- 1Behavior Rehabilitation Training Research Institution, School of Psychology, Northwest Normal University, Lanzhou, China
- 2Lanzhou University Second Hospital, Lanzhou, China
- 3School of Psychology, Beijing Normal University, Beijing, China
Behavioral inhibitory control has been shown to play an important role in a variety of addictive behaviors. A number of studies involving the use of Go/NoGo and stop-signal paradigms have shown that smokers have reduced response inhibition for cigarette-related cues. However, it is not known whether male light smokers’ response inhibition for cigarette-related cues is lower than that of non-smokers in the two-choice oddball paradigm. The objective of the current study was to provide further behavioral evidence of male light smokers’ impaired response inhibition for cigarette-related cues, using the two-choice oddball paradigm. Sixty-two male students (31 smokers, 31 non-smokers), who were recruited via an advertisement, took part in this two-choice oddball experiment. Cigarette-related pictures (deviant stimuli) and pictures unrelated to cigarettes (standard stimuli) were used. Response inhibition for cigarette-related cues was measured by comparing accuracy (ACC) and reaction time (RT) for deviant and standard stimuli in the two groups of subjects. An analysis of variance (ANOVA) showed that in all the participants, ACC was significantly lower for deviant stimuli than for standard stimuli. For deviant stimuli, the RTs were significantly longer for male light smokers than for male non-smokers; however, there was no significant difference in RTs for standard stimuli. Compared to male non-smokers, male light smokers seem to have a reduced ability to inhibit responses to cigarette-related cues.
Introduction
The sense of craving for smoking triggered by cigarette-related cues promotes the maintenance of smoking behavior, thus affecting cigarette withdrawal (Waters et al., 2004). Currently, relevant studies indicate that smokers have impaired response inhibition for cigarette-related cues (Spinella, 2002; Brody et al., 2004; Gallinat et al., 2006; McBride et al., 2006; Xu et al., 2008; Luijten et al., 2011; MacKillop et al., 2011). Response inhibition is an important executive function. It refers to the suppression of inappropriate or no longer relevant behavior, which allows flexible, intentional, behavioral reactions to the environment (Groman et al., 2009; Verbruggen and Logan, 2009). In general, for smokers, cigarette-related cues increase the craving for smoking, which leads to automatic attentional biases to smoking cues (Johnsen et al., 1997; Waters and Feyerabend, 2000; Ehrman et al., 2002). Consequently, the attentional bias toward cigarette-related cues in smokers occupies their cognitive resources and affects their performance on response inhibition tasks (Ryan, 2002; Franken, 2003).
In recent years, studies using event-related potentials (ERPs) have found that cigarette-related cues can impair smokers’ response inhibition (Luijten et al., 2011; Impey et al., 2013; Buzzell et al., 2014; Logemann et al., 2014). Studies have found that compared to controls, reduced NoGo-N2 amplitudes in smokers were accompanied by decreased task performance, whereas no differences between groups were found in the case of P3 amplitudes. This was found to represent a general lack of response inhibition in smokers (Luijten et al., 2011; Buzzell et al., 2014). The studies suggested that NoGo-N2 reflects a top–down inhibition mechanism, that is, it inhibits inappropriate response tendencies before the movement execution (Kok et al., 2004). The inability to inhibit response tendencies during early processing is likely to reflect that smokers are affected by the nicotine intake because of their tendency to inhibit inappropriate responses before movement execution (Luijten et al., 2011). Recent structural and functional brain imaging studies have found that the anterior cingulated cortex exhibited differential activation during exposure to cigarette-related, as opposed to neutral cues, and that sub-regions of the prefrontal cortex showed cue-elicited activation that was modulated by smoking expectancy. In addition, the volume and density of smokers’ gray matter in the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex are significantly lower than those of non-smokers. The volume of gray matter in the left dorsal anterior cingulated cortex of smokers and the density of the gray matter they have in the right cerebellar hemisphere are also lower than those of non-smokers (Brody et al., 2004; Gallinat et al., 2006). Because the dorsolateral prefrontal cortex plays an important role in working memory and other cognitive domains such as information maintaining and processing, researchers believe that structural defects can lead to cognitive defects in smokers (Azizian et al., 2008; Xu et al., 2008).
Previous studies have mostly used Go/NoGo tasks (Spinella, 2002; Evans et al., 2009; Luijten et al., 2011; Impey et al., 2013; Longo et al., 2013; Buzzell et al., 2014; Rass et al., 2014) and stop-signal tasks (Monterosso et al., 2005; Billieux et al., 2010; de Ruiter et al., 2012; Logemann et al., 2014) to investigate response inhibition for cigarette-related cues in smokers. These studies have shown that commission errors and reaction times (RTs), which reflect response inhibition for cigarette-related cues, were significantly high in smokers compared to non-smokers (Spinella, 2002; Billieux et al., 2010; Nestor et al., 2011; de Ruiter et al., 2012). More precisely, Luijten et al. (2011) used cigarette-related and cigarette-unrelated pictures and a Go/NoGo task to compare the response inhibition of moderate smokers and non-smokers for cigarette-related cues, and found that accuracy in response to NoGo stimuli, which reflects suppression ability, was significantly low in moderate smokers compared to non-smokers. In addition, a stop-signal task was used to assess smokers’ response inhibition ability. Logemann et al. (2014) found no difference between smokers and non-smokers with regard to RTs for Go stimuli, whereas the stop signal RT was dramatically longer in smokers than in non-smokers. However, some studies have found that there was no difference between smokers and non-smokers under neutral conditions in commission error and RT using Go/NoGo task and stop-signal task (Monterosso et al., 2005; Evans et al., 2009; de Ruiter et al., 2012; Buzzell et al., 2014; Rass et al., 2014). For example, Buzzell et al. (2014) compared commission error on NoGo trials and omission error on Go trials between smokers and non-smokers using alphabetic Go/No-Go task, it has been found that there was no significant difference in behavioral results. The results of this research are consistent with Evans et al.’s (2009) study, the stimuli were presented for 800 ms in the Go/No-Go task. The entire experiment consisted of 900 trials, 83 of which were NoGo trials. No differences were found between smokers and non-smokers, with regard to performance accuracy in the NoGo trials and RT in the Go trials.
