- 1Clinical Neuropsychology, Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 2Center for Lifespan Psychology, Max Planck Institute for Human Development, Berlin, Germany
- 3Department of Psychiatry and Psychotherapy, University of Lübeck, Lübeck, Germany
- 4Department of Clinical Psychology and Psychotherapy, Institute of Psychology, University of Bern, Bern, Switzerland
- 5Asklepios Medical Center Hamburg-North–Wandsbek, Department of Psychiatry and Psychotherapy, Hamburg, Germany
The majority of patients with schizophrenia display neurocognitive deficits (e.g., memory impairment) as well as inflated cognitive biases (e.g., jumping to conclusions). Both cognitive domains are implicated in the pathogenesis of the disorder and are known to compromise functional outcome. At present, there is a dearth of effective treatment options. A total of 90 patients with schizophrenia were recruited online (a diagnosis of schizophrenia had been confirmed in a large subgroup during a previous hospital admission). Subsequent to a baseline assessment encompassing psychopathology, self-reported cognition as well as objective memory and reasoning tests, patients were randomized to one of three conditions: standard cognitive remediation (mybraintraining), metacognition-augmented cognition remediation (CR) condition (variant of mybraintraining which encouraged patients to reduce speed of decision-making and attenuate response confidence when participants made high-confidence judgements and hasty incorrect decisions) and a waitlist control group. Patients were retested after 6 weeks and again 3 months after the second assessment. Groups did not differ on psychopathology and neurocognitive parameters at any timepoint. However, at follow-up the metacognitive-augmented CR group displayed a significant reduction on jumping to conclusions and overconfidence. Treatment adherence correlated with a reduction of depression; gains in the training exercises from the standard mybraintraining condition were correlated with improved objective memory performance. The study suggests that metacognition-augmented CR may ameliorate cognitive biases but not neurocognition. The study ties in well with prior research showing that neurocognitive dysfunctions are rather resistant to change; the failure to detect significant improvement of CR or metacognition-augmented CR on psychopathology and neurocognition over time may partly be attributed to a number of methodological limitations of our study (low psychopathology and chronicity of participants, low “dosage,” narrow range of tests, self-report psychopathology scales).
Introduction
Schizophrenia is frequently accompanied by neuropsychological deficits which are spread across a wide range of cognitive functions (Heinrichs and Zakzanis, 1998; Keefe and Harvey, 2012). Memory and attention problems in concert with social cognitive impairments (Fett et al., 2011) are a major predictor for disability and low functional outcome in the disorder (Green, 1996; Green et al., 2004; Lepage et al., 2014). Neurocognitive deficits are also a risk factor for poor symptomatic outcome. First, memory problems aggravate medication non-adherence as patients may fail to remember the rationale for drug administration or forget to take their medication (Moritz et al., 2013b), particularly due to prospective memory failure (Moritz et al., 2004). In addition, compromised attention, reasoning, and memory capacity may limit the comprehension and internalization of knowledge and skills acquired during cognitive therapy and thus impede transfer to everyday life.
The causes underlying neurocognitive deficits in schizophrenia are multi-facetted. Apart from early (neurodevelopmental) deficits that already manifest prior to the onset of the disorder (Bang et al., 2014; Corigliano et al., 2014), avolition/lack of effort and a restricted non-challenging environment/hospitalization may compromise cognition. Some recent studies suggest that (conventional) antipsychotics impair brain functioning (Ukai et al., 2004; Ho et al., 2011; Gasso et al., 2012), which in turn hampers neurocognition. While antipsychotic-induced cognitive deficits are clearly non-desired and thus usually considered a side-effect, there is emerging, albeit not yet conclusive, evidence that such secondary cognitive deficits may in fact be one mechanism through which antipsychotics reduce positive symptoms (“effect by defect” hypothesis; Moritz et al., 2013a). In other words, there may be two sides of the same coin: doubt and reduced speed of information processing induced by antipsychotics may be a prerequisite for the dissolution of delusions.
Currently, there is a dearth of potent treatment options against cognitive deficits. Early claims that atypical neuroleptics may act as cognitive enhancers have not lived up to its expectations (Keefe et al., 2007; Davidson et al., 2009; Keefe and Harvey, 2012). Atypical neuroleptics leave cognition uncompromised at best. It should also be taken into account that side effects such as extrapyramidal symptoms (Fervaha et al., 2015) and concomitant medication, particularly anticholinergic drugs (Vinogradov et al., 2009) and tranquilizers/benzodiazepines (Deckersbach et al., 2011) are known to aggravate neurocognitive deficits, too.
Cognitive remediation (CR) has shown some promise; meta-analyses indicate that CR exerts a (small-to-moderate) effect on neurocognition (McGurk et al., 2007; Wykes et al., 2011) but does not have a lasting impact on symptomatology (Wykes et al., 2011). However, this promising evidence has to be weighed against the effort that needs to be invested to produce those changes (e.g., one-on-one training, tailored material). Recently, low-threshold group CR trainings have shown some beneficial effect. A meta-analysis on 36 studies reveals that Integrated Psychological Therapy (IPT), a program at the interface of neurocognition and social cognition, exerts significant positive effects relative to control interventions on neurocognition, social cognition, psychosocial functioning, and negative symptoms (Roder et al., 2011). In a recent study we were able to show that a CR group improved attention after 3 years relative to a metacognition group (Moritz et al., 2014c).
Apart from “cold” cognitive deficits mirroring brain dysfunction in psychosis, particularly in the frontal and temporal lobes, there is an emerging interest in cognitive biases. Cognitive biases are not deficits per se but represent alterations or styles in the perception and processing of information, for example a preference to remember positive versus negative information. Cognitive biases are not pathological as such; some cognitive biases can even promote psychological well-being (e.g., self-serving bias, “Pollyanna effect”; Bentall, 1992; Pohl, 2004). Among other cognitive distortions, studies have implicated jumping to conclusions (Garety et al., 1991) and overconfidence in errors (Moritz et al., 2003) in the formation and maintenance of psychosis. To summarize, a plethora of studies suggest that patients with schizophrenia are hastier in gathering information (for reviews, see Garety and Freeman, 1999, 2013; Fine et al., 2007) and are more confident in erroneous responses pertaining to memory (Moritz and Woodward, 2006a; Gaweda et al., 2012; Peters et al., 2013) and social cognition (Kother et al., 2012; Moritz et al., 2012b) relative to non-clinical and psychiatric controls. Recent evidence suggests that this extends to perception (Moritz et al., 2014b). Both biases foster the formation of momentous false decisions that under some contextual factors may promote delusions (Moritz and Woodward, 2006b; Garety and Freeman, 2013). To illustrate, jumping to conclusions may lead a person with a history of psychosis to infer that a friend who is not calling back within 2 days has turned his back on him and is not trustworthy anymore. This along with overconfidence in errors may later turn the initial benign suspicion into a serious false belief (e.g., that the friend is a police spy who has gathered sufficient information against the patient so that they can terminate surveillance). Once such ideas have systematized, judgments are usually not validated or questioned anymore and the person is no longer open to disconfirmatory evidence, the latter representing another prominent cognitive bias (Woodward et al., 2006, 2008; Veckenstedt et al., 2011).
The present study examined the efficacy of CR training versus a CR training combined with a bias modification approach. To this end, a low-threshold online CR training called mybraintraining Professional (from here on “mybraintraining”) was administered. Mybraintraining intends to improve neurocognitive functioning by training four major faculties: calculation, logic, memory, and vision. We set up two experimental CR conditions which were tested against a waitlist control group. In the standard CR condition, patients were encouraged to avoid making errors when performing cognitive tasks that were presented under time restriction. In the metacognition-augmented CR condition the same exercises were presented but patients additionally had to rate their responses in terms of confidence, that is, whether they were certain or not that their responses were correct. Whenever a subject made a high-confident error and/or an error committed with very short reaction time (i.e., less than half of the allocated time used) they were advised to attenuate their confidence and to take more time if not fully confident for the remaining trials. The aim of this metacognition-augmented CR condition was to sensitize participants to the disadvantages of high-confident and hasty decision-making suggesting that “gut feelings” may be faulty. We hypothesized that the conventional CR condition may improve subjective and perhaps even objective cognitive impairment. The metacognition-enhanced CR condition was hypothesized to additionally improve the jumping to conclusions bias and to attenuate response confidence (as measured by a memory task).
