- 1Developmental Cognitive Neuroscience Laboratory, School of Psychology, University of Central Lancashire, Preston, UK
- 2School of Psychological Sciences, University of Manchester, Manchester, UK
In recent years there has been increasing interest in the neural mechanisms underlying altered emotional processes in children and adolescents with psychopathology. This review provides a brief overview of the most up-to-date findings in the field of event-related potentials (ERPs) to facial and vocal emotional expressions in the most common child psychopathological conditions. In regards to externalizing behavior (i.e., ADHD, CD), ERP studies show enhanced early components to anger, reflecting enhanced sensory processing, followed by reductions in later components to anger, reflecting reduced cognitive-evaluative processing. In regards to internalizing behavior, research supports models of increased processing of threat stimuli especially at later more elaborate and effortful stages. Finally, in autism spectrum disorders abnormalities have been observed at early visual-perceptual stages of processing. An affective neuroscience framework for understanding child psychopathology can be valuable in elucidating underlying mechanisms and inform preventive intervention.
Introduction
The worldwide prevalence of mental disorders in children and adolescents is about 13% and continues to rise (Polanczyk et al., 2015). As the majority of adult mental health disorders begin in childhood and adolescence, it is important to gain a better understanding of the causal mechanisms as well as the factors reducing risk and increasing resilience in the young to help develop effective prevention strategies. In the recent years, there has been a renewed interest in emotion dysregulation as a mechanism increasing the risk for a range of psychopathological conditions (Kret and Ploeger, 2015). Understanding the neurobiology of emotion processing in child psychopathology can advance knowledge of underlying mechanisms and aid the identification of intervention targets (Pine, 2007).
Understanding other’s emotions is critical in social interaction. Theoretical debates have focused on whether brain structures are specialized for processing social information or whether social cognition is part of general cognitive processes applied to social behavior (Adolphs, 2009). Empirical research has supported the proposal that there is a network of specific brain areas preferentially involved in the processing of social information, a network often referred to as the ‘social brain’ (Brothers, 1990; Johnson et al., 2005; Adolphs, 2009). Developmental psychology has demonstrated that the ability to understand other’s feelings and mental states develops in the first 4 years of life (Frith and Frith, 2003). Developmental neuroscience frameworks can be valuable for the study of emotion processing. Development provides a unique opportunity to study the neural correlates of emotion processing as they emerge at different ages (De Haan et al., 2003; Grossmann et al., 2007). This approach can provide answers to the question of ‘when’ the developing brain begins to become ‘tuned’ to its social environment. Event-related potentials (ERPs) represent a useful, non-invasive methodology to understand the timing (in a millisecond resolution) of the sensory, perceptual, and cognitive processes underlying social information processing (Nelson and Luciana, 2001). As neural substrates implicated in social processing become more specialized over development (Johnson et al., 2009), ERPs can inform our understanding of whether neurally separate components have the potential to be specialized for processing emotional information (De Haan and Gunnar, 2009). Finally, ERP methods are useful in conceptualizing not only typical but also atypical development as they can reveal individual differences which may not be evident in observable behavior. Developmental transitions in particular, such as early childhood and adolescence, represent important landmarks in mental health trajectories and are accompanied with a unique set of opportunities and challenges (Blakemore, 2010) which overlap with important neurobiological changes in emotion processing.
This mini-review aims to briefly summarize the ERP components implicated in facial and vocal emotion recognition in typical and atypical development. For this mini-review, computerized searches of articles published until 2015 were conducted using the PubMed, Psycinfo, Science Direct and Nature journals online databases. The following terms ERPs, facial, vocal, emotion recognition, child, adolescent, psychopathology, externalizing, internalizing, ADHD, CD, ASD, anxiety, depression, were entered into the databases. In addition, the table of contents of journals that often publish articles relevant to this topic were reviewed including Journal of Child Psychology and Psychiatry, Frontiers in Neuroscience, Human Brain Mapping, Biological Psychiatry, Nature Neuroscience, Developmental Science, Social Neuroscience and American Journal of Psychiatry. Finally, the reference lists of relevant articles were scanned for pertinent studies. Only studies written in English were included (see Table 1).
