- 1Cognitive Brain Research Unit, Institute of Behavioural Sciences, University of Helsinki, Helsinki, Finland
- 2Cicero Learning, University of Helsinki, Helsinki, Finland
Remediation programs for language-related learning deficits are urgently needed to enable equal opportunities in education. To meet this need, different training and intervention programs have been developed. Here we review, from an educational perspective, studies that have explored the neural basis of behavioral changes induced by auditory or phonological training in dyslexia, specific language impairment (SLI), and language-learning impairment (LLI). Training has been shown to induce plastic changes in deficient neural networks. In dyslexia, these include, most consistently, increased or normalized activation of previously hypoactive inferior frontal and occipito-temporal areas. In SLI and LLI, studies have shown the strengthening of previously weak auditory brain responses as a result of training. The combination of behavioral and brain measures of remedial gains has potential to increase the understanding of the causes of language-related deficits, which may help to target remedial interventions more accurately to the core problem.
Introduction
Finding the most effective techniques to remediate language-related impairments, such as dyslexia, specific language impairment (SLI), or language-learning impairment (LLI, cf. Tallal, 2001), would be of crucial importance to educators, who try to help children struggling with these learning difficulties. This raises a question, whether understanding the neurobiological underpinnings of language impairments facilitates their efficient treatment. In this review, we discuss how neuroscience illuminates the effects of auditory or phonological intervention on dyslexia, SLI, and LLI. We focus on auditory or phonological interventions, because in many cases dyslexia, SLI, and LLI are all characterized by phonological (or auditory) deficits (Tallal, 2001; Shaywitz and Shaywitz, 2005; Pennington and Bishop, 2009; Ramus et al., 2013), despite their complex etiology. Whereas detailed brain areas influenced by reading interventions can be found in a recent meta-analysis by Barquero et al. (2014), here we address whether neuroscientific research on the remediation of language-related deficits is useful for educators and whether it has something to add over behavioral research from an educational perspective.
In the current review, the selection of publications was based on the following criteria: the research should concern dyslexia, SLI, or LLI, include testing before and after an auditory or phonological intervention or training, involve brain research measures [(functional) magnetic resonance imaging (MRI/fMRI), magnetic source imaging (MSI) or magnetoencephalography (MEG), event-related potentials (ERP), or electroencephalography (EEG)], and compare two or more groups of participants to control for the effects of repeated testing and maturation (McArthur, 2009). Searches from Web of Science and PubMed (keywords dyslexia/SLI/LLI, intervention/remediation/training, fMRI/MEG/ERP) were used in finding literature. Additional publications were found in the reference lists of relevant studies.
Is Neuroscientific Research Useful for Educators?
Research on remedial interventions for learning deficits may have important applicability to education (Tallal, 2012). In this area, collaboration between education and neuroscience could result in mutual benefits (Sigman et al., 2014). However, the value of the neuroscientific approach in such research has been questioned by Bishop (2013) because of methodological and interpretive reasons. She argued that neuroscientific studies often use small subject groups, which may decrease their reliability and result in small statistical power (cf. Button et al., 2013). Furthermore, Bishop (2013) noted that some studies lack an adequate control group, which is important to control for the effects of repeated testing and maturation (see also McArthur, 2009). Indeed, future intervention studies should not only aim at having larger subject groups (Bishop, 2013) and adequate control groups (McArthur, 2009; Bishop, 2013), but also control for placebo effects (Boot et al., 2013).
Bishop (2013) also argued that the critical test of the effectiveness of interventions is the change of behavior rather than that of brain function; changes in the brain should not be considered more important than changes in behavior. However, rather than emphasizing the brain over behavior, neuroscientific intervention studies typically aim to determine the links between brain function and behavior. Importantly, understanding the link or correlation between brain activation and skills as a result of training may help to explain how and why remedial gains take place. Since the combination of neuroscientific and behavioral measures has been shown to be a better predictor of reading skills than behavioral measures alone (Hoeft et al., 2007; Maurer et al., 2009), this combination has potential to outperform mere behavioral measures in the study of remedial gains. Cognitive neuroscience has, in our opinion, also some advantages over behavioral research that were not mentioned by Bishop (2013). Especially when working with children whose motivation and skills can affect their performance considerably, a possibility to study the effects of intervention without subject’s active effort or attention is a clear advantage. This is possible, for example, by recording mismatch negativity (MMN) brain response (Näätänen et al., 2007; Kujala and Näätänen, 2010).
