- 1 Department of Psychiatry, University of North Carolina, Chapel Hill, NC, USA
- 2 Department of Genetics, University of North Carolina, Chapel Hill, NC, USA
The etiology of complex psychiatric disorders results from both genetics and the environment. No definitive environmental factor has been implicated, but studies suggest that deficits in maternal care and bonding may be an important contributing factor in the development of anxiety and depression. Perinatal mood disorders such as postpartum depression occur in approximately 10% of pregnant women and can result in detriments in infant care and bonding. The consequences of impaired maternal–infant attachment during critical early brain development may lead to adverse effects on socioemotional and neurocognitive development in infants resulting in long-term behavioral and emotional problems, including increased vulnerability for mental illness. The exact mechanisms by which environmental stressors such as poor maternal care increase the risk for psychiatric disorders are not known and studies in humans have proven challenging. Two inbred mouse strains may prove useful for studying the interaction between maternal care and mood disorders. BALB/c (BALB) mice are considered an anxious strain in comparison to C57BL/6 (B6) mice in behavioral models of anxiety. These strain differences are most often attributed to genetics but may also be due to environment and gene by environment interactions. For example, BALB mice are described as poor mothers and B6 mice as good mothers and mothering behavior in rodents has been reported to affect both anxiety and stress behaviors in offspring. Changes in gene methylation patterns in response to maternal care have also been reported, providing evidence for epigenetic mechanisms. Characterization of these two mouse inbred strains over the course of pregnancy and in the postpartum period for behavioral and neuroendocrine changes may provide useful information by which to inform human studies, leading to advances in our understanding of the etiology of anxiety and depression and the role of genetics and the environment.
Introduction
Maternal psychiatric illness during pregnancy and following childbirth (the perinatal period) is both common and morbid; if untreated, it can result in potentially devastating consequences to the mother and her baby. Both antenatal and postpartum maternal anxiety and depression have been shown to have adverse effects on the offspring (O’Hara and Swain, 1996; Bennett et al., 2004; Flynn et al., 2004; Marmorstein et al., 2004; Gavin et al., 2005; Gaynes et al., 2005; Feldman et al., 2009; Ross and Dennis, 2009; Field, 2010) and increase vulnerability to psychiatric disorders in adulthood (Gluckman et al., 2008). Increased risk for psychiatric disorders in offspring may be due, in part, to genetic predisposition. However, mothering behavior has also been shown to be an important factor. Maternal anxiety and depression may lead to unresponsive or inconsistent care by the mother toward the child leading to insecure attachment (NECCR, 1999; Campbell et al., 2004) which has been linked to increased risk for anxiety and depression in offspring (Wan and Green, 2009; Brumariu and Kerns, 2010a,b). Recent studies in rodents support these findings (Francis et al., 1999b; Champagne et al., 2003; Weaver et al., 2004). Studies indicate that perinatal maternal stress, which often manifests clinically as anxiety and depression, may also mediate these persistent effects on health in offspring (Talge et al., 2007). The biological mechanisms underlying maternal anxiety and depression have been difficult to define. Furthermore, identifying the means by which maternal anxiety and depression impact the health of the offspring has been even more challenging.
Efforts to identify biomarkers that explain the pathophysiology of early adverse life events are complicated by a variety of factors including ethical considerations, lack of experimental control over the subject’s environment and genetic background and inaccessibility of primary tissues required for analysis. Therefore, the use of animal models provides a complementary approach for understanding the processes by which maternal behavior during pregnancy and the postpartum period influences the physiological and psychological health of offspring, resulting in the development of behavioral and emotional disorders. Clearly, this is a highly complex area of study, as there are a multitude of psychosocial, environmental and biological processes involved in parenting behavior. Furthermore, it is likely that a combination of genetic, epigenetic, and environmental processes will most accurately explain the link between insecure mother–infant attachment and the development of psychiatric disorders in offspring.
The overarching goal of this review is to examine the usefulness of animal models to disentangle the role of genetics, epigenetics, and the environment on maternal anxiety and depression with regard to maternal care and its effects on offspring. We examine the similarities between humans and rodent models in this vulnerable period of development and discuss how these similarities present an opportunity to inform human research and result in clinically relevant and translational discoveries. We will briefly review the epidemiology, presentation, and pathogenesis of perinatal mood disorders, clinical syndromes that encompass both anxiety and depression in humans, and describe their putative role on impaired mothering behaviors and the resulting adverse effects in offspring. Finally, we discuss specific inbred mouse strains that may serve as a model for the complex genetic, environmental, and epigenetic mechanisms that mediate maternal mental illness during the perinatal period including the subsequent influence on maternal behavior and infant outcomes.
Perinatal Mood and Anxiety Disorders: Epidemiology, Clinical Presentation, and Pathogenesis
Maternal behavior during pregnancy and the postpartum period is influenced by many factors. However, it is important to consider the contributions of perinatal depression on maternal behavior during this vulnerable time. Perinatal depression is an episode of major depressive disorder (MDD) occurring during pregnancy or within the first 6 months postpartum (Gavin et al., 2005; Gaynes et al., 2005). Perinatal depression is common, has a prevalence of 10–15% in women of childbearing age and is associated with significant morbidity and mortality including increased risk for maternal suicide and infanticide (Lindahl et al., 2005). Perinatal depression and anxiety have been linked to poor childbirth outcomes such as preterm delivery and low birth weight (Rahman et al., 2004; Smith et al., 2011), adverse effects on maternal sensitivity in the postpartum period (NECCR, 1999; Campbell et al., 2004), decreased maternal engagement with the infant (Weinberg and Tronick, 1998; NECCR, 1999; Campbell et al., 2004) and a decrease in healthy child development behaviors (Paulson et al., 2006).
The literature shows that maternal antenatal stress is associated with adverse neurobehavioral outcomes in offspring including both social/emotional and cognitive functioning during childhood and later in life (Talge et al., 2007) and may be a mechanism by which perinatal anxiety and depression results in increased risk for mood disorders in offspring. One working hypothesis suggests that negative maternal emotions during pregnancy can be considered behavioral teratogens; in other words, high levels of maternal stress, anxiety, or depression can trigger a cascade of physiological events in the mother, the placenta, and the fetus that lead to deleterious affects on fetal and postnatal neurobehavioral development (Van den Bergh and Marcoen, 2004). Support for this hypothesis is illustrated by examples in the literature. Infants of mothers reporting higher levels of depression and anxiety during pregnancy show increased levels of negative affect and motor activity when presented with novel toys (Davis et al., 2004) and this infant behavioral profile may continue into later childhood and is manifested by shyness and anxiety disorders (Kagan et al., 1987). Prospective studies document increased rates of ADHD and other anxiety disorders persisting into later childhood and early adulthood in children exposed to maternal antenatal stress (O’Connor et al., 2002a,b; Van den Bergh and Marcoen, 2004; Van den Bergh et al., 2005). In sum, these studies suggest that children who are exposed to maternal antenatal stress and anxiety suffer from a range of adverse outcomes although they can be quite variable. Other maternal/child effects may contribute to these associations including severity of or length of exposure to stress, genetic influences, and other indirect mechanisms.
Although the pathogenesis of perinatal depression is unknown, it is an active area of research. The transition from pregnancy to the postpartum period is characterized by an enormous state of hormonal flux (Mastorakos and Ilias, 2003). In humans, the third trimester of pregnancy is characterized by high estrogen and progesterone levels and a hyperactive hypothalamic–pituitary adrenal (HPA) axis with resulting high plasma cortisol (Nolten et al., 1980) that is stimulated in part by the high levels of estrogen and progesterone (Bloch et al., 2003). At childbirth and during the transition to the postpartum period, levels of estrogen and progesterone fall rapidly and there is blunted HPA axis activity due to suppressed hypothalamic corticotrophin-releasing hormone secretion (Magiakou et al., 1996). Estrogen and progesterone have profound interactions with the HPA axis and may trigger HPA axis abnormalities in susceptible women. Despite normal levels of reproductive hormones, women with postpartum depression (PPD) have an abnormal response to changes in estrogen and progesterone (Bloch et al., 2000, 2005).