In a stop-signal task, the reaction that is about to be stopped has been under processing, which has already reached a certain level and which is way beyond the level of the processing of a NoGo stimuli. There is evidence that the basal ganglia have an important effect on the launch and inhibition of the action response in stop-signal tasks (Eagle and Robbins, 2003). The basal ganglia, a group of subcortical nuclei in the forebrain, include the caudate nucleus, putamen, globus pallidus, subthalamic nucleus, and substantia nigra (Alexander and Crutcher, 1990). In the nucleus of the basal ganglia, the subthalamic nucleus may be a critical structure involved in the processing of response inhibition (Cohen and Frank, 2009; Ray et al., 2012; Wiecki and Frank, 2013). Aron and Poldrack (2006) studied the relationship between the subthalamic nucleus and stop signal RT; they found that the stronger the subthalamic nucleus activation, the shorter was the stop signal RT of the participants. Research comparing the structure of the brain mechanism of smokers and non-smokers has revealed that compared to non-smokers, the size and density of the gray matter in the thalamus, cerebellum, and nigra are less in smokers than in non-smokers. This is because the competition between reaction and non-reaction occurs in the nucleus of the basal ganglia, which, once stimulated and damaged, influence the response inhibition (Chambers et al., 2006).
Because in a stop-signal task, participants need to stop their response when they see the stop signal, to maintain a high rate of successful inhibition, they have to pay more attention to the stop signal and consciously wait for it. Consequently, the measurement of RT for Go stimuli may be inaccurate (Verbruggen and Logan, 2008). However, in a Go/NoGo task, the participants only have to respond to Go, and not NoGo stimuli; as Go trials require motor responses, and NoGo trials do not, the inhibitory control effects observed in studies using the Go/NoGo paradigm are likely to be contaminated by response-related processes (Yuan et al., 2008). That is, Go/NoGo tasks may not provide an effective behavioral indicator of response inhibition. Therefore, the present study involved the use of a two-choice oddball task that required the participants to respond to both standard and deviant stimuli by pressing different keys as quickly and accurately as possible, rather than only responding to Go stimuli in a Go/NoGo task. A two-choice oddball task requires responses to both standard and deviant stimuli so that the results are not contaminated by motor response-related processes. As a result, the difference between the RTs for deviant and standard stimuli is the behavioral index of response inhibition (Yuan et al., 2008).
In the present study, the two-choice oddball paradigm was used to further investigate the influence of cigarette-related cues on male light smokers’ ability to inhibit responses. The two-choice oddball paradigm was based on the traditional oddball paradigm. The participants were asked to press different buttons quickly and accurately in response to high probability standard stimuli and low probability deviant stimuli. The response to standard stimuli became the dominant response because the standard stimuli were presented more often than deviant stimuli. Consequently, when the deviant stimuli appeared, the participants needed to inhibit the dominant standard stimuli response to perform accurately.
Materials and Methods
Participants
As it was difficult to recruit female smokers, 62 male (31 light smokers, 31 non-smokers) undergraduate students were recruited as participants in the experiment. The data of one smoker was not considered because he misunderstood the task. The final group consisted of 30 smokers and 31 non-smokers. As paid volunteers, all the participants signed an informed consent form prior to participating in the experiment. The experiment was approved by the Academic Committee of the School of Psychology, Northwest Normal University, China. The participants also completed questionnaires eliciting demographic information as well as information about their medical and smoking history; further, they completed the Fagerstrom test for Nicotine Dependence (FTND), Beck Depression Inventory (BDI), and the Barratt Impulsiveness Scale-11(BIS-11; Table 1).
Prior to participating in the study, the participants were administered a short questionnaire that was designed for use in this study, and if they fulfilled one or more of certain conditions, they were excluded from the study. These conditions were if they smoked regularly for <2 years; if they were, at that time, under any medications that may affect cognition; if they were suffering from any medical or psychiatric condition that could affect brain function; if they were <18 years; if they had a history of head trauma; if they smoked >1 marijuana cigarette per week; if they consumed >10 standard drinks of alcohol per week; or if they regularly abused substances other than alcohol or marijuana.
At the time of the experiment, the participants were non-deprived smokers. The non-smokers had no current or previous history of cigarette smoking, and the number of ambidextrous (i.e., right- and left-handed) participants was not significant in either of the groups χ2 (61) = 0.669, p = 0.414. All the participants had normal or corrected-to-normal vision.
Instruments
The Fagerstrom Test for Nicotine Dependence (FTND)
The FTND is a six-item revised version of the Fagerström Tolerance Questionnaire (Heatherton et al., 1991), and it includes six of the Fagerström Tolerance Questionnaire’s eight items. With its high reliability and validity, the FTND has been used in many countries to test nicotine dependence. The six-item questionnaire includes questions such as “How many cigarettes do you smoke a day?” “How soon after waking up do you smoke your first cigarette?” and “Do you smoke if you are so ill that you are in bed for most of the day?” FTND scores range from 0 to 10; higher scores indicate greater nicotine dependence. A score of 1–3 indicates low nicotine dependence, a score of 4–5 indicates moderate dependence, and a score of 6 indicates high dependence. The revised Chinese version of the FTND, which has been shown to be valid and reliable, was used (Huang et al., 2006).
The Beck Depression Inventory (BDI)
The BDI is one of the most widely used self-rating scales of depressive symptoms. It not only assesses the presence of depressive symptoms and their degree of severity but also evaluates depression symptoms in control groups. The BDI was developed by Baker in 1961 (Erbauch, 1961), and the revised Chinese version has been shown to have good validity and reliability (Shek, 1990).
Barratt Impulsiveness Scale-11 (BIS-11)
The BIS-11 is a self-rating instrument that is often used to assess impulsiveness. It has three factors (Attentional Impulsiveness, Motor Impulsiveness, and Non-planning Impulsiveness) measured by 26 items. Each factor consists of two sub-scales: Attentional Impulsiveness includes attention and cognitive impulsivity, Motor Impulsiveness includes sports impulsivity and perseverance, and Non-planning Impulsiveness includes automation and cognitive complexity. Items are scored based on the frequency of the appearance of symptoms. The scores range from 1 to 4 (1 = hardly/never, 2 = occasionally, 3 = often, 4 = almost always/always), and 11 items are reverse-scored. Higher scores indicate higher levels of impulsiveness (Patton et al., 1995). The Chinese version of the BIS-11 has good reliability and validity (Zhou, 2006).
