- 1 Faculty of Social Sciences, Psychology I, University of Kaiserslautern, Kaiserslautern, Germany
- 2 Georgetown University, Washington, DC, USA
Visual stimuli can be classified so rapidly that their analysis may be based on a single sweep of feedforward processing through the visuomotor system. Behavioral criteria for feedforward processing can be evaluated in response priming tasks where speeded pointing or keypress responses are performed toward target stimuli which are preceded by prime stimuli. We apply this method to several classes of complex stimuli. (1) When participants classify natural images into animals or non-animals, the time course of their pointing responses indicates that prime and target signals remain strictly sequential throughout all processing stages, meeting stringent behavioral criteria for feedforward processing (rapid-chase criteria). (2) Such priming effects are boosted by selective visual attention for positions, shapes, and colors, in a way consistent with bottom-up enhancement of visuomotor processing, even when primes cannot be consciously identified. (3) Speeded processing of phobic images is observed in participants specifically fearful of spiders or snakes, suggesting enhancement of feedforward processing by long-term perceptual learning. (4) When the perceived brightness of primes in complex displays is altered by means of illumination or transparency illusions, priming effects in speeded keypress responses can systematically contradict subjective brightness judgments, such that one prime appears brighter than the other but activates motor responses as if it was darker. We propose that response priming captures the output of the first feedforward pass of visual signals through the visuomotor system, and that this output lacks some characteristic features of more elaborate, recurrent processing. This way, visuomotor measures may become dissociated from several aspects of conscious vision. We argue that “fast” visuomotor measures predominantly driven by feedforward processing should supplement “slow” psychophysical measures predominantly based on visual awareness.
Complex Stimuli, Binding, and the Fast Feedforward Sweep
Much has been learned in the last decades about the flow of information within a hierarchy of visual areas. A classical view of visual perception is that early visual areas encode local object features that are passed on to higher-level areas for more integrated processing of color, shape, or motion (Hubel and Livingstone, 1987; Livingstone and Hubel, 1987; see Sincich and Horton, 2005, for an update). Beyond that level, more and more intricate aspects of visual objects are extracted, until areas in the inferotemporal cortex are reached that encode complex objects or faces (Tanaka, 1996; Quiroga et al., 2005), either by specialized single cells or by sparse coding (Barlow, 1972; DeCharms and Zador, 2000). Finally, entire scenes are processed where objects have to be recognized in the context of other objects (Bar and Aminoff, 2003; Bar, 2004).
This system faces a set of complicated tasks. One of the most basic ones is feature binding, the challenge to assign separately encoded features to the appropriate objects without creating false combinations (Treisman, 1996; Wolfe and Cave, 1999). Classical evidence that the visual system faces a binding problem comes from visual search experiments, which have specifically shown that it is difficult to find conjunction targets (e.g., a green X) in a clutter of non-targets sharing the defining target features (e.g., red X’s and green O’s), and that single features may be miscombined to form illusory conjunctions (Treisman and Gelade, 1980; Treisman, 1996; Wolfe and Cave, 1999). Of course, this problem especially pertains to the encoding of multi-feature objects, such as complex artificial stimuli or natural images. Similar integration problems arise in visual grouping and figure–ground segmentation, where feature integration occurs across space (Roelfsema, 2006), or in object constancy, where integration occurs across time (Turnbull et al., 1997).
The exact neural foundations of binding are still debated (Schmidt, 2009). The classical view of the visual system as a stepwise processing hierarchy describes input–output relations between visual areas, but basically ignores temporal aspects, such as the speed of information transfer from one area to the next, the role of re-entrant information, and the role of recurrent processing loops (for reviews, see Bullier, 2004; Gilbert and Sigman, 2007). In recent years, the temporal dynamics of processing have received much more attention, which led to a fundamentally revised view of the visual system. Most importantly, several authors have stressed a distinction between two radically different types of visual processing: a rapid feedforward process where visual activation proceeds in bottom-up direction through the visual system, and a slower, recurrent process developing in the immediate wake of this “fast feedforward sweep” (Lamme and Roelfsema, 2000; also see Bullier, 2001; VanRullen and Thorpe, 2002). Many solutions that have been proposed for the binding problem involve the slower, recurrent type of processing, e.g., binding by spike synchrony (Gray and Singer, 1989; Gray, 1999; but see Shadlen and Movshon, 1999), or by visual attention operating on spatial and feature maps (Treisman, 1996; Roelfsema, 2006). Most of these must be considered time-consuming, requiring the integration of information over separate brain areas and several feedback cycles. Faster binding would be achieved by feedforward cascades involving specialized cells (or sparsely coded cell assemblies) directly coding for feature combinations (DeCharms and Zador, 2000; VanRullen, 2009). Consistent with that idea, evidence from the rapid discrimination of natural images suggests that elaborate categorization of objects is possible during the first feedforward sweep of visual processing (Thorpe et al., 1996; VanRullen and Thorpe, 2001; Kirchner and Thorpe, 2006), a finding which is also in accord with neuronal network modeling (VanRullen et al., 1998, 2001; Serre et al., 2007).
The distinction between feedforward and recurrent processing is crucial for many aspects of mid-level vision, particularly figure–ground segmentation, which is a key step in human vision. Lamme et al. (1999; see also Roelfsema et al., 2002; Supèr and Lamme, 2007; Scholte et al., 2008; Supèr et al., 2010) employed figure–ground displays consisting of textures of oriented line elements, showing that neurons in primary visual cortex first respond selectively to local line orientations in their receptive field. After about 100 ms, however, responses are selectively enhanced when the receptive field lies on a figure as opposed to the background, and responses even become independent of the orientation of local line elements. In other words, those cells change from local feature detectors to figure–ground detectors during the course of stimulation. This groundbreaking discovery suggests that the response properties of the cells are radically changed by re-entrant signals from downstream visual areas, even while the cells are already responding to the stimulus. These findings also highlight the need to understand the time course of processing in feedforward as opposed to recurrent networks, recognizing that any area might serve different functions at different points in time.
Response Priming Independent of Visual Awareness
One of the major tenets of this paper is that the role of feedforward processing of complex stimulus displays can be assessed by tracing the time courses of speeded motor responses. A paradigm especially suited to examine rapid response activation by visual stimuli is response priming (Klotz and Wolff, 1995; Klotz and Neumann, 1999). In response priming, participants perform a speeded response to a target stimulus that is preceded by a prime stimulus triggering either the same response as the target (consistent prime) or the opposite response (inconsistent prime). In typical experiments, the targets serves the additional purpose of reducing the visibility of the prime by backward masking (Breitmeyer and Ögğmen, 2006). Nevertheless, consistent primes will speed up responses to the target while inconsistent primes will slow down responses, and this priming effect increases with increasing time interval between prime onset and target onset (stimulus-onset asynchrony, SOA; Vorberg et al., 2003).
Generally, response priming effects occur because the prime activates the response assigned to it, inducing a motor conflict if prime and target are inconsistent. This has first been demonstrated in the time course of lateralized readiness potentials (LRPs), which represent an electroencephalographic measure of selective preparation of right- or left-hand responses. Typically, these potentials start out time-locked to the prime, first develop in the direction specified by the prime, and only later proceed in the direction specified by the actual target (Eimer and Schlaghecken, 1998; Leuthold and Kopp, 1998; Verleger et al., 2004; Klotz et al., 2007; Vath and Schmidt, 2007). An alternative way to trace the prime’s motor impact over time is the kinematic analysis of primed pointing responses (Schmidt, 2002; Brenner and Smeets, 2004; Song and Nakayama, 2009). These experiments show that inconsistent primes are able to mislead pointing movements into the wrong direction, such that the initial finger movement is time-locked to the prime, first proceeds in the direction specified by the prime, and only then proceeds in target direction (Schmidt, 2002; Schmidt et al., 2006). These effects clearly show that the priming effect increases with prime–target SOA because the prime has progressively more time to direct the response into the correct or incorrect direction, even to the point where an inconsistent prime provokes a full-fledged response error (see Vorberg et al., 2003, for a mathematical model of these response activation processes). Consequently, response errors almost exclusively occur in inconsistent trials, and there increase in frequency with increasing SOA.
Strikingly, response priming effects are independent of visual awareness of the prime: the time course of priming is invariant no matter whether the prime can be identified perfectly or not at all, and no matter whether prime identification performance increases or decreases with SOA1, which implies that priming and awareness can actually change in opposite directions (Vorberg et al., 2003; see also Mattler, 2003; Albrecht et al., 2010). Under mild measurement assumptions, such double dissociations between priming and awareness indicate that visual awareness is based on processing mechanisms distinct from those leading to response priming (Schmidt and Vorberg, 2006)2. Specifically, Lamme and Roelfsema (2000) propose that conscious perception is possible only with recurrent processing of the stimulus, whereas feedforward processing alone is insufficient for generating visual awareness (Lamme and Roelfsema, 2000; Lamme, 2006; cf. Dehaene and Naccache, 2001; Dehaene, 2009). In contrast to many other theories of consciousness, Lamme and Roelfsema’s theory is making strong and testable predictions. Supporting evidence comes from studies indicating that visual awareness is suppressed if re-entrant loops from extrastriate visual areas through primary visual cortex are disrupted at critical points in time, for instance, by transcranial magnetic stimulation (Pascual-Leone and Walsh, 2001; Ro et al., 2003), or by backward masking (DiLollo et al., 2000; Lamme et al., 2002; Bacon-Macé et al., 2005; Haynes et al., 2005; Fahrenfort et al., 2007; cf. Macknik and Livingstone, 1998; Macknik and Haglund, 1999).
Rapid-Chase Theory
Lamme and Roelfsemas (2000) theory can easily explain the major findings in response priming: priming could directly reflect sequential sweeps of feedforward processing initiated by primes and targets, and thus precede the recurrent processes leading to visual awareness. However, the notion of a feedforward processing stage in human vision is controversial. Feedback mechanisms within and between visual areas can be rapid (Bullier, 2001, 2004; Sillito et al., 2006; Roland, 2010), and information might be processed at different rates in parallel streams (Merigan and Maunsell, 1993; Chen et al., 2007), with plenty of opportunity for visual signals to cross or overtake each other. Because the feedforward sweep is unobservable on a single-cell level, behavioral evidence is needed to show that the notion is plausible for human information processing.
Indeed, several studies have shown that the early time course of primed pointing movements (Schmidt et al., 2006) and LRPs (Vath and Schmidt, 2007) depends only on prime but not on target characteristics, as would be predicted for a simple feedforward system processing primes and targets in strict sequence. This is most easily seen in the time course of primed pointing movements, which can be measured with higher spatial and temporal precision than EEG signals. Schmidt et al. (2006) studied primed pointing responses to color stimuli, using primes and targets of either high or low color saturation as well as different types of targets. The initial time courses of the priming effects depended exclusively on color saturation of the primes but were independent of target type and target onset time. Similarly, Vath and Schmidt (2007) studied keypress responses with primes and targets that could independently be high or low in color saturation. Again, initial priming effects in LRPs depended only on prime saturation but were independent of target saturation or target onset time. In both studies, only later segments of the priming effects were influenced by characteristics of the actual target.