Rationale for Use of Animal Models: A Complementary Approach
Currently, reliable biomarkers for human PPD do not exist and efforts to elucidate state biomarkers for MDD have proven difficult and frustrating (Rich-Edwards et al., 2008; Meltzer-Brody et al., 2011). Human studies to identify biomarkers for reproductive mood disorders are complicated by a variety of factors including lack of experimental control over the subject’s environment and genetic background and inaccessibility of brain tissue required for analysis. The use of animal models, and particularly rodents, has been helpful in this regard. Animal models provide a complementary approach for understanding the processes by which maternal behavior during pregnancy and the postpartum period influences the physiological and psychological health of offspring, resulting in the development of behavioral and emotional disorders that may persist into adulthood. Identification of discrete genetic or pathophysiological pathways in animal models can serve to narrow the genomic search space, resulting in a more directed approach to gene identification and the effects of environmental factors in human studies.
Obviously, rodent gestation is substantially different from human pregnancy – the gestation period is shorter and regularly results in a litter of two or more offspring rather than a single infant. In addition, mouse pups are less developed at birth than human infants – the developmental changes that occur from birth to weaning in mice are roughly equivalent to development occurring in the third trimester in humans (Clancy et al., 2001). However, the endocrine changes throughout pregnancy and immediately postpartum are similar with one deviation – withdrawal of progesterone from the maternal circulation in rodents is necessary to induce parturition while in humans, plasma progesterone remains high throughout the latter part of pregnancy and decreases sharply after parturition (Mitchell and Taggart, 2009). Regardless of these differences, however, rodents recapitulate many of the endocrinological and HPA axis changes observed in humans during pregnancy including, for example, increasing estrogen and progesterone throughout gestation and high basal HPA activity in mid to late pregnancy. In addition, many of the same neuropeptides and hormones that promote maternal care and behavior in humans serve the same function in rodents (Brunton and Russell, 2010).
Using Mice to Model Anxiety and Depression
Complex neuropsychiatric syndromes like MDD and PPD are multifactorial, and result from a combination of genetic and non-genetic factors. Their complex nature, along with the obvious ethical and logistical impediments to research in humans, has impacted the ability to identify the specific genetic and environmental components that predispose individuals to develop these disorders. While it will never be possible to mimic all facets of a complex human psychiatric syndrome in a rodent model, specific features can be modeled. Historically, rodent models of psychiatric endophenotypes have focused on models with good predictive validity, that responded to commonly used anxiolytics or antidepressants. However, recent commentary has recommended modeling the underlying pathophysiology and neurobiology of the disease rather than using behavioral models based on specific clinical symptoms or response to current pharmacotherapies (Insel et al., 2010; Nestler and Hyman, 2010; Cuthbert and Insel, 2011).
Functional MRI studies have implicated neural circuitry and brain regions involved in postpartum anxiety and depression and maternal bonding (Silverman et al., 2007; Swain et al., 2007; Swain, 2008). Many of the same brain regions and neurotransmitter systems have been implicated in rodents (Tarantino and Bucan, 2000; O’Mahony et al., 2010a,b; Krishnan and Nestler, 2011). Hormonally mediated behavioral changes in animal models of anxiety and depression also mimic those seen in humans (Yan et al., 2010; Krishnan and Nestler, 2011; O’Mahony et al., 2011) indicating that animal models of anxiety, depression, and maternal behavior may share common etiology with human disorders. Therefore, animal model research has the potential to provide information that can be directly translated to humans and, eventually, result in improvements in both the diagnosis and treatment of these devastating disorders.
C57BL/6 and BALB/c Inbred Strains as Models of Maternal Care, Anxiety, and Depression
Inbred mice have recently emerged as the primary model for studying genetic and genomic aspects of human disease (Cryan et al., 2005). This is due, in large part, to the availability of a vast array of resources for such endeavors in the mouse. The power of the mouse as a genetic model lies in the availability of hundreds of inbred strains, millions of cataloged genetic variants (e.g., single nucleotide polymorphisms, SNPs), inexpensive, and high-throughput ways to assess hundreds of thousands of SNPs, and genomic sequence data available for increasing numbers of inbred strains.
Mouse inbred strains have been used with great success for the past 50 years to investigate genetic influences on behavior. Inbred strains are genetically homogeneous within a strain, but vary widely both genetically and phenotypically across strains. These reference populations represent a stable genetic resource that can be tested, analyzed, and compared across laboratories and across time. Inbred strain studies have provided a vast amount of information on anxiety and depression-related behaviors.
Two strains in particular, C57BL/6 (B6) and BALB/c (BALB), have repeatedly shown dramatically different behavioral profiles in anxiety and are commonly referred to as low (B6) and high (BALB) anxiety strains. The results of studies on anxiety-related behaviors, which generally have been shown to be somewhat labile (Crabbe et al., 1999), are surprisingly consistent in these two strains. BALB mice exhibit reduced locomotor activity and less time in unprotected and brightly lit areas in the open field (Carola et al., 2002; Tang et al., 2002; Francis et al., 2003; Priebe et al., 2005; Depino and Gross, 2007; Post et al., 2011) and light:dark assays (Crawley and Davis, 1982; Beuzen and Belzung, 1995; Griebel et al., 2000; Millstein and Holmes, 2007; O’Mahony et al., 2010b) in comparison to B6 mice with very few exceptions. Results from the elevated plus maze have been less consistent with at least one study showing no strain differences (Griebel et al., 2000) and some showing what appears to be less anxiety in the BALB strain (Trullas and Skolnick, 1993; Rogers et al., 1999; Post et al., 2011). These inconsistencies may be due to substrain differences (Trullas and Skolnick, 1993) or might reflect a strain-specific response to a particular apparatus that confounds interpretation of the results.
Stress Reactivity in B6 and BALB Mice
Stressful life events have been shown to play an important role in the development and manifestation of psychiatric illnesses (Kendler et al., 1999; Charney and Manji, 2004; Anisman and Matheson, 2005). Interestingly, B6 and BALB mice also differ in response to stressful stimuli. BALB mice have consistently shown stressor-provoked hyperactivation of the HPA axis as reflected by stress-induced increases in corticosterone release (Shanks et al., 1994; Priebe et al., 2005; Prakash et al., 2006) and ACTH as well as basal differences in CRH (Anisman et al., 1998a). BALB mice have also been shown to exhibit stress-induced differences in CRH receptor immunoreactivity (Anisman et al., 2007) as well as basal and stress-induced differences in expression of GABAA receptor subunits (Poulter et al., 2010). Interestingly, GABAA receptor subunits have also been implicated in a mouse model of PPD (Maguire and Mody, 2008). O’Mahony et al. (2010b) have observed that BALB mice show blunted stress-induced brain activation (as measured by c-Fos expression) in the prefrontal cortex in comparison to B6 mice, indicating that dysregulation in response to stress may be driving behavioral differences between these two strains.
Exposure to both acute and chronic stress differentially modulates depressive-like behavior in BALB mice as well. BALB mice showed significantly greater anhedonia (as measured by decreased sucrose consumption) following both acute and chronic stress in comparison to B6 mice (Poulter et al., 2010). BALB mice also show longer latencies to escape a shuttle box in which they have previously experienced an inescapable footshock (Shanks and Anisman, 1988) and exhibit decreased responding for reward after exposure to stress (Zacharko and Anisman, 1989).
Mothering Behavior in Animal Models
Behavioral differences among inbred strains of mice are frequently studied with regard to their genetic origins. However, environmental factors also play a significant role in the development of behavior in rodents. As has been demonstrated in humans, deficiencies in maternal care during the postpartum period have been shown to result in anxiety, stress, and depression-related behaviors in adult rodent offspring.
