- 1Psychological Medicine Laboratory, Faculty of Medicine, Université Libre de Bruxelles, Brussels, Belgium
- 2Department of Psychology, Brain and Creativity Institute, University of Southern California, Los Angeles, CA, USA
According to the triadic neurocognitive model of addiction to drugs (e.g., cocaine) and non-drugs (e.g., gambling), weakened “willpower” associated with these behaviors is the product of an abnormal functioning in one or more of three key neural and cognitive systems: (1) an amygdala-striatum dependent system mediating automatic, habitual, and salient behaviors; (2) a prefrontal cortex dependent system important for self-regulation and forecasting the future consequences of a behavior; and (3) an insula dependent system for the reception of interoceptive signals and their translation into feeling states (such as urge and craving), which in turn plays a strong influential role in decision-making and impulse control processes related to uncertainty, risk, and reward. The described three-systems account for poor decision-making (i.e., prioritizing short-term consequences of a decisional option) and stimulus-driven actions, thus leading to a more elevated risk for relapse. Finally, this article elaborates on the need for “personalized” clinical model-based interventions targeting interactions between implicit processes, interoceptive signaling, and supervisory function aimed at helping individuals become less governed by immediate situations and automatic pre-potent responses, and more influenced by systems involved in the pursuit of future valued goals.
Introduction
Once an individual has lost control over drug or non-drug (e.g., gambling) use, rising negative consequences do not necessarily lead to any adjustment in their maladaptive choices and actions (1). Reasons for the transition from controlled use to addiction are numerous and include individual vulnerabilities in attention, reward, emotion, decision-making, self-regulation, pain, and stress [for a review, see Ref. (2)]. Additional reasons include the neurotoxic and neuroadaptive effects of drugs and withdrawal on the central nervous system [e.g., Ref. (3)].
Historically, the research on addiction has attracted studies that focus on only one neural system at a time. For instance, during the 1980s, the research focused on the role of the striatum and mesolimbic dopamine system, as well as the amygdala, in drug reward [e.g., see Ref. (4) for a review]. During the 1990s, the prefrontal cortex attracted most of the research on addiction, and its role in various mechanisms of decision-making and impulse control. More recently, there has been a rising interest in the role of the insular cortex in addiction [e.g., Ref. (4, 5)]. In an attempt to unify the concept of addiction that integrates both experimental and clinical perspectives, we present here the triadic neurocognitive model of addiction, which takes into account all the neural systems that have been implicated historically in the neurobiology of addiction, including “willpower,” the ability to self-control, and the capacity to choose according to long-term, rather than short-term, outcomes [see also (6)]. We argue that in addiction to drugs (e.g., cocaine) and non-drugs (e.g., gambling), the weakened “willpower” associated with most addictive behaviors is the product of an abnormal functioning in the interaction between three key neural and cognitive systems: (a) an amygdala-striatum dependent neural system, which promotes automatic cognitions and habitual actions; we have referred to this neural system as the “impulsive” system, and it becomes hyperactive in states of addiction; and (b) a prefrontal cortex dependent neural system, which is important for decision-making, forecasting the future consequences of a behavior, and inhibitory control and self-awareness; we have referred to this neural system as the “reflective” system, and it may become hypoactive in states of addiction; and (c) the insular cortex, which plays a key role in modulating the dynamics between the “impulsive” and “reflective” systems. More specifically, the (anterior) insula is important for translating bottom-up, interoceptive signals into subjective experiences (e.g., craving), which in turn potentiates the activity of the impulsive system, and/or weakens or hijacks the goal-driven cognitive resources needed for the normal operation of the reflective system. At the cognitive level, the characteristics of the impulsive and reflective neural systems mirror those from the dual-processing accounts; one is fast, automatic, and unconscious and the other is slow, deliberative, and conscious (7–9). Automatic or poorly controllable processes include what has been addressed in the literature as biased attention processing toward addiction-related cues (e.g., a bottle of beer, a slot machine, a syringe), implicit memory associations (e.g., alcohol-pleasant associations), and approach/avoidance responses to addiction-related cues (10). However, habits and impulsive behaviors can be brought under control by controlled processes related to goal-directed actions, at least to some extent. These automatic processes can be modified through the engagement of self-regulation processes. In case of addictive behaviors, a number of executive functions either cool (e.g., working memory) or hot (e.g., redirecting attention from addiction- to non-addiction-related cues, inhibiting addiction-related pre-potent responses) are viewed as compromised and unable to exert an effective control of behaviors elicited by automatic processes [e.g., Ref. (11)].
