- 1Department of Psychology, Emory University, Atlanta, GA, USA
- 2Department of Psychology, University of Wisconsin–Madison, Madison, WI, USA
- 3Department of Psychiatry, University of Wisconsin–Madison, Madison, WI, USA
Impairments in social motivational processes may partially explain the differences in social interaction seen among individuals with autism spectrum disorder (ASD). The social motivation hypothesis would predict an association between reduced hedonic capacity and ASD. However, to date, findings have been mixed regarding hedonic deficits among individuals with ASD; adults report lower levels of both social and physical pleasure whereas adolescents only report experiencing lower social pleasure. Moreover, very few studies examining the association between anhedonia and autistic traits have used measures of hedonic response or taken temporal aspects of pleasure into account. The present study examined associations between autistic traits and the experience of pleasure using a non-clinical sample of young adults to further clarify the nature of hedonic deficits in the broader autism phenotype (BAP). Results revealed that autistic traits were negatively associated with both the experience of social pleasure as well as general pleasure, although the association was stronger for social pleasure. Regression analyses revealed that reduced social pleasure was a better predictor of autistic traits than general pleasure. Together these findings suggest that reduced social hedonic capacity is associated with autistic traits in the general population and should be included in conceptualizations of the BAP.
Introduction
Autism spectrum disorders (ASD) are phenomenologically and etiologically heterogeneous neurodevelopmental disorders characterized by social, communicative, and cognitive impairments as well as repetitive and restrictive behaviors or interests (American Psychiatric Association, 2013). Research suggests that from early on, individuals with ASD differ from typically developing individuals. For example, rather than orienting to biological motion, toddlers with ASD prefer non-social contingencies (Klin et al., 2009). In an auditory attention task, preschool children with ASD preferred non-speech signals to motherese speech (Kuhl et al., 2005). Moreover, both children and adults with ASD show increased preference for objects instead of people in social scenes (Klin et al., 2002). These early social deficits often persist throughout development, resulting in impairments in more complex social cognitive processes such as Theory of Mind (ToM; Baron-Cohen et al., 1985; Williams and Happé, 2010), social perception (Klin et al., 2002), and emotion recognition (Balconi and Carrera, 2007; Kennedy and Adolphs, 2012). Numerous theories have been proposed to explain these features of the autism spectrum. More recently, the social motivation theory has gained empirical support as a partial explanation for these differences (Chevallier et al., 2012b).
The social motivation theory of autism posits that deficits in motivational processes constitute a primary impairment in ASD (Chevallier et al., 2012b). Early-onset deficits in social attention contribute to developmental processes that disrupt social learning, cognition, and skill development. These deficits in social attention are thought to reflect reduced social interest and motivation. Thus, the theory asserts that because individuals with ASD do not find social interactions rewarding, they are not interested in pursuing these interactions. Reduced social attention and low interest/motivation over time impairs social cognitive abilities due to inadequate frequency of learning opportunities. Within this framework, research has demonstrated that social orienting, social seeking and liking, as well as social maintaining are all impaired in individuals with ASD (see Chevallier et al., 2012b, for review).
In addition to the behavioral studies that have demonstrated findings consistent with the social motivation theory, some research findings provide evidence that the autism spectrum is characterized by a different response toward socially salient rewards compared to non-social rewards. Using monetary and social incentive delay tasks, individuals with ASD showed decreased activation of the dorsal striatum compared to controls in response to social rewards, but not to monetary rewards, suggesting discrepant responses contingent on the type of reward (Delmonte et al., 2012). When participating in two versions of a rewarded implicit learning task, children with ASD displayed diminished neural responses to both social and monetary rewards, with a more pronounced reduction in response to social rewards, particularly in frontostriatal regions compared to typically developing (TD) children (Scott-Van Zeeland et al., 2010). Moreover, adults with ASD showed atypical amygdala and insular cortex activation while processing social rewards (Dichter et al., 2012). Conversely, other neuroimaging studies found evidence of a general reward dysfunction rather than a deficit specific to social reward (Kohls et al., 2013). However, these inconsistent findings may be due to a lack of ecologically valid social reward paradigms. In a novel behavioral paradigm, namely, the choose-a-movie task (Dubey et al., 2015), investigators provided some intriguing findings. First, they observed that among TD adults, there is a reliable preference for social (smiling faces directly gazing at one) stimuli rather than non-social stimuli (either averted gaze or object). A second study by the same group revealed that adults with ASD showed less preference for the social stimuli (i.e., direct gaze faces) than the TD adults; these findings are consistent with the social motivation hypothesis of autism. In contrast, children and adolescents with ASD did not display any differences in the value placed on social stimuli in a behavioral econometric choice task (Watson et al., 2015). However, the children and adolescents with ASD placed more value on non-social images related to restricted interests (e.g., trains and electronics). These mixed findings highlight the need for further research to clarify the nature of reward-related differences associated with the autism spectrum.
Hedonic capacity is a component of the reward processing system that also includes motivation (i.e., wanting or incentive salience) and learning (Berridge and Robinson, 2003). Although hedonic response is a crucial component in our understanding of the possible reward-related processes that underlie social deficits, thus far relatively few studies have examined hedonic response among individuals with ASD.