As discussed above, there is an extensive literature in humans demonstrating the link between maternal care and bonding and increased risk for development of mood disorders in offspring. This research extends to rat models where it has been shown that offspring of mothers that exhibit higher levels of arched back nursing (ABN) and licking and grooming (LG) exhibit reduced endocrine responses to stress as measured by adrenocorticotropic hormone and corticosterone and decreased anxiety behaviors (for a review see Francis and Meaney, 1999; Francis et al., 1999b; Champagne et al., 2003). Offspring of low LG mothers also exhibit decreased oxytocin receptor binding that is likely related to decreased levels of estrogen receptor alpha expression in the hypothalamus. Oxytocin is believed to promote mother–infant bonding in humans (Bartels and Zeki, 2004; Galbally et al., 2011). Interestingly, differences in maternal behavior have also been shown to be transmitted across generations (Francis et al., 1999a).
Daily removal of pups from the home cage for short periods of handling (3–15 min), results in decreases in anxiety-related behaviors. This observation seems counterintuitive but can be explained by the observation that dams of handled pups show increased ABN and LG when pups are returned to the cage (Liu et al., 1997). Pups exposed to maternal separation for longer periods (3–6 h daily) show increased endocrine responses to stress and increased behavioral reactivity to novelty (Champagne and Meaney, 2007; Curley et al., 2011). The effects of postnatal handling and maternal separation have been shown to change expression levels of genes involved in HPA reactivity including corticotropin releasing factor, glucocorticoid receptor, and subunits of the GABAA receptor (Francis and Meaney, 1999; Francis et al., 1999b; Curley et al., 2011).
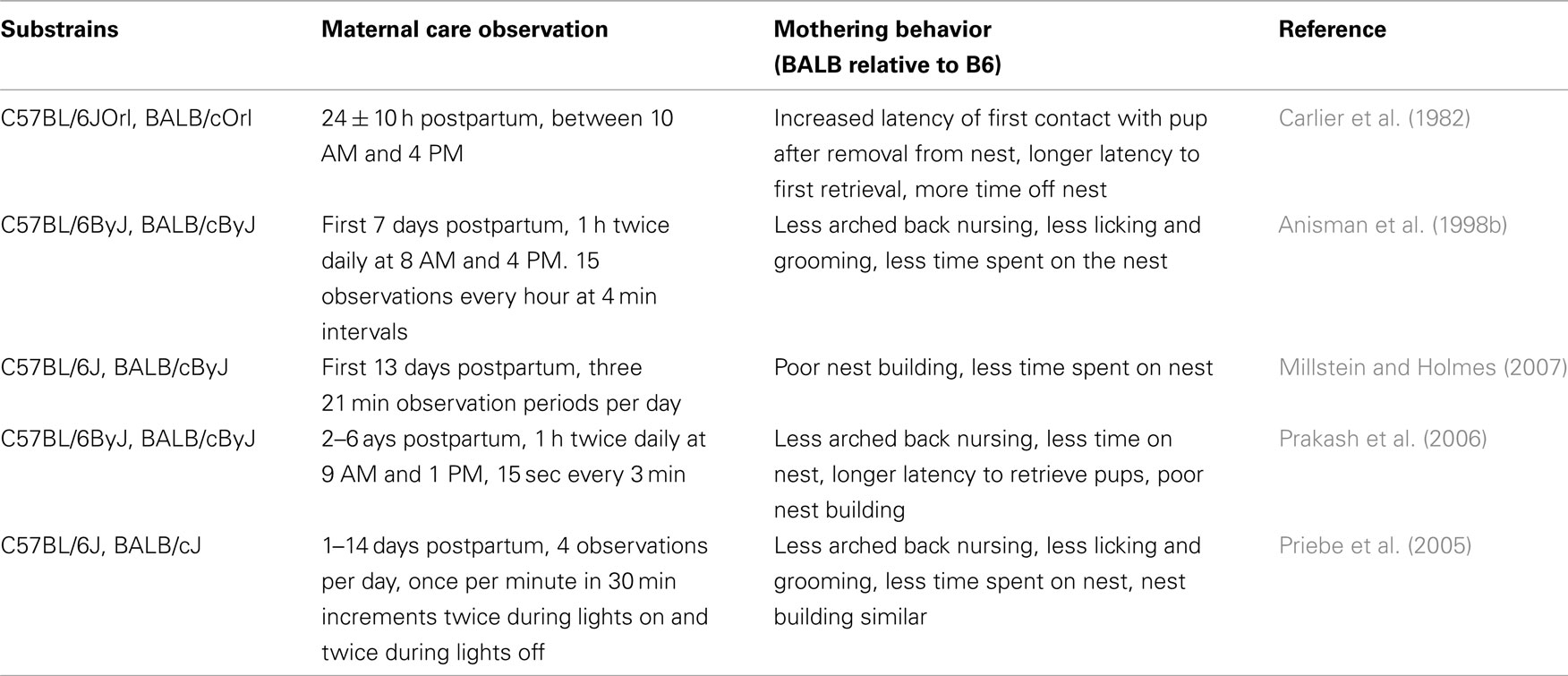
Significant differences in mothering behavior have been observed in B6 and BALB strains of mice (Carlier et al., 1982; Anisman et al., 1998b; Priebe et al., 2005; Prakash et al., 2006) leading to the hypothesis that maternal care differences act separately from, or in conjunction with, genetic background to result in the observed behavioral differences between these strains. BALB mice have consistently been shown to perform less ABN, spend less time on the nest, exhibit less LG, and have longer latencies to retrieve pups that have been removed from the nest (Table 1). Very few studies have examined maternal separation or handling in mouse models. B6 mice have been exposed to both maternal separation and handling with mixed results. Male, but not female B6 mice show increased anxiety behaviors in both the EPM and OF after maternal separation from days 1 to 9 postpartum (Romeo et al., 2003) but showed no change in the defensive withdrawal test – an anxiety assay similar to the emergence test – after handling and under a similar schedule of maternal separation (Parfitt et al., 2004). A study by Millstein and Holmes (2007) examined strain differences in response to both handling and maternal separation and found that neither had an effect on B6 or BALB mice in multiple tests of anxiety and depression. These results indicate that the maternal separation and handling models of anxiety and depression may not be useful in mice, may be strain dependent or may be sensitive to procedural differences. However, too few studies in mice have been conducted to draw a conclusion (for a review see Millstein and Holmes, 2007).
The observation that B6 and BALB mice differ significantly for anxiety, stress, and mothering behaviors has led to research focused on the effects of both genetics and mothering on behavioral differences in these two strains.
Nature or Nurture – It’s Likely both
The use of animal models to disentangle the roles of nature and nurture in the development of anxiety, stress and depression has advantages over human studies – in particular, the ability to control both environmental factors and genetics and perform experimental manipulations during pregnancy and the postpartum period as well as having access to brain tissue for analysis. However, this endeavor is not as straightforward as it may seem. Studies in humans have shown that mothers suffering from PPD demonstrate less attachment to their infants (Fleming and Corter, 1988), which can, in turn, lead to increased risk for psychiatric disorders in the offspring. However, genetic factors also play a role. Mothers contribute half of the genetic material to offspring, therefore, individuals with mothers who suffer from PPD already have an increased genetic risk making it difficult to separate genetic and environmental factors, both of which can act additively or interact to result in increased risk. Adoption studies in humans allow for partitioning of genetic and environmental influences on disease risk but these approaches often suffer from inadequate sample sizes (Merikangas and Low, 2004) along with other drawbacks common to human studies.