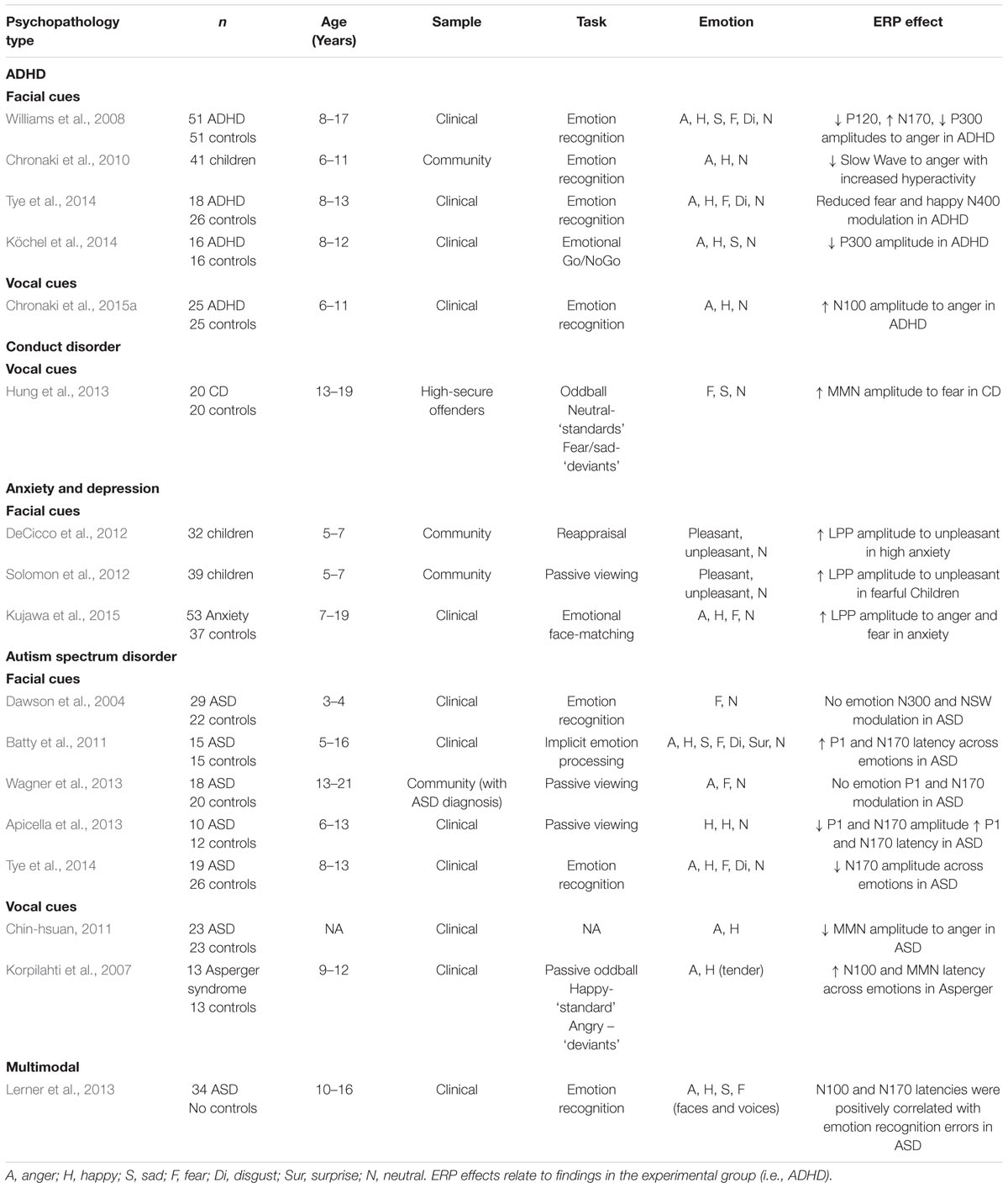
TABLE 1. A summary of empirical findings of altered ERP responses to facial and vocal emotional stimuli in children and adolescents with psychopathology.
Typical Development
Theoretical models for recognizing facial emotional expressions emphasize that conceptual knowledge of emotion signaled by the face is preceded by early perceptual processes by salient stimuli (Bruce and Young, 1986; Haxby et al., 2000). The N170 is an occipitotemporal potential traditionally linked to sensitivity in processing information from human faces (Bentin et al., 1996; Taylor et al., 1999). Some studies have shown that the N170 is sensitive to facial emotion in adults (Batty and Taylor, 2003; Blau et al., 2007), although other studies have not found facial emotion modulation of the N170 (Eimer and Holmes, 2002; Herrmann et al., 2002; Eimer et al., 2003). Infant research has identified the N290 as a developmental precursor to the adult N170 (Halit et al., 2003, 2004). Emotion effects on the N170 have been observed in older (14–15-years-old) compared to younger (4–12-years-old) children, with N170 amplitudes being larger for negative (anger, sad) compared to positive (happy) and neutral faces in emotion recognition tasks (Batty and Taylor, 2006). Compared to the N170 proposed to index ‘fine-grained’ sensitivity to facial emotion emerging during adolescence, a parietal–occipital P1 component (∼120 ms) has been suggested to reflect global and ‘superficial’ processing of facial emotion that is present in younger children (Batty and Taylor, 2006; Vlamings et al., 2010). Beyond early components, later components such as the late positive potential (LPP), a parietal–occipital component evident from around 300 ms, show sensitivity to the emotional content of human faces and are proposed to signify elaborative or effortful processing of emotionally significant stimuli in healthy adults (Hajcak et al., 2010). The LPP has been shown to be sensitive to facial emotion in children. In particular, the LPP was larger in amplitude to angry compared to happy faces in 7-year-old children in emotion recognition tasks (Kestenbaum and Nelson, 1992) and sad compared to neutral faces at occipital areas in 6-year-old children in a passive viewing paradigms (Kujawa et al., 2012).