Materials and Methods
Participants
The present study was approved by the ethics committee of the German Society for Psychology (DGPs). Participants were recruited from various sources. A total of 223 former patients of the Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf (Germany) with verified diagnostic status (schizophrenia or schizoaffective disorder) were informed about the study via email. All participants had given explicit permission to be contacted for future studies. Furthermore, 309 emails were sent to clinicians asking them to pass on information about the study to patients meeting inclusion criteria. Finally, upon the approval of webmasters study invitations were posted in several guided self-help internet networks pertaining to schizophrenia and psychosis (these websites provided reliable information on the disorder and fostered the exchange of individuals affected with psychosis).
The following inclusion criteria were applied: age between 18 and 65 years, willingness to provide electronic informed consent and to participate in anonymous (internet-based) surveys as well as a diagnosis of schizophrenia or schizoaffective psychosis.
All posts and emails contained a weblink directing interested parties to the baseline survey. The trial was created using Questback® which does not store IP addresses. Group allocation was carried out at random.
The first page of the online survey essentially repeated the information of the email (random assignment to either the mybraintraining standard version, metacognition-augmented mybraintraining, or waitlist control group; inclusion criteria) in everyday language. It was announced that all participants would receive free access to the online program (mybraintraining) for 1 year, either immediately or after a 6-week delay. Moreover, all completers would receive a manual containing mindfulness exercises at the end of the study.
Multiple log-ins via the same computer were prevented by means of “cookies.” The survey consisted of the following parts: invitation, informed consent (mandatory), optional consent to contact the patient’s clinician in order to verify diagnostic status (to do this, participants had to provide their own name as well as the name and address of the clinician), demographic section (e.g., gender, age), medical information (e.g., medication, psychiatric diagnoses), assessment of psychopathology I (see questionnaire section below), encoding memory phase, assessment of psychopathology II (see questionnaire section below), memory recognition test, fish task (jumping to conclusions), and request for an email address (to match baseline and post survey data). Then, we asked participants to endorse whether or not they had responded honestly. Finally, participants were given the opportunity to leave comments.
No monetary compensation was offered for participation. Individuals who were randomly assigned to the waitlist condition were informed that they would receive access after completing the follow-up survey 6 weeks later.
Participants in two experimental groups were given access to one of two versions of mybraintraining within 24 h. This email also contained information about the rationale of mybraintraining or metacognition-augmented mybraintraining. Participants in the experimental groups received weekly email reminders encouraging them to use the program on a regular basis.
Procedure
Six weeks after the baseline assessments, participants were invited via email to participate in the post survey. Up to two reminders were dispatched in case subjects failed to complete the post assessment. Three months after the post assessment, invitations for a follow-up assessment were sent. Again, up to two reminders were dispatched if subjects did not fill out the final assessment.
Post Assessment
For the post survey, individuals were requested to enter their email address to allow matching post data with baseline data. The post assessment consisted of the following parts: introduction, current treatment and medication, assessment of psychopathology I, encoding memory phase, assessment of psychopathology II, memory recognition test, fish test (jumping to conclusions), and evaluation of the online training (see below). Similar to the baseline assessment, we asked participants whether or not they had responded honestly and gave them the opportunity to leave comments.
Subsequent to completion of the post assessment, all participants received a manual on relaxation and mindfulness exercises. Participants in the waitlist condition also received access to the standard CR condition. Patients in the standard mybraintraining condition did not receive the metacognition-augmented CR training and vice versa.
Follow-Up Assessment
Three months after the post assessment, participants were invited to a follow-up assessment. This final assessment was not part of our initial study design. As participants in the waitlist group received access to the mybraintraining standard version subsequent to completion of the post assessment, this final follow-up assessment did not allow comparison of the three groups. Hence, the follow-up analysis compared the standard CR group (immediate or delayed) with the metacognition-augmented CR group. As an incentive for continued participation, individuals received a manual with exercises derived from “Acceptance and Commitment Therapy.” The follow-up assessment was a shorter version of the post assessment and involved a selection of previously used scales (see below). As the follow-up was not announced from the start, we expected a higher non-completion rate.
Questionnaires (Online Assessment)
Participants were asked to complete the following questionnaires (the survey proceeded only after all items had been answered):
Paranoia Checklist (Freeman et al., 2005)
The Paranoia Checklist is an 18 item questionnaire assessing paranoid beliefs and suspiciousness. The psychometric properties are good (Freeman et al., 2005; Lincoln et al., 2010a,b). In our slightly adapted version, participants are asked to rate their present symptom severity on a five-point Likert scale ranging from 1 (not at all) to 5 (extremely).
Center for Epidemiologic Studies-Depression Scale (CES-D)
The Center for Epidemiologic Studies-Depression Scale (CES-D) is a 20 item questionnaire covering depressive symptoms; the reliability and validity of the CES-D have been established previously (Radloff, 1977; Hautzinger and Brähler, 1993). In the present study, CES-D items were presented intermixed with items from the Paranoia Checklist.
Launay-Slade Hallucination Scale-Revised (LSHS-R; Bentall and Slade, 1985)
The Launay-Slade Hallucination Scale-Revised (LSHS-R) is a 16 item questionnaire covering sleep-related hallucinations, vivid daydreams, intrusive thoughts, and auditory hallucinations. Its reliability has been demonstrated elsewhere (Goodarzi, 2009). Psychosis patients with hallucinations usually score higher than remitted patients, and the latter in turn reach higher scores than patients who never experienced hallucinations (Varese et al., 2012). The LSHS-R was not included in the follow-up assessment.
Beck Cognitive Insight Scale (BCIS) – Extended
The Beck Cognitive Insight Scale (BCIS; Beck et al., 2004) is a 15-item scale that measures the degree of patients’ self-reflectiveness and overconfidence in the interpretation of experiences. Principal component analysis (Beck et al., 2004) suggests a two-dimensional structure (self-reflectiveness and self-certainty). According to the original article (Beck et al., 2004), the BCIS demonstrates good convergent, discriminant, and construct validity. The psychometric properties of the German translation used in the present study are good as well (Mass et al., 2012). We complemented the BCIS with a number of self-developed novel items asking for subjective cognitive deficits (e.g., “I have trouble learning new things”). The BCIS was not administered in the follow-up assessment.
Jumping to Conclusions
We administered an online version of the probabilistic reasoning task (Speechley et al., 2010; Moritz et al., 2012a), which slightly differs from the original beads task as it employs a different scenario (lakes with fish instead of jars with beads). Three parallel versions were set up to avoid practice effects. In each version, two lakes with colored fish in opposing likelihood (e.g., 80% orange vs. 20% gray fish, and vice versa) were presented to the participant. Following each “catch,” participants were asked to make two judgments: (1) a probability judgment about the likelihood that the fish was/were being caught from lake A versus lake B, and (2) whether the available amount of information would justify a decision or not. The instruction emphasized that the fisherman would not change the lake throughout the task. The ratio of fish in each lake was shown at the bottom of each slide along with previously caught fish (the last catch was indicated with an arrow). In total, 10 fish were presented; one lake was strongly suggested by the chain of events (D–D–D–N–D–D–D–D–N–D; D = dominant color of fish; N = non-dominant color of fish). Jumping to conclusions was defined as a decision after one or two fish. We also computed the number of draws to decisions.