From educators’ perspective, neuroscientific research is seldom directly applicable in the assessment of remedial interventions. Importantly, however, educators may benefit from neuroscientific research by obtaining a more detailed picture of relevant processes underlying behavior. For example, brain measures may help to disentangle whether behaviorally observed improvement is due to the normalization of the core deficit or some compensatory strategy (e.g., Eden et al., 2004; Shaywitz et al., 2004), which is not evident in behavioral data. If, hypothetically, some intervention resulted in the formation of a compensatory function to solve some task, it may improve behavior to a certain degree but might not compete in effectiveness with the optimal function for solving that task. Still, in a large subject group, this compensatory improvement in behavior may be taken to reflect a successful intervention, if statistically significant improvement is achieved. Thus, neuroscientific research can potentially give some valuable information to educators about the deficits, which may help to target the contents of interventions more accurately.
Overview of Studies on Neurobiological Changes Following Phonological or Auditory Interventions
As shown by Tables 1 and 2, the majority of studies on phonological or auditory interventions focused on dyslexia or related problems in reading, writing, or spelling. Furthermore, the majority of studies have focused on children. Older age groups should not be neglected in remediation and its research, however: as noted by Eden et al. (2004), most dyslexics are adults, who may suffer from the socio-economic consequences of their reading deficit. There seem to be no constraints with respect to brain plasticity that would hinder remediation in adults or older children (Simos et al., 2002; Eden et al., 2004). Nevertheless, the earlier the interventions are conducted, the more benefit to individuals is gained, because learning is cumulative. The early gains may help to prevent difficulties not only in academic but socio-emotional domain. The optimal timing of intervention is, however, determined by maturity and acquired skills. For example, if a new skill is scaffolded by previous skills, it cannot be adapted before they are mastered (cf. Jolles and Crone, 2012).

TABLE 1. Publications including neuroscientific research on phonological or auditory remediation of dyslexia (or its risk).
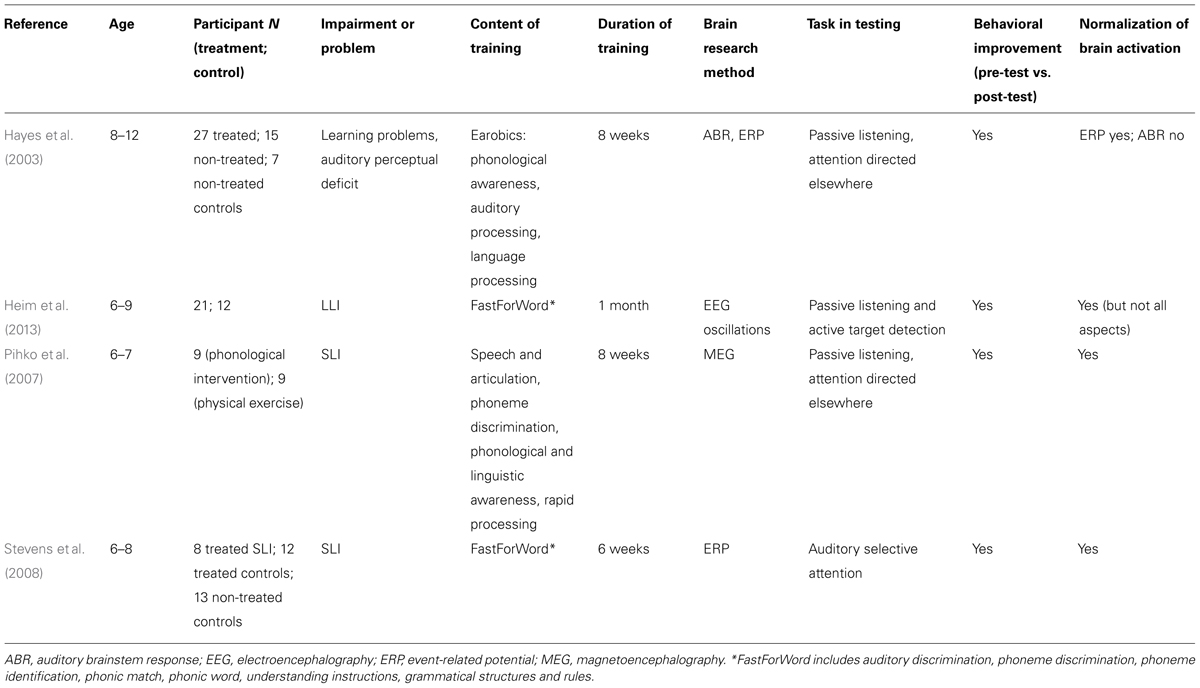
TABLE 2. Publications including neuroscientific research on phonological or auditory remediation of specific language impairment (SLI) or language-learning impairment (LLI).