Despite a number of studies using facial stimuli, considerably less is known about the neural development of vocal emotion processing. This is surprising given the prominent role of vocal emotional expressions in children’s social interactions. Brain potentials in response to voice compared to non-voice sounds emerge between 160 and 200 ms on frontocentral (positivity) and occipital (negativity) sites in healthy adults (Charest et al., 2009). This suggests that the neural processing of voices and faces (‘face-specific’ N170) occur at similar time points explaining the integration of such signals in real-life social interactions (Campanella and Belin, 2007). In healthy adults, the recognition of emotion from vocal signals (i.e., ‘prosody’) is represented in the brain by a series of ERP components. According to a three-process model of emotional prosodic-processing, a temporal N100 component is suggested to reflect early sensory processing of vocal expressions, followed by a P200 component, proposed to reflect integration of prosodic acoustic cues and finally, frontal late latency components (i.e., P300, N400) reflecting cognitive-evaluative judgments such as labeling emotional expressions (Schirmer and Kotz, 2006). In adults, vocal emotion effects have consistently been observed in the N400 component (Bostanov and Kotchoubey, 2004; Paulmann and Kotz, 2008). The human brain begins to become sensitive to vocal signals of emotion from the first months of life (review by Grossmann and Johnson, 2007). Despite a number of infant studies, very little is known about the neural development of vocal emotion processing in childhood. In typically developing 6–11-year-old children differential ERPs to distinct vocal expressions of emotion (angry, happy, and neutral) have been identified in an emotion recognition task (Chronaki et al., 2012). These consisted of an early, N100 (90–180 ms) and a later, N400 (380–500 ms) component observed in more posterior (parietal–occipital) regions compared to adults (Chronaki et al., 2012). Further research is needed in the neural development of vocal emotion processing in children and adolescents.
Atypical Development
An emerging body the ERP literature supports the idea that sensory, perceptual, and cognitive processing stages of emotion recognition may be altered in children with psychopathology. The section that follows reviews some landmark studies in children with externalizing and internalizing problems and autism spectrum conditions.
Attention-Deficit/Hyperactivity Disorder
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder characterized by developmentally inappropriate levels of inattention, hyperactivity, and impulsivity (Americal Psychiatric Association [APA], 2013). Motivational processes (Sonuga-Barke and Fairchild, 2012) are implicated in ADHD and emotion dysregulation is recognized as an important clinical feature of the condition (Shaw et al., 2014; Bunford et al., 2015).
Although, some theories suggest that emotion processing difficulties in children with ADHD may result from general inattention or impulsiveness, socio-cognitive models have argued in favor of emotion-specific difficulties (review by Uekermann et al., 2010). Behavioral studies have shown that individuals with ADHD present deficits in the recognition of emotions (especially negative emotions) from facial expressions (see Uekermann et al., 2010) and that these deficits can be independent of cognitive functions such as attention (Bisch et al., 2016) and performance in non-emotion tasks (Rapport et al., 2002). Emotion recognition deficits are associated with behavior problems already in preschool (Chronaki et al., 2015b) and school-aged children (Pelc et al., 2006; Yuill and Lyon, 2007). ERP correlates of these deficits have only recently been identified. Adolescents with ADHD have been shown to display reduced occipital P120, followed by increased N170 and reduced temporal P300 amplitudes to anger and fear in a facial emotion recognition task (Williams et al., 2008). These findings may suggest reduction in occipital activity during the early perceptual processing of anger (120 ms), followed by increased activity during structural encoding stages (∼170 ms) and later reduction in temporal activity reflecting context processing of anger (∼300 ms). Similarly, hyperactivity was negatively associated with occipital Slow Wave amplitudes to facial anger in an emotion recognition task in a community sample of 6–11-year-old children (Chronaki et al., 2010). Similar work has shown that impairments in response inhibition to angry faces have been associated with reduced P300 amplitudes in a Go/Nogo task in boys with ADHD compared to controls (Köchel et al., 2014).
The only ERP study to date using vocal stimuli has shown enhanced N100 and attenuated P300 amplitudes to vocal anger in 6–11-years-old with ADHD in an emotion recognition task using pure prosodic stimuli (Chronaki et al., 2015a). The N100 effect persisted after excluding children with comorbid Conduct Disorder. This pattern of results possibly reflects hyper-vigilance to vocal anger in ADHD at early and almost automatic processing stages consistent with an automatic and less controlled processing style in ADHD (Oades et al., 1996). These findings are consistent with near-infrared spectroscopy work showing stronger supramarginal gyrus activation to sentences with angry intonation in children with ADHD (Köchel et al., 2015) and functional magnetic resonance imaging (fMRI) work showing enhanced frontal and posterior cingulate cortex activation to anger from facial expressions in 10–17-years-old with ADHD compared to controls (Marsh et al., 2008). Results should be interpreted in the context of recent conceptual models of emotional dysregulation in ADHD involving a circuitry underpinning deficits in rapid early orienting to emotion (i.e., ventral striatum, amygdala; Shaw et al., 2014).
Conduct Disorder
Conduct disorder (CD) is a condition at the severe end of a continuum of oppositional defiant behaviors (Americal Psychiatric Association [APA], 2013). The majority of studies in emotion processing in CD and associated conditions have employed behavioral and fMRI methods and have shown pervasive deficits in the recognition of a range of emotions from facial and vocal modalities (meta-analysis by Dawel et al., 2012). A recent ERP study has shown that young offenders with CD displayed stronger mismatch negativity (MMN) to fearful syllables in a passive listening task with no difference found in controls. This findings may reflect enhanced pre-attentive auditory change detection for distressful stimuli in youth with CD (Hung et al., 2013). Despite methodological differences, these results are generally inconsistent with evidence from behavioral (Blair et al., 2005; Dadds et al., 2008; Fairchild et al., 2009) and functional neuro-imaging (Jones et al., 2009) studies which show reduced sensitivity to fearful facial expressions in active-attention tasks. These findings should be considered in the context of theoretical frameworks suggesting that failure to inhibit antisocial behaviors may be the result of lower sensitivity to distress-related cues from others such as fear (Blair, 2001).