Memory Test
Three parallel versions of a newly developed memory recognition test were composed. The test was modeled after the Auditory Verbal Learning Memory Test (AVLT) but did not encompass active recall. In the (incidental) encoding phase (i.e., unlike in the AVLT participants were not instructed that their later task would be to memorize the items), participants were presented 15 nouns [each five words that were pre-classified by the authors as positive (e.g., cake), negative (e.g., accident) or neutral (e.g., table)] and requested to appraise each noun as either positive, neutral or negative (valence). Later, participants were presented the previously presented 15 words intermingled with 15 distractor words of different valence in random order (recognition phase). Participants were asked to rate if the respective word had been presented before (i.e., in the valence task) and how confident they were in the correctness of their judgment. Items had to be endorsed on a four-point Likert scale (1 = old word, certain; 2 = old word, uncertain; 3 = new word, uncertain; 4 = new word, certain). There was an equal number (n = 15) of (pre-defined) negative, positive, and neutral words, both with respect to old (studied) and new (distractor) words.
Mybraintraining Professional
Mybraintraining is a CR program which is available online (no local installation on PC necessary) at http://www.mybraintraining.com/. The program can be used both as a self-help or conventional treatment device (i.e., guided treatment by neuropsychologist or occupational therapist). The program encompasses 30 exercises aimed at stimulating executive functioning. Exercises fall into four broad categories: calculation, logic, memory, and vision. The exercises were designed during development of the “Train your Brain with Dr. Kawashima” program in cooperation with the Industry University Research Project with Professor Dr. Ryūta Kawashima. According to the developers (personal communication), performance of each exercise had to be accompanied by activation of the frontal lobe (presented in the “Scientific Details” part of each exercise).
The difficulty of the sessions automatically adapts to the patients’ performance. mybraintraining includes motivating elements as used commonly in video games in order to increase fun and adherence. The administrator can define individual training plans and adapt exercises to each patient’s needs (e.g., level of difficulty, varied time limits, etc.). This tool also compiles statistics (e.g., to compare one patient with reference group, number of sessions completed, training success). Data protection and security comply with industry standards.
For the present study, we used the “daily test” tool of mybraintraining Professional which encourages patients to perform a random string of four exercises, one from each category (calculation, logic, memory, and vision).
In addition to the conventional version of mybraintraining Professional, a condition termed metacognitive-augmented CR condition was constructed, which aimed to reduce overconfidence and jumping to conclusions. This version asked participants to make a confidence judgment (certain versus uncertain) after each trial. The program then provided feedback in case of hasty and/or high-confident errors (see Figure 1). Since the termination of the study, this additional option is now part of the standard program.
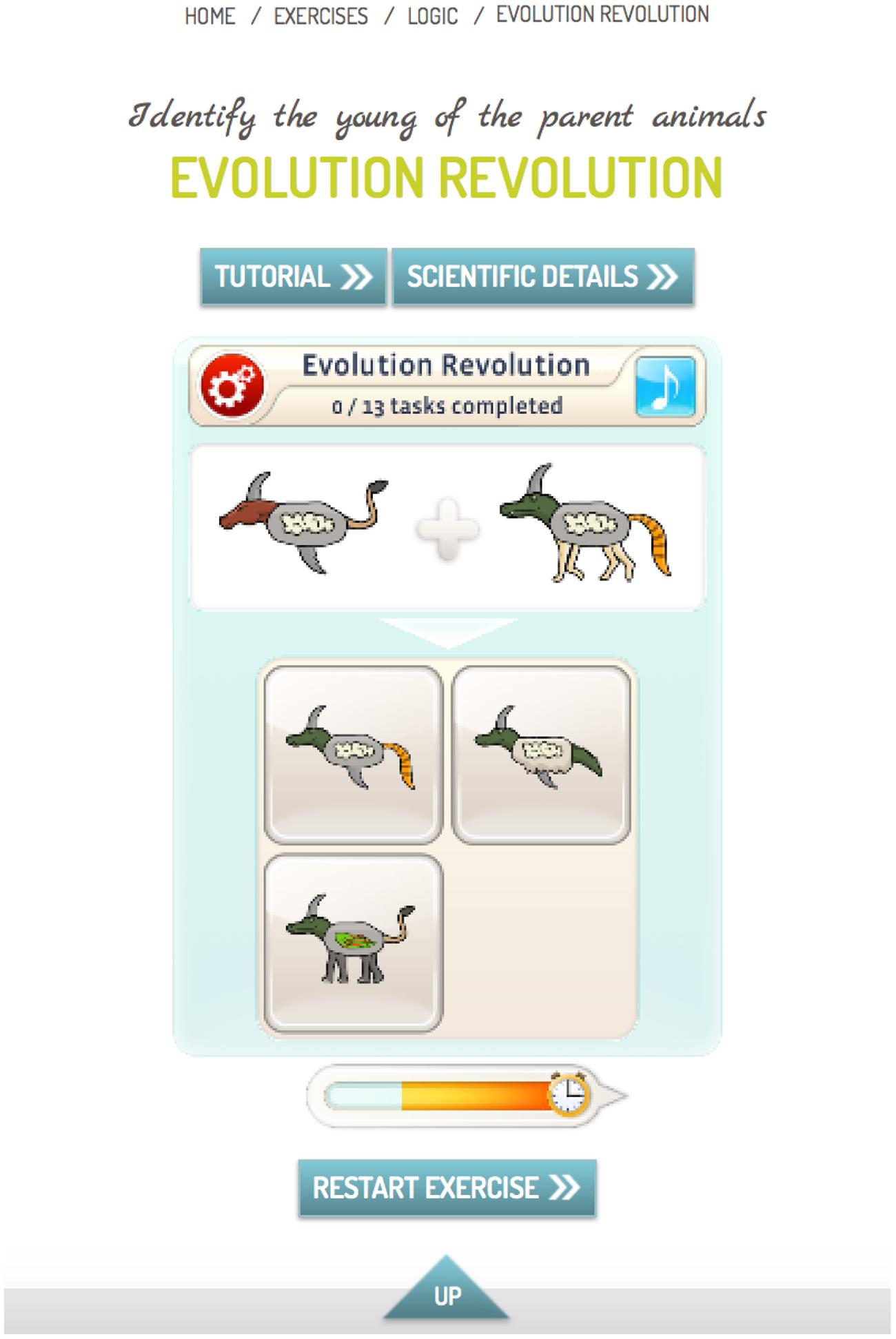
FIGURE 1. Example for an exercise from category “Logic.” The participant had to identify the young of the parent animals (the upper left response option is correct). In the standard version, the participant had to indicate his or her choice and was then informed about the outcome (correct versus incorrect). In the metacognitive-augmented condition, the participant was asked after each response whether he or she was certain that the response was correct. In case of a very fast incorrect response (less than half of the allotted time indicated by the time bar; see bar left to clock symbol) or a high-confident incorrect response, patients were encouraged by automatic feedback to either take more time before completing an item and/or to attenuate response confidence if the available evidence was insufficient.
Strategy of Data Analysis
Simple cross-sectional analyses were performed using t-tests for metric (e.g., age) and cross table statistics for nominal data (e.g., gender distribution). For group comparisons over time we used mixed ANOVAs with Group as the between-subject factor and Time as the within-subject factor when using metric data. In case of binary data (e.g., rate of jumping to conclusions) a generalized estimating equations procedure was performed which was deemed more appropriate than a conventional repeated-measures ANOVA.