The studies listed in Tables 1 and 2 suggest that in addition to behavior, the remedial gains of phonological or auditory interventions are consistently reflected in different aspects of brain functioning. These include increased or normalized brain activation as a result of training in previously hypoactive areas as measured with fMRI (Aylward et al., 2003; Temple et al., 2003; Eden et al., 2004; Shaywitz et al., 2004; Gaab et al., 2007; Meyler et al., 2008; Heim et al., 2014) and MSI or MEG (Simos et al., 2002; Pihko et al., 2007) during different cognitive tasks. MRI-based proton MR spectroscopy has shown normalized metabolism in certain brain areas after interventions (Richards et al., 2000, 2002). Training-induced changes in strength and timing of neural responses to stimulation have been demonstrated with ERPs (Kujala et al., 2001; Hayes et al., 2003; Stevens et al., 2008, 2013; Jucla et al., 2010; Lovio et al., 2012; Hasko et al., 2014). Also the time-frequency analysis of EEG has revealed amplitude increases in the oscillatory brain activity after training (Heim et al., 2013). In addition to brain function, interventions have been found to change brain anatomy, such as white matter integrity (Keller and Just, 2009). Tables 1 and 2 also show that remedial gains, if any, consistently manifest in both behavioral and brain measures: in 16 out of 17 studies of Table 1 and in all four studies of Table 2, remedial gains were found in both brain activation and skills targeted by intervention (note that Jucla et al., 2010, failed to find different behavioral improvement and similar brain response patterns between their treatment group and controls). The strong coupling of training gains in behavior and brain activation suggests that most likely the observed changes in the brain drive the changes in the behavior. As neuroscientific research may reveal the neural dynamics of processes related to behavioral performance and allows localize the deficient brain functions, it may enable to specify the neural mechanisms underlying language-related impairments and to determine brain functions and areas altered by interventions, which surface in behavior as improved skills. However, it is noteworthy that Tables 1 and 2 lists published studies, whereas studies failing to find changes in behavior or brain activation may remain unpublished. This may cause bias toward systematically finding the coupling between neural and behavioral gains.
