- 1 Department of Psychiatry and Behavioral Sciences, Center for Neurobehavioral Research on Addiction, University of Texas Health Science Center at Houston, Houston, TX, USA
- 2 National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, U.S. Department of Health and Human Services, Baltimore, MD, USA
- 3 Biotie Therapies, San Francisco, CA, USA
- 4 Department of Psychiatry, University of Utah, Salt Lake City, UT, USA
- 5 Department of Diagnostic and Interventional Imaging, University of Texas Health Science Center at Houston, Houston, TX, USA
Background: Positron Emission Tomography imaging studies provide evidence of reduced dopamine function in cocaine dependent subjects in the striatum, which is correlated with prefrontal cortical glucose metabolism, particularly in the orbitofrontal cortex. However, whether enhancement of dopamine in the striatum in cocaine dependent subjects would be associated with changes in prefrontal cortical brain activation is unknown. One novel class of medications that enhance dopamine function via heteromer formation with dopamine receptors in the striatum is the selective adenosine A2A receptor antagonists. This study sought to determine the effects administration of the selective adenosine A2A receptor antagonist SYN115 on brain function in cocaine dependent subjects. Methodology/Principle Findings: Twelve cocaine dependent subjects underwent two fMRI scans (one after a dose of placebo and one after a dose of 100 mg of SYN115) while performing a working memory task with three levels of difficulty (3, 5, and 7 digits). fMRI results showed that for 7-digit working memory activation there was significantly greater activation from SYN115 compared to placebo in portions of left (L) lateral orbitofrontal cortex, L insula, and L superior and middle temporal pole. Conclusion/Significance: These findings are consistent with enhanced dopamine function in the striatum in cocaine dependent subjects via blockade of adenosine A2A receptors producing increased brain activation in the orbitofrontal cortex and other cortical regions. This suggests that at least some of the changes in brain activation in prefrontal cortical regions in cocaine dependent subjects may be related to altered striatal dopamine function, and that enhancement of dopamine function via adenosine A2A receptor blockade could be explored further for amelioration of neurobehavioral deficits associated with chronic cocaine use.
Introduction
Cocaine dependence is a clinical disorder believed to be heavily influenced by the neurotransmitter dopamine. Cocaine is an inhibitor of dopamine transport, with acute effects of increasing extracellular concentrations of dopamine (Bradberry et al., 2000). In human cocaine users there is evidence that chronic effects of cocaine result in dopamine depletion or a hypo-dopaminergic state (Parsons et al., 1991). PET studies of cocaine dependent subjects have shown reduced dopamine D2 receptor function (Volkow et al., 2004). Other studies of cocaine dependent subjects have shown reduced dopamine release in the striatum in response to methylphenidate (Volkow et al., 1997), and reduced amphetamine induced dopamine release in the striatum (Martinez et al., 2007), both of which are lower in patients who do not respond to psychosocial treatment (Martinez et al., 2011). It has been postulated that the reduction in the number of striatal dopamine D2 receptors combined with a reduction in dopamine cell activity in cocaine abusers would lead to a reduced sensitivity to natural rewards, predisposing cocaine dependent individuals to drug seeking as a means of temporarily providing stimulation of this system (Volkow et al., 2003). PET studies have indicated a significant correlation between reduction in D2 receptor binding and reduced metabolism in the orbitofrontal cortex, cingulate cortex, and dorsolateral prefrontal cortex (Volkow et al., 1993). This reduction in prefrontal cortical activity may also be important for cocaine addiction, as these brain regions are involved in working memory, decision making, and inhibitory control, which are impaired in cocaine dependent subjects (Moeller et al., 2002; Fernandez-Serrano et al., 2010; Kjome et al., 2010). Dopamine is known to be a key neurotransmitter in frontal–striatal circuits (Fineberg et al., 2010) and frontal–striatal connectivity has been shown to be reduced in cocaine dependent subjects (Hanlon et al., 2011). It is unknown whether enhancing dopamine function (directly or indirectly) in the striatum in cocaine dependent subjects would affect prefrontal cortical brain function and the processes subserved by the prefrontal cortex, including working memory and inhibitory control.
Adenosine A2A receptors in the brain are localized mainly in the striatum (both dorsal and ventral; Schiffmann et al., 2007), where they have been shown to form functional units with dopamine receptors known as heteromers (Zoli et al., 1993; Ferre et al., 1997). Through heteromer formation, A2A receptor activation blocks D2 receptor-mediated decrease in excitability of the striato-pallidal neuron, while A2A receptor antagonists produce the opposite effect (Ferre et al., 1993; Azdad et al., 2009). In animal models of Parkinson’s disease, selective A2A antagonists alone or in combination with dopamine agonists show antiparkinsonian activity (Kanda et al., 2000). This mechanism is the basis for the use of selective A2A antagonists to enhance the efficacy of dopamine precursors or agonists in Parkinson’s disease (Ferre et al., 2001). Accordingly, clinical trials of the selective A2A antagonist istradefylline have provided some evidence that A2A antagonists may be helpful in the treatment of Parkinson’s disease (Hauser et al., 2008); however, not all studies with istradefylline have been positive (Fernandez et al., 2010). Because adenosine A2A receptors are localized primarily in the striatum, and adenosine A2A antagonists have been shown to enhance dopamine function, selective adenosine A2A antagonists provide a unique tool to examine the effect of enhancement of striatal dopamine function on prefrontal cortical brain function in cocaine dependence.
Materials and Methods
Objectives
The objective of this study was to determine the effect of an acute dose of a selective adenosine A2A antagonist (SYN115) on brain activation during a working memory task in cocaine dependent subjects. The hypothesis of the study was that SYN115 would increase brain activation in prefrontal cortical brain regions previously associated with reduced dopamine function in cocaine dependent subjects.
Working memory was chosen for the task as it is well documented that dopamine plays a key role in working memory (Goldman-Rakic, 1996), and previous baseline studies of BOLD fMRI during working memory found significantly lower BOLD activation in cocaine dependent subjects compared to non-drug using controls (Tomasi et al., 2007; Moeller et al., 2010; Bustamante et al., 2011).
