- Formerly affiliated with Grupo de Conducta Adaptativa e Interacción, Psychology Faculty, University of Barcelona, Barcelona, Spain
A society is a complex system composed of individuals that can be characterized by their own attributes that influence their behaviors. In this study, a specific analytical protocol based on social network analysis was adopted to investigate the influence of four attributes (gender, age, matriline, and hierarchical rank) on affiliative (allogrooming) and agonistic networks in a non-human primate species, Macaca sylvanus, at the park La Forêt des Singes in France. The results show significant differences with respect to the position (i.e., centric, peripheral) and role (i.e., implication in the network cohesiveness) of an individual within a social network and hence interactional patterns. Females are more central, more active, and have a denser ego network in the affiliative social network tan males; thus, they contribute in a greater way to the cohesive structure of the network. High-ranking individuals are likely to receive fewer agonistic behaviors than low-ranking individuals, and high-ranking females receive more allogrooming. I also observe homophily for affiliative interactions regarding all attributes and homophily for agonistic interactions regarding gender and age. Revealing the positions, the roles, and the interactional behavioral patterns of individuals can help understand the mechanisms that shape the overall structure of a social network.
Introduction
Animal societies are complex systems in which individuals have non-random and complex interactions, and are likely to develop behavioral strategies (Dunbar, 1989). This leads to the formation of a multilayered and multi-behavioral structure. However, questions persist about the fundamental evolutionary process by which a society emerges, stabilizes, and adapts.
Previous studies of animal species, including human and non-human primates, have investigated the behavioral differences and interactions among individuals according to attributes such as gender (Fedigan, 1982), age (Wey and Blumstein, 2010), body size (Archie et al., 2006), social status (Bergman and Moore, 2003), reproductive state (Cavigelli and Pereira, 2000), and kinship (Widdig et al., 2001). This study focuses on four specific attributes: gender, age, matriline (matrilineal kinship), and hierarchical rank.
Differences in gender lead to contrasting reproductive (Fragaszy and Mitchell, 1974; Fedigan and Baxter, 1984; Pereira, 1988; Cords, 2002) and behavioral strategies (Fedigan, 1982), and in particular, the expression of aggressiveness and allogrooming.
Age, or more precisely, ontogenesis (i.e., the development of an organism), influences the evolution and development of social relations and species-specific behaviors that are largely affected by interactional experiences with congeners (Harlow and Suomi, 1974; Hinde, 1974; Wilson, 1980; Shimada and Sueur, 2014). For example, hierarchical rank acquisition appears to be closely related to age (Borries et al., 1991) and the early experiences of juveniles (Mitchell et al., 1967; Olds et al., 1997). However, this influence differs according to species and gender (Sosa, 2015). Additionally, older individuals and females in particular are more likely to experience social exclusion (i.e., decrease in social interactions) (Hauser and Tyrrell, 1984).
One major kinship phenomenon among the animal kingdom is the matrilineal rank inheritance (MRI) (Kawamura, 1958) observed in macaques. It consists of the transmission of hierarchical rank from mother to daughter; the latter acquires the hierarchical rank directly below that of her mother. In addition, as according to the youngest ascendancy rule, young females outrank their older sisters (Thierry et al., 2004). The MRI process is made possible by nepotism, in that related females support each other during conflicts against non-kin females and help juvenile females outrank their older sisters (Cheney, 1977; Datta, 1983; Chapais and Gauthier, 1993). Furthermore, an adult female can outrank her mother when she is old and subsequently lacks kin support and has limited physical ability (Chapais and Berman, 2004). See Chapais and Berman (2004) and Hepper (2005) for an overview.
Social network analysis (SNA) is one approach used to analyze systems (Sueur et al., 2011) as complex as animal societies. SNA was first applied in psychological studies and, for a few decades, in animal social research (see Prell, 2011 and Brent et al., 2011 for an overview of SNA epistemology). However, certain methodological precautions must be taken when using any of the various analytical techniques based on SNA (Wasserman and Faust, 1994; Krause et al., 2009; Brent et al., 2011). In this study, I describe an analytical protocol based on SNA tools that compensates for the intrinsic limitations of animal behavioral data (i.e., dependency of data) and allows the analysis of weighted networks (network with weighted links).
Several studies have used SNA tools to examine the position and role of group members in non-human primates and other animal species. Lusseau and Newman (2004) revealed that central individuals are key players in maintaining social cohesion and have greater knowledge of their environment. In some non-human primate societies, central individuals are high-ranking animals (Kanngiesser et al., 2011). Using an interspecific comparative approach, several studies have analyzed network metric variations and succeeded in linking them to variability in social structure and dominance style (Sade, 1972; Voelkl and Noe, 2008; Sueur et al., 2011). Previous studies have also found that individuals from the philopatric gender are more central within a network (Smuts, 1985; Matsuda et al., 2012). In this way, central individuals play an important role in group cohesion and their position depends on several individual characteristics. Thus, identifying these central individuals according to their attributes could allow us to better understand how a social structure is shaped.
SNA research also addresses the principles of homophily and heterophily that refer to preferential interactions between similar (homophily) or dissimilar (heterophily) individuals (Lazarsfeld and Merton, 1954). These phenomena have been observed in many animal species: cetaceans (Lusseau and Newman, 2004), fishes (Croft et al., 2005), marmots (Wey and Blumstein, 2010), and human (McPherson et al., 2001) and non-human primates (Silk, 2001; Cords, 2002; Carter et al., 2015). However, animal research has generally disclosed the existence of homophily for one behavior as related to a single attribute. In this study, I examine the existence and level of homophily as related to a variety of behaviors and attributes. Moreover, revealing such a phenomenon may help us understand how individuals build their networks depending on the attributes of other individuals.
Macaca (Macaca sp.) societies are characterized by their common social organization, but they are also known for their different social styles. Extensive research has shown that dominance hierarchies vary greatly in the macaque genus (i.e., dominance styles) (De Waal and Luttrell, 1989; Thierry et al., 2000; Sueur et al., 2011). Furthermore, the hierarchical structure of females in the Macaca taxon is a well-studied phenomenon that appears to be entirely dependent on the MRI (Thierry et al., 2000). In contrast, each Macaca species has stable multi-male, multi-female, and multi-generational social groups in which females are philopatric and males migrate. These common characteristics allow the elucidation of the influence of individual attributes on the interactions between individuals and represents an excellent biological model for this study.
In this study, I use SNA tools to determine individual positions and interactional patterns according to four specific attributes (age, gender, matriline, and hierarchical rank) in affiliative and agonistic networks in M. sylvanus. Based on previous studies, several assumptions can be made in response to the following questions:
(1) Who are the most central individuals? As in many cercopithecines, M. sylvanus females are the philopatric gender, which should increase their ability to form denser, stronger, and more perennial networks than males (Smuts, 1985). Thus, they can be expected to be the most active and central individuals in the affiliative network. Exploring such functions could reveal the significance of their role in facilitating group cohesion. Males are generally the more aggressive individuals (Gray, 1971), and therefore should be particularly active and central in the agonistic network.
(2) How age and gender influence the positions and roles of individuals? According to our extensive knowledge of the MRI process, we can expect to observe age-related behavioral variations in females that are highly correlated with their reproductive status (Borries et al., 1991; Chapais, 2004) and matriline. The social activity of young females would therefore be more intense (affiliatively and agonistically), with a decrease in activity during their latter ontogenesis, which in some cases may lead to social exclusion at an advanced age. In males, a minimum hierarchical level for older individuals may exist that enables them to maintain a certain ranking (Sosa, 2015). Such kinetics among males reduces their chances of experiencing social exclusion and thus they may face only a minor decrease in social activity, position, and role.
(3) Do common interactional patterns exist among individuals according to their attributes? One sociological model predicts attractiveness to high-ranking females (Seyfarth, 1977). However, this model appears subject to variability. It is mainly observed in despotic societies, and attractiveness to low-ranking individuals has been reported in other species (Schino, 2001; Sueur and Petit, 2008). According to these findings, Seyfarth’s model should not apply to M. sylvanus. I also expect to observe homophily related patterns such as allogrooming that target same-gender and same-age individuals and kin (Hirsch et al., 2012). As for agonistic behaviors, it is difficult to form any hypothesis, but heterophily can be suspected.
