- 1 The Cluster of Excellence “Languages of Emotion”, Freie Universität Berlin, Berlin, Germany
- 2 Neurocognitive Psychology, Freie Universität Berlin, Berlin, Germany
- 3 Dahlem Institute for Neuroimaging of Emotion, Freie Universität Berlin, Berlin, Germany
- 4 Department of Linguistics, Institut für Deutsche und Niederländische Philologie, Freie Universität Berlin, Berlin, Germany
- 5 Department of Psychology, Ruhr-Universität Bochum, Bochum, Germany
This study investigates the neuronal correlates of empathic processing in children aged 4–8 years, an age range discussed to be crucial for the development of empathy. Empathy, defined as the ability to understand and share another person’s inner life, consists of two components: affective (emotion-sharing) and cognitive empathy (Theory of Mind). We examined the hemodynamic responses of preschool and school children (N = 48), while they processed verbal (auditory) and non-verbal (cartoons) empathy stories in a passive following paradigm, using functional Near-Infrared Spectroscopy. To control for the two types of empathy, children were presented blocks of stories eliciting either affective or cognitive empathy, or neutral scenes which relied on the understanding of physical causalities. By contrasting the activations of the younger and older children, we expected to observe developmental changes in brain activations when children process stories eliciting empathy in either stimulus modality toward a greater involvement of anterior frontal brain regions. Our results indicate that children’s processing of stories eliciting affective and cognitive empathy is associated with medial and bilateral orbitofrontal cortex (OFC) activation. In contrast to what is known from studies using adult participants, no additional recruitment of posterior brain regions was observed, often associated with the processing of stories eliciting empathy. Developmental changes were found only for stories eliciting affective empathy with increased activation, in older children, in medial OFC, left inferior frontal gyrus, and the left dorsolateral prefrontal cortex. Activations for the two modalities differ only little, with non-verbal presentation of the stimuli having a greater impact on empathy processing in children, showing more similarities to adult processing than the verbal one. This might be caused by the fact that non-verbal processing develops earlier in life and is more familiar.
Introduction
As social interaction is central to the life of human beings, paying attention to and trying to understand the cognitive and affective processes of others is important for the prediction and interpretation of their behavior. These skills are studied under the label of social cognition. Studies concerning the neural basis of social cognition have mainly focused on adults (Amodio and Frith, 2006). Thus, relatively little is known about the neural processing of socio-emotional information in children, although it is well known that changes in many domains of cognition occur with development (Durston and Casey, 2006). For example, the development of higher-level cognitive processes is discussed to covary with maturation of the prefrontal cortex throughout childhood and adolescence (Casey, 1999). In particular, the most anterior part of the prefrontal cortex, the orbitofrontal cortex (OFC), is supposed to support the processing of social cognition, while it is known to mature last in ontogeny (Barbey et al., 2009).
Empathy, the ability to understand and share another person’s inner life, is an essential process in social cognition. It is a complex form of psychological inference, in which observation, memory, knowledge, and reasoning as well as affective sharing are combined (Ickes, 1997). Empathy describes both, sharing as well as understanding the emotional state of others in relation to oneself (Decety et al., 2008). Thus, previous research focused on two main approaches to empathy:
(1) The ability to understand the intentions of another person, so-called cognitive empathy (Baron-Cohen and Wheelwright, 2004; Shamay-Tsoory et al., 2008), sometimes also termed perspective-taking (Lamm et al., 2007), “Theory of Mind” (ToM; Wimmer and Perner, 1983), or mentalizing (Frith and Frith, 2006a, 2006b).
(2) In contrast, affective empathy focuses on the affective response to another person’s presumed affective state (Eisenberg and Miller, 1987) and is sometimes also called affective sharing (Decety and Jackson, 2006). Although empathy and affective empathy are sometimes used synonymously in literature, we will use the term “affective empathy” in the following to distinguish it from “cognitive empathy.”
Cognitive empathy is defined as the ability to imagine or “experience” a situation from another person’s point of view. At the age of two, typically developing children understand another person’s intentions, independently from their own intentions (Leslie, 1987; Flavell, 1999). Understanding another person’s beliefs, for example that a person is thinking wrongly about something, develops at the age of about four (see Wellman et al., 2001, for an overview). Children between two and a half and almost 4 years make the so-called “false belief”-mistake (Baron-Cohen et al., 1985). They are unaware of the fact that their knowledge is different from another person’s knowledge and do not yet understand that different people, depending on their perspective, can have different thoughts about the same situation (“first-order false belief”). With the age of four, most children solve the “false belief” task successfully and 5- to 6-year-olds are able to give correct answers in 90% of the cases (Baron-Cohen et al., 1985; Perner et al., 1987), and solve even higher-order abstraction tasks (Perner and Wimmer, 1985).
Affective empathy is defined as an affective response, derived from the apprehension and comprehension of another person’s affective state, which is identical or very similar to what the other person is expected to feel (Eisenberg, 2000). To empathize with another person does not only mean understanding why the other person is happy or sad, but also being able to feel with her or him, i.e., to mentally “simulate” the other person’s feelings. Affective empathy develops very early in life, and the mechanism underlying affective sharing is discussed to be present from birth on (Decety and Meyer, 2008). The earliest form of empathy is “reactive crying” (emotional contagion) in newborns, presumably lacking any cognitive component. Hamlin et al. (2007) suggested that already at about 6 months of age infants engage in rudimentary forms of social evaluation and preferentially interact with an agent who helped rather than hindered the actions of another character. Altruistic helping as a form of pro-social behavior also emerges early in childhood. Behavioral studies demonstrate that by 12 months of age infants begin to comfort victims of distress, and 14- to 18-month-old infants exhibit spontaneous, unrewarded instrumental helping behaviors (Warneken and Tomasello, 2009). These naturally emerging behaviors are thought to be motivated by sympathetic emotion or concern for others’ well-being. Affective empathy develops and is at its highest level when a person is able to empathize with another person’s experiences and feelings beyond the immediate situation (Hoffman, 2000).
At present, an important argument for distinguishing the two different approaches are the different roles affective and cognitive empathy play in psychiatric disorders like autism and psychopathy: While patients with autism spectrum disorder often show impairment in ToM (Baron-Cohen et al., 1985), affective empathy may be preserved (Blair, 2005; Dziobek et al., 2008). In contrast, psychopaths show a great lack of affective, but mostly no impairment in cognitive empathy (e.g., Baron-Cohen et al., 1985; Soderstrom, 2003). These findings support the assumption that the two concepts, besides sharing similar features and common neural networks, may have specific neuronal correlates, distinct from each other.
Most imaging studies on cognitive empathy have examined mentalizing-tasks that did not include any affective component (Decety and Jackson, 2004). Only few studies have been carried out linking the two components by directly contrasting cognitive and affective empathy tasks in adults (e.g., Hynes et al., 2006; Völlm et al., 2006; Shamay-Tsoory et al., 2008), while studies in children are lacking. The relationship of affective and cognitive empathy has yet to be further determined, especially referring to their neuronal correlates in children.
A neural network often described in empathy research is the frontal mirror neuron system (MNS), including the pars opercularis of the inferior frontal gyrus (IFG; e.g., Singer, 2006; Pfeifer et al., 2008; Hooker et al., 2009). The main function associated with the MNS is that of a simulation mechanism: Perceiving the actions of another person elicits activity in neurons that are also active when we perform those actions ourselves (Gallese and Goldman, 1998), which makes it a suitable mechanism underlying both, affective and cognitive empathic processes (Völlm et al., 2006).
Based on studies on autism and psychopathy, some researchers still argue for a dissociation and define empathy as a general term for a collection of specific neurocognitive functions (Blair, 2005, 2008). The temporoparietal junction (Frith and Frith, 2006a; Decety and Lamm, 2007; Decety et al., 2008; Hooker et al., 2009), for example, is discussed to only support the processing of cognitive empathy and according to a recent meta-analysis on the neural correlates of cognitive empathy (Carrington and Bailey, 2009), OFC, temporoparietal junction and the superior temporal sulcus (Hein and Singer, 2008) are commonly found to be activated by cognitive empathy tasks. Somewhat surprisingly, the same study could not identify a single region that was consistently activated across all analyzed studies (Carrington and Bailey, 2009). This could partly be due to the diversity of the paradigms, but is also evidence for the complexity of social cognition. Moreover, orbitofrontal regions have been found active in both, affective (Hynes et al., 2006; Decety and Meyer, 2008; Decety et al., 2008) and cognitive empathy tasks (Carrington and Bailey, 2009). OFC functioning is known to be critical for social cognition processes, moral decisions, and emotion control.
In the present study we investigate the contribution of the OFC to empathic processing, both, affective and cognitive, in young children.
There is a great lack of developmental studies on empathy and a need for investigating the different levels of empathic responding in further detail, in particular with respect to their underlying neural networks. A neurodevelopmental approach on cognitive and affective empathy may therefore help to better dissociate the two mechanisms (Singer, 2006). A recent functional magnetic resonance imaging (fMRI) study examined the neural correlates of ToM judgments in 8- to 12-year-old children and in adults, using verbal and non-verbal cartoons. The results suggest that children and adults both activate the temporoparietal junction and the IFG for ToM judgments, but differ in their activation patterns depending on task modality, e.g., children have higher activation in left IFG when processing non-verbal cartoons, whereas adults show higher activation in left IFG when processing verbal stimuli. Kobayashi et al. (2007) suggest that children adopt different strategies, and that the observed interactions with age may be linked to age-related refinement of the inferior frontal and posterior temporal regions. A further study by Pfeifer et al. (2008) focused on the role of simulation in affective empathy in 10-year-old children. Their findings show that activity in the posterior IFG (pars opercularis) correlated significantly and positively with empathy and social skills.
The present study examines the processing of affective and cognitive empathy in children between 4 and 8 years of age in a passive empathy recognition paradigm. Most studies on empathy have examined adults and those studies that investigated empathy processing in children examined samples aged seven (Decety et al., 2008; Decety and Michalska, 2010) and older (Kobayashi et al., 2007; Pfeifer et al., 2008). Morphometric studies have demonstrated that structural brain changes in gray and white matter are present at preschool age (Giedd et al., 1999). Similarly, the first and main developmental steps of cognitive empathy are completed about the age of four and affective empathy and forms of pro-social behavior are present from early childhood on.