Materials
The two-choice oddball paradigm was used to assess inhibition ability. There were two kinds of stimuli: standard stimuli (cigarette-unrelated pictures) and deviant stimuli (cigarette-related pictures). Stimuli were selected from the International Affective Pictures System and the Internet, and included 27 cigarette-unrelated and 27 cigarette-related pictures. Photoshop 7.0 software was used to edit the pictures. To prevent the participants from guessing the objective of the experiment, a colored frame surrounded the pictures (blue frames for cigarette-related pictures and yellow frames for cigarette-unrelated pictures). The participants were told to press a button to indicate whether the frame was blue or yellow. The pictures were assessed by 61 male students (30 light smokers, 31 non-smokers), who were not participants of the present study but who were recruited from the same population of students as were the participants [age: F(1,120) = 0.505, p = 0.479, η2 = 0.004; height: F(1,120) = 1.495, p = 0.224, η2 = 0.012; weight: F(1,120) = 1.622, p = 0.205, η2= 0.013]. These 54 pictures were rated in terms of valence (1 = very pleasant, 9 = very unpleasant), arousal (1 = drowsy, 9 = excited), dominance (1 = picture control, 9 = you own pictures), and their degree of relatedness to cigarettes (1 = picture has nothing to do with cigarettes, 9 = picture is related to cigarettes). The participants who participated in the picture assessment task were not allowed to participate in the main experiment.
The results showed that there was no difference between cigarette-related pictures (valence: M = 4.56, SD = 2.15; arousal: M = 4.67, SD = 1.99; dominance: M = 5.34, SD = 2.05) and cigarette-unrelated pictures (valence: M = 5.21, SD = 1.42; arousal: M = 4.98, SD = 1.09; dominance: M = 5.29, SD = 1.52) with regard to valence [F(1,59) = 3.075, p = 0.085, η2 = 0.049], arousal [F(1,59) = 0.955, p = 0.332, η2= 0.016], and dominance [F(1,59) = 0.024, p = 0.878, η2 < 0.001]. The degree of relatedness to cigarettes was significantly higher for cigarette-related pictures (M = 7.45, SD = 1.66) than it was for cigarette-unrelated pictures [M = 2.76, SD = 1.48, F(1,59) = 184.56, p < 0.001, η2 = 0.755].
Task and Procedure
A modified oddball task was used, and the experiment had two blocks of 200 trials. In each block, 170 standard and 30 deviant stimuli (85% vs. 15%, respectively) were presented. In addition, all the pictures were identical in term of size and resolution (210 × 210 pixels).
The participants were seated in an acoustically isolated room at approximately 60 cm from a computer screen. Each block began with a 1000 ms presentation of a small black cross on a gray computer screen; then, a blank screen whose duration varied randomly from 500 to 1500 ms was presented, and then, a stimulus was presented for 500 ms (see Figure 1). In the current study, the participants were instructed to make a standard/deviant categorization by pressing different keys as accurately and, then, as quickly as possible. Within a sequence, the order of the presentation was random, with the restriction that the deviant stimuli could only be presented twice in succession. Additionally, accurate responses to both the standard and deviant stimuli were emphasized during the task. Because the standard stimuli were presented much more frequently than were the deviant stimuli, to make a correct response to the deviant stimuli, the participants consequently had to inhibit dominate responses to the standard stimuli during the onset of the deviant stimuli.
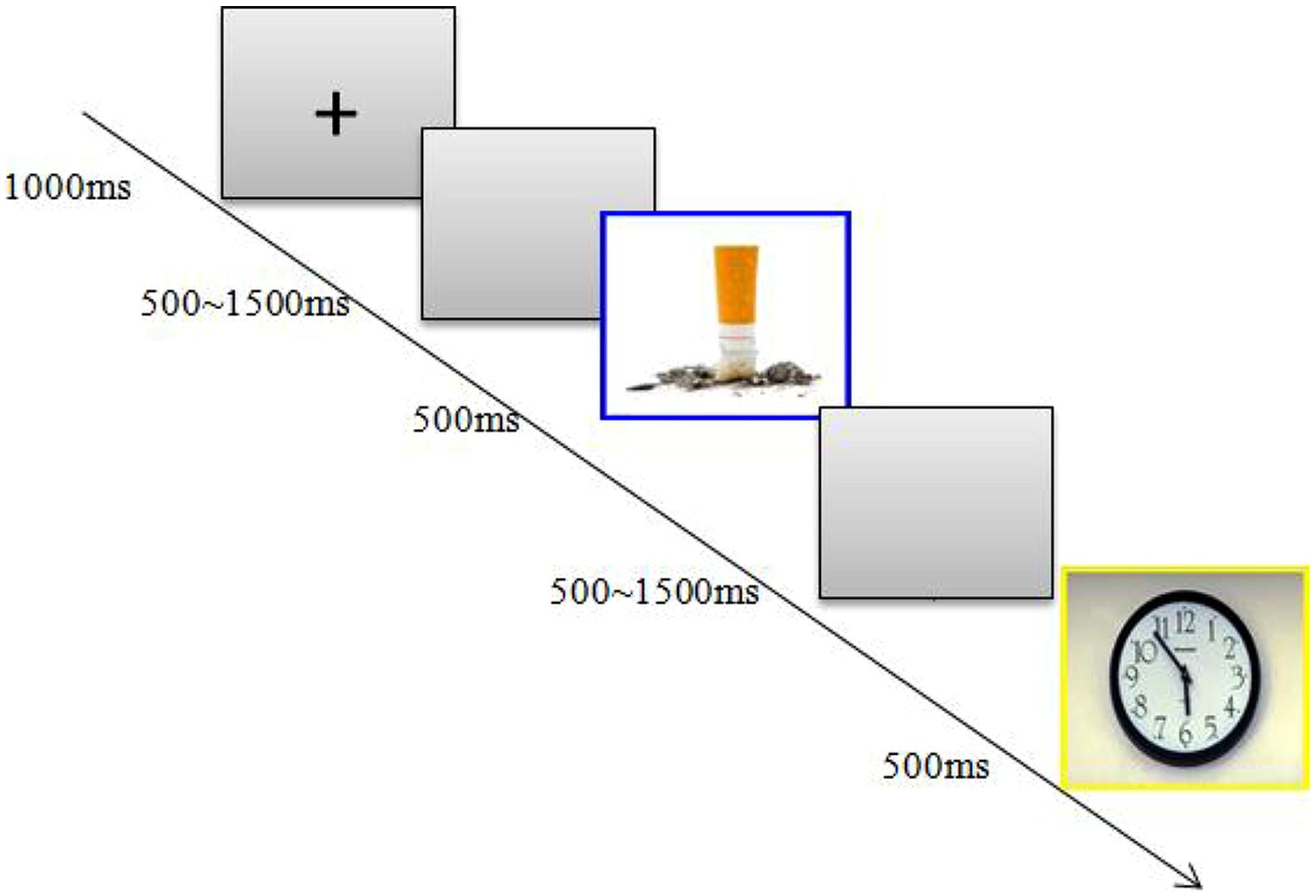
FIGURE 1. Study design. Participants were shown neutral pictures frequently and smoking-related pictures infrequently.
