- 1Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, China
- 2Department of Economic Plants and Biotechnology, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, China
- 3Forestry Bureau of Dongchuan County, Kunming, China
It is believed that high levels of mesophyll conductance (gm) largely contribute to the high rates of photosynthesis in herbaceous C3 plants. However, some sclerophyllous C3 plants that display low levels of gm have high rates of photosynthesis, and the underlying mechanisms responsible for high photosynthetic rates in sclerophyllous C3 plants are unclear. In the present study, we examined photosynthetic characteristics in two high-photosynthesis plants (the sclerophyllous Eucalyptus camaldulensis and the herbaceous Nicotiana tabacum) using measurements of gas exchange and chlorophyll fluorescence. Under saturating light intensities, both species had similar rates of CO2 assimilation at 400 μmol mol−1 CO2 (A400). However, E. camaldulensis exhibited significantly lower gm and chloroplast CO2 concentration (Cc) than N. tabacum. A quantitative analysis revealed that, in E. camaldulensis, the gm limitation was the most constraining factor for photosynthesis. By comparison, in N. tabacum, the biochemical limitation was the strongest, followed by gm and gs limitations. In conjunction with a lower Cc, E. camaldulensis up-regulated the capacities of photorespiratory pathway and alternative electron flow. Furthermore, the rate of alternative electron flow was positively correlated with the rates of photorespiration and ATP supply from other flexible mechanisms, suggesting the important roles of photorespiratory pathway, and alternative electron flow in sustaining high rate of photosynthesis in E. camaldulensis. These results highlight the different mechanisms used to maintain high rates of photosynthesis in the sclerophyllous E. camaldulensis and the herbaceous N. tabacum.
Introduction
In C3 plants, rates of photosynthesis differ widely among species. For individual leaves or whole plants, photosynthetic capacity mainly depends upon their biochemical composition and morphology. Generally, plants with high rates of CO2 assimilation have higher levels of cytochrome f, ATP synthase, Rubisco, and other Calvin-Benson cycle enzymes (Evans, 1987; Terashima and Evans, 1988; Hikosaka, 1996; Hikosaka and Terashima, 1996; Yamori et al., 2010, 2011). The rate of CO2 assimilation is maximized in leaves that usually have high levels of stomatal conductance (gs) and mesophyll conductance (gm), which increase CO2 diffusion into the chloroplasts (Yamori et al., 2010, 2011). Herbaceous plants, e.g., tobacco (Nicotiana tabacum), spinach (Spinacia oleracea), rice (Oryza sativa), and Triticum aestivum have commonly been used for studying the mechanism of photosynthetic acclimation and the rate-limiting step for CO2 assimilation. In herbaceous C3 plants, the rate-limiting step of photosynthesis depends on leaf N content and is mainly determined by N portioning between Rubisco and photosynthetic electron transport (Yamori et al., 2011). However, little is known about the coordination of photosynthetic electron flow and gas exchange in sclerophyllous plants that have high rates of photosynthesis. What is more, it is still not well understood whether the mechanisms responsible for high rates of photosynthesis vary among sclerophyllous and herbaceous C3 plants.
Leaf anatomy plays an important role in determining photosynthetic capacity. The area of chloroplasts facing the intercellular space largely determines the light-saturated rate of photosynthesis (Oguchi et al., 2003, 2005). High-photosynthesis herbaceous plants usually have thinner cell walls, leading to high values of gm. By comparison, leaves of sclerophyllous plants have thicker cell walls, with a high leaf dry mass per area (LMA) (Hassiotou et al., 2009). As an important morphological trait, LMA is inversely related to gm in sclerophyllous plants (Hassiotou et al., 2009). Therefore, sclerophyllous plants usually have low gm and slow rates of photosynthesis (Loreto et al., 1992). For example, a sclerophyllous species Quercus guyavifolia has a low rate of the maximum photosynthesis being 13 μmol CO2 m−2 s−1 (Huang et al., 2016). As we known, the sclerophyllous species Eucalyptus camaldulensis has been introduced for production of paper in China due to its high rate of photosynthesis. The high value of LMA for leaves of E. camaldulensis is assumed to result in low gm. Mesophyll conductance plays an important role in determining the rate of photosynthesis in C3 plants (Flexas et al., 2008; Carriqui et al., 2015). A low gm value increases the resistance of CO2 conductance to the chloroplasts, leading to a decline in the chloroplast CO2 concentration (Cc) and, thus, restricted CO2 assimilation (Loreto et al., 1992; Hanba et al., 2002; Flexas et al., 2012; Gago et al., 2013; Carriqui et al., 2015). Therefore, for the sclerophyllous E. camaldulensis, other mechanisms favoring CO2 diffusion must be used to increase Cc because it is essential for the maintenance of high CO2 assimilation.
The net rate of CO2 assimilation (An) is largely dependent upon the value of Cc because the latter directly determines the affinity of Rubisco to CO2 or O2 (Farquhar et al., 1980; von Caemmerer, 2000). An increased Cc increases the rate of RuBP carboxylation and, thus, results in a rise in the photosynthetic rate. As shown by the calculation of Cc = Ci − An/gm, Cc is mainly determined by three factors: intercellular CO2 concentration (Ci), An, and gm. During the steady-state phase under high light, An and gm reach the steady-state values, the value of Ci determines Cc principally but is mainly influenced by gs. Stomata are the channels for gas exchange between leaf and atmosphere. During periods of drought or high temperatures, a decrease in gs leads to an inhibition of the Calvin-Benson cycle (Flexas et al., 2002; Flexas and Medrano, 2002). In herbaceous plants of high values of gm, high rates of photosynthesis are usually accompanied by high values of gs (Yamori et al., 2010, 2011). However, it is unclear whether the high-photosynthesis sclerophyllous plant E. camaldulensis elevate gs to remedy the deficiency of gm.
According to the C3 photosynthesis model, photosynthesis can be limited by RuBP carboxylation and/or RuBP regeneration (Farquhar et al., 1980). When Cc is higher than Ctrans (the chloroplast CO2 concentration at which the transition from RuBP carboxylation limitation to RuBP regeneration limitation occurs), then CO2 assimilation is limited by RuBP regeneration (Yamori et al., 2010, 2011). Once Cc is lower than Ctrans, CO2 assimilation tends to be limited by RuBP carboxylation. In herbaceous N. tabacum plants grown at high nitrogen concentration, the rate-limiting step of CO2 assimilation is RuBP regeneration because Cc is greater than Ctrans (Yamori et al., 2010, 2011). In the sclerophyllous E. camaldulensis, the high rate of photosynthesis and low gm can cause Cc to be less than Ctrans. Consequently, the rate of CO2 assimilation is probably limited by RuBP carboxylation in E. camaldulensis. If this occurs, then the reduced Cc drives increased photorespiration (or RuBP oxygenation). The photorespiratory pathway is essential for photosynthesis at normal atmospheric CO2 concentrations (Chastain and Ogren, 1989; Eisenhut et al., 2007), and impairment of that pathway decreases the rate of photosynthesis under such CO2 conditions (Somerville and Ogren, 1980, 1981, 1983; Takahashi et al., 2007).