Empathy development and its neural correlates in preschool children are poorly understood. Therefore, the age range examined in the current study includes preschoolers as well as young school children. One reason for the lack of developmental studies on neural correlates of affective and cognitive empathy is that fMRI studies with children are relatively rare. It is still a challenge to perform experiments with small children in the noisy and unfamiliar environment of an fMRI scanner. Another problem is the high level of motion artifacts, occurring during the measurement of young children (Karmiloff-Smith, 2010).
To measure the neural correlates of empathy processing in children, we used functional near-infrared spectroscopy (fNIRS). This is a non-invasive, functional optical imaging method, assessing changes in cortical oxygenation by applying near-infrared light to measure changes in tissue attenuation. It monitors the brain function by measuring changes in the concentrations of oxygenated [oxy-Hb] and deoxygenated hemoglobin [deoxy-Hb] and is based on the fact that hemoglobin changes its color when the oxygen content changes (Obrig and Villringer, 2003; Hofmann et al., 2008). In particular, brain activation is indicated by an increase in [oxy-Hb] and a decrease in [deoxy-Hb], and it is shown that the latter is highly correlated with an increase in fMRI blood oxygen level dependent (BOLD) response (Steinbrink et al., 2006). Near-infrared light is emitted into the cortex by light-sources and the re-emitted light is collected by another set of optic probes (so-called detectors) at a distance of 3 cm, making it possible to detect activated brain regions, which are approximately 1.5 cm under the cranium. NIRS is a high-potential tool in developmental research due to high light penetration depth in children’s brains. fNIRS lowers the sensitivity to motional artifacts and is a less stressful procedure than fMRI (Lloyd-Fox et al., 2010).
There are no studies on empathy using fNIRS so far and generally imaging studies on empathy in children are rare. Therefore, a first aim of this study is to introduce fNIRS to the field of neurodevelopmental research on empathy processing. Functional brain responses in orbitofrontal and posterior temporal brain regions are investigated under affective and cognitive empathy conditions to reveal similarities and differences in the underlying cortical networks. Furthermore, empathic responses in young children were examined in two stimulus modalities, a non-verbal set of cartoon stories and a set of verbal, auditorily presented stories. The aim was to control for modality-specific effects which allows us to identify modality-independent and -specific activations. Thus, we expected that brain regions that are activated in a modality-independent manner, like the posterior temporal regions reported by Kobayashi et al. (2007) for visual processing of ToM judgments, could also be identified across a visual and an auditory recording session related either to affective or cognitive empathy. Finding brain regions that are activated under both empathy conditions and across the different sensory channels would argue for a more general, supervisory role of these regions in empathy.
As most studies investigated adults, we had to derive our hypotheses mainly from those results, tentatively assuming analogous processing in children. The OFC supports affective as well as cognitive empathy, thus we expect specific activations in this region in both conditions. Hynes et al. (2006) already investigated the adult OFC’s role in affective versus cognitive perspective-taking by means of fMRI. They observed neural activations in both conditions in bilateral OFC, but far more for the emotional than for the cognitive condition, especially concerning the medial OFC. Thus, we expect to observe neural activations for cognitive empathy in lateral and for affective empathy in medial OFC.
According to ToM developmental research, older children have developed higher empathy skills and should thus show different activation-patterns than younger subjects. As affective empathy starts to develop very early but seems to change in quality with cognitive development, one would expect age-related differences in both, affective and cognitive empathy responses. In particular, Decety and colleagues (Decety and Michalska 2010; Decety, 2011) hypothesize that a higher involvement of executive functioning in empathy processing develops (relatively slowly) in parallel to brain maturation, pointing to a role of frontal regions in modulating empathetic responses (see also Hoffman, 2000). Focusing on a relatively young sample of participants provides the opportunity to investigate developmental differences in children’s empathy after the age of four, when basic abilities are present but still develop further. Still, because of sparse experimental results, we are reluctant to hypothesize on age differences in more detail.
Materials and Methods
Sample
Forty-eight participants (22 male/26 female) aged between 4;0 and 8;8 (mean age 6;2) were recruited from daycare centers and primary schools in Berlin. To examine developmental changes, two age groups were formed relative to the age of 6;6. Because in the German educational system children start attending primary school at the age of 6, and one can assume that formal education has an important impact on social and cognitive development, the younger group mainly consisted of preschool children aged 4;0–6;6 (N = 24; mean age = 5;0) and the older group consisted of school children aged 6;6 and older (N = 24; mean age = 7;6).
All participants were native German speakers, never had injuries or operations on the brain, were not taking any medication and did not show behavioral or neurological conspicuities. Their parents received an information-brochure and obtained 20 Euros for participation. Both, children and parents were informed about the background of the study and its procedure and gave their agreement. Parents attended the whole session and could observe it via video from a second laboratory room. The recruitment and experimental procedure was performed in accordance with the local ethical guidelines and consent and assent were obtained from the parent(s) or legal guardian(s) and the child.
A short form of the Kaufman assessment battery for children (K-ABC; Kaufman and Applegate, 1988) was used to make sure none of the participants had an estimated IQ lower than 85, which is 1 SD below the mean. The mean score was 109.38 (range: 85–134.10, SD = 11.90) for the mental processing composite (MPC) and 111.42 (range: 88.40–132.35, SD = 8.45) for the Achievement Scales (ACH). The two age groups did not differ significantly in K-ABC scores (young children: mean MPC score = 107.52, SD = 12.34; mean ACH score = 113.38, SD = 8.78; older children: mean MPC score = 111.23, SD = 11.40; mean ACH score = 111.42, SD = 8.45), as proven by a t-test [MPC: t(46) = −1.038, p = 0.285; ACH: T(46) = 1.631, p = 0.110].
Material
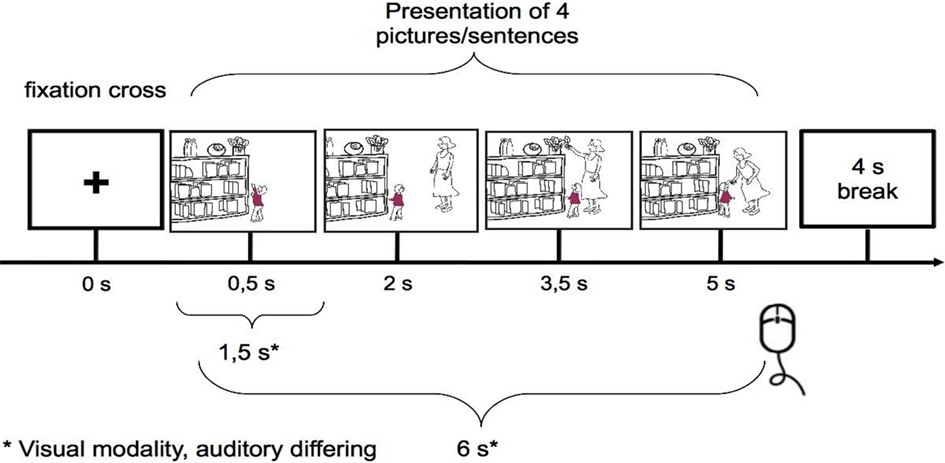
The material consisted of two sets of stimuli, a visual set of non-verbal cartoon stories and an auditory set of verbal listening stories. Every cartoon consisted of four pictures and every story consisted of four sentences, which were presented in a fixed order (see Figure 2). Four conditions were presented during the study:
1. Affective empathy (two characters)
2. Cognitive empathy (one character)
3. Neutral stories with one character
4. Neutral stories with two characters
The material is based on a study by Völlm et al. (2006). A professional illustrator was engaged to adapt these cartoons for the current study. Additionally, new stories were developed. The cartoon stories were drawn in black and white. Only the main character was wearing something colored to direct the attention to her or him (see Figures 1A–D, see also Appendix).
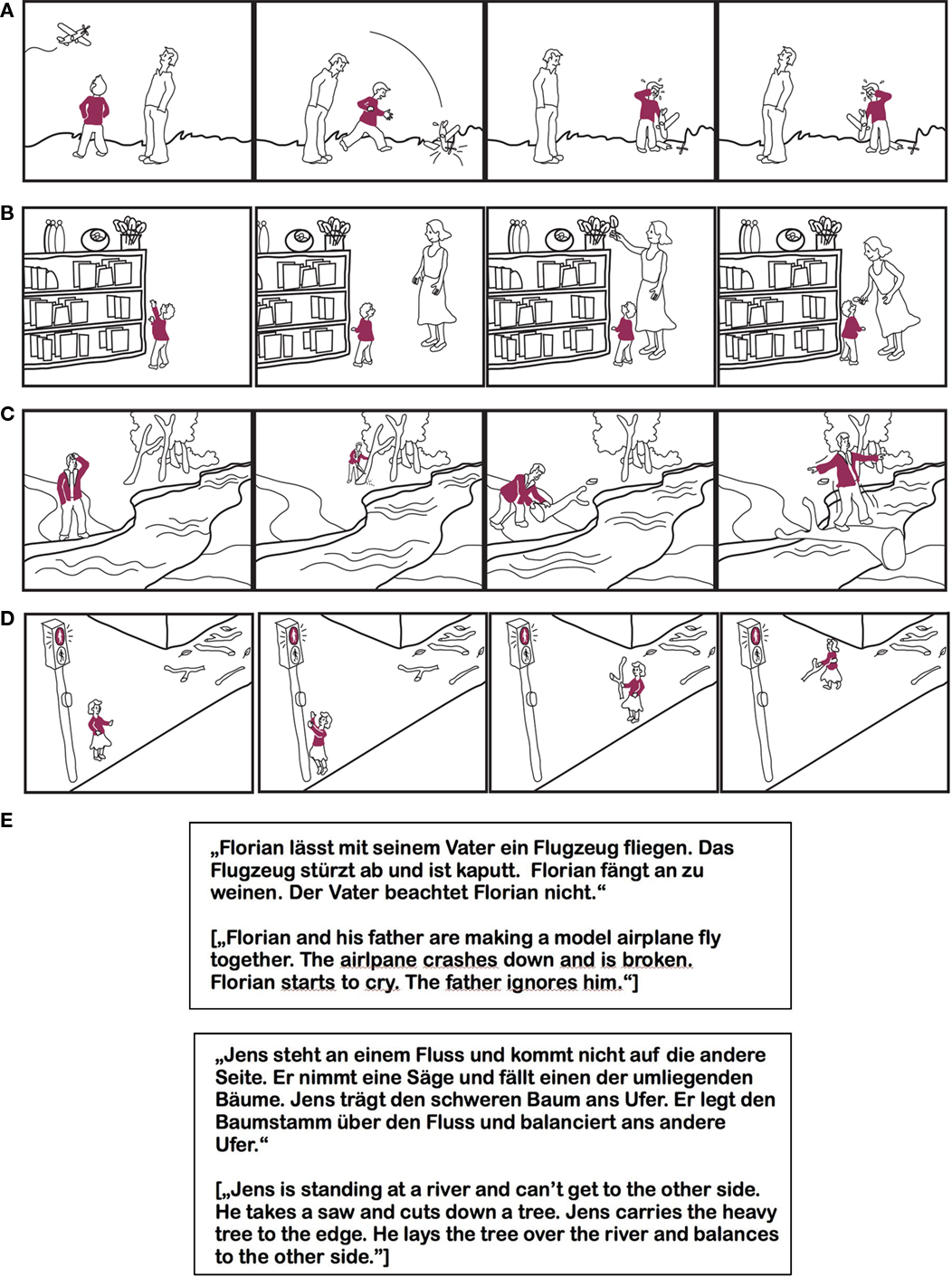
Figure 1. (A) Example of a cartoon stimulus from the negative affective empathy condition. (B) Example of a cartoon stimulus from the positive affective empathy condition. (C) Example of a cartoon stimulus from the logical cognitive empathy condition. (D) Example of a cartoon stimulus from the non-logical cognitive empathy condition. (E) Examples of auditory stimuli: negative affective and logical cognitive story, in German and English.
