- 1 Monash Alfred Psychiatry Research Centre, School of Psychology and Psychiatry, The Alfred and Monash University, Melbourne, VIC, Australia
- 2 Department of Psychiatry, Centre for Addiction and Mental Health, University of Toronto, Toronto, ON, Canada
Transcranial direct current stimulation (tDCS) is a brain stimulation technique that has the potential to improve working memory (WM) deficits in many clinical disorders. The aim of this study was to investigate the role of current strength on the ability of anodal tDCS to improve WM, and secondly to investigate the time course of effects. Twelve healthy participants underwent three stimulation sessions consisting of 20 min of either 1 mA anodal tDCS, 2 mA anodal tDCS, or sham tDCS to the left dorsolateral prefrontal cortex (DLPFC) localized via F3, all whilst completing a WM task. Intra-stimulation and post-stimulation WM performances were measured using the n-back and Sternberg tasks respectively. Results revealed no significant improvements in participants’ accuracy, but a significant interaction was found with respect to current strength and time for accurate reaction time. The finding provides partial support for the hypothesis, in that it appears current strength may affect aspects of WM performance. However, more research is needed, and a higher difficulty level of WM tasks is one of the suggestions discussed for future research.
Introduction
Working memory (WM) is the capacity to temporarily store and manipulate information in mind so as to carry out complex cognitive abilities, and as such WM plays an integral part in a number of key processes, including language comprehension, learning, and reasoning (Mull and Seyal, 2001; Baddeley, 2007). WM impairment is known to be a core feature in numerous neurological and psychiatric disorders such as Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, depression, and schizophrenia (Lawrence et al., 1998; Barch et al., 2003; Bertolino et al., 2003; Logie et al., 2004; Barch and Smith, 2008; Wu et al., 2008; Reppermund et al., 2009). Cognitive impairment is difficult to treat, and traditional approaches such as pharmacotherapy and cognitive rehabilitation have resulted in limited improvements at best (Rund and Borg, 1999; Lee and Park, 2005; Reilly et al., 2006, 2007; Barch and Smith, 2008). Therefore, it is crucial that novel approaches to improving WM be explored. Neuroimaging studies exploring WM processes in the brain have suggested that the prefrontal cortex (Duncan and Owen, 2000; Muller et al., 2002; Passingham and Sakai, 2004; Postle, 2006), and more specifically, the dorsolateral prefrontal cortex (DLPFC) is a critical region involved in WM processes and deficits (Smith and Jonides, 1997, 1999; Callicott et al., 1999; Mull and Seyal, 2001; Passingham and Sakai, 2004; Cohen and Floel, 2007). Therefore, techniques that could directly target and modulate DLPFC activity could have significant potential for improving WM.
Over the last 5 years or so, there has been increasing research looking at the efficacy of non-invasive brain stimulation techniques in improving WM performance (Been et al., 2007; Cohen and Floel, 2007; Hoy and Fitzgerald, 2010). Transcranial direct current stimulation (tDCS) in particular, has shown promising results (Fregni et al., 2005; Boggio et al., 2006; Ohn et al., 2008; Andrews et al., 2011). tDCS is a non-convulsive and non-invasive technique which has been found to be safe with very few side-effects (Poreisz et al., 2007; Nitsche et al., 2008). It has been proposed to modulate the excitability of neurons by shifting the membrane potential of superficial neurons in a de- or hyper-polarizing direction, causing brain cells to be more or less likely to fire respectively (Nitsche and Fregni, 2007). tDCS stimulation has been shown to alter cortical excitability in targeted areas so as to enhance (via anodal stimulation) or inhibit (via cathodal stimulation) brain functioning (Nitsche et al., 2008). While the focality of tDCS is somewhat limited, the direct functional effects of tDCS appear to be restricted to the area directly under the electrodes (Nitsche et al., 2009). This has been shown behaviorally (moving electrodes a few centimeters dramatically alters the effects of tDCS) and physiologically (the electrical field strength has been shown to be relatively consistent under the electrodes, and to diminish exponentially with distance; Nitsche et al., 2009). The overall efficacy and direction of the excitability induced by tDCS is dependent on the strength of the current, the electrode size, and the duration of stimulation, as well as the stimulation polarity (Purpura and McMurtry, 1965; Nitsche and Fregni, 2007; Nitsche et al., 2008). tDCS has also been shown to produce persistent changes post-stimulation (beyond 30 min), indicating a potential for highly relevant therapeutic effects which could be used to treat cortical abnormalities (Bindman et al., 1964; George et al., 1996; Sharma and Antonova, 2003; Been et al., 2007). Initial studies of tDCS have been predominantly on the motor and visual cortex (Nitsche and Paulus, 2000; Liebetanz et al., 2002; see review Nitsche et al., 2008), however, a growing number of studies are investigating the effects of tDCS on the DLFPC.