Results
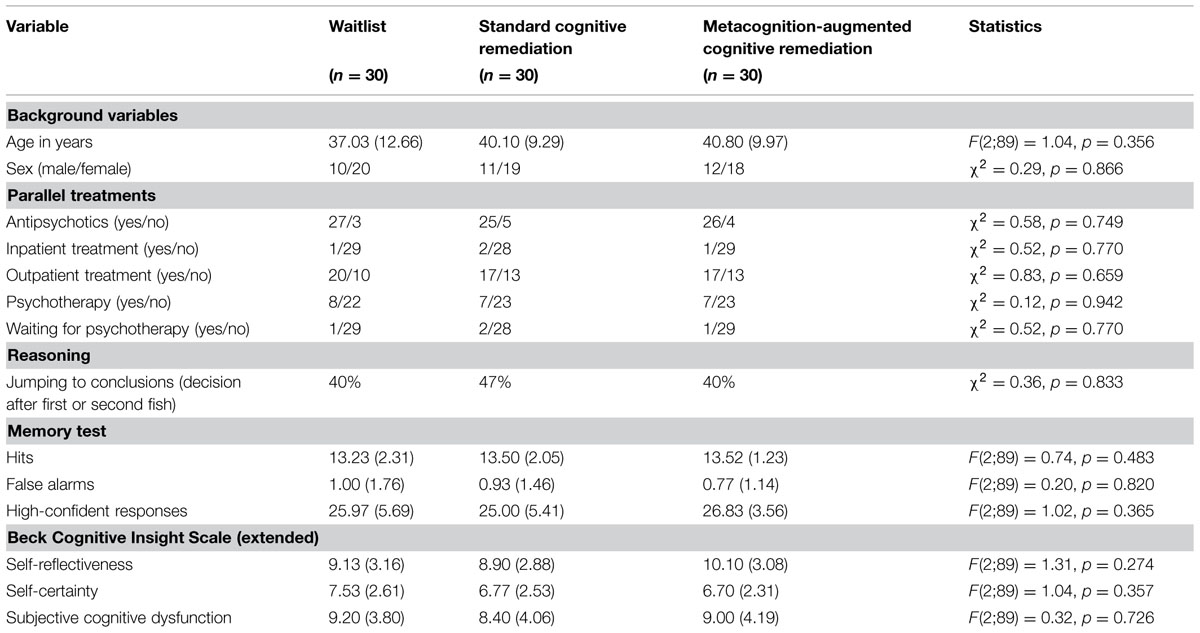
Table 1 shows the baseline characteristics of the sample, of which 76 patients could be reached for the post assessment and 38 for the follow-up. No significant differences emerged for any demographic, psychopathological, or cognitive variable.
Across time, medication status did not change between groups (p > 0.3). At baseline, 87% of the participants were medicated with antipsychotics, at post (85%) and follow-up (87%) the rate was almost identical. Likewise, treatment status [yes (i.e., outpatient, inpatient, day clinic, practitioner) versus no] did not change between groups across time (p > 0.5). Most patients were treated as outpatients (pre: 60%, post: 57.5%, follow-up: 53.3%). Rates did not differ among groups at any point in time (p > 0.6).
Pre versus Post
Table 2 shows between-group differences from pre to post for the per protocol sample (i.e., participants in the CR conditions had logged into mybraintraining at least once). Groups did not differ significantly on any symptoms, and cognition measures.
Pre versus Follow-Up
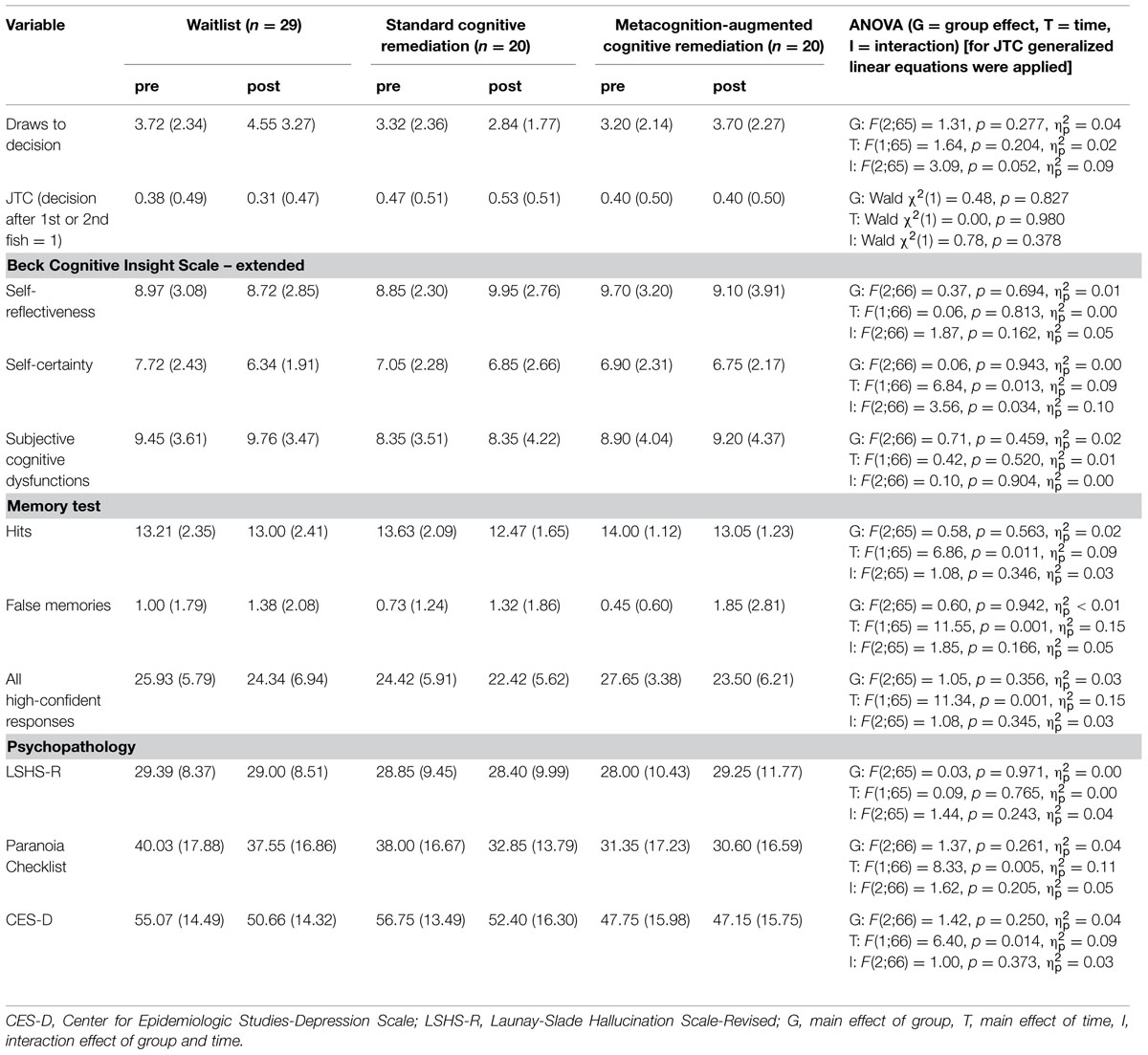
At follow-up, 38 individuals underwent another assessment [metacognition-augmented mybraintraining: n = 14; standard mybraintraining (immediate or delayed): n = 24]. For draws to decision, the effect of time achieved statistical trend level, F(1;36) = 3.46, p = 0.071, = 0.09, while the group effect was insignificant, F(1;36) = 2.44, p = 0.127, = 0.06. This was qualified by a significant interaction, F(1;36) = 5.82, p = 0.021, = 0.14; Figure 2 shows that participants in the metacognition-augmented condition showed delayed decision-making while participants in the standard condition showed a tendency toward more jumping to conclusions. Likewise, using generalized estimating equations to fit a repeated-measures logistic regression to jumping to conclusions data (decision after fish 1 or 2), a significant interaction occurred favoring the metacognition-augmented condition, Wald χ2(1) = 4.55, p = 0.033.
