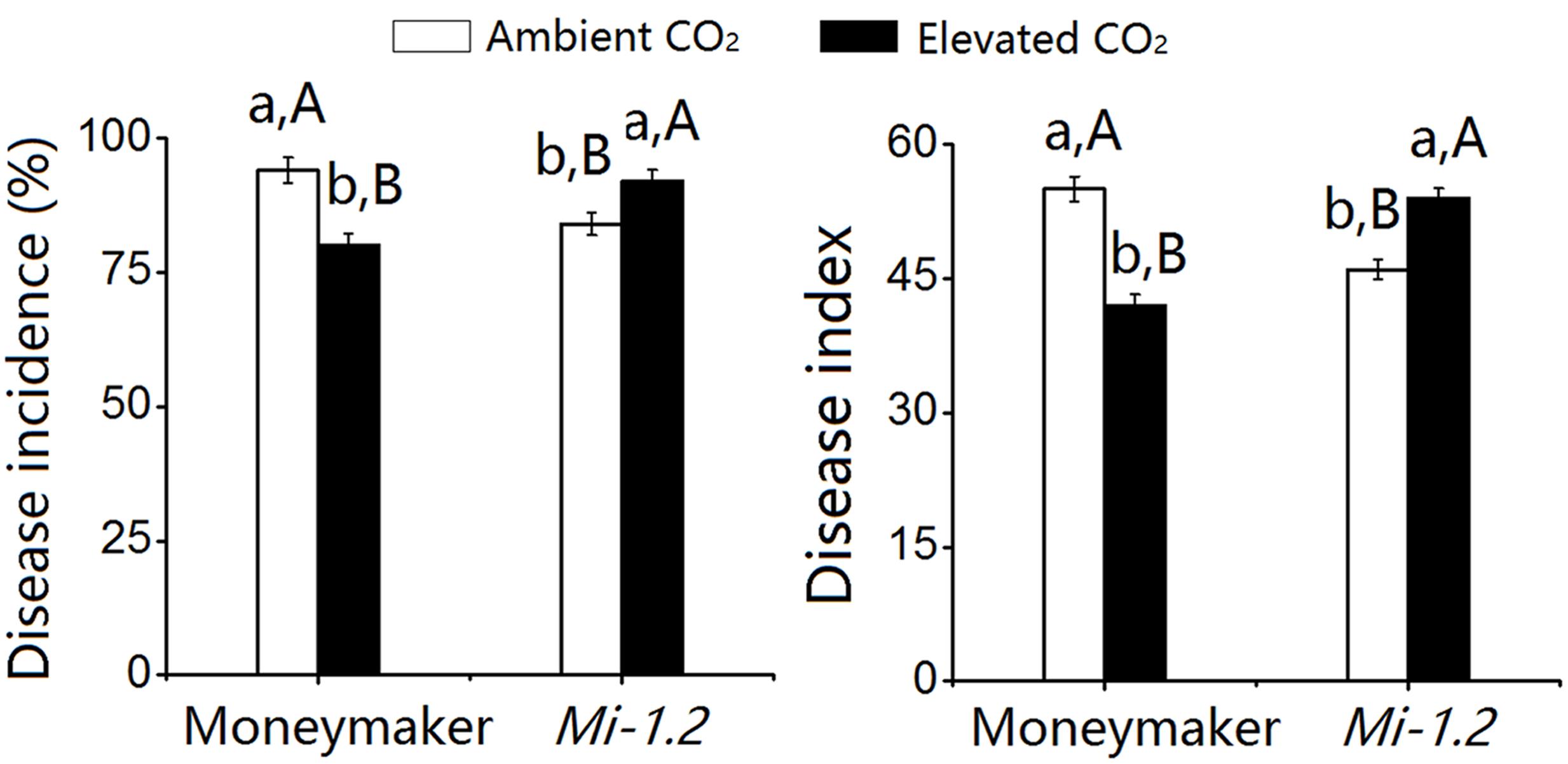
FIGURE 2. Tomato yellow leaf curl virus (TYLCV) disease incidence and index values in two tomato genotypes (Moneymaker and Mi-1.2) that were agroinoculated with the virus and then grown under ambient CO2 and elevated CO2. Different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 within the same genotype. Different uppercase letters indicate significant differences between genotypes within the same CO2 treatment. Means were compared with Tukey’s multiple range test at P < 0.05.
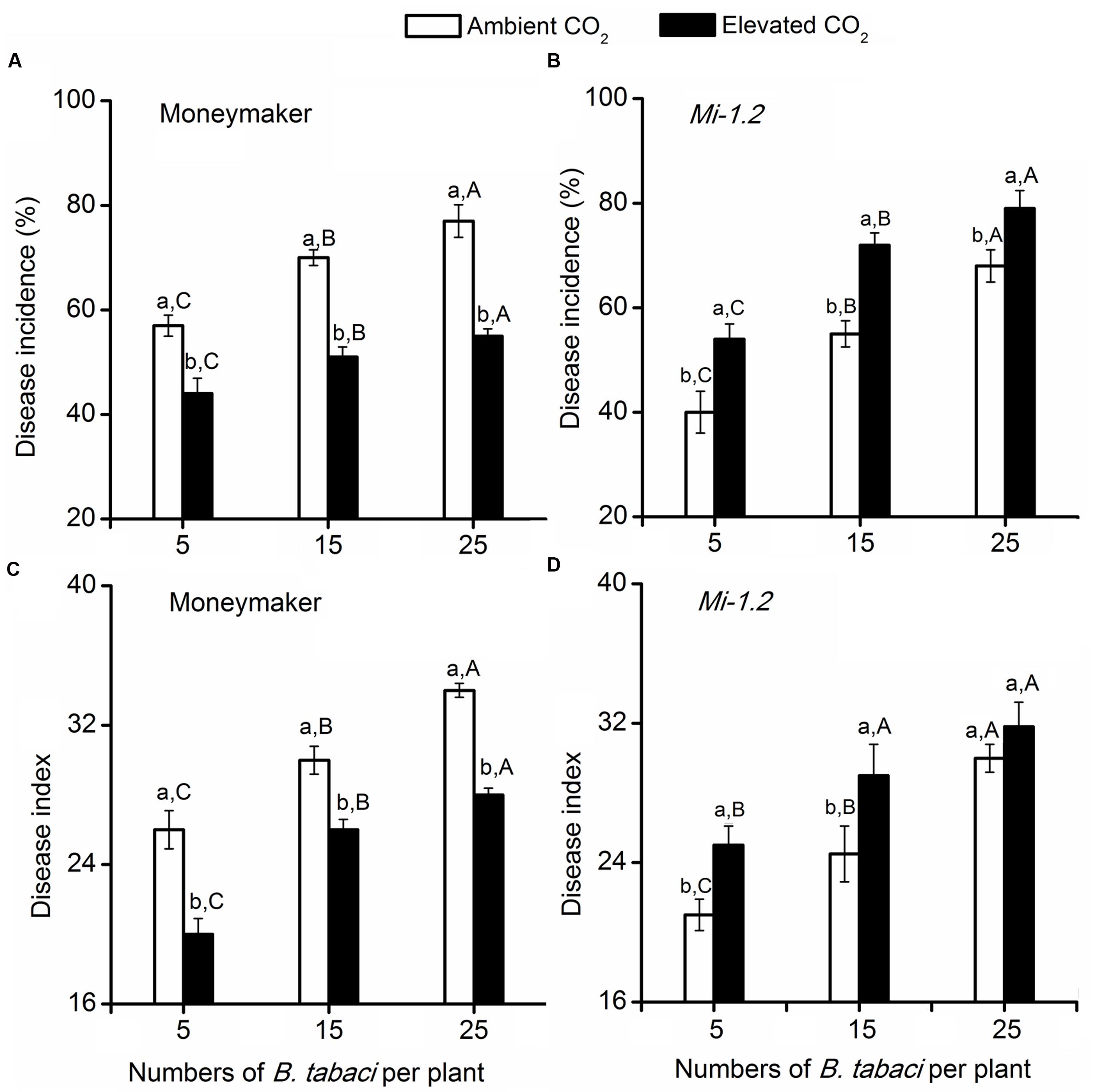
FIGURE 3. Tomato yellow leaf curl virus disease incidence and index values in two tomato genotypes (Moneymaker and Mi-1.2) that were infested with different numbers of viruliferous Bemisia tabaci and grown under ambient CO2 and elevated CO2. (A) Disease incidence of Moneymaker, (B) Disease incidence of Mi-1.2, (C) Disease index of Moneymaker, and (D) Disease index of Mi-1.2. Different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 within the same B. tabaci density. Different uppercase letters indicate significant differences among B. tabaci densities within the same CO2 treatment.