Participants
Twenty-three non-treatment seeking cocaine dependent subjects were recruited using advertisements in newspapers. After providing written informed consent, subjects underwent screening with a physical examination, complete blood count, urinalysis, urine pregnancy test (females), serum chemistries, and HIV test. All subjects underwent a psychiatric interview using the Structured Clinical Interview for Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition (DSM-IV; First et al., 2002). Inclusion criteria were: age 18–50 years, meeting DSM-IV criteria for current cocaine dependence and not treatment seeking. Exclusion criteria were current psychiatric disorder (other than cocaine and cannabis dependence) or past psychiatric disorder (other than substance abuse or dependence or substance-induced mood disorder). Medical exclusion criteria were any clinically significant medical disorder or a disorder requiring medication that could affect the central nervous system. Subjects were excluded if they had a positive breath alcohol screen or a positive urine drug screen other than cocaine or marijuana on the day of the scan. Subjects were also excluded if they were pregnant, had anemia, claustrophobia, or had any history of metal fragments in eyes or other soft tissue, or had any clinically significant abnormalities on structural scans as read by a board certified radiologist (co-author L. A. Kramer). All subjects who had positive urine drug screen for cocaine on the day of the scan were screened by a psychiatrist for symptoms of cocaine intoxication. No subjects had DSM-IV (American Psychiatric Association, 2000) symptoms of cocaine intoxication. All subjects had an EKG on the day of the scan prior to the first dose of medication, and a repeat EKG after completion of the scanning. Subjects were admitted overnight to the Clinical Research Unit of the Center for Clinical and Translational Sciences of the University of Texas Health Science Center at Houston for observation after the scan. Vital signs and repeat EKGs were performed during the observation period until discharge at 4:30 PM the following day.
Description of Procedures or Investigations Undertaken
Subjects and staff performing rating scales were blinded to medication received. Subjects received 5 placebo capsules 30 min before the first fMRI session and 5 (20 mg) capsules of SYN115 180 min before the second fMRI session. SYN115 [4-Hydroxy-4-methyl-piperidine-1-carboxylic acid-(4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl)-amide] (chemical structure in Supplementary Material) is a selective adenosine A2A receptor antagonist with 110- to 260-fold selectivity for the human A2A receptor compared to other adenosine receptors, and 1900-fold selectivity for the A2A receptor compared to 67 other receptors, neurotransmitter transporters and ion channels (Synosia-Therapeutics, 2008). From studies in healthy controls, the half-life of a single dose of 100 mg SYN115 was 15.9 h, with time to peak blood levels of 4.1 h (Synosia-Therapeutics, 2008). SYN115 was administered under IND# 102990 (Moeller).
Scanning Sessions
All subjects underwent two scanning sessions on a Philips 3 T Intera system with an eight-channel receive head coil (Philips Medical Systems, Best, Netherlands). The Eloquence upgrade to the Integrated Functional Imaging System-Stand Alone system (Invivo Corporation, Orlando, FL, USA) was used for stimulus presentation and recording of performance. Spin-echo echo planar imaging (EPI), rather than gradient-echo EPI, was used for fMRI to avoid signal losses caused by through-slice dephasing in regions that are affected by strong magnetic susceptibility gradients at 3 T magnetic field strength (Kruger et al., 2001; Norris et al., 2002; Wang et al., 2004). The regions affected by strong susceptibility gradients near air-tissue interfaces include (but not limited to) medial orbitofrontal cortex, which is a region that was hypothesized to be activated in the current study. In addition to its advantage of eliminating signal loss, spin-echo EPI has potentially more specific spatial localization than gradient-echo EPI (Thulborn et al., 1997; Norris et al., 2002) at 3 T magnetic field strength. Our published fMRI studies (Moeller et al., 2010; Ma et al., 2011) and other cognitive fMRI studies using spin-echo EPI at 3 T (Norris et al., 2002), have successfully obtained significant activation in medial orbitofrontal cortex in addition to elsewhere throughout the cortex in predicted areas, using similar numbers of subjects as in the current study. For the fMRI series, images were acquired in the transverse plane using single shot spin-echo EPI with SENSE factor = 2.0, repetition time = 2200 ms, echo time = 75 ms, flip angle = 90°, number of slices = 22, field-of-view = 240 mm × 240 mm, in-plane resolution = 3.75 mm × 3.75 mm, slice thickness = 3.75 mm, gap between slices = 1.25 mm, repetitions = 294, 10 dummy acquisitions, run duration = 10 min 47 s. Each subject had 2 runs in each session, separated by 1 min rest. A high-resolution T1-weighted 3D-MPRAGE scan (0.938 mm × 0.938 mm × 1 mm) was acquired for co-registration with the fMRI scans, and FLAIR and T2-weighted scans were also acquired that were read by a radiologist (co-author L. A. Kramer) on all subjects to rule out incidental brain abnormalities.
FMRI Behavioral Protocol
The immediate memory task/delayed memory task (IMT/DMT) block-design fMRI protocol (Dougherty et al., 1998; Moeller et al., 2010; Ma et al., 2011) was used as the working memory test. The IMT and DMT are delayed-matching-to-sample tasks. In both IMT and DMT, each stimulus consists of a horizontal string of numbers (e.g., 42563) that is displayed in black font on white background for 0.5 s, followed by an inter-stimulus interval (blank white screen) of 0.5 s. Thus, the rate of stimulus presentation is 1 s−1. In DMT, the target and probe stimuli are separated by distracter stimuli, consisting of a string of all zeros (e.g., 00000) that is repeated three times at the same rate and duration as the target and probe stimuli. Thus, in DMT, the memory delay between the end of the target stimulus and beginning of the probe stimulus is 3.5 s. The IMT is a control condition to control for non-specific features of the experimental design. In IMT, there are no distracter stimuli, and thus the memory delay between target and probe is 0.5 s. A non-salient stimulus consisting of all ones (e.g., 11111) is presented once after each DMT trial and four times after each IMT trial. Therefore the sum of the distracter stimuli and the non-salient inter-trial stimuli is the same (four) during DMT and IMT conditions, and hence the number of trials is same (seven) for IMT blocks and DMT blocks. In both IMT and DMT, the probability of a match and the probability of a catch are both 50%. In a catch, the probe differs from the target in only one of the digits. Subjects were instructed to press a button using the right index finger only when the probe matches the target. The A′ score (Donaldson, 1992) was used as an accuracy measure. The A′ score ranges from 0.5 to 1.0, corresponding to chance to perfect discriminability, respectively. The IMT/DMT fMRI protocol is a block design. There are 12 blocks alternating between IMT and DMT within each run. The number of digits in the stimulus string can be 3, 5, or 7 digits and is held constant within each block. The digit-length conditions represent three different levels of digit-length load. All digit-length conditions are presented within each run, in counterbalanced order between runs and subjects. The duration of each block is 42.5 s; there are 10 s rest between blocks and 20 s rest at the beginning of each run. All subjects completed training in a “mock” scanner while listening to a recording of MRI sounds prior to the first MRI session.