Responding to these questions allows to reveal how individual attributes and social structure (gender philopatry) produce behavioral divergences that lead to different positions and roles within the group (question 1), how these deviations evolve with the ontogenesis of an individual (question 2), and by which mechanisms individuals interact among themselves (question 3). This multilevel approach allows for a better understanding of how these different levels shape the overall structure of the society in M. sylvanus.
Materials and Methods
Study Site and Subjects
The current study was conducted over a period of 4 months (July to October 2011) in the park La Forêt des singes, in Rocamadour, France. The 141 M. sylvanus individuals in the park are divided into three groups and live in semi-free ranging conditions (Sugiyama, 2015) in a 20 hectare forest. They are fed in foraging areas twice per day and have water ad libitum. For more details on the management of the park, refer to de Turckheim and Merz (1984). The demographic data (gender, age, and matriline) were provided by the scientific director of the park, Ms. Ellen Merz. The study focused on one of the three groups. Four newborns were excluded from the observations (three males and one female), so that the number of individuals observed was N = 52. The group had a balanced gender ratio of 25 females and 27 males, with an age range between 1 and 25 years old. The individuals were previously identified during 1 month through their tattoos. The observations were conducted with the approval of the park management, an agreement that was subject to the specific condition that I would not directly contact nor handle individuals. As I performed simple observations without any type of intervention, I did not require authorization from the French National Advisory Ethics Committee.
Behavioral Observations
Observations were conducted by repeated focal samplings of 30 min per individual. Each individual was observed approximately 30 times (15 ± 2 h), for 786 observation hours of 52 individuals. Focal sampling time was determined after 2 months of pre-observation. To trade with bias of observation in time of day and feeding time, individuals were observed randomly from 8 am to 5 pm over the 4 months. During the observations, I registered allogrooming and agonistic behaviors (threatening face or growl, charge, avoidance, attack, chase, and aggressive slap, grab, or bite). A complete description of the ethogram of M. sylvanus can be found in Hesler and Fischer (2007). An iPad 1 tablet (Inc, 1976) computer and the WhatISee2.0 application (Inc, 2009) were used to register the individuals involved, and the direction, frequency, and duration of the behaviors. Directed and weighted agonistic and allogrooming matrices were built using the obtained behavioral frequencies (Figure 1). The overall observation yields a total of 5867 agonistic interactions and 1281 grooming interactions. The agonistic matrices allow us to calculate the hierarchical rank of each individual using David’s Score (David, 1987) with R 3.0.1 (Ihaka and Gentleman, 1996) package steepness (de Vries et al., 2006).
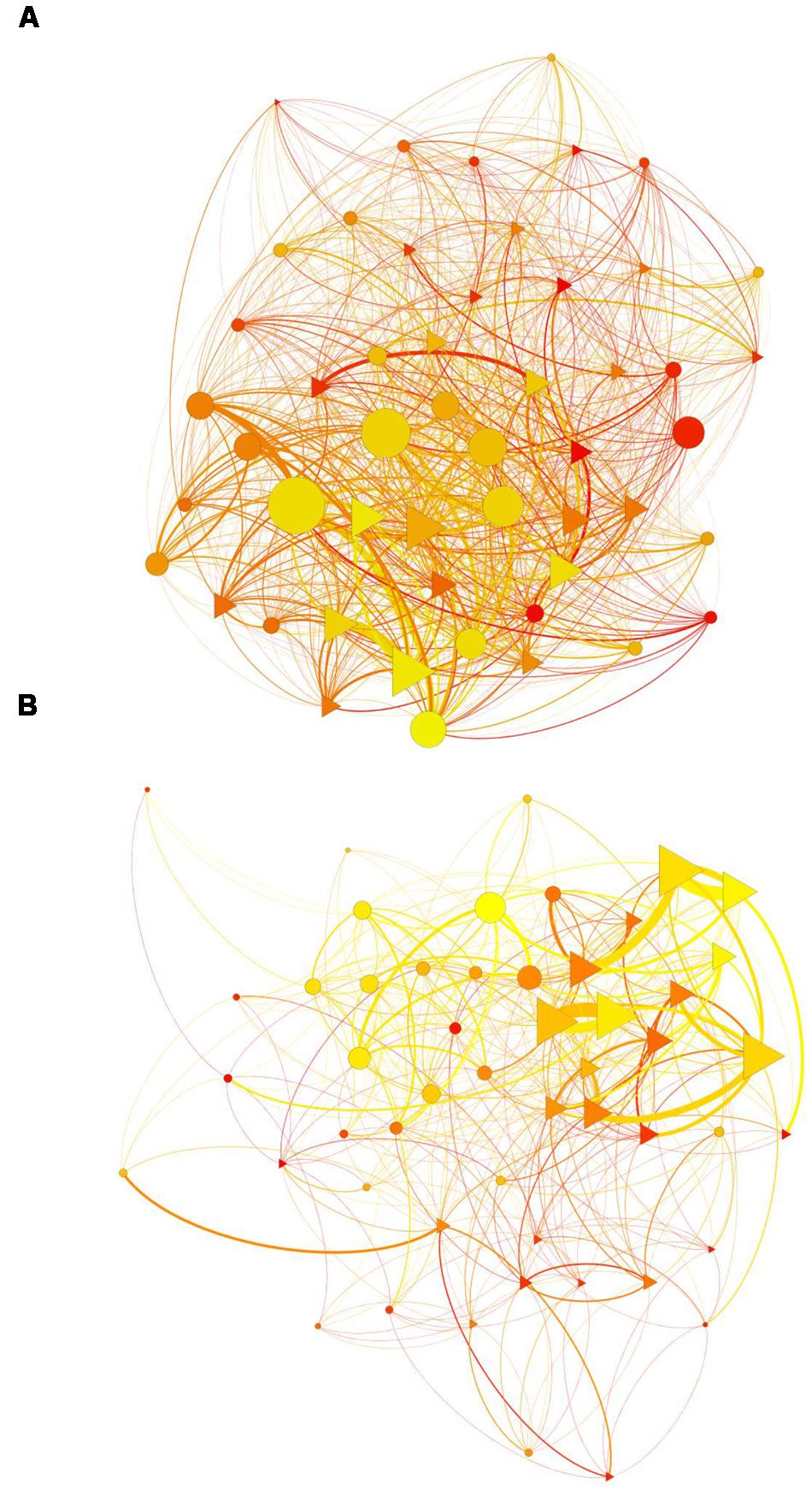
FIGURE 1. Social networks: (A) agonistic network, (B) allogrooming network. Yifan Hu layout. Gradient color of vertices (from yellow to red) represents individuals’ age (from youngest to oldest). Shape of vertices represents individuals’ gender (females: triangles; males: circles). Size of vertices represents individuals’ degree. Size of edges represents the strength of interactions and the color is in accordance with the age of the individual that gives the behavior.
Social Network Analyses
Building Matriline Categories
Kinship bonds among individuals were determined using two methods. First, data were provided by the park officials who, along with scientists, have been monitoring the population in the park. Second, matrilines were determined through genetic analyses of mitochondrial DNA using eight microsatellite markers. The collection and analysis of DNA samples were performed by the park authorities. The poor quality of DNA samples made some DNA results uncertain. For this reason, matriline groups were built only with individuals whose relatedness was confirmed based on direct observations and genetic analyses. To conserve only close kinship relationships, only the individuals with the same mother were considered related for each mitochondrial haplotype (Figure 2). Thus, individuals whose matrilines were uncertain did not belong to any matriline group (eight males and one female). In addition, matriline results must be carefully considered, as not all individuals were taken into account owing to a lack of information on their kinship bonds.