Enhancement of the photorespiratory pathway leads to a considerably improved net photosynthetic rate in Arabidopsis thaliana (Timm et al., 2012, 2015). Therefore, we might also speculate that E. camaldulensis enhances the capacity of that pathway to favor the Calvin-Benson cycle. Furthermore, if the photorespiratory pathway is up-regulated in plants of E. camaldulensis, then the stoichiometry of the ATP/NADPH energy demand by primary metabolism will increase. Therefore, such plants must utilize other flexible mechanisms to balance the ATP/NADPH ratio, e.g., cyclic electron flow (CEF) around photosystem I (PSI) or the water-water cycle (Makino et al., 2002; Walker et al., 2014). The WWC channels electrons obtained from splitting of water molecules at PSII. These electrons are transported to oxygen via the Cyt b6/f complex and PSI, resulting in the formation of a proton gradient across the thylakoid membranes (Asada, 1999, 2000). However, little is known about how the WWC functions in the high-photosynthesis sclerophyllous plant E. camaldulensis.
N. tabacum is regarded as a model plant to study the mechanisms of photosynthetic regulation for herbaceous plants. However, it is not known how the sclerophyllous plant E. camaldulensis obtain a high rate of photosynthesis. Here, we compared gs, gm, CO2 assimilation, photorespiration, and alternative electron flow between N. tabacum and E. camaldulensis. Our objective was to examine the potential differences in mechanisms underlying high rates of photosynthesis between herbaceous and sclerophyllous plants.
Materials and Methods
Plant Materials and Growth Conditions
We compared the photosynthetic characteristics of N. tabacum and Eucalyptus camaldulensis Dehnh. The latter is a fast-growing species native to Australia that has been widely introduced into China for forest plantations. For this study, Eucalyptus samples were collected from plants grown in an open field at an elevation of 700 m in Dongchuan County, Kunming City, Yunnan Province, China. The monthly air temperature, total radiation and precipitation were displayed in Figure 1 (data were collected from 1961 to 1980). Seedlings of N. tabacum cv. k326 were cultivated in plastic pots in a phytotron at Kunming Institute of Botany, Yunnan, China. Growing conditions were 24/18°C (day/night), 60% relative humidity, and an atmospheric CO2 concentration maintained at 400 μmol mol−1. The phytotron used sunlight as the source of illumination, and plants were exposed to approximately 95% of full sunlight (maximum at noon ≈ 1990 μmol photons m−2 s−1). Photosynthetic parameters were measured in June of 2014. Measurements were made using four mature leaves from four independent plants per species. Fully expanded mature leaves on 13-week-old plants of N. tabacum were used for photosynthetic measurements. For E. camaldulensis, mature leaves that flushed in the spring on 3-year-old plants were used for measurements.
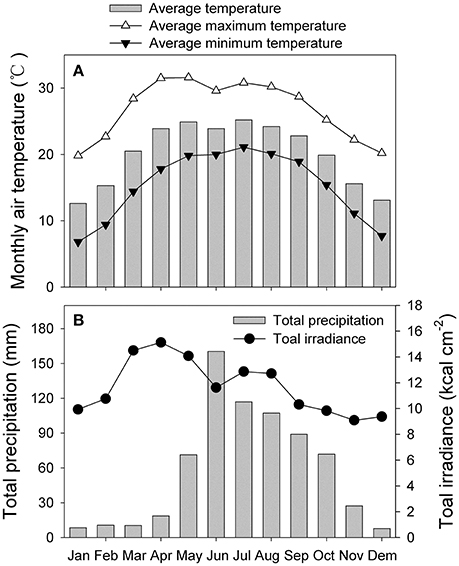
Figure 1. Monthly climatic data collected from 1961 to 1980. (A) monthly average air temperature, monthly average maximum and minimum temperatures; (B) monthly precipitation and total irradiance.
Measurements of Gas Exchange and Chlorophyll Fluorescence
Photosynthetic parameters for gas exchange and chlorophyll fluorescence were monitored with an open gas exchange system that incorporated infrared CO2 and water vapor analyzers (Li-6400XT; Li-Cor Biosciences, Lincoln, NE, USA) and a 2-cm2 measuring head (6400-40 Leaf Chamber Fluorometer; Li-Cor Biosciences). Data were recorded at 25°C and a relative air humidity of 60–70%. The atmospheric CO2 concentration was maintained at 400 μmol mol−1 by the Li-6400XT. Both gs and the CO2 assimilation rate peaked after plants were exposed to saturating light (2000 μmol photons m−2 s−1) for 20 min. Immediately, light response curves were evaluated at 2-min intervals at different light intensities. For N. tabacum, light response curves were measured at a photosynthetic photon flux density (PPFD) of 2000, 1600, 1200, 1000, 800, 600, 400, 300, 200, 150, 100, 50, or 0 μmol photons m−2 s−1. For E. camaldulensis, light response curves were measured at a photosynthetic photon flux density (PPFD) of 2000, 1600, 1200, 1000, 800, 500, 300, 150, 100, 50, or 0 μmol photons m−2 s−1.
The fluorescence parameters Fm′ and Fs were evaluated as previously described (Baker and Rosenqvist, 2004), with Fm′ representing the maximum fluorescence after light-adaption and Fs being the light-adapted steady-state fluorescence. The effective quantum yield of PSII was calculated as ΦPSII = (Fm′ − Fs)/Fm′ (Genty et al., 1989). The maximum fluorescence after dark adaptation (Fm) was examined after 30 min of dark adaptation following measurement of the light response curve. Non-photochemical quenching was calculated as NPQ = (Fm − Fm′)/Fm′.
Estimation of Photosynthetic Electron Flow
Using the data of chlorophyll fluorescence parameters, total photosynthetic electron flow through PSII is calculated as follows (Krall and Edwards, 1992):
where ΦPSII is the effective quantum yield of PSII and Labs represents leaf absorbance. We applied the constant of 0.5 based on the assumption that photons were equally distributed between PSI and PSII.
Using the basic equation of leaf carbon dioxide gas exchange and Rubisco specificity for carboxylation relative to oxygenation (von Caemmerer and Farquhar, 1981; Sharkey, 1988; Walker et al., 2016), the rate of Rubisco carboxylation (Vc) and that of Rubisco oxygenation (Vo) are calculated according to.
