- 1 Neuroscience Program, Wake Forest University School of Medicine, Winston–Salem, NC, USA
- 2 Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Winston–Salem, NC, USA
- 3 Department of Behavioral Neuroscience, Oregon Health and Science University, Portland, OR, USA
Evidence for an interaction between alcohol consumption and the serotonin system has been observed repeatedly in both humans and animal models yet the specific relationship between the two remains unclear. Research has focused primarily on the serotonin transporter (SERT) due in part to its role in regulating extracellular levels of serotonin. The hippocampal formation is heavily innervated by ascending serotonin fibers and is a major component of the neurocircuitry involved in mediating the reinforcing effects of alcohol. The current study investigated the effects of chronic ethanol self-administration on hippocampal SERT in a layer and field specific manner using a monkey model of human alcohol consumption. [3H]Citalopram was used to measure hippocampal SERT density in male cynomolgus macaques that voluntarily self-administered ethanol for 18 months. Hippocampal [3H]citalopram binding was less dense in ethanol drinkers than in controls, with the greatest effect observed in the molecular layer of the dentate gyrus. SERT density was not correlated with measures of ethanol consumption or blood ethanol concentrations, suggesting the possibility that a threshold level of consumption had been met. The lower hippocampal SERT density observed suggests that chronic ethanol consumption is associated with altered serotonergic modulation of hippocampal neurotransmission.
Introduction
Alcohol abuse and dependence are widespread disorders that constitute a significant public health concern, with the prevalence of alcohol abuse in the United States increasing in recent years (Grant et al., 2004). Alcohol use has been identified as the third leading preventable cause of death in the United States (Mokdad et al., 2004) and its financial burden has increased in magnitude over time (Harwood, 2000). Identifying the neurobiological consequences of excessive alcohol consumption and illuminating the mechanisms underlying those effects can provide a framework from which to develop new treatment strategies aimed at reducing the significant human and financial costs associated with this disease.
A long history of research using animal models has reported a role for the serotonin system in regulating virtually every aspect of the progression to alcohol dependence, including intake, preference, tolerance, and withdrawal (Myers and Veale, 1968; Griffiths et al., 1974; Frankel et al., 1975; Richardson and Novakovski, 1978). Despite an abundance of studies, the relationship between ethanol and serotonin remains unclear (for review LeMarquand et al., 1994; Kenna, 2010; Sari et al., 2011). In an effort to better understand the specific effects of chronic ethanol on this system, much research has focused on the serotonin transporter (SERT), as it is the main cellular constituent controlling extracellular levels of serotonin.
Although frequently thought of as a single functional entity, the hippocampal formation is comprised of a discrete set of subregions, each of which make a unique contribution to the classical trisynaptic pathway characteristic of this region. Input to the hippocampal formation comes through projections from the entorhinal cortex to the dentate gyrus via the perforant path. Mossy fibers projecting from the dentate gyrus terminate on CA3 pyramidal neurons, which then project via the Schaffer collaterals to CA1 pyramidal neurons. An additional projection is evident from CA1 to the subiculum, the hippocampal formation’s major source of information output (Amaral and Lavenex, 2007). This brain region serves as a major biological substrate mediating the reinforcing effects of drugs of abuse including alcohol (Haber and Knutson, 2009; Belujon and Grace, 2011) and is heavily innervated by ascending serotonergic fibers emanating from the raphe nuclei (Wilson and Molliver, 1991). Despite this, most studies examining the effects of ethanol on the SERT have focused on other regions involved in the mesocorticolimbic dopamine pathway.
The few studies that have investigated the effects of ethanol on hippocampal SERT report conflicting results. For example, genetically bred lines of ethanol-preferring and non-preferring rats exhibit no differences in hippocampal SERT regardless of ethanol self-administration history (Chen and Lawrence, 2000; Casu et al., 2004). Interestingly, Wistar rats exposed to ethanol in their drinking water for a period of 6 weeks exhibit a reduction in hippocampal SERT immunoreactivity (Tagliaferro et al., 2002), whereas greater SERT immunoreactivity has been reported in CA1–CA3 of mice made dependent following 9 days of ethanol vapor chamber exposure (Shibasaki et al., 2010).
The human literature is equally conflicted. In vivo imaging studies have reported no differences in hippocampal SERT availability in two populations of abstinent alcoholics (Brown et al., 2007; Martinez et al., 2009). Analyses of SERT density in human postmortem hippocampal brain tissue have reported either increases or decreases as a function of alcohol consumption. Gross-Isseroff and Biegon (1988) reported greater SERT density in the stratum oriens of the CA fields in individuals with detectable blood ethanol concentrations at time of death. In contrast, Chen et al. (1991) reported lower postmortem hippocampal SERT density in individuals classified only as heavy drinkers compared to controls.
Rodent models have provided the foundation of our current understanding of serotonergic involvement in acute and chronic ethanol self-administration. However, comparatively few studies have examined similar changes in the primate brain. Non-human primates more closely model human physiology, brain structure, and alcohol drinking patterns than rodents. While the same general fields and functions are present in both the rodent and primate hippocampal formation, there are important species differences that illustrate how the monkey hippocampus more closely resembles that of humans. For example, greater differentiation and laminar organization is observed within the primate entorhinal cortex along with a thicker and more laminated principal cell layer within the CA fields of primates (Amaral and Lavenex, 2007). In addition, substantial differences in dentate gyrus intrinsic neurocircuitry have been noted between primates and rodents (Amaral et al., 1984). Similarly, the distribution of serotonergic fibers innervating the hippocampal formation differs significantly between rodents and primates (Azmitia and Gannon, 1986).