The verbal listening stories were derived from the cartoon stories by describing each picture by one sentence (Figure 1E). These stories were then read in by an actress. In analogy to Völlm et al. (2006), all stories were constructed in two versions, which differed only in the last picture/sentence. The affective empathy stories had either a positive ending or a negative one. The cognitive empathy stories ended logically or illogically (i.e., the behavior of the main character was reasonable or not). All neutral stories relied on the understanding of physical causalities and were shown in correct or scrambled order, the fourth picture being necessary to recognize the difference. Every story was only seen and heard in one version by each subject.
The final material was derived from a larger set of stories by means of a rating study. Sixteen to 24 adults rated all cartoon stories online, evaluating how strongly the course of the story elicits affective empathy on a scale from 1 (not at all) to 5 (very strongly), to control for the affective empathy elicited by these stories.
The final stimulus set consisted of 48 stories in each stimulus modality, 12 of each experimental condition, half of them positive, logical, or in correct order and half negative, not logical, or scrambled. For the cartoon condition, an ANOVA revealed a significant main effect of affective empathy ratings [F(3,44) = 25.206, p < 0.001]. Pairwise post hoc comparisons using Scheffé’s test revealed that the affective empathy cartoons (M = 3.81) differed from all other cartoon conditions (cognitive empathy: M = 2.60; neutral stories with one character: M = 2.47; neutral stories with two characters: M = 1.97). Similarly, an ANOVA for the verbal listening stories revealed a main effect of affective empathy [F(3,44) = 34.917, p < 0.001], again driven by higher ratings for affective empathy stories (M = 3.78) as compared to cognitive empathy (M = 2.00), neutral with one character (M = 2.01), and neutral with two characters (M = 1.86). The length of the spoken sentences and the average number of words of the verbal stories were balanced across conditions (mean number of words: 37.3, range 21–50; mean length: 11.64 s).
Experimental Procedure
The stimuli were presented on a standard 17′ PC screen, which was placed 60 cm in front of the participants. The experiment started with an instruction to listen to in the verbal session or watch the stories carefully in the non-verbal session, respectively, followed by an example story introducing each block of one of the four experimental conditions. Verbal listening stories were presented via earphones. Stimulus presentation and timing in both modalities were controlled using OGAMA (version 2.0; Voßkühler et al., 2008).
After clicking the mouse, a colored screen appeared in both modalities, and after a second mouse click, an example story started. Following the example, a question was asked (“Did you understand?”). This allowed time for more detailed instructions and answering questions. When all questions were answered to the satisfaction of the child, clicking the mouse once again started the actual setting.
For the non-verbal modality, each trial consisted of four pictures, each presented for 1.5 s, so that one cartoon story lasted 6 s. After the fourth picture, a fixation cross (+) appeared in the middle of the screen for 4 s, and the participants were asked to simply click the mouse. This mouse clicking after each story did not have an influence on the speed of presentation, but was used to keep the children concentrated.
The four blocks (affective, cognitive, neutral with one character, and neutral with two characters), each containing 12 stories, were presented in random order. Inside of each block the two types of stories were presented in equal number and pseudorandomized order: an affective empathy block contained six stories with positive and six stories with negative ending; a cognitive empathy block contained six stories with logical and six stories with non-logical ending, and in neutral blocks six stories were presented in correct and six in scrambled order. Between the blocks a colored screen appeared, so that the participants could take a break before continuing by clicking.
The verbal session worked exactly the same way, except that a fixation cross was displayed while the participants listened to the four sentences. The average length of one sentence was 2.9 s. The two runs (two modalities) were presented one after the other, with a short break in between. Half of the subjects started with the cartoons, the other half with the listening stories.
Every cartoon story took 6 s plus break, thus every block took 2 min (8 min for all four blocks) plus breaks and examples for the visual trials; the auditory blocks took 3.13 min on average each, 12.51 min altogether plus breaks and examples.
Throughout the whole measuring procedure, a supervisor stayed in the room, read instructions to the children and answered questions.
Parallel to the experimental sessions, all parents answered a German version of the “Griffith empathy measure (GEM)” scale (Dadds et al., 2008), a brief parent-report measure of child empathy. Overall, the children varied widely in their resulting empathy scores with a GEM total of 15.54 (SD = 18.24). For the affective empathy sub-scale, the mean score was 3.56 (SD = 7.24), for the cognitive empathy sub-scale it was 3.91 (SD = 9.14). A comparison of the younger and older children did not reveal significant differences [GEM total: T(46) = 0.564, p = 0.575; GEM affective T(46) = 0.500, p = 0.620; GEM cognitive: T(46) = 0.413, p = 0.681].
NIRS Data Acquisition
The fNIRS measurements were performed by a DYNOT System (32 sources/32 detectors, NIRx Medizintechnik, GmbH, Berlin, Germany) operating at two wavelengths (760 and 830 nm) at a sampling rate of 4.13 Hz. An optode-set of 11 sources and 21 detectors was used, making 39 source detector pairs (channels; see Figure 3B). The output ends of the sources and the input ends of the detector fibers were inserted into the electrode holes of a 52-cm Easycap (M16, equidistant system; Falk Minow Services), which was placed on the participants’ heads. The optodes were placed on the orbitofrontal and temporal regions of the head with 3 cm averaged source detector-distance (equidistant system). To capture the orbitofrontal regions, the Easycap was turned by 180°, so that optodes could be placed on the forehead, positioned more orbital than usual. Synchronization with the experimental procedures was provided by marker signals (TTL), sent via the parallel port of the stimuli presenting computer, using OGAMA software.
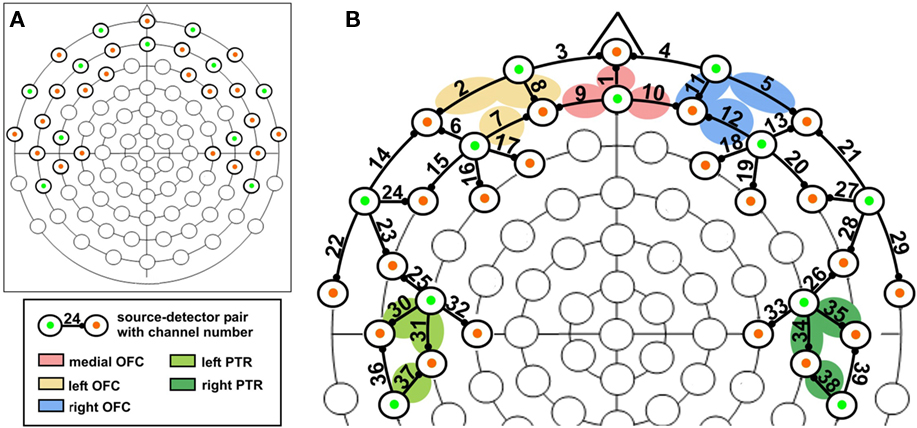
Figure 3. (A) Optode positions on an equidistant 88-channel EEG cap. Note that the EEG cap was turned by 180° to place optodes on the forehead to be able to record orbitofrontal activation; green: sources, orange: detectors; (B) location of the channels superimposed on a equidistant 88-channel EEG cap together with the approximate location of the selected regions of interest.
In order to avoid time-consuming optode positioning procedures, the Easycap was prepared in advance by placing the optodes on it. Some sources and detectors had to be optimized by fixing hair with a small amount of gel (EASYCAP Supervisc, high-viscosity electrolyte-gel) to make sure no hair absorbed the light.
Data Analysis
Preprocessing
The NILAB toolbox (Koch et al., 2009) and Matlab (The MathWorks) were used for fNIRS-data analysis. The time courses of detected light intensities at both wavelengths were transformed into time courses of concentration changes of oxygenated [oxy-Hb] and deoxygenated [deoxy-Hb] hemoglobin by using the modified Beer Lambert law (Cope and Delpy, 1988). We used the following extinction coefficients: 830 nm oxy-Hb 2.3214 mM−1cm−1, deoxy-Hb 1.4866 mM−1cm−1, 760 nm oxy-Hb 1.7917 mM−1cm−1, deoxy-Hb 3.8437 mM−1cm−1 (using data from: W. B. Gratzer, Med. Res. Council Labs, Holly Hill, London), and a photon differential path-length factor of 5.98 cm for 830 nm and 7.15 for 760 nm. The obtained time series of [oxy-Hb] and [deoxy-Hb] concentration changes were low-pass filtered with a cut-off frequency of 0.4 Hz and visually inspected to correct for artifacts related to the subjects’ motion. The trial was excluded from further analysis, if simultaneous step-like changes were observed for both, [oxy-Hb] and [deoxy-Hb], in at least two neighboring channels. Due to this procedure 21.03% (24.67/16.57% in younger/older group) of all data in the visual and 21.19% (21.57/20.78% in younger/older group) in the auditory condition were discarded.