There is a striking lack of empirical studies on the temporal processing of emotion in youth with CD. Further research is necessary before drawing any conclusions. In addition, given the high rates of comorbidity between CD and ADHD, future research should examine the electrophysiological correlates of emotion processing in ADHD, ADHD+CD, and CD to clarify the role of common or distinct neural pathways.
Anxiety and Depression
The experience of negative affect (i.e., anxiety and depression) in children and adolescents has been closely associated with emotion processing (Hadwin and Field, 2010). Behavioral work in this area has predominantly been guided by theoretical frameworks of attentional biases to threat (Bar-Haim et al., 2007). The ERP literature points to the direction of enhanced neural response to threat (i.e., anger) stimuli in anxious children, as reflected by larger amplitudes of the LPP component, proposed to reflect elaborative or effortful processing of emotional stimuli (Schupp et al., 2000; Hajcak et al., 2010). Recently, Kujawa et al. (2015) found that relative to healthy controls, 7–19-year-old diagnosed with social anxiety, separation anxiety, and generalized anxiety disorders showed enhanced LPP amplitudes to angry and fearful faces during an emotional face-matching task. This is consistent with earlier research using pictorial stimuli which has found increased processing of unpleasant compared to neutral pictures (reflected by the posterior LPP amplitudes) in a community sample of 5–7-year-old with high anxiety (DeCicco et al., 2012). Similar results have been found in 5–7-year-old children with inhibited and fearful behavior (Solomon et al., 2012). ERP research in emotion processing in childhood depression is more limited. In the study by Kujawa et al. (2015), higher depressive symptoms were associated with reduced LPP amplitudes to angry faces in 7–19-years-old diagnosed with an anxiety disorder (Kujawa et al., 2015). Results partly support adult studies linking depression to blunted or reduced emotional response (as reflected by the LPP), consistent with theories suggesting disengagement from emotional stimuli more generally in depression (Proudfit et al., 2015). In summary, preliminary findings support the LPP as a neural marker of neurobiological vulnerability to threat in childhood internalizing symptoms. Future work should aim to disentangle the role of anxiety and depression in the neural processing of threat and explore whether existing effects generalize to vocal modalities.
Autism Spectrum Disorder
Autism spectrum disorder (ASD) refers to a range of conditions characterized by impairment in social interaction and communication (Americal Psychiatric Association [APA], 2013). Children with ASD find social stimuli less salient than non-social stimuli (Stavropoulos and Carver, 2014) and present difficulties in recognizing other people’s emotions (see meta-analysis by Uljarevic and Hamilton, 2013). However, not all studies have supported emotion processing deficits in ASD (Jones et al., 2011). Further, it is not clear from behavioral studies whether existing deficits are emotion-specific or whether they are secondary to domain-general processing abnormalities (i.e., attention, sensory-perceptual processing).
Event-related potentials research has partly supported an atypical pattern of facial emotion processing in ASD. Typically developing 3–4-years-old displayed larger N300 amplitudes to fearful than neutral faces, while children with ASD did not show this effect in a passive viewing task (Dawson et al., 2004). Similarly, the amplitude of the face-sensitive N170 component varied with emotional expression only in typically developing adolescents aged 13–21 but not in adolescents with ASD who showed reduced neural differentiation between angry, fearful, and neutral facial expressions in a passive viewing task (Wagner et al., 2013). In an implicit emotional task, 10-years-old children with autism displayed longer P100 and N170 latencies and smaller P100 amplitudes to facial expressions of emotion including anger, disgust, happiness, sadness, surprise and fear. In this study, only the P1 amplitude remained affected in autism, after children with autism were matched by verbal equivalent age to controls, suggesting abnormalities at early stages of rapid visual perceptual processing (Batty et al., 2011). These findings are consistent with a slowed neural speed of face processing (McPartland et al., 2004) already present at 3 years in ASD (Webb et al., 2006). Recent research has shown that relative to controls, 6–13 years-old with ASD presented delayed latencies and reduced amplitudes of early components (P100, N170) regardless of emotion type in an implicit face-perception task whereby children viewed fearful, happy, and neutral faces and were asked to press a button when a cartoon stimulus was presented (Apicella et al., 2013). Results are consistent with fMRI work showing no impairments in the cognitive labeling of basic facial emotions in adolescents with ASD (Wang et al., 2004). More recently, children with ASD and comorbid ADHD have been shown to display reduced N170 amplitude across a range of facial emotions and particularly for fearful compared to neutral expressions in an emotion discrimination task (Tye et al., 2014), confirming work showing abnormalities at an early structural encoding processing stage.
Few studies have investigated the neural processing of vocal emotion in children with ASD, although recent infant fMRI work suggests that some infants at high-risk for ASD may present atypical neural responses to emotional (i.e., sad) vocalizations (Blasi et al., 2015). A first study has shown lower Mismatch negativity (MMN) amplitudes in response to angry but not happy voices in individuals with ASD, possibly reflecting atypical early sensory processing of negative emotion (Chin-hsuan, 2011). A second study examined the electrophysiological correlates of vocal emotion processing in 10–16-years-old with ASD using an emotion recognition task. Stimuli consisted of the phrase “I’m leaving the room now, but I’ll be back later” spoken in happy, angry, sad and fearful tone of voice (Lerner et al., 2013). This study found a significant correlation between emotion recognition errors and N100 latency, suggesting that participants with longer N100 latencies made more recognition errors. The findings were interpreted as indicating difficulties with speed of sensory processing of social information in ASD as reflected by N100 latency (Lerner et al., 2013). An important limitation of this study, however, was that it lacked a group of typically developing children to inform our understanding of the degree of abnormality of this processing. Similar work has investigated the neural correlates of vocal anger processing in 14 9–12-years-old boys with Asperger syndrome (AS) and 13 controls using a passive oddball paradigm (Korpilahti et al., 2007). Vocal stimuli consisted of the word ‘Anna!’ spoken with tender and angry tone of voice. Although the study did not report a differential neural response to emotion condition in any group, the N100 component peaked later in children with AS compared to controls (Korpilahti et al., 2007).