While in vivo imaging is a useful tool, particularly for studies in humans, data using PET and SPECT can be difficult to interpret due to competition of the radiotracer with the endogenous ligand, in this case, serotonin. Studies utilizing human postmortem tissue have also been informative but are often plagued by small sample sizes and diverse alcohol dependent populations that vary in age, sex, ethnicity, and alcoholic subtype. Human studies are frequently further confounded by variables that may directly impact various biochemical measures and are often impossible to control (e.g., comorbid psychiatric conditions, periodicity, and chronicity of alcohol consumption, polysubstance use especially nicotine, and time in recovery). In addition to these limitations, the majority of studies investigating the effects of ethanol on hippocampal SERT have examined the structure as a single entity. While this strategy is often useful from a technical perspective, it fails to recognize the unique regional and laminar organization that makes up functionally and molecularly distinct components of the underlying circuitry within the system (Small et al., 2011). Without this type of analysis, the functional implications of any findings remain limited.
Using a well-established non-human primate model of human alcohol drinking (Vivian et al., 2001; Grant et al., 2008) we examined the effects of chronic ethanol self-administration on hippocampal SERT density. Based on previous data reporting decreased serotonin levels (Murphy et al., 1982; Borg et al., 1985; Fils-Aime et al., 1996) and serotonin fiber number (Halliday et al., 1993; Zhou et al., 1994) in alcoholics and alcohol-preferring rats, we hypothesized that ethanol drinkers would exhibit lower hippocampal SERT density than controls. Furthermore, using in vitro receptor autoradiography we were able to examine SERT density in a layer by field specific manner allowing us to identify distinct areas of the hippocampal formation that may be more or less vulnerable to the effects of chronic ethanol.
Materials and Methods
Subjects
Adult male cynomolgus macaques (Macaca fascicularis) were individually housed in quadrant cages (1.6 m × 0.8 m × 0.8 m) allowing visual, auditory, and olfactory contact with conspecifics, in a room with constant temperature (68–72°F), humidity (65%), and a 12-h light cycle (lights on at 7:00 AM). At approximately 6 years of age (range = 5.67–6.58 years) nine monkeys were allowed to voluntarily self-administer water and ethanol as described previously (Grant et al., 2008). Briefly, monkeys were trained to self-administer food, water, and ethanol using an operant panel permanently attached to the side of their home cage. Animals were initially exposed to ethanol in progressively increasing doses of 0.5, 1.0, and 1.5 g/kg using a schedule-induced polydipsia technique with scheduled food delivery of one 1 gram banana-flavored pellet (Research Diets Incorporated, New Brunswick, NJ, USA) every 5 min. Following induction, all animals were allowed to voluntarily self-administer ethanol, food, and water during daily 22 h sessions for a period of 12 months. Each animal’s intake varied throughout the 22-h phase of the study with daily average consumption ranging from 1.17 to 4.25 g/kg (Table 1). Intake correlated positively with blood ethanol concentration (r2 = 0.70; p = 0.005). Imaging and neuroendocrine data not reported here were collected following this 12 month period putting the total duration of drinking at approximately 18 months for each animal (range = 18–20 months). Additional data from these animals are reported elsewhere (Morrow et al., 2006; Porcu et al., 2006; Cheng et al., 2010; Freeman et al., 2010, 2011; Lebold et al., 2010; Helms et al., 2011).
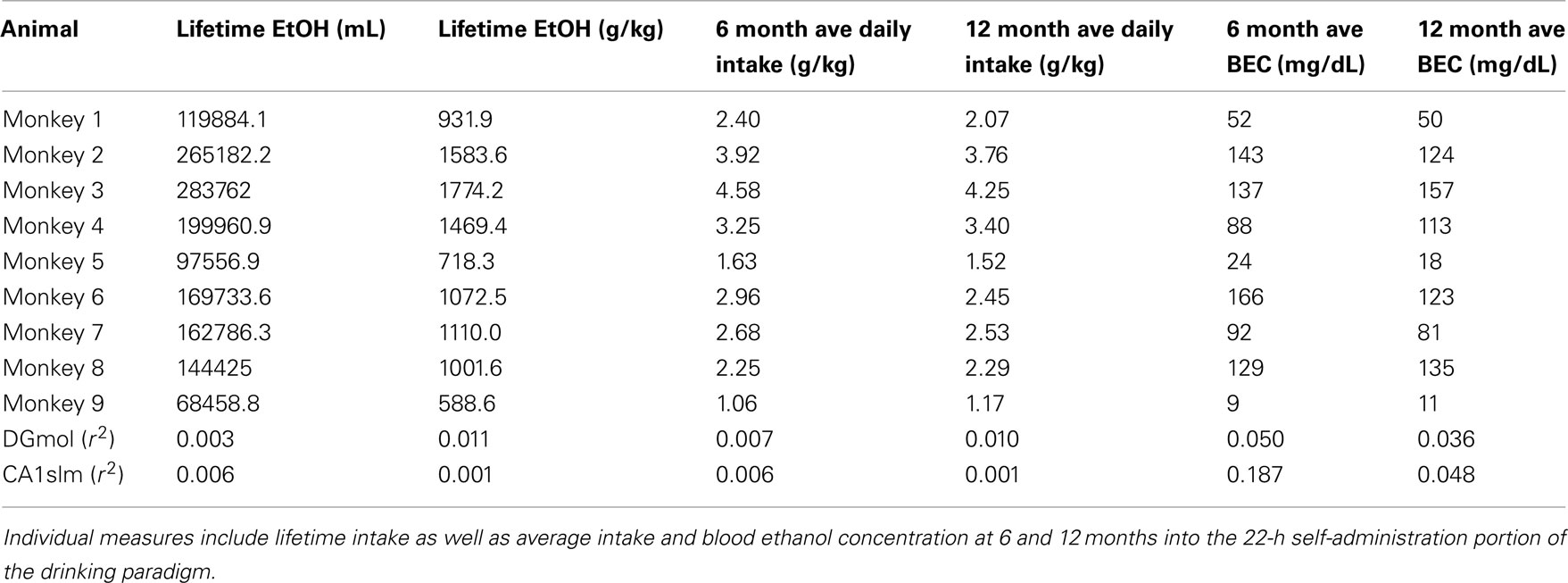
Table 1. Correlation coefficients for hippocampal [3H]citalopram binding and measures of ethanol consumption and blood ethanol concentration (BEC).