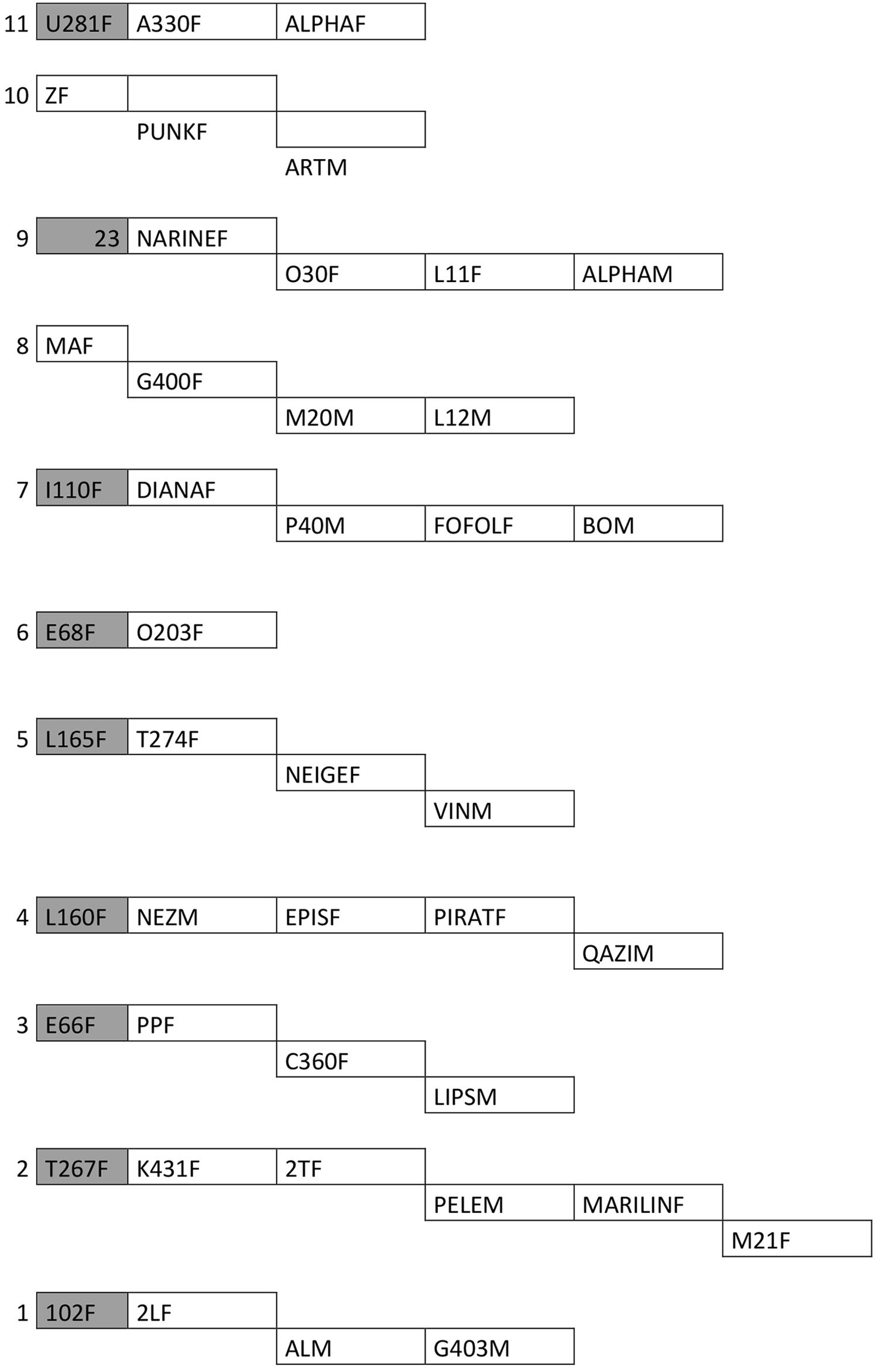
FIGURE 2. Matrilines. This scheme represents individuals which were kept for kinship analysis. Gray cells are dead individuals. Numbers represent the different matrilines. Each subline represents an offspring of the corresponding mother.
Data Consideration before Analysis
Collecting data from all members of the same social group led us to the construction of two social networks (agonistic and affiliative) through the existence of multiple interactions. The intrinsic nature of the collected data (interactions between same-group individuals) underlies the non-independence of the data required by inferential statistical techniques (Wasserman and Faust, 1994; Krause et al., 2009; Brent et al., 2011). Several possibilities exist to deal with this fact. Link filtering is commonly used in animal SNA to delete interactions that can be attributed to random or “chance” events (Croft et al., 2008). However, at present, this filtering process has not been submitted to any formal methodology and has two major limitations: (1) the non-consideration of weak ties (Granovetter, 1973) and thus the loss of important information (Croft et al., 2011); and (2) the sensitivity to data errors such as misidentification.
The approach adopted in this study is based on permutation tests and may help standardize the analysis of animal behavioral data obtained using SNA. In this study, I assumed an approach that allows analysis without the need to filter the links. To this end, the weight of the links must be taken into consideration, which can be done by using weighted social network metrics (Opsahl, 2009; Brent et al., 2011). Frequency-based data are less prone to sampling biases, yet by themselves, they do not solve the issue of data dependency. Therefore, I used weighted network metrics with Null Hypothesis Significant Tests (NHST) involving a permutation-based approach (Manly, 2006). This method generates a set of random values based on the real data set and creates the null hypothesis that the real structural measure X is not different from the random one. This hypothesis is accepted or rejected by comparing the observed value X to the random one. If the observed value is greater than the random one from 95%, then the null hypothesis is rejected. The use of permutation tests in the study of animal societies is discussed in details by Whitehead (2008) and Croft et al. (2011).
The following analyses were performed on both weighted allogrooming and agonistic matrices with 10000 permutations.
Network Metrics
For each individual, I calculated the following weighted network metrics: indegree, outdegree, degree, eigenvector centrality, and clustering coefficient with Ucinet 6.375 (Borgatti et al., 2002). Briefly, the degree corresponds to the total number of individuals that directly interact with one given individual (Freeman, 1979). The weighted version takes into account the weight of the links. I also differentiated between indegree (incoming ties) and outdegree (outgoing ties). This metric is historically the first and conceptually the simplest centrality network metric, and in this case, can also be considered as the activity, or “involvement,” of an individual. The eigenvector centrality index is the sum of the connections to neighbors weighted by their degree. This index provides a metric that determines the individual centrality relative to the rest of the network and the “influence” of an individual on the network (i.e., connection to high-degree nodes) and thus, on the social structure. Additionally, it would appear to be a more pertinent centrality metric for non-human primate groups (Kasper and Voelkl, 2009). The weighted clustering coefficient gives weight to the neighborhood densities proportionate to their size and indicates the contribution of each individual in the connectivity and thus, in the cohesion of the network structure (Watts, 2003; Hanneman and Riddle, 2005). For an overview of the weighted network metrics and calculations, see: Wasserman and Faust (1994), Croft et al. (2008) and Whitehead (2008).
Statistical Analyses
Individual Level
For the first analysis, I aimed to study gender, matriline, hierarchical rank, and age-related changes in each network metric. To this end, I used general linear mixed models (GLMM) in which weighted degrees, indegrees, outdegrees, eigenvectors, and clustering coefficients are the dependent variables in separate models. Exact ages, genders, hierarchical ranks, and matrilines are the independent variables.
To offset the non-independence of these data, I realized GLMM with permutation. The consequent biological null hypothesis was that any individual could have any network metric value. Opting for this method has several advantages. First, it takes into account the non-independency of the data; second, it is a better option than multiple t-tests and ANOVA (which both need discrete variables and would increase the number of tests) with permutation or simple correlations; and finally, it facilitates the analysis of the interactions between factors.
Initially, I created two types of models: those with no interactions with the dependent variables and those with gender interacting with other individual attributes to examine whether age, matriline, and hierarchical rank dissimilarly influence individuals according to their gender. Only factors estimated higher than 0.009 were considered significant. This threshold is arbitrary and aims to consider only significant effects with sufficient weight. These analyses were performed using SPSS 17 (SPSS, 2008) GLM Procedure with Bootstrap option of p-value = 0.05.
Group Level
The aim of the second analysis was to examine homophily and heterophily, for which I used NHST (Stephens et al., 2007) with permutation.
The principle of homophily, or the preferential interactions between same-attribute individuals, consequently determines whether the links within a same-attribute group have greater frequencies than the links between groups. Thus, to study homophily between genders, I used a simple t-test with permutation for comparing the mean of the links between and within the groups depending on gender.