fMRI Processing
For each voxel during an fMRI run, the AFNI software (Cox, 1996) module “3dDespike” was used to automatically replace any extreme outlier in the raw data by the mean of its two neighboring time points. Extreme outlier was defined as an MRI signal value greater than 12 standard deviations from the mean (i.e., greater than twice the average range of the time series (Tippett, 1925). The images underwent further preprocessing using Statistical Parametric Mapping (SPM5) software from the Wellcome Department of Cognitive Neurology, London, UK, implemented in Matlab 7.1 (MathWorks Inc., Sherborn, MA, USA). After slice-timing correction, the fMRI series was realigned to correct head motion. Runs with head motion greater than one voxel (3.75 mm translation) or 3.75° rotation were eliminated from the analysis. For each subject, the first run without excessive motion and that met criteria for greater than chance accuracy (i.e., with A′ score greater than 0.5) in the majority of blocks was included in the analysis. The 3D-MPRAGE image was coregistered to the mean fMRI image and then transformed to Montreal Neurological Institute (MNI; Mazziotta et al., 2001) atlas coordinates using the SPM5 Normalize module. This transformation was applied to the fMRI images to convert them to MNI coordinates, and the fMRI images were resliced to 2 mm isotropic and smoothed with a Gaussian filter of 8 mm isotropic full width at half maximum.
Ethics
All subjects provided written informed consent prior to participation in this study. This study was approved by the Committee for the Protection of Human Subjects (CPHS), which is the Institutional Review Board for the University of Texas Health Science Center at Houston.
Statistical Methods
fMRI statistical parametric mapping analysis
Statistical analysis of the fMRI data was conducted using SPM5. The fMRI time series was high-pass filtered with a cut-off period of 330 s determined by Fourier transformation of the experimental model, which showed that the experimental signal was preserved at 330 s, but that shorter cut-off periods would eliminate most of the signal from the experimental condition. The IMT and DMT blocks for each digit condition were modeled by boxcar functions convolved with the SPM5 hemodynamic response function. The parameters for each condition were estimated using the General Linear Model at each voxel without global normalization. Activation for each digit-length condition was defined as the contrast of DMT minus IMT parameter estimates for that condition (i.e., 3-digit DMT minus 3-digit DMT; 5-digit DMT minus 5-digit IMT, and 7-digit DMT minus 7-digit IMT). For each subject, a contrast image of SYN115 minus placebo activation was entered into the SPM5 second-level (Random Effects) analysis with a one-sample t-test for each digit-length activation condition. The cluster-defining threshold was voxel t = 3.0. All cluster P values that are reported for the fMRI analysis in this paper were corrected for multiple comparisons by SPM5 to control the family wise error (FWE) rate to be less than 0.05 (Friston et al., 1994). In addition, the reported p values were further corrected to account for two tails (i.e., SYN115 greater than Placebo, and SYN115 less than Placebo). Approximate anatomical labels for regions of activation were determined using the Anatomical Automatic Labeling (Tzourio-Mazoyer et al., 2002) toolbox for SPM and direct inspection of the activation clusters. The mean activation across all voxels within a cluster was computed using MarsBaR Toolbox.
Statistical analysis of behavioral and cardiovascular data
Statistical analysis of behavioral and cardiovascular data used repeated measures analysis of variance (ANOVA) after graphical inspection of the residuals indicated normal distributions. Systolic and diastolic blood pressure and heart rate were compared between pre- and post-dose for placebo and SYN115. Data collected at the time of estimated peak blood concentration (4 h) for SYN115 was used as a comparison to data collected at 2 h post placebo because 4 h post placebo data was not obtained. Behavioral performance during the scan was analyzed with the SAS 9.2 GLIMMIX procedure implementation of repeated measures ANOVA, and correlations were computed using Spearman non-parametric CORR procedure.
Results
Screening and Demographic Results
Of the 23 subjects who provided written informed consent and met initial screening criteria, 3 subjects withdrew from the study prior to receiving SYN115 due to claustrophobia in the MRI scanner, and 1 subject withdrew due to being physically uncomfortable in the scanner related to a prior hip injury. Two subjects were excluded due to excessive head motion, one subject was excluded due to artifacts in the fMRI series, three subjects were excluded due to clinically significant abnormalities on structural MRI scans, and one subject was excluded due to discovery of a previously unreported diagnosis of Bipolar Disorder. All exclusion decisions were conducted blind to fMRI results for those subjects.
Twelve cocaine dependent subjects were included in the final analysis. The mean age of the subjects who were included was 39.7 ± 5.3 years with a range of 29–49. Eleven of these subjects were male and 1 was female. All subjects were right handed. Nine of the subjects had a positive urine drug screen for cocaine, two subjects had a positive urine drug screen for both cocaine and marijuana, and one subject had a negative urine drug screen at the time of the scan. Subjects reported cocaine use for an average of 17.3 ± 6.6 years (ranging from 8 to 28 years). Last use of cocaine was 3.5 ± 5.0 days prior to the scan (ranging from 0.8 to 19 days). None of the subjects had symptoms of cocaine intoxication at the time of the scan. All subjects had zero breath alcohol levels at the time of the scan. Eight of the 12 subjects were daily cigarette smokers, smoking 8.6 ± 7.6 cigarettes per day (range 0–20). Five of these subjects were administered a nicotine patch to avoid nicotine withdrawal and did not smoke on the day of the scan. For regular cigarette smokers, the last nicotine (other than nicotine patch) prior to the scan was 4 ± 5 h prior to the scan (range 1–13). No subjects consumed caffeine on the morning of the scan.