For that reason, Schmidt et al. (2006; Vath and Schmidt, 2007) proposed a rapid-chase theory of response priming where primes and targets elicit feedforward sweeps that traverse the visuomotor system in strict sequence, without any temporal overlap. Prime and target sweeps are able to directly initiate the motor responses assigned to them, with no need for conscious control (the principle of direct parameter specification, Neumann, 1990). The prime signal reaches motor areas first, initiating a response and continuing to drive the response on its own until the target signal takes over response control. Priming effects, as well as error rates in inconsistent trials, increase with prime–target SOA because the prime has more time to drive the response on its own when the target is further delayed. The feedforward properties of such a system would show in the time course of the motor response as follows (rapid-chase criteria, Schmidt et al., 2006):
(1) prime rather than target signals should determine the onset and initial direction of the response (initiation criterion);
(2) target signals should be able to influence the response before it is completed (takeover criterion);
(3) movement kinematics should initially depend on prime characteristics only and be independent of all target characteristics (independence criterion)3.
Note that the initiation and independence criteria describe exclusive initial response control by the prime, while the takeover criterion is merely needed to make sure that the target is not altogether ignored. A stimulus–response system meeting these functional criteria would be behaviorally equivalent to a simple feedforward system. Note that the notion of a rapid-chase is milder than that of a feedforward sweep: whereas the feedforward sweep entails the possibility of rapid chases but excludes the possibility of local recurrent activity, the rapid-chase account allows for local recurrence as long as sequential signals still lead to strictly sequential motor output. Further note that rapid-chase processing implies that the first sweep must be independent of the second sweep, but not vice versa. Therefore, the criteria demand that initial processing be controlled exclusively by the prime, but not that later processing be controlled exclusively by the target.
In the following sections, we review our recent research on response priming by complex visual stimuli. We start by showing that the classification of natural images (into animal vs. non-animal pictures) satisfies the rapid-chase criteria, establishing response priming by such images as a rapid-chase process (Schmidt and Schmidt, 2009). We then show how the deployment of visual attention just in time before onset of the prime can boost visuomotor priming, both for spatial and feature-based attention (Schmidt and Seydell, 2008; Schmidt and Schmidt, 2010), in a way consistent with enhanced bottom-up processing of incoming stimuli. Interestingly, similar boosting occurs on a much longer time-scale in individuals with specific phobias (e.g., for spiders or snakes) when processing phobic stimuli, probably as a result of life-long perceptual learning (Haberkamp et al., in preparation). Finally, we investigate priming effects by dark vs. bright primes under illumination and transparency illusions that alter the perceived brightness of the primes. Surprisingly, priming effects in speeded keypress responses can systematically contradict subjective brightness judgments, such that one prime appears brighter than the other but activates motor responses as if it was darker (Schmidt et al., 2010; Weber and Schmidt, in preparation). We will argue that response priming is dominated by the output of the first feedforward pass of visual signals through the visuomotor system, and that this output lacks some characteristic features of more elaborate, recurrent processing. This way, visuomotor measures may dissociate from several aspects of conscious vision.
Rapid-Chase Processing of Natural Images
Our visual system is able to categorize images of natural visual scenes at remarkable speed (Hegdé, 2008). Evidence stems from go–nogo tasks (e.g., Thorpe et al., 1996; VanRullen and Thorpe, 2002; VanRullen and Koch, 2003) as well as from two-alternative forced-choice paradigms (Bacon-Macé et al., 2007). Perhaps the most impressive set of data comes from Kirchner and Thorpe (2006), whose participants had to decide which of two pictures contained an animal by performing a speeded saccade toward the animal picture. The authors demonstrated that the rate of correct responses begins to exceed the rate of errors at saccade latencies as short as 120 ms, indicating extremely fast image classification and response activation. Based on such findings, several authors have suggested that natural scene processing is occurring in the first sweep of feedforward processing through the visuomotor system (e.g., Thorpe et al., 1996; VanRullen and Thorpe, 2002; VanRullen and Koch, 2003).
Of course, the sheer rapidity of this system is highly suggestive of feedforward processing. But does the system meet the rapid-chase criteria? We employed a primed pointing paradigm featuring natural images, comparing four tasks in two experiments (Schmidt and Schmidt, 2009). In each task, two target images (each from one of two picture categories, e.g., animals and objects) appeared in diagonally opposite quadrants of the display (Figure 1A). Participants pointed from the center of the display toward the picture of the relevant target category (e.g., the animal). Before the targets, two primes appeared at the same positions for 33 ms at prime–target SOAs between 33 and 100 ms. Image categories could either be presented at the same spatial positions in primes as well as targets (consistent trials), or prime categories could be spatially switched with respect to target categories (inconsistent trials). Pointing responses were recorded by a POLHEMUS FASTRAK® magnetic tracking device. Primes in consistent and inconsistent trials were expected to initiate responses toward the direction of the correct target image or the opposite direction, respectively. We employed the following tasks:
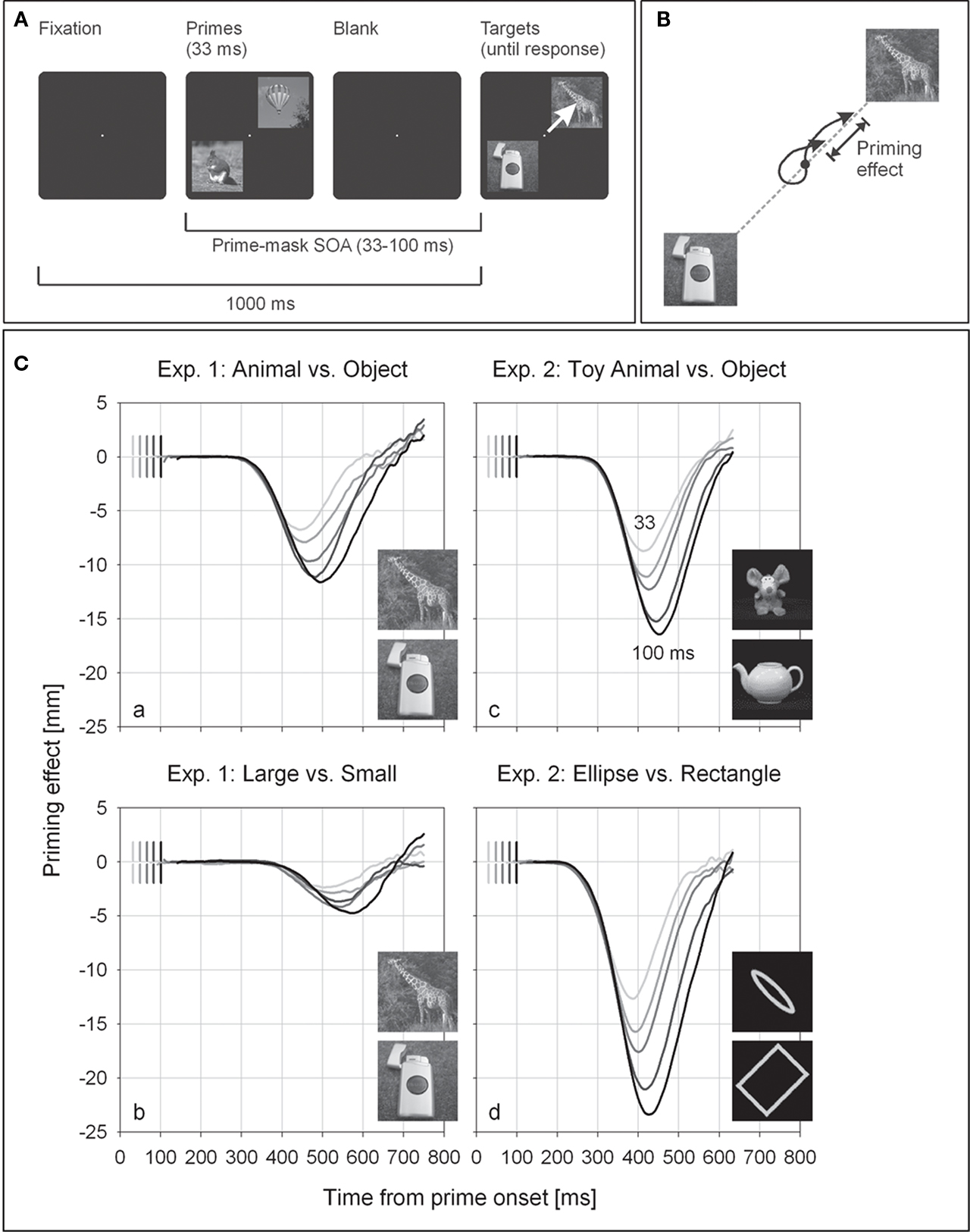
Figure 1. Paradigm and results from Schmidt and Schmidt (2009). (A) Time course of a trial. Participants’ task is to point to the image containing an animal, using a hand-held stylus. The arrow illustrates a correct pointing response. (B) Definition of spatial priming effects. Sensor positions in consistent and inconsistent trials were projected onto the target–non-target axis and subtracted. The resulting measure of priming is negative when the sensor position in consistent trials leads the sensor position in inconsistent trials. (C) Spatial priming functions. Only correct responses are shown; functions are locked to prime onset. Reproduced with permission from Psychonomic Society Inc.
(1) In the animal–object task of Experiment 1, image categories were animals and non-animals presented as gray-scale photographs in front of their natural cluttered backgrounds. Participants pointed as quickly as possible from the display center toward the animal target. This task was designed to match the standard conditions in most studies on rapid image classification (e.g., Thorpe et al., 1996).
(2) In the large–small task of Experiment 1, exactly the same pictures were presented, but participants had to point toward the item that would be larger in real life.
(3) The toy animal–object task of Experiment 2 was identical to that of Experiment 1 but used novel stimuli (mainly pictures of toy animals and household goods, selected from a database by Geusebroek et al., 2005). These appeared at high color and luminance contrast against a black background to provide a stronger feedforward signal and yield larger priming effects than those observed in Experiment 1 with gray-scale images.
(4) In the ellipse–rectangle task of Experiment 2, one ellipse and one rectangle were presented as targets (preceded by ellipses and rectangles as primes), and participants had to point toward the ellipse target. These stimuli should yield unequivocal evidence for rapid-chase processing and approximate an upper limit on classification speed against which the animal–object tasks could be compared.
All tasks yielded large priming effects, with the exception of Experiment 1’s large–small task (discussed below). To analyze response priming in pointing movements, we looked at the entire time course of the primed pointing responses. As observed in previous experiments (e.g., Schmidt, 2002; Schmidt et al., 2006), responses started at a fixed time following prime onset and initially went into the direction specified by the primes, suggesting that the primes initiated the response instead of the actual targets. When primes and targets were consistent, this initial direction was correct, and the movement simply proceeded in the correct direction until the response was completed. However, in inconsistent trials, the movement initially detoured into the direction of the misleading prime. The longer the SOA, the longer and farther the finger traveled toward the misleading prime before the movement reversed and finally proceeded in the correct direction, obviously because the response was now controlled by the targets. Even in the absence of overt detours, inconsistent primes delayed response onset, and this delay increased with prime–target SOA. The time course of primed pointing responses shows that the first two of the rapid-chase criteria are clearly met: it is the primes, not the targets, that elicit the first response and determine its initial direction (initiation criterion), and the targets are able to take over response control before the response is finished (takeover criterion).
To evaluate the independence criterion (initial movements must be controlled exclusively by the primes and independent of all properties of the targets), we examined the time course of priming effects defined in the spatial domain, i.e., the spatial lag of the pointing movements in inconsistent compared to consistent trials at corresponding points in time (see Figure 1B for definition). Spatial priming functions describe spatial lag as a function of time from prime onset.