To study homophily according to age (a continuous attribute), I used the Moran statistic which indexes the differences between the score of an actor and the mean, and then weights the cross products (Moran, 1950). Permutations are used to create a sampling distribution in which scores on the attribute are randomly assigned to actors. As for any permutation test, the real structural measure (the Moran statistic in this case) is compared to the random one (Hanneman and Riddle, 2005). The Moran “I” statistic of autocorrelation ranges from -1.0 (perfect negative correlation) through 0 (no correlation) to +1.0 (perfect positive correlation). These analyses were performed using Ucinet 6.375 (Borgatti et al., 2002).
Results
Individual Level
In the agonistic social network, results show that the higher the matriline, the more central (eigenvector: 0.015, p < 0.05) and active (degree: 17.866, p < 0.05) its members, and the more they receive agonistic behaviors (indegree: 9.857, p < 0.05) and contribute to network cohesion (clustering coefficient: 0.077, p < 0.01). The results also reveal that the higher the hierarchical rank of an individual, the more it gives agonistic behaviors (outdegree: 6.075, p < 0.05), but the less it receives them (indegree: -14.698, p < 0.01). Finally, we observe that with age, individuals are less active (degree: -12.529, p < 0.01), give less (outdegree: -3.568, p < 0.05) and receive fewer (indegree: -8.961, p < 0.01) agonistic behaviors. These results are synthetized in Table 1.
The results of the agonistic social network model for gender interactions with other individual attributes are as follows (synthetized in Table 2, Figure 3, Appendix 2 and 3):
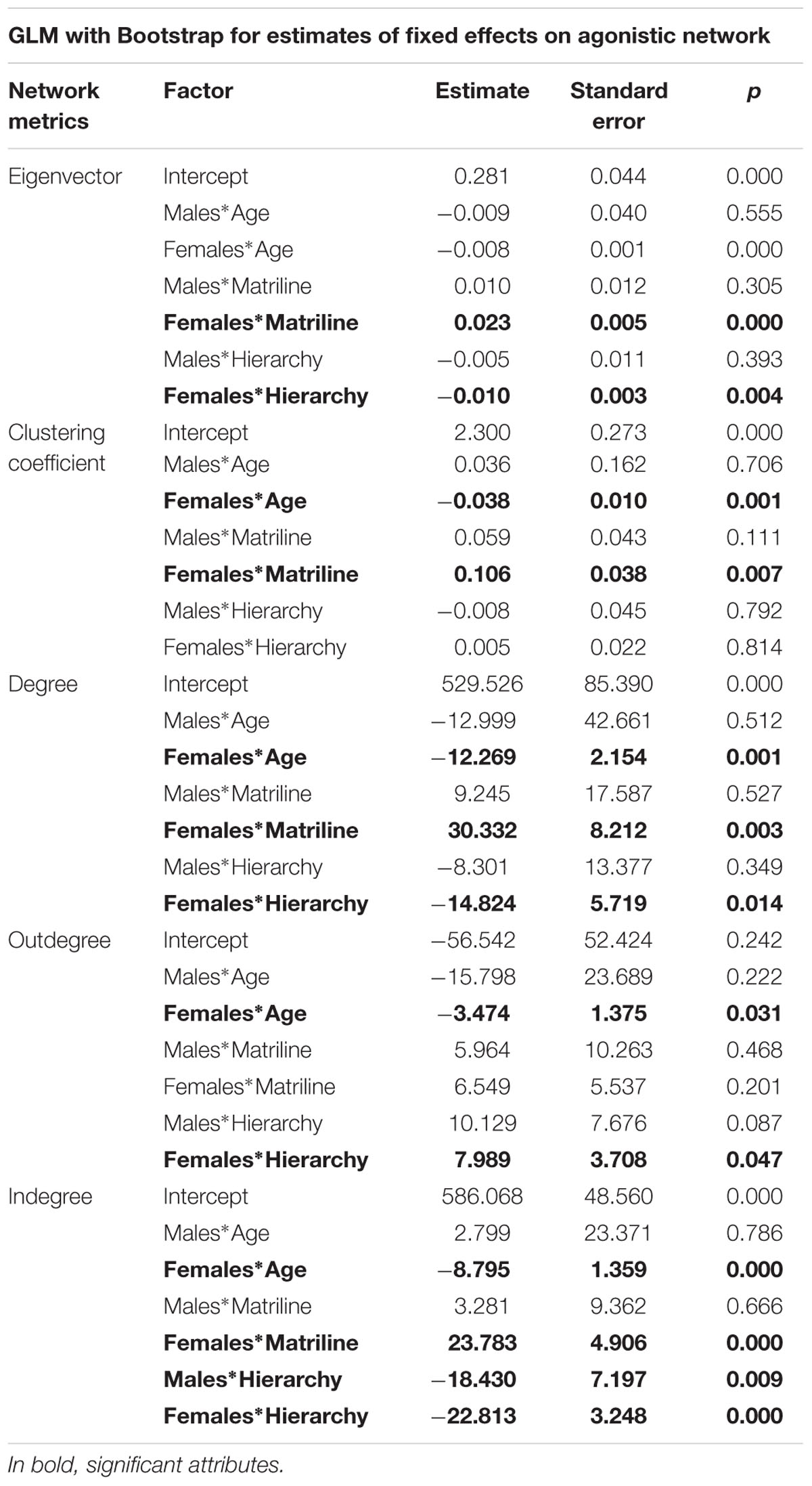
TABLE 2. General linear mixed models for agonistic network metrics for interactions between gender and other individual attributes.
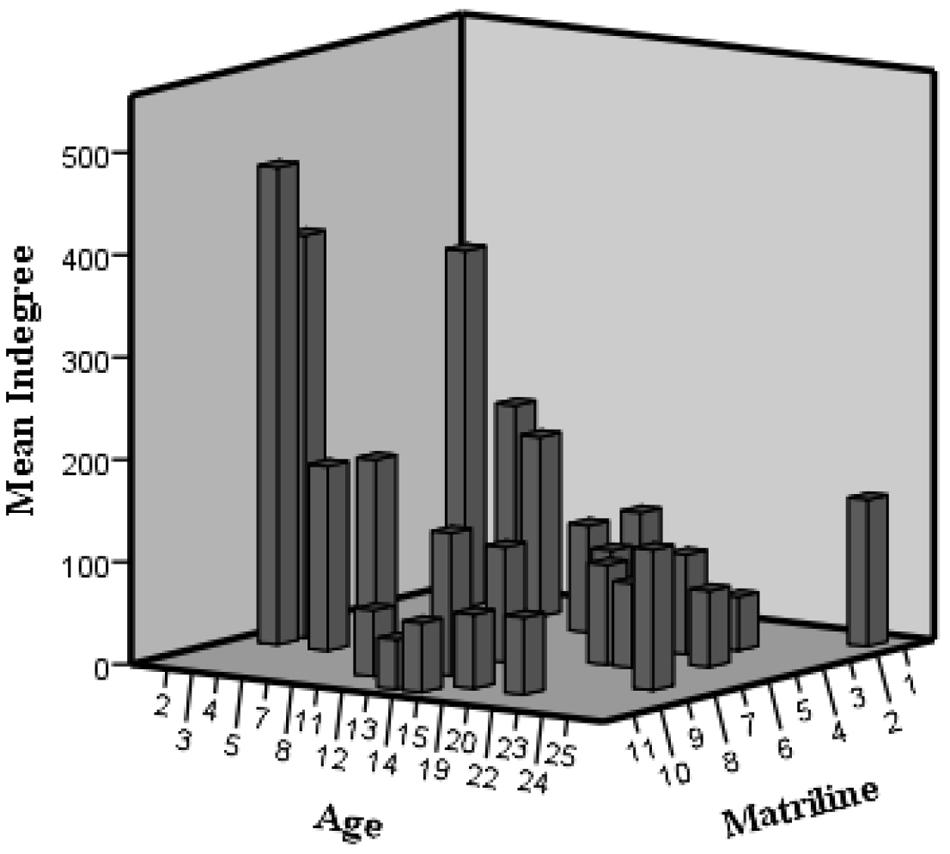
FIGURE 3. 3D histogram of indegree variation according to age and matriline in the agonistic network.
– The eigenvector model shows that for females, the higher the matriline, the more central the individual (0.023, p < 0.01). Furthermore, the centrality of females also decreases with their individual hierarchical rank (-0.010, p < 0.01).