In rodents, cross fostering can be used to assess the role of genes and environment on the development of behavior. Cross-fostering involves removal of pups from their biological mother at birth and placing them with a foster mother. This manipulation can be used to study the effects of maternal care on behavior. In cross-fostering studies with rats, low LG offspring fostered to high LG dams exhibit decreased fear and stress behaviors similar to those observed in high LG offspring reared by their biological mothers. Conversely, high LG offspring fostered to low LG dams exhibited increases in fear and stress related behavior (Francis et al., 1999a). These data substantiate the role of early maternal care on subsequent behavior and, importantly, the non-genomic transmission of individual differences.
The cross-fostering approach has also been used with B6 and BALB mice in several studies with mixed results. Several studies have reported an increase in anxiety behaviors and corticosterone release in response to stress in B6 mice fostered by BALB mothers (Francis et al., 2003; Priebe et al., 2005) but other groups observed no change (Anisman et al., 1998b). BALB mice fostered to B6 mothers have been reported to show decreased anxiety in certain behavioral tests like the elevated plus maze (Priebe et al., 2005) and also improved performance in the Morris water maze (Anisman et al., 1998b), but no change in the open field (Priebe et al., 2005). These results indicate that the effects of maternal care are not sufficient to explain the behavioral differences between these strains, and variations in maternal care result in different outcomes depending upon which behavioral test is employed (even tests within the same domain).
It is likely that both genetics and environment interact to produce the behavioral differences observed in these inbred strains. The interaction of genes and the environment to produce phenotypic outcomes has been acknowledged and accepted for quite some time in the scientific community. However, the exact mechanism by which the environment can act on genetic material has only recently begun to be investigated in a more systematic manner.
A Role for Epigenetics in the Link between Maternal Care and Behavioral Outcomes in Animal Models
The observation that the behavioral effects of maternal care were associated with gene expression changes that persisted into adulthood and could be transmitted across generations suggested a potential role for epigenetic DNA modifications. The term “epigenetics” was coined in the 1940s to describe gene by environment interactions, but as the molecular mechanisms of those interactions have been better characterized, the term has evolved to be more specific. The modern definition of epigenetics is the study of DNA modification leading to changes in gene expression caused by a mechanism other than changes to the DNA sequence (Adrian, 2007). Epigenetic modifications can include DNA methylation, histone modification, and non-coding RNA as well as more recently identified mechanisms such as hydroxymethylcytosine residues in the brain (Kriaucionis and Heintz, 2009; Skinner et al., 2010). Of these, DNA methylation has been the most actively studied due to its role in developmental silencing of genes through imprinting or X-inactivation. DNA methylation refers to the process by which a methyl group attaches to DNA via cytosine at specific locations in the genome called CpG sites (Razin, 1998). The bond formed between the DNA cytosine and the methyl group is strong, causing a stable but potentially reversible change in gene expression (Jones and Taylor, 1980). At its most basic functional level, methylation results in the silencing of a gene, but recent evidence indicates that methylation may also be associated with gene activation (Metivier et al., 2008). It was commonly thought that DNA methylation changes that occurred during development were stable and unchangeable later in life. However, recent evidence suggests that DNA methylation is a dynamic process that allows the genome to adapt to alterations in the environment throughout life (Meaney and Szyf, 2005) providing a mechanism by which early life experiences can leave an indelible mark on the brain and influence behavior and health (Weaver et al., 2004).
Weaver et al. (2004) provided the first direct evidence of an epigenetic change in response to mothering behavior with the observation that rats exposed to poor maternal care exhibited increased methylation at a 5′ CpG site in the promoter region of the glucocorticoid receptor gene. The increase in methylation effectively reduced the number of receptors and resulted in heightened response to stress (Weaver et al., 2004). Champagne et al. (2006) also reported increased methylation in response to maternal care in the signal transducer and activator of transcription 5 (Stat5) binding site in the estrogen receptor alpha (Esr) gene promoter region and cross-fostering reversed this effect. Esr is involved in the regulation of oxytocin receptor binding.
Franklin et al. (2010) have extended these studies to C57BL/6 mice and demonstrated that the stress of chronic and unpredictable early life maternal separation in male offspring alters the profile of DNA methylation in the promoter of several candidate genes including the Mecp2 gene, a transcriptional regulator that binds methylated DNA, the cannabinoid receptor 1 (Cb1) that has been associated with emotionality in rodents and corticotropin releasing hormone receptor 2 (Crhr2), a stress hormone receptor (Franklin et al., 2010).
In sum, neurobehavioral epigenetics holds great promise for the future. The ability to conduct whole genome methylation analysis (Pokholok et al., 2005; Jeddeloh et al., 2008; Butcher and Beck, 2010; Li et al., 2010) along with the availability of novel resources in mouse systems genetics (Churchill et al., 2004) provides the starting point for further research into the complex genetic, environmental, and epigenetic mechanisms that mediate maternal mental illness during the perinatal period including the subsequent influence on maternal behavior and infant outcomes.
Future Directions: Expanding Knowledge of Gene by Environment Interactions in B6 and BALB Mice
Based on the behavioral and neuroendocrine data reviewed above, BALB and B6 mice may prove to be an excellent model for the effects of perinatal anxiety and depression on maternal care and bonding and subsequent behavior in offspring. Research in humans indicates that women who suffer from MDD and anxiety are more likely to develop PPD (Kammerer et al., 2006). It is clear that BALB mice display an increased basal level of anxiety. However, no studies have examined changes in anxiety- or depression-related behaviors in BALB mice during pregnancy or the postpartum period. In both humans and rodents, pregnancy is characterized as a period of high basal HPA activity (Brunton and Russell, 2008) – a phenomenon that has also not been characterized specifically in pregnant BALB mice who show significant basal differences in HPA reactivity.
Neuroendocrine changes during pregnancy and immediately postpartum have been shown to be important for the expression of maternal behavior in both human and rodent models. For example, oxytocin, which is essential for lactation, also plays a major role in facilitating maternal behavior and bonding (Neumann, 2008). Interestingly, oxytocin administered centrally or peripherally, has also been shown to have anxiolytic effects (Ring et al., 2006) and attenuate stress-induced activity of the HPA axis (Neumann, 2002). Prolactin, which also plays a role in milk production, has been shown to be involved in maternal behavior as indicated by the severe maternal behavior deficits observed in prolactin receptor knockout mice (Lucas et al., 1998). However, little or no published information exists regarding levels of oxytocin or prolactin during pregnancy and postpartum in either B6 or BALB mice specifically. Unpublished gene expression data from our own laboratory as well as publicly available data from multiple sources show decreased expression of oxytocin in BALB mice in comparison to B6 (Figure 1). Although these results have not yet been confirmed and gene expression differences by no means translate automatically to functional differences, these data offer tantalizing evidence regarding differences in the neuroendocrine systems of these two strains that may contribute to behavioral profiles.
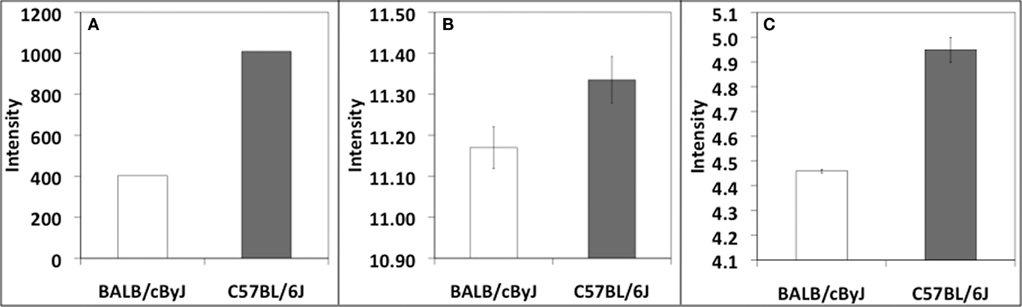
Figure 1. Differential expression of Oxt in (A) hypothalamus, Wiltshire and Tarantino (unpublished), (B) whole brain, PhenoGen Informatics, University of Colorado at Denver Health Sciences Center (Bhave et al., 2007), and (C) hippocampus, Williams et al. (unpublished). Datasets (B,C) are available at http://webqtl.org, accessions GN123, GN273, respectively. The Wiltshire et al. data utilized the Affymetrix 430 v2 array. The Williams et al. data utilized the Affymetrix Mouse Exon 1.0 ST Array. The 3′ UTR probeset from the Exon 1.0 ST Array was utilized for comparison purposes.