Abundance and Fecundity of B. tabaci as Affected by Plant Genotype, CO2 Level, and TYLCV Infection (Group 3)
Elevated CO2 did not significantly affect the abundance or fecundity of B. tabaci on either healthy or virus-infected plants regardless of plant genotype (Figure 4; Supplementary Table S5). Fecundity was lower on healthy Mi-1.2 plants than on healthy Moneymaker plants under ambient CO2. Under elevated CO2, in contrast, neither B. tabaci fecundity nor abundance significantly differed between the two plant genotypes. B. tabaci abundance and fecundity were lower on TYLCV-infected plants than on healthy plants regardless of CO2 level or plant genotype (Figure 4).
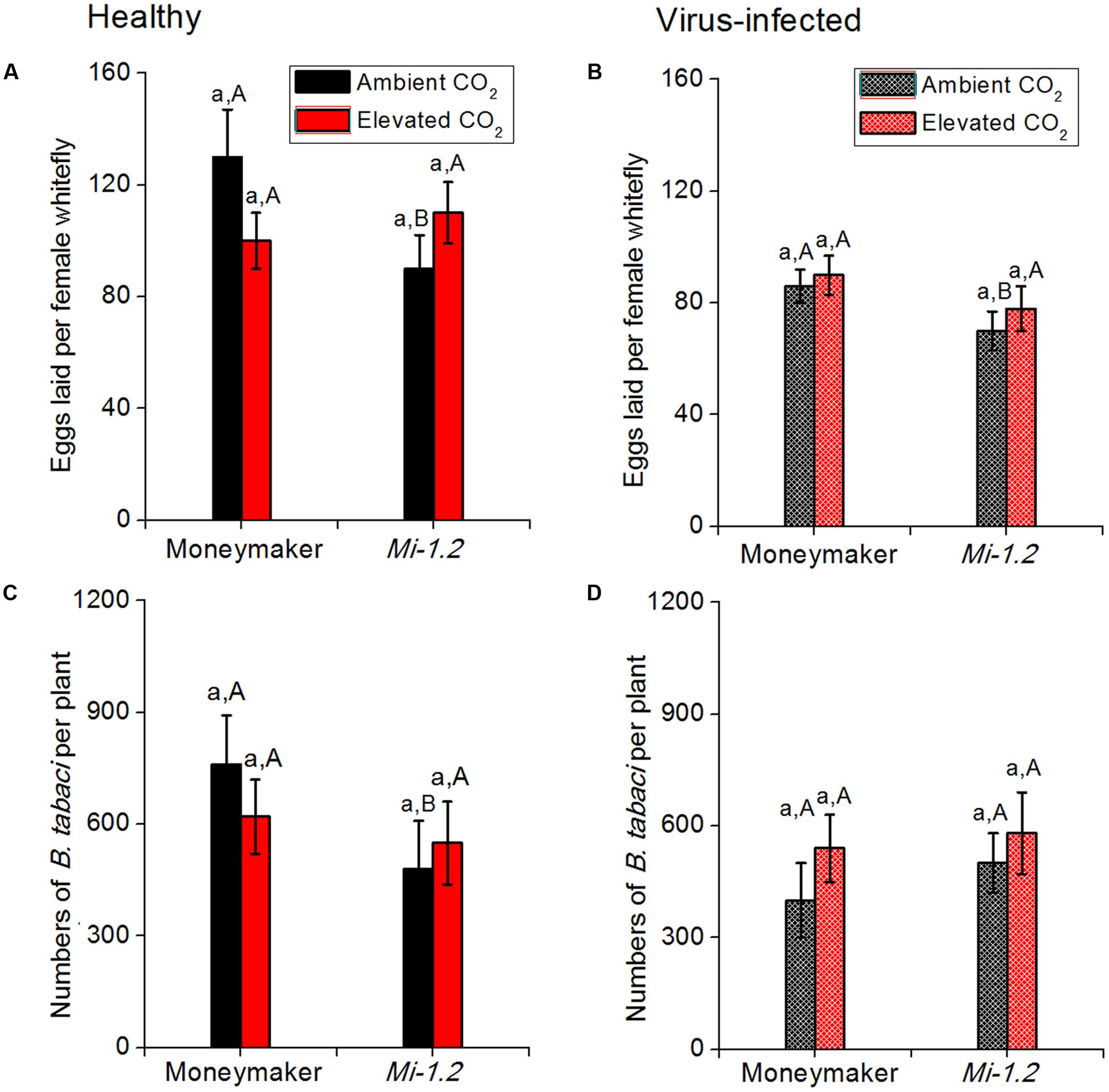
FIGURE 4. Fecundity and abundance of B. tabaci on tomato plants (Moneymaker and Mi-1.2) that were agroinoculated or not infected with TYLCV and grown under ambient CO2 or elevated CO2. (A,B) Fecundity of B. tabaci on healthy and virus-infected plants; (C,D) Abundance of B. tabaci on healthy and virus-infected plants. Different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 within the same genotype. Different uppercase letters indicate significant differences in B. tabaci numbers within the same CO2 treatment.
Acquisition and Transmission of TYLCV by B. tabaci as Affected by Plant Genotype and CO2 Level (Group 4)
After whiteflies had fed on the TYLCV-infected plants for 2, 24, or 48 h, the number of TYLCV-DNA copies per B. tabaci was significantly lower under elevated CO2 than under ambient CO2 in the case of Moneymaker plants but the opposite was true in the case of Mi-1.2 plants (Figures 5A,B). Under ambient CO2, B. tabaci contained fewer TYLCV-DNA copies when reared on TYLCV-infected Mi-1.2 plants than on TYLCV-infected Moneymaker plants (Figures 5A,B). Under elevated CO2, B. tabaci contained a higher number of TYLCV-DNA copies when reared on TYLCV-infected Mi-1.2 plants than on TYLCV-infected Moneymaker plants (Figures 5A,B; Supplementary Table S6), which is consistent with the TYLCV disease incidence and index of both genotypes before whitefly acquired TYLCV from plants (Supplementary Figure S2).
