- 1Research and Innovation Center, Fondazione Edmund Mach, San Michele all’Adige, Italy
- 2Genome Research, Department of Biology, Bielefeld University, Bielefeld, Germany
- 3Department of Developmental Genetics, Centre for Plant Molecular Biology, University of Tübingen, Tübingen, Germany
Yellow raspberry fruits have reduced anthocyanin contents and offer unique possibility to study the genetics of pigment biosynthesis in this important soft fruit. Anthocyanidin synthase (Ans) catalyzes the conversion of leucoanthocyanidin to anthocyanidin, a key committed step in biosynthesis of anthocyanins. Molecular analysis of the Ans gene enabled to identify an inactive ans allele in a yellow fruit raspberry (“Anne”). A 5 bp insertion in the coding region was identified and designated as ans+5. The insertion creates a premature stop codon resulting in a truncated protein of 264 amino acids, compared to 414 amino acids wild-type ANS protein. This mutation leads to loss of function of the encoded protein that might also result in transcriptional downregulation of Ans gene as a secondary effect, i.e., nonsense-mediated mRNA decay. Further, this mutation results in loss of visible and detectable anthocyanin pigments. Functional characterization of raspberry Ans/ans alleles via complementation experiments in the Arabidopsis thaliana ldox mutant supports the inactivity of encoded protein through ans+5 and explains the proposed block in the anthocyanin biosynthetic pathway in raspberry. Taken together, our data shows that the mutation inside Ans gene in raspberry is responsible for yellow fruit phenotypes.
Introduction
Fruit pigmentation in raspberries (Rubus idaeus L., Rosaceae) is a complex phenomenon and one of the most important traits for breeding and consumer choice where a range of color patterns from deep purple to yellow exists. Anthocyanins and carotenoids are considered to be the main pigments involved in coloration of raspberries (de Ancos et al., 1999; Carvalho et al., 2013b). Anthocyanins are water soluble polyphenolic pigments responsible for the colors of many flowers (Chung et al., 2010; Luo et al., 2015; Sundaramoorthy et al., 2016), fruits (Saito et al., 1999; Debes et al., 2011; Liu et al., 2013; Ben-Simhon et al., 2015), and other plant tissues (Gould et al., 2000; Kim et al., 2004; Zhou et al., 2010). Their biochemical role in plants is not fully understood, however, they have been considered to protect tissues from biotic and abiotic stresses, delay in senescence, assist in photosynthetic machinery, delay over-ripening and increase shelf-life of fruits and act as scavengers of reactive oxygen intermediates (Zhang et al., 2013, 2016; Landi et al., 2015; Yousuf et al., 2016). They have also been described to have potential health beneficial effects in humans against cardiovascular and coronary heart diseases, beneficial effect on eye function properties, cancer, aging, inflammation, obesity, and neurodegenerative diseases (Cassidy et al., 2013; Lim et al., 2013; van der Heijden et al., 2016; Yousuf et al., 2016). Aside from the health benefits, anthocyanins play an important role as an indicator of fruit quality and consumer acceptance (Espín et al., 2007).
Genes encoding specific enzymes of the entire anthocyanin pathway have been well characterized in several plant species (Holton and Cornish, 1995; Broun, 2005; Lepiniec et al., 2006; Ferreyra et al., 2012). Structural and regulatory genes control the biosynthesis of anthocyanins while color mutants of structural and regulatory genes of the pathway have extensively been studied and characterized in many plant species (Kim et al., 2004; Kobayashi et al., 2004; Ma et al., 2009; Chung et al., 2010; Ben-Simhon et al., 2015).
Despite the interest in raspberry anthocyanins, little is known about the genetic control and their regulation processes during fruit development. It is supposed to be a complex trait involving not only the amount but also the type of pigments and co-pigments (Giusti et al., 1999; Castañeda-Ovando et al., 2009). Efforts have been made to identify and map the genes associated with anthocyanins in raspberry (Kassim et al., 2009; McCallum et al., 2010). Previous investigations carried out to explore the role of genes influencing pigmentation patterns propose that a dominant form of gene T plays crucial role in regulating the synthesis of anthocyanins (Crane and Lawrence, 1931). However, in yellow raspberries it seems to be a block of their biosynthesis (Määttä-Riihinen et al., 2004; Carvalho et al., 2013a). Many yellow varieties have originated from crosses between two red fruited varieties such as “Anne” (Amity × Glen Gerry), “Fall Gold” (NH-R7 × Taylor × Rubus pungens var. oldhamii), “Lumina” (Autumn Bliss × Tulameen), “Autumn Amber” (Polka × EMR earliest breeding line), “Zheltyi Gigant” (Maroseika × Ivanovskaya), and also some orange fruit varieties like “Orange Marie” [(Autumn Bliss × Fallgold) × Fallgold] and “Valentina” (EM6225/11 × EM5588/81). It has been discussed that there might be recessive form tt of gene T responsible for yellow phenotype of fruits (Crane and Lawrence, 1931), however, involvement of other genes has not been excluded which might affect the phenotype in the absence of dominant T allele (Britton et al., 1959; Macha, 1966; Jennings and Carmichael, 1975). Though the plants have been characterized in terms of chemical composition of fruits of yellow and red raspberry genotypes (Carvalho et al., 2013a,b) and the data obtained did not predict significant differences between red and yellow genotypes besides the presence or absence of anthocyanins.
Genetic studies have shown several genes such as chalcone synthase (Chs), flavanone 3β-hydroxylase (F3h), dihydroflavonol 4-reductase (Dfr), anthocyanidin synthase (Ans; synonym leucoanthocyanidin dioxygenase, Ldox), and UDPG-flavonoid-glycosyltransferase (Ufgt) control the formation of anthocyanins in fruits of Rosaceae species (Manning, 1998; Moyano et al., 1998; Takos et al., 2006; Ravaglia et al., 2013). Previous reports show that the amount of anthocyanins is strongly related to the level of expression of Ans gene (Almeida et al., 2007; Chen et al., 2012). In yellow raspberries there seems to be a block of anthocyanin biosynthesis, even though there is no evidence on where this block might occur (Määttä-Riihinen et al., 2004; Carvalho et al., 2013a) and knowledge of the molecular genetics is still scanty for this species. Against this backdrop, the present study provides new insights and improves the basic knowledge of origin of yellow raspberries as a result of genetic block in the anthocyanin pathway.
Materials and Methods
Plant Materials
Raspberry fruits of varieties “Anne” and “Tulameen” were collected in 2011 from 2 to 10 different plants per variety. Fruits were collected at different ripening stages from initial fruit formation (stage 1) to fully ripened fruits (stage 5) as described in Carvalho et al. (2013b). Young and old leaves of Rubus (“Anne”, “Tulameen”, “Golden Queen”, and “Heritage”) and Fragaria (Fragaria × ananassa and Fragaria vesca) were also collected. All samples were immediately frozen in liquid nitrogen and stored at -80°C until further use. Samples from different plants were kept and analyzed separately.
DNA and RNA Extraction
Total RNA was isolated from leaves and different fruit stages using Spectrum Plant Total RNA kit (Sigma, Germany) according to the manufacturer’s instructions. Total RNA content and purity was assessed by Nanodrop 8000 (Thermo Scientific, USA) before proceeding to reverse transcription. Total RNA from 10 independent fruits of each stage was reverse-transcribed using SuperScript® VILOTM cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Total genomic DNA was also extracted from young and old leaves according to instructions of NucleoSpin® Plant II kit (Macherey-Nagel, Düren, Germany).
Quantitative Real-Time PCR
Gene-specific primers for quantitative real-time PCR (qPCR) were designed using Primer Express 3.0 (PE Corporation, Foster City, CA, USA). Forward and reverse primer sequences for Chs, F3h, Dfr, Ans, Ufgt, and Adh (alcohol dehydrogenase) genes are listed in Supplementary Table S1. Real-time PCR was carried out in triplicates using C1000TM Thermal Cycler CFXTM (Bio-Rad Laboratories, Hercules, CA, USA) with the iQTM Sybr® Green supermix (Bio-Rad Laboratories) under the following conditions: 98°C, 5 s followed by 44 cycles at 98°C 5 s, 58°C 5 s, 60°C 5 s, 76°C 10 s. After a denaturation step at 98°C for 30 s the melting curve analysis was done increasing the temperature of 0.2°C, from 65 to 95°C, each 10 s. The expression levels of different genes were normalized to the constitutive expression of Adh (GenBank Accession Number XM_004290519).
Cloning of Ans Gene
Gene-specific primer “Ans-utr-fwd” and “Ans-utr-rev” (listed in Supplementary Table S1) were designed to amplify full length Ans gene. The amplified products from genomic DNA of both “Anne” and “Tulameen” were cloned into pCRTM4-TOPO® vector by following the instructions of TOPO® TA Cloning® Kit for sequencing (Invitrogen). The cloned Ans gene from “Anne” and “Tulameen” was named as “pCRTM4-1840” and “pCRTM4-1835”, respectively. Then, Ans transcripts of “Anne” and “Tulameen” were cloned using the primer set (“Ans-orf-fwd” and “Ans-orf-rev”; Supplementary Table S1) specific to coding region. Coding regions of Ans of “Tulameen” and “Anne” were amplified and cloned into pENTRTM D-TOPO® vector by following the instructions of pENTRTM Directional TOPO® Cloning Kit (Invitrogen). The nucleotide sequences of cloned genes were verified by Sanger sequencing. Multiple sequence alignment was made using BioEdit (Hall, 1999).