There have been a number of informative findings from the research to date on the effects of DLPFC tDCS on WM. The WM modulatory effects of tDCS have been shown to be both site and polarity-specific, namely produced following anodal DLPFC tDCS (Fregni et al., 2005). Studies have also shown what appears to be a time-dependent effect of tDCS, in that improvement in WM increases with increased stimulation duration, and that effects appear to last for a period of up to 30 min post-stimulation (Ohn et al., 2008). In one of the relatively few patient studies of tDCS and WM modulation, Boggio et al. (2006) found that only 2 mA, but not 1 mA or sham tDCS, resulted in a significant improvement on accuracy on a WM task in Parkinson’s disease; suggesting that current strength may play an important role in tDCS effects. A dose effect with DLFPC tDCS stimulation has also been suggested by findings in other cognitive domains, however, there has yet to be a systematic investigation of the effect of current strength on cognitive ability in healthy controls (Iyer et al., 2005). Finally, the performance of a WM task concurrent with stimulation has been shown to result in greater post-stimulation WM performance, compared with stimulation alone (Andrews et al., 2011). While such research has provided important information regarding the effects of tDCS on WM, there are still a number of fundamental questions to be answered in order to determine the stimulation parameters and protocols that will produce the greatest effects.
The current study aimed to primarily investigate the effect of one of the most fundamental stimulation parameters yet to be systematically explored in healthy controls, that is, optimal current strength. We compared 1–2 mA tDCS to determine the most effective method of WM enhancement. In addition, we looked at the time course of effects: whether changes were produced during stimulation, after stimulation, or both.
Materials and Methods
Participants
Fourteen healthy participants were recruited for the study, but two withdrew due to time commitments. Participants were excluded if they had a history of seizure or mental illnesses, or any neurological or serious medical condition, or were currently pregnant. Suitability was determined via interview which included the administration of the Mini International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998). Written consent was obtained from the participants prior to the commencement of the study. Ethical approval was granted by Monash University and the Alfred Hospital ethics committees.
Design
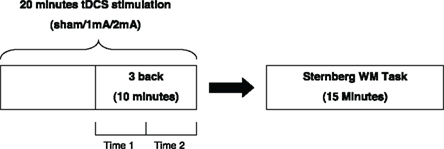
This study was designed as a double-blind repeated measures experiment. Each participant undertook 20 min of each of the three stimulation conditions and completed the 3-back WM task in the final 10 min of stimulation. Participants then undertook the Sternberg WM task immediately following stimulation. The dependent variables were the accuracy and reaction times of participants’ performance in the 3-back task (during stimulation for two time periods; namely, at 10–15 and 15–20 min) and the Sternberg WM task (post-stimulation). The order of conditions was randomized and counter-balanced to account for learning effects. Sham tDCS was included as a control condition. The three sessions were also separated by at least a 1-week washout period to ensure there were no carry over effects. This design is shown in Figure 1.
Materials
Transcranial direct current stimulation
Transcranial direct current stimulation was applied using an Eldith Stimulator Plus from neuroConn GBH. The battery-driven DC-Stimulator was used to deliver a constant direct current through two surface electrodes, the anode (positive) and the cathode (negative). To reduce resistance and minimize discomfort during application (Nitsche et al., 2008), the electrodes were covered in 35 cm2 saline-soaked sponges. Anodal stimulation was applied according to the 10–20 international system for EEG electrode placement, over F3 of the DLPFC, while the cathode was placed over the contralateral supraorbital area (Nitsche et al., 2008; Fitzgerald et al., 2009). The stimulator was set for three conditions, 1, 2 mA, and sham, each for 20 min, and a fade-in and fade-out of 15 s at the start and end of the stimulation. As the sham stimulation was set to have a 2-mA fade-in and fade-out before turning off, participants would still feel the tingling sensations usually produced at the start of an active tDCS stimulation session. The setting of conditions was also carried out by an independent researcher (KH) who assigned participants codes for their active and sham tDCS. The codes were then entered into the tDCS machine at the start of the session by the experimenter (FT). Hence, both participants and experimenter (FT) were effectively blinded as to whether the tDCS was active or sham stimulation.