The experimental time courses were block-averaged over six repetitions of every condition in the interval between −10 and +20 s around the stimulus onset of the last sentence (to cover the length of the hemodynamic responses to each presented story as a whole) and detrended to correct for linear baseline drift.
General linear modeling
Preprocessed data were subjected to a general linear model (GLM) analysis. Each of the four conditions – cognitive empathy, affective empathy, neutral story with one character, neutral story with two characters – was modeled with two predictors: The first predictor of each condition modeled cerebral activation at the onsets of the first three sentences/pictures of the story, the second predictor of each condition modeled the onsets of the last sentence/picture. Delta functions of the sentence/picture onsets were convolved with a hemodynamic response function (sum of two gamma functions with time constants of 5 and 16 s and with weights of 1 and −1/6). The predictors were subjected to the same block average procedure as the experimental time courses, accounting for blocks dismissed due to motion correction. An example of block-averaged data together with predictor time course is shown in Figure 4. Since each of the four conditions was presented in non-overlapping blocks, they were analyzed separately. Contrasts of interest were computed from the estimated beta values of the predictor of the last sentence/picture and subjected to a second level analysis. Results for non-verbal cartoon stories and verbal stories and for both hemoglobin species are reported.
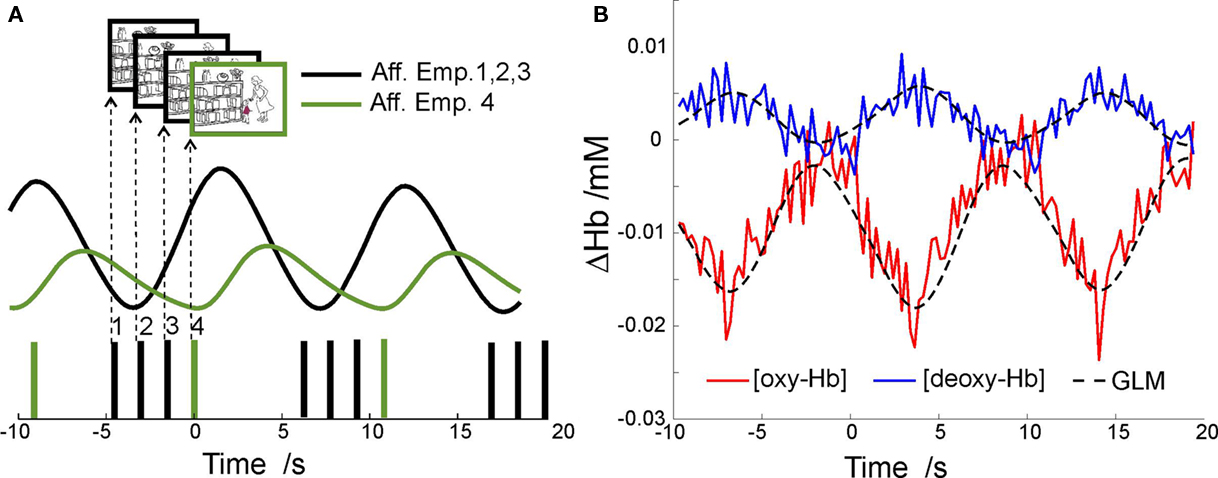
Figure 4. (A) Example time course of the predictors used for GLM-analysis of block-averaged fNIRS-data in the interval between −10 to +20 s around onset of the fourth picture (sentence) of one subject. Displayed are the delta functions and the two predictors that model hemodynamic responses induced by the first three or the fourth picture (sentence) of the story presented at t = 0s. (B) Example time course of [oxy-Hb] and [deoxy-Hb] concentration changes for left OFC for the visual affective empathy condition averaged over all block repetitions and over subject population, overlaid with corresponding GLM-model. Note that the time courses show a relative deactivation for the visual affective empathy condition with decreasing [oxy-Hb] and increasing [deoxy-Hb] due to the superposition of the first three and the fourth cartoon slides. The relative deactivation of the lateral orbitofrontal regions during processing of the stories is in agreement with fMRI results on similar material (Hynes et al., 2006, Figure 2). Besides this overall deactivation during the processing of the empathy stories, activation was revealed for the respective contrasts (see results, Table 2).
Optode locations as a basis for neuroanatomical labeling of the NIRS channels were obtained by means of an anatomical MRI template. Since the brain volume and geometry changes only slightly after the age of two (Faria et al., 2010), we acquired an anatomical T1 scan of an adult subject with a head circumference of 52 cm, the same circumference our sample had on average, wearing the Easycap (3T, SIEMENS-Trio, MPRAGE-Sequence, resolution 1 mm × 1 mm × 1 mm). The fiducial markers were placed in all optode positions and reference Cz. The anatomical volume was normalized to standard Talairach 3D space using BrainVoyager QX (v 1.7; Brain Innovation, Maastricht, The Netherlands). The channel-positions were defined, computing the exact midway between source and detector and the most probable anatomical label was obtained using the NFRI-toolbox (Okamoto et al., 2004; Singh et al., 2005).
Channels were clustered to define regions of interest, based on our initial hypotheses. One cluster comprised the average signal of three defining channels (see Figure 3; Table 1). Five clusters were defined: medial OFC, left OFC, right OFC, left PTR, and right PTR. In addition to this clustered region-of-interest analysis, significant activations of single channels which exceed a conservative threshold of p < 0.01 are reported, too. The positions of the channels and clusters can be seen in Figure 3.
For each stimulus modality one-sample t-tests (two-tailed) were computed at the second level for the following three main contrasts:
(1) Affective empathy > Neutral (two characters): subtracting activations during the processing of neutral stimuli from those during processing affective empathy stimuli to reveal hemodynamic responses related to affective empathy processing only (Note that both conditions contained stories with two characters).
(2) Cognitive empathy > Neutral (one character): subtracting activations during the processing of neutral stimuli from those during processing cognitive empathy stimuli to reveal hemodynamic responses related to cognitive empathy only.
(3) [Affective empathy – Neutral (two)] > [Cognitive empathy – Neutral (one)]: to directly contrast affective and cognitive empathy processing, contrast 1 and 2 are subtracted. As a result, activation increases are related to affective empathy processing, whereas decreases are related to cognitive empathy processing.
To detect effects of age, paired t-tests comprising “age group” as factor were computed. In addition, to identify whether or not neural activity in our a priori regions of interest correlated with the parent-reported empathy scores, a correlational analysis across all subjects was performed between the activity in the three contrasts and the affective and cognitive GEM sub-scores. For all analyses, significant results are reported that exceed a conservative threshold of p < 0.01 (uncorrected).
Results
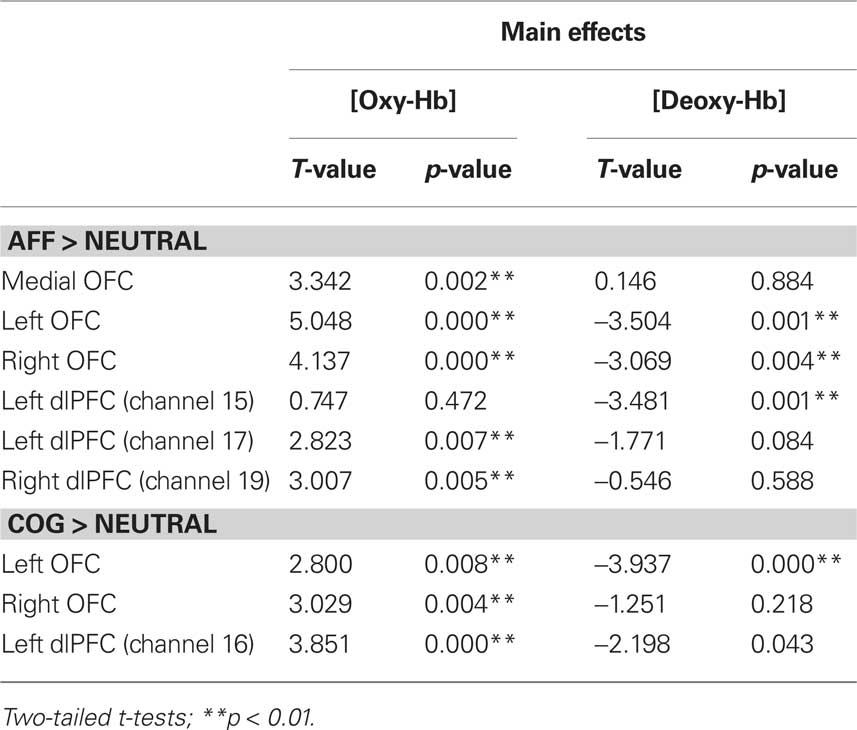
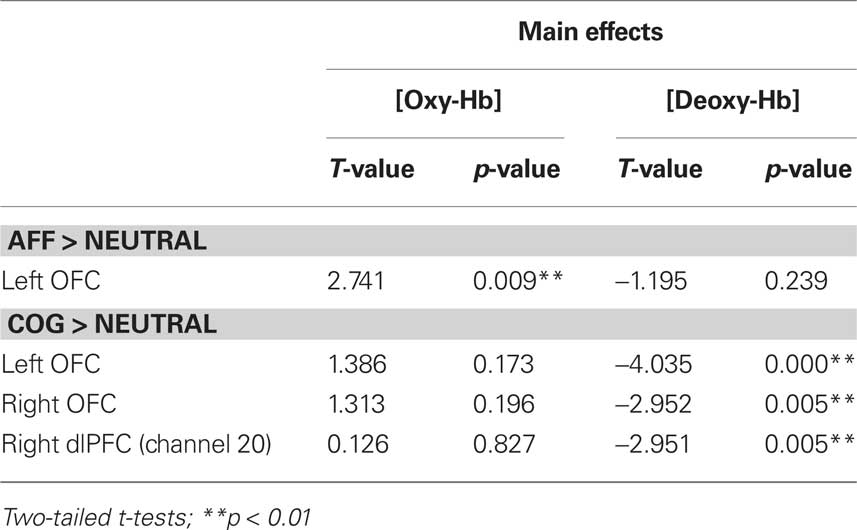
Due to unusually high residual variance in some subjects, data for seven cartoon and four listening sessions had to be excluded from further analysis. Cartoon (N = 40) and auditory listening story (N = 44) data were analyzed separately. Significant changes in oxygenated [oxy-Hb] and deoxygenated blood [deoxy-Hb] during the trials are given in Table 2 for visual and Table 3 for auditory presentation.