Copy Number of Ans Gene in Rubus
A qPCR, an alternative to Southern blot can successfully be utilized by normalizing any gene copies to that of the single copy gene by following the procedure as described earlier (Bustin, 2000; Solomon et al., 2008). For this purpose, full length “Anne” Ans gene cloned into pCRTM4-TOPO® vector (“pCRTM4-1840”) was used for creating standard curve by following the instructions of Applied Biosystems (Life Technologies Corporation, Carlsbad, CA, USA). The standard curve was created by knowing the mass of “pCRTM4-1840” plasmid and running the respective serial dilutions (D1–D5) as described in protocol (http://www6.appliedbiosystems.com/support/tutorials/pdf/quant_pcr.pdf). For this purpose Ans target fragment was amplified by designing universal oligonucleotides (“RubUni-fwd” and “RubUni-rev”; Supplementary Table S1) aimed at species with known (F. vesca, n = x = 7; Fragaria × ananassa Duch, n = 4x = 28) and unknown (Rubus, n = x = 7) Ans copy number in comparison to the normalized plasmid reference. Reactions were performed using iQTM Sybr® Green supermix (Bio-Rad Laboratories) by following the program, 98°C 10 s (denaturation), 49°C 5 s and 51°C 5 s (annealing) 76°C 10 s (extension), 39 cycles from denaturation to extension, 98°C 30 s (final extension) and melting curve 65–95°C with 0.2°C increment in C1000TM Thermal Cycler CFXTM Real-Time System (Bio-Rad Laboratories). All the reactions were carried out with the same set of established conditions in biological and technical triplicates.
Transformation of Arabidopsis
The Ans gene from “Tulameen” (1242 bp) and “Anne” (1247 bp) present in pENTRTM D-TOPO® vector was sub-cloned into binary pLEELA vector by performing LR reaction (Invitrogen) and named as “pLEELA-1242” and “pLEELA-1247”, respectively. Both binary vectors containing Ans gene driven by 2x35S promoter harboring Bar gene were introduced into Arabidopsis ldox mutant line (tds4-2; tt18-1) resistant for kanamycin marker using floral dip technique (Clough and Bent, 1998) via Agrobacterium tumefaciens strain GV3101. Seeds obtained were selected on half MS media (Murashige and Skoog, 1962) containing kanamycin (40 μg ml-1) and basta (25 μg ml-1) as selection markers. Homozygous F2 lines of F1 Arabidopsis seeds were obtained. The Arabidopsis transgenes were grown in growth chamber with the following conditions of 16/8 h light (100 μmol photons m-2 s-1), 70% humidity and 24°C temperature. Wild-type (Col-0) and control (tds4-2) plants were also grown with the same conditions in growth chamber. The transgenic status of several Arabidopsis lines obtained was evaluated by PCR amplification using “Ans-orf-fwd” and “Ans-orf-rev” primers (Supplementary Table S1) for Ans gene.
Inducing Anthocyanins in Arabidopsis Seedlings
In order to evaluate the anthocyanins accumulation in complemented mutant lines of Arabidopsis, sterilized seeds were grown in half MS liquid media containing 5% sucrose under shaking (60 rpm). Sucrose stress promotes anthocyanins accumulation in emerging seedlings. Seeds were also placed in media without sucrose as control. Anthocyanin-based phenotype was observed in 5 days seedlings and 3 weeks old plantlets. Furthermore, plantlets were shifted to greenhouse for anthocyanin extraction and seed production. Anthocyanins were extracted in methanol (1% HCl) and subjected to HPLC analysis. The separation was accomplished under gradient conditions on a Nucleodur C18ec column (250/4; Macherey-Nagel) with solvent A 1% phosphoric acid in water and solvent B 1% phosphoric acid in acetonitrile. Gradient starts with 100% A to 50% A in 25 min, plateau of 3 min, up to 100% A in 7 min and final plateau of 5 min with flow rate of 1 ml min-1 and monitored at 280 and 515 nm.
Establishment of Probe-Based Marker
Unlabeled oligonucleotides (“RubUni-fwd” and “RubUni-rev”) and a 15 bp TaqMan FAM dye-labeled probe (Supplementary Table S1) were designed using Primer Express 3.0 (PE Corporation) for detection of unique mutation inside Ans gene. All samples including non-template control (NTC) were run in triplicates in a volume of 12.5 μl containing 6.25 μl iQ Multiplex (Bio-Rad Laboratories), 0.25 μM each PCR primer, 0.5 μM FAM-probe and 20 ng genomic DNA or RNA/cDNA. Reactions were incubated in the C1000TM Thermal Cycler CFXTM Real-Time System (Bio-Rad Laboratories) for 3 min at 95°C (one cycle) followed by 39 cycles consisting of 10 s at 95°C, 10 s at 51°C and 30 s at 72°C.
Results
Expression Analysis of Anthocyanin Pathway Genes
Structural genes of the anthocyanin biosynthetic pathway play key role in the biogenesis of anthocyanins in fruit tissues. Anthocyanins in raspberry fruits become visible starting from fruit stage 3 (“turning”). The expression pattern of the anthocyanin pathway genes of fruit development stages 3 and 4 in both varieties “Anne” and “Tulameen” are presented in Figure 1. Chs, F3h, Dfr, and Ufgt genes are upregulated from stage 3 to 4 at different levels in both varieties. In fruits of red variety “Tulameen” the apparent RNA accumulation of Ans gene is coordinated with the other biosynthetic genes examined (Chs, F3h, Dfr, Ufgt) as it shows a dramatic increase from stage 3 to 4. However, the increase in expression was not detected in yellow fruits of “Anne”. The expression profile shows that only the Ans gene is not upregulated onward fruit turning stage in “Anne” among all the other tested anthocyanin pathway genes (Figure 1). Therefore, further analysis of the Ans gene was performed to ascertain the possible block at the genetic level.
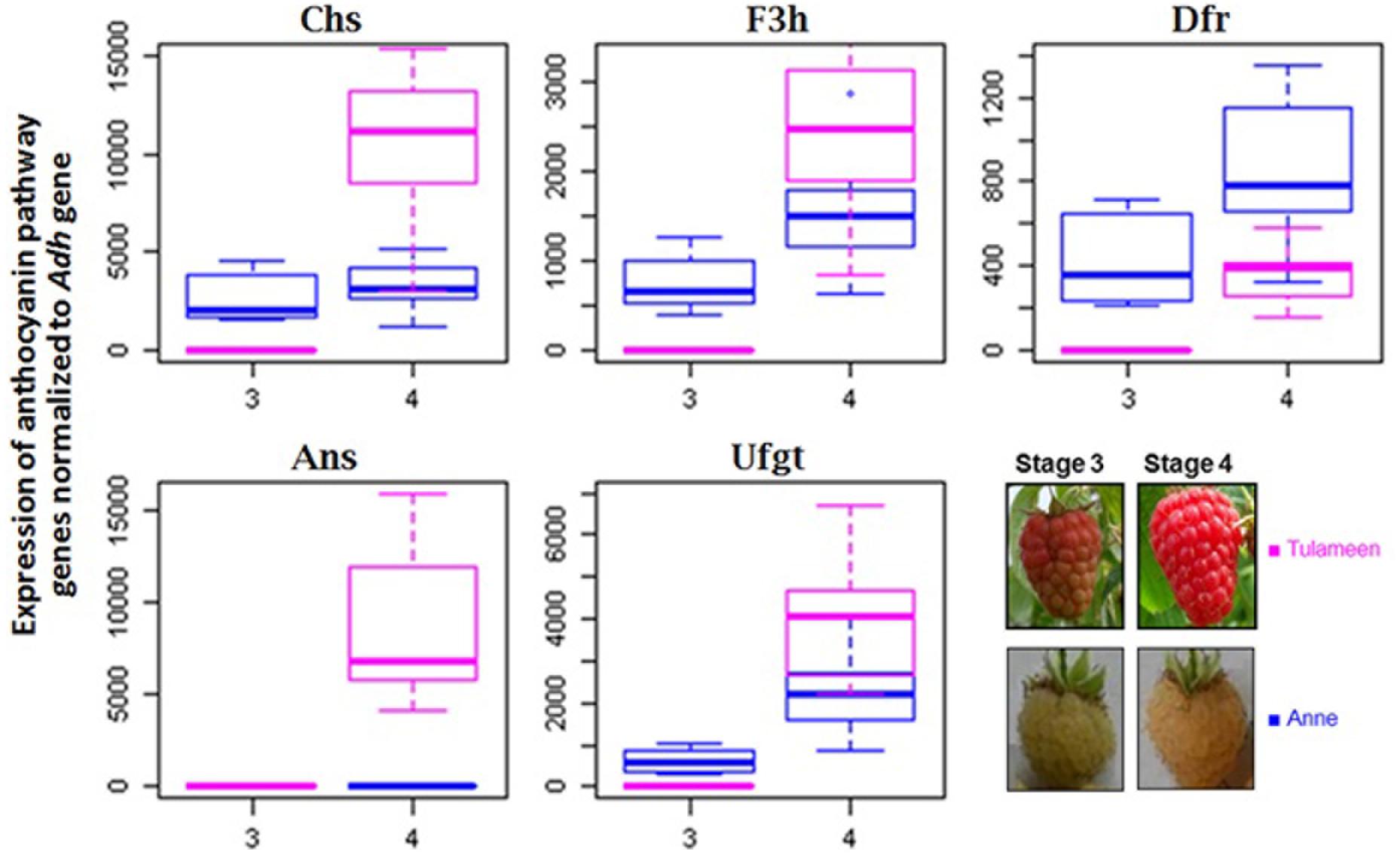
FIGURE 1. Expression analysis of biosynthetic flavonoid pathway genes in cv. “Tulameen” and “Anne”. Expression of Chs. chalcone synthase; F3h. flavanone 3β-hydroxylase; Dfr, dihydroflavonol 4-reductase; Ans, anthocyanidin synthase; Ufgt UDPG-flavonoid-glycosyltransferase at fruiting stage 3 and 4 in yellow variety “Anne” and red variety “Tulameen”, respectively. The inset picture shows an example of a red and a yellow raspberry at the respective fruit ripening stages. Box plots show the mean values of the expression level with standard deviation, while dots give the sample outline.