In summary, findings provide some evidence that impaired automatic discrimination of facial and vocal expressions may be a candidate neural marker of the social impairments observed in ASD. A limitation in existing research using vocal stimuli is that they include semantic or lexical confounds in the tasks. This raises the possibility that findings are influenced by differences in language comprehension (Paul et al., 2005). Future studies should employ pure prosodic stimuli devoid of semantic or lexical content.
Implications for Early Detection and Preventive Intervention
Future research should aim to elucidate the sensory, perceptual and cognitive processes (i.e., mechanisms) underlying emotion processing. Emotion-specific neural markers can be useful in identifying individuals most in need of preventive intervention as well as identifying risk and resilience factors for disorder-specific outcomes. Targeted prevention programs can help children read emotions in others successfully or help implement strategies to compensate for emotion-related abnormalities. This can help reduce the risk for later emerging psychopathology. It is critical to intervene early in order to prevent a number of problems before they manifest and help reduce the economic and social burden of mental disorders for individuals and society.
Author Contribution
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
The author would like to acknowledge funding support from the School of Psychology, University of Central Lancashire.
References
Adolphs, R. (2009). The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716. doi: 10.1146/annurev.psych.60.110707.163514
Americal Psychiatric Association [APA] (2013). Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: Americal Psychiatric Association.
Apicella, F., Sicca, F., Federico, R. R., Campatelli, G., and Muratori, F. (2013). Fusiform gyrus responses to neutral and emotional faces in children with autism spectrum disorders: a high density ERP study. Behav. Brain Res. 251, 155–162. doi: 10.1016/j.bbr.2012.10.040
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., and van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 133, 1–24. doi: 10.1037/0033-2909.133.1.1
Batty, M., Meaux, E., Wittemeyer, K., Rogé, B., and Taylor, M. J. (2011). Early processing of emotional faces in children with autism: an event-related potential study. J. Exp. Child Psychol. 109, 430–444. doi: 10.1016/j.jecp.2011.02.001
Batty, M., and Taylor, M. J. (2003). Early processing of the six basic facial emotional expressions. Cogn. Brain Res. 17, 613–620. doi: 10.1016/S0926-6410(03)00174-5
Batty, M., and Taylor, M. J. (2006). The development of emotional face processing during childhood. Dev. Sci. 9, 207–220. doi: 10.1111/j.1467-7687.2006.00480.x
Bentin, S., Allison, T., Puce, A., Perez, E., and McCarthy, G. (1996). Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 8, 551–565. doi: 10.1162/jocn.1996.8.6.551
Bisch, J., Kreifelts, B., Bretscher, J., Wildgruber, D., Fallgatter, A., and Ethofer, T. (2016). Emotion perception in adult attention-deficit hyperactivity disorder. J. Neural Transm. (Vienna) doi: 10.1007/s00702-016-1513-x [Epub ahead of print].
Blair, R. J., Budhani, S., Colledge, E., and Scott, S. (2005). Deafness to fear in boys with psychopathic tendencies. J. Child Psychol. Psychiatry 46, 327–336. doi: 10.1111/j.1469-7610.2004.00356.x
Blair, R. J. R. (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry 71, 727–731. doi: 10.1136/jnnp.71.6.727
Blakemore, S. J. (2010). The development of the social brain: implications for education. Neuron 65, 744–747. doi: 10.1016/j.neuron.2010.03.004
Blasi, A., Lloyd-Fox, S., Sethna, V., Brammer, M. J., Mercure, E., Murray, L., et al. (2015). Atypical processing of voice sounds in infants at risk for autism spectrum disorder. Cortex 71, 122–133. doi: 10.1016/j.cortex.2015.06.015
Blau, V. C., Maurer, U., Tottenham, N., and McCandliss, B. D. (2007). The face-specific N170 component is modulated by emotional facial expression. Behav. Brain Funct. 3, 7. doi: 10.1186/1744-9081-3-7
Bostanov, V., and Kotchoubey, B. (2004). Recognition of affective prosody: continuous wavelet measures of event-related brain potentials to emotional exclamations. Psychophysiology 41, 259–268. doi: 10.1111/j.1469-8986.2003.00142.x
Brothers, L. (1990). The neural basis of primate social communication. Motiv. Emot. 14, 81–91. doi: 10.1007/bf00991637
Bruce, V., and Young, A. (1986). Understanding face recognition. Br. J. Psychol. 77(Pt 3), 305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x
Bunford, N., Evans, S., and Wymbs, F. (2015). ADHD and emotion dysregulation among children and adolescents. Clin. Child Fam. Psychol. Rev. 18, 185–217. doi: 10.1007/s10567-015-0187-5
Campanella, S., and Belin, P. (2007). Integrating face and voice in person perception. Trends Cogn. Sci. 11, 535–543. doi: 10.1016/j.tics.2007.10.001
Charest, I., Pernet, C. R., Rousselet, G. A., Quinones, I., Latinus, M., Fillion-Bilodeau, S., et al. (2009). Electrophysiological evidence for an early processing of human voices. BMC Neurosci. 10:127. doi: 10.1186/1471-2202-10-127
Chin-hsuan, L. (2011). Atypical Automatic Processing of Emotional Voices in Autism Spectrum Disorders: A MMN Study. Graduate Institute of Cognitive Neuroscience. Available at: http://ir.lib.ncu.edu.tw:88/thesis/view_etd.asp?URN=972207001#
Chronaki, G., Benikos, N., Fairchild, G., and Sonuga-Barke, E. J. S. (2015a). Atypical neural responses to vocal anger in attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 56, 477–487. doi: 10.1111/jcpp.12312
Chronaki, G., Broyd, S., Garner, M., Hadwin, J. A., Thompson, M. J. J., and Sonuga-Barke, E. J. S. (2012). Isolating N400 as neural marker of vocal anger processing in 6–11-year old children. Dev. Cogn. Neurosci. 2, 268–276. doi: 10.1016/j.dcn.2011.11.007
Chronaki, G., Garner, M., Hadwin, J., Thompson, M., Sonuga-Barke, E., and Broyd, S. (2010). Electrophysiological correlates of emotion processing in children with ADHD. Paper Presented at the Eunethydis 1st International ADHD Conference: From Data to Best Clinical Practice, Amsterdam.