Intra-stimulation WM task (n-back)
As previous research has shown that engagement in a WM task during stimulation can enhance the effects of tDCS (Andrews et al., 2011), the n-back WM task was undertaken during the period of tDCS stimulation. The n-back was chosen as the intra-stimulation task as it requires continual WM engagement. In this computer-based task, a series of random letters (A–J) were presented consecutively for participants to remember and respond to with a click of a button when a letter that was presented was the same as n letters before. The n-back WM task was developed using the E-Prime 2.0 program to generate the stimuli. Each letter was presented for 1 s with a fixation dot for 1.5 s in between. The WM task consisted of 10 min of 3-back during the second half of the 20-min stimulation period. Alternate forms of the task were used for each administration.
Post-stimulation WM task (Sternberg)
Post-stimulation WM performance was measured using the Sternberg WM task (Sternberg, 1966). The Sternberg WM task is a well validated measure of WM and has been used extensively as an outcome measure for tDCS research. This task was again administered with the E-Prime 2.0 program. This computer-based task generates a fixation cross (2 s) before presenting a memory set (4 s) of eight random consonants for participants to remember. After a 3-s retention period, a single consonant probe (2 s) appears, during which time participants can respond to with a click of a button as to whether the letter was present in the memory set before. For each session, there were three different blocks of the Sternberg WM task which took approximately 15 min to complete with a 2-min break in between each block. Alternate forms of the Sternberg task were used for each administration.
Procedure
Participants were reminded of the experimental procedures at the start of each session. After the participant was comfortably seated in an arm chair, the laptop with the WM tasks was then placed on a board resting over the arms of the chair in front of the participant. A button on the keyboard for responding was indicated by a yellow sticker and participants were then given instructions and practice trails for both the n-back and Sternberg to familiarize themselves with the WM tasks. Next, the location of DLPFC was determined and the surface electrodes were then secured with a headband and net. Stimulation was then administered for 20 min, immediately after which the Sternberg WM task was administered. The duration of each session was approximately 45 min.
Statistical Analysis
The results were analyzed using the statistical software SPSS 17.0. First, for the intra-effects of tDCS, a 3 × 2 repeated measures ANOVA was carried out for accuracy (number of correct responses) and accurate reaction time (reaction time for correct responses) of the 3-back task, with current (sham, 1, 2 mA) and time (10–15 min into tDCS and 15–20 min into tDCS) as within-subjects factors. For further analysis, paired t-tests were also used to carry out post hoc tests. For analysis of the effects of current strength on overall post-stimulation WM performance, a one-way repeated measures ANOVA was performed for the accuracy and accurate reaction time of the Sternberg task, with current (sham, 1, 2 mA) as the within-subjects factor. All results were assessed using two-tailed tests, and multiple comparisons were controlled for by utilizing an alpha level of 0.01. All assumptions for the statistical analyses used were met.
Results
The 12 participants consisted of 5 men and 7 women. Participants ranged in age from 22 to 55 years, with a mean age of 27.23 and SD of 9.18. Ten were right-handed and two were left-handed. All participants had a tertiary level of education.