Cardiovascular Effects
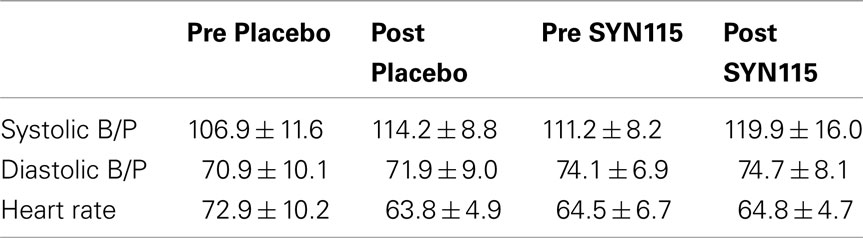
No clinically significant adverse events were reported by the subjects. Vital signs pre and post placebo and SYN115 are shown in Table 1.
Systolic blood pressure showed an effect of time after both placebo and SYN115 administration (F = 13.74, p = 0.003), and a trend effect for an overall difference between drugs (SYN115 vs. placebo; F = 4.1, p = 0.068), but no significant drug by time interaction (F = 0.077, p = 0.787). For diastolic blood pressure there was no significant effect of time (F = 0.278, p = 0.61), but there was a significant overall difference between drugs (F = 7.42, p = 0.02). There was no significant drug by time interaction (F = 0.019, p = 0.892). For heart rate, there was a significant effect of time (F = 6.38, p = 0.028), drug (F = 7.66, p = 0.018), and a drug by time interaction (F = 18.24, p = 0.001), with heart rate decreasing between baseline and post placebo and remaining stable between baseline and post SYN115.
Effects of SYN115 on IMT/DMT Performance
A mixed effects model (Proc GLIMMIX; SAS v. 9.2) evaluated IMT and DMT separately based on A′ score (a metric of signal discriminability and correct response execution) as a function of two within-subject factors: drug and digit-length. Analyses examined main effects, polynomial trends, and interactions. Observed values are displayed in Figures 1A,B.

Figure 1. (A) IMT A’ as a function of digit-length and drug condition with 95% confidence intervals. (B) DMT A’ as a function of digit-length and drug condition with 95% confidence intervals.
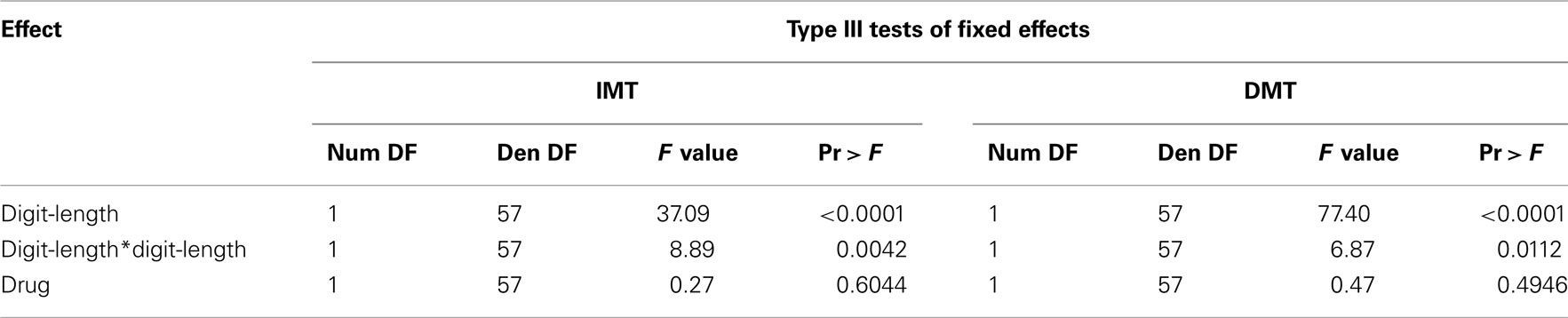
For both IMT and DMT, initial model fitting identified linear and quadratic components for digit-length but failed to find any reliable relations for drug (Table 2).
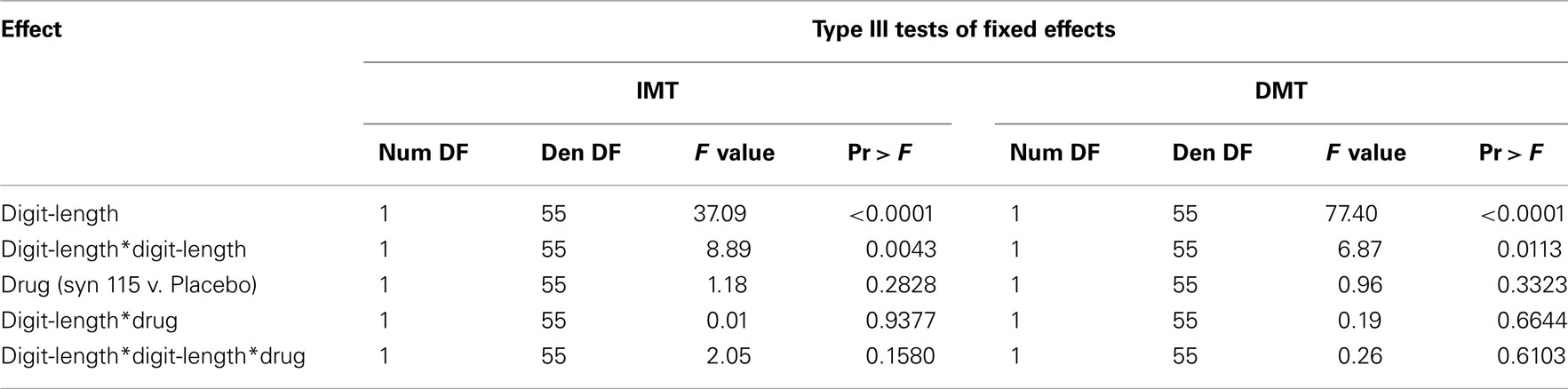
Inspection of the interactions between the linear trend for digit-length and drug, as well as for the quadratic trends for digit-length and drug, failed to increment the prediction of the model (Table 3) for either IMT or DMT. The model predicting IMT A′ score indicated that for every two additional digits in digit-length, A′ score decreased by 0.040 points.