Chronaki, G., Garner, M., Hadwin, J. A., Thompson, M. J. J., Chin, C. Y., and Sonuga-Barke, E. J. S. (2015b). Emotion-recognition abilities and behavior problem dimensions in preschoolers: evidence for a specific role for childhood hyperactivity. Child Neuropsychol. 21, 25–40. doi: 10.1080/09297049.2013.863273
Dadds, M. R., El Masry, Y., Wimalaweera, S., and Guastella, A. J. (2008). Reduced eye gaze explains “Fear Blindness” in childhood psychopathic traits. J. Am. Acad. Child Adolesc. Psychiatry 47, 455–463. doi: 10.1097/CHI.0b013e31816407f1
Dawel, A., O’Kearney, R., McKone, E., and Palermo, R. (2012). Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci. Biobehav. Rev. 36, 2288–2304. doi: 10.1016/j.neubiorev.2012.08.006
Dawson, G., Webb, S. J., Carver, L., Panagiotides, H., and McPartland, J. (2004). Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Dev. Sci. 7, 340–359. doi: 10.1111/j.1467-7687.2004.00352.x
De Haan, M., and Gunnar, M. R. (2009). Handbook of Developmental Social Neuroscience. New York, NY: The Guildford Press.
De Haan, M., Johnson, M. H., and Halit, H. (2003). Development of face-sensitive event-related potentials during infancy: a review. Int. J. Psychophysiol. 51, 45–58. doi: 10.1016/S0167-8760(03)00152-1
DeCicco, J. M., Solomon, B., and Dennis, T. A. (2012). Neural correlates of cognitive reappraisal in children: an ERP study. Dev. Cogn. Neurosci. 2, 70–80. doi: 10.1016/j.dcn.2011.05.009
Eimer, M., and Holmes, A. (2002). An ERP study on the time course of emotional face processing. Neuroreport 13, 427–431. doi: 10.1097/00001756-200203250-00013
Eimer, M., Holmes, A., and McGlone, F. (2003). The role of spatial attention in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cogn. Affect. Behav. Neurosci 3, 97–110. doi: 10.3758/cabn.3.2.97
Fairchild, G., Van Goozen, S. H. M., Calder, A. J., Stollery, S. J., and Goodyer, I. M. (2009). Deficits in facial expression recognition in male adolescents with early-onset or adolescence-onset conduct disorder. J. Child Psychol. Psychiatry 50, 627–636. doi: 10.1111/j.1469-7610.2008.02020.x
Frith, U., and Frith, C. (2003). Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. 358, 459–473. doi: 10.1098/rstb.2002.1218
Grossmann, T., and Johnson, M. H. (2007). The development of the social brain in human infancy. Eur. J. Neurosci. 25, 909–919. doi: 10.1111/j.1460-9568.2007.05379.x
Grossmann, T., Striano, T., and Friederici, A. D. (2007). Developmental changes in infants’ processing of happy and angry facial expressions: a neurobehavioral study. Brain Cogn. 64, 30–41. doi: 10.1016/j.bandc.2006.10.002
Hadwin, J., and Field, A. (2010). “An introduction to the study of information processing biases in childhood anxiety: theoretical and methodological issues,” in Information Processing Biases and Anxiety: A Developmental Perspective, eds J. Hadwin and A. Field (Chichester: Wiley-Blackwell), 1–17.