In their comparison of adolescents with and without ASD, Chevallier et al. (2012a) found that the groups did not differ in terms of their self-reported experience of physical pleasure, though they reported experiencing significantly less pleasure from social situations. These findings suggest that ASD may be associated with a selective hedonic deficit in the social domain (i.e., social anhedonia). In contrast, Berthoz et al. (2013) found that adults with ASD endorsed both greater physical and social anhedonia than controls. Clearly, there is a need for further research to clarify the nature of hedonic deficits in the autism spectrum.
Most research that has examined hedonic deficits in the autism spectrum has done so by examining associations between autistic traits and measures of negative schizotypy such as the Schizotypal Personality Questionnaire (Raine, 1991) and the Oxford-Liverpool Inventory of Feelings and Experiences (Mason et al., 1995). These studies have consistently shown positive associations between autistic traits and anhedonia as a feature of schizotypy in both clinical (Spek and Wouters, 2010) and non-clinical populations (Rawlings and Locarnini, 2008; Claridge and McDonald, 2009; Russell-Smith et al., 2011).
However, little is known about associations between autism spectrum traits and measures of hedonic response that are not particular to schizophrenia. One study that investigated the association between autistic traits and hedonic response was conducted by Foulkes et al. (2015). In their online investigation of adults aged 18–65 (mean age of 35), Foulkes et al. (2014) examined associations between autistic traits and several aspects of social reward using the Social Reward Questionnaire. The SRQ assesses six unique domains of social reward, including sociability, the enjoyment of group interactions. Results revealed a negative association between autistic traits and sociability, which was not accounted for by alexithymia, another construct measured in their study. Overall, few studies have examined the specific nature of social/interpersonal pleasure in relation to autism spectrum traits, particularly using validated measures of hedonic capacity. Furthermore, the measures used in previous studies of autistic traits only assessed social anhedonia and did not differentiate the anticipatory and consummatory components of the experience of pleasure.
In the past few decades, a significant amount of research has focused on examining the autism spectrum below the clinical threshold. With the dimensional nature of autistic traits, the broader autism phenotype (BAP) refers to the presence of autistic characteristics in the general population, including siblings and other relatives of individuals diagnosed with ASD (Piven, 2001; Sucksmith et al., 2011). Investigations into the nature of hedonic deficits within the BAP using non-clinical samples could provide evidence for whether or not reduced hedonic capacity is associated with risk for ASD.
Although autistic traits are thought to be distributed continuously in the general population (Constantino and Todd, 2003; Ruzich et al., 2015), social anhedonia appears to be follow a more skewed distribution in the normal population, so that it is a relatively uncommon trait. Thus, it is beneficial to examine the association between autistic traits and social anhedonia in a non-clinical sample. One of the advantages of studying this association in a non-clinical sample is that one can avoid the possible confounds associated with the disorder, such as impaired communication. Another advantage is that by investigating the association between autistic traits and social anhedonia in a non-clinical sample, any correlation may be less likely to be attributable to atypical social experiences resulting from having a developmental disorder, possibly being placed in different educational settings and/or different social contexts such as group homes. In this way, studying the association between autistic traits and social anhedonia in TD individuals may enable us to gain insights regarding whether lack of enjoyment of social/interpersonal interactions is secondary to lack of exposure to certain types of interactions, reflective of social skills deficits, or more related to an attentional difficulty, broadly defined. Finally, taking a dimensional approach allows us examine whether there is a threshold effect, i.e., identify whether a certain level of functioning is necessary in order for individuals to possibly benefit from certain psychosocial interventions intended to ameliorate social anhedonia.
The aim of the present study was to examine relations between autism spectrum traits and the self-reported experience of pleasure to provide further support for the social motivation theory of autism using validated measures of hedonic capacity, namely the Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS; Gooding and Pflum, 2011, 2014b) and the Temporal Experience of Pleasure Scale (Gard et al., 2006). Moreover, we sought to further clarify the nature of this association by using measures that not only discriminate between social and general pleasure, but also the anticipatory and consummatory aspects of the experience of pleasure. We hypothesized that autism spectrum traits would be negatively associated with both social and general pleasure. We also hypothesized that lower scores on a measure of social pleasure would be a stronger predictor of autism spectrum traits than lower scores of general pleasure. Consistent with previous studies (Gard et al., 2006; Gooding and Pflum, 2014a), we expected females to report experiencing more social as well as general pleasure than males.
Materials and Methods
Participants
This was a non-clinical sample of English speaking undergraduate students at a large Midwestern university. The participants are part of a larger ongoing research endeavor. Two-hundred and sixty-five students enrolled in introductory psychology courses over six semesters were recruited to participate and complete a battery of questionnaires which included the measures in the present study. No information was gathered regarding the psychiatric history of the participants.