Controls were adult male cynomolgus monkeys that had lived at the Oregon National Primate Research Center for 3 years prior to assignment to this protocol. A set of four formed a group of “caloric controls” and were housed in the same room as the ethanol monkeys. Each monkey was allowed to self-administer a volume of a maltose–dextrin solution on a daily basis that was matched (yoked) in calories to the ethanol-caloric intake of an assigned ethanol monkey matched by body weight. Caloric controls were provided the same banana-flavored food pellets through the panels as their primary food source. The laboratory personnel interacted with these monkeys daily in the same manner as the ethanol monkeys for 10 months prior to necropsy.
Additional controls were assembled from a separate study because a sufficient number of caloric control animals were not available at this time. Therefore, a second set of four formed a group of “housing control” monkeys who were housed individually in quadrant cages in a room identical to the ethanol self-administration room (light cycle, temperature, humidity) and remained on regular laboratory diet (Purina monkey chow). The laboratory personnel interacted with the monkeys daily for 4 months prior to necropsy.
All procedures were approved by the Wake Forest University School of Medicine and Oregon Health and Sciences University Institutional Animal Care and Use Committees and adhered to NIH’s Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Necropsy
All monkeys were necropsied at the end of their final 22 h self-administration session at 9:00 AM. Each monkey was sedated with ketamine (15 mg/kg intramuscular) and brought to a deep surgical plane of anesthesia with intravenous pentobarbital administered to effect (30–50 mg/kg). Following a complete craniotomy to speed access to the brain, each subject was perfused transcardially with ice-cold, oxygenated Krebs-Henseleit buffer (in mM: NaCl 137, Na2HPO4 6.5, Na2PO4 1.4, KCl 2.7, CaCl2 0.3, MgCl2 1.0, glucose 5.0; pH = 7.4) for 1.5 min. The brain was then quickly removed from the skull and blocked. Blocks were frozen either in isopentane at −35°C for 15 min or between aluminum slabs cooled on dry ice and subsequently stored at −80°C until ready for sectioning. Blocks containing the hippocampus were sectioned coronally at 20 μm using a cryostat maintained at −20°C. Sections were thaw mounted to frost plus slides, placed immediately on wet ice and then in a desiccator overnight at 4°C before being placed at −80°C until ready to be processed for in vitro receptor autoradiography.
In vitro Receptor Autoradiography
SERT density was determined at two separate levels within the rostrocaudal extent of the hippocampus corresponding to approximately A8-10 (mid rostrocaudal) and A4-6 (caudal) in the atlas of Szabo and Cowan (1984). SERT was labeled with [3H]citalopram (PerkinElmer, Inc., Boston, MA, USA) according to procedures adapted from Strazielle et al. (1996). Briefly, sections from each animal were preincubated for 15 min at room temperature in 50 mM Tris–HCl buffer containing 120 mM NaCl and 5 mM KCl (pH 7.4). Two adjacent sections per animal per level were used to determine total binding by incubation for 60 min at room temperature in the same buffer containing 2 nM [3H]citalopram (70.0 Ci/mmol) while non-specific binding was determined using the same conditions with the addition of 10 μM fluoxetine (one adjacent section per animal per level). Sections were subsequently washed in ice-cold preincubation buffer (2 × 10 min) followed by a rinse in ice-cold distilled water and then dried overnight in a hood under a stream of cool air. Dried sections and [3H] standards (American Radiolabeled Chemicals, Inc., St. Louis, MO, USA) were apposed to [3H] Hyperfilm for 5 weeks. Films were subsequently developed with Kodak GBX developer, stopbath, and Kodak Rapid Fixer.
Autoradiograms were analyzed by quantitative densitometry using MCID (Imaging Research, St. Catharines, ON, Canada). Standard curves from [3H] standards were used to convert optical density values to tissue equivalent values (fmol/mg wet weight tissue). Two adjacent measurements were taken in each region analyzed on each section measuring total binding (i.e., four measurements per region per level per animal). Specific binding was determined by subtracting non-specific binding from total binding in adjacent sections. Adjacent nissl stained sections were used to confirm the anatomical placement of the measurements taken in each field and layer.
Statistical Analysis
Prism 5 for Mac OS X (San Diego, CA, USA) was used for statistical analyses. Two-way analysis of variance was employed for each region measured with group (ethanol versus control) and level (mid rostrocaudal versus caudal) as the factors. Linear regression was used to identify the relationship between [3H]citalopram binding and drinking measures.
Results
For all animals, [3H]citalopram binding was greatest in the molecular layer of the dentate gyrus (DGmol) with moderate binding in the stratum lacunosum moleculare of CA1 (CA1slm) reflecting the normal distribution of serotonin fibers innervating the primate hippocampus (Figure 1; Wilson and Molliver, 1991).
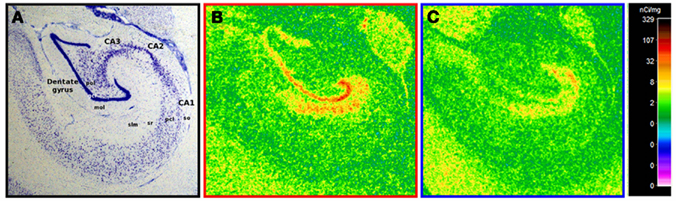
Figure 1. Representative [3H]citalopram binding in ethanol drinkers and controls. (A) A nissl stain of the non-human primate hippocampus in coronal view with relevant regions and layers labeled. (B) A representative example of hippocampal [3H]citalopram binding in a healthy control. (C) A representative example of hippocampal [3H]citalopram binding following chronic ethanol self-administration. Note that lower binding is visually apparent in the ethanol drinker. Abbreviations: gcl, granule cell layer; mol, molecular layer; pcl, pyramidal cell layer; pol, polymorphic layer; slm, stratum lacunosum moleculare; so, stratum oriens; sr, stratum radiatum.