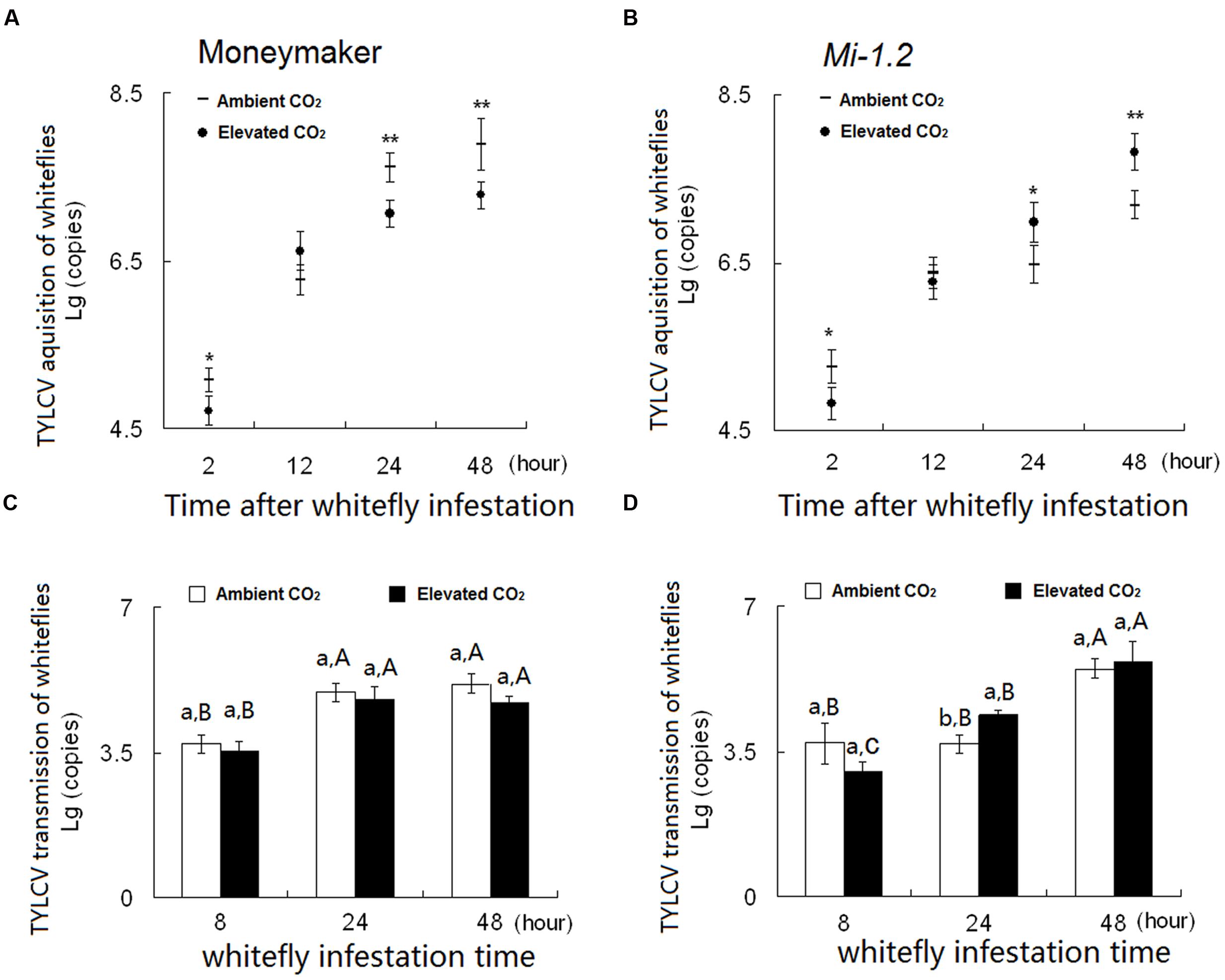
FIGURE 5. Number of TYLCV-DNA copies acquired per B. tabaci when feeding on (A) Moneymaker plants and on (B) Mi-1.2 plants. Number of TYLCV-DNA copies per gram of tissue in (C) Moneymaker plants and (D) Mi-1.2 plants infested with viruliferous B. tabaci. Within (A) and (B), ∗ and ∗∗ indicate a significant difference in copy number between ambient and elevated CO2 at the same time point at P < 0.05 and 0.01, respectively. Within (C) and (D), different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 at the same time point, and different uppercase letters indicate significant differences within the same CO2 treatment at P < 0.05. In all cases, means were compared with Tukey’s multiple range test.
After viruliferous B. tabaci had fed on plants for 24 h, numbers of TYLCV-DNA copies in Moneymaker plants were unaffected by CO2 level but were higher in Mi-1.2 plants under elevated CO2 than under ambient CO2 (Figures 5C,D). After a 48 h transmission access period, Mi-1.2 plants contained fewer TYLCV-DNA copies than Moneymaker plants under ambient CO2 but contained higher numbers of TYLCV-DNA copies under elevated CO2 (Figures 5C,D).
SA and JA Content and Defensive Enzyme Activity
In Moneymaker plants that were not infected by TYLCV, elevated CO2 increased SA content and PAL activity but decreased JA content and LOX activity (Figure 6; Supplementary Table S7). Elevated CO2 increased SA content and decreased JA content of Mi-1.2 plants (Figures 6A,B). After agroinoculation of TYLCV infection for 48 h, elevated CO2 increased the SA and JA contents and PAL and LOX activities of Moneymaker plants. In contrast, elevated CO2 decreased SA and PAL activity but increased JA content and LOX activity of Mi-1.2 plants (Figure 6). Under ambient CO2, SA content and PAL activity were lower in infected Moneymaker plants than in infected Mi-1.2 plants. Under elevated CO2, however, SA content and PAL activity were lower in the Mi-1.2 plants than in Moneymaker plants regardless of TYLCV infection.