Within each group, half the participants were instructed to press the “F” key with their index finger (as accurately and quickly as possible) if the standard picture appeared and to press the “J” key if the deviant picture appeared. For the remaining participants, the assignment of response hands was reversed for controlling the influence of response hands. The stimulus picture ceased to appear on the screen if a key was pressed or after the picture had appeared for 1000 ms. Therefore, the participants were informed that they had to respond within 1000 ms. Each response was followed by a blank screen (which appeared randomly from 500 to 1500 ms). Pre-training with 20 practice trials was conducted before the experiment to familiarize the participants with the procedure, and the pictures used during the practice were not used in the experimental block. All the participants achieved 85% accuracy during practice.
All the participants provided basic information and informed consent, completed the FTND, BDI, and BIS-11 using a paper and pencil, and then started the main experiment. The entire experiment lasted for 15 min.
Results
The accuracy and RT for deviant and standard stimuli were analyzed to assess response inhibition. The data for less than 15% of the trials (i.e., trials for which RTs were less than 150 ms) were not considered (Meule et al., 2012). The main statistical methods in the database—repeated measures ANOVA were used. In the experimental task, the between-subjects variable was group (male light smokers vs. non-smokers), and the within-subject variable was stimulus type (deviation stimuli vs. standard stimuli).
Accuracy
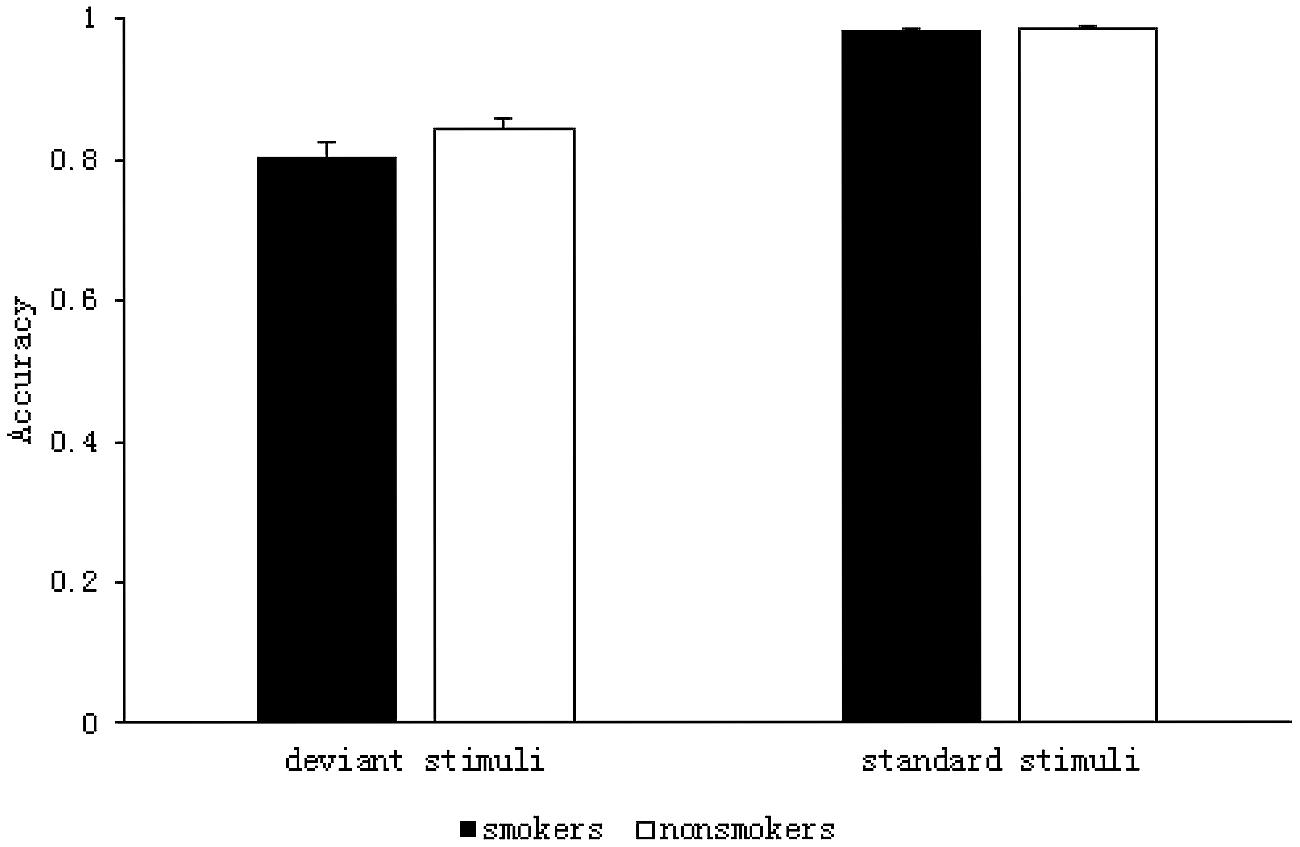
The results of the 2 (group: smoker, non-smoker) × 2 (stimulus type: deviant, standard) repeated measures ANOVA are shown in Figure 2. The main effect of group was not significant F(1,59) = 2.191, p = 0.144, η2= 0.036. However, there was a significant main effect of stimulus type F(1,59) = 181.384, p < 0.001, η2 = 0.755; ACC was significantly lower for the deviant stimuli (M = 0.82, SD = 0.01) than it was for the standard stimuli (M = 0.99, SD = 0.001) in both the groups. There was no significant interaction between group and stimulus type F(1,59) = 1.941, p = 0.169, η2= 0.032.