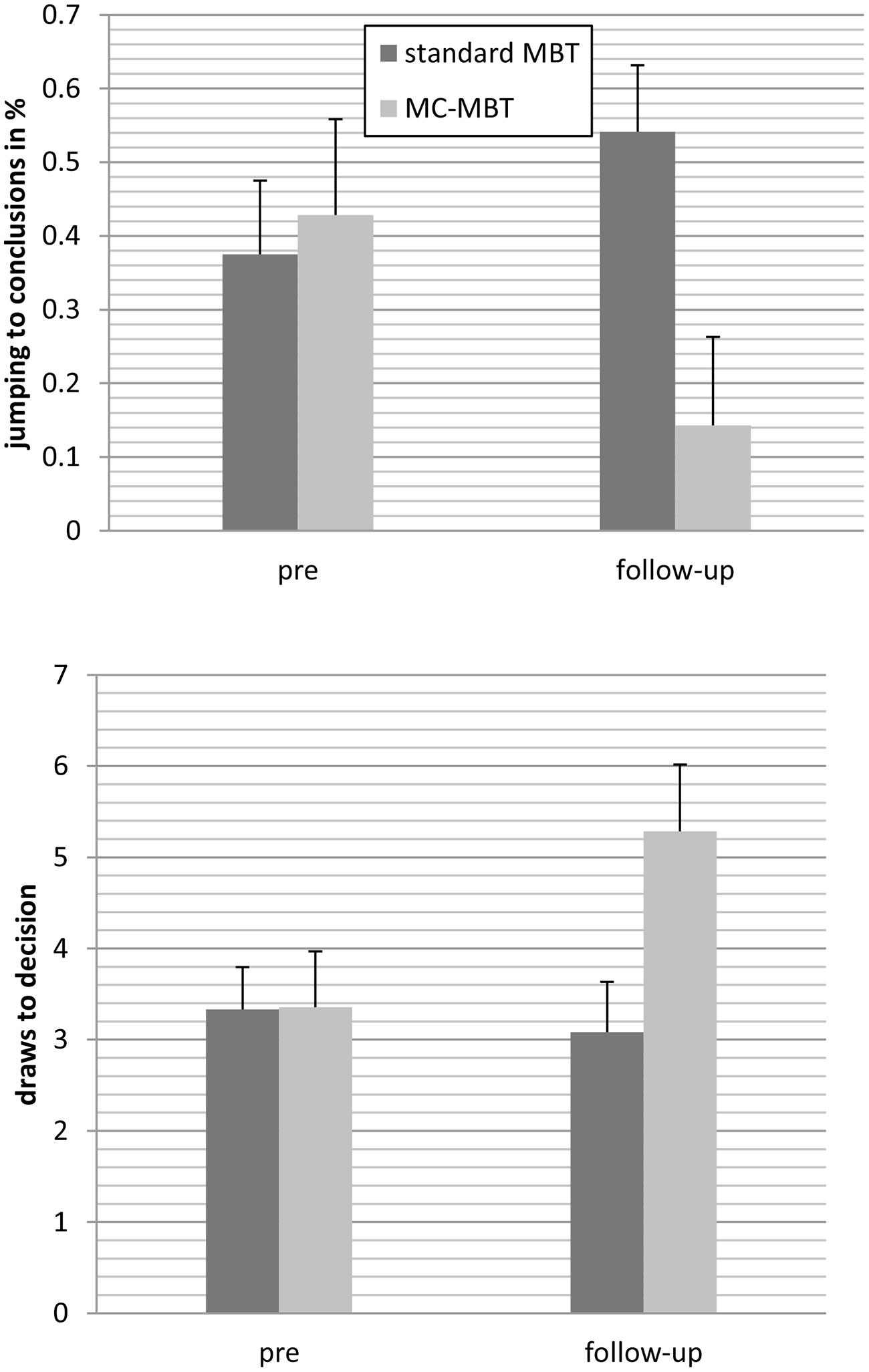
FIGURE 2. Patients who underwent the metacognition-augmented cognitive remediation program (MC-MBT) showed less jumping to conclusions from baseline to follow-up (upper) and delayed decision-making (lower) relative to participants who received the standard version (MBT; immediately or delayed), respectively.
For the number of high-confident responses on the memory test the effect of time, F(1;36) = 5.12, p = 0.030, = 0.125 but not group, F(1;36) = 0.11, p = 0.737, < 0.01 were significant, which was qualified by significant interaction at an almost large effect size, F(1;36) = 5.59, p = 0.024, = 0.13. As can be seen in Figure 3 the number of high-confident responses remained stable in the standard CR group but declined in the metacognition-augmented group.
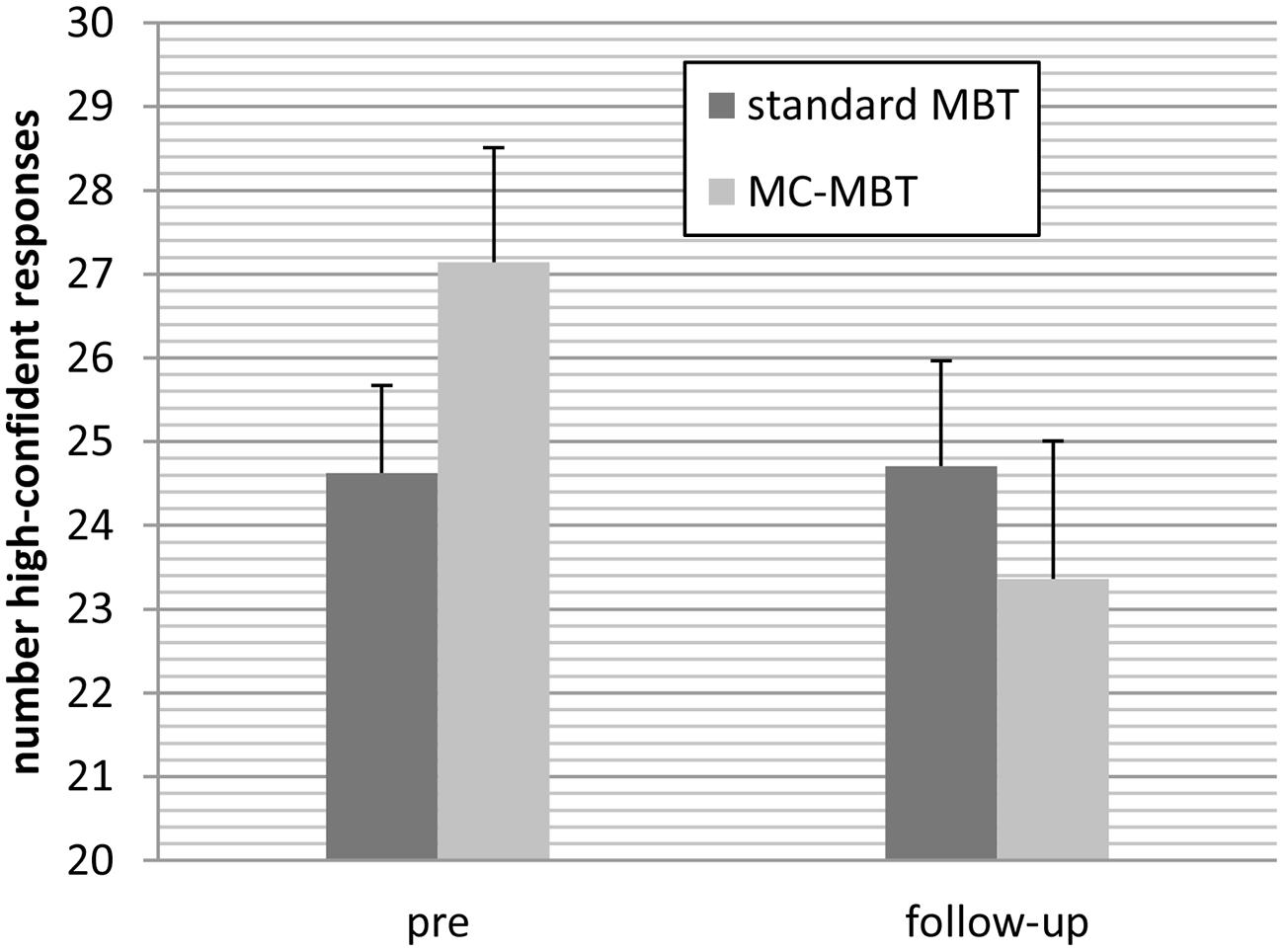
FIGURE 3. Patients in the metacognition-augmented cognitive remediation condition (MC-MBT) attenuated confidence ratings from baseline to the follow-up period relative to the standard CR group (MBT).