Studies in rats have examined the development of mothering behavior in virgin females and suggest that animals that are stressed or anxious are less likely and take longer to display mothering behaviors (Bridges et al., 1972; Pereira et al., 2005; Mann and Gervais, 2011). These results are fairly intuitive based on the observation that oxytocin, which is known to induce mothering behavior, is also anxiolytic. Expanding upon these studies in mice may allow for more direct evidence in BALB for the role of innate anxiety and its effects on mothering behavior.
The environment in which an animal is raised can also have an effect on subsequent behaviors. Francis et al. (2002) have shown that environmental enrichment from weaning until adulthood ameliorates HPA axis reactivity and stress behaviors in rats that have been exposed to maternal separation as pups. Environmental enrichment in BALB mice has been shown to decrease anxiety-related behaviors as well (Chapillon et al., 1999). Moreover, Curley et al. (2009) have also shown that social enrichment in BALB mice enhances maternal care and reduces anxiety behaviors and that these effects may be mediated by increased receptor densities of both oxytocin and vasopressin (V1a). These studies highlight the impact of early environment as a protective influence as well and present the potential for studying mechanisms that “rescue” phenotypes resulting from the detrimental environments early in life.
Conclusion
Postpartum depression is debilitating to women who experience it and potentially damaging to their offspring. Although the etiology of PPD remains unclear, headway is being made toward a better understanding of the complicated interplay of reproductive steroids with the HPA axis and other neuroregulatory systems implicated in depressive illness. Further study of alterations in the HPA axis during the transition from pregnancy to the postpartum period may provide new insights into the pathophysiology of PPD. Moreover, understanding the pathophysiology of PPD can potentially lead to the discovery of biomarkers specific for PPD so that prospective identification of those at risk may become feasible. This would have enormous implications for both the prevention and treatment of women at risk for PPD as well as the transmission of adverse sequelae to offspring exposed to mothers with PPD including adverse effects on neurobehavioral development and increased risk for psychiatric illness.
Although humans and mice evolved with very different sets of reproductive and selective pressures, complete genome sequencing has revealed that mouse and human genomes are highly conserved (Mouse Genome Sequencing Consortium et al., 2002), thereby substantiating the use of mouse models to study genetic aspects of human disease. Animal studies have been used successfully to model perinatal maternal behavior and to study the pathogenesis of perinatal anxiety, stress, and depression. Although animal models do not fully recapitulate human psychiatric syndromes and behaviors, many of the key characteristics observed in human disorders can be observed in mice and many of the same genetic and neuroendocrine factors are involved.
In particular, B6 and BALB mice exhibit divergent phenotypes for stress, anxiety, and mothering behaviors. Moreover, these phenotypic differences have been shown to be at least partially genetically determined, can be modulated by environmental manipulations and result in behavioral changes in adult offspring. The mechanism by which early environmental stress results in lasting changes in behavior and increased risk for psychiatric disorders has not yet been resolved. We believe that more detailed behavioral and neuroendocrine studies using genetic reference populations like B6 and BALB inbred mice will provide insight into the mechanisms by which early environment shapes later behavior and, more generally, into the etiology of anxiety and depression. Furthermore, the rapidly growing field of behavioral epigenetics offers an intriguing area of study that may provide new insights into the nature of gene–environment interactions during development. The dramatic expansion of genomic data and genetic resources in the mouse provides the perfect opportunity to expand on the building evidence for environmentally mediated epigenetic changes and their role, along with genetic predisposition, in increasing risk for psychiatric disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Anisman, H., Lacosta, S., Kent, P., McIntyre, D. C., and Merali, Z. (1998a). Stressor-induced corticotropin-releasing hormone, bombesin, ACTH and corticosterone variations in strains of mice differentially responsive to stressors. Stress 2, 209–220.
Anisman, H., Zaharia, M. D., Meaney, M. J., and Merali, Z. (1998b). Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 16, 149–164.
Anisman, H., and Matheson, K. (2005). Stress, depression, and anhedonia: caveats concerning animal models. Neurosci. Biobehav. Rev. 29, 525–546.
Anisman, H., Prakash, P., Merali, Z., and Poulter, M. O. (2007). Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav. Brain Res. 181, 180–190.
Bartels, A., and Zeki, S. (2004). The neural correlates of maternal and romantic love. Neuroimage 21, 1155–1166.
Bennett, H. A., Einarson, A., Taddio, A., Koren, G., and Einarson, T. R. (2004). Prevalence of depression during pregnancy: systematic review. Obstet. Gynecol. 103, 698–709.
Beuzen, A., and Belzung, C. (1995). Link between emotional memory and anxiety states: a study by principal component analysis. Physiol. Behav. 58, 111–118.
Bhave, S. V., Hornbaker, C., Phang, T. L., Saba, L., Lapadat, R., Kechris, K., Gaydos, J., McGoldrick, D., Dolbey, A., Leach, S., Soriano, B., Ellington, A., Ellington, E., Jones, K., Mangion, J., Belknap, J. K., Williams, R. W., Hunter, L. E., Hoffman, P. L., and Tabakoff, B. (2007). The PhenoGen informatics website: tools for analyses of complex traits. BMC Genet. 8, 59. doi:10.1186/1471-2156- 8-59
Bloch, M., Daly, R. C., and Rubinow, D. R. (2003). Endocrine factors in the etiology of postpartum depression. Compr. Psychiatry 44, 234–246.
Bloch, M., Rubinow, D. R., Schmidt, P. J., Lotsikas, A., Chrousos, G. P., and Cizza, G. (2005). Cortisol response to ovine corticotropin-releasing hormone in a model of pregnancy and parturition in euthymic women with and without a history of postpartum depression. J. Clin. Endocrinol. Metab. 90, 695–699.
Bloch, M., Schmidt, P. J., Danaceau, M., Murphy, J., Nieman, L., and Rubinow, D. R. (2000). Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157, 924–930.
Bridges, R., Zarrow, M. X., Gandelman, R., and Denenberg, V. H. (1972). Differences in maternal responsiveness between lactating and sensitized rats. Dev. Psychobiol. 5, 123–127.
Brumariu, L. E., and Kerns, K. A. (2010a). Mother-child attachment patterns and different types of anxiety symptoms: is there specificity of relations? Child Psychiatry Hum. Dev. 41, 663–674.
Brumariu, L. E., and Kerns, K. A. (2010b). Parent-child attachment and internalizing symptoms in childhood and adolescence: a review of empirical findings and future directions. Dev. Psychopathol. 22, 177–203.
Brunton, P. J., and Russell, J. A. (2008). The expectant brain: adapting for motherhood. Nat. Rev. Neurosci. 9, 11–25.
Brunton, P. J., and Russell, J. A. (2010). Endocrine induced changes in brain function during pregnancy. Brain Res. 1364, 198–215.
Butcher, L. M., and Beck, S. (2010). AutoMeDIP-seq: a high-throughput, whole genome, DNA methylation assay. Methods 52, 223–231.
Campbell, S. B., Brownell, C. A., Hungerford, A., Spieker, S. I., Mohan, R., and Blessing, J. S. (2004). The course of maternal depressive symptoms and maternal sensitivity as predictors of attachment security at 36 months. Dev. Psychopathol. 16, 231–252.
Carlier, M., Roubertoux, P., and Cohen-Salmon, C. (1982). Differences in patterns of pup care in Mus musculus domesticus l-Comparisons between eleven inbred strains. Behav. Neural Biol. 35, 205–210.