While this dual-process model may explain many aspects of complex decision-making, self-control, and “willpower” to resist the temptation of addictive stimuli, we argue that the model is not sufficient to explain all aspects of addictive behaviors, especially those related to deprivation from the reward stimulus or withdrawal, stress, anxiety, and other homeostatic disturbances. For example, while an individual with alcoholism may manage to exert self-control over his or impulse to have a drink for a period of time, the mere sight of a bottle, or experiencing a stressful situation, can throw this control completely out of balance, and the old automatic and habit systems take over. We argue that this drastic weakening of control systems brought about by changes in the internal body state of the organism play a key role in modulating the strengths of impulsive and reflective systems, and incorporating this influential role of internal states is not part of the dual-process models. For this reason, we propose a key role for a third neural system, namely the insula [e.g., see its role in nicotine dependence, (12)]. The insula is viewed as a “gate” system involved in the way a person feels at a particular point in time, which responds to homeostatic perturbations (13), and in turn modulates the interaction between these two systems, i.e., the impulsive and reflective systems (4, 5, 14). Specifically, engagement of the insula exacerbates activity of the impulsive system and hijack activity of the reflective system; thus creating a severe imbalance between the two. At the neuroanatomic level, strong afferent projections connect the insula to the ventral striatum (15). In addition, insular hyperactivity during addiction-related cue exposure may result in compromising self-control efficiency. In light of well-documented links between stressor exposure and addiction-related experiences (e.g., relapse), stressor exposure modulate cue reactivity to drug cues [for a review, see Ref. (16)] and thus may dramatically impact the balance between the impulsive and reflective systems. Besides, the imbalance found in individuals struggling with their compulsive behaviors often accompanies blunted sensitivity to non-drug rewards (17); thus making addiction-related activities highly attractive.
Finally, in this article, we will elaborate on clinical model-based interventions targeting implicit processes, interoceptive processing, and supervisory functioning aimed at helping individuals become less governed by immediate situations and automatic pre-potent responses, and more influenced by systems involved in the pursuit of future valued goals. We argue that this pathway may restore some kind of “free will,” thus making the initiation and the maintenance of a change process of addictive behaviors more likely and more effective.
The Impulsive System, and Its Implicit Cognitive Determinants of Addictive Behaviors
Over the course of the development of an addiction, related behaviors become progressively controlled by addiction-associated information that have acquired, through Pavlovian and instrumental learning mechanisms, the property to automatically elicit substance-related (or gambling) actions (18, 19). In this section, we describe some neural and cognitive properties of what we have called “impulsive system,” and focus on some of its properties that are associated with addictive behaviors.
These fast and poorly deliberated responses triggered by competent cues present in the environment (e.g., the view of a bottle of beer by a drinker) depend on basal ganglia neural circuits (20). Critically, the amygdala-striatal (dopamine dependent) neural system is a key structure for the incentive motivational effects of a variety of non-natural rewards (e.g., psychoactive drugs) and natural rewards (e.g., food) (21). This stimulus bound rigid and automatic-habit decision-making system requires little mental elaboration (22), and it is modified by drugs, alcohol, and gambling through changes in the phasic characteristics of dopamine activity in reward signaling, and the tonic function of dopamine levels in permitting and facilitating a large variety of motor and cognitive functions (23, 24). Increased mesolimbic dopamine activity, stimulated by drugs of abuse, reinforces the repetition of behaviors, influencing learning and attentional processes, and the strengthening of associations of reinforcing effects (10, 25, 26). Through intensive practice and operant conditioning processes, instrumental performance (e.g., a rat pressing a lever to receive cocaine) could easily switch from goal-directed action-outcome associations, which requires a representation of the outcome as a goal, to actions more independent of the current value of the goal (27); thus characterizing a state of compulsivity (28). The transition between goal-directed and compulsive behaviors was associated with specific aspects of synaptic structural plasticity in both dorsal (29–31) and ventral striatal regions (31). This transition process is accelerated by the sensitization of dopaminergic systems (32). At the cognitive processing level, the impulsive system processes information automatically/implicitly, which describes a number of properties such as goal independence, absence of intentionality, uncontrollability, lack of awareness of one or more aspects of the process (e.g., stimuli, origins, attributes, behavioral effects), efficiency (effectiveness under processing load), and operation even under time constraints (33).