This decrease in A′ score was further accelerated by 0.010 points for every two additional digits in digit-length (Figure 1A). Similarly, the model predicting DMT performance indicated that for every two increments in digit-length, A′ score decreased by 0.060 points. This decrease in A′ score was further accelerated by 0.010 points for every two additional digits in digit-length (Figure 1B). In summary, digit-length exerted systematic effects on performance, measured by A′, but there were no effects of drug condition or drug × digit-length interactions.
fMRI Results
SPM5 second-level (Random effects) analysis resulted in no significant clusters in which placebo produced greater activation than SYN115 for any (3, 5, or 7) digit-length condition (two-tailed FWE-corrected cluster p > 0.05). For 3-digit, and 5-digit conditions, no significant clusters were found in which SYN115 produced greater activation than placebo (two-tailed FWE-corrected cluster p > 0.05). For the 7-digit condition, there was a cluster of 362 voxels in which SYN115 produced significantly greater activation (two-tailed FWE-corrected cluster p = 0.010) compared to placebo (Table 4; Figure 2). The significant cluster was found in portions of left lateral orbitofrontal cortex, left insula, and left superior and middle temporal poles.
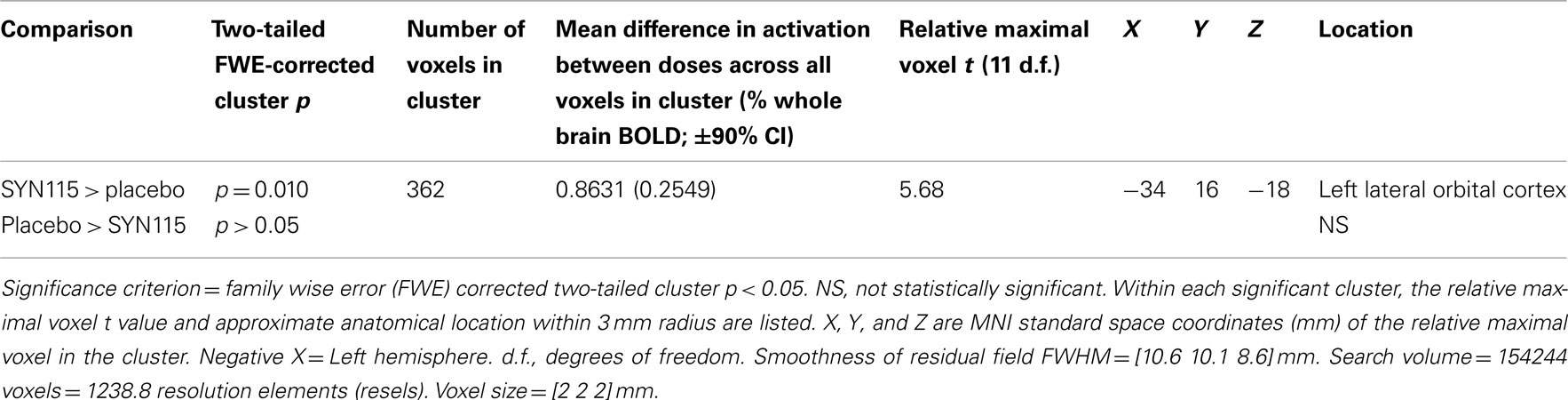
Table 4. SPM5 random effects comparison of SYN15 vs. placebo for 7-digit working memory activation within 12 cocaine dependent subjects.
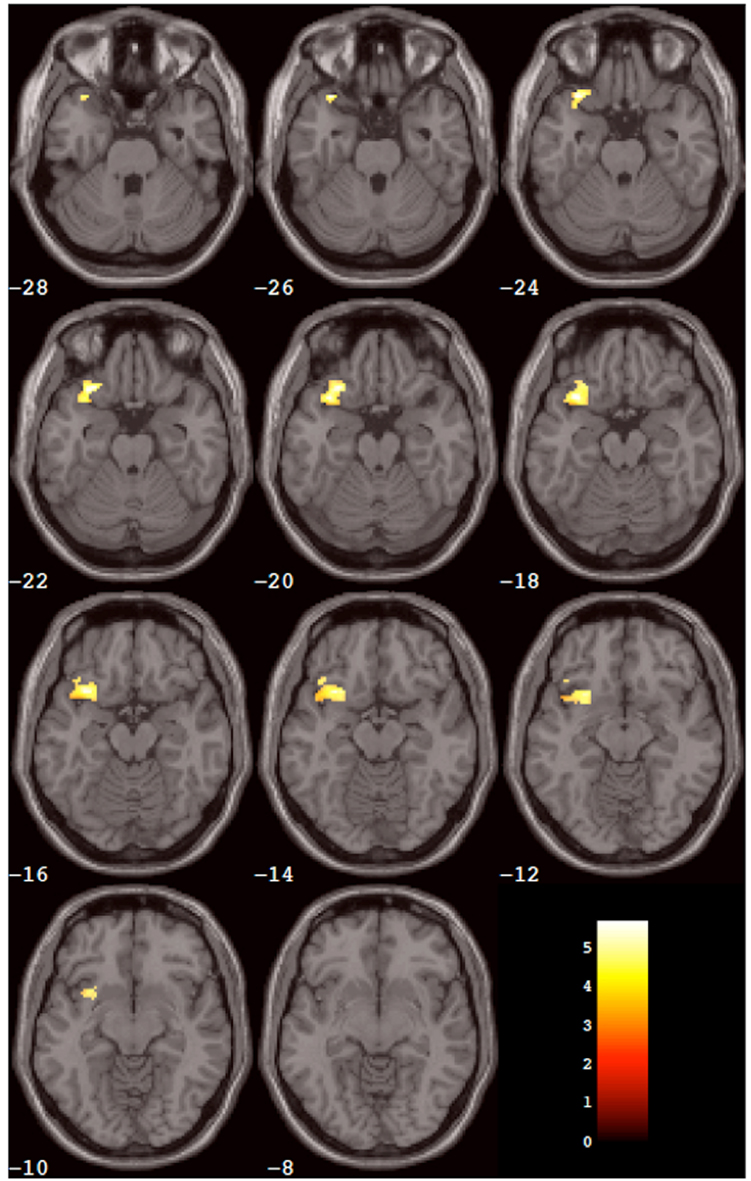
Figure 2. Results of the within-group analysis. The cluster of voxels (from Table 2) that showed significantly greater activation after SYN115 compared to placebo within the SYN group is depicted in color overlaid on a montage of slices from the standard MNI brain in gray. The slices progress from inferior slices in the left upper corner of the window to superior slices in the right lower corner of the window. The number in white below each slice is the MNI Z coordinate in mm for that slice. The left hemisphere is on the reader’s left-hand side of each slice. The color scale is in the right lower corner of the window and is in units of the t statistic.