In silico Mining and Genomic Structure of Rubus Ans
In silico search of Rubus genome draft version 1.08 (cv. “Heritage”) and in house 454 EST library of fruit stages of “Tulameen” (unpublished data) with Ans gene of Fragaria × ananassa “Queen Elisa” (GenBank Accession Number AY695817) as template enabled the assembly of a putative Ans gene of Rubus. An Ans gene, spanning the full coding region was cloned with the help of assembled contigs (Supplementary Figure S1). Gene-specific primer were designed based on the sequence assembly for PCR amplification of a 1835 bp genomic Ans fragment from “Tulameen” that includes the entire coding region with one 446 bp intron, and 118 bp of the 5′UTR and 29 bp of the 3′UTR (GenBank Accession Number KX950789). Subsequently, the same approach was used to amplify the respective Ans gene of “Anne” (GenBank Accession Number KX950788) resulting in a 1840 bp amplicon. Direct comparison of these two alleles revealed a 5 bp insertion (GGCCT) in the second exon at position 730 bp of the “Anne” allele (ans; Figure 2). This insertion was designated as ans+5.
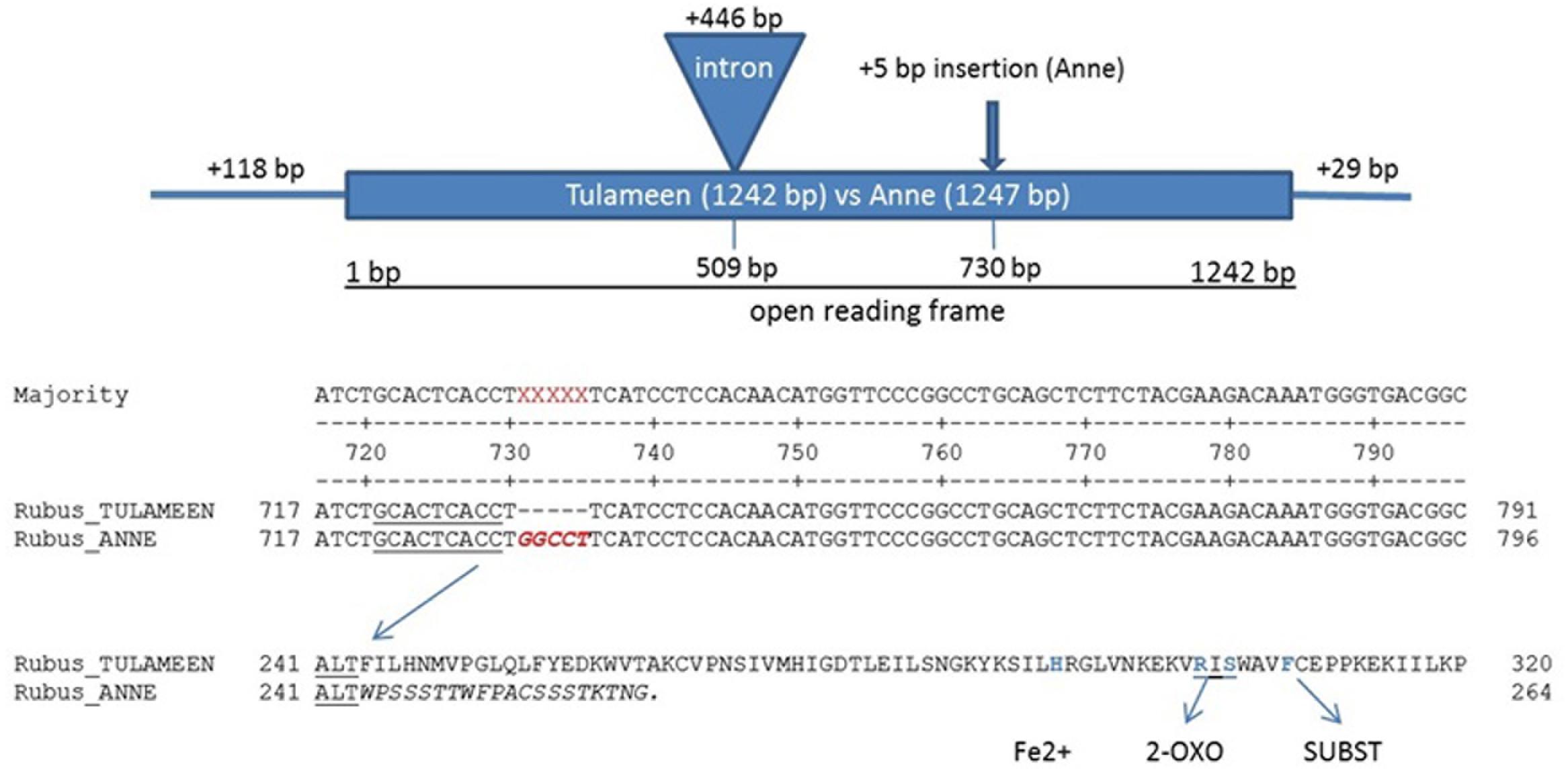
FIGURE 2. Analyses of anthocyanidin synthase alleles in cv. “Tulameen” and “Anne”. Comparison indicate a 5 bp insertion (ans+5) in the second exon at position 730 of “Anne” allele (ans) causing a frameshift and thus leading to a truncated protein in the yellow variety, missing conserved regions for substrate-, co-substrate, and iron binding sites. The Ans fragment from “Anne” and “Tulameen” includes the entire coding region with one 446 bp intron at position 509, and 118 bp of the 5′UTR and 29 bp of the 3′UTR.
Sequence Analysis of Rubus Ans Gene
A 1254 bp cDNA fragment of Ri-Ans was obtained by PCR amplification using primers designed from the predicted coding sequence using cDNA of “Tulameen” as template. The open reading frame (orf) contained 1242 bp, encoding a polypeptide of 414 amino acid (aa) residues and showing 92% similarity to the Fragaria × ananassa ANS protein. Comparison of Ans sequences revealed the same ans+5 in “Anne” cDNA as in genomic DNA sequences. This extra 5 bp sequence resulted in a frame-shift and creates a premature stop codon after 20 aa from this mutation point. It is predicted to result in a much shorter ANS protein of “Anne” of only 264 aa. The shorter “Anne” ANS protein lacks the essential conserved amino acids, known to be responsible for substrate and co-substrate binding (Figure 2), giving strong support for its inactivity and therefore the genetic block in the pathway.
Sequence alignment showed that the putative Rubus ANS grouped together with other known ANS from Rosaceae, but also other plant families and is separated from other flavonoid pathway 2-oxoglutarate-dependent dioxygenases (2-ODDs) such as flavonol synthase and flavanone 3β-hydroxylase (Supplementary Figure S2). It is obvious that most of the catalytical important residues are missing only in ANS of “Anne” in comparison to 2-ODDs (Supplementary Figure S2).
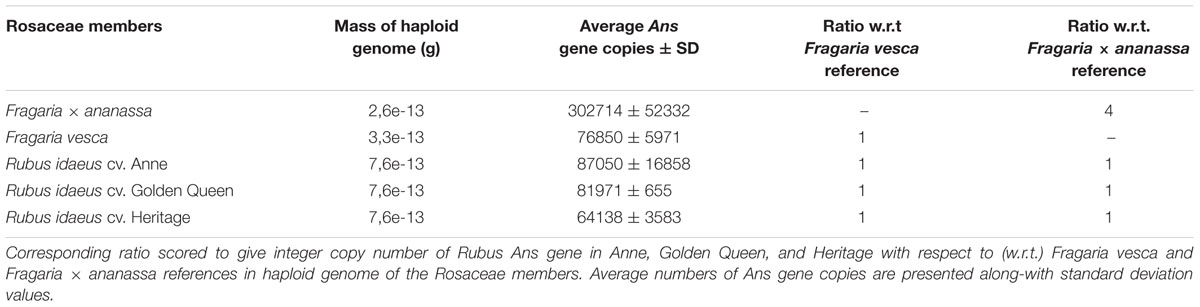
Copy Number Analysis of Ans Gene
As the genome of Rubus is not fully sequenced yet (89%; Ward et al., personal communication) estimation of Ans gene copies is desirable. Here, in order to determine the copy number of Ans gene in Rubus we exploited the genome of diploid species F. vesca (2n = 2x = 14) with a single copy of Ans gene, and used Fragaria × ananassa Duch (octoploid; 2n = 8x = 56) with four copies of Ans gene (Almeida et al., 2007) as an additional endorsement to the methodology. To determine the genomic complexity, mass of “pCRTM4-1840” plasmid (5795 bp) was determined as 6.35e-18 g. Then, high quality standard curve was obtained by making serial dilutions (D1–D5). Similarly, mass of haploid genomes of F. vesca (n = x = 7; 120 Mb) and Fragaria × ananassa Duch (n = 4x = 28; 349 Mb) and Rubus (n = x = 7; approximately 150 Mb) was calculated as 3,288e-13 g, 2,630e-13 g, and 7,650e-13 g, respectively. Now, Ans target from Rubus and other reference species was put under comparative copy number analysis to the normalized single Ans copy (“pCRTM4-1840”). The corresponding ratio of Ans gene copies in haploid genome of F. vesca, Fragaria × ananassa, and different raspberry varieties is given in Table 1. It is evident that average numbers of Ans gene copies in Rubus varieties are closely correlated to the known Ans copies in F. vesca genome. Haploid genome of all the tested Rubus varieties (“Anne”, “Golden Queen”, and “Heritage”) contain single copy of Ans gene in comparison to haploid genome of F. vesca normalized to that of the single Ans copy gene. Furthermore, F. vesca and all the raspberry varieties present the same ratio (single copy of Ans gene) in comparison to the additional reference Fragaria × ananassa with four Ans copies (Table 1). Based on the results, single copy number of Ans gene in Rubus is suggested.