A vast literature has investigated the relationship between automatic/implicit processes and behaviors and reached the conclusion that those processes could modify action in the absence of any decision (34). For instance, in experiments investigating priming effects, participants who were primed with words such as “wrinkles” or “gray” walked more slowly when leaving the experiment (35). In this case, one could expect that conscious deliberation be not impacted by this experimental condition; thus highlighting a direct implicit cognition/action pathway. In other cases, viewing a beer advertisement in the television may result in making the decision to go to the fridge and grasping a beer, it seems reasonable to posit that some automatic processes resulted in biasing the decision possibly through the signaling of the immediate rewarding aspects of the experience (e.g., pleasant taste of the beer), sometimes at the detriment of differed consequences (e.g., fatigue). In line with this idea, is the experiment showing that altering the effort required to reach foods by manipulating their proximity can increase the selection of easier-to-reach food options (36). With respect to drug use and gambling, addiction-related cues are progressively flagged as salient and grab the addicts’ attention (i.e., attentional bias) (37). The attentional processing of such stimuli trigger implicit “wanting” and generate automatic approach behaviors (10).
Addicts exhibit a modified attentional processing for addiction-relevant stimuli (25). These attentional biases occur at both early and later levels of attentional processing, that is, at both automatic, and at more conscious and deliberate, levels (37). Indeed, early level of attentional processing (e.g., attentional encoding; initial orientation of attention) depends primarily on automatic-habit processes (38, 39), whereas later attentional processes (i.e., maintenance of attention; disengagement of attention) involve higher level of conscious control processes (39). Attentional bias at the level of attentional encoding has been studied with the attentional blink (AB) paradigm (40). The AB phenomenon refers to the observation that the second of two-masked targets (T1 and T2), which appears in a rapid serial visual presentation (RSVP) stream of distracters, is usually poorly identified when it is presented within a short time interval after T1 [e.g., within a several 100 ms; (40)]. Using this task, recent studies [e.g., Ref. (41, 42)] have shown that, in addicts, addiction-related words were less affected by interference of other RSVP items within a short time interval after T1, as compared with neutral words. These results suggest that individuals suffering from addiction are more likely to identify addiction-related words than neutral words under conditions of limited attentional resources, which is consistent with an enhanced attentional bias for addiction cues at the encoding level. Attentional bias at the engagement and the maintenance/disengagement levels of attention has been highlighted through the monitoring of eye-movements during performance of attentional tasks [for a review, see Ref. (37)]. For instance, using a change detection task, Brevers et al. (43) showed that, compared with their controls, problem gamblers were faster to detect gambling-related than neutral-related stimulus change. In addition, these investigators observed that problem gamblers directed their first eye-movements (i.e., attentional engagement) more frequently toward gambling-related than toward neutral stimuli, exhibited more gaze fixation counts (i.e., attentional maintenance) on gambling stimuli and spent more time looking (i.e., attentional maintenance) at gambling-related than neutral stimuli.
Implicit addiction-related association refers to spontaneous associations between addiction-related cues and affective, arousal, motivational representation in memory. This association tends to reveal automatic, impulsive cognitive-motivational mental processes, which are sparsely independent of, or not available to, conscious awareness (10). In other words, implicit association could be defined as an introspectively unidentified (or inaccurately identified) trace of past experience that mediate feeling, thought, or action (44). The Implicit Association Task [IAT; Ref. (45)] is a paradigm commonly used to assess implicit association. In a typical IAT, stimuli belonging to one of four possible categories are presented one by one on a computer screen. On each trial, participants categorize as fast as they can the presented stimulus by pressing one of two keys. The assumption underlying the IAT effect is that two concepts that are more closely related in memory should facilitate responses (faster responses and less errors) when they share the same response key, and impair responses when they do not share the same response key. Hence, they should be faster in the first categorization than in the second. For instance, when classifying names of alcohol or soft drinks (i.e., target stimuli), and positive or negative words (i.e., attribute stimuli), people who hold stronger alcohol-positive affect associations than soft drink positive affect associations should be faster when alcohol and positive words are assigned to one key, and soft drinks and negative words to the second key, when compared to the condition in which soft drinks and positive words are assigned to one key, and alcohol and negative words to the other. Using this task, researches have shown that appetitive associations (i.e., positive, arousal) predict alcohol and substance use (46). For instance, Thush and Wiers (47) observed that positive affect and arousal associations predict the level of drinking in adolescents for the following year.