A recent meta-analysis of neuroscientific research exploring reading networks in the brain has suggested that dyslexia is characterized by the dysfunction of left occipito-temporal cortex, left inferior frontal gyrus, and the inferior parietal lobule (Richlan, 2012; see also Richlan et al., 2011). These brain areas are involved in phonological encoding, phonological representations, and attention, respectively (Richlan, 2012). Barquero et al.’s (2014) meta-analysis of the neuroimaging of reading interventions, in turn, suggests intervention-induced functional changes in the left thalamus, left middle occipital gyri, bilateral inferior frontal gyri, right insula, and right posterior cingulate gyrus. Thus, both Richlan’s (2012) and Barquero et al.’s (2014) findings point toward the central role of inferior frontal and occipito-temporal/occipital dysfunction in dyslexia. Correspondingly, the neuroscientific dyslexia studies included in Table 1, involving auditory or phonological intervention, have shown normalized brain activation, metabolism, or anatomy as a result of interventions in the occipito-temporal (Aylward et al., 2003; Heim et al., 2014) and inferior frontal (Richards et al., 2000, 2002; Aylward et al., 2003; Shaywitz et al., 2004; Heim et al., 2014) areas. In addition, normalized activation following interventions has been repeatedly observed in inferior parietal (Temple et al., 2003; Eden et al., 2004; Meyler et al., 2008, see also Richlan, 2012), superior parietal (Aylward et al., 2003; Eden et al., 2004; Meyler et al., 2008), and temporal (Simos et al., 2002; Aylward et al., 2003; Temple et al., 2003; Shaywitz et al., 2004) areas. Although inferior frontal and occipito-temporal/occipital dysfunctions, linked with phonological representations and processes (Richlan et al., 2011; Richlan, 2012), seem to be the robustest effects in dyslexia, the effects in the other areas need not to be spurious. The fact that studies use different training techniques and experimental tasks in the scanner during neuroimaging may account for finding remedial changes in different brain functions and areas (Heim et al., 2014).
Neuroimaging research on the effects of auditory and phonological intervention is complemented by ERPs, reflecting the dynamics of neural responses. Studies on dyslexia (Table 1) have shown that treatment strengthens brain responses, such as MMN (Kujala et al., 2001; Lovio et al., 2012), attention-related ERP (Stevens et al., 2013), and N400 (Hasko et al., 2014). Lovio et al. (2012) observed also a treatment-induced shortening of the MMN latency across groups receiving grapheme-phoneme or number-knowledge training. In SLI and LLI (Table 2), the remedial gains of interventions have been observed as the strengthening of oscillatory brain activity (Heim et al., 2013) and auditory cortical responses, including MMN (Pihko et al., 2007) and attention-related ERPs (Stevens et al., 2013), which are accompanied by improved performance in behavioral language tasks. Training has also been shown to shorten the latency of auditory P1-N2 complex, resulting in a more mature response pattern (Hayes et al., 2003). As the MMN study by Pihko et al. (2007) involved passive listening, where participants’ attention was directed elsewhere, enhanced MMN responses indicate remedial effects on low-level, pre-attentive auditory processing that is modified by phonetic representations.
Does the Content of the Intervention Matter?
From educators’ perspective, it would be important to conduct interventions that tap the core deficit rather than induce compensatory improvements. Direct comparisons using the same experimental tasks but different interventions could clarify, whether the optimal method of remediating language-related deficits can be found. To this end, Shaywitz et al. (2004) have compared the effects of a targeted experimental intervention and a community intervention on behavioral performance and brain activation in children with reading difficulties. The experimental intervention focused specifically on phonological skills with different kinds of tasks, whereas community intervention consisted of activities commonly provided in school, such as remedial reading (see Shaywitz et al., 2004, for details). As a result of interventions, the experimental intervention group had achieved significant gains in reading fluency and showed an increased activation of left-hemisphere brain regions, whereas no such gains were observed after community intervention. This result emphasizes that the nature of intervention is critical for its success (however, see Boot et al., 2013, for discussion on the expectations about improvement).
Besides showing a correspondence between improvements in skills and changes in neural function, neuroscientific measures can illuminate the specific effects of interventions on brain areas subserving distinct cognitive functions. Heim et al. (2014) compared three different kinds of training that focused on phonology, attention, and visual word recognition (reading). They divided school-aged dyslexic children into three training groups according to their cognitive profiles. All training methods improved children’s reading skills to a similar degree. During a reading task in an fMRI scanner, all training programs resulted in the increased activation of the visual word form area, located in the left fusiform gyrus. In some other brain areas, however, the training programs had different effects on brain activation: phonological and reading training increased activation in bilateral parietal areas, whereas attention training increased activation in the left temporal cortex. Thus, different training programs had both shared and specific effects on brain activation, which would not have been evident on the basis of behavior alone.