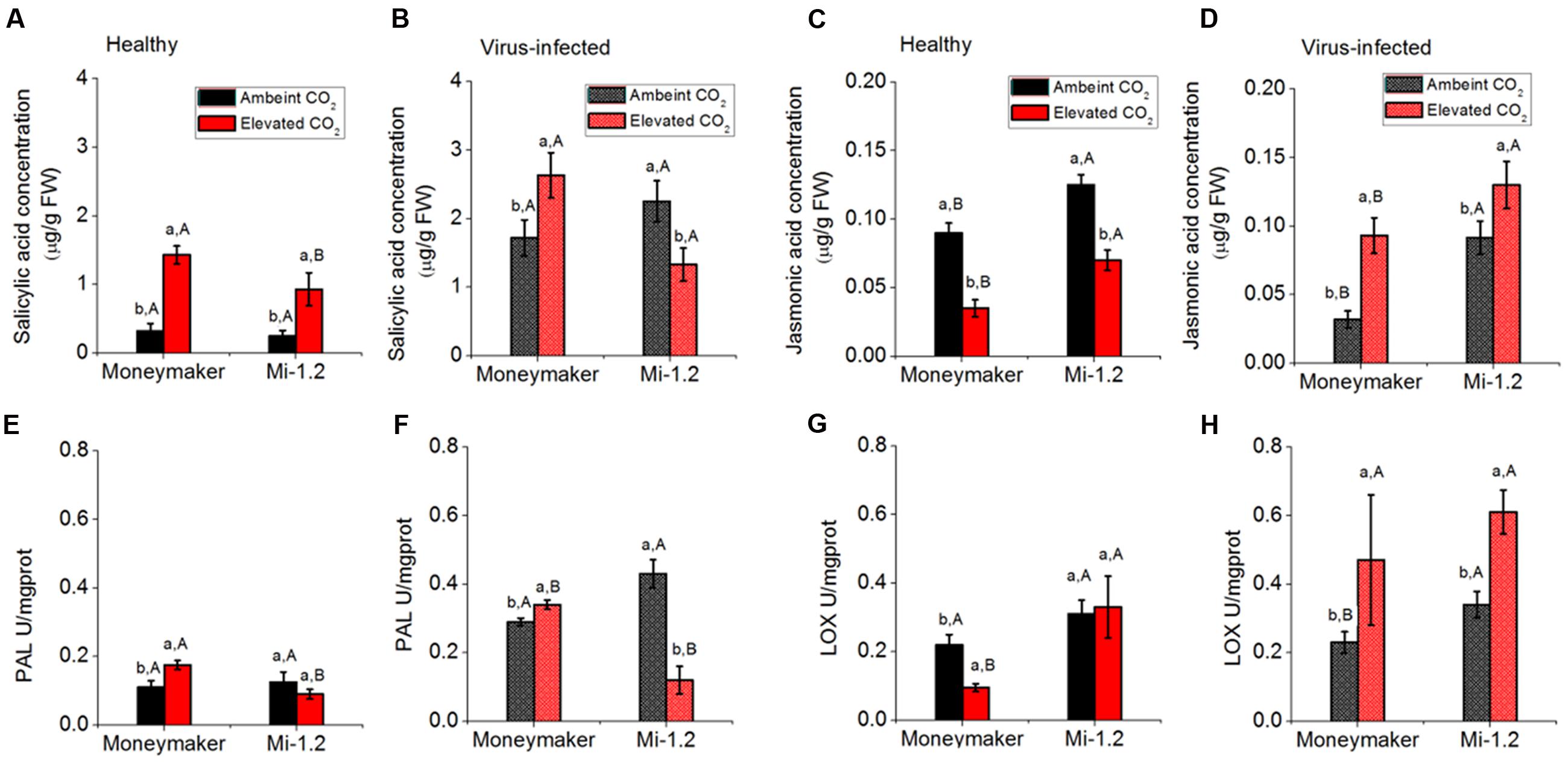
FIGURE 6. Contents of phytohormones and activities of enzymes involved in the JA and SA signaling pathways of two tomato genotypes grown under ambient CO2 and elevated CO2 with and without TYLCV infection. (A,B) SA concentration in healthy and virus-infected plants; (C,D) JA concentration in healthy and virus-infected plants; (E,F) PAL activity in healthy and virus-infected plants; and (G,H) LOX activity in healthy and virus-infected plants. Different lowercase letters indicate significant differences between ambient CO2 and elevated CO2 within the same genotype. Different uppercase letters indicate significant differences in B. tabaci numbers within the same CO2 treatment.
Expression of Genes Involved in the SA- and JA-Signaling Pathways
From 8 to 48 h post-infection with TYLCV artificially, elevated CO2 increased the expression of genes encoding PAL5 and PR1a involved in the SA-signaling pathway of Moneymaker plants but decreased their expression in Mi-1.2 plants (Figures 7A,B; Supplementary Table S8). The expression of genes encoding LOX2 and PI1-1 in the JA-signaling pathway, however, was not greatly affected by elevated CO2 (Figures 7C,D; Supplementary Table S8). TYLCV infection tended to up-regulate the expression of genes encoding PAL5 and PR1a but to down-regulate the expression of LOX2 and PI1-1 regardless of plant genotype (Figure 7). Compared with Moneymaker plants, Mi-1.2 plants had a higher expression of genes encoding PAL5 and PR1a under ambient CO2 but the reverse was true under elevated CO2 (Figure 7). The expression pattern of genes involved in the SA- signaling pathway across the treatments suggested that the SA-signaling pathway is an important part of plant response to TYLCV infection.
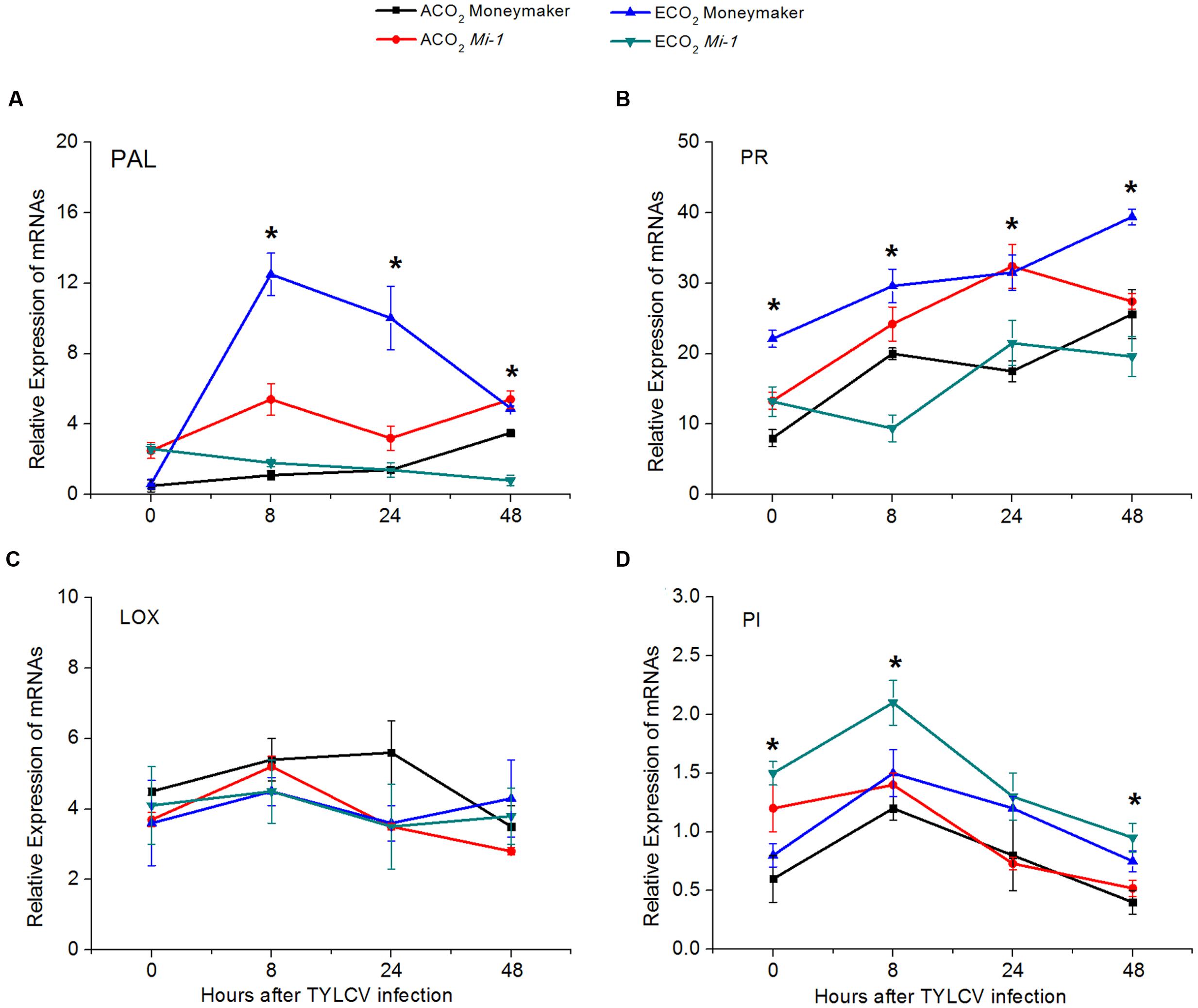
FIGURE 7. Expression of key genes in the JA- and SA-signaling pathways of two tomato genotypes that were grown under ambient CO2 and elevated CO2 and that were infected with TYLCV for 0 to 48 h. (A) Phenylalanine ammonia lyase (PAL); (B) Pathogenesis-related protein 1 (PR); (C) Lipoxygenase 2 (LOX); and (D) Proteinase inhibitor (PI). Significant differences among different treatments in the same time point at P < 0.05 are indicated by an asterisk.