Priming functions for all four tasks and different SOA conditions are depicted in Figure 1C. It is evident that spatial priming functions in each task follow an invariant initial time course before individual priming functions branch off one after another in the order of increasing prime–target SOAs. (Again, the only exception is the large–small task of Experiment 1.) This striking invariance means that the initial priming effects are exclusively determined by the primes and that only later phases of the process are affected by the actual targets. The spatial priming functions thus satisfy the rapid-chase criteria, establishing response priming of animal–non-animal classifications in natural images as a rapid-chase process.
Our findings also show that the type of image classification task is crucial for determining whether the classification can be performed as a rapid-chase process. A case in point is the large–small task of Experiment 1, which yielded slow responses, small priming effects, and little evidence for rapid-chase processing. It thus seems that this task cannot be performed in a single feedforward sweep and requires more extensive recurrent processing, or even cognitive control to overrule a natural tendency to classify the images based on an animal–object distinction.
For the remaining tasks, however, rapid-chase processing links visuomotor priming to the feedforward processing of images (Lamme and Roelfsema, 2000), thereby supporting conclusions from previous studies (e.g., Thorpe et al., 1996; VanRullen and Koch, 2003; Hung et al., 2005; Kirchner and Thorpe, 2006; Bacon-Macé et al., 2007; Serre et al., 2007). However, there is no indication that natural image classification is extraordinarily fast, or “ultra-rapid” (VanRullen and Thorpe, 2001; Kirchner and Thorpe, 2006), at least when compared with basic classifications of geometric stimuli, like ellipses and rectangles. The important point is that even though the different tasks reported here represent a wide range of processing speeds, they all meet strict functional criteria for the behavior of a rapid-chase system. Meeting those functional criteria is not simply a matter of sheer rapidity of processing but of its temporal dynamics (i.e., whether stimulus signals remain separate when traversing the visuomotor system in sequence), even for comparatively low rates of processing.
Response Priming Enhanced by Visual Attention: Dissociations between Motor Activation, Attention, and Awareness
Rapid-chase theory maintains that primes and targets should not be able to trigger attentional modulation of their own feedforward processing, which would outpace any such modulatory influence. However, visual attention deployed in time before the occurrence of the primes should be able to modulate subsequent feedforward processing of primes and targets. In a first study (Schmidt and Seydell, 2008), our goal was to create a task where visual selective attention was a necessary precondition for performing the task at all, and also necessary for allowing a priming effect to develop. We studied the impact of endogenous spatial attention (Yantis and Serences, 2003) on primed pointing movements by presenting a circular configuration of 10 possible targets preceded by 10 primes at the same positions (Figure 2, upper panel). Stimulus configurations were such that neighboring stimuli would be alternately red and green, and a red target or prime would always lie opposite to a green one. Primes were either all consistent with the targets (meaning that at each of the 10 positions, prime and target had the same color) or all inconsistent to the targets. Participants responded to one pair of opposite targets by pointing to the target of appointed color. To know which of the target pairs to respond to, they had to process a spatial cue presented just before prime onset, which indicated the relevant pair of opposing targets.
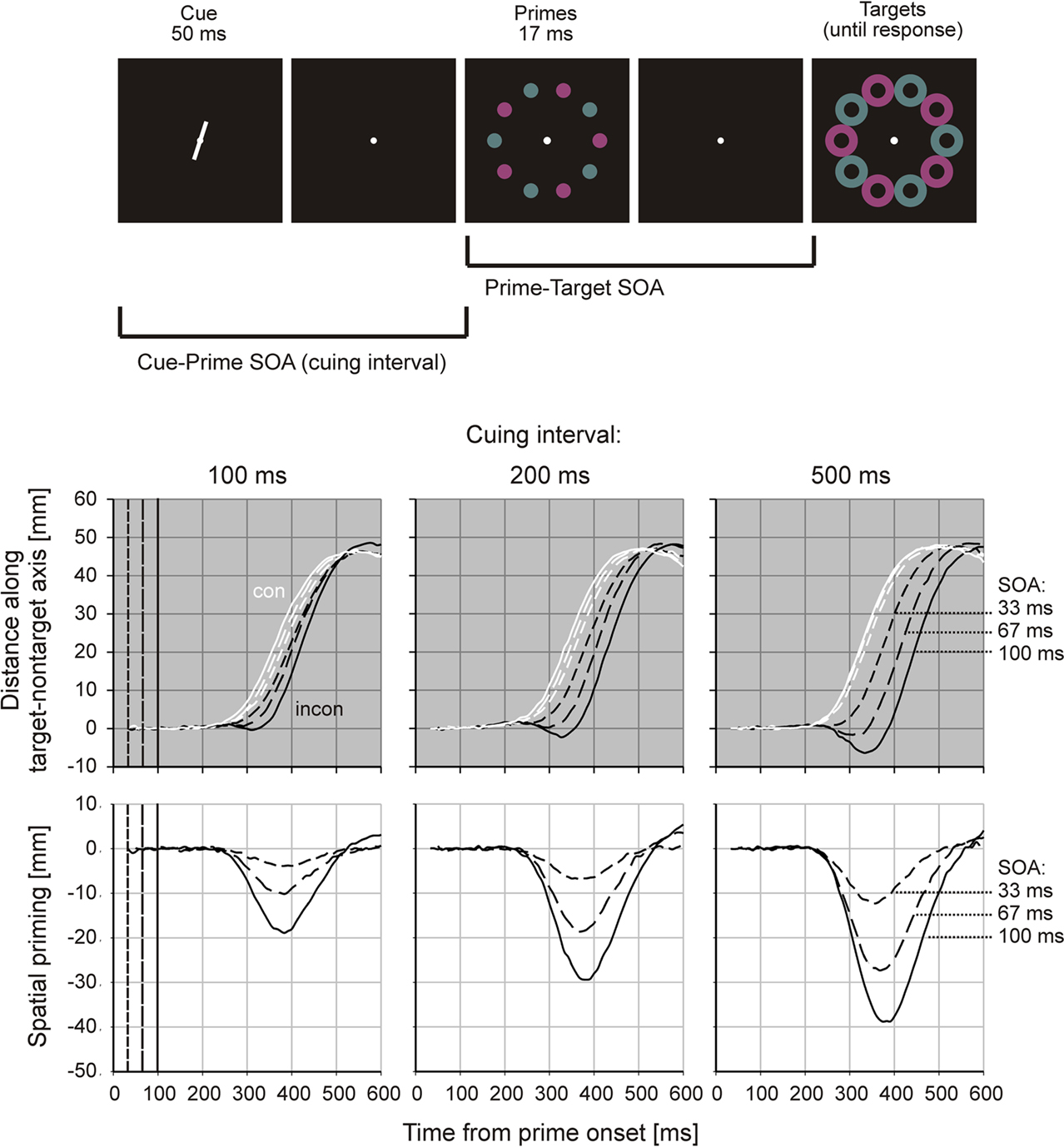
Figure 2. Paradigm and results from Schmidt and Seydell(2008; Experiment 2). Upper panel: time course of a trial. Participants point from the center of the display to the red one of the two targets indicated by the position cue. Middle panel: finger position along the target–non-target axis, with positive values indicating the direction of the correct target. Note the detours toward the incorrect target in inconsistent trials. Lower panel: spatial priming functions for different cuing intervals. Conventions as in Figure 1.
In this paradigm, selective attention is controlled by the cuing interval, i.e., the amount of time available for processing the cue before the prime is presented. The idea is that the longer the cuing interval, the more time is available for deploying selective attention to the cued stimulus locations. Primes and targets presented at those locations should then have a processing advantage, leading to more vigorous visuomotor processing and larger priming effects. Note that this design has two peculiarities that make it attractive for studying the deployment of visual attention. Firstly, participants are forced to spatially select stimuli in order to respond to the correct target. This is why we named the design a “selection-for-action” paradigm (after Allport, 1989). Secondly, and more importantly, any finding of a spatially directed priming effect implies that spatial selection must have taken place, because without spatial selection, the circular array of primes surrounding the initial finger position would not be expected to induce a movement in any particular direction.
Figure 2 shows how different cuing intervals affect spatial priming effects. Pointing trajectories developed in the typical manner described above (Figure 2, middle panel). In consistent as well as inconsistent trials, response onset was time-locked to the prime and first proceeded in the direction specified by the prime, repeatedly leading to detours in inconsistent trials. Again, the duration and spatial extent of those detours increased with prime–target SOA. Spatial priming functions (Figure 2, lower panel) were also time-locked to the prime, and their early time course was largely invariant except for the shortest SOA. Even though the rapid-chase criteria do not strictly apply to this class of experiment because the prime–target sequence encounters a system already engaged in cue processing and the deployment of attention, the overall time courses are similar to those shown in Figure 1, and are reasonably consistent with a rapid-chase system.
Most importantly, spatial priming was clearly modulated by the cuing interval: all aspects of the motor response were enhanced as the cuing interval became longer, including the onset time of the spatial priming effect, the peak priming amplitude, and several movement velocity parameters. Moreover, priming effects were spatially directed as shown by the overt detours in the direction of the cued prime pair, which implies that spatial selection must have taken place. Together, these findings strongly suggest that participants indeed use the cue to deploy selective attention to the cued positions, and that the visuomotor feedforward processing of primes and targets at those positions is enhanced relative to the uncued positions. We found similar results for an experiment where attention was summoned involuntarily by valid or invalid exogenous cues (Schmidt and Seydell, 2008; Experiment 1). Our results are in line with a study by Sumner et al. (2006), who showed that response priming can be enhanced by exogenous attention.
Fascinatingly, all aspects of selective attention in the spatial domain can be generalized to selection in the domains of color and shape features. Schmidt and Schmidt (2010) employed the selection-for-action paradigm to study the effects of feature-based attention on priming. Instead of using a precue that would directly point at the relevant pair of stimulus positions, we employed a shape cue that would indicate the shape of the relevant target stimuli (Figure 3, upper panel). Participants were then instructed to point as quickly as possible from the display center to the cued shape of appointed color (e.g., to the red one of the two squares). Findings were very similar to the ones by Schmidt and Seydell (2008), with spatially directed detours indicating that spatial selection had taken place on the basis of feature-based attention. Enhancement of priming effects was optimal at cuing intervals of 500 ms. With the caveats mentioned above, priming functions were consistent with the rapid-chase criteria, even for the shortest SOAs, suggesting that the attentional modulation directly affected feedforward processing of primes and targets (Figure 3, middle panel). Similar results were obtained when the shape and color domains were swapped, i.e., when participants processed a color cue telling them to respond to the cued color stimulus of appointed shape (Schmidt and Schmidt, 2010; Experiment 1).