Complementation of Arabidopsis ldox Mutant
Transgenic lines obtained from ldox mutant of Arabidopsis harboring Ri-Ans coding sequences from “Anne” and “Tulameen” driven by the constitutive CaMV35S promoter were termed as “ldox::35S:Ans_Anne” and “ldox::35S:Ans_Tulameen”, respectively, in connection to control tds4-2 (“ldox:KO”) line. The transgenic status of Arabidopsis lines was confirmed by PCR amplification of Ri-Ans genes using DNA as template (Figure 3). Eight of 13 “ldox::35S:Ans_Tulameen” lines and five out of six “ldox::35S:Ans_Anne” lines were tested for functional activity. One representative line is shown from all genotypes (Figure 4).
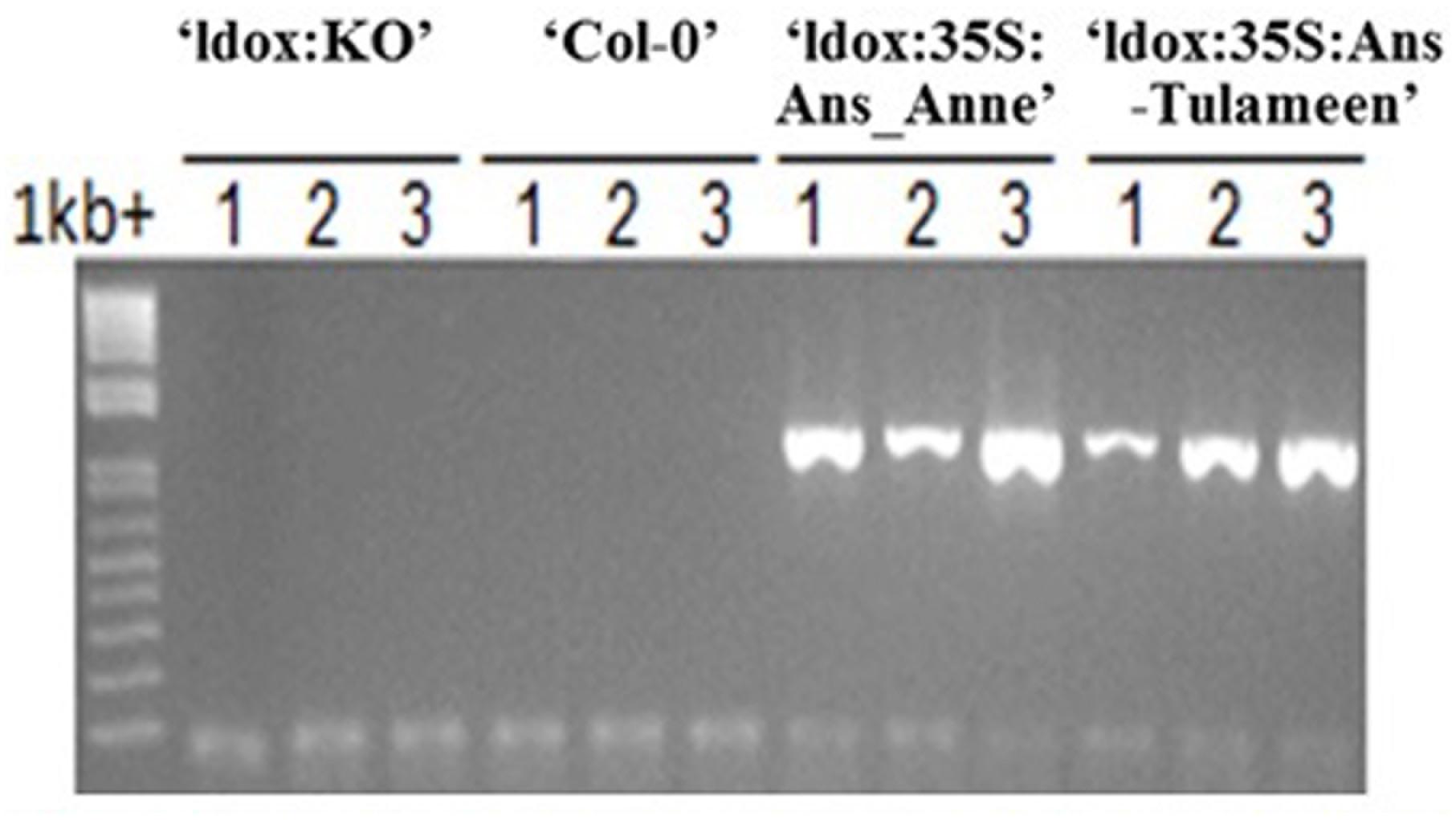
FIGURE 3. PCR analysis, detecting Ri-Ans transgenes from “Anne” and “Tulameen”. Independent transgenic (‘ldox::35S:Ans_Anne’ and ‘ldox::35S:Ans_Tulameen’) and independent non-transgenic (‘Col-0’ and ‘ldox:KO′) Arabidopsis lines.

FIGURE 4. Flavonoid phenotype of Arabidopsis transgenes in comparison to wild-type (′Col-0′) and Idox knockout line (tds4-2; ‘ldox:KO’). Control, wild-type, ‘ldox::35S:Ans_Anne’ and ‘ldox::35S:Ans_Tulameen’ seedlings grown in half MS without sucrose (Suc-), 5 days old seedlings (A1, C1, E1, G1) and 2 weeks old plantlets (A2, C2, E2, G2), respectively. Control, wild-type, ‘ldox::35S:Ans_Anne’ and ‘ldox::35S:Ans_Tulameen’ seedlings grown on half MS with 5% sucrose (Suc+), 5 days old seedlings (B1, D1, F1, H1) and 2 weeks old plantlets (B2, D2, F2, H2), Respectively. Formation of anthocyanins was also obtained in 4 week old plants wild-type (B3) and ‘ldox::35S:Ans_Tulameen’ (D3) in comparison to control ‘ldox:KO’ (A3) and ‘ldox::35S:Ans_Anne’ (C3) lines. The seed coat color associated with presence of PA (B4,D4) or in absence of PA (A4,C4).
Wild-type “Col-0” shows anthocyanins accumulation in 5 days old seedlings in response to sucrose stress induction as compared to seedlings with no anthocyanins in sucrose-less media. The control “ldox:KO” line could not produce anthocyanins in seedlings, irrespective of sucrose treatment. However, sucrose-mediated, wild-type-like anthocyanin phenotype of “ldox::35S:Ans_Tulameen” lines can clearly be noticed in hypocotyl and cotyledons of developing 5 days old seedlings. On the other hand, “ldox::35S:Ans_Anne” did not complement the tds4-2 phenotype (Figures 4A1–H1). The absence of anthocyanins in “ldox::35S:Ans_Anne” line clearly demonstrates the non-functional status of “Anne” ANS protein in contrary to functional “Tulameen” ANS. None of the Arabidopsis lines presents phenotype with anthocyanins accumulation in their 5 days old seedlings in sucrose-less media. The phenotype of 2 weeks old plantlets in growth chamber in response to sucrose induction (Figures 4A2–H2) and 4 weeks old plants remained unchanged, i.e., “Col-0” and “ldox::35S:Ans_Tulameen” lines depict the presence of anthocyanins while “ldox:KO” and “ldox::35S:Ans_Anne” lines remained anthocyanin-less (Figures 4A3–D3).
Homozygous lines of “ldox::35S:Ans_Tulameen” showed a restored characteristic brown seed color as found in the wild-type “Col-0” whereas “ldox::35S:Ans_Anne” have the same yellowish color as the “ldox:KO” control line. The brown seed coat color of “ldox::35S:Ans_Tulameen” lines is associated with the presence of proanthocyanidins as found in the wild-type while seed coat of “ldox::35S:Ans_Anne” lines is yellow which is more likely comprised of mainly flavonols as in control “ldox:KO” line (Figures 4A4–D4). Furthermore, HPLC analysis showed the presence and a similar pattern of anthocyanins from extracts of all “ldox::35S:Ans_Tulameen” lines with wild-type Arabidopsis on the contrary to the plants of “ldox::35S:Ans_Anne” and “ldox:KO” lines (Supplementary Figure S3). Hence, functional characterization and complementation in planta provide an additional strong proof of inactive ANS protein in “Anne” as compared to functional ANS protein in “Tulameen”.
Development of DNA/RNA-Based Probe Marker
A big germplasm collection of Rubus is available at various platforms worldwide. It would be of a great interest in breeding programs to screen the Rubus varieties for Anne-like mutation inside Ans gene. Therefore, a DNA- or RNA-based probe marker was developed to detect ans+5 mutation. The probe was not only tested on independent samples of genomic DNA but also on RNA/cDNA of different ripening stages of fruits of “Anne” and “Tulameen”, respectively. In order to confirm the probe specificity, full length Ans gene from genomic DNA of the both varieties cloned into pCRTM4-TOPO® vector (“pCRTM4-1835” and “pCRTM4-1840”) was also included in the analysis. As probe is designed on the basis of mutation inside Ans gene, it will not work on the samples that do not contain the mutation. Therefore, to validate the amplification, same primers were also tested on genomic DNA template of “Anne” and “Tulameen” in reaction mix exempting probe. The probe assay, as indicated in Figure 5A, shows that FAM dye-labeled probe containing 3 bp of ans+5 (GGCCT) was successfully applied to “Anne” Ans (“pCRTM4-1840”; violet circles) but not on “Tulameen” Ans (“pCRTM4-1835”; pink squares on baseline). Similarly, the FAM probe applied on various independent samples based on genomic DNA gave fluorescent signals for all the “Anne” samples (blue circles) but none of the “Tulameen” sample (pink square on baseline). However, not only “Anne” (blue lines) but all the “Tulameen” samples (pink lines) based on genomic DNA showed the amplification without probe (Figure 5A). The fluorescently labeled probe applied on RNA/cDNA also detected the mutation from the fruit stages of “Anne” (blue lines as shown in Figure 5B) and none of the fruit stage of “Tulameen” (pink) showed probe-based signals. NTC is also indicated along the baseline (green) as “Tulameen” samples. So it is clear from the probe analysis that the FAM probe is specific for mutation/insertion in “Anne” but work neither on genomic DNA nor RNA/cDNA of “Tulameen” samples (Figures 5A,B).