No significant interaction emerged for depression, F(1;36) = 0.14, p = 0.91, < 0.01, paranoia, F(1;36) = 0.64, p = 0.428, = 0.02, hits, F(1;36) = 0.78, p = 0.785, < 0.01, and false memories, F(1;36) = 1.23, p = 0.276, = 0.03.
Retrospective Assessment (Post)
Feasibility and comprehensibility of the training were rated high by respondents and did not differ between the two CR conditions (Table 3). Patients were able to perform the tasks alone and rated the exercises as helpful, although only a minority reported symptom improvement.
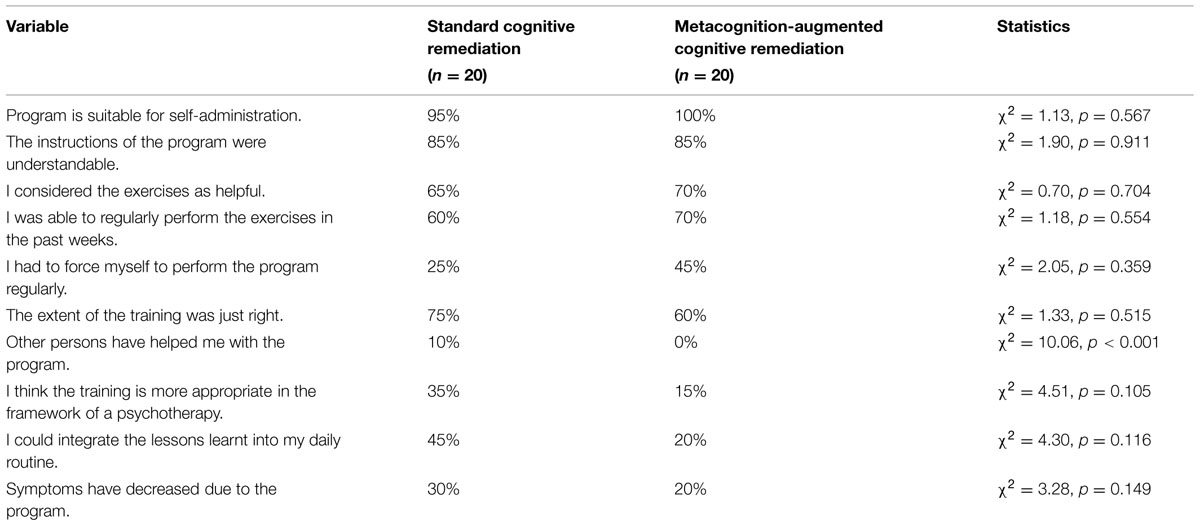
TABLE 3. Retrospective subjective assessment (“fully applies” and “rather applies” were combined) at post.
Correlations between Performance and Adherence with Symptomatology
We examined whether adherence and progress on the CR program impacted outcome variables. Progress in performance in CR memory exercises (slope change measure) was correlated at r = 0.61 (p = 0.026) with improvement in the memory test from pre to post for the standard mybraintraining group (no other variables turned significant). Gain in overall performance (all exercises combined) in the metacognition-augmented mybraintraining condition correlated with more draws to decision in the fish task, r = 0.54, p = 0.021 and less jumping to conclusions at trend level, r = -0.42, p = 0.079. Similarly, the number of exercises performed (objective measure) in the metacognition-augmented mybraintraining condition correlated with less jumping to conclusions significantly (r = -0.398, p = 0.040) and less draws to decision over time at trend level (r = 0.353, p = 0.071). Adherence in the standard condition (number of days the CR program was used) was associated with reduction of depression over time (r = 0.467, p = 0.028). Likewise, number of exercises performed (objective) was correlated with decline of depressive symptoms (r = 0.482, p = 0.023), again for the standard version only.
Test–Retest Reliability of the Data and Plausibility Checks
Test–retest reliability was determined for pre–post scores only due to the low number of participants at follow-up. Consistency of the psychopathological scales was excellent (CES-D: pre–post: r = 0.817, p < 0.001; Paranoia Checklist: r = 0.891, p < 0.001, LSHS-R: r = 0.936, p < 0.001). The recognition test showed low reliability from pre to post (r = 0.255, p = 0.024). The correlation between subjective adherence (number of days exercises were performed: 0–7 days/week) and objective number of exercises performed (data extracted from log files) was good (r = 0.817, p < 0.001).
Discussion
The study set out to examine the effectiveness of conventional as well as metacognition-augmented CR training. Most patients were on antipsychotic medication and in outpatient treatment. Treatment status did not change substantially across time. Special precautions were taken to verify diagnostic status. Speaking for the quality of the data, the test-retest reliability of the questionnaires was very high. Further, subjective and objective adherence were highly correlated.
We used a low-threshold online CR training termed mybraintraining targeting four cognitive domains which according to the developers (personal communication, unpublished data) are linked with metabolic changes in frontal lobe areas. Patients carried out the exercises on their home computer. The program was delivered unguided; no individual adaption was performed apart from automatic adjustments pertaining to difficulty. Our hypotheses were partly confirmed. Group comparisons indicate that conventional CR did not impact any outcome measure suggesting that cold cognitive functioning is quite resistant to cognitive training interventions, at least in a rather chronic and subacute psychosis population. At the same time, the CR version showed some interesting correlations with depression: the number of completed sessions was correlated with a reduction on the CES-D which could hint at (but is no proof for) the possibility that training improves well-being. This would be a potentially important finding as neither antipsychotic (Leucht et al., 2009) nor antidepressant medication (Kishi and Iwata, 2014) exert substantial effects on depression in psychosis. Likewise, psychotherapy with cognitive-behavioral therapy only yields a small-to-medium effect according to a meta-analysis (Wykes et al., 2008). However, an opposite causal relationship cannot be fully dismissed: Improvement of well-being may enhance fidelity to perform the tasks. Further, performance gains on the memory task were correlated with improvements on the objective memory test, speaking for the ecological validity of the task. Again, however, group differences were not significant.
At follow-up, the metacognition-enhanced CR training yielded the expected significant effects on the JTC bias (i.e., delayed decision-making) and reduced overconfidence. These findings are noteworthy since both biases are implicated in the formation of psychosis and JTC is rather resistant to change (Ross et al., 2011; So et al., 2012a,b). This delayed effect is interesting and may indicate that the newly acquired skills need some time to settle before they become manifest. At post, we found substantial correlations between fish task parameters with adherence and performance gain.
At first sight, the results are sobering in face of recent reviews indicating that CR tasks may yield at least small-to-medium effects on objective neurocognitive functioning (McGurk et al., 2007; Wykes et al., 2011). A number of factors may have prevented the hypothesized pattern of results from emerging. First, the training was self-paced, that is, individuals were encouraged to perform the tests daily but in fact many did not perform the tasks on a regular basis. In contrast, in many clinical trials on CR there are frequent appointments and homework is checked by therapists. A certain (cued) participation frequency may be necessary to show an effect. Our weekly email reminders may not have been sufficiently strong cues. Second, the group was not severely ill (mainly outpatient treatment) and self-help was performed predominantly at home as patients were not hospitalized. A chronic and more remitted sample is likely to show less benefit from training than an acute and hospitalized sample (e.g., because of regression to the mean). Thus, a first-episode and CR-naive treatment group may show better outcome. Third, the outcome measures did not cover the full range of domains targeted. In fact, we had only one objective memory test with rather low reliability. Perhaps the training exerted effects on functions not covered by our battery. Future studies should therefore administer a wider range of behavioral tests. Finally, while the initial sample was rather large and we had a good retention rate for the post phase, less than 50% participated in the follow-up.