Reaction Time
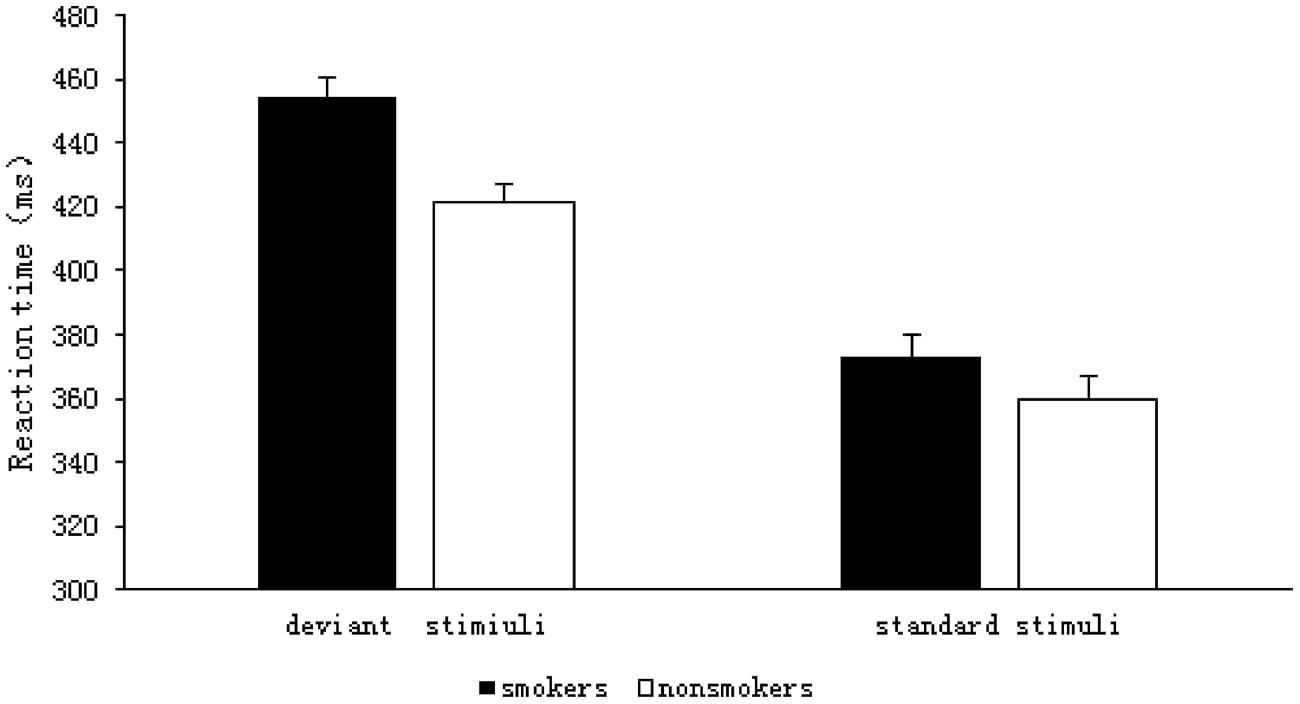
The results of the 2 (group: smoker, non-smoker) × 2 (stimulus type: deviant, standard) repeated measures ANOVA are shown in Figure 3. The RT analysis indicated a significant main effect of group F(1,59) = 8.512, p = 0.005, η2= 0.126, such that the RTs were longer for the smokers (M = 413.71, SD = 5.65) than they were for the non-smokers (M = 390.58, SD = 5.56). There was also a significant main effect of stimulus type F(1,59) = 228.05, p < 0.001, η2= 0.794; RTs were significantly longer for the deviant stimuli (M = 438.07, SD = 4.10) than they were for the standard stimuli (M = 366.22, SD = 5.09) in both the groups. There was a significant interaction between group and stimulus type F(1,59) = 4.213, p = 0.045, η2= 0.067, and a simple effect analysis showed that the RTs for deviant stimuli were significantly longer for smokers (M = 454.52, SD = 5.85) than they were for non-smokers (M = 421.62, SD = 5.75), F(1,59) = 16.091, p < 0.001, η2= 0.214. In contrast, the RTs for standard stimuli did not differ between the groups (smokers: M = 372.91, SD = 7.26; non-smokers: M = 359.54, SD = 7.14, F(1,59) = 1.723, p = 0.194, η2 = 0.028).
Discussion
The aim of the present study was to explore the differences between male light smokers and non-smokers with regard to the ability to inhibit responses to cigarette-related cues, using the two-choice oddball paradigm. The results showed that compared to non-smokers, male light smokers have a poor ability to inhibit responses. Specifically, male smokers showed a longer delay effect for deviant cigarette-related stimuli. The results of this study are consistent with those of previous researches (Powell et al., 2004; Bekker et al., 2005; Musso et al., 2007; Billieux et al., 2010; Luijten et al., 2011). For deviant stimuli, the RTs were significantly longer for smokers than they were for non-smokers. The results indicated that cigarette-related stimuli had a greater impact on response inhibition in male light smokers than they did for response inhibition in male non-smokers. In other words, compared to non-smokers, the response inhibition of male light smokers is closely associated with cigarette-related cues.