Discussion
The Mi-1.2 gene in tomato mediates resistance to insect vectors by triggering an array of defense responses that could in turn affect virus infection (Tameling et al., 2002). Resistance against nematodes conferred by the Mi-1.2 gene can be reduced by elevated temperature and other environmental variables (Holtzman, 1965). In the current study, we determined the effects of elevated CO2 on Mi-1.2 gene-mediated resistance against TYLCV and its vector, B. tabaci. Inconsistent with our hypotheses that elevated CO2 would increase the resistance of plants to TYLCV in both genotype, we discovered that the effects of elevated CO2 on TYLCV infection differed between Moneymaker and Mi-1.2 plants. Under elevated CO2, the responses of the SA-signaling pathway differed between the plant genotypes, which suggested that the SA-signaling pathway may help explain the differences in plant responses to TYLCV under elevated CO2.
Elevated CO2 is expected to affect plant–virus interactions by altering both plant physiology and vector transmission ability (Malmström and Field, 1997; Rúa et al., 2013). In the present study, we found that elevated CO2 decreased the severity of disease caused by TYLCV on agroinoculated, Moneymaker plants, which is consistent with previous studies (Matros et al., 2006; Huang et al., 2012). The Mi-1.2 plants, which were previously reported to be resistant to B. tabaci (Nombela et al., 2003), were also resistant to TYLCV, i.e., they were less diseased than the Moneymaker plants under ambient CO2. Under elevated CO2, however, the Mi-1.2 plants had a higher disease index and severity values than wild-type plants whether they were agroinoculated with the virus or inoculated by B. tabaci. This result indicated that elevated CO2 tends to increase the resistance of Moneymaker plants but decrease the resistance of Mi-1.2 plants against TYLCV.
In plant–virus interactions, the SA-signaling pathway is thought to provide efficient resistance against plant viruses. For example, exogenous application of SA reduces the levels of Tobacco mosaic virus and Potato virus X coat proteins in infected Nicotiana benthamiana leaves (Lee et al., 2011). In N. tabacum and Arabidopsis, the activation of the SA-signaling pathway inhibits the systemic movement of Cucumber Mosaic Virus (Alazem and Lin, 2015). Our results showed that tomato plants rapidly up-regulated the activity of enzymes and the expression of genes involved in the SA-signaling pathway to defend against TYLCV infection regardless of plant genotype under ambient CO2. The SA-signaling pathway was also found to be involved in Mi-mediated resistance in plants when against nematodes and aphids (Branch et al., 2004; Li et al., 2006). In the current study, Mi-1.2 plants had a higher SA content and greater SA signaling-related enzyme activity and gene expression than Moneymaker plants under ambient CO2 when infected by TYLCV, which suggests that Mi-1.2 plants have greater resistance against TYLCV infection than Moneymaker plants. Interestingly, we found that elevated CO2 increased SA-signaling-related enzyme activity and gene expression in virus-infected Moneymaker plants but had the opposite effect in virus-infected Mi-1.2 plants. To our knowledge, this is the first report that the effects of elevated CO2 on the SA-signaling pathway differ greatly between plant genotypes differing in R gene-mediated resistance when those genotypes are infected by a plant virus.
Under natural conditions, TYLCV is mainly transmitted by whiteflies in a persistent-circulative, non-propagative manner (Hogenhout et al., 2008). Previous research has demonstrated that vector-borne viruses can modify vector behavior and fitness and thereby enhance virus spread by altering the host plant traits. For example, the virus could increase the nutritional quality of infected host plants, decrease the resistance of infected host plants, or increase the attractiveness of infected plants to their vectors (Jiménez-Martínez et al., 2004; Luan et al., 2013; Trêbicki et al., 2016). Infection by TYLCCNV, for example, suppresses JA-induced defenses in tomato plants, which increases the feeding and the fitness of the whitefly vector, which in turn enhances the transmission of the virus (Zhang et al., 2012). In current study, we did not observe a positive effect of TYLCV infection on B. tabaci performance, even though TYLCV infection suppressed JA content and the expression level of PI in both tomato genotypes.
Most of the insects that vector plant viruses, like aphids, whiteflies, and planthoppers, have piercing-sucking mouthparts. The piercing-sucking insects could directly suppress plant efficient defense and subsequently increase the virus transmission (Zarate et al., 2007; Walling, 2008). The fitness of sap-sucking insects could be easily affected by abiotic environment. As reviewed by Sun et al. (2016), elevated CO2 tends to increase the feeding efficiency of some aphids by decreasing JA-mediated resistance and by increasing nutrition content of host plants. As an exception, elevated CO2 decreased the feeding efficiency of Myzus persicae on bell pepper. Thus, the decreased performance of M. persicae led to a twofold decrease in virus transmission under elevated CO2 (Dáder et al., 2016). The current study showed that, regardless of plant genotype, elevated CO2 had little effect on the abundance and fecundity of B. tabaci. As a result, elevated CO2 did not affect TYLCV transmission by viruliferous B. tabaci regardless of plant genotype. The levels of TYLCV acquired by B. tabaci were positively correlated with the levels of virus in the plants (Lapidot et al., 2001). Thus, during the virus acquisition process, elevated CO2 decreased the numbers of TYLCV-DNA copies in B. tabaci feeding on Moneymaker plants but increased the numbers in B. tabaci feeding on Mi-1.2 plants (Figure 5).
Plants have evolved sophisticated mechanisms to perceive biotic stress caused by herbivorous insects and virus pathogens (Dangl and Jones, 2001). Although tomato plants with Mi-1.2 are resistant to sap-sucking vector whiteflies, aphids and pysllids and root-knot nematodes, the mechanisms are distinct. For instance, once infested by B. tabaci, the increased resistance of Mi-1.2 prolonged the pathway stage prior to establishment of feeding site (Jiang et al., 2001). With respect to aphids, they feed for shorter periods on Mi-1.2 plants, apparently perishing due to dehydration or starvation (Kaloshian et al., 2000). In contrast, psyllids exhibited a host selection preference and higher survival for the susceptible variety Moneymaker relative to the resistant Mi-1.2 plants (Casteel et al., 2006). These may suggest that the effect of Mi-conferred resistance on different feeding stage of vector insects could further affect their virus transmission ability. In current study, although the TYLCV severity in Mi-1.2 genotype was lower than Moneymaker, the mechanisms of defense may differ between the virus and its vector. For whiteflies, the Mi-1.2 gene of tomato can directly recognize the elicitor and up-regulate Sgt1 (suppressor of G-two allele of Skp1) and Hsp90 (heat shock protein 90) to induce hypersensitive response (HR)-mediated effector-triggered immunity (ETI) if the same signaling mechanisms are used by Mi-1.2 in response to aphids and whiteflies (Bhattarai et al., 2007). In contrast, the defense of Mi-1.2 plants against TYLCV involves the up-regulation of SA-mediated resistance.