Discussion
To our knowledge, this is the first study to examine the effects of a selective adenosine A2A receptor antagonist on brain function in cocaine dependent subjects. The main finding is that there was a significant increase in brain activation in left lateral orbitofrontal cortex, left insula, and left temporal pole after SYN115 compared to placebo. Prior imaging studies have shown evidence of connectivity between the striatum and insula (Postuma and Dagher, 2006), which is consistent with neuroanatomical studies in non-human primates (Chikama et al., 1997). Likewise, studies in non-human primates have shown reciprocal connections between the temporal pole and orbital prefrontal cortex (Kondo et al., 2003). The temporal pole, insula, and orbitofrontal cortex comprise the insulo-orbito-temporopolar component of the paralimbic brain as described by Mesulam and Mufson (1982), which is thought to be important in the integration between external stimuli and internal states (Chabardes et al., 2002).
Cocaine dependent subjects have been shown to have altered brain function in paralimbic regions associated with cocaine craving (Kilts et al., 2001; Risinger et al., 2005).
The increase in brain activation after SYN115 was not accompanied by a significant change in performance on the working memory task. This finding is not uncommon in fMRI research of cognition using a single dose of medication. In a study by Tomasi et al. (2011) a single dose of 20 mg of methylphenidate produced a significant increase in brain activation in healthy controls in parietal and prefrontal cortical regions but had no significant effect on performance of a working memory task. Likewise, acute doses of the cholinesterase inhibitor galantamine did not produce a significant behavioral effect on a facial recognition task in patients with Alzheimer’s disease but did produce a significant increase in brain activation (Goekoop et al., 2006). In another study by Ye et al. (2011) an acute dose of pramipexole did not significantly affect performance on a monetary incentive delay task but did produce a significant increase in brain activation in the nucleus accumbens. These studies support brain activation as being more sensitive to effects of single doses of medication than cognitive performance.
Significant differences were only observed on trials with 7-digit stimuli. This may be due to the relatively few errors in the 3- and 5-digit stimuli, creating a ceiling effect on medication related changes in both performance and brain activation.
Based on the work of Volkow et al. (1993) showing a significant reduction in orbitofrontal metabolism in cocaine dependent subjects, which was correlated with reduced striatal D2 receptor availability, the results of the current study are consistent with SYN115 enhancing dopamine function in the striatum leading to increased brain activation in orbitofrontal cortex and other brain regions. In the study of Volkow et al. (1993), left and right orbitofrontal cortex were combined as a region of interest showing a significant correlation with striatal D2 receptor availability. The current study found a significant increase in left orbitofrontal cortical activation. This finding could be related to the task used in the current study, as prior studies have shown a dominance of left hemisphere in verbal working memory (Binder and Urbanik, 2006; Oztekin et al., 2009) and number processing tasks (Dehaene et al., 2003). Other studies have shown that medication that enhances dopamine can affect brain activation in prefrontal regions without significant activation in the striatum. During a working memory task, methylphenidate significantly increased brain activation in prefrontal regions without a significant activation effect on the striatum in healthy controls (Tomasi et al., 2011), and methylphenidate increased brain activation in the anterior cingulate without significantly affecting striatal activation in cocaine users performing a salient cognitive task (Goldstein et al., 2010).
The effects of dopamine enhancement on activation in prefrontal brain regions without significantly altering activation in the striatum may be related to the findings of previous preclinical studies showing that energy metabolism changes are greatest at the site of increased synaptic activity, instead of the site of increased spiking activity (Buxton, 2009).
Limitations
A limitation of this study is that in order to avoid carry over effects of SYN115, all subjects received placebo on the first fMRI scan and SYN115 on the second fMRI scan. This raises the possibility that some of the differences in brain activation could be related to practice or time of day effects. However, the increased brain activation after SYN115 occurred in a relatively localized brain region, which overlaps with regions known to be correlated with reduced D2 receptor availability in cocaine abusers. This suggests that at least some significant portion of the brain activation results can be attributed to the acute effects of SYN115. Bearing in mind this limitation, the observed brain activation changes in this study suggest that enhancing dopamine function in the striatum increases brain activation in the orbitofrontal cortex in cocaine dependent subjects via effects of the A2A antagonist SYN115. The lateral orbitofrontal cortex is associated with executive control of behavior, which is critical in the initiation and maintenance of addictions (Volkow et al., 2011).
Data from clinical trials support the use of dopamine enhancing medications for cocaine dependence. Medications showing some evidence of reduction of cocaine use in clinical trials include those that inhibit dopamine reuptake or metabolism (e.g., bupropion, disulfiram; Carroll et al., 2004; Poling et al., 2006), replenish dopamine stores (e.g., levodopa; Schmitz et al., 2008), or indirectly enhance dopaminergic function via effects on dopamine release (e.g., dextroamphetamine; Grabowski et al., 2004) and methamphetamine (Mooney et al., 2009). The adenosine A2A receptor may be a target for treatment of cocaine dependence given its involvement in critical brain regions affecting dopamine function (Ferre et al., 2007). Based on the approach of dopamine enhancement as a potential treatment for cocaine dependence (Moeller et al., 2008), adenosine A2A antagonists could be investigated further as a potential treatment for cocaine dependence.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by National Institute on Drug Abuse Grants P50DA009262 and K0200403. Sergi Ferre was supported by the intramural funds of the National Institute on Drug Abuse.
References
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR Fourth Edition (Text Revision). Washington, DC: American Psychiatric Publishing, Inc.
Azdad, K., Gall, D., Woods, A. S., Ledent, C., Ferre, S., and Schiffmann, S. N. (2009). Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34, 972–986.
Binder, M., and Urbanik, A. S. (2006). Material-dependent activation in prefrontal cortex: working memory for letters and texture patterns – initial observations. Radiology 238, 256–263.
Bradberry, C. W., Barrett-Larimore, R. L., Jatlow, P., and Rubino, S. R. (2000). Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. J. Neurosci. 20, 3874–3883.
Bustamante, J. C., Barros-Loscertales, A., Ventura-Campos, N., Sanjuan, A., Llopis, J. J., Parcet, M. A., and Avila, C. (2011). Right parietal hypoactivation in a cocaine-dependent group during a verbal working memory task. Brain Res. 1375, 111–119.