Intra-Stimulation Effects: 3-Back
Reaction time
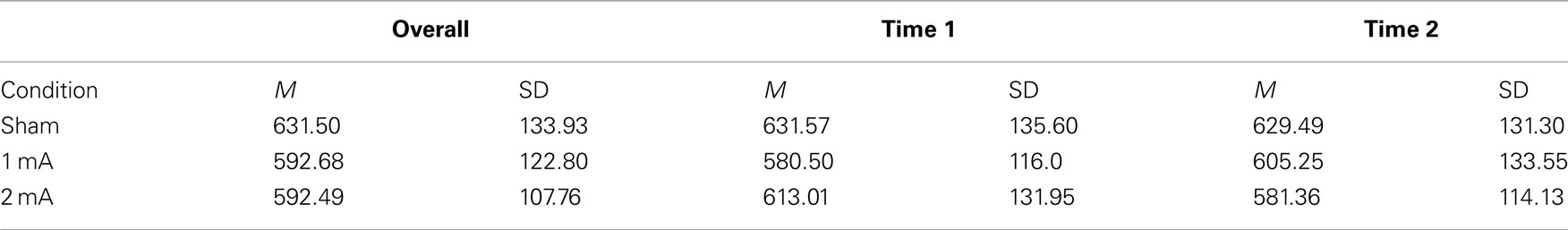
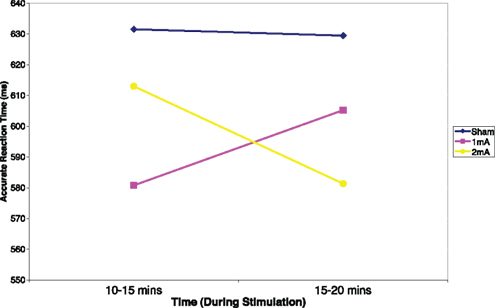
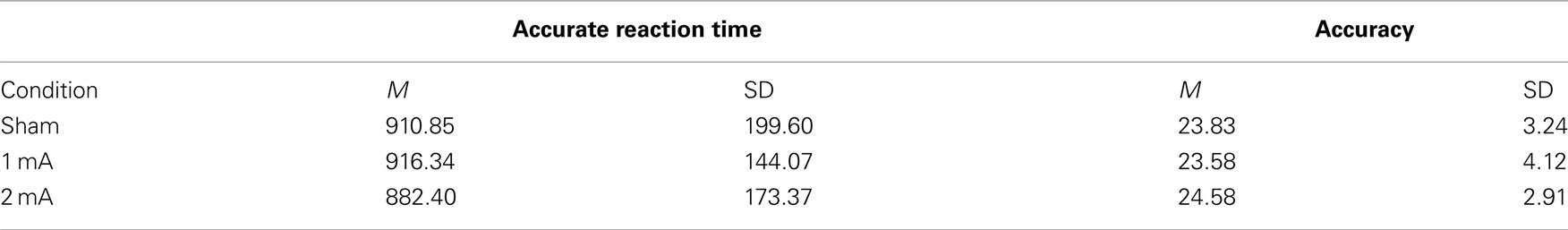
Mean and SD of reaction time for the 3-back task can be found in Table 1. There was a significant interaction effect between condition and time, F (2, 22) = 5.36, p = 0.01, n2 = 0.33 (Figure 2). There was no significant main effect of condition, F (2, 22) = 2.04, p = 0.15 or time, F (1, 11) = 0.08, p = 0.78. Post hoc tests revealed a significant difference between the sham and 2 mA conditions during the final 5 min of stimulation, t (11) = 3.41, p = 0.00, r2 = 0.51, but no differences between 1 and 2 or 1 mA and sham. A trend toward significance was also found between the sham and 1 mA conditions in the 10–15 min interval during stimulation, t (11) = 2.16, p = 0.05.
Accuracy
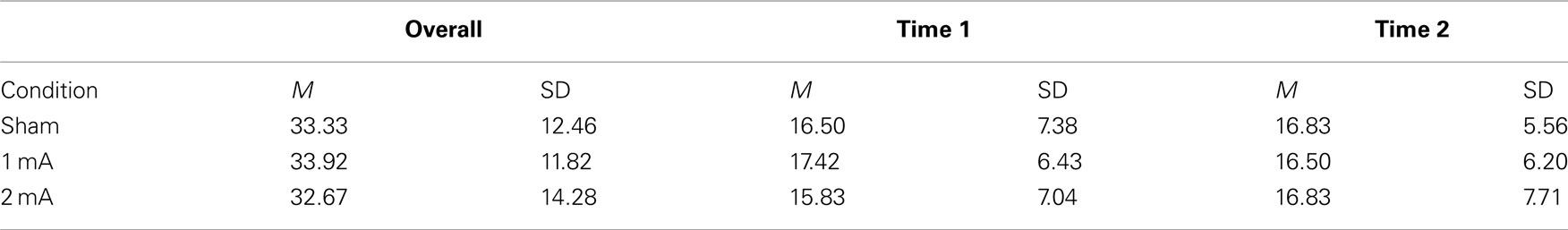
Mean and SD of accuracy for the 3-back task are shown in Table 2. There was no effect of condition on overall accuracy, F (2, 22) = 0.16, p = 0.84. There was also no interaction between condition and accuracy across the two time periods, F (2, 22) = 0.87, p = 0.48.