where An represented the net rate of CO2 assimilation, Rd was the rate of mitochondrial respiration as measured after 30 min of dark adaptation, Γ* was the CO2 compensation point in the absence of daytime respiration (Farquhar et al., 1980; Brooks and Farquhar, 1985), and Cc was the chloroplast CO2 concentration. The electron flow for photorespiratory carbon oxidation can be expressed as:
The NADPH demands from CO2 assimilation and photorespiration were calculated according to the models of Farquhar et al. (1980). Using the data from gas exchange measurements, we determined the rate of electron transport for NADPH required by carboxylation and oxygenation of RuBP (Jg) as follows (Zivcak et al., 2013; Walker et al., 2014)
where Ci was the intercellular CO2 concentration. The alternative electron flow was calculated as follows (Makino et al., 2002; Zivcak et al., 2013; Huang et al., 2016):
Estimations of Mesophyll Conductance and Chloroplast CO2 Concentration
Values for mesophyll conductance (gm) were estimated through a combination analysis of gas exchange and chlorophyll fluorescence, and according to the following equation (Harley et al., 1992; Loreto et al., 1992; Warren and Dreyer, 2006; Yamori et al., 2010, 2011):
Using the estimated gm, we calculated the chloroplast CO2 concentration (Cc) according to the following equation (Long and Bernacchi, 2003; Warren and Dreyer, 2006; Yamori et al., 2010, 2011):
The response of net CO2 assimilation rate to CO2 concentration was examined at 2000 μmol photons m−2 s−1 and 25°C. Before A/Ci measurement, leaves were light adapted at 2000 μmol photons m−2 s−1 and 400 μmol mol−1 CO2 concentration for at least 20 min to obtain the maximum values of gs and An. Afterwards, the CO2 concentrations were set to 50 μmol mol−1 and increased stepwise. For E. camaldulensis, CO2 concentrations were set to 0, 50, 100, 150, 200, 300, 400, 600, 800, 1000, and 1200 μmol mol−1. The CO2 concentrations in A/Ci measurement in N. tabacum were set to 0, 50, 100, 150, 200, 300, 400, 600, 800, 1000, 1200, 1600, 2000 μmol mol−1. Each stepwise measurement was completed within 2–3 min. Using A/Ci curves, we calculated the maximum rates of RuBP regeneration (Jmax) and RuBP carboxylation (Vcmax) according to the method of Long and Bernacchi (2003). To identify the limiting step of CO2 assimilation, we determined the chloroplast CO2 concentration at which the transition from RuBP carboxylation to RuBP regeneration occurred (Ctrans) as follows (Yamori et al., 2010, 2011):
where Kc (μmol mol−1) and Ko (mmol mol−1) were the Michaelis constants for CO2 and O2, respectively (Farquhar et al., 1980), and were assumed to be 406.8 μmol mol−1 and 277 mmol mol−1, respectively, at 25°C (Long and Bernacchi, 2003); O was the partial pressure of O2 and was assumed to be 210 (Farquhar et al., 1980); Jmax was the maximum rate of RuBP regeneration; and Vcmax was the maximum rate of RuBP carboxylation. The rate-limiting step for CO2 assimilation was then determined by comparing the values of Cc and Ctrans.
Modeling ATP Supplied via Flexible Mechanisms
The total amount of ATP demand from Rubisco carboxylation and oxygenation was obtained with the following formula (Walker et al., 2014):
Assuming that the stoichiometry of ATP/NADPH produced by LEF (electron transport from PSII to NADP+) is 1.29 (Sacksteder et al., 2000; Seelert et al., 2000; Walker et al., 2014), the amount of ATP produced by LEF was calculated as:
Rates of ATP supply from other flexible mechanisms were determined by subtracting the amount of ATP produced by LEF from vATP according to:
Quantitative Limitation Analysis of An
Photosynthetic limitations in E. camaldulensis and N. tabacum were assessed according to the method of Grassi and Magnani (2005) and Carriqui et al. (2015). The values for stomatal (ls), mesophyll conductance (lmc), and biochemical (lb) limitations represented measures of the relative importance of stomatal diffusion, mesophyll diffusion, and photosynthetic biochemistry in setting the observed value of An. Relative photosynthetic limitations were calculated as follows (Grassi and Magnani, 2005; Carriqui et al., 2015):
where gtot was total conductance to CO2 between the leaf surface and carboxylation sites (calculated as 1/gtot = 1/ gs + 1/ gm).
Statistical Analysis
All results were displayed as mean values of four independent measurements. We used one-way ANOVA and SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) to examine differences between the two species. Those differences were considered significant at P < 0.05.
Results
A/Ci Curves and the Rate-Limiting Step of CO2 Assimilation
The A/Ci curves indicated that the maximum rate of photosynthesis was higher in N. tabacum than in E. camaldulensis (Figure 2A). Values for the maximum rate of RuBP regeneration (Jmax) and RuBP carboxylation (Vcmax) were significantly higher in N. tabacum (Figure 2B). Both species showed similar ratios of Jmax/Vcmax (Figure 1B) as well as the same value for A400 at 400 μmol mol−1 CO2. Because the CO2 compensation point in the absence of daytime respiration (Γ*) has an important impact on gm, Cc, Ctrans, and limitations on the stomata (ls), mesophyll conductance (lmc), and biochemical functions (lb), we conducted a sensitivity analysis and examined the rate-limiting step for CO2 assimilation to Γ* (range from 30 to 40 μmol mol−1). For E. camaldulensis, lmc was the most important constraining factor for photosynthesis, followed by ls and lb (Figure 2C). By contrast, biochemical limitations were the most significant in N. tabacum, followed by lmc and ls (Figure 2C). Furthermore, irrespective of Γ*, the value of Cc at 400 μmol mol−1 CO2 was significantly lower than Ctrans in E. camaldulensis (Table 1), suggesting that the rate-limiting step of A400 tended to be RuBP carboxylation. By comparison, in N. tabacum the value of Cc at 400 μmol mol−1 CO2 was significantly higher than Ctrans (Table 1), indicating A400 tended to be limited by RuBP regeneration.
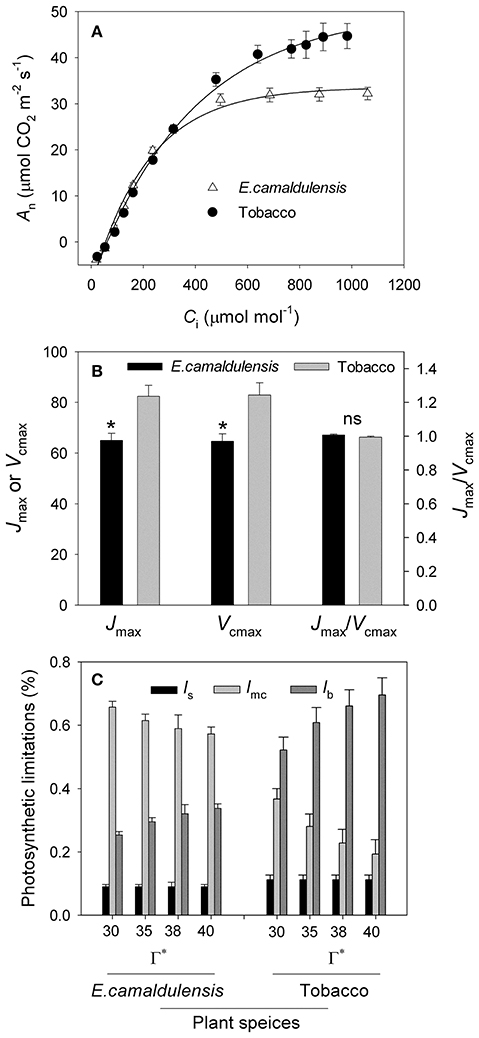
Figure 2. Analysis of A/Ci curves and quantitative limitation analysis of An in Eucalyptus camaldulensis and N. tabacum. (A) Intercellular CO2 concentration (Ci) response of CO2 assimilation rate (An) in Eucalyptus camaldulensis and N. tabacum measured at 25°C and 1500 μmol photons m−2 s−1. (B) Values for Jmax, Vcmax and Jmax/Vcmax ratio. Jmax represents the maximum rate of RuBP regeneration; Vcmax indicates the maximum rate of RuBP carboxylation. (C) Sensitivity analyses of relative stomatal (ls), mesophyll conductance (lmc) and biochemical (lb) limitations for photosynthesis to CO2 compensation point under the absence of respiration condition (Γ*) at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
Light Response Changes in CO2 Assimilation and Photosynthetic Electron Flow
The light response curves demonstrated that the response of An to incident light was similar between E. camaldulensis and N. tabacum (Figure 3A). However, the maximum value of gs was much higher in E. camaldulensis (Figure 3B), while Ci was slightly higher in that species (Figure 3C). When Γ* was assumed to be 40 μmol mol−1, then Cc under saturating light was much lower in E. camaldulensis (Figure 3D). At 2000 μmol photons m−2 s−1 in light response curves, the value of Cc was 124 μmol mol−1 in E. camaldulensis and 226 μmol mol−1 in N. tabacum (Figure 3D). This large difference of Cc between E. camaldulensis and N. tabacum was principally caused by the contrast in gm.