Contrast (1): Affective > Neutral with Two Characters
Cartoons
Significant activations were observed in medial OFC and bilateral OFC. In addition, channels in dorsolateral prefrontal cortex (dlPFC) revealed significant activations: channel 15 [deoxy-Hb] and 17 [oxy-Hb] of the left and channel 19 [oxy-Hb] of the right dlPFC (see Table 2; Figure 5).
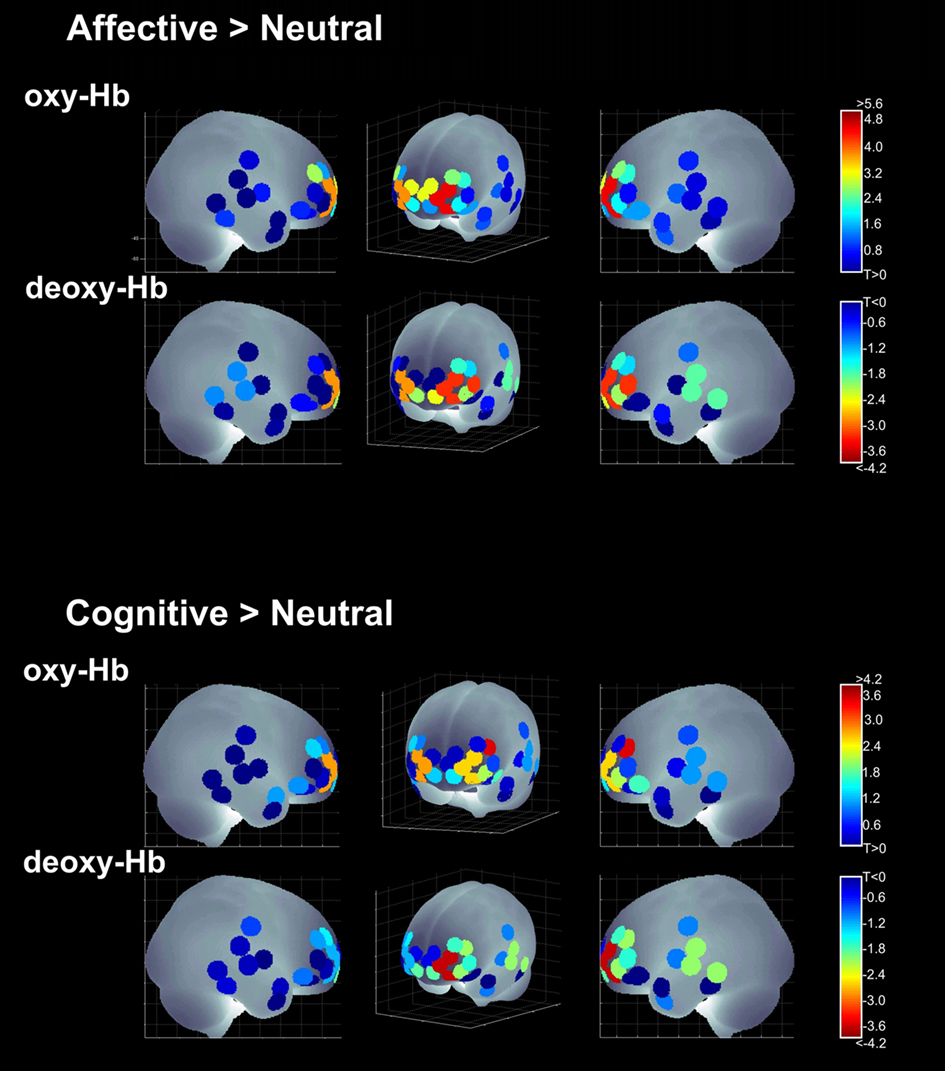
Figure 5. Activations for the visual condition; [oxy-Hb] and [deoxy-Hb] results across all four main contrasts in the sagittal and a frontal view.
The t-test for age-group differences showed a significant age-effect in channel 17 [oxy-Hb], left dlPFC, demonstrating that older children activated this region to a greater degree than younger ones [T(38) = −3.737; p = 0.001].
Listening stories
The left OFC was significantly activated for the affective condition. Age differences were observed in [deoxy-Hb] channel 24 [T(42) = 3.728; p = 0.001] in the anterior left IFG. Again, older children showed higher activations (increased deactivations in [deoxy-Hb]) revealing age-related differences in left IFG involvement in affective empathy processing (see Table 3; Figure 6).
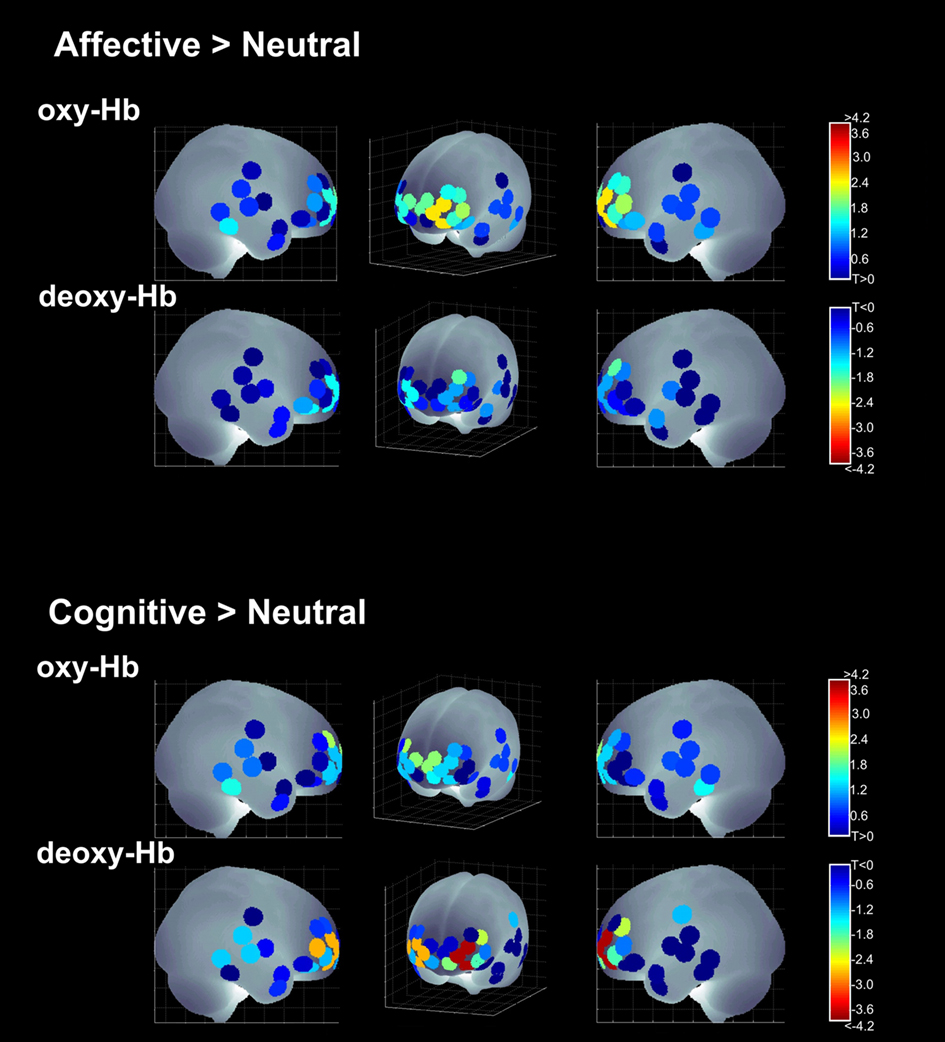
Figure 6. Activations for the auditory condition; [oxy-Hb] and [deoxy-Hb] results across all four main contrasts in the sagittal and a frontal view.
Contrast (2): Cognitive > Neutral with One Character
Cartoons
Significant activations related to cognitive empathy processing were elicited in right and left OFC, as well as in channel 16 [oxy-Hb] of the left dlPFC. No significant age differences were observed in this contrast.
Listening stories
Significant activations were observed in left and right OFC. Additionally [deoxy-Hb] channel 20 of the right dlPFC showed a significant activation. No age-group differences were found.
Contrast (3): (Affective – Neutral with One Character) > (Cognitive – Neutral with Two Characters)
Cartoons
No region reached the level of significance in the direct contrast. No age differences were found.
Listening stories
No region reached the level of significance. A significant effect of age group was observed in the medial OFC ([oxy-Hb]; T(42) = −2.925; p = 0.006). The effect was due to larger positive concentration changes in [oxy-Hb] in the older children’s group in the affective empathy condition compared with higher activation of medial OFC in the cognitive empathy condition in younger children.
The correlational analysis with the affective and cognitive GEM scores revealed significant correlations with the parent-rated affective empathy measure only. Significant positive correlations were found for the non-verbal cartoon-modality in the medial OFC in contrast (1) ([Affective > Neutral]; [deoxy-Hb]; r = 0.409, p = 0.009) as well as in contrast (2) ([Cognitive > Neutral]; [deoxy-Hb]; r = 0.460; p = 0.003), revealing activation decreases in children with higher affective GEM scores in the medial OFC.
Discussion
The present study investigated the neural correlates of affective and cognitive empathy in young children in orbitofrontal and posterior temporal regions. We measured hemodynamic responses of empathy processing in a non-verbal cartoon and a verbal story-listening task using fNIRS and observed activations related to empathy processing in medial and bilateral OFC for both types of stimuli.