With respect to insect vectors, elevated CO2 may accelerate the breakdown of R gene-mediated resistance in Rubus idaeus when that plant is attacked by the aphid Amphorophora idaei (Martin and Johnson, 2011). In contrast, we did not find any significant effect of elevated CO2 on the resistance of Mi-1.2 plants against B. tabaci whether the insect was feeding on virus-infected or healthy plants. With respect to the plant virus, elevated CO2 decreased the SA-signaling pathway of Mi-1.2 plants and therefore decreased the resistance against TYLCV. The different response of B. tabaci and TYLCV to elevated CO2 on Mi-1.2 plants suggests that the resistance mechanism in plants that contain R genes differs for pathogens vs. herbivorous insects and that those mechanisms may be respond differently to changes in the environment.
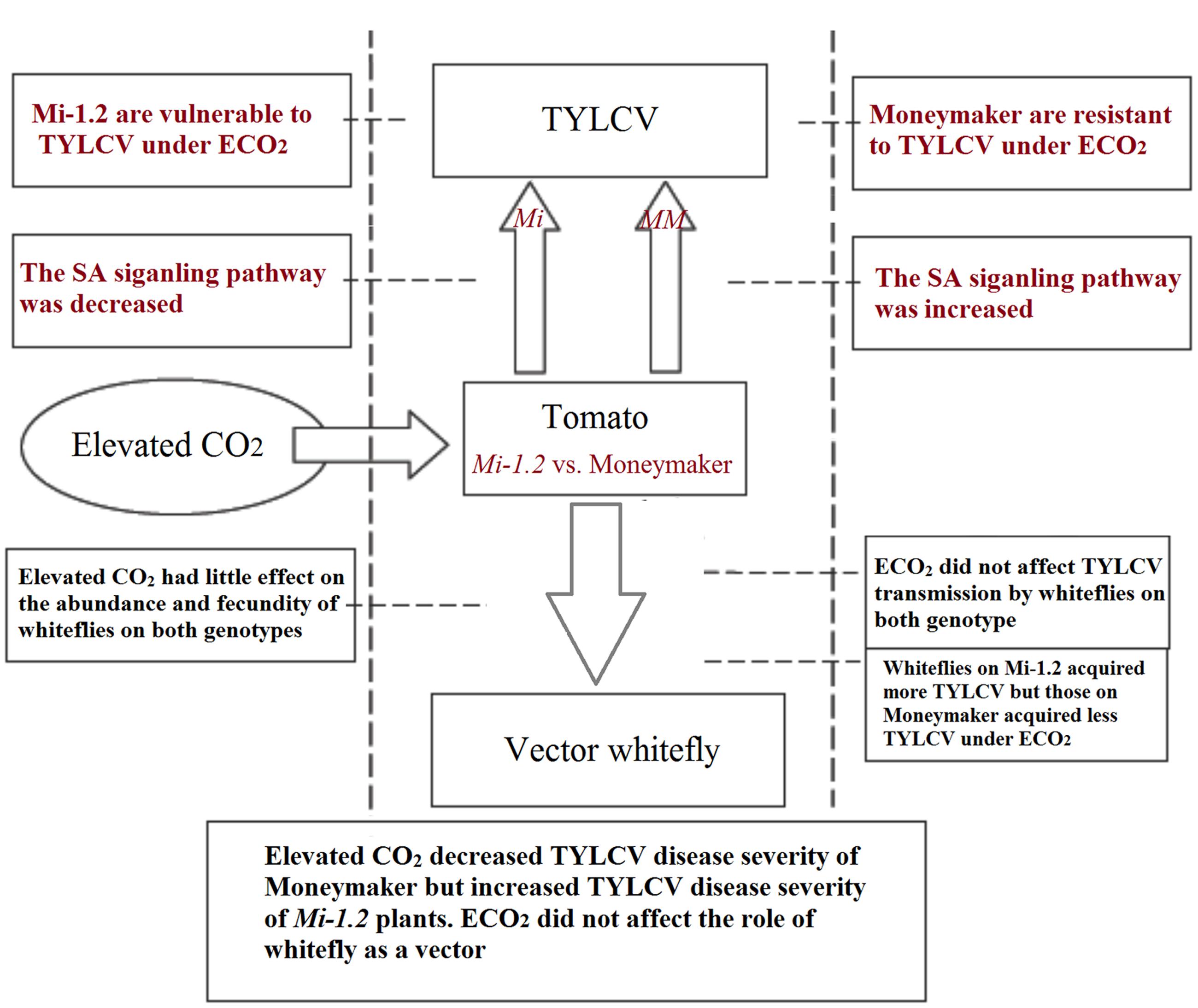
In summary, this study showed that the effects of elevated CO2 on TYLCV transmission and infection differed greatly between tomato genotypes with and without the R gene Mi-1.2, i.e., elevated CO2 decreased TYLCV disease severity of Moneymaker plants but increased TYLCV disease severity of Mi-1.2 plants. The genotype-specific responses were closely related to the expression pattern of the SA-signaling pathway (Figure 8). Elevated CO2 did not affect the role of B. tabaci as a vector. The results indicate that Mi-1.2 plants are more vulnerable than Moneymaker plants to TYLCV and may suffer greater virus damage if atmospheric CO2 levels continue to increase. The outcomes of this study have important implications for agricultural pest control and for transgenic breeding of resistant plants under future elevated CO2 conditions.
Author Contributions
HG contribute to data analysis and article writing. LH design and do the experiment. YS wrote and revised this article. HG performed the technical work. FG conceived the project.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no.XDB11050400) and the National Nature Science Fund of China (nos.31500332 and 31221091).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01680/full#supplementary-material
References
Ainsworth, E. A., Leakey, A. D., Ort, D. R., and Long, S. P. (2008). FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated CO2 impacts on crop yield and food supply. New Phytol. 179, 5–9. doi: 10.1111/j.1469-8137.2008.02500.x
Ainsworth, E. A., and Long, S. P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372. doi: 10.1111/j.1469-8137.2004.01224.x
Al Abdallat, A. M., Al Debei, H. S., Asmar, H., Misbeh, S., Quraan, A., and Kvarnheden, A. (2010). An efficient in vitro-inoculation method for Tomato yellow leaf curl virus. Virol. J. 7:84. doi: 10.1186/1743-422X-7-84
Alazem, M., and Lin, N. S. (2015). Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 16, 529–540. doi: 10.1111/mpp.12204
Belliure, B., Janssen, A., Maris, P. C., Peters, D., and Sabelis, M. W. (2005). Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 8, 70–79. doi: 10.1111/j.1461-0248.2004.00699.x
Bhattarai, K. K., Li, Q., Liu, Y., Dinesh-Kumar, S. P., and Kaloshian, I. (2007). The Mi-1-mediated pest resistance requires Hsp90 and Sgt1. Plant Physiol. 144, 312–323. doi: 10.1104/pp.107.097246
Branch, C., Hwang, C. F., Navarre, D. A., and Williamson, V. M. (2004). Salicylic acid is part of the Mi-1-mediated defense response to root-knot nematode in tomato. Mol. Plant Microbe Interact. 17, 351–356. doi: 10.1094/MPMI.2004.17.4.351
Butler, G. D., Kimball, B. A., and Mauney, J. R. (1986). Populations of Bemisia tabaci (Homoptera: Aleyrodidae) on cotton grown in open-top field chambers enriched with CO2. Environ. Entomol. 15, 61–63. doi: 10.1093/ee/15.1.61
Casteel, C. L., Walling, L. L., and Paine, T. D. (2006). Behavior and biology of the tomato psyllid, Bactericera cockerelli, in response to the Mi-1.2 gene. Entomol. Exp. Appl. 121, 67–72.