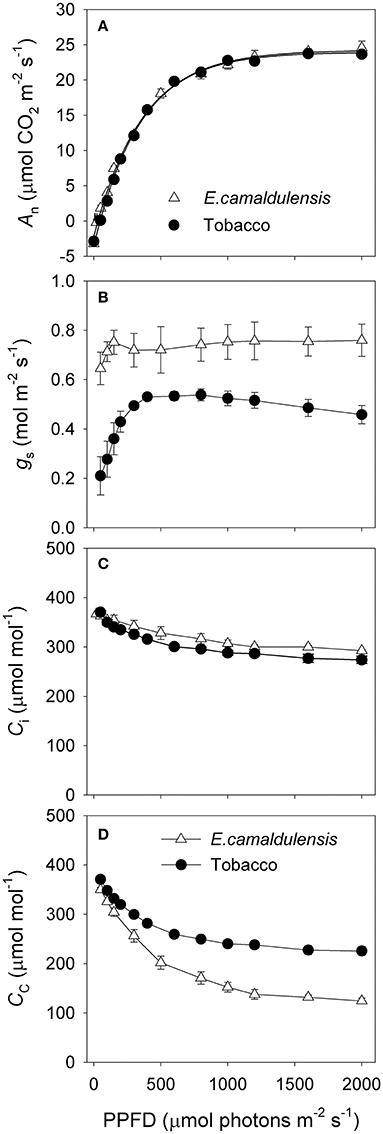
Figure 3. Light response changes in (A) photosynthetic rate (An), (B) stomatal conductance (gs), (C) intercellular CO2 concentration (Ci), and (D) chloroplast CO2 concentration (Cc) for leaves of Eucalyptus camaldulensis and N. tabacum. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. The value of Cc was based on the calculation of gm on assumptions of Γ* being 40 μmol mol−1 and Labs being 0.85. Values are means ± SE (n = 4).
Under all light intensities, E. camaldulensis had significantly higher effective quantum yield of PSII (ΦPSII) compared to N. tabacum, especially under high light (Figure 4A). Concomitantly, NPQ values were lower in E. camaldulensis than N. tabacum (Figure 4B). According to the data of ΦPSII, E. camaldulensis had significantly higher values of total electron flow through PSII (JT) than N. tabacum (Figure 5A). Meanwhile, the ratios of the rate of Rubisco carboxylation (Vc) to that of Rubisco oxygenation (Vo) under saturating light intensities were much higher in E. camaldulensis (Figure 5B). On assumptions of Γ* being 40 μmol mol−1 and leaf absorbance being 0.85 in E. camaldulensis and N. tabacum, at 2000 μmol photons m−2 s−1, comparative values for JT and the Vo/Vc ratio in E. camaldulensis vs. N. tabacum were 280 vs. 178 and 0.63 vs. 0.38, respectively (Figures 5A,B). Furthermore, under light intensities above 300 μmol photons m−2 s−1, electron flux for photorespiratory carbon oxidation [Je(PCO)] were significantly higher in E. camaldulensis (Figure 5C). Values for Je(PCO) at 2000 μmol photons m−2 s−1 were 100 and 48 μmol electrons m−2 s−1 in E. camaldulensis and N. tabacum, respectively. Plotting the Vo/Vc ratio and Cc indicated that the difference in the Vo/Vc ratio between E. camaldulensis and N. tabacum was mainly determined by the difference in Cc (Figure 6), which in turn caused by the change in gm.
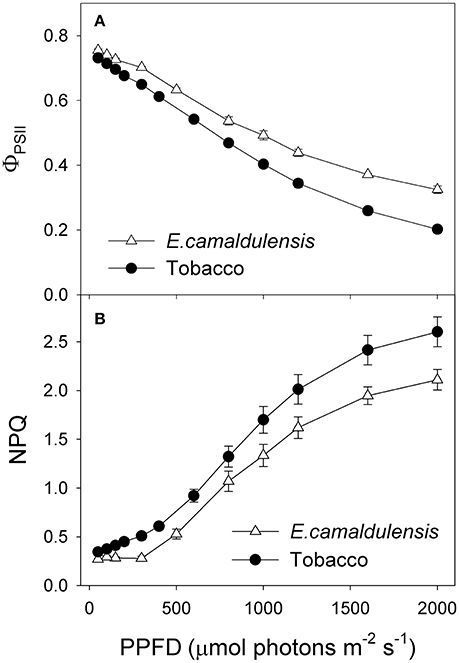
Figure 4. Light response changes in (A) effective quantum yield of PSII [Y(II)], and (B) non-photochemical quenching (NPQ) for leaves of Eucalyptus camaldulensis and N. tabacum. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
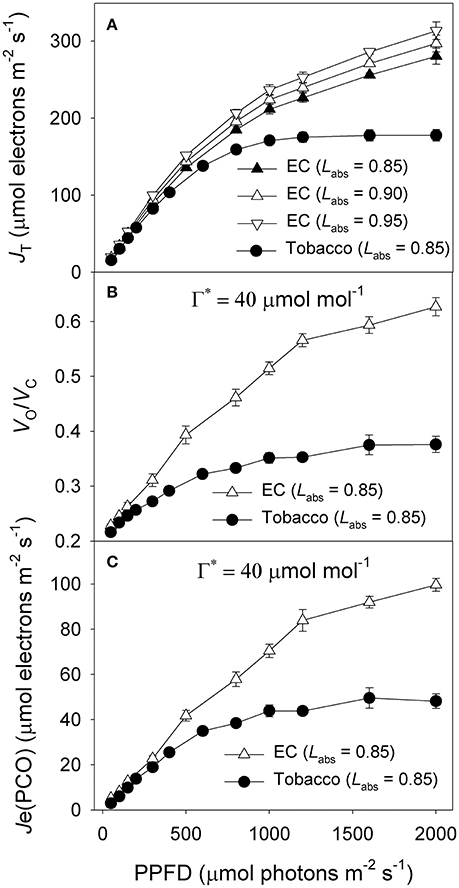
Figure 5. Light response changes in (A) photosynthetic electron flow through PSII (JT), and (B) the ratio of the rate of Rubisco carboxylation (Vc) to that of Rubisco oxygenation (Vo), and (C) electron flux for photorespiratory carbon oxidation [Je(PCO)] for leaves of Eucalyptus camaldulensis and N. tabacum. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
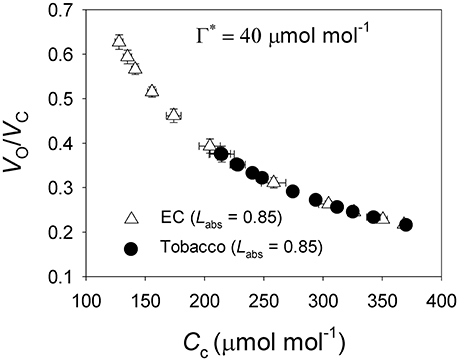
Figure 6. The ratio of Rubisco oxygenation to carboxylation (Vo/Vc) as a function of Cc for leaves of Eucalyptus camaldulensis and N. tabacum. The data of Vo/Vc and Cc were referred from Figures 2D, 4B, respectively. Values are means ± SE (n = 4).