There were no significant differences in [3H]citalopram binding in either DGmol or CA1slm between the two control groups, one of which drank only water and remained on standard monkey chow, and the other, which received a calorically matched volume of maltose dextrin and ate the pelleted diet (p = 0.22 and p = 0.39 respectively; data not shown). As a result, data from both control groups were pooled for subsequent analyses.
[3H]Citalopram binding was less dense in ethanol drinkers than in controls. In CA1slm a main effect of group approached significance [F(1, 30) = 3.370, p = 0.076] with no effect of level [F(1, 30) = 0.008, p = 0.783] (Figure 2A). The effect was significant in DGmol, again with a main effect of group [F(1, 30) = 8.636, p = 0.006] and no effect of level [F(1, 30) = 0.802, p = 0.378; Figure 2B).
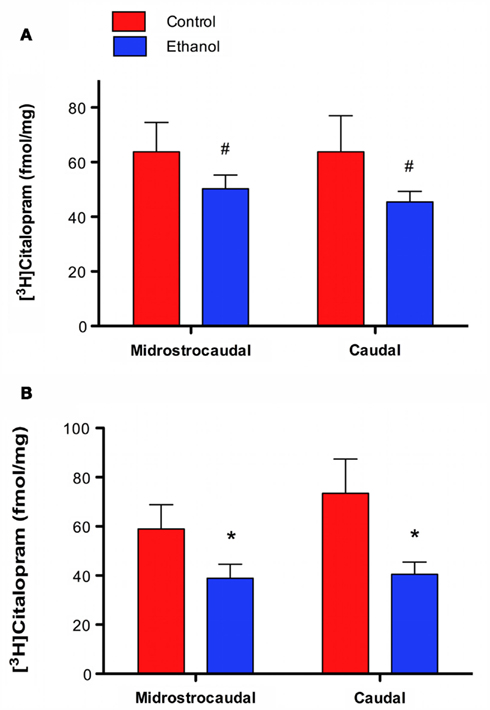
Figure 2. [3H]Citalopram binding is lower in animals that chronically self-administered ethanol. (A) CA1slm [3H]citalopram binding is lower in ethanol drinkers than controls. (B) DGmol [3H]citalopram binding is significantly lower in ethanol drinkers than controls. #p = 0.08; *p < 0.01.
[3H]Citalopram binding was not significantly correlated with measures of ethanol intake or blood ethanol concentration over the 12-month 22 h drinking period (Table 1).
Discussion
Chronic ethanol self-administration was associated with lower hippocampal SERT density in cynomolgus macaques, an effect that was most pronounced in the molecular layer of the dentate gyrus. This is the first study to investigate the effects of chronic ethanol self-administration on hippocampal SERT density in a layer by region specific manner in a macaque model that closely resembles human physiology, neurobiology, and voluntary alcohol drinking patterns. In addition, we have shown that self-administration and human interaction had no significant effect on hippocampal SERT density suggesting that housing controls are sufficient for future studies.
The lower hippocampal SERT density observed in ethanol drinkers may be the result of a compensatory down-regulation of the transporter secondary to decreased serotonin concentrations associated with chronic ethanol consumption (Borg et al., 1985; Fils-Aime et al., 1996). Indeed, the hippocampus appears to be particularly vulnerable to decreased serotonergic neurotransmission following chronic ethanol consumption (Wu et al., 1986). Alternatively, lower hippocampal SERT density may indicate a loss of serotonergic innervation. The human literature both supports and challenges this suggestion. Halliday et al. (1993) reported degeneration of midbrain serotonergic neurons identified by tryptophan hydroxylase (TPH) immunoreactivity in the brains of alcoholics with and without Wernicke–Korsakoff’s syndrome. This study was contradicted by a subsequent study in which midbrain serotonergic neuron degeneration was not observed in alcoholics without Wernicke–Korsakoff’s (Baker et al., 1996). Recent studies suggest a more complex picture. In a comprehensive analysis, Underwood et al. (2007) investigated a heterogenous population of alcoholics and reported no differences in dorsal raphe serotonin neuron number or size but greater TPH immunoreactivity and density of TPH+ neuron processes in the dorsal raphe of alcoholics than controls. Similar findings were reported by Bonkale et al. (2006) who observed greater TPH immunoreactivity in a single subnucleus of the dorsal raphe of depressed alcoholic suicides than in healthy controls. Unfortunately, it is difficult to make comparisons among these studies due to small sample sizes and sample heterogeneity, but overall they suggest that chronic alcohol consumption is associated with serotonergic neuroplasticity. Importantly, however, recent work has shown that the SERT is a more accurate marker of serotonin positive neurons than serotonergic biosynthetic enzymes (Nielsen et al., 2006), the use of which can frequently produce false negatives. In addition, differences (or lack thereof) in raphe neuron number are not necessarily indicative of the density of serotonergic projections to regions outside the midbrain, making it possible that previously reported results are region specific. Further investigation will be required to determine the impact of serotonergic neurodegeneration and the extent that brain regions receiving these midbrain serotonergic projections are affected.