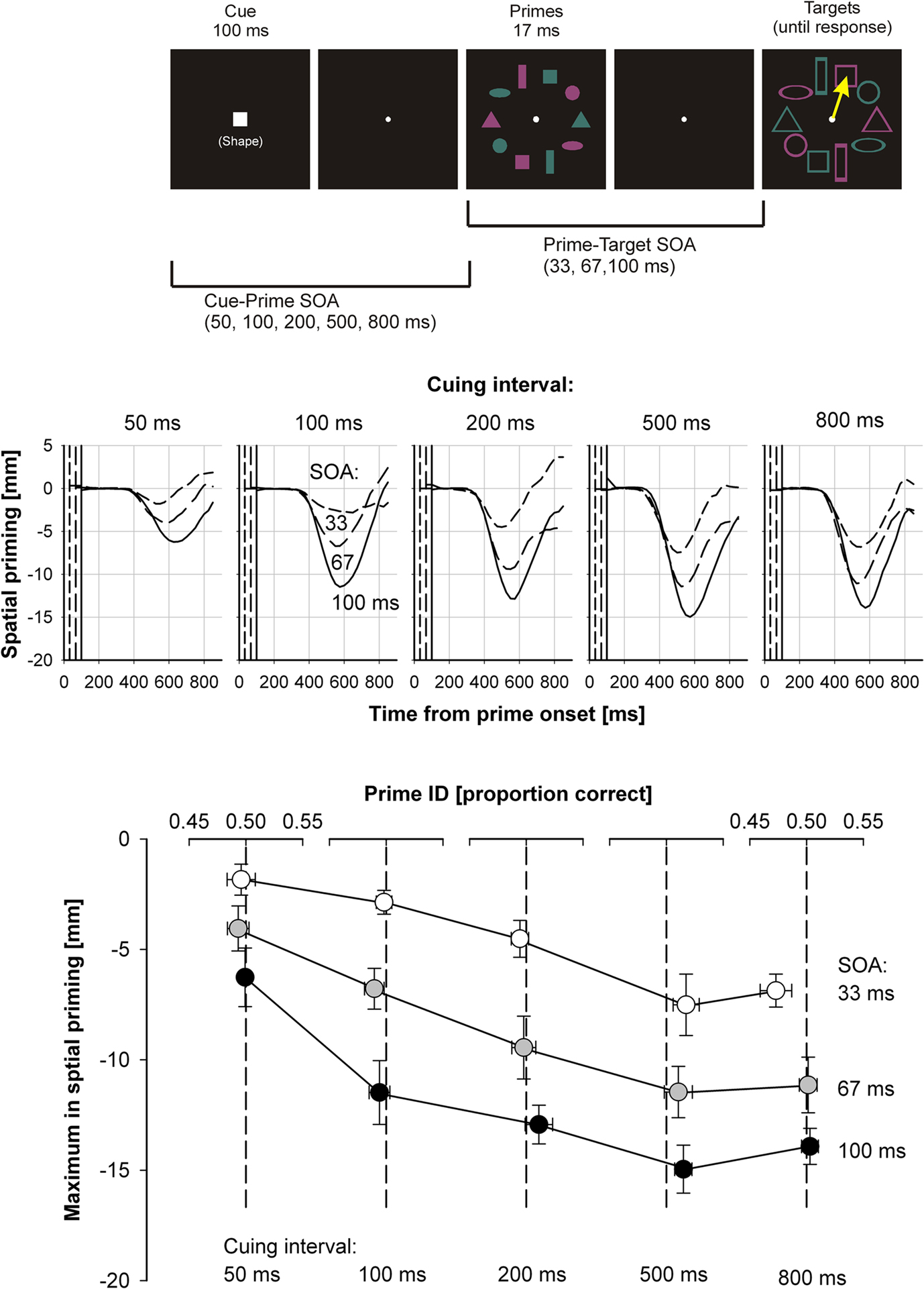
Figure 3. Paradigm and results from Schmidt and Schmidt(2010; Experiment 2). Upper panel: time course of a trial. Participants point from the center of the display to the red one of the two targets indicated by the shape cue (here, a square). Middle panel: spatial priming functions for different cuing intervals. Conventions as in Figure 1. Lower panel: response accuracy in the prime identification task plotted against maximum spatial priming effects in the target identification task. Here and in all further figures, error bars denote standard errors of the mean with pure intersubject variance removed (Cousineau, 2005).
Several recent studies have demonstrated effects of selective attention on stimuli that do not reach awareness (e.g., Melcher et al., 2005; Kanai et al., 2006; Kentridge et al., 2008; Zhaoping, 2008; Shin et al., 2009). Therefore, in addition to priming effects, we also measured prime visibility in a task where participants saw the exact same stimuli as before but had to point without time pressure to the position of the cued prime of appointed color (Schmidt and Schmidt, 2010). Figure 3 (lower panel) plots performance in prime identification against the maximum amplitude of the spatial priming functions. Clearly, priming amplitude is strongly modulated by both prime–target SOA and cuing interval, even though in all conditions, prime identification performance is close to chance level. This finding not only indicates that visuomotor priming processes are independent of prime visibility, but that the attentional enhancement of prime processing also is. In other words, feature-based attention enhanced the processing of stimuli which themselves remained invisible to the participants, suggesting that different neurophysiological processes are underlying attention and awareness (Lamme, 2003, 2005; Koch and Tsuchiya, 2007).
Enhanced Processing of Phobic Images
The studies described above show that visual attention deployed in time before the occurrence of the prime can boost visuomotor priming processes. Interestingly, however, a similar enhancement of visuomotor processing occurs on a much longer time-scale in participants with specific phobias. Current studies suggest that people with specific phobias initially display an involuntary attentional bias toward threat stimuli (Mogg and Bradley, 2006; Rinck and Becker, 2006; Fox et al., 2007; for a review see Mathews and MacLeod, 2005). Recent studies also reported a subsequent and probably voluntary avoidance of those stimuli by phobic individuals (e.g., Rinck and Becker, 2006). Both phenomena, the early and involuntary attentional bias toward a threatening stimulus as well as the later intentional avoidance, might contribute to the maintenance of specific phobias in patients. However, despite the many studies focusing on the spontaneous attentional response to phobic images, no study we know of directly addressed the speed with which phobic and non-phobic stimuli are processed.
Working from the assumption that the speeded classification of images into specific response categories is a feedforward process (Thorpe et al., 1996; Kirchner and Thorpe, 2006; Schmidt and Schmidt, 2009), our goal was to explore whether fear-related images give rise to larger response priming effects than neutral images, and whether this processing advantage is stronger in participants specifically fearful of those images, compared to non-anxious control participants (Haberkamp et al., in preparation). We distinguished downright phobic pictures (e.g., spiders for spider-fearful participants, snakes for snake-fearful participants) from merely aversive ones (spiders and snakes in general) as well as from neutral, non-aversive pictures (flowers and mushrooms). Spider-fearful, snake-fearful, and non-phobic individuals performed speeded keypress responses to target pictures of snakes, spiders, mushrooms, and flowers, which were preceded by prime pictures from the same image categories (Figure 4A). In each trial, the prime was either consistent or inconsistent with the target with respect to the required motor response. Participants performed two tasks (Figure 4B): they either had to discriminate spider and snake targets from mushroom and flower targets (animal vs. non-animal task) or spider and mushroom targets from snake and flower targets (spider vs. snake task). By comparing these two tasks, we not only can address the processing difference between aversive and neutral images (i.e., the difference between animal and non-animal images), but also the difference between aversive and phobic images (e.g., the difference between spider and snake images in spider-fearful individuals). Using this two-task design, most meaningful comparisons can be made within individual participants.
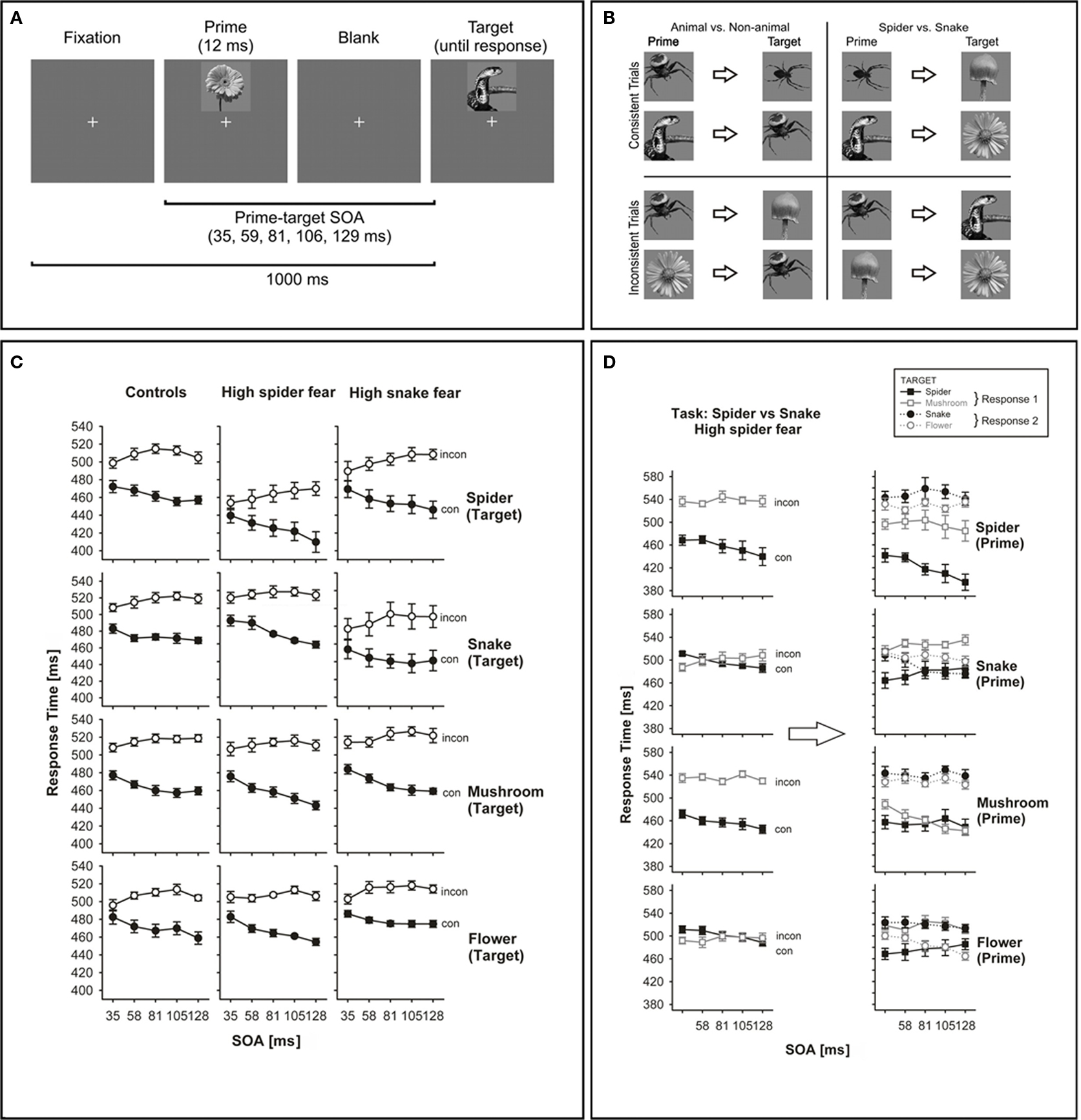
Figure 4. Paradigm and results from Haberkamp et al. (in preparation). (A) Time course of a trial. Participants classify the target picture by keypress according to different tasks. (B) Stimulus–response mappings. Participants either had to discriminate spider and snake targets from mushroom and flower targets (animal vs. non-animal task) or spider and mushroom targets from snake and flower targets (spider vs. snake task). (C) Priming effects in each group separated by targets, pooled across tasks. (D) Priming effects for spider-fearful individuals in the spider vs. snake task. The left panel shows effects for response-consistent and response-inconsistent primes, the right panel shows effects for all prime categories.