Measures
Autism Spectrum Quotient (AQ)
The Autism Spectrum Quotient (AQ; Baron-Cohen et al., 2001) is a self-report measure designed to assess autism spectrum traits in individuals from the general population with average or above IQs. The AQ consists of 50 items, each rated on a 4-point Likert-based scale, ranging from “strongly disagree” to “strongly agree.” The responses are scored dichotomously, whereby an endorsement in the direction of the autistic trait (either mildly or strongly) is scored “+1” and the opposite response is scored as a “0.” The maximum score on the AQ is 50, with higher scores indicating the presence of more autistic traits. Baron-Cohen et al. (2001) suggested that a score of 32 or higher on the AQ indicated the presence of an ASD. Subsequently, classifications based upon AQ scores became more fine-grained. Wheelwright et al. (2010) operationally defined BAP based upon AQ scores, whereby the BAP is 1 to 2 SDs above the mean (AQ between 23 and 28), the medium autism phenotype is used to describe AQ scores that are 2 to 3 Ss above the mean (i.e., AQ between 29 and 35) and individuals classified in the “narrow autism phenotype” have scores falling 3 SDs or above the mean (i.e., AQ scores greater than 35). The total score has been shown to have moderate to high internal consistency with coefficient alphas ranging from 0.67 to 0.82 (Austin, 2005; Hurst et al., 2007). In the present sample, the AQ demonstrated moderate internal consistency (coefficient α = 0.73). The total AQ score as well as the three-factors proposed by Austin (2005) were used to examine associations with hedonic capacity. This includes a social skills factor, a details and patterns factor, and a communication/mind reading factor. In the factor analysis conducted by Austin (2005), some items did not load on a factor and were left out.
Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS)
The ACIPS is a 17-item self-report measure designed to assess one’s capacity to experience pleasure in the interpersonal and social domains (Gooding and Pflum, 2011, 2014b). Items are scored on a 6-point Likert scale, ranging from 1 (very false for me) to 6 (very true for me), with lower scores indicating social anhedonia, or reduction in the capacity to experience interpersonal pleasure. Gooding and Pflum (2014a) showed that the items load onto four factors: (1) general social interactions, (2) close relationships, (3) bonding over shared interests and experiences, and (4) family-related interactions. The psychometric properties of the ACIPS have been described elsewhere. Briefly, the ACIPS has high internal consistency, temporal stability, convergent, and discriminative validity (Gooding and Pflum, 2014a,b; Gooding et al., 2015). In the present sample, the ACIPS demonstrated high internal consistency (coefficient α = 0.90).
Temporal Experience of Pleasure Scale (TEPS)
The TEPS is an 18-item self-report measure used to assess individual experiences of anticipatory and consummatory pleasure (Gard et al., 2006). Items are rated on a scale of 1 (very false for me) to 6 (very true for me), with 10 of them assessing anticipatory and eight assessing consummatory pleasure. Because most of the items on the TEPS assess non-social pleasure, it was included to be a comparison of the ACIPS as a measure of more general pleasure. The TEPS has been shown to have good internal consistency and test–retest reliability (Gard et al., 2006). In the present sample, the TEPS demonstrated good internal consistency (coefficient α = 0.80).
Statistical Analyses
Although the TEPS and ACIPS were administered in one document, the items were separated and the two measures were scored separately. Linear regression was used to examine hedonic response as a predictor of autistic traits. Bivariate correlations were used to examine associations between scales. An alpha of p < 0.001 determined significance after applying a Bonferroni correction to account for multiple correlations. When comparing associations between the two scales with the AQ, Steiger’s r-to-z transformation was used (Steiger, 1980). Independent samples t-tests were used to examine possible sex differences. All p-values are two-tailed. All data preparation and analyses were performed using SPSS version 21.
Procedure
The present study was approved by the University of Wisconsin–Madison Social and Behavioral Sciences Institutional Review Board. Written informed consent was obtained before the study began. All participants were at least 18 years of age. Participants were administered a battery of questionnaires (the “Mass Survey”) during class. The total administration time was 30 min. Participants received extra credit points in exchange for their participation.
Results
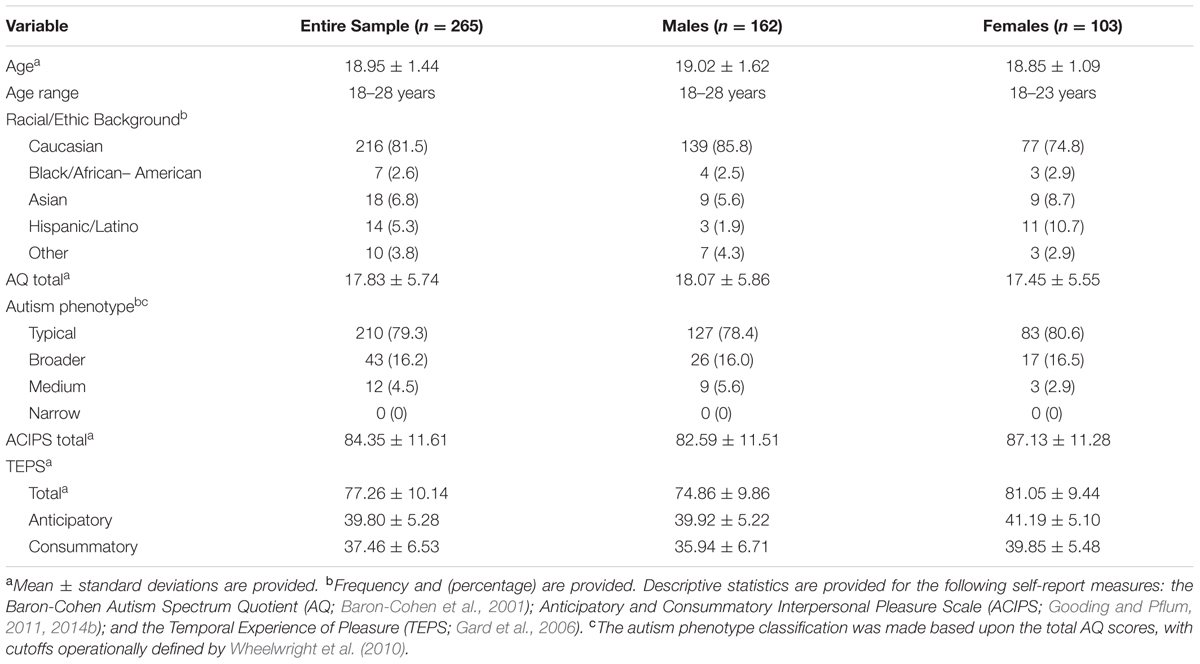
Table 1 provides detailed demographic description of the sample. Briefly, the sample consisted of 265 students (162 males, 103 females) with a mean age of 18.95 (±1.44) years. Consistent with the racial and ethnic makeup of the undergraduate population, 81.5% of the sample identified as Caucasian.