Alternative Electron Flow and ATP Synthesis from Flexible Mechanisms
Under high light, E. camaldulensis had significantly increased alternative electron flow, as indicated by the higher values of Ja and the Ja/Jg ratio (Figure 7). To examine the relationship between the alternative electron flow and photorespiration, we evaluated possible associations between Ja and Je(PCO) under light intensities higher than 300 μmol photons m−2 s−1. Interestingly, Ja was positively and linearly correlated with Je(PCO) (Figure 7A, P < 0.0001). Similarly, the Ja/Jg ratio was positively and linearly correlated with Vo/Vc ratio (Figure 7B, P < 0.0001). According to photosynthesis model, an increase in the Vo/Vc ratio requires a higher ATP/NADPH energy demand from other flexible mechanisms such as cyclic electron flow and alternative electron flow. Increased alternative electron flow promotes the formation of a proton gradient across the thylakoid membrane (ΔpH), which can be used for activating NPQ and ATP synthesis. We found that under high light the rate of ATP supplied from alternative electron sinks [vATP(Flex)] were much higher in E. camaldulensis than in N. tabacum (Figure 8A), and the rate of alternative electron flow was positively correlated to vATP(Flex) (Figure 8B, P < 0.0001). Furthermore, NPQ values under high light were significantly lower in E. camaldulensis than in N. tabacum (Figure 4B), suggesting that the main role for alternative electron flow in E. camaldulensis is not to activate NPQ but to provide extra ATP. The greater capacity of the photorespiratory pathway in E. camaldulensis plants is sustained by the enhanced alternative electron flow.
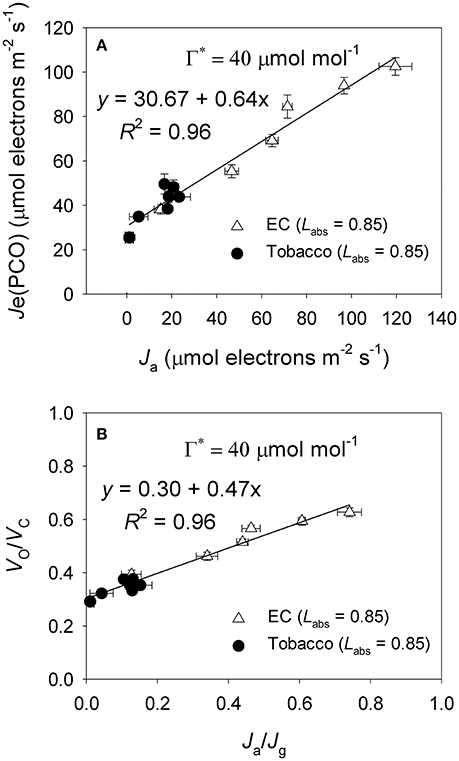
Figure 7. (A) The value of Je(PCO) as a function of Ja (electron flux for alternative electron sinks) for leaves of Eucalyptus camaldulensis and tobacco; (B) The value of Vo/Vc as a function of Ja/Jg for leaves of Eucalyptus camaldulensis and tobacco. Data were obtained from light response curves (light intensities higher than 300 μmol photons m−2 s−1) measured at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4). The coefficient of correlation (r) and significance of correlation (P) are 0.98 and <0.0001 for Figure 6A, respectively. The coefficient of correlation (r) and significance of correlation (P) are 0.98 and <0.0001 for Figure 6B, respectively.
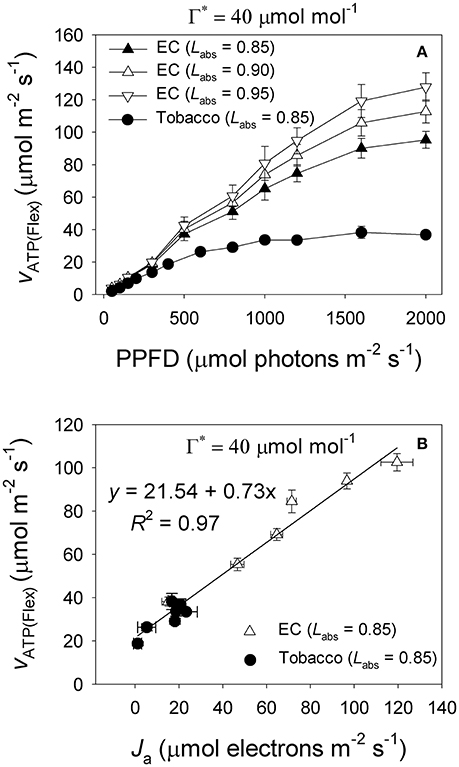
Figure 8. (A) Light response change in the rate of ATP supplied from other flexible mechanisms rather than electron flow from water to NADP+ [vATP(Flex)] for leaves of Eucalyptus camaldulensis and tobacco; (B) Relationship between the electron flux for alternative electron sinks (Ja) and vATP(Flex) for leaves of Eucalyptus camaldulensis and tobacco, and data were obtained from light response curves (light intensities higher than 300 μmol photons m−2 s−1) measured at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4). The coefficient of correlation (r) and significance of correlation (P) are 0.98 and <0.0001 for Figure 7B, respectively.
Discussion
In herbaceous C3 crop species such as N. tabacum, spinach, rice and wheat, high stomatal and mesophyll conductance are essential for their strong photosynthetic capacities (Yamori et al., 2010, 2011). However, for sclerophyllous Eucalyptus camaldulensis, we found that its high rate of CO2 assimilation (Figure 3A) was accompanied with a low gm (≈0.12 molm−2 s−1) (Table 1). Thus, high levels of mesophyll conductance do not appear to be a common mechanism for high rates of photosynthesis in C3 plants. Surprisingly, in the sclerophyllous E. camaldulensis, the high rates of photosynthesis occurred at the low levels of Cc (Figure 3D), which directly increased the Vo/Vc ratio (Figure 5B). What is more, E. camaldulensis showed increased capacities of photorespiratory pathway (Figure 5C) and electron flow to alternative sinks (Figure 7). Enhancement of photorespiratory pathway increased the release of CO2 in mitochondria. This photorespired CO2 can be trapped and reassimilated by chloroplast, thereby boosting photosynthesis (Busch et al., 2013). Concomitantly, the increased photorespiratory pathway in E. camaldulensis needs more extra ATP supply from alternative electron sinks rather than electron transfer from water to NADP+. Interestingly, the increased alternative electron flow provided essential extra ATP to balance the energy budget and sustain the high rate of photorespiratory pathway, then increasing the rate of photosynthetic CO2 assimilation. These results highlight that the sclerophyllous E. camaldulensis enhanced the capacities of photorespiratory pathway and alternative electron flow to sustain a high rate of photosynthesis.