No correlational relationship was observed between hippocampal SERT density and individual measures of ethanol intake or blood ethanol concentration. It is possible that the neurobiological adaptations observed are reached at a lower threshold level of alcohol intake. The animals used in the present study had an average daily ethanol intake ranging from 1.16 to 4.20 g/kg (Grant et al., 2008) suggesting that changes in hippocampal SERT occur at alcohol intakes lower than 1.16 g/kg/day (about an average of four drinks per day). Several studies have reported correlations between amount of ethanol intake and neurobiological endpoints (Adell and Myers, 1995; Heinz et al., 1998; Acosta et al., 2010; Cuzon Carlson et al., 2011). However, in extrapolating to the human literature, accurate measures of intake are difficult to ascertain whereas duration of drinking is more quantifiable. Chronicity of drinking may in fact be a more accurate predictor of the neurobiological adaptations induced by of chronic ethanol consumption, as has been reported in previous studies examining effects on the serotonergic system (Preuss et al., 2000; Berggren et al., 2002; Johnson et al., 2008). While the monkeys in the present study varied in overall levels of consumption, the duration of drinking was identical, which may explain the lack of correlation between intake and blood ethanol concentration measures and hippocampal SERT density.
With the identification of a SERT polymorphism affecting transcriptional control of the protein product (5-HTTLPR), a significant amount of research has investigated whether basal SERT levels play a role in the risk for alcohol dependence. The 5-HTTLPR is a 44 base pair insertion/deletion with the short allele conferring reduced SERT mRNA, density, and activity compared to the long variant (Lesch et al., 1996). The alcohol literature is replete with contradictory findings, but a recent meta-analysis revealed a modest association between dependence and the presence of at least one short allele of the 5-HTTLPR polymorphism. Importantly, however, the study also uncovered the potential for publication bias toward positive results (McHugh et al., 2010). We are unable to conclusively determine whether lower hippocampal SERT density is a risk factor for heavy or compulsive ethanol self-administration, but the data we report here suggest that basal SERT density may not be a risk factor for the amount an individual drinks. All of the subjects in this study were randomly assigned to drinking and control groups so there is no reason to expect that drinkers, as a group, would have lower SERT densities before they were exposed to ethanol. In addition, the absence of any correlation between individual consumption and hippocampal SERT density, while not conclusive, does not support the hypothesis that basal SERT density is a risk factor for or predictor of the amount consumed.
Our findings contradict those of Shibasaki et al. (2010) who reported greater hippocampal SERT immunoreactivity in mice exposed to ethanol chronically using a vapor chamber apparatus, but support Tagliaferro et al. (2002) who observed lower hippocampal SERT immunoreactivity in rats exposed to ethanol in their drinking water for 6 weeks. These contradictory results are likely due to strain/species differences, exposure methods, and perhaps the stress induced by involuntary ethanol exposure. Various rodent species and strains exhibit marked differences in neurochemistry and neuroanatomy (Ingram and Corfman, 1980; Nguyen et al., 2000; Chen et al., 2006; Kapasova and Szumlinski, 2008). Furthermore, voluntary drug self-administration can produce drastically different effects from non-contingent drug exposure (Hemby et al., 1997; Jacobs et al., 2003). Both of these factors may contribute to the disparate findings reported in rodent models.
Two studies have assessed the effects of alcohol on hippocampal SERT density in humans. The first examined the hippocampal formation in a regionally specific manner and reported greater SERT density in individuals with a positive blood ethanol concentration at time of death as measured by [3H]imipramine binding (Gross-Isseroff and Biegon, 1988). These results are difficult to attribute to an effect of chronic alcohol consumption, however, because [3H]imipramine binds to both the SERT and a second, low affinity binding site (D’Amato et al., 1987) and subjects were assigned to the alcohol group simply on the basis of the presence of alcohol at time of death, which is not necessarily indicative of chronic consumption. In contrast, a more recent study reported lower SERT density in the brains of alcoholics than controls using [3H]paroxetine (Chen et al., 1991). These authors, however, failed to examine the hippocampus by its functionally distinct subregions as reported in the present study.
Other studies have reported lower SERT binding or availability in brain regions outside the hippocampus in chronic drinkers (Heinz et al., 1998, 2000; Szabo et al., 2004; Storvik et al., 2006a,b, 2007). It is tempting to suggest that SERT density is lower globally in animals and humans exposed to chronic ethanol, but comparisons between the existing literature and our own data are difficult to make due to significant species and methodological differences, most notably the heterogeneity of the samples investigated. Further work is needed to determine whether the differences observed in the hippocampal formation are also observed elsewhere in the brain, how these differences may affect neurotransmission and the responsivity to pharmacotherapeutics aimed at reducing drinking.
If the lower hippocampal SERT density we observed in the present study following chronic ethanol consumption is related to serotonergic fiber degeneration, then the hippocampus may be operating under a serotonin deficit in chronic ethanol drinkers. The dentate gyrus, which we found was most vulnerable to this effect, is the first step in the unidirectional circuit that makes up the hippocampal formation (Insausti and Amaral, 2004), receiving all incoming sensory information from the entorhinal cortex (Insausti and Amaral, 2004). Because of the complexity of the serotonin system and the abundance of functionally distinct receptors in the serotonin family (Barnes and Sharp, 1999) it is difficult to speculate on the functional consequences of the changes we observed. The 5-HT1A receptor, however, is particularly abundant in the hippocampal formation in the same regions where the SERT is localized (Stuart et al., 1986). The 5-HT1A receptor is coupled to Gi/o and consequently inhibits adenylate cyclase ultimately resulting cellular inhibition and decreased neurotransmission (De Vivo and Maayani, 1986; Pugliese et al., 1998). Lower serotonin concentrations would likely reduce the activation of the 5-HT1A receptor and ultimately result in a relief of the inhibitory modulation that the hippocampal circuit is typically under. An alteration at the beginning of this circuit is likely to have downstream effects and ultimately modify the circuit’s final output.