In line with Heim et al.’s (2014) conclusions on shared effects induced by different training types, very different kinds of interventions have resulted in significant behavioral and neural gains in individuals with language-related deficits. For example, many studies (see Tables 1 and 2) have shown the remedial gains of phonological training with FastForWord, including auditory discrimination, phoneme discrimination, phoneme identification, phonic match, phonic word, understanding instructions, and grammatical structures and rules. Significant remedial gains in brain activation and behavior have, however, been obtained also with non-linguistic tasks that, at first sight, might seem to have a less obvious link to language-related deficits. Kujala et al. (2001) presented dyslexics with an intervention with non-linguistic audiovisual training, including matching a sequence of non-speech sounds with a sequence of visual shapes. As a result of intervention, reading accuracy had improved and MMN brain responses to tone-order reversals had increased. The change in reading skills and MMN amplitude significantly correlated, suggesting an association between reading abilities and non-linguistic processing. In a similar vein, Gaab et al. (2007) used non-speech stimuli with rapid transitions to remediate dyslexia. After training, language and reading skills had improved and prefrontal regions associated with the processing of rapid transitions were more strongly activated than before training. The remedial gains for reading skills from very different kinds of intervention tasks allude to the possibility that they tap some common, domain-general process involved in, and perhaps necessary for, reading and language skills and contribute to remedial gains along with domain-specific effects.
A candidate function that may, in concert with others, participate in domain-general remedial gains is attention. Stevens et al. (2008) studied whether linguistic intervention would improve selective attention in children with SLI. As a result of training, measures of receptive language had improved and previously attenuated event-related brain responses reflecting selective attention had normalized. Stevens et al. (2013) have also suggested that children at risk for reading difficulty show atypical brain measures of selective attention, which can be remediated by reading intervention. These findings suggest that language skills and auditory attention are strongly connected, complementing the earlier findings on the role of visual attention in dyslexia (Facoetti et al., 2000; Valdois et al., 2004; Shaywitz and Shaywitz, 2008; Vidyasagar and Pammer, 2010; Franceschini et al., 2012; Vogel et al., 2012). This is in line with models of dyslexia proposing the dysfunction of the inferior parietal lobule, which has been linked to attention (Richlan, 2012), and the observations of normalized training-induced activation in the inferior parietal areas (Temple et al., 2003; Eden et al., 2004; Meyler et al., 2008).
How Long-Lasting are the Remedial Gains in the Brain?
Interventions aim at long-lasting gains. The dynamics of neural changes induced by intervention can be explored with follow-up neuroimaging studies, which enable to specify brain functions that show long-term effects. Shaywitz et al.’s (2004) experimental intervention group of dyslexic children returned to an fMRI scan 1 year after the intervention. The normalization of activation pattern was seen both immediately after intervention as well as 1 year after it, suggesting long-lasting remedial effects. Similarly, Meyler et al. (2008) observed hypoactivation of parietal areas before intervention in poor readers and increased activation of these areas immediately after intervention. Interestingly, when they conducted a follow-up 1 year after the intervention, they found that the activation of the parietal areas had continued to increase. Thus, the activation pattern of previously hypoactive areas had normalized, probably reflecting cumulative learning effects following intervention. These follow-up studies thus show that treatment-induced neurobiological changes, coupled with improvement in behavioral performance, can be long-lasting and may enable cumulative gains in language-related skills.
Conclusion
Interventions and training programs involving phonological and auditory tasks have repeatedly gained remedial effects in dyslexia, SLI, and LLI. Neuroscientific research has demonstrated that improved behavioral performance is coupled with changes in both brain function and brain anatomy. Neuroimaging has revealed normalized training-induced brain activation patterns, whereas electrophysiological measures have demonstrated the normalization of strength and timing of brain responses and oscillatory activity after training. Training effects have been observed also in white matter. Especially in the study of dyslexia, neuroscientific studies have illuminated the location of aberrant brain functions, which has enabled to specify the models of the impairment. Neuroimaging studies have also highlighted partly similar and partly specific patterns of neural activation as a result of different training programs. Gains from very different phonological and auditory tasks as well as training effects in the parietal cortex support the models that propose the involvement of some domain-general neural mechanisms, such as attention, in language-related impairments.