Chakraborty, S., and Datta, S. (2003). How will plant pathogens adapt to host plant resistance at elevated CO2 under a changing climate? New Phytol. 159, 733–742. doi: 10.1046/j.1469-8137.2003.00842.x
Chen, F., Wu, G., Ge, F., Parajulee, M. N., and Shrestha, R. B. (2005). Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance, and feeding of an insect herbivore, the cotton bollworm. Entomol. Exp. Appl. 115, 341–350. doi: 10.1111/j.1570-7458.2005.00258.x
Colvin, J., Omongo, C. A., Govindappa, M. R., Stevenson, P. C., Maruthi, M. N., Gibson, G., et al. (2006). Host-plant viral infection effects on arthropod-vector population growth, development and behaviour: management and epidemiological implications. Adv. Virus Res. 67, 419–452. doi: 10.1016/S0065-3527(06)67011-5
Curvers, K., Seifi, H., Mouille, G., de Rycke, R., Asselbergh, B., Van Hecke, A., et al. (2010). Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 154, 847–860. doi: 10.1104/pp.110.158972
Czosnek, H., and Laterrot, H. (1997). A worldwide survey of tomato yellow leaf curl viruses. Arch. Virol. 142, 1391–1406. doi: 10.1007/s007050050168
Dáder, B., Fereres, A., Moreno, A., and Trêbicki, P. (2016). Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6:19120. doi: 10.1038/srep19120
Dangl, J. L., and Jones, J. D. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. doi: 10.1038/35081161
Eastburn, D. M., McElrone, A. J., and Bilgin, D. D. (2011). Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathol. 60, 54–69. doi: 10.1111/j.1365-3059.2010.02402.x
Friedmann, M., Lapidot, M., Cohen, S., and Pilowsky, M. (1998). A novel source of resistance to tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J. Am. Soc. Hortic. Sci. 123, 1004–1007.
Guo, H., Sun, Y., Ren, Q., Zhu-Salzman, K., Kang, L., Wang, C., et al. (2012). Elevated CO2 reduces the resistance and tolerance of tomato plants to Helicoverpa armigera by suppressing the JA signaling pathway. PLoS ONE 7:e41426. doi: 10.1371/journal.pone.0041426
Hogenhout, S. A., Ammar, E. D., Whitfield, A. E., and Redinbaugh, M. G. (2008). Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46, 327–359. doi: 10.1146/annurev.phyto.022508.092135
Holtzman, O. V. (1965). Effect of soil temperature on resistance of tomato to root-knot nematode (Meloidogyne incognita). Phytopathology 55, 990–992.
Huang, L., Ren, Q., Sun, Y., Ye, L., Cao, H., and Ge, F. (2012). Lower incidence and severity of tomato virus in elevated CO2 is accompanied by modulated plant induced defence in tomato. Plant Biol. 14, 905–913. doi: 10.1111/j.1438-8677.2012.00582.x
IPCC (2013). “Summary for policymakers,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G. K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge: Cambridge University Press), 3–32.
Jiang, Y. X., Nombela, G., and Muñiz, M. (2001). Analysis by DC–EPG of the resistance to Bemisia tabaci on an Mi-tomato line. Entomol. Exp. Appl. 99, 295–302. doi: 10.1046/j.1570-7458.2001.00828.x
Jiménez-Martínez, E. S., Bosque-Pérez, N. A., Berger, P. H., Zemetra, R. S., Ding, H., and Eigenbrode, S. D. (2004). Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 33, 1207–1216. doi: 10.1603/0046-225X-33.5.1207
Jiu, M., Zhou, X. P., Tong, L., Xu, J., Yang, X., Wan, F. H., et al. (2007). Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2:e182. doi: 10.1371/journal.pone.0000182
Jwa, N. S., and Walling, L. L. (2001). Influence of elevated CO2 concentration on disease development in tomato. New Phytol. 149, 509–518. doi: 10.1046/j.1469-8137.2001.00063.x
Kaloshian, I., Kinsey, M. G., Williamson, V. M., and Ullman, D. E. (2000). Mi-mediated resistance against the potato aphid Macrosiphum euphorbiae (Hemiptera: Aphididae) limits sieve element ingestion. Environ. Entomol. 29, 690–695. doi: 10.1603/0046-225X-29.4.690
Kaloshian, I., Lange, W. H., and Williamson, V. M. (1995). An aphid-resistance locus is tightly linked to the nematode-resistance gene, Mi, in tomato. Proc. Natl. Acad. Sci. U.S.A. 92, 622–625. doi: 10.1073/pnas.92.2.622
Kobayashi, T., Ishiguro, K., Nakajima, T., Kim, H. Y., Okada, M., and Kobayashi, K. (2006). Effects of elevated atmospheric CO2 concentration on the infection of rice blast and sheath blight. Phytopathology 96, 425–431. doi: 10.1094/PHYTO-96-0425
Lapidot, M., Friedmann, M., Pilowsky, M., Ben-Joseph, R., and Cohen, S. (2001). Effect of host plant resistance to Tomato yellow leaf curl virus (TYLCV) on virus acquisition and transmission by its whitefly vector. Phytopathology 91, 1209–1213. doi: 10.1094/PHYTO.2001.91.12.1209
Li, Q., Xie, Q. G., Smith-Becker, J., Navarre, D. A., and Kaloshian, I. (2006). Mi-1-mediated aphid resistance involves salicylic acid and mitogen-activated protein kinase signaling cascades. Mol. Plant Microbe Interact. 19, 655-664. doi: 10.1094/MPMI-19-0655
Lee, W. S., Fu, S. F., Verchot-Lubicz, J., and Carr, J. P. (2011). Genetic modification of alternative respiration in Nicotiana benthamiana affects basal and salicylic acid-induced resistance to potato virus X. BMC Plant Biol. 11:41. doi: 10.1186/1471-2229-11-41
Liu, B., Preisser, E. L., Chu, D., Pan, H., Xie, W., Wang, S., et al. (2013). Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and tomato yellow leaf curl virus. J. Virol. 87, 4929–4937. doi: 10.1128/JVI.03571-12
Luan, J. B., Yao, D. M., Zhang, T., Walling, L. L., Yang, M., Wang, Y. J., et al. (2013). Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16, 390–398. doi: 10.1111/ele.12055
Malmström, C. M., and Field, C. B. (1997). Virus-induced differences in the response of oat plants to elevated carbon dioxide. Plant Cell Environ. 20, 178–188. doi: 10.1046/j.1365-3040.1997.d01-63.x
Martin, P., and Johnson, S. N. (2011). Evidence that elevated CO2 reduces resistance to the European large raspberry aphid in some raspberry cultivars. J. Appl. Entomol. 135, 237–240. doi: 10.1111/j.1439-0418.2010.01544.x
Martinez de Ilarduya, O., Xie, Q., and Kaloshian, I. (2003). Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 16, 699–708. doi: 10.1094/MPMI.2003.16.8.699