Autism Spectrum Traits and Hedonic Capacity
Table 1 also provides descriptive statistics for each of the self-report measures. Participants were grouped according to the different autism phenotypes, based upon AQ totals, with cutoffs operationally defined by Wheelwright et al. (2010). Although none of the sample met criteria for the narrow autism phenotype, approximately 20% of the participants fell into either the broader or medium autism spectrum phenotype category.
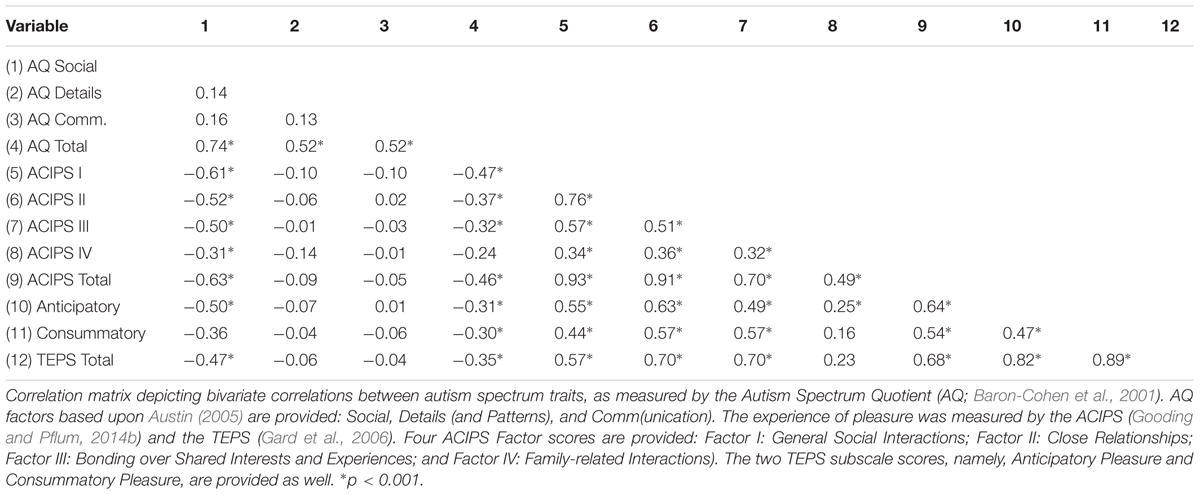
Table 2 provides the Pearson correlation coefficients for associations between autism spectrum traits and various aspects of pleasure. Autism spectrum traits, as measured by the AQ total score, were negatively associated with the experience of social/interpersonal pleasure as measured by the ACIPS total score, r = -0.47 p < 0.001. Autism spectrum traits were also significantly and inversely associated with the four empirically derived factor loadings from the ACIPS, namely: general social interactions (r = -0.47), close relationships (r = -0.37), bonding over shared interests and experiences (r = -0.32), and family-related interactions (r = -0.24), ps < 0.001, respectively. When we examined the AQ factor scores and the ACIPS scores (see Table 2), the only AQ factor scores that were significantly associated with the ACIPS total score was the AQ social factor (r = -0.63, p < 0.001). Autism spectrum traits were also negatively associated with TEPS anticipatory pleasure (r = -0.31, p < 0.001) and TEPS consummatory pleasure (r = -0.30, p < 0.001). Steiger’s r-to-z transformation revealed a significant difference in the strength of the correlations between autism spectrum traits and the two measures of pleasure. The association between autism spectrum traits and social/interpersonal pleasure was significantly greater than the association between those traits and general pleasure, z = -2.49, p < 0.01. There was no difference in the strength of the association between AQ scores and anticipatory versus consummatory pleasure, z = -0.17, p = 0.87.
In order to determine whether correlations between the ACIPS and AQ were due to items assessing social pleasure in the AQ, items that referred to the enjoyment of social interactions were removed from the social factor proposed by Austin (2005). This included items #17 (“I enjoy social chit-chat”), 44 (“I enjoy social occasions”), and 47 (“I enjoy meeting new people”). After removing the three aforementioned items, the association between the ACIPS total score and the AQ social factor remained significant (r = -0.59, p < 0.001), suggesting that these items were not solely driving the association.