Drugs targeting the serotonin system, including selective serotonin reuptake inhibitors (SSRIs), which increase extracellular serotonin levels in the brain by blocking SERT activity, have been proposed for the treatment of alcohol dependence (Johnson, 2004). Unfortunately, these drugs have had only limited success despite an abundance of promising preclinical data (Kranzler et al., 1995; Kabel and Petty, 1996). This preclinical data has relied, however, mainly on rodent models, which exhibit important serotonergic differences compared to primates (Azmitia and Gannon, 1986; Duncan et al., 1998). As such, the field can benefit from work using primate models, including the present study, to better understand the interaction between chronic alcohol consumption and serotonergic perturbations in the brain. The results reported here suggest that continued exploration of drugs targeting the serotonin system is warranted for the treatment of alcohol use disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by NIH grants AA018901 (E. J. Burnett), AA013510 (K. A. Grant), and AA14106 (D. P. Friedman).
References
Acosta, G., Hasenkamp, W., Daunais, J. B., Friedman, D. P., Grant, K. A., and Hemby, S. E. (2010). Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 1318, 144–154.
Adell, A., and Myers, R. D. (1995). Selective destruction of midbrain raphe nuclei by 5,7-DHT: is brain 5-HT involved in alcohol drinking in Sprague-Dawley rats? Brain Res. 693, 70–79.
Amaral, D. G., Insausti, R., and Cowan, W. M. (1984). The commissural connections of the monkey hippocampal formation. J. Comp. Neurol. 224, 307–336.
Amaral, D. G., and Lavenex, P. (2007). “Hippocampal neuroanatomy,” in The Hippocampus Book, eds P. Anderson, R. Morris, D. Amaral, T. Bliss, and J. O’Keefe (New York: Oxford University Press), 37–114.
Azmitia, E. C., and Gannon, P. J. (1986). The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Adv. Neurol. 43, 407–468.
Baker, K. G., Halliday, G. M., Kril, J. J., and Harper, C. G. (1996). Chronic alcoholics without Wernicke-Korsakoff syndrome or cirrhosis do not lose serotonergic neurons in the dorsal raphe nucleus. Alcohol. Clin. Exp. Res. 20, 61–66.
Barnes, N. M., and Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152.
Belujon, P., and Grace, A. A. (2011). Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann. N. Y. Acad. Sci. 1216, 114–121.
Berggren, U., Eriksson, M., Fahlke, C., and Balldin, J. (2002). Is long-term heavy alcohol consumption toxic for brain serotonergic neurons? Relationship between years of excessive alcohol consumption and serotonergic neurotransmission. Drug Alcohol Depend. 65, 159–165.
Bonkale, W. L., Turecki, G., and Austin, M. C. (2006). Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse 60, 81–85.
Borg, S., Kvande, H., Liljeberg, P., Mossberg, D., and Valverius, P. (1985). 5-Hydroxyindoleacetic acid in cerebrospinal fluid in alcoholic patients under different clinical conditions. Alcohol 2, 415–418.
Brown, A. K., George, D. T., Fujita, M., Liow, J., Ichise, M., Hibbeln, J., Ghose, S., Sangare, J., Hommer, D., and Innis, R. B. (2007). PET [11C]DASB imaging of serotonin transporters in patients with alcoholism. Alcohol. Clin. Exp. Res. 31, 28–32.
Casu, M., Pisu, C., Lobina, C., and Pani, L. (2004). Immunocytochemical study of the forebrain serotonergic innervation in Sardinian alcohol-preferring rats. Psychopharmacology (Berl.) 172, 341–351.
Chen, F., and Lawrence, A. J. (2000). 5-HT transporter sites and 5-HT1A and 5-HT3 receptors in Fawn-Hooded rats: a quantitative autoradiography study. Alcohol. Clin. Exp. Res. 24, 1093–1102.
Chen, H.-T., Casanova, M. F., Kleinman, J. E., Zito, M., Goldman, D., and Linnoila, M. (1991). 3H-paroxetine binding in brains of alcoholics. Psychiatry Res 38, 293–299.
Chen, X. J., Kovacevic, N., Lobaugh, N. J., Sled, J. G., Henkelman, R. M., and Henderson, J. T. (2006). Neuroanatomical differences between mouse strains as shown by high-resolution 3D MRI. Neuroimage 29, 99–105.
Cheng, H., Grant, K. A., Han, Q., Daunais, J. B., Friedman, D. P., Masutani, S., Little, W. C., and Cheng, C. (2010). Up-regulation and functional effect of cardiac β3-adrenoreceptors in alcoholic monkeys. Alcohol. Clin. Exp. Res. 34, 1171–1181.
Cuzon Carlson, V. C., Seabold, G. K., Helms, C. M., Garg, N., Odagiri, M., Rau, A. R., Daunais, J., Alvarez, V. A., Lovinger, D. M., and Grant, K. A. (2011). Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528.
D’Amato, R. J., Largent, B. L., Snowman, A. M., and Snyder, S. H. (1987). Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. J. Pharmacol. Exp. Ther. 242, 364–371.
De Vivo, M., and Maayani, S. (1986). Characterization of the 5-hydroxytryptamine1a receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J. Pharmacol. Exp. Ther. 238, 248–253.
Duncan, G. E., Knapp, D. J., Breese, G. R., Crews, F. T., and Little, K. Y. (1998). Species differences in regional patterns of 3H-8-OH-DPAT and 3H-zolpidem binding in the rat and human brain. Pharmacol. Biochem. Behav. 60, 439–448.
Fils-Aime, M. L., Eckardt, M. J., George, D. T., Brown, G. L., Mefford, I., and Linnoila, M. (1996). Early-onset alcoholics have lower cerebrospinal fluid 5-hydroxyindoleacetic acid levels than late-onset alcoholics. Arch. Gen. Psychiatry 53, 211–216.
Frankel, D., Khanna, J. M., LeBlanc, A. E., and Kalant, H. (1975). Effect of p-chlorophenylalanine on the acquisition of tolerance to ethanol and pentobarbital. Psychopharmacologia 44, 247–252.
Freeman, W. M., Salzberg, A. C., Gonzales, S. W., Grant, K. A., and Vrana, K. E. (2010). Classification of alcohol abuse by plasma protein biomarkers. Biol. Psychiatry 68, 219–222.