Quantitative Limitation Analysis of An
In N. tabacum leaves, lb was the most important constraining factor for photosynthesis, followed by lmc and ls, being consistent with high levels of stomatal and mesophyll conductance. By comparison, in E. camaldulensis, lmc had the greatest influence while ls was the least limiting factor. This was consistent with the higher gs and lower gm measured for that species. These results confirmed that, the major limiting factor for photosynthesis differs intrinsically between high-photosynthesis herbaceous and sclerophyllous plants. Diffusional limitations of CO2 are the main reason for lower photosynthetic rates in ferns than in angiosperms (Gago et al., 2013; Carriqui et al., 2015). Mesophyll conductance is the most constraining factor for photosynthesis in ferns (Carriqui et al., 2015). These findings suggested that, not only in low-photosynthesis species (e.g., ferns) but also in some high-photosynthesis sclerophyllous species such as E. camaldulensis, mesophyll conductance is the primary limiting factor for photosynthesis. The difference of gm between E. camaldulensis and N. tabacum is probably determined by their leaf anatomy (Terashima et al., 2006). The thicker cell walls lead to a lower gm in E. camaldulensis when compared with N. tabacum.
The Rate-Limiting Step of CO2 Assimilation
The rate of CO2 assimilation can be limited by RuBP carboxylation and/or RuBP regeneration in C3 plants (Farquhar et al., 1980; Yamori et al., 2010, 2011). The specific rate-limiting step of CO2 assimilation is determined by the relative values of Cc and Ctrans. When the value of Cc is higher than Ctrans, the CO2 assimilation rate is mainly limited by RuBP regeneration. Otherwise, the CO2 assimilation rate is limited by RuBP carboxylation when Cc is lower than Ctrans. In N. tabacum leaves, the value of Cc under saturating light was higher than Ctrans, and thus the rate-limiting step of CO2 assimilation tended to be RuBP regeneration (Table 1). On the contrary, the value of Cc under saturating light was lower than Ctrans in E. camaldulensis, and thus the rate of CO2 assimilation was limited by RuBP carboxylation (Table 1). According to the calculation of Cc = Ci − An/gm, the value of Cc can be affected by three parameters Ci, An, and gm. Here, because An and Ci values under saturating light were similar in E. camaldulensis and N. tabacum (Figures 3A,C), Because the value of Cc can be largely determined by gm, the difference in the main rate-limiting step of CO2 assimilation between N. tabacum and E. camaldulensis was mainly caused by the contrasts in their mesophyll conductance.
In the herbaceous N. tabacum, which has thin, flexible leaves, gm can reach 0.5 mol m−2 s−1 when plants are exposed to high nitrogen concentrations (Yamori et al., 2011). By comparison, sclerophyllous plants have relatively lower gm values that range between 0.09 and 0.25 mol m−2 s−1 (Lloyd et al., 1992; Hassiotou et al., 2009). Conductance in the mesophyll can be influenced by leaf anatomical traits such as the cell surface area and the chloroplast surface area that is exposed to intercellular air spaces (Evans et al., 1994; Oguchi et al., 2005; Terashima et al., 2011), chloroplast rearrangements (Tholen et al., 2008), cell wall thickness (Terashima et al., 2006, 2011; Flexas et al., 2012), and LMA (Flexas et al., 2008; Hassiotou et al., 2009). The value of LMA for in E. camaldulensis is approximately 169.5 g m−2 (Suganuma et al., 2006), which is much higher than N. tabacum (approximately 25 g m−2) (Yamori et al., 2010). The large differences of leaf anatomical traits between E. camaldulensis and N. tabacum might result in the diversity of gm.
Photorespiration
We found that the capacity of the photorespiratory pathway was greater for E. camaldulensis than for N. tabacum (Figures 5B,C). Rubisco is a dual functional enzyme that catalyzes the carboxylation of RuBP, but also oxygenates RuBP in photorespiration. A lower Cc in E. camaldulensis would increase the rate of RuBP oxygenation by Rubisco. Photorespiration begins with RuBP oxygenation that generates glycolate-2-phosphate and glycerate-3-phosphateare. To maintain a steady-state high rate of photosynthesis in E. camaldulensis, the RuBP pool must remain stable. In chloroplast, the steady-state of RuBP pool depends on two different RuBP regeneration pathways: the Calvin-Benson cycle and the photorespiratory pathway. Impairment of photorespiratory pathway induced a gradient decrease in photosynthetic rate at atmospheric CO2 concentration (Takahashi et al., 2007). Importantly, during steady-state phases, the rates of RuBP oxygenation and RuBP regeneration through the photorespiratory pathway must be balanced. Under high light, E. camaldulensis accelerate the photorespiratory pathway to favor the regeneration of RuBP via glycerate-3-phosphate, thereby preventing the RuBP pool from shrinking.
A reduction in Cc also accelerates the production of photorespiratory intermediates, e.g., glycine and glycerate, which inhibit the Calvin-Benson cycle (Chastain and Ogren, 1989; Eisenhut et al., 2007; Timm et al., 2012, 2015). In Arabidopsis thaliana plants with elevated glycine decarboxylase activity, the rapid acceleration of the photorespiratory pathway lowers the accumulation of photorespiratory metabolites including those which impair Rubisco activation and possibly the activity of other enzymes of the Calvin-Benson cycle, increasing the performance of the Calvin-Benson cycle (Timm et al., 2012, 2015). Furthermore, N. tabacum plants grown under high light and high nitrogen concentration up-regulate photorespiratory pathway to maintain high rates of photosynthesis (Huang et al., 2014, 2016). To overcome those detrimental effects, the photorespiratory pathway should be enhanced in E. camaldulensis. In addition, although CO2 is released in the mitochondria in the photorespiratory pathway, C3 plants can trap photorespired CO2 within individual mesophyll cell. This causes chloroplast CO2 concentrations to rise and, ultimately, improves the rate of photosynthesis in C3 plants (Busch et al., 2013). Taking together, the enhanced photorespiratory capacity strongly contributes to the high rate of CO2 assimilation in E. camaldulensis.
Alternative Electron Flow
We were surprised to learn that, under saturating illumination, alternative electron flow was enhanced in E. camaldulensis when compared with N. tabacum. The water-water cycle, nitrate reduction and malate shunt are potential candidates responsible for this increase in alternative electron flow (Yi et al., 2014). When plants are illuminated at atmospheric CO2 and O2, most of this alternative flow accounts for the electron flux to oxygen and oxidized ascorbic acid (Miyake and Yokota, 2000; Makino et al., 2002). Thus, we concluded that the WWC activity was greater in E. camaldulensis. During the early phase of photosynthetic induction in rice, the WWC first generates a ΔpH across the thylakoid membranes to form NPQ and supply ATP for carbon assimilation (Neubauer and Yamamoto, 1992; Miyake and Yokota, 2000; Makino et al., 2002). However, when photosynthesis reaches a steady-state rate, the WWC no longer maintains a high NPQ but instead provides additional ATP for primary metabolism in rice leaves (Makino et al., 2002). Our light response curves indicated that NPQ values under high light were significantly lower in E. camaldulensis (Figure 4B). Because the NPQ activation relies on the acidification of thylakoid lumen, and which also depresses electron transport through Cyt b6/f complex via “photosynthetic control,” this result suggested the higher levels of lumen acidification in leaves of N. tabacum when illuminated at high light. Therefore, the steady-state rate of WWC under intense illumination in E. camaldulensis mainly contributed to additional ATP synthesis rather than lumen acidification, which is consistent with previous studies on the role of the WWC (Makino et al., 2002; Huang et al., 2016).