Post-Stimulation Effects: Sternberg
Reaction time
Mean and SD of accurate reaction time for the Sternberg task are shown in Table 3. There was no significant difference in participants’ reaction times between the sham, 1, and 2 mA conditions, F (2, 24) = 0.41, p = 0.68.
Accuracy
Mean and SD for accuracy of the Sternberg task are summarized in Table 3. There was no significant difference in participants’ correct responses between the sham, 1, and 2 mA conditions. F (2, 22) = 1.32, p = 0.28.
Discussion
The current study investigated the role of current strength on the ability of tDCS to improve WM with regards to participants’ accuracy and response time in the 3-back and Sternberg tasks. The study also looked at the time-dependent effects of tDCS on WM. Overall, the hypotheses were not supported in terms of accuracy for WM performance. The results however did provide support for tDCS improvements in WM performance in terms of reaction time. In particular, there was no effect of current strength or time course on participants’ WM performance in terms of accuracy in either of the WM tasks. However there was a significant current by time interaction found for reaction time in the 3-back WM task. Post hoc tests revealed that participants were in fact reacting quicker to produce accurate responses in the 2-mA condition compared to the sham condition in the last 5 min of stimulation, with a trend toward significance in the preceding 5 min (i.e., 10–15 min) for the 1-mA condition compared to the sham condition. This finding lends partial support to the hypotheses that participants’ WM performance may improve with application of anodal tDCS in a current and time-dependent manner.
The findings that WM performance did not improve in terms of accuracy, either during or following anodal tDCS, are largely inconsistent with past research. Unlike Fregni et al. (2005) and Ohn et al. (2008), 1 mA of anodal tDCS in this study did not significantly improve healthy participants’ WM performance in terms of accuracy. Andrews et al. (2011) also showed a significant improvement in participants’ WM accuracy following 1 mA anodal tDCS during the engagement of a WM task. One potential explanation for the lack of significant improvement in WM performance accuracy in the current study relates to the difficulty to detect changes in WM performance when participants do not have a pre-existing deficit. The primary outcome measure in this study, i.e., the Sternberg WM task, was possibly too easy as many participants were performing at near-optimal levels in the sham session. Therefore, more sensitive measures of WM performance were needed in this study to detect WM improvements. Consistent with this explanation, the current study did find some improvements in the 3-back WM task on reaction time, which has been shown to be a more sensitive measure of WM performance compared to accuracy (Prinzmetal et al., 2005). In addition, the lack of significant findings for the Sternberg task may be due to the use of non-optimal tDCS parameters. Stimulation may need to be applied for longer durations or in different current profiles (i.e., random, intermittent, or alternating, as opposed to direct) to produce a robust enough effect to enhance accuracy on a behavioral task such as the Sternberg.
While participants’ post-stimulation WM performance in terms of reaction time was found not to differ with respect to strength of current or time following anodal tDCS, the current study did find a significant interaction of current and time for accurate reaction time during stimulation. Further analysis using post hoc tests showed that participants’ reaction times were improved with increasing current strength as stimulation duration increased. Although Ohn et al. (2008) study found significance only in participants’ accuracy performance, a similar pattern could be observed in the time-dependent results found in the current study. The significant result found in the 2-mA condition in patients with Parkinson’s disease in the study by Boggio et al. (2006) was also a result of 20 min anodal tDCS stimulation. Both studies, as well as the present one, only found significant improvements in WM performance following longer stimulation durations (around 20 min), which supports the suggestion that longer and stronger stimulation may be needed to produce a more robust WM enhancement. In addition, the trend effect of the 1-mA resulting in a longer accurate reaction time when compared to the sham condition in the 10–15 min interval of the stimulation may be indicative of the need for both a stronger current and longer durations to produce reliable enhancement effects. The types of improvements seen in the current study, i.e., increased response rates, are of some clinical relevance. There have been a number of findings showing a relationship between increased speed of information processing and improved functioning (i.e., vocational performance) in illnesses such as schizophrenia (Gold et al., 2002; Evans et al., 2004). In addition, the enhancement of more broad cognitive functions such as speed of information processing can assist patients to more effectively engage in cognitive remediation programs, which may lead to greater gains (Demaree et al., 1999).