Freeman, W. M., VanGuilder, H. D., Guidone, E., Krystal, J. H., Grant, K. A., and Vrana, K. E. (2011). Plasma proteomic alterations in non-human primates and humans after chronic alcohol self-administration. Int. J. Neuropsychopharmacol. 14, 899–911.
Grant, B. F., Dawson, D. A., Stinson, F. S., Chou, S. P., Dufour, M. C., and Pickering, R. P. (2004). The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 74, 223–234.
Grant, K. A., Leng, X., Green, H. L., Szeliga, K. T., Rogers, L. S. M., and Gonzales, S. W. (2008). Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol. Clin. Exp. Res. 32, 1824–1838.
Griffiths, P. M., Littleton, J. M., and Ortiz, A. (1974). Effect of p-chlorophenylalanine on brain monoamines and behaviour during ethanol withdrawal in mice. Br. J. Pharmacol. 51, 307–309.
Gross-Isseroff, R., and Biegon, A. (1988). Autoradiographic analysis of [3H]imipramine binding in the human brain postmortem: effects of age and alcohol. J. Neurochem. 51, 528–534.
Haber, S. N., and Knutson, B. (2009). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26.
Halliday, G., Ellis, J., Heard, R., Caine, D., and Harper, C. (1993). Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. J. Neuropathol. Exp. Neurol. 52, 567–579.
Harwood, H. (2000). Updating Estimates of the Economic Costs of Alcohol Abuse in the United States: Estimates, Update Methods, and Data. Rockville, MD: Report prepared for the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Heinz, A., Jones, D. W., Mazzanti, C., Goldman, D., Ragan, P., Hommer, D., Linnoila, M., and Weinberger, D. R. (2000). A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol. Psychiatry 47, 643–649.
Heinz, A., Ragan, P., Jones, D. W., Hommer, D., Williams, W., Knable, M. B., Gorey, J. G., Doty, L., Geyer, C., Lee, K. S., Coppola, R., Weinberger, D. R., and Linnoila, M. (1998). Reduced central serotonin transporters in alcoholism. Am. J. Psychiatry 155, 1544–1549.
Helms, C. M., Messaoudi, I., Jeng, S., Freeman, W. M., Vrana, K. E., and Grant, K. A. (2011). A longitudinal analysis of circulating stress-related proteins and chronic ethanol self-administration in cynomolgus macaques. Alcohol. Clin. Exp. Res. doi: 10.1111/j.1530-0277.2011.01685.x. [Epub ahead of print].
Hemby, S. E., Co, C., Koves, T. R., Smith, J. E., and Dworkin, S. I. (1997). Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl.) 133, 7–16.
Ingram, D. K., and Corfman, T. P. (1980). An overview of neurobiological comparisons in mouse strains. Neurosci. Biobehav. Rev. 4, 421–435.
Insausti, R., and Amaral, D. G. (2004). “Hippocampal formation,” The Human Nervous System, eds G. Paxinos, and J. Mai (San Diego, CA: Elsevier Academic Press), 871–914.
Jacobs, E. H., Smit, A. B., de Vries, T. J., and Schoffelmeer, A. N. (2003). Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol. Sci. 24, 566–573.
Johnson, B. A. (2004). Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs 18, 1105–1118.
Johnson, B. A., Javors, M. A., Roache, J. D., Seneviratne, C., Bergeson, S. E., Ait-Daoud, N., Dawes, M. A., and Ma, J. Z. (2008). Can serotonin transporter genotype predict serotonergic function, chronicity, and severity of drinking? Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 209–216.
Kabel, D. I., and Petty, F. (1996). A placebo-controlled, double-blind study of fluoxetine in severe alcohol dependence: adjunctive pharmacotherapy during and after inpatient treatment. Alcohol. Clin. Exp. Res. 20, 780–784.
Kapasova, Z., and Szumlinski, K. K. (2008). Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol. Clin. Exp. Res. 32, 617–631.
Kenna, G. A. (2010). Medications acting on the serotonergic system for the treatment of alcohol dependent patients. Curr. Pharm. Des. 16, 2126–2135.
Kranzler, H. R., Burleson, J. A., Korner, P., Del Boca, F. K., Bohn, M. J., Brown, J., and Liebowitz, N. (1995). Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am. J. Psychiatry 152, 391–397.
Lebold, K. M., Grant, K. A., Freeman, W. M., Wiren, K. M., Miller, G. W., Kiley, C., Leonard, S. W., and Traber, M. G. (2010). Individual differences in hyperlipidemia and vitamin E status in response to chronic alcohol self-administration in cynomolgus monkeys. Alcohol. Clin. Exp. Res. 35, 474–483.
LeMarquand, D., Pihl, R. O., and Benkelfat, C. (1994). Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol. Psychiatry 36, 395–421.
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., Benjamin, J., Müller, C. R., Hamer, D. H., and Murphy, D. L. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531.
Martinez, D., Slifstein, M., Gil, R., Hwang, D.-R., Huang, Y., Perez, A., Frankle, W. G., Laruelle, M., Krystal, J., and Abi-Dargham, A. (2009). Positron emission tomography imaging of the serotonin transporter and 5-HT1A receptor in alcohol dependence. Biol. Psychiatry 65, 175–180.
McHugh, R. K., Hofmann, S. G., Asnaani, A., Sawyer, A. T., and Otto, M. W. (2010). The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug Alcohol Depend. 108, 1–6.
Mokdad, A. H., Marks, J. S., Stroup, D. F., and Gerberding, J. L. (2004). Actual causes of death in the United States, 2000. JAMA 291, 1238–1245.
Morrow, A. L., Porcu, P., Boyd, K. N., and Grant, K. A. (2006). Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin. Neurosci. 8, 463–477.
Murphy, J. M., McBride, W. J., Lumeng, L., and Li, T.-K. (1982). Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats. Pharmacol. Biochem. Behav. 16, 145–149.