– The degree model shows that degree significantly decreases with age for females (-12.269, p < 0.01), but not for males. Individual degree increases with matriline (30.332, p < 0.01) and decreases with hierarchical rank for females only (-14.824, p < 0.05).
– The outdegree model shows that outdegree significantly decreases with age (-3.474, p < 0.05) for females only. Individual outdegree increases with hierarchical rank for females (7.989, p < 0.05).
– The indegree model shows that indegree significantly decreases with age for females (-8.795, p < 0.01). Individual indegree increases with matriline (23.783, p < 0.05) for females. However, indegree decreases with hierarchical rank for both males and females (males: -18.430, p < 0.01; females: -22.813, p < 0.01).
– The clustering coefficient model shows that clustering coefficient significantly decreases with age (-0.038, p < 0.01) and increases with matriline (0.106, p < 0.01) for females only.
The allogrooming social network is primarily influenced by gender and age, with matriline having no significant effect. We also observe that the higher the hierarchical rank, the more an individual receives allogrooming (indegree: 1.366, p < 0.05). Furthermore, with age, individuals are less central (eigenvector: -0.014, p < 0.01), less active (degree: -5.625, p < 0.01), and give (outdegree: -3.082, p < 0.01) and receive (indegree: -2.064, p < 0.01) less allogrooming. Interestingly, whereas no significant difference was observed between males and females in the agonistic network, in the allogrooming network, females are more central (eigenvector: 0.231, p < 0.01) and more active (degree: 71.708, p < 0.01) than males. Allogrooming behaviors are mostly given (outdegree: 32.018, p < 0.01) and received by females (indegree: 39.691, p < 0.01). These results are synthetized in Table 3.
The results of the allogrooming social network model for gender interactions with other individual attributes are as follows (synthetized in Table 4, Appendix 4 and 5):
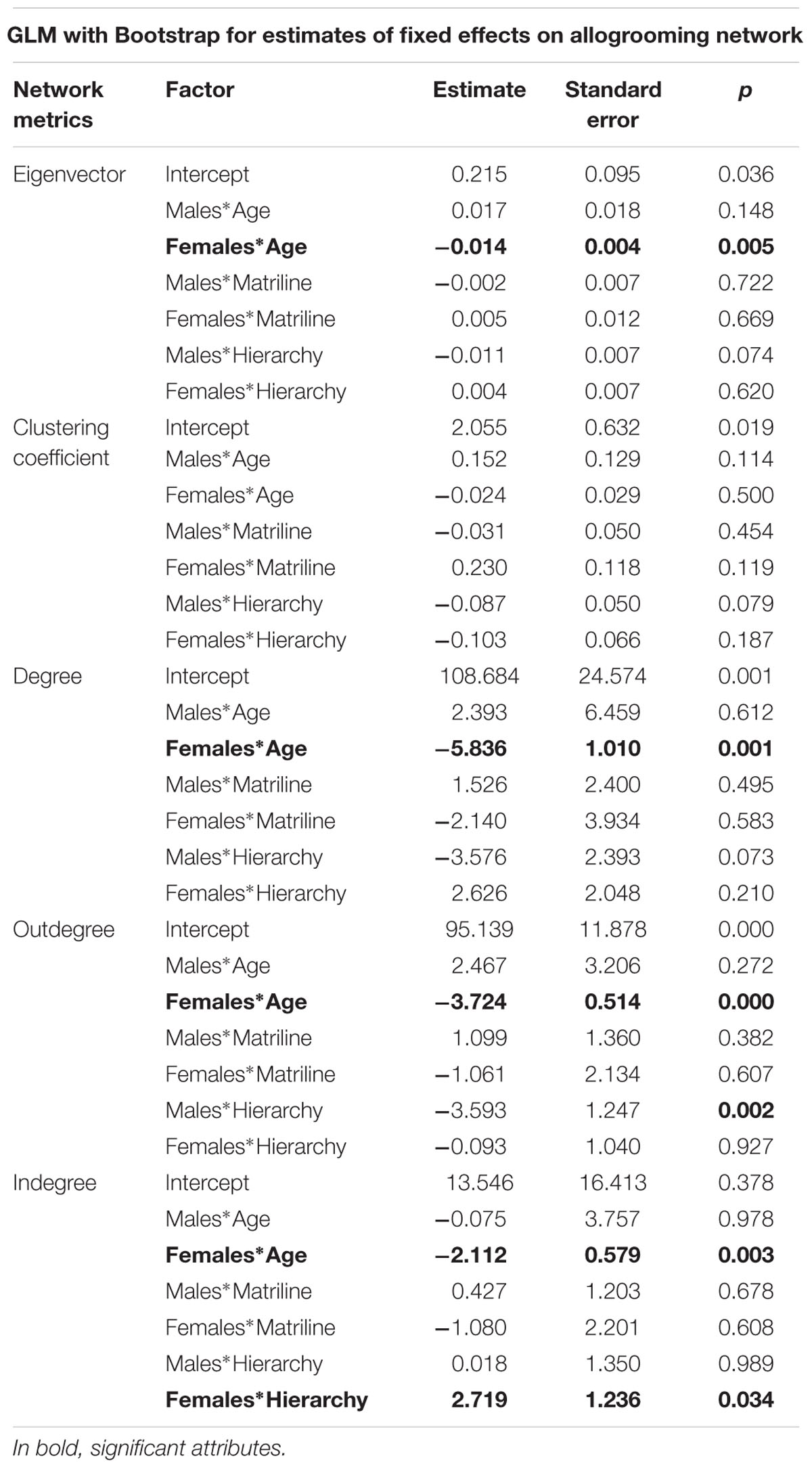
TABLE 4. General linear mixed models for allogrooming network metrics for interactions between gender and other individual attributes.
– The eigenvector model shows that eigenvector significantly decreases with age for females (-0.014, p < 0.01), but not for males.
– The degree model shows that degree significantly decreases with age for females (-5.836, p < 0.01).
– The outdegree model shows that outdegree significantly decreases with age for females (-3.724, p < 0.01).
– The indegree model shows that for females, indegree significantly decreases with age (-2.112, p < 0.01) and increases with hierarchical rank (2.719, p < 0.05).
– The clustering coefficient model shows non-significant results with any individual attribute.
Group Level
With respect to agonistic behaviors, we obtain homophily for gender (difference in means: -1.651, p < 0.05) and for age (I = 0.273, p < 0.05). Testing genders separately, we obtain homophily by age for females (I = 0.336, p < 0.05) and for males (I = 0.211, p < 0.05). The results for matriline and individual hierarchical rank were non-significant.
For allogrooming, homophily is observable for gender (difference in means: -1.942, p < 0.05) and for age (I = 0.318, p < 0.05). Testing genders separately to analyze if there are homophilic differences between genders according to age, we obtain homophily by age for females (I = 0.565, p < 0.05), but we do not obtain significant results for males according to age (I = 0.100, p = 0.106). Homophily is also observed by matriline (I = 0.321, p < 0.05) Testing genders separately, we observe significant homophily by matriline for females (0.458, p < 0.01), but not for males. Finally, we also observe homophily by hierarchical rank (I = 0.228, p < 0.05), yet testing genders separately, we do not observe significant results in either gender.
Discussion
In this study, I established an analytical protocol that balances the inter-dependency of the data without filtering the links and that considers the weight of the links, and I analyzed the effects of several factors (gender, age, matriline, and hierarchical rank) at different levels of social organization in a non-human primate species, M. sylvanus. These findings reveal to what extent SNA facilitates the investigation of various aspects of animal societies by studying: (1) the position and influence of individuals according to their attributes; (2) the attribute-related network; and (3) the interactional dynamics reflected by homophily. In this way, I demonstrated that the sociogenesis process (rank acquisition) is intimately linked to ontogenesis (i.e., it is age-related), and differs between genders. Hence, individuals with common attributes have similar positions and roles in the group. I also stressed the existence of homophily in several behaviors, reflecting common individual behavioral patterns, including: (1) the acquisition of status within an age-related category, leading to intra-generational conflicts; (2) high-ranking individuals preferably groom similar-rank and opposite-gender individuals to secure better protection and support; and (3) the existence of homophily in grooming behaviors by gender, age, hierarchical rank, and matriline. The results suggest six main findings.