Carola, V., D’Olimpio, F., Brunamonti, E., Mangia, F., and Renzi, P. (2002). Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 134, 49–57.
Champagne, F. A., Francis, D. D., Mar, A., and Meaney, M. J. (2003). Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol. Behav. 79, 359–371.
Champagne, F. A., and Meaney, M. J. (2007). Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav. Neurosci. 121, 1353–1363.
Champagne, F. A., Weaver, I. C., Diorio, J., Dymov, S., Szyf, M., and Meaney, M. J. (2006). Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147, 2909–2915.
Chapillon, P., Manneche, C., Belzung, C., and Caston, J. (1999). Rearing environmental enrichment in two inbred strains of mice: 1. Effects on emotional reactivity. Behav. Genet. 29, 41–46.
Charney, D. S., and Manji, H. K. (2004). Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE 2004, re5.
Churchill, G. A., Airey, D. C., Allayee, H., Angel, J. M., Attie, A. D., Beatty, J., Beavis, W. D., Belknap, J. K., Bennett, B., Berrettini, W., Bleich, A., Bogue, M., Broman, K. W., Buck, K. J., Buckler, E., Burmeister, M., Chesler, E. J., Cheverud, J. M., Clapcote, S., Cook, M. N., Cox, R. D., Crabbe, J. C., Crusio, W. E., Darvasi, A., Deschepper, C. F., Doerge, R. W., Farber, C. R., Forejt, J., Gaile, D., Garlow, S. J., Geiger, H., Gershenfeld, H., Gordon, T., Gu, J., Gu, W., de Haan, G., Hayes, N. L., Heller, C., Himmelbauer, H., Hitzemann, R., Hunter, K., Hsu, H. C., Iraqi, F. A., Ivandic, B., Jacob, H. J., Jansen, R. C., Jepsen, K. J., Johnson, D. K., Johnson, T. E., Kempermann, G., Kendziorski, C., Kotb, M., Kooy, R. F., Llamas, B., Lammert, F., Lassalle, J. M., Lowenstein, P. R., Lu, L., Lusis, A., Manly, K. F., Marcucio, R., Matthews, D., Medrano, J. F., Miller, D. R., Mittleman, G., Mock, B. A., Mogil, J. S., Montagutelli, X., Morahan, G., Morris, D. G., Mott, R., Nadeau, J. H., Nagase, H., Nowakowski, R. S., O’Hara, B. F., Osadchuk, A. V., Page, G. P., Paigen, B., Paigen, K., Palmer, A. A., Pan, H. J., Peltonen-Palotie. L, Peirce. J., Pomp, D., Pravenec, M., Prows, D. R., Qi, Z., Reeves, R. H., Roder, J., Rosen, G. D., Schadt, E. E., Schalkwyk, L. C., Seltzer, Z., Shimomura, K., Shou, S., Sillanpää, M. J., Siracusa, L. D., Snoeck, H. W., Spearow, J. L., Svenson, K., Tarantino, L. M., Threadgill, D., Toth, L. A., Valdar, W., de Villena, F. P., Warden, C., Whatley, S., Williams, R. W., Wiltshire, T., Yi, N., Zhang, D., Zhang, M., Zou, F., and Complex Trait Consortium. (2004). The collaborative cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 36, 1133–1137.
Clancy, B., Darlington, R. B., and Finlay, B. L. (2001). Translating developmental time across mammalian species. Neuroscience 105, 7–17.
Crabbe, J. C., Wahlsten, D., and Dudek, B. C. (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284, 1670–1672.
Crawley, J. N., and Davis, L. G. (1982). Baseline exploratory activity predicts anxiolytic responsiveness to diazepam in five mouse strains. Brain Res. Bull. 8, 609–612.
Cryan, J. F., Mombereau, C., and Vassout, A. (2005). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 29, 571–625.
Curley, J. P., Davidson, S., Bateson, P., and Champagne, F. A. (2009). Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front. Behav. Neurosci. 3, 25. doi: 10.3389/neuro.08.025.2009
Curley, J. P., Jensen, C. L., Mashoodh, R., and Champagne, F. A. (2011). Social influences on neurobiology and behavior: epigenetic effects during development. Psychoneuroendocrinology 36, 352–371.
Cuthbert, B., and Insel, T. (2011). The data of diagnosis: new approaches to psychiatric classification. Psychiatry 73, 311–314.
Davis, E. P., Snidman, N., Wadhwa, P. D., Glynn, L. M., Schetter, C. D., and Sandman, C. A. (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy 6, 319–331.
Depino, A. M., and Gross, C. (2007). Simultaneous assessment of autonomic function and anxiety-related behavior in BALB/c and C57BL/6 mice. Behav. Brain Res. 177, 254–260.
Feldman, R., Granat, A., Pariente, C., Kanety, H., Kuint, J., and Gilboa-Schechtman, E. (2009). Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J. Am. Acad. Child Adolesc. Psychiatry 48, 919–927.
Field, T. (2010). Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav. Dev. 33, 1–6.
Fleming, A. S., and Corter, C. (1988). Factors influencing maternal responsiveness in humans: usefulness of an animal model. Psychoneuroendocrinology 13, 189–212.
Flynn, H. A., Davis, M., Marcus, S. M., Cunningham, R., and Blow, F. C. (2004). Rates of maternal depression in pediatric emergency department and relationship to child service utilization. Gen. Hosp. Psychiatry 26, 316–322.
Francis, D., Diorio, J., Liu, D., and Meaney, M. J. (1999a). Non-genomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155–1158.
Francis, D. D., Champagne, F. A., Liu, D., and Meaney, M. J. (1999b). Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann. N. Y. Acad. Sci. 896, 66–84.
Francis, D. D., Diorio, J., Plotsky, P. M., and Meaney, M. J. (2002). Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 22, 7840–7843.
Francis, D. D., and Meaney, M. J. (1999). Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 9, 128–134.
Francis, D. D., Szegda, K., Campbell, G., Martin, W. D., and Insel, T. R. (2003). Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 6, 445–446.
Franklin, T. B., Russig, H., Weiss, I. C., Gräff, J., Linder, N., Michalon, A., Vizi, S., and Mansuy, I. M. (2010). Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68, 408–415.
Galbally, M., Lewis, A. J., Ijzendoorn, M., and Permezel, M. (2011). The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv. Rev. Psychiatry 19, 1–14.
Gavin, N. I., Gaynes, B. N., Lohr, K. N., Meltzer-Brody, S., Gartlehner, G., and Swinson, T. (2005). Perinatal depression: a systematic review of prevalence and incidence. Obstet. Gynecol. 106(5 Pt 1), 1071–1083.
Gaynes, B. N., Gavin, N., Meltzer-Brody, S., Lohr, K. N., Swinson, T., Gartlehner, G., Brody, S., and Miller, W. C. (2005). Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid. Rep. Technol. Assess. (Summ.) 119, 1–8.
Gluckman, P. D., Hanson, M. A., Cooper, C., and Thornburg, K. L. (2008). Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73.
Griebel, G., Belzung, C., Perrault, G., and Sanger, D. J. (2000). Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl.) 148, 164–170.
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., Quinn, K., Sanislow, C., and Wang, P. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167, 748–751.
Jeddeloh, J. A., Greally, J. M., and Rando, O. J. (2008). Reduced-representation methylation mapping. Genome Biol. 9, 231.
Jones, P. A., and Taylor, S. M. (1980). Cellular differentiation, cytidine analogs and DNA methylation. Cell 20, 85–93.
Kagan, J., Reznick, J. S., and Snidman, N. (1987). The physiology and psychology of behavioral inhibition in children. Child Dev. 58, 1459–1473.
Kammerer, M., Taylor, A., and Glover, V. (2006). The HPA axis and perinatal depression: a hypothesis. Arch. Womens Ment. Health 9, 187–196.
Kendler, K. S., Karkowski, L. M., and Prescott, C. A. (1999). Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841.