We performed linear regression analyses to examine whether social pleasure as measured by the ACIPS was a significant predictor of autism spectrum traits. The results revealed that the ACIPS total score was a significant predictor of autism spectrum traits, β = -0.462, p < 0.001, accounting for 21% of the variance. When the TEPS anticipatory and consummatory scores were added as predictors in the second block, the ACIPS remained a significant predictor, β = -0.420, p < 0.001. However, the TEPS anticipatory and consummatory scores were not significant predictors of autism spectrum traits, β = -0.013, p = 0.86 and β = -0.062, p = 0.35, respectively.
In a subsequent model in which the TEPS scores were entered first, the model was significant, indicating that the TEPS anticipatory and consummatory scores were significant predictors of autism spectrum traits, β = -0.223, p < 0.01 and β = -0.190, p < 0.01, respectively. However, when the ACIPS was added in the second block, the TEPS anticipatory and consummatory scores were no longer significant predictors, β = -0.013, p = 0.86 and β = -0.062, p = 0.35, respectively. However, the ACIPS total score was a significant predictor, β = -0.420, p < 0.001.
Gender Differences in Self-report Measures
The male and female respondents did not differ in terms of their level of endorsement of autism spectrum traits, t(263) = 0.87, p = 0.39. However, we observed a gender difference in terms of total ACIPS scores, with the female participants reporting significantly higher levels of social pleasure, t(263) = 3.15, p < 0.01. Similarly, we found that females reported both more anticipatory pleasure [t(263) = 3.488, p < 0.001] and consummatory pleasure [t(263) = 4.97, p < 0.001], as measured by the TEPS, than males.
Discussion
The results from the present study suggest that non-clinical individuals with a greater number of autism spectrum traits, as measured by the Autism Spectrum Quotient, are likely to report lower levels of social and interpersonal pleasure, as measured by the ACIPS. The higher the presence of autism spectrum traits, the less pleasure participants reported experiencing from social and interpersonal contexts. This relationship held constant even after removing AQ items that referred to the enjoyment of social interactions from the social factor proposed by Austin (2005).
In this investigation, autism spectrum traits were negatively associated with hedonic capacity. However, we noted that the relationship between autism spectrum traits and pleasure for social interactions, as measured by the ACIPS, was significantly stronger than the relationship between autism spectrum traits and general pleasure, as measured by the TEPS. This relationship was confirmed by regression analyses, which indicated that social pleasure was a better predictor of autism spectrum traits than the experience of general pleasure. Indeed, although TEPS scores predicted autistic traits, this effect was not significant when ACIPS scores were introduced into the model.
These results are consistent with two previous studies that examined the nature of hedonic capacity using clinical samples of individuals with ASD, in which the ASD groups reported experiencing lower levels of social pleasure than controls (Chevallier et al., 2012a; Berthoz et al., 2013). Although both social and general pleasure were negatively associated with autistic traits, the stronger association with social pleasure suggests that reduced social hedonic capacity may be particularly characteristic of the autism spectrum. Given that hedonic capacity and motivation are related components of reward processing, these findings can be viewed as further support for the social motivation theory of autism, which asserts that deficits in social motivational mechanisms contribute to the social deficits experienced by individuals with ASD (Chevallier et al., 2012b). Our present findings appear wholly consistent with a recent report by Foulkes et al. (2015), in which non-clinical adults displayed negative inverse relationships between autistic traits and reported levels of enjoyment of prosocial interactions (“sociability”) as measured by the Social Reward Questionnaire.
Although prior work has demonstrated that individuals ASD report experiencing lower levels of social pleasure than TD individuals, it was important to examine whether the association between autistic traits and diminished social interest would be evident in a non-clinical sample using a modern, validated measure of social/interpersonal hedonic pleasure. The results of the present study can be viewed as further evidence for social anhedonia as a core feature of the BAP, a term used to refer to the presence of subthreshold autistic features and traits in individuals who do not meet criteria for ASD (Piven, 2001).
While the present findings appear consistent with the social motivation theory, there are equally plausible alternative accounts for the association between reduced hedonic capacity and autism spectrum traits. An example of such an alternative is the view that the social anhedonia observed among ASD individuals is secondary to their ToM deficits. According to Krach et al. (2010), mental processing and interpretation of the mental states of one’s social interaction partners is what gives the social interaction its rewarding quality. Hence, according to this view, it is a ToM impairment that renders an individual less able to find social interactions pleasurable, rather than the individual necessarily having less motivation to engage in social interactions per se. Indeed, there seems to be some support for this view, given increasing numbers of reports of loneliness among college-aged and older adults with ASD (see, for example, Jobe and White, 2007). Viewed either way, these findings have treatment implications. Decreased social pleasure has been associated with higher levels of self-reported loneliness and social interaction anxiety, two constructs related to other forms of psychopathology (Gooding et al., 2015). Thus, individuals who endorse higher levels of autism spectrum traits who also report social anhedonia may benefit from psychotherapy focused on improving their social functioning and/or decreasing any anxiety related to social interactions.