Strong and reliable priming effects were observed in response times and error rates for each group and task. In Figure 4C, priming effects are shown separately for each target and group, but averaged over the two tasks. The most important effect was a dramatic increase in response speed in the spider-fearful group when spiders served as targets. In contrast to that, snake-fearful and control participants responded with similar speed to all target categories (spiders, snakes, mushrooms, and flowers). This was a surprise given our expectation that snake-fearful participants, analogous to spider-fearful participants, would respond selectively faster to phobic targets.
Beside faster responses to phobic spider targets, we also found stronger priming effects from phobic primes, especially in participants fearful of spiders. Figure 4D shows the results for the spider-fearful participants in the “spider vs. snake” task, where spider and mushroom pictures are mapped to one response, and snake and flower pictures to the alternative response. The left panel plots priming effects separately for different types of primes. Here, the result is that priming effects are large for spider and mushroom primes, but seem to break down for snake and flower primes. This surprising pattern is due to a strong main effect of target type, as shown in the right panel where effects are displayed separately for each type of prime picture. Clearly, spider-fearful individuals always respond rapidly whenever a spider appears as a target, even if it is inconsistent to the prime. This behavior augments priming effects when primes are response-consistent with the spider (i.e., when primes are spiders or mushrooms), but reduces priming effects when primes are response-inconsistent with the spider (i.e., when primes are snakes or flowers), thus explaining the data pattern in the left panel. Again, this response pattern does not occur in snake-fearful or control participants. One reason for these qualitative difference between the two phobias might be that Germans are much more likely to encounter a wildlife spider than a wildlife snake in everyday life.
Various authors (e.g., Anderson and Phelps, 2001; Phelps et al., 2006; Tamietto and de Gelder, 2010) have proposed that accelerated response times to aversive or phobic stimuli are due to an activation of the limbic system, more precisely to attentional modulation induced by the amygdala. Specifically, the perception of emotionally significant stimuli is supposed to be enhanced by the deployment of initial attention to them, which in turn would lead to increases in performance (Anderson and Phelps, 2001). This hypothesis requires the amygdala to have extraordinary processing abilities, including the ability to classify objects, to outpace other cortical processing routes, and to modulate those processing routes before they finish processing the object. However, our data rule out that enhanced priming is caused by an emotional response in the ongoing trial because spider-fearful and snake-fearful individuals were virtually identical in their reported fear levels but showed marked differences in their processing of phobic stimuli.
In our opinion, it seems more plausible to assume a processing advantage for phobic material due to life-long perceptual learning rather than an ultra-fast attentional modulation by the amygdala. VanRullen (2009) suggests the possibility of “hard-wired” binding of features to which a person is frequently exposed, i.e., an object recognition process that can be accomplished in a feedforward fashion. Extending this view to emotional stimuli, we propose that emotionally significant stimuli are not processed faster because emotional arousal speeds on-line perceptual processes (Öhman et al., 2001), but because emotion promotes perceptual learning that speeds processing at later times, e.g., by establishing hard-wired binding (VanRullen, 2009).
Dissociating Conscious and Preconscious Brightness Processing
The subjective perception of lightness and brightness is the final outcome of a complex processing system that finally leads to an integrated representation of the visual stimuli in conscious vision. Typical psychophysical judgments are based on this conscious representation; examples include judgments of lightness (the subjectively perceived reflectance of a surface) and brightness (the subjectively perceived luminance of a surface). An intriguing property of this representation is lightness constancy, the ability to perceive invariant surface reflectance despite changes in illumination. Lightness constancy demands the extraction of three-dimensional scenic layout from the two-dimensional optic array. It requires the correct assessment of complex information about oriented surfaces, light sources, light and shadow contours, and transparency. Theories of lightness processing agree that this is a multistage process starting from a raw material of local image contrasts (Adelson, 1993; Gilchrist et al., 1999; Li and Gilchrist, 1999; Gilchrist, 2006)4.
Rapid-chase theory suggests that rapid motor measures of perception are based on the first feedforward sweep of image processing. If lightness constancy is the final outcome of elaborate processing (probably involving binding by widespread recurrent processing), is it possible that visuomotor measures of lightness and brightness might tap into earlier representations, maybe those still devoid of lightness constancy? To address this question, we used response priming to compare visuomotor measures of processing with conscious brightness judgments. Our major question was whether visual brightness illusions affect response priming effects in the same way that they affect conscious vision. Alternatively, are there qualitative differences of illusion effects on priming and awareness?
We employed brightness illusions (cf. Adelson, 2000) based on illumination effects (Schmidt et al., 2010) or transparency effects (Weber and Schmidt, in preparation). Stimuli from Schmidt et al. (2010) are shown in Figure 5A. In each trial, a folded plane was presented that was apparently illuminated from the left or right, creating light and shadow zones. Two target patches (one with high-luminance, “bright,” and one with low-luminance, “dark”) were presented in the mid-segment of the plane, preceded by two flanker primes (one bright and one dark) with the same two physical luminance values as the targets (Figure 5B). The flankers’ spatial arrangement could be consistent with the targets’ (e.g., bright flanker on the same side as the bright target) or inconsistent (spatially switched). The twist of the experiment is that flankers are subject to a visual illusion: if the bright flanker is placed on the lighted segment of the plane and the dark flanker on the shadow segment (attenuation condition), the flankers appear more similar than when placed on a neutral background. Conversely, if the bright flanker is placed on the shadow segment and the dark flanker on the lighted segment (enhancement conditions), the flankers appear more dissimilar than under neutral conditions (Figure 5A).
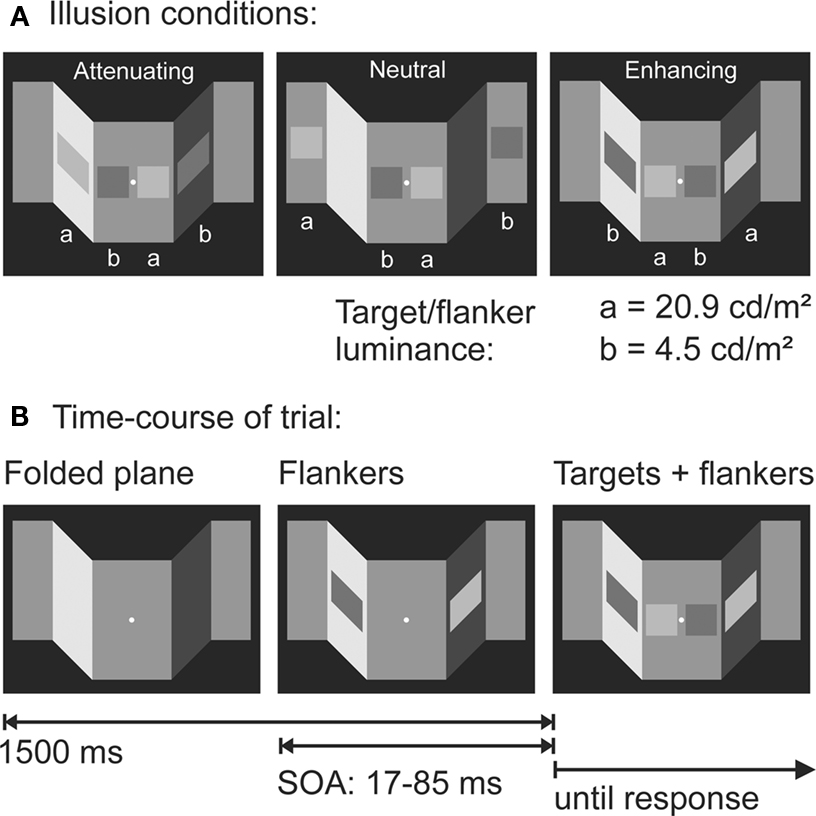
Figure 5. Paradigm from Schmidt et al. (2010). (A) Stimulus examples. Note that target and flanker pairs have the exact same two luminance levels. (B) Time course of a trial. Note that all stimuli remain on screen until the response is complete.
To compare the effects of this illusion on response priming and visual awareness, we designed two tasks. In the priming task, participants had to respond to the target patches presented in the mid-segment of the plane by pressing one of two response keys on the side where the brighter (more luminant) of the two targets was presented. This task was designed to measure rapid visuomotor priming effects from the flankers. In the matching task, we asked participants to adjust the luminance of the targets until their perceived brightness matched that of the adjacent flankers. This task was designed to measure the illusion effect in visual awareness. Carefully note that participants matched the brightness, not the lightness of the flankers: they were not instructed to discard the effect of illuminance when matching the flankers, but to produce a match where targets and flankers looked equally bright.
As expected, the matching task confirmed the effect of the illusion (Figure 6, upper panel). In the enhancing condition, the bright flanker was judged too bright, and the dark flanker was judged too dark, while in the attenuating condition, the bright flanker was judged too dark, and the dark flanker was judged too bright. (Matching was nearly veridical in the neutral condition). Importantly, the more luminant flanker was always judged brighter than the less luminant flanker, and the illusion never led to a reversal of the perceived brightnesses.
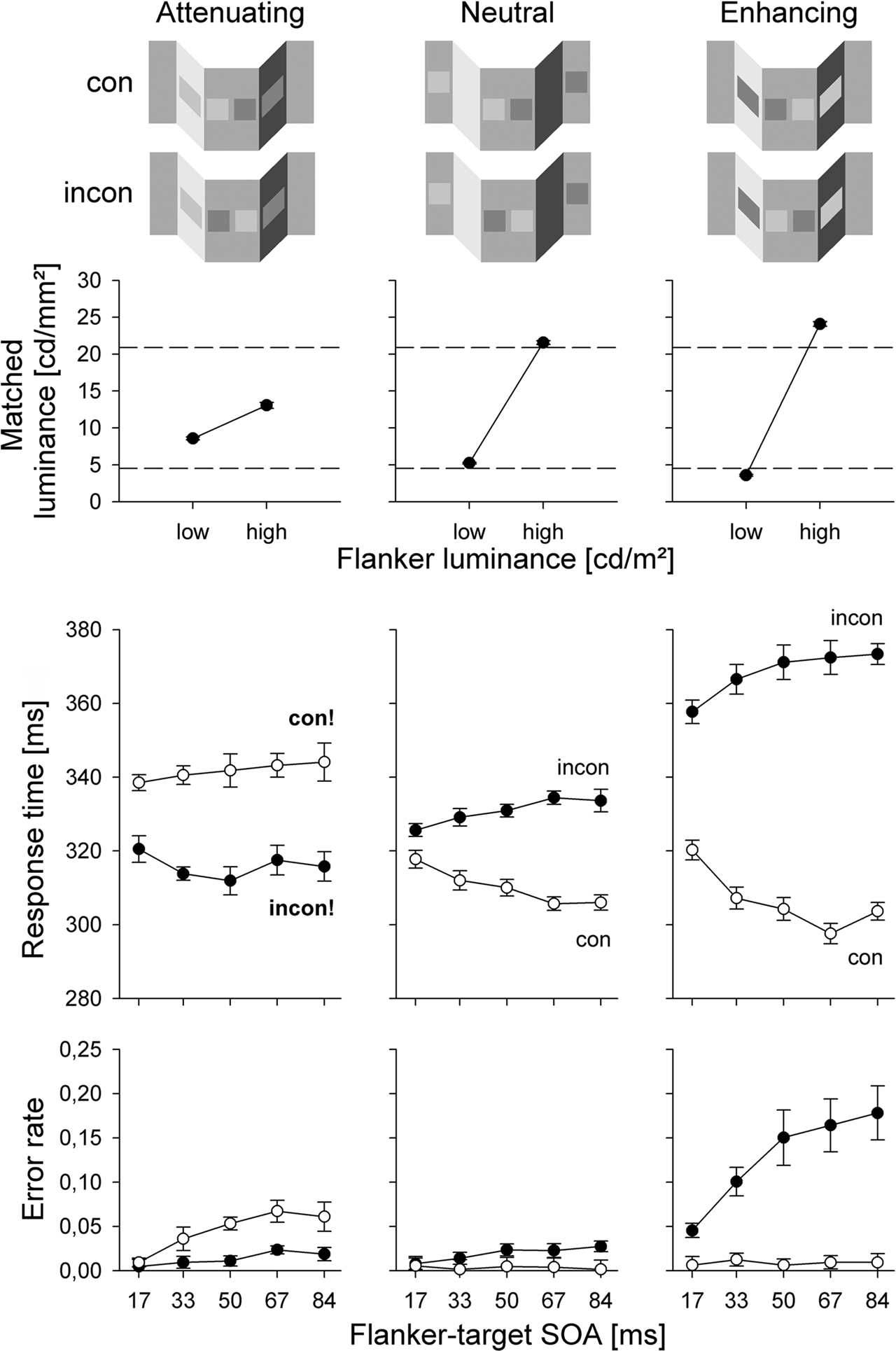
Figure 6. Results from Schmidt et al. (2010). Upper panel: results of the matching task where participants matched the brightness of the targets to the brightness of the adjacent flankers. Stippled lines denote veridical values. Lower panels: priming effects. Note the reversal in priming for response times and error rates under the attenuation condition.