An unexpected result from this study was that the significant interaction effect found in the 3-back task for accurate reaction time in the last 5 min of the 2-mA stimulation did not carry over to the Sternberg task. Based on other studies which have looked into WM impairments in schizophrenia, the Sternberg task has been validated as a reliable form of measure for WM strongly associated with the DLPFC (Ragland et al., 2002; Altamura et al., 2007; Gore et al., 2010). Apart from also being considered a valid measure of WM (Callicott et al., 2003; Glahn et al., 2005); the cognitive processes involved in the n-back have been shown to differ with respect to the Sternberg task. Participants doing the n-back need to continuously maintain relevant information and match it with the stimulus presented, whereas in the Sternberg task, participants may begin abandoning WM maintenance once they begin searching the memory set for a match (Watter et al., 2001). With respect to this study, as well as the past studies which produced significant results for the n-back, it may be the case that the anodal tDCS applied to the DLPFC enhanced WM to a greater extent when the task required more continual consistent cognitive processes to be involved. Hence it could be that the greater cortical excitability needed to perform the n-back task with its more consistent WM load resulted in a cumulative effect, which led to a significant improvement in accurate reaction time performance in the 3-back task – the effects of which did not carry over to the Sternberg task.
There are a number of limitations that need to be considered. First, the small sample size in this study may have resulted in insufficient power to detect changes in WM performance. However, past studies have generally found significant effects with small samples of about 10–18 participants (Fregni et al., 2005; Boggio et al., 2006; Ohn et al., 2008; Andrews et al., 2011). Another possible limitation was that the 10-min of constant engagement in the n-back WM task may have caused fatigue, which could have negatively affected participants’ subsequent accurate reaction time performances in the Sternberg task. Third, as mentioned before, the low difficulty of the Sternberg WM task could have caused a ceiling effect in participants’ accuracy scores, thus limiting the ability of the study to investigate the impact of tDCS on accuracy in WM performance. Despite these limitations, the current study generated interesting and informative findings.
The current results indicate the need for further research into the role of current strength and time-dependence to better understand optimal parameters for administering tDCS to improve WM. There are many unanswered questions with respect to the optimal method of DLPFC tDCS stimulation for WM enhancement. The culmination of findings like those seen in the current study will greatly assist in the evidence-based development of optimal protocols, which will in turn lead to the conduct of larger-scale studies looking at repeated stimulation protocols which will have more direct clinical applications. Developing optimal tDCS parameters for the enhancement of WM could have significant clinical implications, with the possibility that tDCS could ultimately be utilized as an adjunct to enhance the effectiveness of traditional cognitive therapies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Altamura, M., Elvevag, B., Blasi, G., Bertolino, A., Callicott, J. H., Weinberger, D. R., Mattay, V. S., and Goldberg, T. E. (2007). Dissociating the effects of Sternberg working memory demands in prefrontal cortex. Psychiatry Res. 154, 103–114.
Andrews, S. C., Hoy, K. E., Enticott, P. G., Daskalakis, Z. J., and Fitzgerald, P. B. (2011). Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 4, 84–89.
Barch, D. M., Sheline, Y. I., Csernansky, J. G., and Snyder, A. Z. (2003). Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol. Psychiatry 53, 376–384.
Barch, D. M., and Smith, E. (2008). The cognitive neuroscience of working memory: relevance to CNTRICS and schizophrenia. Biol. Psychiatry 64, 11–17.
Been, G., Ngo, T. T., Miller, S. M., and Fitzgerald, P. B. (2007). The use of tDCS and CVS as methods of non-invasive brain stimulation. Brain Res. Rev. 56, 346–361.
Bertolino, A., Sciota, D., Brudaglio, F., Altamura, M., Blasi, G., Bellomo, A., Antonucci, N., Callicott, J. H., Goldberg, T. E., Scarabino, T., Weinberger, D. R., and Nardini, M. (2003). Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. Am. J. Psychiatry 160, 483–489.