Limitations, Strengths, and Future Directions
All of the participants in the present study were college undergraduates. Use of a non-clinical sample therefore limits the external validity of the study findings to community samples of educated young adults. Indeed, no one in the present sample met criteria, based on the threshold suggested by the developers of the AQ, for the presence of an ASD and very few met criteria for even the medium autism phenotype. We have taken care to speak about autism spectrum traits. Research based upon a clinical sample (i.e., individuals diagnosed with ASD) that asks similar questions as those addressed here would be imperative to further address the nature of the relationship between social hedonic capacity and autism spectrum traits. Thus, we are hopeful that this study will serve as a heuristic for future research in this regard.
Another limitation of the present study is our failure to include a measure of alexithymia. Prince and Berenbaum (1993) observed that alexithymia is significantly associated with social anhedonia. In prior research from our lab (Gooding and Tallent, 2003), we observed that 23% (10 of 43) of our socially anhedonic participants were identified as alexithymic on the basis of their elevated scores on the Toronto Alexithymia Scale (TAS-20; Bagby et al., 1994). Prior research indicates that there is a strong and inverse relationship between the Chapman revised Social Anhedonia Scale and the ACIPS (Gooding and Pflum, 2014a,b; Gooding et al., 2015). Moreover, studies of individuals with and without ASD have indicated that alexithymia predicted reduced empathic brain activation (Bird et al., 2010), as well as poor recognition of emotional facial expressions (Cook et al., 2013). Taken together, these facts might lead one to question whether the negative association between autistic traits and hedonic capacity might be accounted for by the elevated levels of alexithymia that may co-occur in individuals with a greater number of autistic traits. While this is a plausible hypothesis, we think it is a less tenable one, given the results of the Foulkes et al. (2015) investigation. In the Foulkes et al. (2015) study, although there was an association between autistic traits, social reward, and alexithymia, alexithymia did not contribute unique variance to the prediction of sociability, enjoyment, or admiration, or prosocial interactions. Prior research has demonstrated positive and significant associations between ACIPS total scores and SRQ Admiration, Prosocial Interaction, and Sociability scores (Gooding et al., 2015). Thus, one cannot conclude that alexithymia can account for the association between autistic traits and hedonic capacity. Further research using measures of hedonic capacity such as the ACIPS is needed in order to more robustly test the relationships between the constructs of alexithymia, capacity for social/interpersonal pleasure, and autistic traits.
While previous studies examining relations between autistic traits and anhedonia yielded consistent results (Rawlings and Locarnini, 2008; Claridge and McDonald, 2009; Berthoz et al., 2013), further exploration was warranted given that most of the earlier studies used measures of schizotypy to assess anhedonia rather than measures of hedonic response. One strength of the present study is that we included two distinct, well-validated measures of pleasure. In this way, we were able to distinguish hedonic capacity for social and interpersonal interactions from hedonic capacity for general stimuli and situations. Moreover, we were also able to distinguish the temporal aspects of hedonic response (i.e., anticipatory versus consummatory pleasure) in the relation with autistic traits. Future research would be enhanced by the inclusion of behavioral paradigms for assessing social motivation, such as the recently developed choose-a-movie paradigm (Dubey et al., 2015). This would be useful in terms of an increased focus on identifying the mechanisms underlying social anhedonia in ASD individuals. Ideally, a multi-method investigation would allow researchers to study pleasure and social interactions while distinguishing social skills from hedonic capacity. It would also be interesting to examine how amenable self-reported social anhedonia is in individuals with various levels of autism spectrum traits.
Conclusion
The present study indicates that individuals with greater number of autism spectrum traits are more likely to report lower levels of social/interpersonal pleasure. These findings are consistent with growing evidence for social anhedonia being part of a BAP. It is critical that future work in this area include clinical samples in order to permit further exploration of the association between autism spectrum traits and social anhedonia. It is also imperative that future studies investigating the association between autism spectrum traits and social anhedonia use measures that specifically assess hedonic capacity for social and interpersonal stimuli and situations, as well as measures that are developmentally appropriate for the age of the sample.
Author Contributions
DCG conceived of the project, designed the project, and revised the manuscript for important intellectual content. She is the primary developer of the ACIPS. DMN collected some of the data, analyzed the data, and prepared the first draft of the manuscript. MJP trained DMN, oversaw all of the data collection, and helped to critically revise the manuscript for important intellectual content. MJP is a co-developer of the ACIPS.
Funding
This research was supported in part by a Leon Epstein Faculty Fellowship to DCG.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors express their gratitude to the individuals who participated in this study. Part of these data were presented at the May 2015 annual meeting of the Association for Psychological Science, New York, NY, USA. We also grateful acknowledge the previous comments of the two reviewers whose critique helped to refine our work.
References
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorder, 5th Edn. Arlington, VA: American Psychiatric Publishing.