Strikingly, the priming task revealed a qualitatively different pattern (Figure 6, lower panels). In the neutral condition, the standard response priming effect was observed in response times and error rates. In the enhancing condition, priming effects were much larger, which was also not surprising. But in the attenuation condition, priming effects unexpectedly reversed, such that responses in inconsistent trials became faster than those in consistent trials (a similar reversal occurred in the error rates). Together, the two tasks indicate that even though one flanker appeared brighter than the other in conscious vision, it primed responses as if it was darker (and vice versa). This constitutes a double dissociation between priming and awareness where an experimental manipulation leads to opposite effects on both measures (Schmidt and Vorberg, 2006).
How can this dissociation be explained? In an extensive follow-up experiment, we showed that the priming effect strictly depends on the flankers’ local contrast values (Schmidt et al., 2010). In fact, the reversal occurs precisely in those conditions where the physically brighter flanker becomes a locally dark stimulus because it appears against a brighter background (and vice versa for the physically darker flanker). As long as the physically brighter flanker remains more luminant than the immediate background, it continues to activate responses as expected from a bright flanker. However, in the attenuation condition, the brighter flanker is darker than the immediate background, and so it activates responses as if it was dark (again, vice versa for the physically darker flanker). In other words, the sign switch in priming under attenuation conditions is due to a sign switch in local contrast. Brightness judgments in the matching task, even though strongly affected by the illusion, are robust against the switch in local contrast sign and thus dissociate from the priming effects.
These effects can be extended to transparency illusions (Weber and Schmidt, in preparation). Figure 7 shows examples where the central targets are flanked by stimuli that seem to be partly occluded by transparent surfaces. By varying the luminance and transparency of those occluders, conditions can be created where the flanker’s local contrast switches sign so that the physically brighter flanker becomes a locally dark stimulus (and vice versa). As soon as this happens (here, only in the “Attenuating 2” condition), priming effects switch sign as well; they remain regular in all the remaining conditions where the flankers’ local contrast signs remain unchanged (Figure 7, lower panels). At the same time, Figure 7 (upper panel) shows that in all conditions tested, including the critical condition with reversed priming, the physically more luminant flanker is judged brighter than the less luminant flanker, establishing the double dissociation between priming and visual awareness.
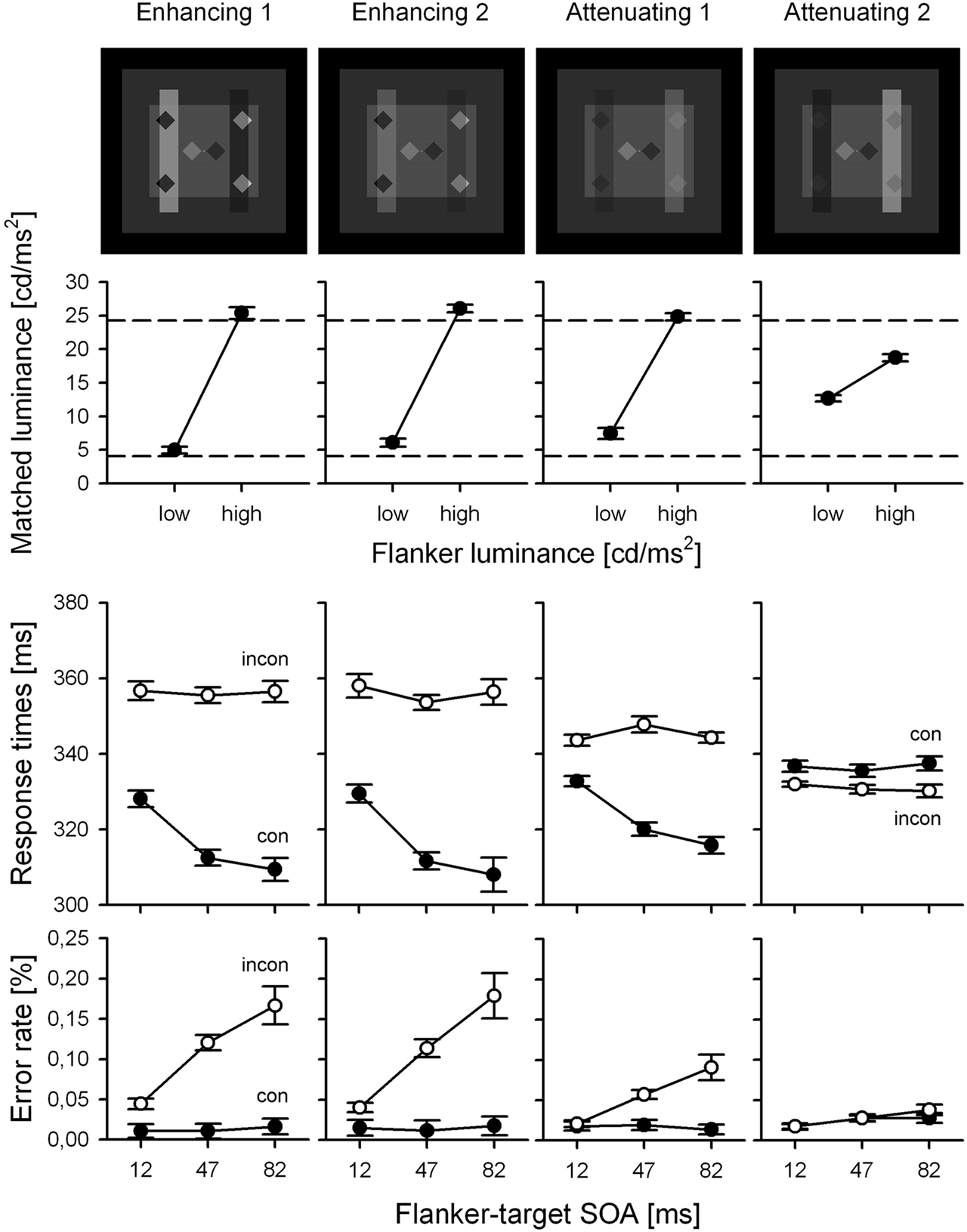
Figure 7. Paradigm and results from Weber and Schmidt (in preparation). Conventions same as in Figure 6. Again, note the reversal in priming under the “Attenuation 2” condition.
This dissociation suggests that the two measures are based on qualitatively different stimulus representations: visual awareness makes use of a highly integrated three-dimensional reconstruction of the visual scene that achieves some degree of lightness/brightness constancy, whereas the representation underlying rapid response activation uses more basic (or more primitive) information, only encoding local contrast values (for a similar but slightly more radical view involving response priming by color stimuli, see Breitmeyer et al., 2004a,b). Rapid-chase theory’s explanation of the dissociation is that response priming is based on fast feedforward processing while lightness constancy requires more time-consuming recurrent processing. Viewed this way, response priming provides a window into preconscious visual processing because it is driven by comparatively early output of the feedforward cascade. This might also hold for other fast reaction-time tasks, such as visual search (Treisman and Gelade, 1980). For instance, in a study by Moore and Brown (2001), visual search for high-luminance and low-luminance targets was performed under conditions in which parts of the display seemed to be viewed through a dark transparent filter. Because visual search was dominated by raw luminance rather than perceived lightness of the targets, the authors concluded that visual search had been initiated before scene processing and lightness constancy were complete5.
Conclusion
Binding problems arise at all stages of human perception – from the extraction of basic visual features to mid-level processes like figure–ground segregation and grouping, and finally to high-level object and scene perception. Different binding tasks are probably solved by different mechanisms: while contour assignment and visual search for conjunction targets might require extensive recurrent processing and selective attention (Roelfsema, 2006), basic object classification tasks might be based on feedforward processes converging on high-level object classifiers (Thorpe et al., 1996). Here, we advocate the idea that visuomotor measures of perception can give new insights into the temporal dynamics of visual processing, and can be employed to identify cases where visuomotor processing can plausibly be attributed to feedforward processing. Most importantly, we maintain that visuomotor measures like response priming can be used to dissociate preconscious and conscious visual processing.
In assessing the dynamics of visuomotor processing, response priming effects can be employed in different ways, some more descriptive, others more theoretically driven. These include the following uses: (1) employing priming effects as indicators that a feature can activate rapid responses; (2) comparing priming functions for different features or attentional states to assess their relative processing dynamics; (3) employing response priming as a measure of feedforward processing, using the rapid-chase criteria; (4) establishing qualitative dissociations between response priming and visual awareness.
Among those four uses of response priming, the first two are largely descriptive and assumption-free. Just establishing a large and rapid response priming effect for a given stimulus feature demonstrates that this feature is able to activate fast motor responses. A next step is to compare two or more time courses of priming: for instance, priming functions can be used to demonstrate faster image processing in phobic images as compared to neutral images (Haberkamp et al., in preparation), or priming effects in pointing trajectories can be examined to compare different states of attention (Schmidt and Seydell, 2008; Schmidt and Schmidt, 2010). This way, the entire priming function can be regarded as a dependent measure for the processing dynamics of the system.
Beyond such descriptive uses, the paradigm becomes theoretically interesting under the assumption that response priming largely reflects the first output of a feedforward processing cascade. An essential requirement for this is to establish that response priming by a specific feature obeys the rapid-chase criteria (Schmidt et al., 2006). For example, we argued above that the classification of natural images into animals or non-animals meets the initiation, takeover, and independence criteria of rapid-chase theory, as does the classification of simple geometric and color stimuli. In contrast to that, classification of objects as “large” or “small” fails those criteria (Schmidt and Schmidt, 2009). When the rapid-chase criteria are met, the system is behaviorally equivalent to a feedforward system where primes and targets control the motor response in strict sequence at high temporal resolution. It is a fascinating and useful working hypothesis to assume that a rapid-chase system in fact is a feedforward system whose initial motor output is still unaffected by recurrent processing. Such a system’s initial motor output would reflect the results of first-pass processing of primes and targets, i.e., the earliest output based on only a single feedforward sweep through the visuomotor system.