Our results provide evidence for the hypothesis that empathy processing in young, healthy children requires a greater involvement of orbitofrontal brain regions, irrespective of whether the task elicits affective or cognitive empathy. As such, the present study extends recent findings from studies examining adult participants that also found orbitofrontal regions involved in empathy processing (Hynes et al., 2006; Decety and Meyer, 2008; Decety et al., 2008). Thus, although it is discussed that anterior frontal regions mature relatively late (until the age of 20, see Giedd et al., 1999), and maturation itself is suggested to be the reason for the late development of complex social cognition skills in ontogeny, our results suggest that also young children’s empathic processing depends on orbitofrontal functioning comparable to what is known from adult studies.
Main Findings
In line with our initial hypothesis, affective as well as cognitive empathy processing activates the OFC bilaterally. The occurrence of similar activation patterns for the two conditions is additionally supported by the finding that the direct (Affective > Cognitive) contrast showed no significant differences.
The role of the OFC has been examined by recent fMRI studies, investigating either affective (e.g., Decety et al., 2008) or cognitive (see Carrington and Bailey, 2009) empathic processing in adults, but without contrasting the two conditions directly. The neuropsychological lesion literature indicates, too, that the critical areas for empathic impairment of empathy processing are located in the OFC, as damage to those regions is associated with deficits in empathy (Eslinger, 1998; Shamay-Tsoory et al., 2004).
Only two fMRI studies, both on adults, were conducted that were comparable: Völlm et al. (2006) as well as Hynes et al. (2006) found OFC activations for both conditions bilaterally. In the latter study, affective empathic processing elicited higher activation and additionally activated the medial OFC (Hynes et al., 2006). Still, these studies differ from the present study in critical aspects and therefore their results are not directly comparable to ours: Both fMRI studies relied on adult participants, who were asked to make explicit empathy judgments, whereas the present study might have triggered empathic responses implicitly, more closely related to the concept of empathy as “Einfühlen,” defining empathy as a mode of inner imitation or “feeling into someone” (Eisenberg and Strayer, 1987; Barnes and Thagard, 1997).
A direct comparison of the auditory and visual modality has so far not been conducted with young participants and it seems likely that their empathic processing might differ from that in adults. Since no additional activations in temporal regions were observed in the present study, differences between children’s and adults’ processing of empathic stories seem to be associated with differences in the engagement of temporal regions, and not the OFC. Still, it should be noted that the present definition of the PTR and its approximate location does not fully overlap with the often reported TPJ area in adults’ cognitive empathy processing. The examined PTR cluster is located in large parts along the superior temporal sulcus. The latter has reciprocal connections to the OFC (Barbas, 1988) and has been implicated in cognitive empathy processing and social cognition (Allison et al., 2000; Hein and Singer, 2008). Figure 5 shows that activations in PTR occurred in both visual empathy conditions, but did not reach our a priori level of significance1. Whether this trend occurred because of a loss in statistical power due to methodological issues or whether it indicates principal differences between an involvement of the posterior superior temporal sulcus and the neighboring TPJ in children’s empathy processing should be examined in future longitudinal neuroimaging studies using similar paradigms in adults and children that can help solving this issue.
The OFC is suggested to be a key region supporting social cognition in general: It was found to be critical for ToM-processing (Carrington and Bailey, 2009) and decision making (Kringelbach, 2005) on the one hand, and moral appraisals (Moll et al., 2002) and affective empathy (Decety and Jackson, 2004; Hynes et al., 2006) on the other hand. Thus, it is likely that social cognition, including empathy, is supported by OFC functioning, independent of how affective or cognitive the process might be.
The observed involvement of the OFC in empathy processing is also in line with theories that propose a hierarchical structure of the prefrontal cortex, postulating more complex cognitive processes, the more anterior a region is located in the frontal lobe (Fuster, 2004; Botvinick, 2008; Barbey et al., 2009). As a first result, thus, complex socio-emotional processing as in the affective and cognitive empathy conditions of our study, demanding the mirroring of different plots, intentions and emotions, seems to require the most anterior part of the brain in young children.
It still remains an open question why other studies on the same issue did not find OFC activations for affective and cognitive empathy (see Hein and Singer, 2008). There is a lot of variance among activated areas within empathy paradigms (Carrington and Bailey, 2009). It is also obvious from the heterogeneity in the literature that there are several ways of defining and assessing empathy and the relationship of its components. For example, “affective ToM,” “affective perspective-taking,” and ”affective empathy” are often used synonymously, but do not always really describe and measure the same process, as these concepts can focus on different aspects. Thus, it can be discussed whether the notion of affective empathy in terms of a social cognition process differs from the notion of affective empathy in terms of pain in others (e.g., Decety et al., 2008; Hein and Singer, 2008).
Finally, the diversity of paradigms and materials may account for some of the variability in the results of different studies: Written scenarios or cartoons (Kobayashi et al., 2007), interpretation of facial expression from the eyes (Baron-Cohen et al., 1999), detection of faux-pas and irony (Shamay-Tsoory et al., 2005), judgments (Völlm et al., 2006), or pain in others (Decety et al., 2008) are all paradigms used in empathy research. The present study was designed to focus on the direct comparison of equivalent processing of affective versus cognitive empathy, while passively following the course of a stimulus story. We think a strength of the present study lies in the parallel measurement of a visual non-verbal and an auditory verbal condition, which shows a remarkably high degree of concordance in the results and thus is able to replicate the OFC findings in each presentation domain. However, across all conditions, cartoon stories elicited overall higher activations, recruited more anterior frontal regions in the OFC, and enhanced activations in dorsolateral regions. This points to the hypothesized visual dominance in younger children’s processing (Guttentag, 1985), and a higher familiarity and external validity of cartoon-like stories. Thus, it seems likely that the visual modality facilitates the processing of its content and therefore the processing of empathic contents, too.
Only small differences are visible between affective and cognitive empathy processing in the present study and those did not survive the direct contrasting. fNIRS can only measure hemodynamic responses in the outer cortical regions. Thus, it is obvious that this method is not able to detect emotion-related differences in subcortical parts of the emotion processing circuits (Dolan, 2002), where further specific activations related to affective processing may be located. Still, our finding of additional medial OFC involvement, specific for affective empathy processing, is in line with the results of Hynes et al. (2006) for adults, even if we observed this activation for the visual modality only. Hynes et al. (2006) report medial OFC and medial PFC activations for affective processing, suggesting this to be the difference between the two empathy-components. Again, it should be noted that medial PFC has not been the focus of the present study due to the low sensitivity of fNIRS to signals in these deeper brain regions along the medial wall.
We further observed an effect of children’s age in medial OFC, related to affective empathy processing: Older children showed higher medial OFC activation for the direct (Affective empathy > Cognitive empathy) contrast than younger ones. We think that this main effect of age indicates a shift toward an involvement of additional medial parts of the anterior frontal lobe with older age in affective but not in cognitive empathy processing. This finding is supported by the neuroimaging literature on adults’ affective empathy processing, who additionally require medial frontal regions (Decety and Jackson, 2004; Hynes et al., 2006; Hein and Singer, 2008). As such, our results show a pattern opposite to that of a recent neurodevelopmental study by Decety and Michalska (2010), who conducted an fMRI study with participants ranging from 7 to 40 years of age. Decety and Michalska (2010) report greater activation in the OFC in response to affective empathy-eliciting scenarios (depicting intentional harm) that was shifted from its medial portion in younger participants, to the lateral portion in older participants. The authors discuss this pattern of developmental change in the OFC as reflecting a gradual shift from the monitoring of somatovisceral responses in young children to the executive control of emotion processing in older participants. Interestingly, Decety and Michalska (2010) report also age-related correlations in lateral frontal regions (enhanced involvement with older age) which seem to be more consistent with age-related findings in left dlPFC and IFG for the affective empathy condition.
Because Decety and Michalska (2010) investigated a much larger age range (with only a small amount of children at the age of our sample) and focused on somatovisceral empathic processing, whereas our study examined affective empathy processing in everyday social contexts, these two studies are not directly comparable. Still, we see the increased activation in medial OFC in older children as evidence in support of the hypothesis of an ongoing development of affective empathy processing in children aged between 4 and 8 years, with a further specialization of medial OFC in social-cognitive affective empathy processing in the older children. Whereas the younger age group shows no differential activation in medial OFC between the affective and cognitive condition, older children have a higher activation in medial OFC during affective empathy processing compared than during cognitive empathy processing2.
Still, one might wonder why this age-effect was only found in the auditory condition. This finding suggests that visual and auditory processing of empathy stories in young children are qualitatively different due to their different everyday familiarity. We suggest this to result from a visual processing dominance in children. Children are probably more used to following visually guided cartoon stories than to listening mindfully to auditory empathy stories (e.g., Hayes and Birnbaum, 1980). Moreover, one could suppose that visual empathic processing along the OFC is developed earlier in ontogeny than the development of auditory empathic processing. For example, when children’s auditory and visual perception of video-clips was tested, the results provide evidence that children, especially preschoolers, payed more attention to and recognized more details in visual than in auditory information (Grieve and Williamson, 1977; Hayes and Birnbaum, 1980). This is supported by the pattern of results for the visual condition which in general more closely mirrors the results obtained in studies with adults. The observed medial OFC activation in the visual affective empathy condition across the whole sample may result from facilitated and further-developed processing in the visual domain. Accordingly, it seems possible that it is easier to detect developmental changes in the auditory domain. Still, the adult fMRI studies relied on visual processing, and processing of empathy has not been investigated with auditory stimuli yet.
A second developmental effect was visible in affective empathy processing of auditory stimuli in the left IFG. As mentioned above, involvement of left IFG is often reported in adult studies on empathy processing and the MNS. These differences in the activation of the left IFG probably point to a similar age-dependent shift in affective empathy processing in older compared with younger children. Older children’s processing of auditory affective empathy stories is associated with higher involvement of the left IFG. Thus, children seem to increase the use of the frontal MNS more with ongoing development.