In recent years, some studies have found that compared to controls, response inhibition ability in smokers were affected by nicotine intake (Monterosso et al., 2005; Luijten et al., 2011; Charles-Walsh et al., 2014). Smokers were further divided into light, moderate and heavy group. Compared with moderate group and heavy group, response inhibition ability in light smokers was not influenced by the nicotine. For example, some studies did not find differences in inhibitory control (measured with Go/No-Go or stop-signal tasks) between non-smokers and light smokers (5–10 cigarettes per day; Dinn et al., 2004; Reynolds et al., 2007). In addition, Robinson et al. (1992) recruited five male smokers, after 48-h abstention from tobacco product use, smoking a leading “light” category cigarette (Control 0.6 mg nicotine) and another cigarette yielding similar amount of “tar” and carbon monoxide (CO), but only 0.06 mg nicotine (Test). The electroencephalogram (EEG) were monitored before, during and after the smoking of each cigarette. The results indicated that smoking the Test cigarette had no effect on the EEG. Smoking the Control cigarette produced a significant increase in beta2 magnitude and a significant decrease in delta magnitude. This study selected male light smokers as participants, and they showed poor response inhibition for cigarette-related cues. Probably because cigarette-related cues lead to a craving for smoking, and this craving leads smokers to automatically attend to cigarette-related cues. Consequently, cognitive resources were occupied, which affected the smokers’ performance on the cognitive task (Johnsen et al., 1997; Waters and Feyerabend, 2000; Ehrman et al., 2002; Ryan, 2002; Franken, 2003). Previous studies have found that the presentation of cigarette-related cues interfered with working memory encoding (Heishman et al., 2006), thus affecting the response speed for visual stimuli (Sayette and Hufford, 1994), reading (Zwaan et al., 2000), and arithmetic tasks (Madden and Zwaan, 2001). In general, as addiction develops, the addictive substance-related stimulus becomes particularly attractive, and can grab individuals’ attention (Field and Cox, 2008). A recent study found that individuals’ attentional bias to addictive substance-related cues is positively related to decreased inhibition control in decision-making, especially when the decisions are related to the addictive substance (Field et al., 2007).
The majority of previous studies have used Go/NoGo and stop-signal tasks to show the effects of nicotine on executive function in smokers (Bekker et al., 2005; Billieux et al., 2010; Luijten et al., 2011). In recent years, researchers have also adopted the two-choice oddball paradigm to investigate response inhibition (Wang et al., 2011; Yuan et al., 2011). Here, the two-choice oddball paradigm was used to explore response inhibition for cigarette-related cues in male light smokers. Cigarette-related pictures and cigarette-unrelated pictures were used as deviant and standard stimuli, respectively. The response inhibition of smokers was compared to that of non-smokers, and so was the difference in results among the Go/No-Go, stop-signal, and two-choice oddball paradigms. Compared to Go/NoGo tasks and stop-signal tasks, in the two-choice oddball paradigm, in addition to perceptual processing, stimulus identification, response selection, and execution, deviant stimuli require the behavioral inhibition of the dominant response. In contrast, behavioral inhibition is not required for standard stimuli (Yuan et al., 2008). Therefore, regardless of whether the cues are cigarette-related or cigarette-unrelated; the RTs were significantly longer for the deviant stimuli than they were for the standard stimuli. Because of the need for response inhibition, all the participants showed a significant delay effect for deviant stimuli, but this was especially pronounced in male light smokers.
We only used the two-choice oddball paradigm to explore male light smokers’ behavioral ability to inhibit responses to cigarette-related cues. The neurophysiological mechanism underlying impaired response inhibition for cigarette-related cues in smokers remains unclear. Luijten et al. (2011) compared ERP components of 19 moderate smokers and 20 non-smokers, in a Go/NoGo task. The results revealed that moderate smokers’ NoGo-N2 amplitude was significantly lower than that of non-smokers, and the two groups showed no significant difference in NoGo-P3. Studies have found that Go/NoGo tasks activate some areas of the left side of the brain, such as the anterior cingulate cortex and pre-supplementary motor areas that are associated with tasks involving intense exercise and behavior choice. This suggests that the inhibition observed in the case of Go/NoGo tasks was mainly due to behavior choice and preparation. Therefore, rather than using Go/NoGo tasks, in the future, researchers should use ERPs in the context of the two-choice oddball paradigm, which will enable them to measure electrical brain activity related to behavioral control, and should examine the differences between ERPs for standard stimuli and those for deviant stimuli (Wei et al., 2002; Yuan et al., 2008). In addition, it may be possible to observe differences in the underlying brain mechanism of smokers and non-smokers in the two-choice oddball paradigm, using functional magnetic resonance imaging. Because only male light smokers were tested in this study, future studies should explore response inhibition in female smokers, and examine gender-based differences in the ability to inhibit responses to cigarette-related cues. Here, we can make a comparison between light smokers and non-smokers; Another important question for future research is whether differences in response inhibition vary on the basis of degree of smoking (moderate, and heavy) when studied in the context of the two-choice oddball paradigm.
Funding
This work was supported by The National Natural Science Foundation of China (31300838, 31560283) and Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning (CNLYB1317).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alexander, G. E., and Crutcher, M. D. (1990). Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. doi: 10.1016/0166-2236(90)90107-L