Kriaucionis, S., and Heintz, N. (2009). The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930.
Krishnan, V., and Nestler, E. J. (2011). Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 7, 121–147.
Li, N., Ye, M., Li, Y., Yan, Z., Butcher, L. M., Sun, J., Han, X., Chen, Q., Zhang, X., and Wang, J. (2010). Whole genome DNA methylation analysis based on high throughput sequencing technology. Methods 52, 203–212.
Lindahl, V., Pearson, J. L., and Colpe, L. (2005). Prevalence of suicidality during pregnancy and the postpartum. Arch. Womens Ment. Health 8, 77–87.
Liu, D., Diorio, J., Tannenbaum, B., Caldji, C., Francis, D., Freedman, A., Sharma, S., Pearson, D., Plotsky, P. M., and Meaney, M. J. (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662.
Lucas, B. K., Ormandy, C. J., Binart, N., Bridges, R. S., and Kelly, P. A. (1998). Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107.
Magiakou, M. A., Mastorakos, G., Rabin, D., Dubbert, B., Gold, P. W., and Chrousos, G. P. (1996). Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: implications for the increase in psychiatric manifestations at this time. J. Clin. Endocrinol. Metab. 81, 1912–1917.
Maguire, J., and Mody, I. (2008). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron 59, 207–213.
Mann, P. E., and Gervais, K. J. (2011). Environmental enrichment delays pup-induced maternal behavior in rats. Dev. Psychobiol. 53, 371–382.
Marmorstein, N. R., Malone, S. M., and Lacono, W. G. (2004). Psychiatric disorders among offspring of depressed mothers: associations with paternal psychopathology. Am. J. Psychiatry 161, 1588–1594.
Mastorakos, G., and Ilias, I. (2003). Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann. N. Y. Acad. Sci. 997, 136–149.
Meaney, M. J., and Szyf, M. (2005). Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 7, 103–123.
Meltzer-Brody, S., Stuebe, A. M., Dole, N., Savitz, D., Rubinow, D., and Thorp, J. (2011). Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). J. Clin. Endocrinol. Metab. 96, E40–E47.
Merikangas, K. R., and Low, N. C. (2004). The epidemiology of mood disorders. Curr Psychiatry Rep 6, 411–421.
Metivier, R., Gallais, R., Tiffoche, C., Le Péron, C., Jurkowska, R. Z., Carmouche, R. P., Ibberson, D., Barath, P., Demay, F., Reid, G., Benes, V., Jeltsch, A., Gannon, F., and Salbert, G. (2008). Cyclical DNA methylation of a transcriptionally active promoter. Nature 452, 45–50.
Millstein, R. A., and Holmes, A. (2007). Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 31, 3–17.
Mitchell, B. F., and Taggart, M. J. (2009). Are animal models relevant to key aspects of human parturition? Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R525–R545.
Mouse Genome Sequencing Consortium, Waterston, R. H., Lindblad-Toh, K., Birney, E., Rogers, J., Abril, J. F., Agarwal, P., Agarwala, R., Ainscough, R., Alexandersson, M., An, P., Antonarakis, S. E., Attwood, J., Baertsch, R., Bailey, J., Barlow, K., Beck, S., Berry, E., Birren, B., Bloom, T., Bork, P., Botcherby, M., Bray, N., Brent, M. R., Brown, D. G., Brown, S. D., Bult, C., Burton, J., Butler, J., Campbell, R. D., Carninci, P., Cawley, S., Chiaromonte, F., Chinwalla, A. T., Church, D. M., Clamp, M., Clee, C., Collins, F. S., Cook, L. L., Copley, R. R., Coulson, A., Couronne, O., Cuff, J., Curwen, V., Cutts, T., Daly, M., David, R., Davies, J., Delehaunty, K. D., Deri, J., Dermitzakis, E. T., Dewey, C., Dickens, N. J., Diekhans, M., Dodge, S., Dubchak, I., Dunn, D. M., Eddy, S. R., Elnitski, L., Emes, R. D., Eswara, P., Eyras, E., Felsenfeld, A., Fewell, G. A., Flicek, P., Foley, K., Frankel, W. N., Fulton, L. A., Fulton, R. S., Furey, T. S., Gage, D., Gibbs, R. A., Glusman, G., Gnerre, S., Goldman, N., Goodstadt, L., Grafham, D., Graves, T. A., Green, E. D., Gregory, S., Guigó, R., Guyer, M., Hardison, R. C., Haussler, D., Hayashizaki, Y., Hillier, L. W., Hinrichs, A., Hlavina, W., Holzer, T., Hsu, F., Hua, A., Hubbard, T., Hunt, A., Jackson, I., Jaffe, D. B., Johnson, L. S., Jones, M., Jones, T. A., Joy, A., Kamal, M., Karlsson, E. K., Karolchik, D., Kasprzyk, A., Kawai, J., Keibler, E., Kells, C., Kent, W. J., Kirby, A., Kolbe, D. L., Korf, I., Kucherlapati, R. S., Kulbokas, E. J., Kulp, D., Landers, T., Leger, J. P., Leonard, S., Letunic, I., Levine, R., Li, J., Li, M., Lloyd, C., Lucas, S., Ma, B., Maglott, D. R., Mardis, E. R., Matthews, L., Mauceli, E., Mayer, J. H., McCarthy, M., McCombie, W. R., McLaren, S., McLay, K., McPherson, J. D., Meldrim, J., Meredith, B., Mesirov, J. P., Miller, W., Miner, T. L., Mongin, E., Montgomery, K. T., Morgan, M., Mott, R., Mullikin, J. C., Muzny, D. M., Nash, W. E., Nelson, J. O., Nhan, M. N., Nicol, R., Ning, Z., Nusbaum, C., O’Connor, M. J., Okazaki, Y., Oliver, K., Overton-Larty, E., Pachter, L., Parra, G., Pepin, K. H., Peterson, J., Pevzner, P., Plumb, R., Pohl, C. S., Poliakov, A., Ponce, T. C., Ponting, C. P., Potter, S., Quail, M., Reymond, A., Roe, B. A., Roskin, K. M., Rubin, E. M., Rust, A. G., Santos, R., Sapojnikov, V., Schultz, B., Schultz, J., Schwartz, M. S., Schwartz, S., Scott, C., Seaman, S., Searle, S., Sharpe, T., Sheridan, A., Shownkeen, R., Sims, S., Singer, J. B., Slater, G., Smit, A., Smith, D. R., Spencer, B., Stabenau, A., Stange-Thomann, N., Sugnet, C., Suyama, M., Tesler, G., Thompson, J., Torrents, D., Trevaskis, E., Tromp, J., Ucla, C., Ureta-Vidal, A., Vinson, J. P., Von Niederhausern, A. C., Wade, C. M., Wall, M., Weber, R. J., Weiss, R. B., Wendl, M. C., West, A. P., Wetterstrand, K., Wheeler, R., Whelan, S., Wierzbowski, J., Willey, D., Williams, S., Wilson, R. K., Winter, E., Worley, K. C., Wyman, D., Yang, S., Yang, S. P., Zdobnov, E. M., Zody, M. C., and Lander, E. S. (2002). Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562.
NECCR, N. (1999). Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. NICHD Early Child Care Research Network. Dev. Psychol. 35, 1297–1310.
Nestler, E. J., and Hyman, S. E. (2010). Animal models of neuropsychiatric disorders. Nat. Neurosci. 13, 1161–1169.
Neumann, I. D. (2002). Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 139, 147–162.
Neumann, I. D. (2008). Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 20, 858–865.
Nolten, W. E., Lindheimer, M. D., Rueckert, P. A., Oparil, S., and Ehrlich, E. N. (1980). Diurnal patterns and regulation of cortisol secretion in pregnancy. J. Clin. Endocrinol. Metab. 51, 466–472.