Austin, E. J. (2005). Personality correlates of the broader autism phenotype as assessed by the autism spectrum quotient (AQ). Pers. Individ. Dif. 38, 451–460. doi: 10.1016/j.paid.2004.04.022
Bagby, R. M., Parker, J. D., and Taylor, G. J. (1994). The twenty-item toronto alexithymia scale: I.Item selection and cross-validation of the factor structure. J. Psychosom. Res. 38, 23–32. doi: 10.1016/0022-3999(94)90005-1
Balconi, M., and Carrera, A. (2007). Emotional representation in facial expression and script: a comparison beween normal and autistic children. Res. Dev. Disabil. 28, 409–422. doi: 10.1016/j.ridd.2006.05.001
Baron-Cohen, S., Leslie, A. M., and Frith, U. (1985). Does the autistic child have a “theory of mind”? Cognition 21, 37–46. doi: 10.1016/0010-0277(85)90022-8
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., and Clubley, E. (2001). The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism. Dev. Disord. 31, 5–17. doi: 10.1023/A:1005653411471
Berridge, K. C., and Robinson, T. E. (2003). Parsing reward. Trends Neurosci. 26, 507–513. doi: 10.1016/S0166-2236(03)00233-9
Berthoz, S., Lalanne, C., Crane, L., and Hill, E. L. (2013). Investigating emotional impairments in in adults with autism spectrum disorders and the broader autism phenotype. Psychiatry Res. 208, 257–264. doi: 10.1016/j.psychres.2013.05.014
Bird, G., Silani, G., Brindley, R., White, S., Frith, U., and Singer, T. (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133, 1515–1525. doi: 10.1093/brain/awq060
Chevallier, C., Grèzes, J., Molesworth, C., Berthoz, S., and Happé, F. (2012a). Brief report: selective social anhedonia in high functioning autism. J. Autism. Dev. Disord. 42, 1504–1509. doi: 10.1007/s10803-011-1364-0
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., and Schultz, R. T. (2012b). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. doi: 10.1016/j.tics.2012.02.007
Claridge, G., and McDonald, A. (2009). An investigation into the relationships between convergent and divergent thinking, schizotypy, and autistic traits. Pers. Individ. Dif. 46, 794–799. doi: 10.1016/j.paid.2009.01.018
Constantino, J. N., and Todd, R. D. (2003). Autistic traits in the general population. Arch. Gen. Psychiatry 60, 524–530. doi: 10.1001/archpsyc.60.5.524
Cook, R., Brewer, R., Shah, P., and Bird, G. (2013). Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychol. Sci. 24, 723–732. doi: 10.1177/0956797612463582
Delmonte, S., Balsters, J. H., McGrath, J., Fitzgerald, J., Brennan, S., Fagan, A. J., et al. (2012). Social and monetary reward processing in autism spectrum disorders. Mol. Autism 3, 7. doi: 10.1186/2040-2392-3-7
Dichter, G. S., Richey, J. A., Rittenberg, A. M., Sabatino, A., and Bodfish, J. W. (2012). Reward circuitry function in autism during face anticipation and outcomes. J. Autism Dev. Disord. 42, 147–160. doi: 10.1007/s10803-011-1221-1
Dubey, I., Ropar, D., de, C., and Hamilton, A. F. (2015). Measuring the value of social engagement in adults with and without autism. Mol. Autism 6, 35. doi: 10.1186/213229-015-0031-2
Foulkes, L., Bird, G., Gøkcen, E., McCrory, E., and Viding, E. (2015). Common and distinct impacts of autistic traits and alexithymia on social reward. PLoS ONE 10:e0121018. doi: 10.1371/journal.pone.0211018
Foulkes, L., Viding, E., Mccrory, E., and Neumann, C. S. (2014). Social reward questionnaire (SRQ): development and validation. Front. Psychol. 5:201. doi: 10.3389/fpsyg.2014.00201
Gard, D. E., Germans Gard, M., Kring, A. M., and John, O. P. (2006). Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 40, 1086–1102. doi: 10.1016/j.jrp.2005.11.001
Gooding, D. C., and Pflum, M. J. (2011). Anticipatory and Consummatory Interpersonal Pleasure Scale (ACIPS). Madison, WI: University of Wisconsin-Madison.