Myers, R. D., and Veale, W. L. (1968). Alcohol preference in the rat: reduction following depletion of brain serotonin. Science 160, 1469–1471.
National Research Council. (1996). Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press.
Nguyen, P. V., Abel, T., Kandel, E. R., and Bourtchouladze, R. (2000). Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn. Mem. 7, 170–179.
Nielsen, K., Brask, D., Knudsen, G. M., and Aznar, S. (2006). Immunodetection of the serotonin transporter protein is a more valid marker for serotonergic fibers than serotonin. Synapse 59, 270–276.
Porcu, P., Rogers, L. S. M., Morrow, A. L., and Grant, K. A. (2006). Plasma pregnenolone levels in cynomolgus monkeys following pharmacological challenges of the hypothalamic-pituitary-adrenal axis. Pharmacol. Biochem. Behav. 84, 618–627.
Preuss, U. W., Soyka, M., Bahlmann, M., Wenzel, K., Behrens, S., de Jonge, S., Krüger, M., and Bondy, B. (2000). Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res 96, 51–61.
Pugliese, A. M., Passani, M. B., and Corradetti, R. (1998). Effect of the selective 5-HT1A receptor antagonist WAY 100635 on the inhibition of e.p.s.ps produced by 5-HT in the CA1 region of rat hippocampal slices. Br. J. Pharmacol. 124, 93–100.
Richardson, J. S., and Novakovski, D. M. (1978). Brain monoamines and free choice ethanol consumption in rats. Drug Alcohol Depend. 3, 253–264.
Sari, Y., Johnson, V. R., and Weedman, J. M. (2011). Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog. Mol. Biol. Transl. Sci. 98, 401–443.
Shibasaki, M., Inoue, M., Kurokawa, K., Ogou, S., and Ohkuma, S. (2010). Expression of serotonin transporter in mice with ethanol physical dependency. J. Pharmacol. Sci. 114, 234–237.
Small, S. A., Schobel, S. A., Buxton, R. B., Witter, M. P., and Barnes, C. A. (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 12, 585–601.
Storvik, M., Tiihonen, J., Haukijarvi, T., and Tupala, E. (2006a). Lower serotonin transporter binding in caudate in alcoholics. Synapse 59, 144–151.
Storvik, M., Tiihonen, J., Haukijarvi, T., and Tupala, E. (2006b). Nucleus accumbens serotonin transporters in alcoholics measured by whole-hemisphere autoradiography. Alcohol 40, 177–184.
Storvik, M., Tiihonen, J., Haukijarvi, T., and Tupala, E. (2007). Amygdala serotonin transporters in alcoholics measured by whole hemisphere autoradiography. Synapse 61, 629–636.
Strazielle, C., Lalonde, R., Riopel, L., Botez, M. I., and Reader, T. A. (1996). Regional distribution of the 5-HT innervation in the brain of normal and lurcher mice as revealed by [3H]citalopram quantitative autoradiography. J. Chem. Neuroanat. 10, 157–171.
Stuart, A. M., Mitchell, I. J., Slater, P., Unwin, H. L., and Crossman, A. R. (1986). A semi-quantitative atlas of 5-hydroxytryptamine-1 receptors in the primate brain. Neuroscience 18, 619–639.
Szabo, J., and Cowan, W. M. (1984). A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J. Comp. Neurol. 222, 265–300.
Szabo, Z., Owonikoko, T., Peyrot, M., Varga, J., Mathews, W. B., Ravert, H. T., Dannals, R. F., and Wand, G. (2004). Positron emission tomography imaging of the serotonin transporter in subjects with a history of alcoholism. Biol. Psychiatry 55, 766–771.
Tagliaferro, P., Vega, M. D., Evrard, S. G., Ramos, A. J., and Brusco, A. (2002). Alcohol exposure during adulthood induces neuronal and astroglial alterations in the hippocampal CA-1 Area. Ann. N. Y. Acad. Sci. 965, 334–342.
Underwood, M. D., Mann, J. J., and Arango, V. (2007). Morphometry of dorsal raphe nucleus serotonergic neurons in alcoholism. Alcohol. Clin. Exp. Res. 31, 837–845.
Vivian, J. A., Green, H. L., Young, J. E., Majerksy, L. S., Thomas, B. W., Shively, C. A., Tobin, J. R., Nader, M. A., and Grant, K. A. (2001). Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol. Clin. Exp. Res. 25, 1087–1097.
Wilson, M. A., and Molliver, M. E. (1991). The organization of serotonergic projections to cerebral cortex in primates: regional distribution of axon terminals. Neuroscience 44, 537–553.
Wu, P. H., Naranjo, C. A., and Fan, T. (1986). Chronic ethanol inhibits rat hippocampal “stimulus-secretion” coupling mechanism for 5-hydroxytryptamine in vitro. Neurochem. Res. 11, 801–812.
Keywords: 5-HT, 5-HTT, SERT, monkey, excessive drinking, alcohol self-administration, heavy drinking, hippocampus
Citation: Burnett EJ, Davenport AT, Grant KA and Friedman DP (2012) The effects of chronic ethanol self-administration on hippocampal serotonin transporter density in monkeys. Front. Psychiatry 3:38. doi: 10.3389/fpsyt.2012.00038
Received: 06 February 2012; Accepted: 10 April 2012;
Published online: 26 April 2012.
Edited by:
Giancarlo Colombo, University of Cagliari, ItalyReviewed by:
Giovanni Addolorato, Catholic University of Rome, ItalyHerminia Alicia Brusco, Instituto de Biologia Celular y Neurociencia (UBA–CONICET), Argentina
Copyright: © 2012 Burnett, Davenport, Grant and Friedman. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: D. P. Friedman, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Blvd, Winston–Salem, NC 27157, USA. e-mail: dfriedmn@wakehealth.edu