Buxton, R. B. (2009). Introduction to Functional Magnetic Resonance Imaging. New York: Cambridge University Press.
Carroll, K. M., Fenton, L. R., Ball, S. A., Nich, C., Frankforter, T. L., Shi, J., and Rounsaville, B. J. (2004). Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch. Gen. Psychiatry 61, 264–272.
Chabardes, S., Kahane, P., Minotti, L., Hoffmann, D., and Benabid, A. L. (2002). Anatomy of the temporal pole region. Epileptic Disord. 4(Suppl. 1), S9–S15.
Chikama, M., Mcfarland, N. R., Amaral, D. G., and Haber, S. N. (1997). Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J. Neurosci. 17, 9686–9705.
Cox, R. W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 29, 162–173.
Dehaene, S., Piazza, M., Pinel, P., and Cohen, L. (2003). Three parietal circuits for number processing. Cogn. Neuropsychol. 20, 487–506.
Dougherty, D. M., Steinberg, J. L., Wassef, A. A., Medearis, D., Cherek, D. R., and Moeller, F. G. (1998). Immediate versus delayed visual memory task performance among schizophrenic patients and normal control subjects. Psychiatry Res. 79, 255–265.
Fernandez, H. H., Greeley, D. R., Zweig, R. M., Wojcieszek, J., Mori, A., and Sussman, N. M. (2010). Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat. Disord. 16, 16–20.
Fernandez-Serrano, M. J., Perez-Garcia, M., Schmidt Rio-Valle, J., and Verdejo-Garcia, A. (2010). Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J. Psychopharmacol. (Oxford) 24, 1317–1332.
Ferre, S., Diamond, I., Goldberg, S. R., Yao, L., Hourani, S. M., Huang, Z. L., Urade, Y., and Kitchen, I. (2007). Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog. Neurobiol. 83, 332–347.
Ferre, S., Fredholm, B. B., Morelli, M., Popoli, P., and Fuxe, K. (1997). Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 20, 482–487.
Ferre, S., O’connor, W. T., Fuxe, K., and Ungerstedt, U. (1993). The striopallidal neuron: a main locus for adenosine-dopamine interactions in the brain. J. Neurosci. 13, 5402–5406.
Ferre, S., Popoli, P., Gimenez-Llort, L., Rimondini, R., Muller, C. E., Stromberg, I., Ogren, S. O., and Fuxe, K. (2001). Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat. Disord. 7, 235–241.
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., Sahakian, B. J., Robbins, T. W., Bullmore, E. T., and Hollander, E. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35, 591–604.
First, M. B., Gibbon, M., Spitzer, R. L., and Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute.
Friston, K. J., Worsley, K. J., Frackowiak, R. S. J., Mazziotta, J. C., and Evans, A. C. (1994). Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapp. 1, 210–220.
Goekoop, R., Scheltens, P., Barkhof, F., and Rombouts, S. A. (2006). Cholinergic challenge in Alzheimer patients and mild cognitive impairment differentially affects hippocampal activation – a pharmacological fMRI study. Brain 129, 141–157.
Goldman-Rakic, P. S. (1996). Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. U.S.A. 93, 13473–13480.
Goldstein, R. Z., Woicik, P. A., Maloney, T., Tomasi, D., Alia-Klein, N., Shan, J., Honorio, J., Samaras, D., Wang, R., Telang, F., Wang, G. J., and Volkow, N. D. (2010). Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc. Natl. Acad. Sci. U.S.A. 107, 16667–16672.
Grabowski, J., Rhoades, H., Stotts, A., Cowan, K., Kopecky, C., Dougherty, A., Moeller, F. G., Hassan, S., and Schmitz, J. (2004). Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology 29, 969–981.
Hanlon, C. A., Wesley, M. J., Stapleton, J. R., Laurienti, P. J., and Porrino, L. J. (2011). The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 115, 240–243.
Hauser, R. A., Shulman, L. M., Trugman, J. M., Roberts, J. W., Mori, A., Ballerini, R., and Sussman, N. M. (2008). Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov. Disord. 23, 2177–2185.
Kanda, T., Jackson, M. J., Smith, L. A., Pearce, R. K., Nakamura, J., Kase, H., Kuwana, Y., and Jenner, P. (2000). Combined use of the adenosine A(2A) antagonist KW-6002 with L-DOPA or with selective D1 or D2 dopamine agonists increases antiparkinsonian activity but not dyskinesia in MPTP-treated monkeys. Exp. Neurol. 162, 321–327.
Kilts, C. D., Schweitzer, J. B., Quinn, C. K., Gross, R. E., Faber, T. L., Muhammad, F., Ely, T. D., Hoffman, J. M., and Drexler, K. P. (2001). Neural activity related to drug craving in cocaine addiction. Arch. Gen. Psychiatry 58, 334–341.
Kjome, K. L., Lane, S. D., Schmitz, J. M., Green, C., Ma, L., Prasla, I., Swann, A. C., and Moeller, F. G. (2010). Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Res. 178, 299–304.
Kondo, H., Saleem, K. S., and Price, J. L. (2003). Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J. Comp. Neurol. 465, 499–523.
Kruger, G., Kastrup, A., and Glover, G. H. (2001). Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn. Reson. Med. 45, 595–604.
Ma, L., Steinberg, J. L., Hasan, K. M., Narayana, P. A., Kramer, L. A., and Moeller, F. G. (2011). Working memory load modulation of parieto-frontal connections: evidence from dynamic causal modeling. Hum. Brain Mapp.. [Epub ahead of print].
Martinez, D., Carpenter, K. M., Liu, F., Slifstein, M., Broft, A., Friedman, A. C., Kumar, D., Van Heertum, R., Kleber, H. D., and Nunes, E. (2011). Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry 168, 634–641.
Martinez, D., Narendran, R., Foltin, R. W., Slifstein, M., Hwang, D. R., Broft, A., Huang, Y., Cooper, T. B., Fischman, M. W., Kleber, H. D., and Laruelle, M. (2007). Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am. J. Psychiatry 164, 622–629.
Mazziotta, J., Toga, A., Evans, A., Fox, P., Lancaster, J., Zilles, K., Woods, R., Paus, T., Simpson, G., Pike, B., Holmes, C., Collins, L., Thompson, P., Macdonald, D., Iacoboni, M., Schormann, T., Amunts, K., Palomero-Gallagher, N., Geyer, S., Parsons, L., Narr, K., Kabani, N., Le Goualher, G., Boomsma, D., Cannon, T., Kawashima, R., and Mazoyer, B. (2001). A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 1293–1322.