In electron flow from PSII to NADP+, the stoichiometry of the ATP/NADPH ratio is thought to be 1.29 (Sacksteder et al., 2000; Seelert et al., 2000). By comparison, each Rubisco oxygenation consumes 3.5 ATP and 2 NADH equivalents in total. Therefore, the occurrence of photorespiratory pathway needs a higher ATP/NADPH ratio than 1.29 to maintain primary metabolism (Edwards and Walker, 1983; Walker et al., 2014). Under high light and ambient CO2, the ATP/NADPH ratio required by CO2 assimilation, photorespiration, and nitrite assimilation is approximately 1.6 (Walker et al., 2014). Furthermore, the ATP/NADPH energy demand for primary metabolism will rise as photorespiration increases. As a result, the rate of ATP supplied from other flexible pathways must be higher in E. camaldulensis due to its higher rate of photorespiratory pathway. Cyclic electron flow and the WWC are the main flexible pathways that contribute to extra ATP synthesis under high light and atmospheric CO2 concentrations (Makino et al., 2002; Walker et al., 2014; Huang et al., 2015, 2016). Here, we found that the greater rate of photorespiration was accompanied by higher alternative electron flow (Figure 7), and the rate of ATP supplied from other flexible mechanisms was positively correlated to the rate of electron flow to alternative sinks (Figure 8B). Therefore, it appears that E. camaldulensis enhances the alternative electron flow to balance the ATP/NADPH energy demand for high rates of photorespiration.
Conclusions
In order to illustrate the potential different mechanisms underlying the high rates of photosynthesis in sclerophyllous and herbaceous C3 plants. Gas exchange and chlorophyll fluorescence were measured in E. camaldulensis (sclerophyllous) and N. tabacum (herbaceous). Although E. camaldulensis and N. tabacum had similar An under saturating light, the value of gm differed largely between N. tabacum and E. camaldulensis. In N. tabacum, a higher gm increased the value of Cc, resulting in the rate-limiting step of CO2 assimilation tended to be RuBP regeneration. On the contrary, RuBP carboxylation was the main rate-limiting step of CO2 assimilation in E. camaldulensis because CO2 diffusion to the chloroplasts was restricted by a lower gm. Therefore, the rate-limiting step of CO2 assimilation appears to be more related to gm rather than gs in high-photosynthesis species. The lower Cc aggravated RuBP oxygenation in E. camaldulensis. Meanwhile, increased flux through the photorespiratory pathway minimizes the accumulation of photorespiratory metabolites, benefiting photosynthetic CO2 fixation in the Calvin-Benson cycle in E. camaldulensis. In order to balance the ATP/NADPH energy demand for high rates of photorespiration, E. camaldulensis up-regulated alternative electron flow to provide extra ATP. Thus, coordination of photorespiratory pathway and alternative electron flow is crucial for the high rates of CO2 assimilation in E. camaldulensis. These results highlight the different mechanisms responsible for high rates of photosynthesis in the sclerophyllous plant E. camaldulensis and the herbaceous plant N. tabacum.
Author Contributions
WH and YT conceived and designed research. WH conducted experiments. WH, GY, and WY analyzed data. WH wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 31300332), and Youth Innovation Promotion Association of the Chinese Academy of Sciences.
References
Asada, K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 50, 601–639. doi: 10.1146/annurev.arplant.50.1.601
Asada, K. (2000). The water–water cycle as alternative photon and electronsinks. Phil. Trans. R. Soc. Lond. B. 355, 1419–1431. doi: 10.1098/rstb.2000.0703
Baker, N. R., and Rosenqvist, E. (2004). Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 55, 1607–1621. doi: 10.1093/jxb/erh196
Brooks, A., and Farquhar, G. D. (1985). Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. doi: 10.1007/BF00392238
Busch, F. A., Sage, T. L., Cousins, A. B., and Sage, R. F. (2013). C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 36, 200–212. doi: 10.1111/j.1365-3040.2012.02567.x
Carriqui, M., Cabrera, H. M., Conesa, M. Á., Coopman, R. E., Douthe, C., Gago, J., et al. (2015). Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 38, 448–460. doi: 10.1111/pce.12402
Chastain, C. J., and Ogren, W. L. (1989). Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation state in vivo. Plant Cell Physiol. 30, 937–944.
Edwards, G. E., and Walker, D. A. (1983). C3, C4: Mechanisms, and Cellular and Environmental Regulation, of Photosynthesis. Oxford; London: Blackwell Scientific.
Eisenhut, M., Bauwe, H., and Hagemann, M. (2007). Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS. Microbiol. Lett. 277, 232–237. doi: 10.1111/j.1574-6968.2007.00960.x
Evans, J. R. (1987). The relationship between electron transport components and photosynthetic capacity in pea leaves grown at different irradiances. Aust. J. Plant Physiol. 14, 157–170. doi: 10.1071/pp9870157
Evans, J. R., von Caemmerer, S., Setchell, B. A., and Hudson, G. S. (1994). The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Funct. Plant Biol. 21, 475–495.
Farquhar, G. D., von Caemmerer, S., and Berry, J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. doi: 10.1007/BF00386231
Flexas, J., Barbour, M. M., Brendel, O., Cabrera, H. M., Carriqui, M., Díaz-Espejo, A., et al. (2012). Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci. 193–194, 70–84. doi: 10.1016/j.plantsci.2012.05.009
Flexas, J., Bota, J., Escalona, J. M., Sampol, B., and Medrano, H. (2002). Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471. doi: 10.1071/pp01119
Flexas, J., and Medrano, H. (2002). Energy dissipation in C3 plants under drought. Funct. Plant Biol. 29, 1209–1215. doi: 10.1071/fp02015
Flexas, J., Ribas-Carbó, M., Díaz-Espejo, A., Galmés, J., and Medrano, H. (2008). Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31, 602–621. doi: 10.1111/j.1365-3040.2007.01757.x
Gago, J., Coopman, R. E., Cabrera, H. M., Hermida, C., Molins, A., Conesa, M. A., et al. (2013). Photosynthesis limitations in three fern species. Physiol. Plant 149, 599–611. doi: 10.1111/ppl.12073
Genty, B., Briantais, J. M., and Baker, N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 99, 87–92. doi: 10.1016/s0304-4165(89)80016-9
Grassi, G., and Magnani, F. (2005). Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 28, 834–849. doi: 10.1111/j.1365-3040.2005.01333.x
Hanba, Y. T., Kogami, H., and Terashima, I. (2002). The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ. 25, 1021–1030. doi: 10.1046/j.1365-3040.2002.00881.x
Harley, P. C., Loreto, F., Marco, G. D., and Sharkey, T. D. (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98, 1429–1436. doi: 10.1104/pp.98.4.1429
Hassiotou, F., Ludwig, M., Renton, M., Veneklaas, E. J., and Evans, J. R. (2009). Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J. Exp. Bot. 60, 2303–2314. doi: 10.1093/jxb/erp021
Hikosaka, K. (1996). Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.). grown horizontally to avoid mutual shading of leaves. Planta 198, 144–150.
Hikosaka, K., and Terashima, I. (1996). Nitrogen partitioning among photosynthetic components and its consequence in sun and shade plants. Funct. Ecol. 10, 335–343.