Aron, A. R., and Poldrack, R. A. (2006). Cortical and subcortical contributions to stop signal response inhibition: role of the subthalamic nucleus. J. Neurosci. 26, 2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006
Azizian, A., Monterosso, J. R., Brody, A. L., Simon, S. L., and London, E. D. (2008). Severity of nicotine dependence moderates performance on perceptual-motor tests of attention. Nicotine Tob. Res. 10, 599–606. doi: 10.1080/14622200801979159
Bekker, E. M., Böcker, K., Van Hunsel, F., Van Den Berg, M. C., and Kenemans, J. L. (2005). Acute effects of nicotine on attention and response inhibition. Pharmacol. Biochem. Behav. 82, 539–548. doi: 10.1016/j.pbb.2005.10.009
Billieux, J., Gay, P., Rochat, L., Khazaal, Y., Zullino, D., and Van der Linden, M. (2010). Lack ofinhibitory control predicts cigarette smoking dependence: evidence from a non-deprived sample of light to moderate smokers. Drug Alcohol Depen. 112, 164–167. doi: 10.1016/j.drugalcdep.2010.06.006
Brody, A. L., Mandelkern, M. A., Jarvik, M. E., Lee, G. S., Smith, E. C., Huang, J. C., et al. (2004). Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry 55, 77–84. doi: 10.1016/S0006-3223(03)00610-3
Buzzell, G. A., Fedota, J. R., Roberts, D. M., and McDonald, C. G. (2014). The N2 ERP component as an index of impaired cognitive control in smokers. Neurosci. Lett. 563, 61–65. doi: 10.1016/j.neulet.2014.01.030
Chambers, C., Bellgrove, M., Stokes, M., Henderson, T., Garavan, H., Robertson, I., et al. (2006). Executive “brake failure” following deactivation of human frontal lobe. J. Cogn. Neurosci. 18, 444–455. doi: 10.1162/089892906775990606
Charles-Walsh, K., Furlong, L., Munro, D. G., and Hester, R. (2014). Inhibitory control dysfunction in nicotine dependence and the influence of short-term abstinence. Drug Alcohol Depen. 143, 81–86. doi: 10.1016/j.pscychresns.2014.04.007
Cohen, M. X., and Frank, M. J. (2009). Neurocomputational models of basal ganglia function in learning, memory and choice. Behav. Brain Res. 199, 141–156. doi: 10.1016/j.bbr.2008.09.029
de Ruiter, M. B., Oosterlaan, J., Veltman, D. J., van den Brink, W., and Goudriaan, A. E. (2012). Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depen. 121, 81–89. doi: 10.1016/j.drugalcdep.2011.08.010
Dinn, W. M., Aycicegi, A., and Harris, C. L. (2004). Cigarette smoking in a student sample: neurocognitive and clinical correlates. Addict. Behav. 29, 107–126. doi: 10.1016/j.addbeh.2003.07.001
Eagle, D. M., and Robbins, T. W. (2003). Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav. Brain Res. 146, 131–144. doi: 10.1016/j.bbr.2003.09.022
Ehrman, R. N., Robbins, S. J., Bromwell, M. A., Lankford, M. E., Monterosso, J. R., and O’Brien, C. P. (2002). Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug Alcohol Depen. 67, 185–191. doi: 10.1016/S0376-8716(02)00065-0
Evans, D. E., Park, J. Y., Maxfield, N., and Drobes, D. J. (2009). Neurocognitive variation in smoking behavior and withdrawal: genetic and affective moderators. Genes Brain Behav. 8, 86–96. doi: 10.1111/j.1601-183X.2008.00445.x
Field, M., and Cox, W. M. (2008). Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depen. 97, 1–20. doi: 10.1016/j.drugalcdep.2008.03.030
Field, M., Rush, M., Cole, J., and Goudie, A. (2007). The smoking Stroop and delay discounting in smokers: effects of environmental smoking cues. J. Psychopharmacol. 21, 603–610. doi: 10.1177/0269881106070995
Franken, I. H. (2003). Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progr. Neuro Psychopharmacol. Biol. Psychiatry 27, 563–579. doi: 10.1016/S0278-5846(03)00081-2
Gallinat, J., Meisenzahl, E., Jacobsen, L. K., Kalus, P., Bierbrauer, J., Kienast, T., et al. (2006). Smoking and structural brain deficits: a volumetric MR investigation. Eur. J. Neurosci. 24, 1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x
Groman, S. M., James, A. S., and Jentsch, J. D. (2009). Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder. Neurosci. Biobehav. Rev. 33, 690–698. doi: 10.1016/j.neubiorev.2008.08.008
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., and Fagerstrom, K. O. (1991). The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 86, 1119–1127.
Heishman, S. J., Boas, Z. P., Hager, M. C., Taylor, R. C., Singleton, E. G., and Moolchan, E. T. (2006). Effect of tobacco craving cues on memory encoding and retrieval in smokers. Addict. Behav. 31, 1116–1121. doi: 10.1016/j.addbeh.2005.08.007
Huang, C., Lin, H., and Wang, H. (2006). The psychometric properties of the Chinese version of the Fagerstrom Test for Nicotine Dependence. Addict. Behav. 31, 2324–2327. doi: 10.1016/j.addbeh.2006.02.024
Impey, D., Chique-Alfonzo, M., Shah, D., Fisher, D. J., and Knott, V. J. (2013). Effects of nicotine on visuospatial attentional orienting in non-smokers. Pharmacol. Biochem. Behav. 106, 1–7. doi: 10.1016/j.pbb.2013.02.015
Johnsen, B. H., Thayer, J. F., Laberg, J. C., and Asbjornsen, A. E. (1997). Attentional bias in active smokers, abstinent smokers, and nonsmokers. Addict. Behav. 22, 813–817. doi: 10.1016/S0306-4603(97)00010-5
Kok, A., Ramautar, J. R., De Ruiter, M. B., Band, G. P., and Ridderinkhof, K. R. (2004). ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 41, 9–20. doi: 10.1046/j.1469-8986.2003.00127.x
Logemann, H., Böcker, K., Deschamps, P., Kemner, C., and Kenemans, J. L. (2014). The effect of enhancing cholinergic neurotransmission by nicotine on EEG indices of inhibition in the human brain. Pharmacol. Biochem. Behav. 122, 89–96. doi: 10.1016/j.pbb.2014.03.019
Longo, C. A., Fried, P. A., Cameron, I., and Smith, A. M. (2013). The long-term effects of prenatal nicotine exposure on response inhibition: an fMRI study of young adults. Neurotoxicol. Teratol. 39, 9–18. doi: 10.1016/j.drugalcdep.2014.08.006
Luijten, M., Littel, M., and Franken, I. H. (2011). Deficits in inhibitory control in smokers during a Go/NoGo task: an investigation using event-related brain potentials. PLoS ONE 6:e18898. doi: 10.1371/journal.pone.0018898
MacKillop, J., Amlung, M. T., Few, L. R., Ray, L. A., Sweet, L. H., and Munafò, M. R. (2011). Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology 216, 305–321. doi: 10.1007/s00213-011-2229-0