Viewing response priming effects as first-pass output of a feedforward system allows us to connect response priming to Lamme and Roelfsema’s (2000) proposal that visual awareness requires recurrent processing. If this is true, it implies that first-pass processing is preconscious, in the sense that it precedes visual awareness (cf. Dehaene and Naccache, 2001; Dehaene, 2009). Indeed, the most fascinating feature of response priming is its independence of visual awareness, as best shown by qualitatively different time courses of priming and masking (Mattler, 2003; Vorberg et al., 2003; Albrecht et al., 2010). In fact, rapid-chase theory predicts that response priming effects (at least, effects satisfying the rapid-chase criteria) are always based on preconscious processing, regardless of whether or how the stimuli are later represented in visual awareness. A case in point is the dissociation between psychophysical judgments of flanker brightness and the associated priming effects induced by those flankers (Schmidt et al., 2010; Weber and Schmidt, in preparation). This is clearly an example of a double dissociation between response priming and visual awareness (Schmidt and Vorberg, 2006), but one that occurs despite the fact that all stimuli are clearly visible and remain on screen until the participant has responded. Therefore, the dissociation solely relies on the different time courses of rapid visuomotor activation (the results of first-pass processing) and visual awareness (the result of widespread recurrent processing).
The fundamental distinction between processing based on rapid feedforward cascades and processing based on elaborate recurrent mechanisms raises important new questions about the methodology of behavioral neuroscience in general, especially when it comes to processes of human “mid-level vision” (Hegdé, 2008) like image segmentation, grouping, and binding. We propose that a fundamental distinction should be made between “slow psychophysics,” where participants report the final outcome of processing in visual awareness, and “fast psychophysics,” which are based on reaction-time measures or other indicators of processing speed (Schmidt et al., 2010). Traditionally, both types of measures have been used to tackle similar problems, and are generally expected to lead to consistent results. Even though “slow” psychophysics has had great success in disentangling surprisingly early mechanisms of visual processing (in the case of color vision, right down to different levels of retinal processing), one has to be aware of the fact that slow measures will often be remote from early processing. On occasion, fast psychophysical measures may contradict slow ones by being based on qualitatively different stimulus representations – e.g., the conscious perception of a stimulus measured by slow psychophysics might be affected by masking or lightness constancy, while fast psychophysical measures like response priming may be unaffected by such factors. Being aware of potential differences between traditional slow psychophysics and fast motor measures of processing holds great promise for discovering qualitative differences between conscious and unconscious vision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the German Research Foundation, grant Schm1671-1 to Thomas Schmidt. We thank our student assistants Joline Jochum, Peter Kohl, and Lisa Reiner, for collecting data, and Dirk Vorberg, Steven Scholte, and Rufin VanRullen for helpful discussions.
Footnotes
- ^This holds true as long as visual awareness of the prime is manipulated only by changing the degree of visual masking while leaving the prime stimulus physically intact. Altering the prime stimulus itself of course alters the priming effect (see Schmidt et al., in revision, for further discussion of “Dos and Don’ts in Response Priming Research”).
- ^As analyzed in detail by Schmidt and Vorberg (2006), a double dissociation occurs if an experimental manipulation leads to opposite effects in two measures, for instance, a direct measure of visual awareness (e.g., prime identification performance) and an indirect measure of prime processing per se (e.g., a priming effect). Let Ii(ci, ui) and Di(ci, ui) be indirect and direct measures in experimental condition i, such that both are functions of conscious (c) as well as unconscious (u) sources of visual information. A double dissociation is observed when for two experimental conditions i and j, Ii(ci, ui) > Ij(cj, uj) while Di(ci, ui) < Dj(cj, uj), or vice versa. Schmidt and Vorberg (2006) show that a double dissociation implies non-zero unconscious information (u > 0) in at least one experimental condition. Measurement assumptions required for this conclusion are much weaker than those required for demonstrating zero sensitivity in the direct task.
- ^A corollary of these criteria is that priming effects should be fully present in the fastest motor responses, for example, in the fastest deciles of the response time distributions from consistent and inconsistent trials.
- ^Note that the terms “lightness” and “brightness” have a well-defined meaning only for real-world objects; they are difficult to disentangle when pictorial stimuli are involved. See Schmidt et al. (2010) for a brief discussion.
- ^This conclusion might seem to conflict with evidence that subjective lightness is represented early in the processing hierarchy, e.g., in primary visual cortex or even the lateral geniculate nucleus (Rossi et al., 1996; Rossi and Paradiso, 1999; MacEvoy and Paradiso, 2001; for neural mechanisms of transparency processing, see Qiu and von der Heydt, 2007). However, those studies used a simultaneous contrast illusion, which is not well understood at present (Gilchrist, 2006), and the methods employed are not suited to distinguish between activation by feedforward or re-entrant signals.
References
Adelson, E. H. (2000). “Lightness perception and lightness illusions,” in The New Cognitive Neurosciences, 2nd Edn, ed. M. S. Gazzaniga (Cambridge, MA: MIT Press), 339–351.
Albrecht, T., KlapÖtke, S., and Mattler, U. (2010). Individual differences in metacontrast masking are enhanced by perceptual learning. Conscious. Cogn. 19 656–666.
Allport, D. A. (1989). “Visual attention,” in Foundations of Cognitive Science, ed. M. I. Posner (Cambridge, MA: MIT Press), 631–682.
Anderson, A. K., and Phelps, E. A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411 305–309.
Bacon-Macé, N., Kirchner, H., Fabre-Thorpe, M., and Thorpe, S. J. (2007). Effects of task requirements on rapid natural scene processing: from common sensory encoding to distinct decisional mechanisms. J. Exp. Psychol. Hum. Percept. Perform. 33 1013–1026.
Bacon-Macé, N., Macé, M. J. M., Fabre-Thorpe, M., and Thorpe, S. J. (2005). The time course of visual processing: backward masking and natural scene categorisation. Vision Res. 45 1459–1469.
Barlow, H. B. (1972). Single units and sensation: a neuron doctrine for perceptual psychology? Perception 1, 371–394.
Breitmeyer, B. G., Öğmen, H., and Chen, J. (2004a). Unconscious priming by color and form: different processes and levels. Conscious. Cogn. 13 138–157.
Breitmeyer, B. G., Ro, T., and Singhal, N. S. (2004b). Unconscious color priming occurs at stimulus- not percept-dependent levels of processing. Psychol. Sci. 15 198–202.
Brenner, E., and Smeets, J. B. J. (2004). Color vision can contribute to fast corrections of arm movements. Exp. Brain Res. 158 302–307.
Bullier, J. (2004). “Communications between cortical areas of the visual system,” in The Visual Neurosciences, Vol. 1, eds L. M. Chalupa and J. S. Werner (Cambridge, MA: MIT Press), 522–540.
Chen, C.-M., Lakatos, P., Shah, A. S., Mehta, A. D., Givre, S. D., Javitt, D. C., and Schroeder, C. E. (2007). Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb. Cortex 17 1561–1569.
Cousineau, D. (2005). Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorials Quant. Methods Psychol. 1 42–46.
DeCharms, R. C., and Zador, A. (2000). Neural representation and the cortical code. Annu. Rev. Neurosci. 23 613–647.
Dehaene, S. (2009). Conscious and nonconscious processes: distinct forms of evidence accumulation? Séminaire Poincaré 12, 89–114.
Dehaene, S., and Naccache, L. (2001). Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition 79 1–37.
DiLollo, V., Enns, J. T., and Rensink, R. A. (2000). Competition for consciousness among visual events: the psychophysics of reentrant visual processes. J. Exp. Psychol. Gen. 129 481–507.
Eimer, M., and Schlaghecken, F. (1998). Effects of masked stimuli on motor activation: behavioral and electrophysiological evidence. J. Exp. Psychol. Hum. Percept. Perform. 24 1737–1747.
Fahrenfort, J. J., Scholte, H. S., and Lamme, V. A. F. (2007). Masking disrupts reentrant processing in human visual cortex. J. Cogn. Neurosci. 19, 1488–1497.
Fox, E., Griggs, L., and Mouchlianitis, E. (2007). The detection of fear-relevant stimuli: are guns noticed as quickly as snakes? Emotion 7, 691–696.
Geusebroek, J. M., Burghouts, G. J., and Smeulders, A. W. M. (2005). The Amsterdam library of object images. Int. J. Comput. Vis. 61 103–112.
Gilbert, C. D., and Sigman, M. (2007). Brain states: top-down influences in sensory processing. Neuron 54 677–696.
Gilchrist, A., Kossyfidis, C., Bonato, F., Agostini, T., Cataliotti, J., Li, X., Spehar, B., Annan, V., and Economou, E. (1999). An anchoring theory of lightness perception. Psychol. Rev. 106 795–834.
Gray, C. M. (1999). The temporal correlation hypothesis of visual feature integration: still alive and well. Neuron 24 31–47.
Gray, C. M., and Singer, W. (1989). Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. U.S.A. 86 1698–1702.
Haynes, J.-D., Driver, J., and Rees, G. (2005). Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron 46 811–821.
Hegdé, J. (2008). Time course of visual perception: coarse-to-fine processing and beyond. Prog. Neurobiol. 84 405–439.
Hubel, D. H., and Livingstone, M. S. (1987). Segregation of form, color, and stereopsis in primate area 18. J. Neurosci. 7 3378–3415.
Hung, C. P., Kreiman, G., Poggio, T., and DiCarlo, J. J. (2005). Fast readout of object identity from macaque inferior temporal cortex. Science 310 863–866.
Kanai, R., Tsuchiya, N., and Verstraten, F. (2006). The scope and limits of top-down attention in unconscious visual processing. Curr. Biol. 16 2332–2336.
Kentridge, R. W., Nijboer, T. C. W., and Heywood, C. A. (2008). Attended but unseen: visual attention is not sufficient for visual awareness. Neuropsychologia 46 864–869.
Kirchner, H., and Thorpe, S. J. (2006). Ultra-rapid object detection with saccadic eye movements: visual processing speed revisited. Vision Res. 46, 1762–1776.
Klotz, W., Heumann, M., Ansorge, U., and Neumann, O. (2007). Electrophysiological activation by masked primes: independence of prime-related and target-related activities. Adv. Cogn. Psychol. 3 449–465.
Klotz, W., and Neumann, O. (1999). Motor activation without conscious discrimination in metacontrast masking. J. Exp. Psychol. Hum. Percept. Perform. 25 976–992.
Klotz, W., and Wolff, P. (1995). The effect of a masked stimulus on the response to the masking stimulus. Psychol. Res. 58 92–101.
Koch, C., and Tsuchiya, N. (2007). Attention and consciousness: two distinct brain processes. Trends Cogn. Sci. (Regul. Ed.) 11 16–22.
Lamme, V. A. F. (2003). Why visual awareness and attention are different. Trends Cogn. Sci. (Regul. Ed.) 7, 12–18.
Lamme, V. A. F. (2005). “The difference between visual attention and awareness: a cognitive neuroscience perspective,” in Neurobiology of Attention eds L. Itti, G. Rees, and J. K. Tsotsos (San Diego: Elsevier Academic Press), 167–174.