Hajcak, G., MacNamara, A., and Olvet, D. M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 35, 129–155. doi: 10.1080/87565640903526504
Halit, H., Csibra, G., Volein,Á., and Johnson, M. H. (2004). Face-sensitive cortical processing in early infancy. J. Child Psychol. Psychiatry 45, 1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x
Halit, H., de Haan, M., and Johnson, M. H. (2003). Cortical specialisation for face processing: face-sensitive event-related potential components in 3- and 12-month-old infants. Neuroimage 19, 1180–1193. doi: 10.1016/S1053-8119(03)00076-4
Haxby, J. V., Hoffman, E. A., and Gobbini, M. I. (2000). The distributed human neural system for face perception. Trends Cogn. Sci. 4, 223–233. doi: 10.1016/S1364-6613(00)01482-0
Herrmann, M. J., Aranda, D., Ellgring, H., Mueller, T. J., Strik, W. K., Heidrich, A., et al. (2002). Face-specific event-related potential in humans is independent from facial expression. Int. J. Psychophysiol. 45, 241–244. doi: 10.1016/s0167-8760(02)00033-8
Hung, A.-Y., Ahveninen, J., and Cheng, Y. (2013). Atypical mismatch negativity to distressful voices associated with conduct disorder symptoms. J. Child Psychol. Psychiatry 54, 1016–1027. doi: 10.1111/jcpp.12076
Johnson, M. H., Griffin, R., Csibra, G., Halit, H., Farroni, T., De Haan, M., et al. (2005). The emergence of the social brain network: evidence from typical and atypical development. Dev. Psychopathol. 17, 599–619. doi: 10.1017/S0954579405050297
Johnson, M. H., Grossmann, T., and Kadosh, K. C. (2009). Mapping functional brain evelopment: building a social brain through interactive specialization. Dev. Psychol. 45, 151–159. doi: 10.1037/a0014548
Jones, A. P., Laurens, K. R., Herba, C. M., Barker, G. J., and Viding, E. (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am. J. Psychiatry 166, 95–102. doi: 10.1176/appi.ajp.2008.07071050
Jones, C., Pickles, A., Falcaro, M., Marsden, A. J. S., Happé, F., Scott, S. K., et al. (2011). A multimodal approach to emotion recognition ability in autism spectrum disorders. J. Child Psychol. Psychiatry 52, 275–285. doi: 10.1111/j.1469-7610.2010.02328.x
Kestenbaum, R., and Nelson, C. A. (1992). Neural and behavioral correlates of emotion recognition in children and adults. J. Exp. Child Psychol. 54, 1–18. doi: 10.1016/0022-0965(92)90014-w
Köchel, A., Leutgeb, V., and Schienle, A. (2014). Disrupted response inhibition toward facial anger cues in children with attention-deficit hyperactivity disorder (ADHD): an event-related potential study. J. Child Neurol. 29, 459–468. doi: 10.1177/0883073813476139
Köchel, A., Schöngaßner, F., Feierl-Gsodam, S., and Schienle, A. (2015). Processing of affective prosody in boys suffering from attention deficit hyperactivity disorder: a near-infrared spectroscopy study. Soc. Neurosci. 10, 583–591. doi: 10.1080/17470919.2015.1017111
Korpilahti, P., Jansson-Verkasalo, E., Mattila, M.-L., Kuusikko, S., Suominen, K., Rytky, S., et al. (2007). Processing of affective speech prosody is impaired in Asperger syndrome. J. Autism Dev. Disord. 37, 1539–1549. doi: 10.1007/s10803-006-0271-2
Kret, M. E., and Ploeger, A. (2015). Emotion processing deficits: a liability spectrum providing insight into comorbidity of mental disorders. Neurosci. Biobehav. Rev. 52, 153–171. doi: 10.1016/j.neubiorev.2015.02.011
Kujawa, A., Hajcak, G., Torpey, D., Kim, J., and Klein, D. N. (2012). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J. Child Psychol. Psychiatry 53, 207–215. doi: 10.1111/j.1469-7610.2011.02461.x
Kujawa, A., MacNamara, A., Fitzgerald, K., Monk, C., and Phan, K. L. (2015). Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. J. Abnorm. Child Psychol. 43, 1493–1501. doi: 10.1007/s10802-015-0029-4
Lerner, M. D., McPartland, J. C., and Morris, J. P. (2013). Multimodal emotion processing in autism spectrum disorders: an event-related potential study. Dev. Cogn. Neurosci. 3, 11–21. doi: 10.1016/j.dcn.2012.08.005
Marsh, A. A., Finger, E. C., Mitchell, D. G., Reid, M. E., Sims, C., Kosson, D. S., et al. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry 165, 712–720. doi: 10.1176/appi.ajp.2007.07071145
McPartland, J., Dawson, G., Webb, S. J., Panagiotides, H., and Carver, L. J. (2004). Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J. Child Psychol. Psychiatry 45, 1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x
Nelson, C. A., and Luciana, M. (eds) (2001). Handbook of Developmental Cognitive Neuroscience. Cambridge: The MIT Press.