Madden, C. J., and Zwaan, R. A. (2001). The impact of smoking urges on working memory performance. Exp. Clin. Psychopharm. 9, 418–424. doi: 10.1037/1064-1297.9.4.418
McBride, D., Barrett, S. P., Kelly, J. T., Aw, A., and Dagher, A. (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31, 2728–2738. doi: 10.1038/sj.npp.1301075
Meule, A., Lutz, A., Vögele, C., and Kübler, A. (2012). Women with elevated food addiction symptoms show accelerated reactions, but no impaired inhibitory control, in response to pictures of high-calorie food-cues. Eat. Behav. 13, 423–428. doi: 10.1016/j.eatbeh.2012.08.001
Monterosso, J. R., Aron, A. R., Cordova, X., Xu, J., and London, E. D. (2005). Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depen. 79, 273–277. doi: 10.1016/j.drugalcdep.2005.02.002
Musso, F., Bettermann, F., Vucurevic, G., Stoeter, P., Konrad, A., and Winterer, G. (2007). Smoking impacts on prefrontal attentional network function in young adult brains. Psychopharmacology 191, 159–169. doi: 10.1007/s00213-006-0499-8
Nestor, L., McCabe, E., Jones, J., Clancy, L., and Garavan, H. (2011). Differences in “bottom-up” and “top-down” neural activity in current and former cigarette smokers: evidence for neural substrates which may promote nicotine abstinence through increased cognitive control. Neuroimage 56, 2258–2275. doi: 10.1016/j.neuroimage.2011.03.054
Patton, J. H., Stanford, M. S., and Barratt, E. S. (1995). Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 51, 768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1
Powell, J. H., Pickering, A. D., Dawkins, L., West, R., and Powell, J. F. (2004). Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict. Behav. 29, 1407–1426. doi: 10.1016/j.addbeh.2004.06.006
Rass, O., Fridberg, D. J., and Donnell, B. F. (2014). Neural correlates of performance monitoring in daily and intermittent smokers. Clin. Neurophysiol. 125, 1417–1426. doi: 10.1016/j.clinph.2013.12.001
Ray, N. J., Brittain, J., Holland, P., Joundi, R. A., Stein, J. F., Aziz, T. Z., et al. (2012). The role of the subthalamic nucleus in response inhibition: evidence from local field potential recordings in the human subthalamic nucleus. Neuroimage 60, 271–278. doi: 10.1016/j.neuroimage.2011.12.035
Reynolds, B., Patak, M., Shroff, P., Penfold, R. B., Melanko, S., and Duhig, A. M. (2007). Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp. Clin. Psychopharm. 15, 264–271. doi: 10.1037/1064-1297.15.3.264
Robinson, J. H., Pritchard, W. S., and Davis, R. A. (1992). Psychopharmacological effects of smoking a cigarette with typical “tar” and carbon monoxide yields but minimal nicotine. Psychopharmacology 108, 466–472. doi: 10.1007/BF02247423
Ryan, F. (2002). Detected, selected, and sometimes neglected: cognitive processing of cues in addiction. Exp. Clin. Psychopharm. 10, 67–76. doi: 10.1037/1064-1297.10.2.67
Sayette, M. A., and Hufford, M. R. (1994). Effects of cue exposure and deprivation on cognitive resources in smokers. J. Abnorm. Psychol. 103, 812–818. doi: 10.1037/0021-843X.103.4.812
Shek, D. T. (1990). Reliability and factorial structure of the Chinese version of the Beck Depression Inventory. J. Clin. Psychol. 46, 35–43. doi: 10.1002/1097-4679(199001)46:1<35::AID-JCLP2270460106>3.0.CO;2-W
Spinella, M. (2002). Correlations between orbitofrontal dysfunction and tobacco smoking. Addict. Biol. 7, 381–384. doi: 10.1080/1355621021000005964
Verbruggen, F., and Logan, G. D. (2008). Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 12, 418–424. doi: 10.1016/j.tics.2008.07.005
Verbruggen, F., and Logan, G. D. (2009). Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 33, 647–661. doi: 10.1016/j.neubiorev.2008.08.014
Wang, Y., Yang, J., Yuan, J., Fu, A., Meng, X., and Li, H. (2011). The impact of emotion valence on brain processing of behavioral inhibitory control: spatiotemporal dynamics. Neurosci. Lett. 502, 112–116. doi: 10.1016/j.neulet.2011.07.039
Waters, A. J., and Feyerabend, C. (2000). Determinants and effects of attentional bias in smokers. Psychol. Addict. Behav. 14, 111. doi: 10.1037/0893-164X.14.2.111
Waters, A. J., Shiffman, S., Sayette, M. A., Paty, J. A., Gwaltney, C. J., and Balabanis, M. H. (2004). Cue-provoked craving and nicotine replacement therapy in smoking cessation. J. Consult. Clin. Psychol. 72, 1136–1143. doi: 10.1016/j.jcrs.2006.04.024
Wei, J. H., Chan, T. C., and Luo, Y. J. (2002). A modified oddball paradigm “crossmodal delayed response” and the research on mismatch negativity. Brain Res. Bull. 57, 221–230. doi: 10.1016/S0361-9230(01)00742-0
Wiecki, T. V., and Frank, M. J. (2013). A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol. Rev. 120, 329–335. doi: 10.1037/a0031542
Xu, J., Azizian, A., Monterosso, J., Domier, C. P., Brody, A. L., London, E. D., et al. (2008). Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob. Res. 10, 1653–1661. doi: 10.1080/14622200802412929
Yuan, J., He, Y., Qinglin, Z., Chen, A., and Li, H. (2008). Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology 45, 986–993. doi: 10.1111/j.1469-8986.2008.00693.x
Yuan, J., Xu, S., Yang, J., Liu, Q., Chen, A., Zhu, L., et al. (2011). Pleasant mood intensifies brain processing of cognitive control: ERP correlates. Biol. Psychol. 87, 17–24. doi: 10.1016/j.biopsycho.2011.01.004
Zhou, L. (2006). Reliability and validity of Chinese version of Barratt Impulsiveness Scale-11. Chin. J. Clin. Psychol. 14, 343–344. doi: 10.3969/j.issn.1005-3611.2006.04.005
Keywords: male light smokers, cigarette-related cues, response inhibition, two-choice oddball paradigm
Citation: Xin Z, Ting LX, Yi ZX, Li D and Bao ZA (2015) Response inhibition of cigarette-related cues in male light smokers: behavioral evidence using a two-choice oddball paradigm. Front. Psychol. 6:1506. doi: 10.3389/fpsyg.2015.01506
Received: 17 June 2015; Accepted: 17 September 2015;
Published: 15 October 2015.
Edited by:
Tifei Yuan, Nanjing Normal University, ChinaCopyright © 2015 Xin, Ting, Yi, Li and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao Xin and Zhou A. Bao, Behavior Rehabilitation Training Research Institution, School of Psychology, Northwest Normal University, Lanzhou, China, psyzhaoxin@nwnu.edu.cn; zhouab@nwnu.edu.cn
 Zhao Xin
Zhao Xin Liu X. Ting1
Liu X. Ting1 Zhou A. Bao
Zhou A. Bao