First, we observe that variations in individual attributes have a greater impact on the position, role, and interactional patterns of females than on males. In most cases, these dissimilarities result from the social structure of females in M. sylvanus that is based on philopatry and MRI. Additionally, we observe significant disparities in activity and centrality between males and females. Females are more central and active (for both received and given behaviors) in the allogrooming network. More specifically, they give and receive more allogrooming, mainly with individuals who have similar characteristics, namely females according to homophily results. These findings are in line with the literature that stresses that the philopatric gender plays a key role in affiliative behaviors (Aureli and de Waal, 2000; Silk, 2001). From a biological perspective, the philopatric gender has more time to develop a denser, stronger, and perennial network than the non-philopatric gender. In addition, female matriline homophily results emphasize the relevance of kinship bonds among females in affiliative behaviors. Individuals with high centrality and activity thus preferentially contribute to the establishment of the global network structure (Lusseau and Newman, 2004; Sosa, 2014) and cohesion of the group. In M. sylvanus, these key individuals are unquestionably the females.
Second, we observe that for female M. sylvanus, network metrics decrease with age in the agonistic and allogrooming networks. In the agonistic network, older females are less active (degree, indegree, and outdegree) and less involved in the cohesion of the network (clustering coefficient). In the allogrooming network, older females are less active (degree, indegree, and outdegree) and less central (eigenvector). During the early years, high centrality and activity for allogrooming behaviors is likely related to a long period of mother–infant and kin-related preferential interactions (which is supported by the results of matriline homophily for allogrooming behaviors) that generates kin recognition and later, kin-biased affiliative interactions (Pereira and Fairbanks, 1993). Furthermore, juveniles learn how to interact by relating to their close relatives. The observed decrease of allogrooming centrality and activity with age is likely related to the progressive stabilization of the affiliative networks of females. In addition, the decline with progressing age in the agonistic network (as related to activity and the role in the cohesion of the network) is probably a result of the stabilization of the hierarchical ranks of females when sexual maturity is attained (Chapais, 2004). These results show that the sociogenesis process, or rank acquisition, is intimately linked to ontogenesis (i.e., it is age-related), with the latter being closely related to the reproductive status of females (menarche and postmenopause) (Borries et al., 1991). This ontogenetic process can be characterized into three stages. The first occurs before sexual maturity, when the female has numerous social interactions in order to establish her position within the group. Second, once the female is mature, she has fewer social interactions, which indicates a period of stabilization of her position. The final stage corresponds to the postmenopausal period, which can lead to even fewer social interactions resulting from exclusion (Borries et al., 1991; Sosa, 2015). Unlike females, males are not subject to the phenomenon of declining social interactions, as none of their network metrics significantly decreases with age.
Third, the frequency of given agonistic behaviors increases with the hierarchical rank of a female. More specifically, the higher her hierarchical position, the greater number of submissive individuals with whom to ensure her rank a female has (Tokuda and Jensen, 1969) and the more she intervenes in conflicts to provide support (De Waal and Roosmalen, 1979; Seyfarth and Cheney, 1984; De Waal, 1997). This does not appear to be a response to received agonistic behaviors as the indegree would also increase, which is not the case. Instead, the agonistic indegree, together with the eigenvector, decreases with the hierarchical rank of a female. This decline in agonistic indegree is also observed in males, stressing that high-ranking individuals, regardless of gender, receive fewer agonistic behaviors than low-ranking ones. This reveals the benefits of dominant positions, with the reduction of associated risks (Gartlan, 1968; Bernstein, 1976; Chapais, 1991). Additionally, a significant relationship between the frequency of received allogrooming behaviors and hierarchical rank is observed in females. The absence of such phenomenon among males can be attributed to the fact that attractiveness to high-ranking individuals in allogrooming for males seems species-specific (Watts, 2000). Nonetheless, this phenomenon is observed among females according to GLMM results, which is in accordance with the theory advanced by Seyfarth (1977) in which dominant females should be preferred allogrooming partners as they can provide better protection (Watts, 2000; Cheney and Seyfarth, 2008) and support (Cheney and Seyfarth, 1990).
The fourth finding is that, similar to the hierarchy results, females within the same matriline have similar centralities and activities, and the higher the matriline, the more central and active the female is in the agonistic network. This trend can be attributed to the fact that in the genus Macaca, the hierarchical ranks of females are intimately linked to their matrilines. Furthermore, a closer examination of the previously discussed high agonistic activity of immature females shows that the indegree is more intense for high-born ones (Figure 3). Before sexual maturity, females must settle their dominance relations with lower matriline-ranking females through agonistic interactions (Chapais (2004). Thus, in their early years, females compete to establish their hierarchical rank on multiple fronts: (1) within their own matriline (as supported by the MRI phenomenon); (2) within their age category (in accordance with age homophily results for agonistic behaviors); and (3) toward older lower-ranking females.
Fifth point is that homophily results for the agonistic network show that agonistic interactions are mainly directed within the same age and gender. These findings, combined with GLMM results, yield interesting biological interpretations. Higher activity and connections in the agonistic ego network among young individuals (GLMM results) can be interpreted as a phenomenon of hierarchical rank acquisition. Homophily results highlight that this rank acquisition occurs mainly between same-age and same-gender individuals (Chapais, 1988; Holekamp and Smale, 1991), stressing the existence of a particular phenomenon that we could call the intra-generational conflict. Furthermore, the fact that affiliative behaviors are also primarily directed toward same-age individuals (age homophily results for allogrooming behaviors) underlines the trend of individuals to build their affiliative network within their age category.
Sixth, we observe homophily in allogrooming behaviors according to hierarchical rank, but only when both genders are taken into account. In other words, opposite-gender individuals with similar hierarchical ranks have preferential affiliative interactions. Allogrooming behaviors facilitate the creation of affiliative bonds and potential support in future conflicts (De Waal and Roosmalen, 1979; Seyfarth and Cheney, 1984; De Waal, 1997). Subsequently, this behavioral pattern can explain the decrease of agonistic behaviors received by high-ranking individuals (observed in GLMM results) owing to the high-ranking support of a third party.
Homophily has previously been reported in many species (McPherson et al., 2001; Lusseau and Newman, 2004; Massen and Koski, 2013). Extensive research on human homophily stressed that it is a major mechanism in stranger cooperation (Haun and Over, 2015), social learning (Buttelmann et al., 2013), and cultural and norms transmission (Chudek and Henrich, 2011). Recent studies argue that homophilic preferences may explain the gap between animals and humans regarding these abilities (Haun and Over, 2015). Revealing homophily in several behaviors and as it is influenced by different attributes highlights the importance of these mechanisms in a non-human primate species. However, many methods exist to evaluate the presence or absence of homophily (E-I index, ERGM, assortativity, Moran I statistic), each one of them with inherent pros and cons that would need to be evaluated before determining which of these approaches is more relevant for studying animal societies.
This analytical protocol can be used to study other animal societies and might enable interspecific comparisons. I believe that the important findings of this study might help understand the global patterning of a non-human primate society, and likely other animal societies, from an evolutionary perspective.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
First of all, I would like to formulate special thanks to Marine Huser for her appreciated help during this study. I am grateful to Francesc Salvador Beltran and Vicenç Quera for guiding and advising me throughout this investigation. Many thanks to Ellen Merz who allowed me to lead the research field work at ‘La Forêt des Singes’ and provided me the demographic data; I sincerely thank her for her advice. Salvador Herrando, David Leiva Ureña and Roland Hilgartner provided helpful advice at different stages of the analytical protocol. I would also like to thank Frontiers in Psychology reviewers for their precious suggestions, which largely participated to the improvement of this work. Finally, thanks to all the anonymous who have encouraged me from near or far and allowed the existence of this work.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpsyg.2016.00529
References
Archie, E. A., Morrison, T. A., Foley, C. A., Moss, C. J., and Alberts, S. C. (2006). Dominance rank relationships among wild female African elephants, Loxodonta africana. Anim. Behav. 71, 117–127. doi: 10.1002/zoo.20249
Aureli, F., and de Waal, F. B. M. (2000). Natural Conflict Resolution. Berkeley, CA: University of California Press.