Huang, W., Yang, Y.-J., Hu, H., and Zhang, S.-B. (2015). Different roles of cyclic electron flow around photosystem I under sub-saturating and saturating light intensities in tobacco leaves. Front. Plant Sci. 6:923. doi: 10.3389/fpls.2015.00923
Huang, W., Yang, Y.-J., Hu, H., and Zhang, S.-B. (2016). Different roles of cyclic electron flow around photosystem I under sub-saturating and saturating light intensities in tobacco leaves. Front. Plant Sci. 157, 97–104. doi: 10.3389/fpls.2015.00923
Huang, W., Zhang, S.-B., and Hu, H. (2014). Sun leaves up-regulate the photorespiratory pathway to maintain a high rate of CO2 assimilation in tobacco. Front. Plant Sci. 5:688. doi: 10.3389/fpls.2014.00688
Krall, J. P., and Edwards, G. E. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant 86, 180–187.
Lloyd, J., Syvertsen, J. P., Kriedemann, P. E., and Farquhar, G. D. (1992). Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ. 15, 873–899.
Long, S. P., and Bernacchi, C. J. (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 54, 2393–2401. doi: 10.1093/jxb/erg262
Loreto, F., Harley, P. C., Marco, G. D., and Sharkey, T. D. (1992). Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 98, 1437–1443.
Makino, A., Miyake, C., and Yokota, A. (2002). Physiological functions of the water–water cycle (Mehler reaction). and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol. 43, 1017–1026. doi: 10.1093/pcp/pcf124
Miyake, C., and Yokota, A. (2000). Determination of the rate of photoreduction of O2 in the water-water cycle in watermelon leaves and enhancement of the rate by limitation of photosynthesis. Plant Cell Physiol. 41, 335–343. doi: 10.1093/pcp/41.3.335
Neubauer, C., and Yamamoto, H. (1992). Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxanthin-related fluorescence quenching in intact chloroplasts. Plant Physiol. 99, 1354–1361. doi: 10.1104/pp.99.4.1354
Oguchi, R., Hikosaka, K., and Hirose, T. (2003). Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ. 26, 505–512. doi: 10.1046/j.1365-3040.2003.00981.x
Oguchi, R., Hikosaka, K., and Hirose, T. (2005). Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ. 28, 916–927. doi: 10.1111/j.1365-3040.2005.01344.x
Sacksteder, C. A., Kanazawa, A., Jacoby, M. E., and Kramer, D. M. (2000). The proton to electron stoichiometry of steady-state photosynthesis in living plants: a proton-pumping Q cycle is continuously engaged. Proc. Natl. Acad. Sci. U.S.A. 97, 14283–14288. doi: 10.1073/pnas.97.26.14283
Seelert, H., Poetsch, A., Dencher, N. A., Engel, A., Stahlberg, H., and Müller, D. J. (2000). Proton-powered turbine of a plant motor. Nature 405, 418–419. doi: 10.1111/j.1399-3054.1988.tb09205.x
Sharkey, T. D. (1988). Estimating the rate of photorespiration in leaves. Physiol. Plant 73, 147–152.
Somerville, C. R., and Ogren, W. L. (1980). Inhibition of photosynthesis in Arabidopsis mutants lacking leaf glutamate synthase activity. Nature 286, 257–259.
Somerville, C. R., and Ogren, W. L. (1981). Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol. 67, 666–671.
Somerville, C. R., and Ogren, W. L. (1983). An Arabidopsis thaliana mutant defective in chloroplast dicarboxylate transport. Proc. Natl. Acad. Sci. U.S.A. 80, 1290–1294.
Suganuma, H., Abe, Y., Taniguchi, M., Tanouchi, H., Utsugi, H., Kojima, T., et al. (2006). Stand biomass estimation method by canopy coverage for application to remote sensing in an arid area of Western Australia. Forest Ecol. Manage 222, 75–87. doi: 10.1016/j.foreco.2005.10.014
Takahashi, S., Bauwe, H., and Badger, M. R. (2007). Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 144, 487–494. doi: 10.1104/pp.107.097253
Terashima, I., and Evans, J. R. (1988). Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 29, 143–155.
Terashima, I., Hanba, Y. T., Tazoe, Y., Vyas, P., and Yano, S. (2006). Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57, 343–354. doi: 10.1093/jxb/erj014
Terashima, I., Hanba, Y. T., Tholen, D., and Niinemets, Ü. (2011). Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 155, 108–116. doi: 10.1104/pp.110.165472
Tholen, D., Boom, C., Noguchi, K., Ueda, S., Katase, T., and Terashima, I. (2008). The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant Cell Environ. 31, 1688–1700. doi: 10.1111/j.1365-3040.2008.01875.x
Timm, S., Florian, A., Arrivault, S., Stitt, M., and Fernie, A. R. (2012). Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 586, 3692–3697. doi: 10.1016/j.febslet.2012.08.027
Timm, S., Wittmiß, M., Gamlien, S., Ewald, R., Florian, A., Frank, M., et al. (2015). Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. Plant Cell 27, 1968–1984. doi: 10.1105/tpc.15.00105
von Caemmerer, S. (2000). Biochemical Models of Leaf Photosynthesis. Collingwood, VIC: CSIRO Publishing.
von Caemmerer, S., and Farquhar, G. D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387.
Walker, B. J., Strand, D. D., Kramer, D. M., and Cousins, A. B. (2014). The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant Physiol. 165, 453–462. doi: 10.1104/pp.114.238238
Walker, B. J., VanLoocke, A., Bernacchi, C. J., and Ort, D. R. (2016). The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 67, 107–129. doi: 10.1146/annurev-arplant-043015-111709
Warren, C. R., and Dreyer, E. (2006). Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. J. Exp. Bot. 57, 3057–3067. doi: 10.1093/jxb/erl067
Yamori, W., Evans, J. R., and von Caemmerer, S. (2010). Effects of growth and measurement light intensities on temperature dependence of CO2 assimilation rate in tobacco leaves. Plant Cell Environ. 33, 332–343. doi: 10.1111/j.1365-3040.2009.02067.x
Yamori, W., Nagai, T., and Makino, A. (2011). The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 34, 764–777. doi: 10.1111/j.1365-3040.2011.02280.x
Yi, X.-P., Zhang, Y.-L., Yao, H.-S., Zhang, X.-J., Luo, H.-H., Gou, L., et al. (2014). Alternative electron sinks are crucial for conferring photoprotection in field-grown cotton under water deficit during flowering and boll setting stages. Funct. Plant Biol. 41, 737–747. doi: 10.1071/fp13269
Keywords: alternative electron flow, CO2 assimilation, mesophyll conductance, photorespiration, sclerophyllous
Citation: Huang W, Tong Y-G, Yu G-Y and Yang W-X (2016) The Sclerophyllous Eucalyptus camaldulensis and Herbaceous Nicotiana tabacum Have Different Mechanisms to Maintain High Rates of Photosynthesis. Front. Plant Sci. 7:1769. doi: 10.3389/fpls.2016.01769
Received: 04 August 2016; Accepted: 10 November 2016;
Published: 24 November 2016.
Edited by:
Antonio Ferrante, University of Milan, ItalyReviewed by:
Veronica De Micco, University of Naples Federico II, ItalyDaniele Massa, Council for Agricultural Research and Economics, Italy
Copyright © 2016 Huang, Tong, Yu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Huang, huangwei@mail.kib.ac.cn
 Wei Huang
Wei Huang You-Gui Tong3
You-Gui Tong3