Bindman, L. J., Lippold, O. C., and Redfearn, J. W. T. (1964). The action of brief polarizing currents on the cerebral cortex of the rat (i) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 172, 369–382.
Boggio, P. S., Ferrucci, R., Rigonatti, S. P., Covre, P., Nitsche, M., Pascual-Leone, A., and Fregni, F. (2006). Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J. Neurol. Sci. 249, 31–38.
Callicott, J. H., Mattay, V. S., Bertolino, A., Finn, K., Coppola, R., Frank, J. A., Goldberg, T. E., and Weinberger, D. R. (1999). Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb. Cortex 9, 20–26.
Callicott, J. H., Mattay, V. S., Verchinski, B. A., Marenco, S., Egan, M. F., and Weinberger, D. R. (2003). Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am. J. Psychiatry 160, 2209–2215.
Cohen, L. G., and Floel, A. (2007). Contribution of noninvasive cortical stimulation to the study of memory functions. Brain Res. Rev. 53, 250–259.
Demaree, H. A., DeLuca, J., Gaudino, E. A., and Diamond, B. J. (1999). Speed of information processing as a key deficit in multiple sclerosis: implications for rehabilitation. J. Neurol. Neurosurg. Psychiatr. 67, 661–663.
Duncan, J., and Owen, A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23, 475–483.
Evans, J., Bond, G. R., Meyer, P. S., Kim, K. W., Lysaker, P.H., Gibson, P.J., and Tunis, S. (2004). Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr. Res. 70, 331–342.
Fitzgerald, P. B., Hoy, K., McQueen, S., Maller, J. J., Herring, S., Segrave, R., Been, G., Daskalakis, Z. J., and Kulkarni, J. (2009). A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment resistant depression. Neuropsychopharmacology 34, 1255–1262.
Fregni, F., Boggio, P. S., Nitsche, M., Bermpohl, F., Antal, A., Feredoes, E., Marcolin, M. A., Rigonatti, S. P., Silva, M. T., and Paulus, W. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 166, 23–30.
George, M. S., Wassermann, E. M., and Post, R. M. (1996). Transcranial magnetic stimulation: a neuropsychiatric tool for the 21st century. J. Neuropsychiatry Clin. Neurosci. 8, 373–382.
Glahn, D. C., Ragland, J., Abramoff, A., Barrett, J., Laird, A. R., Bearden, C. E., and Velligan, D. I. (2005). Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum. Brain Mapp. 25, 60–69.
Gold, J. M., Goldberg, R. W., McNary, S. W., Dixon, L. B., and Lehman, A. F. (2002). Cognitive correlates of job tenure among patients with severe mental illness. Am. J. Psychiatry 159, 1395–1402.
Gore, C. D., Banyai, M., Gray, P. J., Diwadkar, V., and Erdi, P. (2010). Pathological effects of cortical architecture on working memory in schizophrenia. Pharmacopsychiatry 43(Suppl. 1), S92–S97.
Hoy, K. E., and Fitzgerald, P. B. (2010). Brain stimulation in psychiatry and its effects on cognition. Nat. Rev. Neurol. 6, 267–275.
Iyer, M. B., Mattu, U., Grafman, J., Lomerev, M., Sato, S., and Wassermann, E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64, 872–875.
Lawrence, A. D., Hodges, J. R., Rosser, A. E., Kershaw, A., ffrench-Constant, C., Rubinsztein, D. C., Robbins, T. W., and Sahakian, B. J. (1998). Evidence for specific cognitive deficits in preclinical Huntington’s disease. Brain 121, 1329–1341.
Lee, J., and Park, S. (2005). Working memory impairments in schizophrenia: a meta-analysis. J. Abnorm. Psychol. 114, 599–611.
Liebetanz, D., Nitsche, M. A., Tergau, F., and Paulus, W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247.
Logie, R. H., Cocchini, G., Delia Sala, S., and Baddeley, A. D. (2004). Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer’s disease. Neuropsychology 18, 504–513.
Mull, B. R., and Seyal, M. (2001). Transcranial magnetic stimulation of left prefrontal cortex impairs working memory. Clin. Neurophysiol. 112, 1672–1675.