In our opinion, neuroscientific studies thus give an important contribution to the treatment of language-related impairments. Specifically, we argue that the use of both neuroscientific and behavioral measures in intervention studies can increase the understanding of how and why interventions change the deficient neural networks, if methodological requirements are met (cf. Bishop, 2013). From educators’ perspective, neuroscientific research methods are seldom directly applicable to the assessment of remedial interventions. However, keeping up-to-date in such research can provide educators with better understanding of the causes of language-related impairments and help them to target interventions more accurately.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work has been funded by the Academy of Finland (projects 131963, 274058, and 276414).
References
Aylward, E. H., Richards, T. L., Berninger, V. W., Nagy, W. E., Field, K. M., Grimme, A. C.,et al. (2003). Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology 61, 212–219. doi: 10.1212/01.WNL.0000068363.05974.64
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barquero, L. A., Davis, N., and Cutting, L. E. (2014). Neuroimaging of reading intervention: a systematic review and activation likelihood estimate meta-analysis. PLoS ONE 9:e83668. doi: 10.1371/journal.pone.0083668
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bishop, D. V. (2013). Research review: Emanuel Miller Memorial Lecture 2012 – neuroscientific studies of intervention for language impairment in children: interpretive and methodological problems. J. Child Psychol. Psychiatry 54, 247–259. doi: 10.1111/jcpp.12034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Boot, W. R., Simons, D. J., Stothart, C., and Stutts, C. (2013). The pervasive problem with placebos in psychology: why active control groups are not sufficient to rule out placebo effects. Perspect. Psychol. Sci. 8, 445–454. doi: 10.1177/1745691613491271
Button, K. S., Ioannidis, J. P., Mokrysz, C., Nosek, B. A., Flint, J., Robinson, E. S.,et al. (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. doi: 10.1038/nrn3475
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Eden, G. F., Jones, K. M., Cappell, K., Gareau, L., Wood, F. B., Zeffiro, T. A.,et al. (2004). Neural changes following remediation in adult developmental dyslexia. Neuron 44, 411–422. doi: 10.1016/j.neuron.2004.10.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Facoetti, A., Paganoni, P., Turatto, M., Marzola, V., and Mascetti, G. G. (2000). Visual-spatial attention in developmental dyslexia. Cortex 36, 109–123. doi: 10.1016/S0010-9452(08)70840-2
Franceschini, S., Gori, S., Ruffino, M., Pedrolli, K., and Facoetti, A. (2012). A causal link between visual spatial attention and reading acquisition. Curr. Biol. 22, 814–819. doi: 10.1016/j.cub.2012.03.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gaab, N., Gabrieli, J. D., Deutsch, G. K., Tallal, P., and Temple, E. (2007). Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor. Neurol. Neurosci. 25, 295–310.
Hasko, S., Groth, K., Bruder, J., Bartling, J., and Schulte-Korne, G. (2014). What does the brain of children with developmental dyslexia tell us about reading improvement? ERP evidence from an intervention study. Front. Hum. Neurosci. 8:441. doi: 10.3389/fnhum.2014.00441
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hayes, E. A., Warrier, C. M., Nicol, T. G., Zecker, S. G., and Kraus, N. (2003). Neural plasticity following auditory training in children with learning problems. Clin. Neurophysiol. 114, 673–684. doi: 10.1016/S1388-2457(02)00414-5
Heim, S., Keil, A., Choudhury, N., Thomas Friedman, J., and Benasich, A. A. (2013). Early gamma oscillations during rapid auditory processing in children with a language-learning impairment: changes in neural mass activity after training. Neuropsychologia 51, 990–1001. doi: 10.1016/j.neuropsychologia.2013.01.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heim, S., Pape-Neumann, J., van Ermingen-Marbach, M., Brinkhaus, M., and Grande, M. (2014). Shared vs. specific brain activation changes in dyslexia after training of phonology, attention, or reading. Brain Struct. Funct. doi: 10.1007/s00429-014-0784-y [Epub ahead of print].