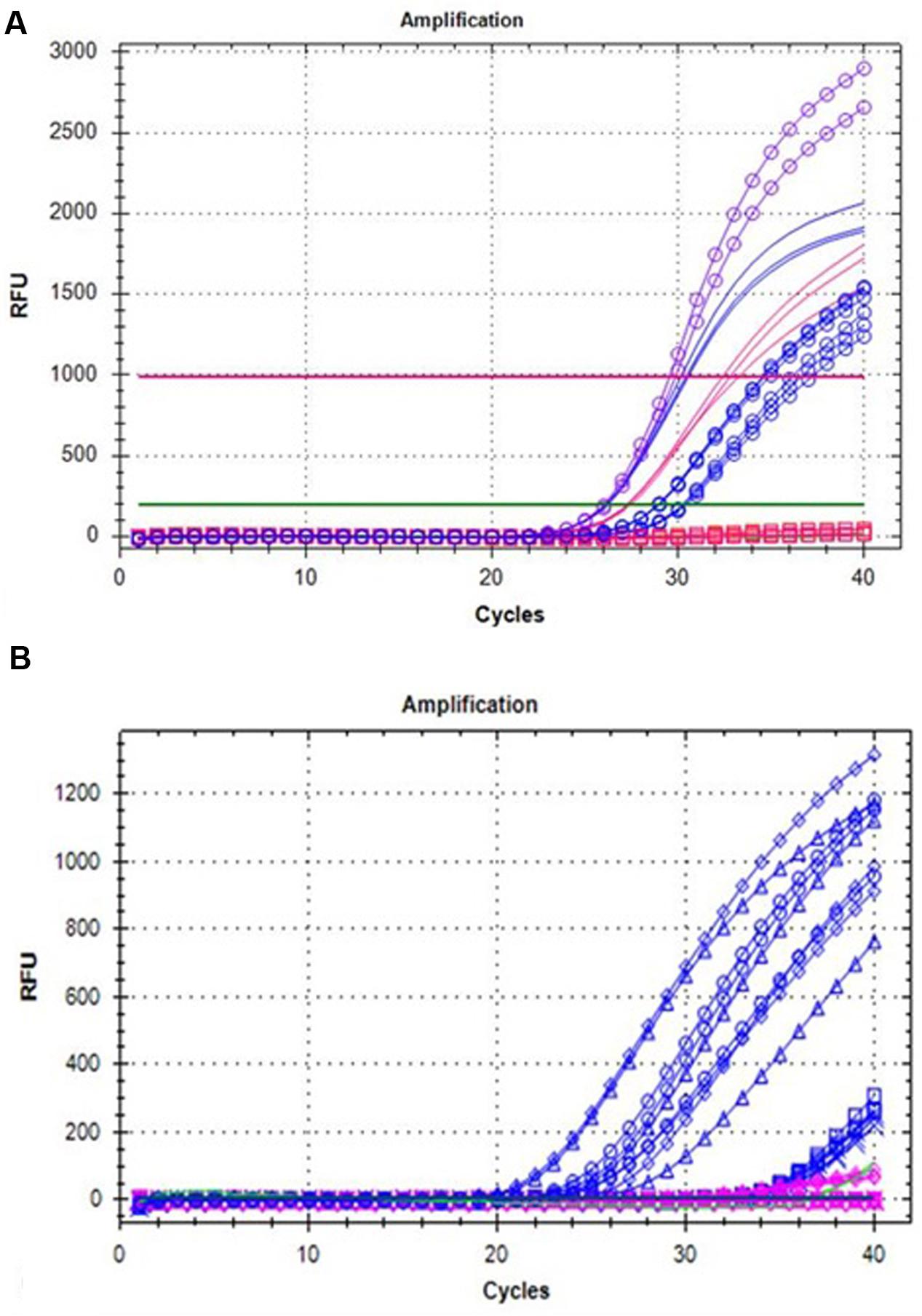
FIGURE 5. (A) Probe analysis on genomic DNA samples of “Anne” (blue) and “Tulameen” (pink) for the 5 bp insertion (ans+5). Probe assay on both genomic DNA (blue circles), ‘pCRTM4-1840’ (violet circles) of “Anne” and genomic DNA, ‘pCRTM4-1835’ (pink squares) of “Tulameen”. Genomic DNA samples amplified from “Anne” (blue lines) and “Tulameen” (pink lines) without probe in reaction mix. (B) Probe analysis on different fruit stages of “Anne” (blue) and “Tulameen” (pink) for the 5 bp insertion (ans+5). Transcripts from different fruit stages of “Anne” exhibits fluorescent signals (blue) but none of the “Tulameen” fruit stage (pink) as indicated by the diamond (Stage 1), cross (Stage 2), triangle (Stage 3), sequence (Stage 4), and circle (Stage 5). NTC is indicated along the baseline (green).
Discussion
The current study provides new details in view of exploring the genetic mechanism controlling the biosynthesis of flavonoids, in particular anthocyanins, in raspberries. Raspberries with yellow or orange pigmentation, which have been described already more than a century ago (Card, 1898), are most probably based on anthocyanin pathway mutants. So far the knowledge is that homozygous recessive alleles tt of gene T are considered to play a fundamental role in determining the yellow color (Crane and Lawrence, 1931; Jennings and Carmichael, 1975). Whether this locus is related to Ans or not cannot be stated here. In the present work, the most sold red fruiting raspberry “Tulameen” was included for comparative study with the important pale yellow fruiting variety “Anne” to elucidate the phenomenon involved in pigmentation. Red raspberry “Tulameen” was selected from a cross “Nootka” × “Glen Prosen”, both bearing red fruits (Daubeny and Anderson, 1991). Yellow variety “Anne” has been selected from a controlled cross of two red varieties, “Amity” × “Glen Gerry” (Swartz et al., 1998); however, the authors did not provide any details on the inheritance of yellow pigmented fruits. In a recent study, biochemical analysis of various red and yellow raspberries including “Tulameen” and “Anne” gave no clear evidence where a block in the anthocyanin pathway might have occurred (Carvalho et al., 2013a), as no intermediate compounds or class of compounds were significantly accumulated in the turning stages (fruiting stage 3–4) of yellow and red fruiting raspberries.
Regulation of Gene Expression
The anthocyanin accumulation in raspberry fruits becomes visible from ripening stage 3–4 (Figure 1). Therefore, expression analysis of the anthocyanin biosynthetic genes was carried out at these fruit developmental stages. As indicated in Figure 1, almost all structural anthocyanin genes show a significant increase in their expression; however, this was not the case for Ans in yellow fruits of “Anne”. The Ans transcripts were not induced in “Anne” when compared to “Tulameen” Ans. This reduction of Ans transcripts in “Anne” might be due to secondary effect of a nonsense mutation inside Ans gene. Such mechanism, known as nonsense-mediated mRNA decay (NMD), has been reported in plants (Schwartz et al., 2006; Wu et al., 2007) by which mutations inside a gene cause premature termination codons (PTCs) and quickly degrade mRNA to inhibit the accumulation of nonsense (inactive) proteins. Similar profiles with a reduced Ans expression among the anthocyanin pathway genes were also found in mock strawberry (Debes et al., 2011) and pomegranate (Zhao et al., 2015), leading to white fruiting anthocyanin-free phenotypes. In contrast, an unchanged expression of Ans was observed in the Arabidopsis loss-of function mutant transparent testa 17 (tt17), where a SNP was found to result in an inactive protein and transparent testa seed phenotype (Appelhagen et al., 2011). However, our findings are in line with a recent study. In a white phenotypic fruit of pomegranates almost no expression of Ans gene was described. An insertion in coding sequence resulted in lack of Pg-ldox transcripts and of red pigments (Ben-Simhon et al., 2015). Similarly, in white fruited mock strawberry and yellow onion bulb no expression was observed of inactive Ans gene in comparison to active Ans gene in red phenotypes (Kim et al., 2004; Debes et al., 2011). Thus, reduced Ans transcripts might suggest a block at Ans level in “Anne”. As accumulation of flavonols and flavonol glycosides was observed in both, red and yellow raspberry fruits (Määttä-Riihinen et al., 2004; Carvalho et al., 2013a), similarly as they were found in the pale yellow Cactaceous and Caryophyllales species (Iwashina et al., 1988; Shimada et al., 2004) and later anthocyanin block was confirmed at Ans level (Shimada et al., 2005).