Gooding, D. C., and Pflum, M. J. (2014a). Further validation of the ACIPS as a measure of social hedonic response. Psychiatry Res. 215, 771–777. doi: 10.1016/j.psychres.2013.11.009
Gooding, D. C., and Pflum, M. J. (2014b). The assessment of interpersonal pleasure: introduction of the anticipatory and consummatory interpersonal pleasure scale (ACIPS) and preliminary findings. Psychiatry Res. 215, 237–243. doi: 10.1016/j.psychres.2013.10.012
Gooding, D. C., and Tallent, K. A. (2003). Spatial, object, and affective working memory in social anhedonia: an exploratory study. Schizophr. Res. 63, 247–260. doi: 10.1016/S0920-9964(02)00326-2
Gooding, D. C., Winston, T. M., Pflum, M. J., and Burgin, C. J. (2015). Individual differences in hedonic experience: further evidence for the construct validity of the ACIPS. Psychiatry Res. 229, 524–532. doi: 10.1016/j.psychres.2015.05.061
Hurst, R. M., Mitchell, J. T., Kimbrel, N. A., Kwapil, T. K., and Nelson-Gray, R. O. (2007). Examination of the reliability and factor structure of the autism spectrum quotient (AQ) in a non-clinical sample. Pers. Individ. Dif. 43, 1938–1949. doi: 10.1016/j.paid.2007.06.012
Jobe, L. E., and White, S. W. (2007). Loneliness, social relationships, and a broader autism phenotype in college students. Pers. Individ. Dif. 42, 1479–1489. doi: 10.1080/00221325.2013.856838
Kennedy, D. P., and Adolphs, R. (2012). Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia 50, 3313–3319. doi: 10.1016/j.neuropsychologia.2012.09.038
Klin, A., Jones, W., Schultz, R., Volkmar, R., and Cohen, D. (2002). Visual fixation patterns during the viewing of naturalistic and social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry 59, 809–816. doi: 10.1001/archpsyc.59.9.809
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G., and Jones, W. (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motions. Nature 459, 257–261. doi: 10.1038/nature07868
Kohls, G., Schulte-Rüther, M., Nehrkorn, B., Müller, K., Fink, G. R., Kamp-Becker, I., et al. (2013). Reward system dysfunction in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 8, 565–572. doi: 10.1093/scan/nss033
Krach, S., Paulus, F. M., Bodden, M., and Kircher, T. (2010). The rewarding nature of social interactions. Front. Behav. Neurosci. 4:22. doi: 10.3389/fnbeh.2010.00022
Kuhl, P. K., Coffey-Corina, S., Padden, D., and Dawson, G. (2005). Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev. Sci. 8, F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x
Mason, O., Claridge, G., and Jackson, M. (l995). New scales for the assessment of schizotypy. Pers. Individ. Dif. 18, 7–13. doi: 10.1016/0191-8869(94)00132-C
Piven, J. (2001). The broad autism phenotype: a complementary strategy for molecular genetic studies of autism. Am. J. Med. Genet. 105, 34–35. doi: 10.1002/1096-8628(20010108)105:1<34::AID-AJMG1052>3.0.CO;2-D
Prince, J. D., and Berenbaum, H. (1993). Alexithymia and hedonic capacity. J. Res. Pers. 27, 15–22. doi: 10.1006/jrpe.1993.1002
Raine, A. (l991). The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 17, 555–564. doi: 10.1093/schbul/17.4.555
Rawlings, D., and Locarnini, A. (2008). Dimensional schizotypy, autism, and unusual word associations in artists and scientists. J. Res. Pers. 42, 465–471. doi: 10.1016/j.jrp.2007.06.005
Russell-Smith, S. N., Maybery, M. T., and Bayliss, D. M. (2011). Relationships between autistic-like and schizotypal traits: an analysis using the autism spectrum quotient and Oxford-Liverpool Inventory of Feelings and Experiences. Pers. Individ. Dif. 51, 128–132. doi: 10.1016/j.paid.2011.03.027
Ruzich, E., Allison, C., Smith, P., Watson, P., Auyeung, B., Ring, H., et al. (2015). Measuring autistic traits in the general population: a systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol. Autism 6, 1–12. doi: 10.1186/2040-2392-6-2
Scott-Van Zeeland, A. A., Dapretto, M., Ghahremani, D. G., Poldrack, R. A., and Bookheimer, S. Y. (2010). Reward processing in autism. Autism Res. 3, 53–67. doi: 10.1002/aur.122
Spek, A. A. and Wouters, S. G. M. (2010). Autism and schizophrenia in high functioning adults: behavioral differences and overlap. Res. Autism Spectr. Disord. 4, 709–717. doi: 10.1016/j.rasd.2010.01.009
Steiger, J. H. (1980). Tests for comparing elements of a correlation matrix. Psychol. Bull. 87:245. doi: 10.1016/j.neuroimage.2010.08.042
Sucksmith, E., Roth, I., and Hoekstra, R. A. (2011). Autistic traits below the clinical threshold: re-examining the broader autism phenotype in the 21st century. Neuropsychol. Rev. 21, 360–389. doi: 10.1007/s11065-011-9183-9
Watson, K. K., Miller, S., Hannah, E., Kovac, M., Damiano, C. R., Sabatino-DiCrisco, A., et al. (2015). Increased reward value of non-social stimuli in children and adolescents with autism. Front. Psychol. 6:1026. doi: 10.3389/fpsyg.2015.01026
Wheelwright, S., Auyeung, B., Allison, C., and Baron-Cohen, S. (2010). Defining the broader, medium, and narrow autism phenotype among parents using the autism spectrum quotient (AQ). Mol. Autism 1, 1–10. doi: 10.1186/2040-2392-1-10
Keywords: pleasure, social interaction, hedonic capacity, autism, ACIPS, TEPS
Citation: Novacek DM, Gooding DC and Pflum MJ (2016) Hedonic Capacity in the Broader Autism Phenotype: Should Social Anhedonia Be Considered a Characteristic Feature? Front. Psychol. 7:666. doi: 10.3389/fpsyg.2016.00666
Received: 08 December 2015; Accepted: 21 April 2016;
Published: 06 May 2016.
Edited by:
Antoine Bechara, University of Southern California, USAReviewed by:
Hamed Qahri-Saremi, University of Illinois at Springfield, USAAndrea Marotta, Sapienza University of Rome, Italy
Copyright © 2016 Novacek, Gooding and Pflum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diane C. Gooding, dgooding@wisc.edu
 Derek M. Novacek
Derek M. Novacek Diane C. Gooding
Diane C. Gooding Madeline J. Pflum
Madeline J. Pflum