Bergman, D. A., and Moore, P. A. (2003). Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 205, 26–35. doi: 10.2307/1543442
Bernstein, I. S. (1976). Dominance, aggression and reproduction in primate societies. J. Theor. Biol. 60, 459–472. doi: 10.1016/0022-5193(76)90072-2
Borgatti, S. P., Everett, M. G., and Freeman, L. C. (2002). Ucinet for Windows: Software for Social Network Analysis. Harvard: Analytic Technologies.
Borries, C., Sommer, V., and Srivastava, A. (1991). Dominance, age, and reproductive success in free-ranging female Hanuman langurs (Presbytis entellus). Int. J. Primatol. 12, 231–257. doi: 10.1007/BF02547586
Brent, L. J., Lehmann, J., and Ramos-Fernández, G. (2011). Social network analysis in the study of nonhuman primates: a historical perspective. Am. J. Primatol. 73, 720–730. doi: 10.1002/ajp.20949
Buttelmann, D., Zmyj, N., Daum, M., and Carpenter, M. (2013). Selective imitation of in-group over out-group members in 14-month-old infants. Child Dev. 84, 422–428. doi: 10.1111/j.1467-8624.2012.01860.x
Carter, A. J., Lee, A. E., Marshall, H. H., Ticó, M. T., and Cowlishaw, G. (2015). Phenotypic assortment in wild primate networks: implications for the dissemination of information. R. Soc. Open Sci. 2:140444. doi: 10.1098/rsos.140444
Cavigelli, S. A., and Pereira, M. E. (2000). Mating season aggression and fecal testosterone levels in male ring-tailed lemurs Lemur catta. Horm. Behav. 37, 246–255. doi: 10.1006/hbeh.2000.1585
Chapais, B. (1988). Experimental matrilineal inheritance of rank in female Japanese macaques. Anim. Behav. 36, 1025–1037. doi: 10.1016/S0003-3472(88)80062-9
Chapais, B. (1991). “Primates and the origins of aggression, power and politics among humans,” in Understanding Behavior: What Primate Studies Tell us About Human Behavior, eds J. Loy and B. Peters (New York, NY: Oxford University Press), 190–218.
Chapais, B. (2004). “How kinship generates dominance structures: a comparative perspective,” in Cambridge Studies in Biological and Evolutionary Anthropology, eds B. Thierry, V. Sing, and W. Kaumanns (Cambridge: Cambridge university press), 186–203.
Chapais, B., and Berman, C. M. (2004). ). Kinship and Behavior in Primates. Oxford: Oxford University Press.
Chapais, B., and Gauthier, C. (1993). “Early agonistic experience and the onset of matrilineal rank acquisition in Japanese macaques,” in Juvenile Primates–Life History, Development, and Behavior, eds M. E. Pereira and L. A. Fairbanks (Oxford: Oxford University Press), 245–258.
Cheney, D., and Seyfarth, R. (1990). How Monkeys See the World: Inside the Mind of Another Specie. Chicago, IL: Chicago University Press.
Cheney, D. L. (1977). The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behav. Ecol. Sociobiol. 2, 303–318. doi: 10.1007/BF00299742
Cheney, D. L., and Seyfarth, R. M. (2008). Baboon Metaphysics: The Evolution of a Social Mind. Chicago, IL: University of Chicago Press.
Chudek, M., and Henrich, J. (2011). Culture–gene coevolution, norm-psychology and the emergence of human prosociality. Trends Cogn. Sci. 15, 218–226. doi: 10.1016/j.tics.2011.03.003
Cords, M. (2002). Friendship among adult female blue monkeys (Cercopithecus mitis). Behaviour 139, 291–314. doi: 10.1163/156853902760102681
Croft, D., James, R., and Krause, J. (2008). Exploring Animal Social Networks. Princeton, NJ: Princeton University Press.
Croft, D., James, R., Ward, A., Botham, M., and Mawdsley, D. (2005). Assortative interactions and social networks in fish. Oecologia 143, 211–219. doi: 10.1007/s00442-004-1796-8
Croft, D. P., Madden, J. R., Franks, D. W., and James, R. (2011). Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507. doi: 10.1016/j.tree.2011.05.012
Datta, S. (1983). “Patterns of agonistic interference,” in Primate Social Relationships: An Integrated Approach, ed. R. A. Hinde (Sunderland, MA: Sinauer Associates), 289–297.
David, H. A. (1987). Ranking from unbalanced paired-comparison data. Biometrika 74, 432–436. doi: 10.1093/biomet/74.2.432
de Turckheim, G., and Merz, E. (1984). Breeding Barbary macaques in Outdoor Open Enclosures. New York, NY: Plenum Press.
de Vries, H., Stevens, J. M., and Vervaecke, H. (2006). Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592. doi: 10.1016/j.anbehav.2005.05.015
De Waal, F. (1997). The chimpanzee’s service economy: food for grooming. Evol. Hum. Behav. 18, 375–386. doi: 10.1016/S1090-5138(97)00085-8
De Waal, F., and Luttrell, L. M. (1989). Toward a comparative socioecology of the genus Macaca: different dominance styles in rhesus and stumptail monkeys. Am. J. Primatol. 19, 83–109. doi: 10.1002/ajp.1350190203
De Waal, F. M., and Roosmalen, A. (1979). Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55–66. doi: 10.1007/BF00302695
Dunbar, R. (1989). “Social systems as optimal strategy sets: the costs and benefits of sociality,” in Comparative Socioecology: the Behavioural Eology of Humans and Animals, eds V. Standen and R. Foley (Oxford: Blackwell Scientific), 73–88.
Fedigan, L. M., and Baxter, M. J. (1984). Sex differences and social organization in free-ranging spider monkeys (Ateles geoffroyi). Primates 25, 279–294. doi: 10.1007/BF02382267
Fragaszy, D. M., and Mitchell, G. (1974). Infant socialization in primates. J. Hum. Evol. 3, 563–574. doi: 10.1016/0047-2484(74)90017-7
Freeman, L. C. (1979). Centrality in social networks conceptual clarification. Soc. Netw. 1, 215–239. doi: 10.1016/0378-8733(78)90021-7
Gartlan, J. (1968). Structure and function in primate society. Folia Primatol. 8, 89–120. doi: 10.1159/000155138
Granovetter, M. S. (1973). The strength of weak ties. Am. J. Sociol. 78, 1360–1380. doi: 10.1086/225469
Gray, J. A. (1971). Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta Psychol. 35, 29–46. doi: 10.1016/0001-6918(71)90029-1
Hanneman, R. A., and Riddle, M. (2005). Introduction to Social Network Methods. Riverside, CA: University of California Riverside.
Harlow, H. F., and Suomi, S. J. (1974). Induced depression in monkeys. Behav. Biol. 12, 273–296. doi: 10.1016/S0091-6773(74)91475-8
Haun, D., and Over, H. (2015). Like me: A homophily-Based Account of Human Culture, Epistemological Dimensions of Evolutionary Psychology. Berlin: Springer, 117–130.
Hauser, M. D., and Tyrrell, G. (1984). Old age and its behavioral manifestations: a study on two species of macaque. Folia Primatol. 43, 24–35. doi: 10.1159/000156168
Hesler, N., and Fischer, J. (2007). “Gestural communication in Barbary macaques (Macaca sylvanus): an overview,” in The Gestural Communication of Apes and Monkeys, eds M. Tomasello and J. Call (New Jersey: Lawence Erlbaaum Associates), 159–195.
Hirsch, B. T., Stanton, M. A., and Maldonado, J. E. (2012). Kinship shapes affiliative social networks but not aggression in ring-tailed coatis. PLoS ONE 7:e37301. doi: 10.1371/journal.pone.0037301
Holekamp, K. E., and Smale, L. (1991). Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Am. Zool. 31, 306–317. doi: 10.1093/icb/31.2.306
Ihaka, R., and Gentleman, R. (1996). R: alanguage for data analysis and graphics. J. Computat. Graph. Statistics 5, 299–314. doi: 10.2307/1390807
Inc, H. (2009). H3Apps Icorporated, Heuser Timothy. App WhatISee 2.0. United States, Louisville, KY.