Molecular Analysis of Ans/ans Alleles
The gene encoding ANS protein, a member of 2-ODD family has been reported for Rosaceae members and other plant species but not from Rubus yet. Among all 2-ODDs the catalytic residues are characterized by highly conserved histidine (His) residues for ferrous-iron coordination, and arginine (Arg) and serine (Ser) residues for binding site of 2-oxoglutarate and phenylalanine (Phe) residue for substrate binding (Saito et al., 1999; Koehntop et al., 2005; Clifton et al., 2006; Gebhardt et al., 2007; Cheng et al., 2014) as shown in Figure 2. Molecular analysis of Ans gene at genomic and mRNA/cDNA level indicates a 5 bp insertion (GGCCT; ans+5) in “Anne” ans alleles (Figure 2; Supplementary Figure S2). Furthermore, the coding region of “Anne” and “Tulameen” Ans contains a 446 bp intron (Figure 2). The Rubus intron comprises consensus “GT” and “AG” sequences at the 5′ and 3′ ends, respectively. This one intron genomic structure is similar to those found in Fragaria × ananassa, F. vesca, Malus, Allium, Theobroma, Arabidopsis, Forsythia, Prunus, and Petunia (Weiss et al., 1993; Rosati et al., 1999; Deng and Davis, 2001; Almeida et al., 2007; Liu et al., 2013; Shen et al., 2014; Kim et al., 2015). The Ri-ANS protein sequence from “Tulameen” showed high (88%) identity to other plant species (Malus ANS, Prunus ANS, and Pyrus ANS) of Rosaceae family. The protein sequence is approximately 93% identical to F. vesca ANS, which is highly homologous to other functionally characterized plant ANSs. For example, it is 83, 78, and 73% identical to ANS from Theobroma, Perilla, and Gerbera, respectively. The known conserved domains were found in the deduced amino acid sequence of ANS from red fruiting raspberry “Tulameen”. However, the ans+5 in Ans gene of “Anne” led to a premature stop codon 20 aa upstream the insertion and the loss of the conserved His (iron binding site) and the RxS motif involved in 2-oxoglutarate binding. Furthermore, it has been described that as long the PTCs are present upstream of 3′ mRNA termini, they act as substrates (cis-acting elements) to activate NMD mechanism (Kertesz et al., 2006; Schwartz et al., 2006; Hori and Watanabe, 2007). Hence Ans PTC in “Anne” which is 455 bp distant from native 3′UTR, strongly supports this phenomenon.
Molecular Marker for Rubus Screening
Molecular DNA- or RNA-based probe markers are common in use nowadays and have been reported for identification of SNPs or small target sequence within particular amplified fragments (Wittwer et al., 1997). Taking advantage of this technique, we designed a fluorogenic-labeled probe that proved to be useful for detection of mutants of Ans gene from genomic DNA and RNA/cDNA of “Anne” (Figures 5A,B). The probe developed here can further be utilized for characterization of specific insertion/mutation inside Ans gene to screen other yellow varieties of raspberry but can also be used as molecular marker for characterization of germplasm collections and offsprings in breeding programs.
Complexity of Ans Gene
As the available genome draft of Rubus is not completed yet, it could not be excluded that more than one copy of Ans gene is present. Therefore, qPCR approach was performed to determine the complexity of the Ans gene, adopted of the method applied earlier to determine gene copy number in filamentous fungi (Solomon et al., 2008). The analysis indicated that there is only a single copy of the Ans gene in the genome of R. idaeus (as presented in Table 1), which is in agreement with the BLAST search of the Rubus genome draft (“Heritage”). The presence of a small gene family of the Ans gene has been reported for some species such as apple, peach, and diploid strawberry. Peach and octoploid strawberry (Fragaria × ananassa) genome encodes two and four copies of Ans gene, respectively (Almeida et al., 2007; Ravaglia et al., 2013). Only one gene copy has been described in diploid strawberry (F. vesca) and apple (Takos et al., 2006; Lin-Wang et al., 2014).
Functional Characterization of Ans Gene
In order to functionally characterize the Rubus Ans genes, transgenic lines were obtained through complementation of ldox mutant of Arabidopsis “ldox:KO” harboring Ri-Ans coding sequences from “Anne” and “Tulameen” expressed under the control of the constitutive CaMV 35S promoter. The plants transformed with “35S::Ans_Tulameen” showed a restored phenotype, while lines transformed with “35S::Ans_Anne” showed no changes when compared to “ldox:KO” line as shown in Figure 4. Chemical analysis confirmed the complementation of anthocyanin pathway due to presence of anthocyanins in transgenic plants transformed with “35S::Ans_Tulameen” (Supplementary Figure S3). However, no anthocyanins were detected from the plants transformed with “35S::Ans_Anne” like the “ldox:KO” line, similar to the well-established complementation reports (Abrahams et al., 2003; Teng et al., 2005; Solfanelli et al., 2006; Kovinich et al., 2014). These results confirm that “Tulameen-Ans” codes for a functional ANS protein while “Anne-Ans” results in a non-functional protein.
Conclusion
We identified a 5 bp insertion (ans+5) in Ans gene of yellow variety “Anne” as compared to red fruiting variety (“Tulameen”) of raspberry. We elucidated the functional role of ANS protein from “Tulameen” that imparts red fruiting color but is non-functional in yellow fruiting raspberry “Anne”. It is therefore considered that the ans+5 (GGCCT) in “Anne” generated a truncated polypeptide lacking the consensus motifs for cofactor and substrate binding, posing a complete loss-of-function in the mutant Ri-Ans. Deletion or insertion of a number of bases that is not a multiple of 3, usually introduces premature STOP codons in addition to lots of amino acid changes ultimately causing loss of function mutation of a protein sequence. We report a block in a step of anthocyanin pathway at ANS level in Rubus resulting in yellow anthocyanin-free fruit phenotype as indicated in the proposed pathway (Figure 6). It also supports the phenomenon that such nonsense mutations can abolish splicing resulting in NMD mechanism.
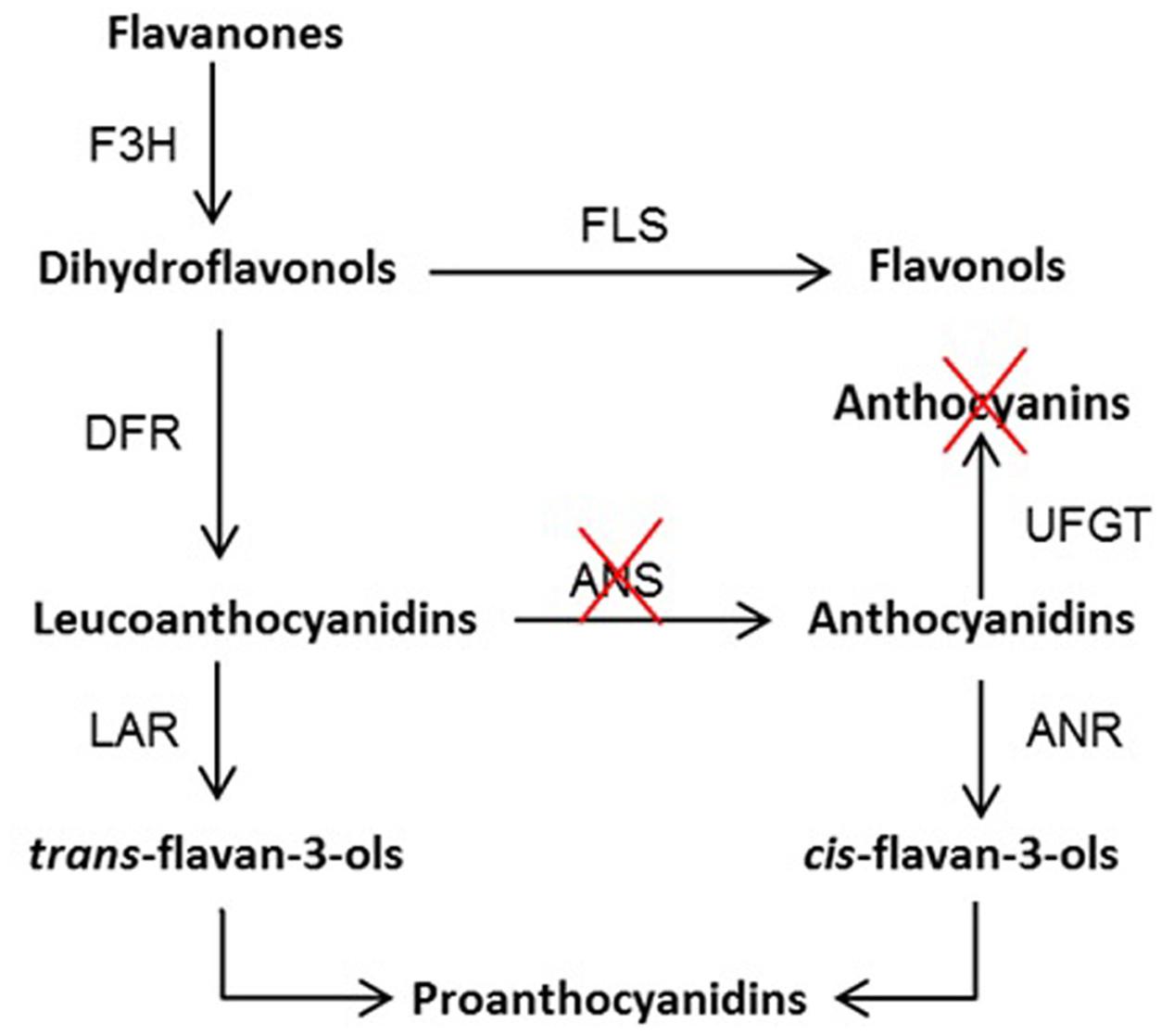
FIGURE 6. Proposed flavonoid biosynthesis pathway in raspberry. F3H, flavanone 3β-hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; UFGT, UDPG-flavonoid-glycosyltransferase; ANR, anthocyanidin reductase; LAR, leucoanthocyanidin reductase; and FLS, flavonol synthase. The red crosses indicate the blocked step of anthocyanin biosynthetic pathway at ANS level leading to the absence of anthocyanin pigments.
The probe developed in the current study can efficiently be utilized to screen other yellow and orange but also red varieties of raspberry for “Anne” like mutation inside Ans gene. In case of reduced anthocyanins, carotenoids are considered to be the main pigments accounting the pigmentation (yellow but also orange) in raspberries. Taken together, the data described here can further be utilized in breeding programs for indirect selection of genetic determinants of a trait of interest. Furthermore, other yellow and orange varieties are also under evaluation for possible flavonoid or carotenoid pathway blocks, eventually leading to develop a general pathway map for pigmentation in raspberry.