Mesulam, M. M., and Mufson, E. J. (1982). Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J. Comp. Neurol. 212, 1–22.
Moeller, F. G., Dougherty, D. M., Barratt, E. S., Oderinde, V., Mathias, C. W., Harper, R. A., and Swann, A. C. (2002). Increased impulsivity in cocaine dependent subjects independent of antisocial personality disorder and aggression. Drug Alcohol Depend. 68, 105–111.
Moeller, F. G., Schmitz, J. M., Herin, D., and Kjome, K. L. (2008). Use of stimulants to treat cocaine and methamphetamine abuse. Curr. Psychiatry Rep. 10, 385–391.
Moeller, F. G., Steinberg, J. L., Schmitz, J. M., Ma, L., Liu, S., Kjome, K. L., Rathnayaka, N., Kramer, L. A., and Narayana, P. A. (2010). Working memory fMRI activation in cocaine-dependent subjects: association with treatment response. Psychiatry Res. 181, 174–182.
Mooney, M. E., Herin, D. V., Schmitz, J. M., Moukaddam, N., Green, C. E., and Grabowski, J. (2009). Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 101, 34–41.
Norris, D. G., Zysset, S., Mildner, T., and Wiggins, C. J. (2002). An investigation of the value of spin-echo-based fMRI using a Stroop color-word matching task and EPI at 3 T. Neuroimage 15, 719–726.
Oztekin, I., Curtis, C. E., and Mcelree, B. (2009). The medial temporal lobe and the left inferior prefrontal cortex jointly support interference resolution in verbal working memory. J. Cogn. Neurosci. 21, 1967–1979.
Parsons, L. H., Smith, A. D., and Justice, J. B. Jr. (1991). Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse 9, 60–65.
Poling, J., Oliveto, A., Petry, N., Sofuoglu, M., Gonsai, K., Gonzalez, G., Martell, B., and Kosten, T. R. (2006). Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch. Gen. Psychiatry 63, 219–228.
Postuma, R. B., and Dagher, A. (2006). Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16, 1508–1521.
Risinger, R. C., Salmeron, B. J., Ross, T. J., Amen, S. L., Sanfilipo, M., Hoffmann, R. G., Bloom, A. S., Garavan, H., and Stein, E. A. (2005). Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage 26, 1097–1108.
Schiffmann, S. N., Fisone, G., Moresco, R., Cunha, R. A., and Ferre, S. (2007). Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 83, 277–292.
Schmitz, J. M., Mooney, M. E., Moeller, F. G., Stotts, A. L., Green, C., and Grabowski, J. (2008). Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 94, 142–150.
Thulborn, K. R., Chang, S. Y., Shen, G. X., and Voyvodic, J. T. (1997). High-resolution echo-planar fMRI of human visual cortex at 3.0 tesla. NMR Biomed. 10, 183–190.
Tippett, L. (1925). On the extreme individuals and the range of samples taken from a normal population. Biometrika 17, 23.
Tomasi, D., Goldstein, R. Z., Telang, F., Maloney, T., Alia-Klein, N., Caparelli, E. C., and Volkow, N. D. (2007). Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Res. 1171, 83–92.
Tomasi, D., Volkow, N. D., Wang, G. J., Wang, R., Telang, F., Caparelli, E. C., Wong, C., Jayne, M., and Fowler, J. S. (2011). Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage 54, 3101–3110.
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., Mazoyer, B., and Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289.
Volkow, N. D., Fowler, J. S., and Wang, G. J. (2003). The addicted human brain: insights from imaging studies. J. Clin. Invest. 111, 1444–1451.
Volkow, N. D., Fowler, J. S., Wang, G. J., Hitzemann, R., Logan, J., Schlyer, D. J., Dewey, S. L., and Wolf, A. P. (1993). Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14, 169–177.
Volkow, N. D., Fowler, J. S., Wang, G. J., and Swanson, J. M. (2004). Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry 9, 557–569.
Volkow, N. D., Wang, G. J., Fowler, J. S., Logan, J., Gatley, S. J., Hitzemann, R., Chen, A. D., Dewey, S. L., and Pappas, N. (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature 386, 830–833.
Volkow, N. D., Wang, G. J., Fowler, J. S., Tomasi, D., and Telang, F. (2011). Quantification of behavior sackler colloquium: addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. U.S.A. 108, 15037–15042.
Wang, J., Li, L., Roc, A. C., Alsop, D. C., Tang, K., Butler, N. S., Schnall, M. D., and Detre, J. A. (2004). Reduced susceptibility effects in perfusion fMRI with single-shot spin-echo EPI acquisitions at 1.5 Tesla. Magn. Reson. Imaging 22, 1–7.
Ye, Z., Hammer, A., Camara, E., and Munte, T. F. (2011). Pramipexole modulates the neural network of reward anticipation. Hum. Brain Mapp. 32, 800–811.
Keywords: cocaine, fMRI, adenosine A2A, orbitofrontal cortex, working memory
Citation: Moeller FG, Steinberg JL, Lane SD, Kjome KL, Ma L, Ferre S, Schmitz JM, Green CE, Bandak SI, Renshaw PF, Kramer LA and Narayana PA (2012) Increased orbitofrontal brain activation after administration of a selective adenosine A2A antagonist in cocaine dependent subjects. Front. Psychiatry 3:44. doi: 10.3389/fpsyt.2012.00044
Received: 03 April 2012; Accepted: 22 April 2012;
Published online: 28 May 2012.
Edited by:
Thomas Kosten, Baylor College of Medicine, USAReviewed by:
Thomas Kosten, Baylor College of Medicine, USARichard De La Garza, Baylor College of Medicine, USA
Copyright: © 2012 Moeller, Steinberg, Lane, Kjome, Ma, Ferre, Schmitz, Green, Bandak, Renshaw, Kramer and Narayana. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: F. Gerard Moeller, Department of Psychiatry and Behavioral Sciences, Behavioral and Biomedical Sciences Building, University of Texas Health Science Center at Houston, 1941 East Road, Houston, TX 77054, USA. e-mail: frederick.g.moeller@uth.tmc.edu