A different view comes from semantic memory research. Left IFG has been shown to be more active in tasks requiring retrieval of semantic knowledge and verbal recoding (Thompson-Schill et al., 1997; Poldrack et al., 1999). Thus, it is also reasonable to argue that younger and older children differ in the amount of verbal processing (e.g., rehearsal or recoding) when listening to affective empathy stories. As the IFG was only activated for age differences, the theory of a developing MNS seems to be a plausible account.
Finally, an age-effect was observed for channel 17 [oxy-Hb], showing higher activation in left dlPFC for older children for affective empathic processing in the visual domain. Apart from a general discussion on the role of dlPFC in working memory and executive functions (Miller and Cohen, 2001; Fuster, 2004; Petrides, 2005), an involvement of dlPFC in empathy processing has previously been reported in a lesion study (Shamay-Tsoory et al., 2003; also see Shamay-Tsoory and Aharon-Peretz, 2007). Patients with dlPFC lesions, as well as patients with ventro-medial PFC lesions, showed higher deficits in different ToM tasks, as compared to healthy control participants. Still, these authors also showed that empathy performance in the dlPFC group correlates with cognitive flexibility measures, again pointing more toward a role of dlPFC in executive functioning as a basis for empathy processing.
Taken together, the age-effects observed in the present study are all related to affective empathy processing, thus indicating developmental changes in the affective empathy component in the age-group investigated. Future research might use the same design in a study on adults to further our knowledge of developmental differences between children and adults.
Additional Findings
Apart from the finding of medial OFC involvement, no specific activations were found for the affective versus cognitive empathy comparisons. Also, no PTR cluster and no other channel in the temporal lobe became significantly activated. Temporal regions are discussed to be specifically activated for cognitive empathic processing in adults (Frith and Frith, 2006a). As Carrington and Bailey (2009) meta-analyzed, many different studies on ToM found different regions, but no single region to be activated concordantly. Only few other functional imaging studies on children’s empathic processing (Kobayashi et al., 2007; Pfeifer et al., 2008; Decety and Michalska, 2010, age seven and above) reported a TPJ activation. In this study, school children processed ToM stories and were asked to judge false beliefs (Kobayashi et al., 2007). Thus, it is likely that passive following of empathic stories in young children, as in the present study, does not recruit additional neural circuits in anterior and posterior temporal regions. Empathic processing in this age range may be restricted to the most anterior parts of the brain.
For the visual modality, correlations of the affective GEM scores with medial OFC activation were found, revealing a negative correlation with affective and cognitive empathic processing, whereas cognitive GEM scores did not correlate significantly with any activated brain region in the present study. These results lead to the question to what extent children who score high in affective GEM activate the medial OFC less for affective and for cognitive empathic processing.
As parent-rated GEM scores and age did not correlate in the present study, this effect cannot simply be attributed to age. The finding suggests that children who are more affectively empathic process affective and cognitive empathy stories differently in the medial OFC (whose activation was only found to be related with affective empathic processing). Although the particular correlations are negative, this result mirrors well the above findings which point to the close relationship between medial OFC and children’s affective empathy processing. Here, less activation in the medial OFC is found for children who are more empathic. This rather paradoxical finding might indicate a shift of empathic processing away from anterior frontal regions in emphatic children, toward subcortical processing (as mirrored by the reported positive correlations in adult studies between empathic traits and anterior insula activation, e.g., Moriguchi et al., 2007; Silani et al., 2008, see Hein and Singer, 2008). However, this aspect remains speculative and should be investigated in future studies using fMRI.
With the present study, we introduce the method of fNIRS to the field of neurodevelopmental studies on empathy processing, and we believe it is a valuable tool for research in this field. fNIRS has several advantages over other imaging methods such as fMRI or PET, first of all, that no fixation of the head is needed. The child can sit on a comfortable chair in front of a screen, communicate with supervisor and parents during preparation and, if necessary, also during the trials. The optodes can easily be prepared beforehand, thus the actual preparation is comfortable and not so time-consuming. Because of its low constraints on the experimental environment, fNIRS is a good tool to investigate higher cognition, whereas an MRI environment may impair children’s concentration and speech perception (Hofmann et al., 2008). It has already been established as a method in studying neuropsychological development in neonates and infants (Peña et al., 2003; Taga et al., 2004; Wartenburger et al., 2007). Additionally, fNIRS might be less prone than, e.g., fMRI to artifacts caused by the proximity to the air-filled sinuses of the OFC, when measuring OFC activations (Kringelbach, 2005). However, fNIRS cannot supply the spatial resolution of fMRI-based approaches (Hofmann et al., 2008).
There are some limitations to our study. Both limitations are based on the fact that we employed a design that was applicable to young children, having in mind not to overtax the children. First of all, the application of a passive following paradigm in children does not allow to conclude that all children processed all stories deeply and with an intention to take the point of view of the main character which might have led to less activations in empathy processing regions. Secondly, one might ask whether the adult ratings to the empathy stories are also valid for children. Children’s ratings are not yet available for the stimulus set and it is questionable to what extend young children are able to explicitly judge their empathic involvement – which is the reason why we had to base the study on adult rating data. Therefore, we cannot be certain, for example, whether the children processed all empathy stories in an affective manner or not. Still, given the post-experimental interviews, we believe, that the children processed the stories in the intended manner, and the high concordance of the results with that of previous adult studies seems to validate this assumption. Further studies are needed which directly address the differences between passively following and explicitly judging of empathy stories in young children.
Conclusion
Taken together, our findings provide evidence for higher medial and bilateral OFC activation in both, affective and cognitive empathy processing in a sample of young children 4–8 years of age. Thus, in a manner similar to what is known from adult OFC recruitment in complex social cognition tasks and empathy processing, orbitofrontal regions were involved in a task in which children passively followed empathic narratives – independently of whether these stories presented social situations where a character experiences affective outcomes of its own action or the plot required the mentalizing and prediction of further actions, and independently of whether these stories were presented visually or auditorily. Hence, our results support the idea that the OFC is a brain region associated with computing and evaluating predictions of other persons’ actions and the comparison of these predictions with subjective states across both affective and non-affective situations.
Furthermore, in contrast to our initial hypotheses, developmental changes with increased brain activation in older children were observed in affective empathy processing as compared to neutral stories in left dlPFC in the visual condition and left anterior IFG in the auditory condition, but no age-related effects were observed in cognitive empathy processing. In contrast, medial OFC showed a higher activation when directly contrasting affective and cognitive processing conditions. Thus, the results support the idea of medial OFC being especially engaged in socio-affective processing and a development of medial OFC functioning toward a higher involvement with older ages during childhood.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^left PTR [deoxy-Hb]: visual (Affective > Neutral) contrast: T(39) = −1.950; p = 0.058; left PTR [deoxy-Hb]: visual (Cognitive > Neutral) contrast: T(39) = −2.176; p = 0.036;
- ^To further evaluate the age-related effect in [oxy-Hb] in contrast 3 (Affective empathy > Cognitive empathy) post hoc simple t-test were computed for each age-group separately. In young children, no significant contrast effect was visible [T(22) = −1.750; p = 0.094], whereas older children showed a significant higher activation in affective empathy processing [T(20) = 2.609; p = 0.017].
References
Allison, T., Puce, A., and McCarthy, G. (2000). Social perception from visual cues: role of the STS region. Trends Cogn. Sci. (Regul. Ed.) 4, 267–278.
Amodio, D. M., and Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277.
Barbas, H. (1988). Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Comp. Neurol. 276, 313–342.
Barbey, A. K., Krueger, F., and Grafman, J. (2009). An evolutionarily adaptive neural architecture for social reasoning. Trends Neurosci. 32, 603–610.
Baron-Cohen, S., Leslie, A. M., and Frith, U. (1985). Does the autistic child have a “theory of mind.” Cognition 21, 37–46.
Baron-Cohen, S., Ring, H. A., Wheelwright, S., Bullmore, E. T., Brammer, M. J., Simmons, A., and Williams, S. C. R. (1999). Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 11, 1891–1898.
Baron-Cohen, S., and Wheelwright, S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 34, 163–175.
Blair, R. J. (2005). Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 14, 698–718.
Blair, R. J. (2008). Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q. J. Exp. Psychol. 61, 157.
Botvinick, M. M. (2008). Hierarchical models of behavior and prefrontal function. Trends Cogn. Sci. (Regul. Ed.) 12, 201–208.
Carrington, S. J., and Bailey, A. J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 30, 2313–2335.
Casey, B. J. (1999). Images in neuroscience. Brain development. XII. Maturation in brain activation. Am. J. Psychiatry 156, 504–504.
Cope, M., and Delpy, D. T. (1988). System for long term measurement of cerebral blood and tissue oxygenation on newborn infants by near infrared transillumination. Med. Biol. Eng. Comput. 26, 289–294.
Dadds, M. R., Hunter, K., Hawes, D. J., Frost, A. D., Vassallo, S., Bunn, P., Merz, S., and El Masry, Y. (2008). A measure of cognitive and affective empathy in children using parent ratings. Child Psychiatry Hum. Dev. 39, 111–122.
Decety, J., and Jackson, P. L. (2004). The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 3, 71–100.
Decety, J., and Jackson, P. L. (2006). A social-neuroscience perspective on empathy. Curr. Dir. Psychol. Sci. 15, 54–58.
Decety, J., and Lamm, C. (2007). The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist 13, 580–593.
Decety, J., and Meyer, M. (2008). From emotion resonance to empathic understanding: a social developmental neuroscience account. Dev. Psychopathol. 20, 1053–1080.
Decety, J., and Michalska, K. J. (2010). Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev. Sci. 13, 886–899.
Decety, J., Michalska, K. J., and Akitsuki, Y. (2008). Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia 46, 2607–2614.
Durston, S., and Casey, B. J. (2006). What have we learned about cognitive development from neuroimaging? Neuropsychologia 44, 2149–2157.
Dziobek, I., Rogers, K., Fleck, S., Bahnemann, M., Heekeren, H. R., Wolf, O., and Convit, A. (2008). Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the multifaceted empathy test (MET). J. Autism Dev. Disord. 38, 464–473.
Eisenberg, N. (2000). “Empathy and sympathy,” in Handbook of Emotions, eds M. Lewis and J. M. Haviland-Jones (New York: Guilford Press), 677–691.