Conclusion
The metacognition-enhanced CR condition showed delayed changes on two prominent cognitive biases which are implicated in the pathogenesis of positive symptoms: jumping to conclusions and overconfidence. The program under investigation now incorporates these additional metacognitive features which are deemed important as prior studies suggest that JTC is quite resistant to change (see above) and is not only tied to positive symptoms but predicts functional outcome to some degree (Andreou et al., 2014). It seems that the training – like metacognitive training (MCT; Moritz et al., 2014a) – successfully “sows the seeds of doubt.” Further studies should investigate whether this leads to a reduction of symptoms in the long run.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Andreou, C., Treszl, A., Roesch-Ely, D., Kother, U., Veckenstedt, R., and Moritz, S. (2014). Investigation of the role of the jumping-to-conclusions bias for short-term functional outcome in schizophrenia. Psychiatry Res. 218, 341–347. doi: 10.1016/j.psychres.2014.04.040
Bang, M., Kim, K. R., Song, Y. Y., Baek, S., Lee, E., and An, S. K. (2014). Neurocognitive impairments in individuals at ultra-high risk for psychosis: who will really convert? Aust. N. Z. J. Psychiatry 49, 462–470. doi: 10.1177/0004867414561527
Beck, A. T., Baruch, E., Balter, J. M., Steer, R. A., and Warman, D. M. (2004). A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr. Res. 68, 319–329. doi: 10.1016/S0920-9964(03)00189-0
Bentall, R. P. (1992). A proposal to classify happiness as a psychiatric disorder. J. Med. Ethics 18, 94–98. doi: 10.1136/jme.18.2.94
Bentall, R. P., and Slade, P. D. (1985). Reliability of a scale measuring disposition towards hallucination: a brief report. Pers. Individ. Dif. 6, 527–529. doi: 10.1016/0191-8869(85)90151-5
Corigliano, V., De Carolis, A., Trovini, G., Dehning, J., Di Pietro, S., Curto, M., et al. (2014). Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res. 220, 129–134. doi: 10.1016/j.psychres.2014.07.067
Davidson, M., Galderisi, S., Weiser, M., Werbeloff, N., Fleischhacker, W. W., Keefe, R. S., et al. (2009). Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST). Am. J. Psychiatry 166, 675–682. doi: 10.1176/appi.ajp.2008.08060806
Deckersbach, T., Moshier, S. J., Tuschen-Caffier, B., and Otto, M. W. (2011). Memory dysfunction in panic disorder: an investigation of the role of chronic benzodiazepine use. Depress. Anxiety 28, 999–1007. doi: 10.1002/da.20891
Fervaha, G., Agid, O., Takeuchi, H., Lee, J., Foussias, G., Zakzanis, K. K., et al. (2015). Extrapyramidal symptoms and cognitive test performance in patients with schizophrenia. Schizophr. Res. 161, 351–356. doi: 10.1016/j.schres.2014.11.018
Fett, A. K., Viechtbauer, W., Dominguez, M. D., Penn, D. L., van Os, J., and Krabbendam, L. (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 35, 573–588. doi: 10.1016/j.neubiorev.2010.07.001
Fine, C., Gardner, M., Craigie, J., and Gold, I. (2007). Hopping, skipping or jumping to conclusions? Clarifying the role of the JTC bias in delusions. Cogn. Neuropsychiatry 12, 46–77. doi: 10.1080/13546800600750597
Freeman, D., Garety, P. A., Bebbington, P. E., Smith, B., Rollinson, R., Fowler, D., et al. (2005). Psychological investigation of the structure of paranoia in a non-clinical population. Br. J. Psychiatry 186, 427–435. doi: 10.1192/bjp.186.5.427
Garety, P. A., and Freeman, D. (1999). Cognitive approaches to delusions: a critical review of theories and evidence. Br. J. Clin. Psychol. 38, 113–154. doi: 10.1348/014466599162700
Garety, P. A., and Freeman, D. (2013). The past and future of delusions research: from the inexplicable to the treatable. Br. J. Psychiatry 203, 327–333. doi: 10.1192/bjp.bp.113.126953
Garety, P. A., Hemsley, D. R., and Wessely, S. (1991). Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J. Nerv. Ment. Dis. 179, 194–201. doi: 10.1097/00005053-199104000-00003
Gasso, P., Mas, S., Molina, O., Bernardo, M., Lafuente, A., and Parellada, E. (2012). Neurotoxic/neuroprotective activity of haloperidol, risperidone and paliperidone in neuroblastoma cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 71–77. doi: 10.1016/j.pnpbp.2011.08.010
Gaweda, L., Moritz, S., and Kokoszka, A. (2012). Impaired discrimination between imagined and performed actions in schizophrenia. Psychiatry Res. 195, 1–8. doi: 10.1016/j.psychres.2011.07.035
Goodarzi, M. A. (2009). Psychometric properties of a Persian translation of the Launay-Slade Hallucination Scale in an Iranian population. Percept. Mot. Skills 109, 911–923. doi: 10.2466/pms.109.3.911-923
Green, M. F. (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry 153, 321–330. doi: 10.1176/ajp.153.3.321
Green, M. F., Kern, R. S., and Heaton, R. K. (2004). Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr. Res. 72, 41–51. doi: 10.1016/j.schres.2004.09.009
Hautzinger, M., and Brähler, M. (1993). Allgemeine Depressionsskala (ADS) [General Depression Scale]. Göttingen: Hogrefe.
Heinrichs, R. W., and Zakzanis, K. K. (1998). Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12, 426–445. doi: 10.1037/0894-4105.12.3.426
Ho, B. C., Andreasen, N. C., Ziebell, S., Pierson, R., and Magnotta, V. (2011). Long-term antipsychotic treatment and brain volumes. A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 68, 128–137. doi: 10.1001/archgenpsychiatry.2010.199
Keefe, R. S., Bilder, R. M., Davis, S. M., Harvey, P. D., Palmer, B. W., Gold, J. M., et al. (2007). Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry 64, 633–647. doi: 10.1001/archpsyc.64.6.633
Keefe, R. S. E., and Harvey, P. D. (2012). “Cognitive impairment in schizophrenia,” in Novel Antischizophrenia Treatments. Handbook of Experimental Pharmacology, eds M. A. Geyer and G. Gross (Berlin: Springer), 11–37. doi: 10.1007/978-3-642-25758-2_2
Kishi, T., and Iwata, N. (2014). Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia. Int. J. Neuropsychopharmacol. 17, 343–354. doi: 10.1017/S1461145713000667
Kother, U., Veckenstedt, R., Vitzthum, F., Roesch-Ely, D., Pfueller, U., Scheu, F., et al. (2012). “Don’t give me that look” - overconfidence in false mental state perception in schizophrenia. Psychiatry Res. 196, 1–8. doi: 10.1016/j.psychres.2012.03.004
Lepage, M., Bodnar, M., and Bowie, C. R. (2014). Neurocognition: clinical and functional outcomes in schizophrenia. Can. J. Psychiatry 59, 5–12.