O’Connor, T. G., Heron, J., Glover, V., and Alspac Study Team. (2002a). Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J. Am. Acad. Child Adolesc. Psychiatry 41, 1470–1477.
O’Connor, T. G., Heron, J., Golding, J., Beveridge, M., and Glover, V. (2002b). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon longitudinal study of parents and children. Br. J. Psychiatry 180, 502–508.
O’Hara, M. W., and Swain, A. M. (1996). Rates and risk of postpartum depression-A meta-analysis. Int. Rev. Psychiatry 8, 37–54.
O’Mahony, C. M., Bravo, J. A., Dinan, T. G., and Cryan, J. F. (2010a). Comparison of hippocampal metabotropic glutamate receptor 7 (mGlu7) mRNA levels in two animal models of depression. Neurosci. Lett. 482, 137–141.
O’Mahony, C. M., Sweeney, F. F., Daly, E., Dinan, T. G., and Cryan, J. F. (2010b). Restraint stress-induced brain activation patterns in two strains of mice differing in their anxiety behaviour. Behav. Brain Res. 213, 148–154.
O’Mahony, C. M., Clarke, G., Gibney, S., Dinan, T. G., and Cryan, J. F. (2011). Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression. Pharmacol. Biochem. Behav. 97, 690–699.
Parfitt, D. B., Levin, J. K., Saltstein, K. P., Klayman, A. S., Greer, L. M., and Helmreich, D. L. (2004). Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res. 1016, 111–118.
Paulson, J. F., Dauber, S., and Leiferman, J. A. (2006). Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics 118, 659–668.
Pereira, M., Uriarte, N., Agrati, D., Zuluaga, M. J., and Ferreira, A. (2005). Motivational aspects of maternal anxiolysis in lactating rats. Psychopharmacology (Berl.) 180, 241–248.
Pokholok, D. K., Harbison, C. T., Levine, S., Cole, M., Hannett, N. M., Lee, T. I., Bell, G. W., Walker, K., Rolfe, P. A., Herbolsheimer, E., Zeitlinger, J., Lewitter, F., Gifford, D. K., and Young, R. A. (2005). Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122, 517–527.
Post, A. M., Weyers, P., Holzer, P., Painsipp, E., Pauli, P., Wultsch, T., Reif, A., and Lesch, K. P. (2011). Gene-environment interaction influences anxiety-like behavior in ethologically based mouse models. Behav. Brain Res. 218, 99–105.
Poulter, M. O., Du, L., Zhurov, V., Merali, Z., and Anisman, H. (2010). Plasticity of the GABA(A) receptor subunit cassette in response to stressors in reactive versus resilient mice. Neuroscience 165, 1039–1051.
Prakash, P., Merali, Z., Kolajova, M., Tannenbaum, B. M., and Anisman, H. (2006). Maternal factors and monoamine changes in stress-resilient and susceptible mice: cross-fostering effects. Brain Res. 1111, 122–133.
Priebe, K., Romeo, R. D., Francis, D. D., Sisti, H. M., Mueller, A., McEwen, B. S., and Brake, W. G. (2005). Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev. Psychobiol. 47, 398–407.
Rahman, A., Iqbal, Z., Bunn, J., Lovel, H., and Harrington, R. (2004). Impact of maternal depression on infant nutritional status and illness: a cohort study. Arch. Gen. Psychiatry 61, 946–952.
Razin, A. (1998). CpG methylation chromatin structure and gene silencing–a three-way connection. EMBO J. 17, 4905–4908.
Rich-Edwards, J. W., Mohllajee, A. P., Kleinman, K., Hacker, M. R., Majzoub, J., Wright, R. J., and Gillman, M. W. (2008). Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J. Clin. Endocrinol. Metab. 93, 1946–1951.
Ring, R. H., Malberg, J. E., Potestio, L., Ping, J., Boikess, S., Luo, B., Schechter, L. E., Rizzo, S., Rahman, Z., and Rosenzweig-Lipson, S. (2006). Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl.) 185, 218–225.
Rogers, D. C., Jones, D. N., Nelson, P. R., Jones, C. M., Quilter, C. A., Robinson, T. L., and Hagan, J. J. (1999). Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav. Brain Res. 105, 207–217.
Romeo, R. D., Mueller, A., Sisti, H. M., Ogawa, S., McEwen, B. S., and Brake, W. G. (2003). Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm. Behav. 43, 561–567.
Ross, L. E., and Dennis, C. L. (2009). The prevalence of postpartum depression amongst women with substance use, an abuse history, or chronic illness: a systematic review. J. Womens Health 18, 475–486.
Shanks, N., and Anisman, H. (1988). Stressor-provoked behavioral changes in six strains of mice. Behav. Neurosci. 102, 894–905.
Shanks, N., Griffiths, J., and Anisman, H. (1994). Central catecholamine alterations induced by stressor exposure: analyses in recombinant inbred strains of mice. Behav. Brain Res. 63, 25–33.
Silverman, M. E., Loudon, H., Safier, M., Protopopescu, X., Leiter, G., Liu, X., and Goldstein, M. (2007). Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr. 12, 853–862.
Skinner, M. K., Manikkam, M., and Guerrero-Bosagna, C. (2010). Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 21, 214–222.
Smith, M. V., Shao, L., Howell, H., Lin, H., and Yonkers, K. A. (2011). Perinatal depression and birth outcomes in a Healthy Start project. Matern Child Health J. 15, 401–409.
Swain, J. E. (2008). Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont) 5, 28–36.
Swain, J. E., Lorberbaum, J. P., Kose, S., and Strathearn, L. (2007). Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J. Child. Psychol. Psychiatry 48, 262–287.
Talge, N. M., Neal, C., Glover, V., and Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health. (2007). Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol. Psychiatry 48, 245–261.
Tang, X., Orchard, S. M., and Sanford, L. D. (2002). Home cage activity and behavioral performance in inbred and hybrid mice. Behav. Brain Res. 136, 555–569.
Tarantino, L. M., and Bucan, M. (2000). Dissection of behavior and psychiatric disorders using the mouse as a model. Hum. Mol. Genet. 9, 953–965.
Trullas, R., and Skolnick, P. (1993). Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl.) 111, 323–331.
Van den Bergh, B. R., and Marcoen, A. (2004). High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 75, 1085–1097.
Van den Bergh, B. R., Mennes, M., Oosterlaan, J., Stevens, V., Stiers, P., Marcoen, A., and Lagae, L. (2005). High antenatal maternal anxiety is related to impulsivity during performance on cognitive tasks in 14- and 15-year-olds. Neurosci. Biobehav. Rev. 29, 259–269.
Wan, M. W., and Green, J. (2009). The impact of maternal psychopathology on child-mother attachment. Arch. Womens Ment. Health 12, 123–134.
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M., and Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854.
Weinberg, M. K., and Tronick, E. Z. (1998). The impact of maternal psychiatric illness on infant development. J. Clin. Psychiatry 59(Suppl. 2), 53–61.
Keywords: perinatal, anxiety, depression, mothering, bonding, genetic, epigenetic, mice
Citation: Tarantino LM, Sullivan PF and Meltzer-Brody S (2011) Using animal models to disentangle the role of genetic, epigenetic, and environmental influences on behavioral outcomes associated with maternal anxiety and depression. Front. Psychiatry 2:44. doi: 10.3389/fpsyt.2011.00044
Received: 15 March 2011;
Accepted: 05 July 2011;
Published online: 18 July 2011.
Edited by:
Sheryl Moy, University of North Carolina, USAReviewed by:
Allan V. Kalueff, Tulane University, USAVaishnav Krishnan, UT Southwestern Medical Center, USA
Copyright: © 2011 Tarantino, Sullivan and Meltzer-Brody. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Lisa M. Tarantino, Department of Psychiatry, University of North Carolina, 1012 Genetic Medicine Building, CB#7361, 120 Mason Farm Road, Chapel Hill, NC 27599, USA. e-mail: lisat@med.unc.edu