Kanngiesser, P., Sueur, C., Riedl, K., Grossmann, J., and Call, J. (2011). Grooming network cohesion and the role of individuals in a captive chimpanzee group. Am. J. Primatol. 73, 758–767. doi: 10.1002/ajp.20914
Kasper, C., and Voelkl, B. (2009). A social network analysis of primate groups. Primates 50, 343–356. doi: 10.1007/s10329-009-0153-2
Kawamura, S. (1958). The matriarchal social order in the Minoo-B group. Primates 1, 149–156. doi: 10.1007/BF01813701
Krause, J., Lusseau, D., and James, R. (2009). Animal social networks: an introduction. Behav. Ecol. Sociobiol. 63, 967–973. doi: 10.1007/s00265-009-0747-0
Lazarsfeld, P. F., and Merton, R. K. (1954). Friendship as a social process: a substantive and methodological analysis. Freedom Control Modern Soc. 18, 18–66.
Lusseau, D., and Newman, M. E. (2004). Identifying the role that animals play in their social networks. Proc. R. Soc. Lon. B Biol. Sci. 271, S477–S481. doi: 10.1098/rsbl.2004.0225
Manly, B. F. (2006). Randomization, Bootstrap and Monte Carlo Methods in Biology. Boca Raton, FL: CRC Press.
Massen, J. J., and Koski, S. E. (2013). Chimps of a feather sit together: chimpanzee friendships are based on homophily in personality. Evol. Hum. Behav. 35, 1–8.
Matsuda, I., Zhang, P., Swedell, L., Mori, U., Tuuga, A., Bernard, H., et al. (2012). Comparisons of intraunit relationships in nonhuman primates living in multilevel social systems. Int. J. Primatol. 33, 1038–1053. doi: 10.1007/s10764-012-9616-1
McPherson, M., Smith-Lovin, L., and Cook, J. M. (2001). Birds of a feather: homophily in social networks. Ann. Rev. Sociol. 27, 415–444. doi: 10.1146/annurev.soc.27.1.415
Mitchell, G., Arling, G., and Moller, G. (1967). Long-term effects of maternal punishment on the behavior of monkeys. Psychon. Sci. 8, 209–210. doi: 10.3758/BF03331624
Moran, P. A. (1950). Notes on continuous stochastic phenomena. Biometrika 37, 17–23. doi: 10.2307/2332142
Olds, D. L., Eckenrode, J., Henderson, C. R., Kitzman, H., Powers, J., Cole, R., et al. (1997). Long-term effects of home visitation on maternal life course and child abuse and neglect. J. Am. Med. Assoc. 278, 637–643. doi: 10.1001/jama.278.8.637
Opsahl, T. (2009). Structure and Evolution of Weighted Networks. London: Queen Mary University of London.
Pereira, M. E. (1988). Effects of age and sex on intra-group spacing behaviour in juvenile savannah baboons, Papio cynocephalus cynocephalus. Anim. Behav. 36, 184–204. doi: 10.1016/S0003-3472(88)80262-8
Pereira, M. E., and Fairbanks, L. A. (1993). Juvenile Primates: Life History, Development and Behavior, with a New Foreword. Chicago, IL: University of Chicago Press.
Prell, C. (2011). Social Network Analysis: History, Theory and Methodology. Thousand Oaks, CA: Sage.
Sade, D. (1972). Sociometrics of Macaca mulatta linkages and cliques in grooming matrices. Folia Primatol. 18, 196–223. doi: 10.1159/000155480
Schino, G. (2001). Grooming, competition and social rank among female primates: a meta-analysis. Anim. Behav. 62, 265–271. doi: 10.1006/anbe.2001.1750
Seyfarth, R. M. (1977). A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698. doi: 10.1016/0022-5193(77)90015-7
Seyfarth, R. M., and Cheney, D. L. (1984). Grooming, alliances and reciprocal altruism in vervet monkeys. Nature 308, 541–543. doi: 10.1038/308541a0
Shimada, M., and Sueur, C. (2014). The importance of social play network for infant or juvenile wild chimpanzees at Mahale Mountains National Park, Tanzania. Am. J. Primatol. 76, 1025–1036. doi: 10.1002/ajp.22289
Silk, I. B. (2001). “Ties that bond: the role of kinship in primate societies,” in New Directions in Anthropological Kinship, ed. L. Stone (New York, NY: Rowman & Lit-tlefiled), 71.
Sosa, S. (2014). Structural architecture of the social network of a non-human primate (Macaca sylvanus): a study of its topology in la forêt des singes. Rocamadour. Folia Primatol. 85, 154–163. doi: 10.1159/000360986
Sosa, S. (2015). Using individual attributes to predict hierarchical position in a Macaca sylvanus group at ‘La forêt des singes’, Rocamadour. Behav. Process. 111, 109–117. doi: 10.1016/j.beproc.2014.12.011
Stephens, P. A., Buskirk, S. W., and del Rio, C. M. (2007). Inference in ecology and evolution. Trends Ecol. Evol. 22, 192–197. doi: 10.1016/j.tree.2006.12.003
Sueur, C., and Petit, O. (2008). Organization of group members at departure is driven by social structure in Macaca. Int. J. Primatol. 29, 1085–1098. doi: 10.1007/s10764-008-9262-9
Sueur, C., Petit, O., De Marco, A., Jacobs, A., and Watanabe, K. (2011). A comparative network analysis of social style in macaques. Anim. Behav. 82, 845–852. doi: 10.1016/j.anbehav.2011.07.020
Sugiyama, Y. (2015). Influence of provisioning on primate behavior and primate studies. Mammalia 79, 255–265. doi: 10.1515/mammalia-2014-0028
Thierry, B., Iwaniuk, A. N., and Pellis, S. M. (2000). The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology 106, 713–728. doi: 10.1046/j.1439-0310.2000.00583.x
Thierry, B., Singh, M., and Kaumanns, W. (2004). Macaque Societies: A Model for the Study of Social Organization. Cambridge: Cambridge University Press.
Tokuda, K., and Jensen, G. D. (1969). Determinants of dominance hierarchy in a captive group of pigtailed monkeys (Macaca nemestrina). Primates 10, 227–236. doi: 10.1007/BF01730344
Voelkl, B., and Noe, R. (2008). The influence of social structure on the propagation of social information in artificial primate groups: a graph-based simulation approach. J. Theor. Biol. 252, 77–86. doi: 10.1016/j.jtbi.2008.02.002
Wasserman, S., and Faust, K. (1994). Social Network Analysis: Methods and Applications. Cambridge, MA: Cambridge university press.
Watts, D. J. (2003). Small Worlds: The Dynamics of Networks Between Order and Randomness. Princeton, NJ: Princeton university press.
Watts, D. P. (2000). Grooming between male chimpanzees at Ngogo, Kibale National Park. I. Partner number and diversity and grooming reciprocity. Int. J. Primatol. 21, 189–210. doi: 10.1023/A:1005421419749
Wey, T. W., and Blumstein, D. T. (2010). Social cohesion in yellow-bellied marmots is established through age and kin structuring. Anim. Behav. 79, 1343–1352. doi: 10.1016/j.anbehav.2010.03.008
Whitehead, H. (2008). Analyzing Animal Societies: Quantitative Methods for Vertebrate Social Analysis. Chicago, IL: University of Chicago Press.
Widdig, A., Nürnberg, P., Krawczak, M., Streich, W. J., and Bercovitch, F. B. (2001). Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc. Natl. Acad. Sci. U.S.A. 98, 13769–13773. doi: 10.1073/pnas.241210198
Keywords: social network analysis, multilevel analysis, non-human primate, allogrooming, antagonism, individual attributes, homophily
Citation: Sosa S (2016) The Influence of Gender, Age, Matriline and Hierarchical Rank on Individual Social Position, Role and Interactional Patterns in Macaca sylvanus at ‘La Forêt des Singes’: A Multilevel Social Network Approach. Front. Psychol. 7:529. doi: 10.3389/fpsyg.2016.00529
Received: 31 October 2015; Accepted: 30 March 2016;
Published: 18 April 2016.
Edited by:
Cédric Sueur, Institut Pluridisciplinaire Hubert Curien, FranceReviewed by:
Ikki Matsuda, Kyoto University, JapanBorbala Foris, Leibniz Institute for Farm Animal Biology, Germany
Copyright © 2016 Sosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Sosa, s.sosa@live.fr
 Sebastian Sosa
Sebastian Sosa