Leucht, S., Arbter, D., Engel, R. R., Kissling, W., and Davis, J. M. (2009). How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol. Psychiatry 14, 429–447. doi: 10.1038/sj.mp.4002136
Lincoln, T. M., Peter, N., Schafer, M., and Moritz, S. (2010a). From stress to paranoia: an experimental investigation of the moderating and mediating role of reasoning biases. Psychol. Med. 40, 169–171. doi: 10.1017/S003329170999095X
Lincoln, T. M., Ziegler, M., Lullmann, E., Muller, M. J., and Rief, W. (2010b). Can delusions be self-assessed? Concordance between self- and observer-rated delusions in schizophrenia. Psychiatry Res. 178, 249–254. doi: 10.1016/j.psychres.2009.04.019
Mass, R., Wolf, K., and Lincoln, T. M. (2012). Associations of the beck cognitive insight scale (BCIS) with poor insight, subjective experiences, and depression. Int. J. Cogn. Ther. 5, 197–210. doi: 10.1521/ijct.2012.5.2.197
McGurk, S. R., Twamley, E. W., Sitzer, D. I., McHugo, G. J., and Mueser, K. T. (2007). A meta-analysis of cognitive remediation in schizophrenia. Am. J. Psychiatry 164, 1791–1802. doi: 10.1176/appi.ajp.2007.07060906
Moritz, S., Andreou, C., Klingberg, S., Thoering, T., and Peters, M. J. (2013a). Assessment of subjective cognitive and emotional effects of antipsychotic drugs. Effect by defect? Neuropharmacology 72, 179–186. doi: 10.1016/j.neuropharm.2013.04.039
Moritz, S., Favrod, J., Andreou, C., Morrison, A. P., Bohn, F., Veckenstedt, R., et al. (2013b). Beyond the usual suspects: positive attitudes towards positive symptoms is associated with medication noncompliance in psychosis. Schizophr. Bull. 39, 917–922. doi: 10.1093/schbul/sbs005
Moritz, S., Andreou, C., Schneider, B. C., Wittekind, C. E., Menon, M., Balzan, R. P., et al. (2014a). Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin. Psychol. Rev. 34, 358–366. doi: 10.1016/j.cpr.2014.04.004
Moritz, S., Ramdani, N., Klass, H., Andreou, C., Jungclaussen, D., Eifler, S., et al. (2014b). Overconfidence in incorrect perceptual judgments in patients with schizophrenia. Schizophr. Res. Cogn. 1, 165–170. doi: 10.1016/j.scog.2014.09.003
Moritz, S., Veckenstedt, R., Andreou, C., Bohn, F., Hottenrott, B., Leighton, L., et al. (2014c). Sustained and “sleeper” effects of group metacognitive training for schizophrenia: a randomized clinical trial. JAMA Psychiatry 71, 1103–1111. doi: 10.1001/jamapsychiatry.2014.1038
Moritz, S., Ferahli, S., and Naber, D. (2004). Memory and attention performance in psychiatric patients: lack of correspondence between clinician-rated and patient-rated functioning with neuropsychological test results. J. Int. Neuropsychol. Soc. 10, 623–633. doi: 10.1017/S1355617704104153
Moritz, S., Van Quaquebeke, N., and Lincoln, T. M. (2012a). Jumping to conclusions is associated with paranoia but not general suspiciousness: a comparison of two versions of the probabilistic reasoning paradigm. Schizophr. Res. Treatment 2012, 384039. doi: 10.1155/2012/384039
Moritz, S., Woznica, A., Andreou, C., and Kother, U. (2012b). Response confidence for emotion perception in schizophrenia using a Continuous Facial Sequence Task. Psychiatry Res. 200, 202–207. doi: 10.1016/j.psychres.2012.07.007
Moritz, S., and Woodward, T. S. (2006a). The contribution of metamemory deficits to schizophrenia. J. Abnorm. Psychol. 115, 15–25. doi: 10.1037/0021-843X.15.1.15
Moritz, S., and Woodward, T. S. (2006b). Metacognitive control over false memories: a key determinant of delusional thinking. Curr. Psychiatry Rep. 8, 184–190. doi: 10.1007/s11920-006-0022-2
Moritz, S., Woodward, T. S., and Ruff, C. C. (2003). Source monitoring and memory confidence in schizophrenia. Psychol. Med. 33, 131–139. doi: 10.1017/S0033291702006852
Peters, M. J., Hauschildt, M., Moritz, S., and Jelinek, L. (2013). Impact of emotionality on memory and meta-memory in schizophrenia using video sequences. J. Behav. Ther. Exp. Psychiatry 44, 77–83. doi: 10.1016/j.jbtep.2012.07.003
Pohl, P. F. (2004). Cognitive Illusions: A Handbook on Fallacies and Biases in Thinking, Judgement and Memory. Hove: Psychology Press.
Radloff, L. S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. doi: 10.1177/014662167700100306
Roder, V., Mueller, D. R., and Schmidt, S. J. (2011). Effectiveness of integrated psychological therapy (IPT) for schizophrenia patients: a research update. Schizophr. Bull. 37(Suppl. 2), S71–S79. doi: 10.1093/schbul/sbr072
Ross, K., Freeman, D., Dunn, G., and Garety, P. (2011). A randomized experimental investigation of reasoning training for people with delusions. Schizophr. Bull. 37, 324–333. doi: 10.1093/schbul/sbn165
So, S. H., Freeman, D., Dunn, G., Kapur, S., Kuipers, E., Bebbington, P., et al. (2012a). Jumping to conclusions, a lack of belief flexibility and delusional conviction in psychosis: a longitudinal investigation of the structure, frequency, and relatedness of reasoning biases. J. Abnorm. Psychol. 121, 129–139. doi: 10.1037/a0025297
So, S. H., Garety, P. A., Peters, E. R., and Kapur, S. (2012b). Change in delusional dimensional dimensions, reasoning biases and emotions in the first eight weeks of antipsychotic treatment. Schizophr. Res. 136(Suppl. 1), S375. doi: 10.1016/S0920-9964(12)71014-9
Speechley, W. J., Whitman, J. C., and Woodward, T. S. (2010). The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J. Psychiatry Neurosci. 35, 7–17. doi: 10.1503/jpn.090025
Ukai, W., Ozawa, H., Tateno, M., Hashimoto, E., and Saito, T. (2004). Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J. Neural Transm. 111, 667–681. doi: 10.1007/s00702-004-0109-z
Varese, F., Barkus, E., and Bentall, R. P. (2012). Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychol. Med. 42, 1025–1036. doi: 10.1017/S0033291711001826
Veckenstedt, R., Randjbar, S., Vitzthum, F., Hottenrott, B., Woodward, T. S., and Moritz, S. (2011). Incorrigibility, jumping to conclusions, and decision threshold in schizophrenia. Cogn. Neuropsychiatry 16, 174–192. doi: 10.1080/13546805.2010.536084
Vinogradov, S., Fisher, M., Warm, H., Holland, C., Kirshner, M. A., and Pollock, B. G. (2009). The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am. J. Psychiatry 166, 1055–1062. doi: 10.1176/appi.ajp.2009.09010017
Woodward, T. S., Moritz, S., Cuttler, C., and Whitman, J. C. (2006). The contribution of a cognitive bias against disconfirmatory evidence (BADE) to delusions in schizophrenia. J. Clin. Exp. Neuropsychol. 28, 605–617. doi: 10.1080/13803390590949511
Woodward, T. S., Moritz, S., Menon, M., and Klinge, R. (2008). Belief inflexibility in schizophrenia. Cogn. Neuropsychiatry 13, 267–277. doi: 10.1080/13546800802099033
Wykes, T., Huddy, V., Cellard, C., McGurk, S. R., and Czobor, P. (2011). A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am. J. Psychiatry 168, 472–485. doi: 10.1176/appi.ajp.2010.10060855
Keywords: psychosis, schizophrenia, neurocognition, cognitive biases, cognitive remediation
Citation: Moritz S, Thoering T, Kühn S, Willenborg B, Westermann S and Nagel M (2015) Metacognition-augmented cognitive remediation training reduces jumping to conclusions and overconfidence but not neurocognitive deficits in psychosis. Front. Psychol. 6:1048. doi: 10.3389/fpsyg.2015.01048
Received: 30 March 2015; Accepted: 09 July 2015;
Published: 03 August 2015.
Edited by:
Gianluca Castelnuovo, Università Cattolica del Sacro Cuore, ItalyReviewed by:
Guido Edoardo D’Aniello, I.R.C.C.S. Istituto Auxologico Italiano, ItalyLaurent Pezard, Aix-Marseille Université, France
Copyright © 2015 Moritz, Thoering, Kühn, Willenborg, Westermann and Nagel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffen Moritz, Clinical Neuropsychology, Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, moritz@uke.uni-hamburg.de
†These authors share first authorship.
 Steffen Moritz
Steffen Moritz Teresa Thoering1†
Teresa Thoering1† Simone Kühn
Simone Kühn Bastian Willenborg
Bastian Willenborg Stefan Westermann
Stefan Westermann Matthias Nagel
Matthias Nagel