Author Contributions
AF, EC, LH, and LP: Sample collection, preparation and expression analysis. EC and MR: In silico mining and cloning of Ans gene. LP and MR: Ans copy number analysis and development of probe-based marker. MM: Provided tissue culture facilities. MR and RS: Cloning, Arabidopsis transformation, and complementation. MR: Manuscript writing. SM: Overall guide as group leader and reviewed by all the co-authors.
Funding
This work was funded by The Autonomous Province of Trento, Italy (ADP 2010-2016).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We express our gratitude to Lara Giongo and Marcella Grisenti [Fondazione Edmund Mach (FEM), San Michele all’Adige, Italy] for helping to make available the Rubus tissue used in the current research. We extend our gratitude to Umberto Salvagnin, FEM for photography for this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01892/full#supplementary-material
References
Abrahams, S., Lee, E., Walker, A. R., Tanner, G. J., Larkin, P. J., and Ashton, A. R. (2003). The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 35, 624–636. doi: 10.1046/j.1365-313X.2003.01834.x
Almeida, J. R. M., Eleonora, D., Anja, P., Fabrizio, C., de Vos, C. H. R., Bettina, D., et al. (2007). Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria × ananassa). Arch. Biochem. Biophys. 465, 61–71. doi: 10.1016/j.abb.2007.04.040
Appelhagen, I., Jahns, O., Bartelniewoehner, L., Sagasser, M., Weisshaar, B., and Stracke, R. (2011). Leucoanthocyanidin dioxygenase in Arabidopsis thaliana: characterization of mutant alleles and regulation by MYB–BHLH–TTG1 transcription factor complexes. Gene 484, 61–68. doi: 10.1016/j.gene.2011.05.031
Ben-Simhon, Z., Judeinstein, S., Trainin, T., Harel-Beja, R., Bar-Ya’akov, I., Borochov-Neori, H., et al. (2015). A “White” anthocyanin-less pomegranate (Punica granatum L.) caused by an insertion in the coding region of the leucoanthocyanidin dioxygenase (LDOX; ANS) gene. PLoS ONE 10:e0142777. doi: 10.1371/journal.pone.0142777
Britton, D. M., Lawrence, F. J., and Haut, I. C. (1959). THE INHERITANCE OF APRICOT FRUIT-COLOR IN RASPBERRIES. Can. J. Genet. Cytol. 1, 89–93. doi: 10.1139/g59-013
Broun, P. (2005). Transcriptional control of flavonoid biosynthesis: a complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 8, 272–279. doi: 10.1016/j.pbi.2005.03.006
Bustin, S. A. (2000). Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25, 169–193. doi: 10.1677/jme.0.0250169
Card, F. W. (1898). Bush-fruits; a Horticultural Monograph of Raspberries, Blackberries, Dewberries, Currants, Gooseberries, and other Shrub-Like Fruits. London: Macmillan.
Carvalho, E., Franceschi, P., Feller, A., Palmieri, L., Wehrens, R., and Martens, S. (2013a). A targeted metabolomics approach to understand differences in flavonoid biosynthesis in red and yellow raspberries. Plant Physiol. Biochem. 72, 79–86. doi: 10.1016/j.plaphy.2013.04.001
Carvalho, E., Fraser, P. D., and Martens, S. (2013b). Carotenoids and tocopherols in yellow and red raspberries. Food Chem. 139, 744–752. doi: 10.1016/j.foodchem.2012.12.047
Cassidy, A., Mukamal, K. J., Liu, L., Franz, M., Eliassen, A. H., and Rimm, E. B. (2013). High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 127, 188–196. doi: 10.1161/CIRCULATIONAHA.112.122408
Castañeda-Ovando, A., Pacheco-Hernández, M., de, L., Páez-Hernández, M. E., Rodríguez, J. A., and Galán-Vidal, C. A. (2009). Chemical studies of anthocyanins: a review. Food Chem. 113, 859–871. doi: 10.1016/j.foodchem.2008.09.001
Chen, Q., Yu, H., Tang, H., and Wang, X. (2012). Identification and expression analysis of genes involved in anthocyanin and proanthocyanidin biosynthesis in the fruit of blackberry. Sci. Hortic. 141, 61–68. doi: 10.1016/j.scienta.2012.04.025
Cheng, A.-X., Han, X.-J., Wu, Y.-F., and Lou, H.-X. (2014). The function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 15, 1080–1095. doi: 10.3390/ijms15011080
Chung, S.-J., Lee, G.-J., Lee, H.-J., Kim, J.-B., Kim, D.-S., and Kang, S.-Y. (2010). Isolation of a leucoanthocyanidin dioxygenase (LDOX) gene from a spray-type chrysanthemum (Dendranthema$∖times$ grandiflorum) and its colored mutants. Korean J. Hortic. Sci. Technol. 28, 818–827.
Clifton, I. J., McDonough, M. A., Ehrismann, D., Kershaw, N. J., Granatino, N., and Schofield, C. J. (2006). Structural studies on 2-oxoglutarate oxygenases and related double-stranded beta-helix fold proteins. J. Inorg. Biochem. 100, 644–669. doi: 10.1016/j.jinorgbio.2006.01.024
Clough, S. J., and Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. doi: 10.1046/j.1365-313x.1998.00343.x
Crane, M. B., and Lawrence, W. J. C. (1931). Inheritance of sex, colour and hairiness in the raspberry,Rubus idaeus. J. Genet. 24, 243–255. doi: 10.1007/BF02983857
de Ancos, B., Gonzalez, E., and Cano, M. P. (1999). Differentiation of raspberry varieties according to anthocyanin composition. Z. Lebensm. Unters. Forsch. 208, 33–38. doi: 10.1007/s002170050371
Debes, M. A., Arias, M. E., Grellet-Bournonville, C. F., Wulff, A. F., Martínez-Zamora, M. G., Castagnaro, A. P., et al. (2011). White-fruited Duchesnea indica (Rosaceae) is impaired in ANS gene expression. Am. J. Bot. 98, 2077–2083. doi: 10.3732/ajb.1000373
Deng, C., and Davis, T. M. (2001). Molecular identification of the yellow fruit color (c) locus in diploid strawberry: a candidate gene approach. Theor. Appl. Genet. 103, 316–322. doi: 10.1007/s001220100648
Espín, J. C., García-Conesa, M. T., and Tomás-Barberán, F. A. (2007). Nutraceuticals: facts and fiction. Phytochemistry 68, 2986–3008. doi: 10.1016/j.phytochem.2007.09.014
Ferreyra, M. L. F., Rius, S. P., and Casati, P. (2012). Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 3:222. doi: 10.3389/fpls.2012.00222
Gebhardt, Y. H., Witte, S., Steuber, H., Matern, U., and Martens, S. (2007). Evolution of flavone synthase I from parsley flavanone 3beta-hydroxylase by site-directed mutagenesis. Plant Physiol. 144, 1442–1454. doi: 10.1104/pp.107.098392
Giusti, M. M., Rodríguez-Saona, L. E., and Wrolstad, R. E. (1999). Molar absorptivity and color characteristics of acylated and non-acylated pelargonidin-based anthocyanins. J. Agric. Food Chem. 47, 4631–4637. doi: 10.1021/jf981271k
Gould, K. S., Markham, K. R., Smith, R. H., and Goris, J. J. (2000). Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J. Exp. Bot. 51, 1107–1115. doi: 10.1093/jexbot/51.347.1107
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Holton, T. A., and Cornish, E. C. (1995). Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7, 1071–1083. doi: 10.1105/tpc.7.7.1071
Hori, K., and Watanabe, Y. (2007). Context analysis of termination codons in mRNA that are recognized by plant NMD. Plant Cell Physiol. 48, 1072–1078. doi: 10.1093/pcp/pcm075
Iwashina, T., Ootani, S., and Hayashi, K. (1988). On the pigmented spherical bodies and crystals in tepals of cactaceous species in reference to the nature of betalains or flavonols. Bot. Mag. 101, 175–184. doi: 10.1007/BF02488894
Jennings, D. L., and Carmichael, E. (1975). A dominant gene for yellow fruit in the raspberry. Euphytica 24, 467–470. doi: 10.1007/BF00028215
Kassim, A., Poette, J., Paterson, A., Zait, D., McCallum, S., Woodhead, M., et al. (2009). Environmental and seasonal influences on red raspberry anthocyanin antioxidant contents and identification of quantitative traits loci (QTL). Mol. Nutr. Food Res. 53, 625–634. doi: 10.1002/mnfr.200800174
Kertesz, S., Kerenyi, Z., Merai, Z., Bartos, I., Palfy, T., Barta, E., et al. (2006). Both introns and long 3’-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 34, 6147–6157. doi: 10.1093/nar/gkl737
Kim, S., Binzel, M. L., Yoo, K. S., Park, S., and Pike, L. M. (2004). Pink (P), a new locus responsible for a pink trait in onions (Allium cepa) resulting from natural mutations of anthocyanidin synthase. Mol. Genet. Genomics 272, 18–27. doi: 10.1007/s00438-004-1041-5
Kim, S., Park, J. Y., and Yang, T.-J. (2015). Characterization of three active transposable elements recently inserted in three independent DFR-A alleles and one high-copy DNA transposon isolated from the Pink allele of the ANS gene in onion (Allium cepa L.). Mol. Genet. Genomics 290, 1027–1037. doi: 10.1007/s00438-014-0973-7
Kobayashi, S., Goto-Yamamoto, N., and Hirochika, H. (2004). Retrotransposon-induced mutations in grape skin color. Science 304:982. doi: 10.1126/science.1095011
Koehntop, K. D., Emerson, J. P., and Que, L. Jr. (2005). The 2-His-1-carboxylate facial triad: a versatile platform for dioxygen activation by mononuclear non-heme iron(II) enzymes. J. Biol. Inorg. Chem. 10, 87–93. doi: 10.1007/s00775-005-0624-x
Kovinich, N., Kayanja, G., Chanoca, A., Riedl, K., Otegui, M. S., and Grotewold, E. (2014). Not all anthocyanins are born equal: distinct patterns induced by stress in Arabidopsis. Planta 240, 931–940. doi: 10.1007/s00425-014-2079-1
Landi, M., Tattini, M., and Gould, K. S. (2015). Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 119, 4–17. doi: 10.1016/j.envexpbot.2015.05.012
Lepiniec, L., Debeaujon, I., Routaboul, J.-M., Baudry, A., Pourcel, L., Nesi, N., et al. (2006). Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 57, 405–430. doi: 10.1146/annurev.arplant.57.032905.105252
Lim, S., Xu, J., Kim, J., Chen, T.-Y., Su, X., Standard, J., et al. (2013). Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 57, 1908–1917. doi: 10.1002/mnfr.201300040
Lin-Wang, K., McGhie, T. K., Wang, M., Liu, Y., Warren, B., Storey, R., et al. (2014). Engineering the anthocyanin regulatory complex of strawberry (Fragaria vesca). Front. Plant Sci. 5:651. doi: 10.3389/fpls.2014.00651
Liu, Y., Shen, X., Zhao, K., Ben, Y., Guo, X., Zhang, X., et al. (2013). Expression analysis of anthocyanin biosynthetic genes in different colored sweet cherries (Prunus avium L.) during fruit development. J. Plant Growth Regul. 32, 901–907. doi: 10.1007/s00344-013-9373-1
Luo, P., Ning, G., Wang, Z., Shen, Y., Jin, H., Li, P., et al. (2015). Disequilibrium of flavonol synthase and dihydroflavonol-4-reductase expression associated tightly to white vs. Red color flower formation in plants. Front. Plant Sci. 6:257. doi: 10.3389/fpls.2015.01257
Ma, H., Pooler, M., and Griesbach, R. (2009). Anthocyanin regulatory/structural gene expression in phalaenopsis. J. Am. Soc. Hortic. Sci. 134, 88–96.