Eisenberg, N., and Miller, P. A. (1987). The relation of empathy to prosocial and related behaviors. Psychol. Bull. 101, 91–119.
Eisenberg, N., and Strayer, J. (1987). “Critical issues in the study of empathy,” in Empathy and its Development, eds N. Eisenberg and J. Strayer (New York: Cambridge University Press), 3–13.
Eslinger, P. J. (1998). Neurological and neuropsychological bases of empathy. Eur. Neurol. 39, 193–199.
Faria, A. V., Zhang, J., Oishi, K., Li, X., Jiang, H., Akhter, K., Hermoye, L., Lee, S., Hoon, A., Stashinko, E., Miller, M. I., van Zijl, P. C. M., and Mor, S. (2010). Atlas-based analysis of neurodevelopment from infancy to adulthood using diffusion tensor imaging and application for automated abnormality detection. Neuroimage 52, 415–428.
Flavell, J. H. (1999). Cognitive development: children’s knowledge about the mind. Annu. Rev. Psychol. 50, 21–45.
Frith, C. D., and Frith, U. (2006b). How we predict what other people are going to do. Brain Res. 1079, 36–46.
Fuster, J. M. (2004). Upper processing stages of the perception–action cycle. Trends Cogn. Sci. (Regul. Ed.) 8, 143–145.
Gallese, V., and Goldman, A. (1998). Mirror neurons and the simulation theory of mind-reading. Trends Cogn. Sci. (Regul. Ed.) 2, 493–501.
Giedd, J. N., Blumenthal, J., Jeffries, N. O., Castellanos, F. X., Liu, H., Zijdenbos, A., Paus, T., Evans, A. C., and Rapoport, J. L. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 2, 861–862.
Grieve, R., and Williamson, K. (1977). Aspects of auditory and visual attention to narrative material in normal and mentally handicapped children. J. Child Psychol. Psychiatry 18, 251–262.
Guttentag, R. E. (1985). A developmental study of attention to auditory and visual signals. J. Exp. Child. Psychol. 39, 546–561.
Hamlin, J. K., Wynn, K., and Bloom, P. (2007). Social evaluation by preverbal infants. Nature 450, 557–559.
Hayes, D. S., and Birnbaum, D. W. (1980). Preschoolers’ retention of televised events: is a picture worth a thousand words. Dev. Psychol. 16, 410–416.
Hein, G., and Singer, T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Curr. Opin. Neurobiol. 18, 153–158.
Hofmann, M. J., Herrmann, M. J., Dan, I., Obrig, H., Conrad, M., Kuchinke, L., Jacobs, A. M., and Falllgatter, A. J. (2008). Differential activation of frontal and parietal regions during visual word recognition: an optical topography study. Neuroimage 40, 1340–1349.
Hooker, C. I., Verosky, S. C., Germine, L. T., Knight, R. T., and D’Esposito, M. (2009). Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 1308, 100–113.
Hynes, C. A., Baird, A. A., and Grafton, S. T. (2006). Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia 44, 374–383.
Karmiloff-Smith, A. (2010). Neuroimaging of the developing brain: taking “developing” seriously. Hum. Brain Mapp. 31, 934–941.
Kaufman, A. S., and Applegate, B. (1988). Short forms of the K-ABC mental processing and achievement scales at ages 4 to 12 1/2 years for clinical and screening purposes. J. Clin. Child Adolesc. Psychol. 17, 359–369.
Kobayashi, C., Glover, G. H., and Temple, E. (2007). Children’s and adults’ neural bases of verbal and nonverbal “theory of mind”. Neuropsychologia 45, 1522–1532.
Koch, S. P., Steinbrink, J., and Obrig, H. (2009). “NILAB – a Matlab based processing tool for functional optical data,” in Optical Imaging Workshop, December 7–10, 2009, Bad Honnef.
Kringelbach, M. L. (2005). The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702.
Lamm, C., Batson, C. D., and Decety, J. (2007). The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J. Cogn. Neurosci. 19, 42–58.
Leslie, A. M. (1987). Pretense and representation: the origins of “theory of mind”. Psychol. Rev. 94, 412–426.
Lloyd-Fox, S., Blasi, A., and Elwell, C. E. (2010). Illuminating the developing brain: the past, present, and future of near infrared spectroscopy. Neurosci. Biobehav. Rev. 34, 269–284.
Miller, E. K., and Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202.
Moll, J., de Oliveira-Souza, R., Eslinger, P. J., Bramati, I. E., Mourão-Miranda, J., Andreiuolo, P. A., and Pessoa, L. (2002). The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. J. Neurosci. 22, 2730–2736.
Moriguchi, Y., Decety, J., Ohnishi, T., Maeda, M., Mori, T., Nemoto, K., Matsuda, H., and Komaki, G. (2007). Empathy and judging other’s pain: an fMRI study of alexithymia. Cereb. Cortex 17, 2223–2234.
Obrig, H., and Villringer, A. (2003). Beyond the visible – imaging the human brain with light. J. Cereb. Blood Flow Metab. 23, 1–18.
Okamoto, M., Dan, H., Sakamoto, K., Takeo, K., Shimizu, K., Kohno, S., Oda, I., Isobe, S., Suzuki, T., Kohyama, K., and Dan, I. (2004). Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21, 99–111.
Peña, M., Maki, A., Kovačić, D., Dehaene-Lambertz, G., Koizumi, H., Bouquet, F., and Mehler, J. (2003). Sounds and silence: an optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U.S.A. 100, 11702–11705.
Perner, J., Leekam, S. R., and Wimmer, H. (1987). Three-year-olds’ difficulty with false belief: the case for a conceptual deficit. Br. J. Dev. Psychol. 5, 125–137.
Perner, J., and Wimmer, H. (1985). “John thinks that Mary thinks that…”: attribution of second-order beliefs by 5-to 10-year-old children. J. Exp. Child Psychol. 39, 437–471.
Petrides, M. (2005). Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 781–795.
Pfeifer, J. H., Iacoboni, M., Mazziotta, J. C., and Dapretto, M. (2008). Mirroring others’ emotions relates to empathy and interpersonal competence in children. Neuroimage 39, 2076–2085.
Poldrack, R. A., Wagner, A. D., Prull, M. W., Desmond, J. E., Glover, G. H., and Gabrieli, J. D. (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10, 15–35.
Shamay-Tsoory, S. G., and Aharon-Peretz, J. (2007). Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia 45, 3054–3067.
Shamay-Tsoory, S. G., Aharon-Peretz, J., and Perry, D. (2008). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132, 617–627.
Shamay-Tsoory, S. G., Tomer, R., Berger, B., and Aharon-Peretz, J. (2003). Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J. Cogn. Neurosci. 15, 324–337.
Shamay-Tsoory, S. G., Tomer, R., Berger, B. D., Goldsher, D., and Aharon-Peretz, J. (2005). Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn. Behav. Neurol. 18, 55–67.
Shamay-Tsoory, S. G., Tomer, R., Goldsher, D., Berger, B. D., and Aharon-Peretz, J. (2004). Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. J. Clin. Exp. Neuropsychol. 26, 1113–1127.
Silani, G., Bird, G., Brindley, R., Singer, T., Frith, C., and Frith, U. (2008). Levels of emotional awareness and autism: an fMRI study. Soc. Neurosci. 3, 97–112.
Singer, T. (2006). The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev. 30, 855–863.
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V., and Dan, I. (2005). Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage 27, 842–851.
Soderstrom, H. (2003). Psychopathy as a disorder of empathy. Eur. Child. Adolesc. Psychiatry 12, 249–252.
Steinbrink, J., Villringer, A., Kempf, F., Haux, D., Boden, S., and Obrig, H. (2006). Illuminating the BOLD signal: combined fMRI–fNIRS studies. Magn. Reson. Imaging 24, 495–505.
Taga, G., Asakawa, K., Hirasawa, K., and Konishi, Y. (2004). Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: a near-infrared optical topography study. Pathophysiology 10, 277–281.
Thompson-Schill, S. L., D’Esposito, M., Aguirre, G. K., and Farah, M. J. (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl. Acad. Sci. U.S.A. 94, 14792–14797.
Voßkühler, A., Nordmeier, V., Kuchinke, L., and Jacobs, A. M. (2008). OGAMA (open gaze and mouse analyzer): open-source software designed to analyze eye and mouse movements in slideshow study designs. Behav. Res. Methods 40, 1150–1162.
Völlm, B. A., Taylor, A. N., Richardson, P., Corcoran, R., Stirling, J., McKie, S., Deakin, J. F. W., and Elliottand, R. (2006). Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage 29, 90–98.
Wartenburger, I., Steinbrink, J., Telkemeyer, S., Friedrich, M., Friederici, A. D., and Obrig, H. (2007). The processing of prosody: evidence of interhemispheric specialization at the age of four. Neuroimage 34, 416–425.
Wellman, H. M., Cross, D., and Watson, J. (2001). Meta-analysis of theory-of-mind development: the Truth about false belief. Child Dev. 72, 655–684.
Keywords: OFC, cognitive empathy, affective empathy, children, fNIRS, verbal, non-verbal
Citation: Brink TT, Urton K, Held D, Kirilina E, Hofmann MJ, Klann-Delius G, Jacobs AM and Kuchinke L (2011) The role of orbitofrontal cortex in processing empathy stories in 4- to 8-year-old children. Front. Psychology 2:80. doi: 10.3389/fpsyg.2011.00080
Received: 30 November 2010;
Paper pending published: 22 December 2010;
Accepted: 13 April 2011;
Published online: 28 April 2011
Edited by:
Judit Gervain, CNRS – Universite Paris Descartes, FranceReviewed by:
Kalina J. Michalska, The University of Chicago, USASarah Lloyd-Fox, Birkbeck, University of London, UK
Copyright: © 2011 Brink, Urton, Held, Kirilina, Hofmann, Klann-Delius, Jacobs and Kuchinke. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Tila Tabea Brink, General and Neurocognitive Psychology, Freie Universität Berlin, Habelschwerdter Allee 45, 14195 Berlin, Germany. e-mail: tila.brink@fu-berlin.de; Lars Kuchinke, Experimental Psychology, Ruhr-Universität Bochum,Universitätsstraße 150, 44801 Bochum, Germany.e-mail: lars.kuchinke@rub.de