Muller, N. G., Machado, L., and Knight, R. T. (2002). Contributions of subregions of the prefrontal cortex to working memory: evidence from brain lesions in humans. J. Cogn. Neurosci. 14, 673–686.
Nitsche, M. A., Boggio, P., Frengi, F., and Pascual Leone, A. (2009). Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp. Neurol. 219, 14–19.
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., Paulus, W., Hummel, F., Boggio, P. S., Fregni, F., and Pascual-Leone, A. (2008). Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 1, 206–223.
Nitsche, M. A., and Fregni, F. (2007). Transcranial direct current stimulation – an adjuvant tool for the treatment of neuropsychiatric diseases? Curr. Psychiatry Rev. 3, 222–232.
Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527, 633–639.
Ohn, S. H., Park, C. I., Yoo, W. K., Ko, M. H., Choi, K. P., Kim, G. M., Lee, Y. T., and Kim, Y. H. (2008). Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 19, 43–47.
Passingham, D., and Sakai, K. (2004). The prefrontal cortex and working memory: physiology and brain imaging. Curr. Opin. Neurobiol. 14, 163–168.
Poreisz, C., Boros, K., Antal, A., and Paulus, W. (2007). Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 72, 208–214.
Postle, B. R. (2006). Working memory as an emergent property of the mind and brain. Neuroscience 139, 23–38.
Prinzmetal, W., McCool, C., and Park, S. (2005). Attention: reaction time and accuracy reveal different mechanisms. J. Exp. Psychol. 134, 73–92.
Purpura, D. P., and McMurtry, J. G. (1965). Intracellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol. 28, 166–185.
Ragland, J. D., Turetsky, B. I., Gur, R. C., Gunning-Dixon, F., Turner, T., Schroeder, L., Chan, R., and Gur, R. E. (2002). Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 16, 370–379.
Reilly, J. L., Harris, M. S., Keshavan, M. S., and Sweeney, J. A. (2006). Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch. Gen. Psychiatry 63, 1189–1197.
Reilly, J. L., Harris, M. S., Khine, T. T., Keshavan, M. S., and Sweeney, J. A. (2007). Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol. Psychiatry 62, 818–821.
Reppermund, S., Ising, M., Lucae, S., and Zihl, J. (2009). Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol. Med. 39, 603–614.
Rund, B. R., and Borg, N. E. (1999). Cognitive deficits and cognitive training in schizophrenic patients: a review. Acta Psychiatr. Scand. 100, 85–95.
Sharma, T., and Antonova, L. (2003). Cognitive function in schizophrenia. Deficits, functional consequences, and future treatment. Psychiatr. Clin. North Am. 26, 25–40.
Sheehan, D. V., Lecrubie, R. Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., Hergueta, T., Baker, R., and Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59(Suppl. 20), 22–33.
Smith, E. E., and Jonides, J. (1997). Working memory: a view from neuroimaging. Cogn. Psychol. 33, 5–42.
Smith, E. E., and Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science 283, 1657–1661.
Watter, S., Geffen, G. M., and Geffen, L. B. (2001). The n-back as a dual-task: P300 morphology under divided attention. Psychophysiology 38, 998–1003.
Keywords: transcranial direct current stimulation, working memory, dorsolateral prefrontal cortex
Citation: Teo F Hoy KE, Daskalakis ZJ, and Fitzgerald PB (2011) Investigating the role of current strength in tDCS modulation of working memory performance in healthy controls. Front. Psychiatry 2:45. doi: 10.3389/fpsyt.2011.00045
Received: 30 May 2011;
Accepted: 06 July 2011;
Published online: 18 July 2011.
Edited by:
F. Andrew Kozel, University of Texas Southwestern Medical Center, USAReviewed by:
Tal Herbsman, University of Wisconsin, USAShawn Michael McClintock, University of Texas Southwestern Medical Center, USA
Patrick John Marsh, University of South Florida, USA
Copyright: © 2011 Teo, Hoy, Daskalakis and Fitzgerald. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Kate E. Hoy, Monash Alfred Psychiatry Research Centre, The Alfred and Monash University, First Floor, Old Baker Building, The Alfred, Commercial Road, Melbourne, VIC 3000, Australia. e-mail: kate.hoy@monash.edu
 Florence Teo1
Florence Teo1