Oades, R. D., Dittmann-Balcar, A., Schepker, R., Eggers, C., and Zerbin, D. (1996). Auditory event-related potentials (ERPs) and mismatch negativity (MMN) in healthy children and those with attention-deficit or Tourette/tic symptoms. Biol. Psychol. 43, 163–185. doi: 10.1016/0301-0511(96)05189-7
Paul, R., Augustyn, A., Klin, A., and Volkmar, F. (2005). Perception and production of prosody by speakers with autism spectrum disorders. J. Autism Dev. Disord. 35, 205–220. doi: 10.1007/s10803-004-1999-1
Paulmann, S., and Kotz, S. A. (2008). An ERP investigation on the temporal dynamics of emotional prosody and emotional semantics in pseudo- and lexical-sentence context. Brain Lang. 105, 59–69. doi: 10.1016/j.bandl.2007.11.005
Pelc, K., Kornreich, C., Foisy, M. L., and Dan, B. (2006). Recognition of emotional facial expressions in attention-deficit hyperactivity disorder. Pediatr. Neurol. 35, 93–97. doi: 10.1016/j.pediatrneurol.2006.01.014
Pine, D. S. (2007). Research review: a neuroscience framework for pediatric anxiety disorders. J. Child Psychol. Psychiatry 48, 631–648. doi: 10.1111/j.1469-7610.2007.01751.x
Polanczyk, G. V., Salum, G. A., Sugaya, L. S., Caye, A., and Rohde, L. A. (2015). Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry 56, 345–365. doi: 10.1111/jcpp.12381
Proudfit, G. H., Bress, J. N., Foti, D., Kujawa, A., and Klein, D. N. (2015). Depression and event-related potentials: emotional disengagement and reward insensitivity. Curr. Opin. Psychol. 4, 110–113. doi: 10.1016/j.copsyc.2014.12.018
Rapport, L. J., Friedman, S. R., Tzelepis, A., and Van Voorhis, A. (2002). Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology 16, 102–110. doi: 10.1037/0894-4105.16.3.369
Schirmer, A., and Kotz, S. A. (2006). Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends Cogn. Sci. 10, 24–30. doi: 10.1016/j.tics.2005.11.009
Schupp, H. T., Cuthbert, B. N., Bradley, M. M., Cacioppo, J. T., Ito, T., and Lang, P. J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37, 257–261. doi: 10.1111/1469-8986.3720257
Shaw, P., Stringaris, A., Nigg, J., and Leibenluft, E. (2014). Emotional dysregulation and Attention-Deficit/Hyperactivity disorder. Am. J. Psychiatry 171, 276–293. doi: 10.1176/appi.ajp.2013.13070966
Solomon, B., DeCicco, J. M., and Dennis, T. A. (2012). Emotional picture processing in children: an ERP study. Dev. Cogn. Neurosci. 2, 110–119. doi: 10.1016/j.dcn.2011.04.002
Sonuga-Barke, E. J. S., and Fairchild, G. (2012). Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol. Psychiatry 72, 126–133. doi: 10.1016/j.biopsych.2012.04.004
Stavropoulos, K. K. M., and Carver, L. J. (2014). Reward anticipation and processing of social versus nonsocial stimuli in children with and without autism spectrum disorders. J. Child Psychol. Psychiatry 55, 1398–1408. doi: 10.1111/jcpp.12270
Taylor, M. J., McCarthy, G., Saliba, E., and Degiovanni, E. (1999). ERP evidence of developmental changes in processing of faces. Clin. Neurophysiol. 110, 910–915. doi: 10.1016/S1388-2457(99)00006-1
Tye, C., Battaglia, M., Bertoletti, E., Ashwood, K. L., Azadi, B., Asherson, P., et al. (2014). Altered neurophysiological responses to emotional faces discriminate children with ASD, ADHD and ASD + ADHD. Biol. Psychol. 103, 125–134. doi: 10.1016/j.biopsycho.2014.08.013
Uekermann, J., Kraemer, M., Abdel-Hamid, M., Schimmelmann, B. G., Hebebrand, J., Daum, I., et al. (2010). Social cognition in attention-deficit hyperactivity disorder (ADHD). Neurosci. Biobehav. Rev. 34, 734–743. doi: 10.1016/j.neubiorev.2009.10.009
Uljarevic, M., and Hamilton, A. (2013). Recognition of emotions in autism: a formal meta-analysis. J. Autism Dev. Disord. 43, 1517–1526. doi: 10.1007/s10803-012-1695-5
Vlamings, P. H. J. M., Jonkman, L. M., and Kemner, C. (2010). An eye for detail: an event-related potential study of the rapid processing of fearful facial expressions in children. Child Dev. 81, 1304–1319. doi: 10.1111/j.1467-8624.2010.01470.x
Wagner, J., Hirsch, S., Vogel-Farley, V., Redcay, E., and Nelson, C. (2013). Eye-tracking, autonomic, and electrophysiological correlates of emotional face processing in adolescents with autism spectrum disorder. J. Autism Dev. Disord. 43, 188–199. doi: 10.1007/s10803-012-1565-1
Wang, A. T., Dapretto, M., Hariri, A. R., Sigman, M., and Bookheimer, S. Y. (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 43, 481–490. doi: 10.1097/00004583-200404000-00015
Webb, S., Dawson, G., Bernier, R., and Panagiotides, H. (2006). ERP evidence of atypical face processing in young children with autism. J. Autism Dev. Disord. 36, 881–890. doi: 10.1007/s10803-006-0126-x
Williams, L. M., Hermens, D. F., Palmer, D., Kohn, M., Clarke, S., Keage, H., et al. (2008). Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: evidence for a neural marker and stimulant effects. Biol. Psychiatry 63, 917–926. doi: 10.1016/j.biopsych.2007.11.022
Keywords: ERPs, emotion, children, adolescents, psychopathology
Citation: Chronaki G (2016) Event-Related Potentials and Emotion Processing in Child Psychopathology. Front. Psychol. 7:564. doi: 10.3389/fpsyg.2016.00564
Received: 07 December 2015; Accepted: 05 April 2016;
Published: 29 April 2016.
Edited by:
Salvatore Campanella, Université Libre de Bruxelles, BelgiumReviewed by:
Patrizia Silvia Bisiacchi, University of Padova, ItalyHarold Mouras, University of Picardie Jules Verne, France
Copyright © 2016 Chronaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgia Chronaki, GChronaki@uclan.ac.uk https://georgiachronaki.wordpress.com/clarrity-3/
 Georgia Chronaki
Georgia Chronaki