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hoeft, F., Ueno, T., Reiss, A. L., Meyler, A., Whitfield-Gabrieli, S., Glover, G. H.,et al. (2007). Prediction of children’s reading skills using behavioral, functional, and structural neuroimaging measures. Behav. Neurosci. 121, 602–613. doi: 10.1037/0735-7044.121.3.602
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jolles, D. D., and Crone, E. A. (2012). Training the developing brain: a neurocognitive perspective. Front. Hum. Neurosci. 6:76. doi: 10.3389/fnhum.2012.00076
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jucla, M., Nenert, R., Chaix, Y., and Demonet, J. F. (2010). Remediation effects on N170 and P300 in children with developmental dyslexia. Behav. Neurol. 22, 121–129. doi: 10.1155/2010/913692
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keller, T. A., and Just, M. A. (2009). Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron 64, 624–631. doi: 10.1016/j.neuron.2009.10.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kujala, T., Karma, K., Ceponiene, R., Belitz, S., Turkkila, P., Tervaniemi, M.,et al. (2001). Plastic neural changes and reading improvement caused by audiovisual training in reading-impaired children. Proc. Natl. Acad. Sci. U.S.A. 98, 10509–10514. doi: 10.1073/pnas.181589198
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kujala, T., and Näätänen, R. (2010). The adaptive brain: a neurophysiological perspective. Prog. Neurobiol. 91, 55–67. doi: 10.1016/j.pneurobio.2010.01.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lovio, R., Halttunen, A., Lyytinen, H., Naatanen, R., and Kujala, T. (2012). Reading skill and neural processing accuracy improvement after a 3-hour intervention in preschoolers with difficulties in reading-related skills. Brain Res. 1448, 42–55. doi: 10.1016/j.brainres.2012.01.071
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maurer, U., Bucher, K., Brem, S., Benz, R., Kranz, F., Schulz, E.,et al. (2009). Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biol. Psychiatry 66, 341–348. doi: 10.1016/j.biopsych.2009.02.031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McArthur, G. M. (2009). Auditory processing disorders: can they be treated? Curr. Opin. Neurol. 22, 137–143. doi: 10.1097/WCO.0b013e328326f6b1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Meyler, A., Keller, T. A., Cherkassky, V. L., Gabrieli, J. D., and Just, M. A. (2008). Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: a longitudinal study of neuroplasticity. Neuropsychologia 46, 2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Näätänen, R., Paavilainen, P., Rinne, T., and Alho, K. (2007). The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol. 118, 2544–2590. doi: 10.1016/j.clinph.2007.04.026
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pennington, B. F., and Bishop, D. V. (2009). Relations among speech, language, and reading disorders. Annu. Rev. Psychol. 60, 283–306. doi: 10.1146/annurev.psych.60.110707.163548
Pihko, E., Mickos, A., Kujala, T., Pihlgren, A., Westman, M., Alku, P.,et al. (2007). Group intervention changes brain activity in bilingual language-impaired children. Cereb. Cortex 17, 849–858. doi: 10.1093/cercor/bhk037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ramus, F., Marshall, C. R., Rosen, S., and van der Lely, H. K. (2013). Phonological deficits in specific language impairment and developmental dyslexia: towards a multidimensional model. Brain 136, 630–645. doi: 10.1093/brain/aws356
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Richards, T. L., Berninger, V. W., Aylward, E. H., Richards, A. L., Thomson, J. B., Nagy, W. E.,et al. (2002). Reproducibility of proton MR spectroscopic imaging (PEPSI): comparison of dyslexic and normal-reading children and effects of treatment on brain lactate levels during language tasks. AJNR Am. J. Neuroradiol. 23, 1678–1685.
Richards, T. L., Corina, D., Serafini, S., Steury, K., Echelard, D. R., Dager, S. R.,et al. (2000). Effects of a phonologically driven treatment for dyslexia on lactate levels measured by proton MR spectroscopic imaging. AJNR Am. J. Neuroradiol. 21, 916–922.