Lamme, V. A. F. (2006). Towards a true neural stance on consciousness. Trends Cogn. Sci. (Regul. Ed.) 10 494–501.
Lamme, V. A. F., Rodriguez-Rodriguez, V., and Spekreijse, H. (1999). Separate processing dynamics for texture elements, boundaries and surfaces in primary visual cortex of the macaque monkey. Cereb. Cortex 9 406–413.
Lamme, V. A. F., and Roelfsema, P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23 571–579.
Lamme, V. A. F., Zipser, K., and Spekreijse, H. (2002). Masking interrupts figure-ground signals in V1. J. Cogn. Neurosci. 14 1044–1053.
Leuthold, H., and Kopp, B. (1998). Mechanisms of priming by masked stimuli: inferences from event-related brain potentials. Psychol. Sci. 9 263–269.
Li, X., and Gilchrist, A. L. (1999). Relative area and relative luminance combine to anchor surface lightness values. Percept. Psychophys. 61 771–785.
Livingstone, M. S., and Hubel, D. H. (1987). Connections between layer 4B and the thick cytochrome oxidase stripes of area 18 in the squirrel monkey. J. Neurosci. 7 3371–3377.
MacEvoy, S. P., and Paradiso, M. A. (2001). Lightness constancy in primary visual cortex. Proc. Natl. Acad. Sci. U.S.A. 98 8827–8831.
Macknik, S. L., and Haglund, M. M. (1999). Optical images of visible and invisible percepts in the primary visual cortex of primates. Proc. Natl. Acad. Sci. U.S.A. 96 15208–15210.
Macknik, S. L., and Livingstone, M. S. (1998). Neuronal correlates of visibility and invisibility in the primate visual system. Nat. Neurosci. 1 144–149.
Mathews, A., and MacLeod, C. (2005). Cognitive vulnerability to emotional disorders. Annu. Rev. Clin. Psychol. 1 167–195.
Mattler, U. (2003). Priming of mental operations by masked stimuli. Percept. Psychophys. 65 167–187.
Melcher, D., Papathomas, T. V., and Vidnyánszky, Z. (2005). Implicit attentional selection of bound visual features. Neuron 46, 723–729.
Merigan, W. H., and Maunsell, J. H. R. (1993). How parallel are the primate visual pathways? Annu. Rev. Neurosci. 16, 369–402.
Mogg, K., and Bradley, B. P. (2006). Time course of attentional bias for fear-relevant pictures in spider-fearful individuals. Behav. Res. Ther. 44 1241–1250.
Moore, C. M., and Brown, L. E. (2001). Preconstancy information can influence visual search: the case of lightness constancy. J. Exp. Psychol. Hum. Percept. Perform. 27 178–194.
Neumann, O. (1990). Direct parameter specification and the concept of perception. Psychol. Res. 52 207–215.
Öhman, A., Flykt, A., and Esteves, F. (2001). Emotion drives attention: detecting the snake in the grass. J. Exp. Psychol. Gen. 130 466–478.
Pascual-Leone, A., and Walsh, V. (2001). Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292 510–512.
Phelps, E., Ling, S., and Carrasco, M. (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol. Sci. 17 292–299.
Qiu, F. T., and von der Heydt, R. (2007). Neural representation of transparent overlay. Nat. Neurosci. 10 283–284.
Quiroga, R. Q., Reddy, L., Kreiman, G., Koch, C., and Fried, I. (2005). Invariant visual representation by single neurons in the human brain. Nature 435 1102–1107.
Rinck, M., and Becker, E. S. (2006). Spider fearful individuals attend to threat, then quickly avoid it: evidence from eye movements. J. Abnorm. Psychol. 115 231–238.
Ro, T., Breitmeyer, B., Burton, P., Singhal, N. S., and Lane, D. (2003). Feedback contributions to visual awareness in human occipital cortex. Curr. Biol. 11 1038–1041.
Roelfsema, P. R. (2006). Cortical algorithms for perceptual grouping. Annu. Rev. Neurosci. 29 203–227.
Roelfsema, P. R., Lamme, V. A. F., Spekreijse, H., and Bosch, H. (2002). Figure-ground segregation in a recurrent network architecture. J. Cogn. Neurosci. 14 525–537.
Roland, P. E. (2010). Six principles of visual cortical dynamics. Front. Syst. Neurosci. 4:28. doi: 10.3389/fnsys.2010.00028
Rossi, A. F., and Paradiso, M. A. (1999). Neural correlates of perceived brightness in the retina, lateral geniculate nucleus, and striate cortex. J. Neurosci. 19 6145–6156.
Rossi, A. F., Rittenhouse, C. D., and Paradiso, M. A. (1996). The representation of brightness in primary visual cortex. Science 273 1104–1107.
Schmidt, F., and Schmidt, T. (2010). Feature-based attention to unconscious shapes and colors. Atten. Percept. Psychophys. 72 1480–1494.
Schmidt, T. (2002). The finger in flight: real-time motor control by visually masked color stimuli. Psychol. Sci. 13 112–118.
Schmidt, T. (2009). “The binding problem and the coherence of perception,” in Encyclopedia of Consciousness, ed. W. P. Banks (Amsterdam: Elsevier), 147–158.
Schmidt, T., Miksch, S., Bulganin, L., Jäger, F., Lossin, F., Jochum, J., and Kohl, P. (2010). Response priming driven by local contrast, not subjective brightness. Atten. Percept. Psychophys. 72 1556–1568.
Schmidt, T., Niehaus, S., and Nagel, A. (2006). Primes and targets in rapid chases: tracing sequential waves of motor activation. Behav. Neurosci. 120 1005–1016.
Schmidt, T., and Schmidt, F. (2009). Processing of natural images is feedforward: a simple behavioral test. Atten. Percept. Psychophys. 71 594–606.
Schmidt, T., and Seydell, A. (2008). Visual attention amplifies response priming of pointing movements to color targets. Percept. Psychophys. 70 443–455.
Schmidt, T., and Vorberg, D. (2006). Criteria for unconscious cognition: three types of dissociation. Percept. Psychophys. 68 489–504.
Scholte, H. S., Jolij, J., Fahrenfort, J. J., and Lamme, V. A. F. (2008). Feedforward and recurrent processing in scene segmentation: electroencephalography and functional magnetic resonance imaging. J. Cogn. Neurosci. 20, 2097–2109.
Serre, T., Oliva, A., and Poggio, T. (2007). A feedforward architecture accounts for rapid categorization. Proc. Natl. Acad. Sci. U.S.A. 104 6424–6429.
Shadlen, M. N., and Movshon, J. A. (1999). Synchrony unbound: a critical evaluation of the temporal binding hypothesis. Neuron 24 67–77.
Shin, K., Stolte, M., and Chong, S. C. (2009). The effect of spatial attention on invisible stimuli. Atten. Percept. Psychophys. 71 1507–1513.
Sillito, A. M., Cudeiro, J., and Jones, H. E. (2006). Always returning: feedback and sensory processing in visual cortex and thalamus. Trends Neurosci. 29 307–316.
Sincich, L. C., and Horton, J. C. (2005). The circuitry of V1 and V2: integration of color, form, and motion. Annu. Rev. Neurosci. 28 303–326.
Song, J.-H., and Nakayama, K. (2009). Hidden cognitive states revealed in choice reaching tasks. Trends Cogn. Sci. (Regul. Ed.) 13 360–366.
Sumner, P., Tsai, P.-C., Yu, K., and Nachev, P. (2006). Attentional modulation of sensorimotor processes in the absence of perceptual awareness. Proc. Natl. Acad. Sci. U.S.A. 103 10520–10525.
Supèr, H., and Lamme, V. A. F. (2007). Strength of figure-ground activity in monkey primary visual cortex predicts saccadic reaction time in a delayed detection task. Cereb. Cortex 17 1468–1475.
Supèr, H., Romeo, A., and Keil, M. (2010). Feed-forward segmentation of figure-ground and assignment of border-ownership. PLoS ONE 5, e10705.doi: 10.1371/journal.pone.0010705
Tamietto, M., and de Gelder, B. (2010). Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11 697–709.
Thorpe, S. J., Fize, D., and Marlot, C. (1996). Speed of processing in the human visual system. Nature 381 520–522.
Treisman, A., and Gelade, G. (1980). A feature-integration theory of attention. Cogn. Psychol. 12 97–136.
Turnbull, O. H., Carey, D. P., and McCarthy, R. A. (1997). The neuropsychology of object constancy. J. Int. Neuropsychol. Soc. 3 288–298.
VanRullen, R. (2009). Binding hardwired versus on-demand feature conjunctions. Vis. Cogn. 17 103–119.
VanRullen, R., Delorme, A., and Thorpe, S. J. (2001). Feed-forward contour integration in primary visual cortex based on asynchronous spike propagation. Neurocomputing 38–40, 1003–1009.
VanRullen, R., Gautrais, J., Delorme, A., and Thorpe, S. J. (1998). Face processing using one spike per neuron. Biol. Syst. 48 229–239.
VanRullen, R., and Koch, C. (2003). Visual selective behaviour can be triggered by a feed-forward process. J. Cogn. Neurosci. 15 209–217.
VanRullen, R., and Thorpe, S. J. (2001). Is it a bird? Is it a plane? Ultra-rapid visual categorisation of natural and artifactual objects. Perception 30 655–668.
VanRullen, R., and Thorpe, S. J. (2002). Surfing a spike wave down the ventral stream. Vision Res. 42 2593–2615.
Vath, N., and Schmidt, T. (2007). Tracing sequential waves of rapid visuomotor activation in lateralized readiness potentials. Neuroscience 145 197–208.
Verleger, R., Jaśkowski, P., Aydemir, A., van der Lubbe, R. H. J., and Groen, M. (2004). Qualitative differences between conscious and nonconscious processing? On inverse priming induced by masked arrows. J. Exp. Psychol. Gen. 133 494–515.
Vorberg, D., Mattler, U., Heinecke, A., Schmidt, T., and Schwarzbach, J. (2003). Different time courses for visual perception and action priming. Proc. Natl. Acad. Sci. U.S.A. 100 6275–6280.
Wolfe, J. M., and Cave, K. R. (1999). The psychophysical evidence for a binding problem in human vision. Neuron 24 11–17.
Yantis, S., and Serences, J. T. (2003). Cortical mechanisms of space-based and object-based attentional control. Curr. Opin. Neurobiol. 13 187–193.
Keywords: visual perception, visual awareness, response priming, rapid-chase, feature-based attention, objects, phobias, lightness
Citation: Schmidt T, Haberkamp A, Veltkamp GM, Weber A, Seydell-Greenwald A and Schmidt F (2011) Visual processing in rapid-chase systems: image processing, attention, and awareness. Front. Psychology 2:169. doi: 10.3389/fpsyg.2011.00169
Received: 02 March 2011;
Accepted: 06 July 2011;
Published online: 15 July 2011.
Edited by:
Rufin VanRullen, Centre de Recherche Cerveau et Cognition, FranceCopyright: © 2011 Schmidt, Haberkamp, Veltkamp, Weber, Seydell-Greenwald and Schmidt. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Thomas Schmidt, Faculty of Social Sciences, Psychology I, University of Kaiserslautern, Erwin-Schrödinger-Strasse, Gebäude 57, D-67633 Kaiserslautern, Germany. e-mail: thomas.schmidt@sowi.uni-kl.de