Matros, A., Amme, S., Kettig, B., Buck-Sorlin, G. H., Sonnewald, U. W. E., and Mock, H. P. (2006). Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. Samsun NN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 29, 126–137. doi: 10.1111/j.1365-3040.2005.01406.x
McKenzie, C. L. (2002). Effect of tomato mottle virus (ToMoV) on Bemisia tabaci biotype B (Homoptera: Aleyrodidae) oviposition and adult survivorship on healthy tomato. Florida Entomol. 85, 367–368. doi: 10.1653/0015-4040 (2002)085[0367:EOTMVT]2.0.CO;2
Melloy, P., Hollaway, G., Luck, J. O., Norton, R. O. B., Aitken, E., and Chakraborty, S. (2010). Production and fitness of Fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Glob. Change Biol. 16, 3363–3373. doi: 10.1111/j.1365-2486.2010.02178.x
Milligan, S. B., Bodeau, J., Yaghoobi, J., Kaloshian, I., Zabel, P., and Williamson, V. M. (1998). The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10, 1307–1319. doi: 10.2307/3870642
Nombela, G., Beitia, F., and Muñiz, M. (2000). Variation in tomato host response to Bemisia tabaci (Hemiptera: Aleyrodidae) in relation to acyl sugar content and presence of the nematode and potato aphid resistance gene Mi. Bull. Entomol. Res. 90, 161–167. doi: 10.1017/S0007485300000274
Nombela, G., Williamson, V. M., and Muñiz, M. (2003). The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol. Plant Microbe Interact. 16, 645–649. doi: 10.1094/MPMI.2003.16.7.645
Pritchard, J., Griffiths, B., and Hunt, E. J. (2007). Can the plant-mediated impacts on aphids of elevated CO2 and drought be predicted? Glob. Change Biol. 13, 1616–1629. doi: 10.1111/j.1365-2486.2007.01401.x
Robinson, E. A., Ryan, G. D., and Newman, J. A. (2012). A meta-analytical review of the effects of elevated CO2 on plant–arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 194, 321–336. doi: 10.1111/j.1469-8137.2012.04074.x
Rúa, M. A., Umbanhowar, J., Hu, S., Burkey, K. O., and Mitchell, C. E. (2013). Elevated CO2 spurs reciprocal positive effects between a plant virus and an arbuscular mycorrhizal fungus. New Phytol. 199, 541–549. doi: 10.1111/nph.12273
Rubinstein, G., and Czosnek, H. (1997). Long-term association of tomato yellow leaf curl virus with its whitefly vector Bemisia tabaci: effect on the insect transmission capacity, longevity and fecundity. J. Gen. Virol. 78, 2683–2689. doi: 10.1099/0022-1317-78-10-2683
Shi, X., Pan, H., Xie, W., Wu, Q., Wang, S., Liu, Y., et al. (2013). Plant virus differentially alters the plant’s defense response to its closely related vectors. PLoS ONE 8:e83520. doi: 10.1371/journal.pone.0083520
Stout, M. J., Thaler, J. S., and Thomma, B. P. (2006). Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Ann. Rev. Entomol. 51, 663–689. doi: 10.1146/annurev.ento.51.110104.151117
Sun, Y., Guo, H., and Ge, F. (2016). Plant–aphid interactions under elevated CO2: some cues from aphid feeding behavior. Front. Plant Sci. 7:502. doi: 10.3389/fpls.2016.00502
Sun, Y., Guo, H., Zhu-Salzman, K., and Ge, F. (2013). Elevated CO2 increases the abundance of the peach aphid on Arabidopsis by reducing jasmonic acid defenses. Plant Sci. 210, 128–140. doi: 10.1016/j.plantsci.2013.05.014
Tameling, W. I., Elzinga, S. D., Darmin, P. S., Vossen, J. H., Takken, F. L., Haring, M. A., et al. (2002). The tomato R gene products I-2 and Mi-1 are functional ATP binding proteins with ATPase activity. Plant Cell 14, 2929–2939. doi: 10.1105/tpc.005793
Trêbicki, P., Vandegeer, R. K., Bosque-Pérez, N. A., Powell, K. S., Dader, B., Freeman, A. J., et al. (2016). Virus infection mediates the effects of elevated CO2 on plants and vectors. Sci. Rep. 6:22785. doi: 10.1038/srep22785
Tripp, K. E., Kroen, W. K., Peet, M. M., and Willits, D. H. (1992). Fewer whiteflies found on CO2-enriched greenhouse tomatoes with high C : N ratios. Hort Sci. 27, 1079–1080.
Vos, P., Simons, G., Jesse, T., Wijbrandi, J., Heinen, L., Hogers, R., et al. (1998). The tomato Mi-1 gene confers resistance to both root-knot nematodes and potato aphids. Nat. Biotech. 16, 1365–1369. doi: 10.1038/4350
Walling, L. L. (2008). Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol. 146, 859–866. doi: 10.1104/pp.107.113142
Wang, G. H., Wang, X. X., Sun, Y. C., and Ge, F. (2014). Impacts of elevated CO2 on Bemisia tabaci infesting Bt cotton and its parasitoid Encarsia formosa. Entomol. Exp. Appl. 152, 228–237. doi: 10.1111/eea.12214
Zarate, S. I., Kempema, L. A., and Walling, L. L. (2007). Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143, 866–875. doi: 10.1104/pp.106.090035
Zhai, Q., Yan, L., Tan, D., Chen, R., Sun, J., Gao, L., et al. (2013). Phosphorylation-coupled proteolysis of the transcription factor MYC2 is important for jasmonate-signaled plant immunity. PLoS Genet. 9:e1003422. doi: 10.1371/journal.pgen.1003422
Zhang, H., Gong, H., and Zhou, X. (2009). Molecular characterization and pathogenicity of tomato yellow leaf curl virus in China. Virus Genes 39, 249–255. doi: 10.1007/s11262-009-0384-8
Zhang, S., Li, X., Sun, Z., Shao, S., Hu, L., Ye, M., et al. (2015). Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 66, 1951–1963. doi: 10.1093/jxb/eru538
Keywords: elevated CO2, resistance, Mi-1.2 gene, tomato, TYLCV, whitefly
Citation: Guo H, Huang L, Sun Y, Guo H and Ge F (2016) The Contrasting Effects of Elevated CO2 on TYLCV Infection of Tomato Genotypes with and without the Resistance Gene, Mi-1.2. Front. Plant Sci. 7:1680. doi: 10.3389/fpls.2016.01680
Received: 27 May 2016; Accepted: 25 October 2016;
Published: 09 November 2016.
Edited by:
Linda Walling, University of California, Riverside, USAReviewed by:
Donato Gallitelli, University of Bari, ItalyMurad Ghanim, Agricultural Research Organization, Volcani Center, Israel
Copyright © 2016 Guo, Huang, Sun, Guo and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Ge, gef@ioz.ac.cn
†These authors have contributed equally to this work.
 Huijuan Guo
Huijuan Guo Lichao Huang
Lichao Huang Yucheng Sun
Yucheng Sun Honggang Guo
Honggang Guo Feng Ge
Feng Ge