Richlan, F. (2012). Developmental dyslexia: dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 6:120. doi: 10.3389/fnhum.2012.00120
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Richlan, F., Kronbichler, M., and Wimmer, H. (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 56, 1735–1742. doi: 10.1016/j.neuroimage.2011.02.040
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shaywitz, B. A., Shaywitz, S. E., Blachman, B. A., Pugh, K. R., Fulbright, R. K., Skudlarski, P.,et al. (2004). Development of left occipitotemporal systems for skilled reading in children after a phonologically- based intervention. Biol. Psychiatry 55, 926–933. doi: 10.1016/j.biopsych.2003.12.019
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shaywitz, S. E., and Shaywitz, B. A. (2005). Dyslexia (specific reading disability). Biol. Psychiatry 57, 1301–1309. doi: 10.1016/j.biopsych.2005.01.043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shaywitz, S. E., and Shaywitz, B. A. (2008). Paying attention to reading: the neurobiology of reading and dyslexia. Dev. Psychopathol. 20, 1329–1349. doi: 10.1017/S0954579408000631
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sigman, M., Pena, M., Goldin, A. P., and Ribeiro, S. (2014). Neuroscience and education: prime time to build the bridge. Nat. Neurosci. 17, 497–502. doi: 10.1038/nn.3672
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Simos, P. G., Fletcher, J. M., Bergman, E., Breier, J. I., Foorman, B. R., Castillo, E. M.,et al. (2002). Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology 58, 1203–1213. doi: 10.1212/WNL.58.8.1203
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stevens, C., Fanning, J., Coch, D., Sanders, L., and Neville, H. (2008). Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Res. 1205, 55–69. doi: 10.1016/j.brainres.2007.10.108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stevens, C., Harn, B., Chard, D. J., Currin, J., Parisi, D., and Neville, H. (2013). Examining the role of attention and instruction in at-risk kindergarteners: electrophysiological measures of selective auditory attention before and after an early literacy intervention. J. Learn. Disabil. 46, 73–86. doi: 10.1177/0022219411417877
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tallal, P. (2001). “Language learning impairment,” in International Encyclopedia of Social & Behavioral Sciences, eds N. J. Smelser and P. B. Baltes (Oxford: Pergamon), 8353–8357. doi: 10.1016/B0-08-043076-7/03600-7
Tallal, P. (2012). Improving neural response to sound improves reading. Proc. Natl. Acad. Sci. U.S.A. 109, 16406–16407. doi: 10.1073/pnas.1214122109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Temple, E., Deutsch, G. K., Poldrack, R. A., Miller, S. L., Tallal, P., Merzenich, M. M.,et al. (2003). Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 100, 2860–2865. doi: 10.1073/pnas.0030098100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Valdois, S., Bosse, M. L., and Tainturier, M. J. (2004). The cognitive deficits responsible for developmental dyslexia: review of evidence for a selective visual attentional disorder. Dyslexia 10, 339–363. doi: 10.1002/dys.284
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vidyasagar, T. R., and Pammer, K. (2010). Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends Cogn. Sci. 14, 57–63. doi: 10.1016/j.tics.2009.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vogel, A. C., Miezin, F. M., Petersen, S. E., and Schlaggar, B. L. (2012). The putative visual word form area is functionally connected to the dorsal attention network. Cereb. Cortex 22, 537–549. doi: 10.1093/cercor/bhr100
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: language deficit, dyslexia, neuroscience, training, remediation, intervention
Citation: Ylinen S and Kujala T (2015) Neuroscience illuminating the influence of auditory or phonological intervention on language-related deficits. Front. Psychol. 6:137. doi: 10.3389/fpsyg.2015.00137
Received: 25 September 2014; Accepted: 26 January 2015;
Published online: 17 February 2015.
Edited by:
Julie Chobert, Centre National de la Recherche Scientifique, FranceReviewed by:
April A. Benasich, Rutgers University, USAAnna J. Simmonds, Imperial College London, UK
L. Robert Slevc, University of Maryland, College Park, USA
Fabio Richlan, University of Salzburg, Austria
Copyright © 2015 Ylinen and Kujala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sari Ylinen, Cognitive Brain Research Unit, Institute of Behavioural Sciences, University of Helsinki, P.O. Box 9, Helsinki, FIN-00014, Finland e-mail: sari.ylinen@helsinki.fi
 Sari Ylinen
Sari Ylinen Teija Kujala
Teija Kujala