Määttä-Riihinen, K. R., Kamal-Eldin, A., and Törrönen, A. R. (2004). Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 52, 6178–6187. doi: 10.1021/jf049450r
Macha, R. L. (1966). Variability Studies of Fruit Color and other Plant Characteristics in Interspecific Crosses of Yellow-Fruited Raspberries. Available at: http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=6326&context=rtd
Manning, K. (1998). Isolation of a set of ripening-related genes from strawberry: their identification and possible relationship to fruit quality traits. Planta 205, 622–631. doi: 10.1007/s004250050365
McCallum, S., Woodhead, M., Hackett, C. A., Kassim, A., Paterson, A., and Graham, J. (2010). Genetic and environmental effects influencing fruit colour and QTL analysis in raspberry. Theor. Appl. Genet. 121, 611–627. doi: 10.1007/s00122-010-1334-5
Moyano, E., Portero-Robles, I., Medina-Escobar, N., Valpuesta, V., Muñoz-Blanco, J., and Caballero, J. L. (1998). A fruit-specific putative dihydroflavonol 4-reductase gene is differentially expressed in strawberry during the ripening process. Plant Physiol. 117, 711–716. doi: 10.1104/pp.117.2.711
Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x
Ravaglia, D., Espley, R. V., Henry-Kirk, R. A., Andreotti, C., Ziosi, V., Hellens, R. P., et al. (2013). Transcriptional regulation of flavonoid biosynthesis in nectarine (Prunus persica) by a set of R2R3 MYB transcription factors. BMC Plant Biol. 13:68. doi: 10.1186/1471-2229-13-68
Rosati, C., Cadic, A., Duron, M., Ingouff, M., and Simoneau, P. (1999). Molecular characterization of the anthocyanidin synthase gene in Forsythia × intermedia reveals organ-specific expression during flower development. Plant Sci. 149, 73–79. doi: 10.1016/S0168-9452(99)00146-6
Saito, K., Kobayashi, M., Gong, Z., Tanaka, Y., and Yamazaki, M. (1999). Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: molecular cloning and functional expression of cDNA from a red forma of Perilla frutescens. Plant J. 17, 181–189. doi: 10.1046/j.1365-313X.1999.00365.x
Schwartz, A. M., Komarova, T. V., Skulachev, M. V., Zvereva, A. S., Dorokhov, I. L., and Atabekov, J. G. (2006). Stability of plant mRNAs depends on the length of the 3’-untranslated region. Biochemistry 71, 1377–1384.
Shen, X., Zhao, K., Liu, L., Zhang, K., Yuan, H., Liao, X., et al. (2014). A role for PacMYBA in ABA-regulated anthocyanin biosynthesis in red-colored sweet cherry cv. Hong Deng (Prunus avium L.). Plant Cell Physiol. 55, 862–880. doi: 10.1093/pcp/pcu013
Shimada, S., Inoue, Y. T., and Sakuta, M. (2005). Anthocyanidin synthase in non-anthocyanin-producing Caryophyllales species: ANS in non-anthocyanin producing plants. Plant J. 44, 950–959. doi: 10.1111/j.1365-313X.2005.02574.x
Shimada, S., Takahashi, K., Sato, Y., and Sakuta, M. (2004). Dihydroflavonol 4-reductase cDNA from non-anthocyanin-producing species in the Caryophyllales. Plant Cell Physiol. 45, 1290–1298. doi: 10.1093/pcp/pch156
Solfanelli, C., Poggi, A., Loreti, E., Alpi, A., and Perata, P. (2006). Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 140, 637–646. doi: 10.1104/pp.105.072579
Solomon, P. S., Ipcho, S. V. S., Hane, J. K., Tan, K. C., and Oliver, R. P. (2008). A quantitative PCR approach to determine gene copy number. Fungal Genet. Rep. 55, 5–8.
Sundaramoorthy, J., Park, G. T., Chang, J. H., Lee, J.-D., Kim, J. H., Seo, H. S., et al. (2016). Identification and molecular analysis of four new alleles at the W1 locus associated with flower color in soybean. PLoS ONE 11:e0159865. doi: 10.1371/journal.pone.0159865
Swartz, H. J., Fiola, J. A., Stiles, H. D., and Smith, B. R. (1998). Raspberry Plant NamedAnne. US Patent PP10,411. Available at: https://www.google.com/patents/USPP10411
Takos, A. M., Ubi, B. E., Robinson, S. P., and Walker, A. R. (2006). Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin. Plant Sci. 170, 487–499. doi: 10.1016/j.plantsci.2005.10.001
Teng, S., Keurentjes, J., Bentsink, L., Koornneef, M., and Smeekens, S. (2005). Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 139, 1840–1852. doi: 10.1104/pp.105.066688
van der Heijden, R. A., Morrison, M. C., Sheedfar, F., Mulder, P., Schreurs, M., Hommelberg, P. P. H., et al. (2016). Effects of anthocyanin and flavanol compounds on lipid metabolism and adipose tissue associated systemic inflammation in diet-induced obesity. Mediat. Inflamm. 2016:2042107. doi: 10.1155/2016/2042107
Weiss, D., van der Luit, A. H., Kroon, J. T., Mol, J. N., and Kooter, J. M. (1993). The petunia homologue of the Antirrhinum majus candi and Zea mays A2 flavonoid genes; homology to flavanone 3-hydroxylase and ethylene-forming enzyme. Plant Mol. Biol. 22, 893–897. doi: 10.1007/BF00027374
Wittwer, C. T., Herrmann, M. G., Moss, A. A., and Rasmussen, R. P. (1997). Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 22, 134–138.
Wu, J., Kang, J.-H., Hettenhausen, C., and Baldwin, I. T. (2007). Nonsense-mediated mRNA decay (n.d.) silences the accumulation of aberrant trypsin proteinase inhibitor mRNA in Nicotiana attenuata. Plant J. 51, 693–706. doi: 10.1111/j.1365-313X.2007.03173.x
Yousuf, B., Gul, K., Wani, A. A., and Singh, P. (2016). Health benefits of anthocyanins and their encapsulation for potential use in food systems: a review. Crit. Rev. Food Sci. Nutr. 56, 2223–2230. doi: 10.1080/10408398.2013.805316
Zhang, N., Sun, Q., Li, H., Li, X., Cao, Y., Zhang, H., et al. (2016). Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 7:197. doi: 10.3389/fpls.2016.00197
Zhang, Y., Butelli, E., De Stefano, R., Schoonbeek, H.-J., Magusin, A., Pagliarani, C., et al. (2013). Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 23, 1094–1100. doi: 10.1016/j.cub.2013.04.072
Zhao, X., Yuan, Z., Feng, L., and Fang, Y. (2015). Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 128, 687–696. doi: 10.1007/s10265-015-0717-8
Keywords: anthocyanidin synthase, yellow raspberry, pigmentation, mutation, complementation, pathway block
Citation: Rafique MZ, Carvalho E, Stracke R, Palmieri L, Herrera L, Feller A, Malony M and Martens S (2016) Nonsense Mutation Inside Anthocyanidin Synthase Gene Controls Pigmentation in Yellow Raspberry (Rubus idaeus L.). Front. Plant Sci. 7:1892. doi: 10.3389/fpls.2016.01892
Received: 26 October 2016; Accepted: 30 November 2016;
Published: 19 December 2016.
Edited by:
Kevin Davies, Plant & Food Research, New ZealandReviewed by:
Keiko Yonekura-Sakakibara, RIKEN Center for Sustainable Resource Science, JapanLaura Jaakola, UiT The Arctic University of Norway, Norway
Copyright © 2016 Rafique, Carvalho, Stracke, Palmieri, Herrera, Feller, Malony and Martens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Martens, stefan.martens@fmach.it
 Muhammad Z. Rafique
Muhammad Z. Rafique Elisabete Carvalho
Elisabete Carvalho Ralf Stracke
Ralf Stracke Luisa Palmieri
Luisa Palmieri Lorena Herrera
Lorena Herrera Antje Feller
Antje Feller Mickael Malnoy
Mickael Malnoy Stefan Martens
Stefan Martens