In addition to attentional bias and implicit association, prominent addiction models posit that automatically activated approach/avoidance tendencies play a critical role in addition. Indeed, the repetition of addiction-related behaviors elicits automatic approach tendencies toward addiction-related cues (10). Several paradigms have been developed to assess this action tendency. Consider, for example, the stimulus-response compatibility task (48), in which participants are instructed, in one block, to move a manikin (i.e., a little man) as fast as they can toward substance-related pictures, and away from neutral pictures (approach substance block). In another block, the task is to move the manikin away from the substance-related pictures and toward neutral pictures (avoid substance block). Through this task, substance abusers (alcohol, cigarettes, marijuana) exhibited relatively fast approach movements toward substance cues (48–50). Hence, by highlighting that addiction-related cues automatically trigger a corresponding impulse, which consists of a behavioral schema to approach it (10), these studies provide strong evidence for the presence of automatic incentive habits in gambling addiction. However, it is important to realize that these tendencies are likely to be highly malleable and context-dependent. For instance, unlike controls, abstaining alcohol-dependent patients revealed a relative avoidance bias rather than relative approach bias and moreover, relapse rates were found to increase as the relative tendency to avoid alcohol increased (51).
Altogether, these cognitive aspects are consistent with the incentive-sensitization theory (19, 52), which suggests that, through repetition of rewarding appetitive experiences, the degree to which addiction-related objects are “wanted,” desired, and their effect anticipated, increases disproportionately when compared with the degree to which they are “liked” (i.e., the actual mood change), and this dissociation may progressively increase with the development of addiction. In addition to the increased salience attributed to cues that predict drug reward, addiction is characterized by relatively reduced sensitivity to natural rewards (17, 53), as seen for instance in cocaine abusers for whom non-addiction rewards generate below normal mesocorticolimbic neural activations as compared to monetary reward (54). Also, while occasional smokers show greater neural responses to money than cigarettes, dependent smokers react equally across conditions (55). We should note here that money reward may be exchanged for smoking or drugs or any other addictive stimulus. For this reason monetary reward seems to exert strong neural responses. Most importantly, addictive stimuli acquire a strong incentive motivational salience (e.g., Robinson and Berridge), which renders it highly more preferable to any other type of reward (except money). This increased reward salience is also evident in animal paradigms. While novelty serves as a strong reward stimulus in animals, those animals that become exposed to drugs begin to prefer the choice of the drug instead of the novelty (56), and even instead of maternal behavior (57), or food (58).
The Reflective System and Its Supervisory Functions in Addiction
While the habit (or impulsive) system, which is key to generating at least the “wanting” component to seek reward, may explain one important aspect of the behaviors associated with approach behaviors, it is clear that it does not explain how one does control his or her behavior. This function refers to the action of what we called the “reflective system,” which is necessary to control these more basic impulses, particularly when the behavior may not be optimal or advantageous, or is perceived as the incorrect thing to do; and it disallows a more flexible pursuit of long-term goals (17). In this section, we review some evidence showing that addicted individuals have abnormalities in this system and have difficulties in exercising willpower to resist the habits and temptations associated with their compulsive actions.
Self-control can be estimated through the capacity to inhibit pre-potent motor responses. This process refers to the ability to deliberately suppress dominant, automatic responses that are no longer relevant or required. This type of inhibitory control can be indexed by the stop-signal [e.g., Ref. (59)] and go/no-go tasks [e.g., Ref. (60)], which require the subject to withhold simple motor responses when a stop-signal occurs (stop-signal task), or when a no-go stimulus is presented (go/no-go task). Impaired response inhibition performance has been previously highlighted in addicts by using the stop-signal task (i.e., prolonged latency of motor response inhibition), and the go/no-go paradigm (i.e., more errors of commission: subject had to withhold a response but pressed a button instead) [for a review of response inhibition impairment in gambling, opiate, and alcohol addiction, see respectively (17, 61, 62)]. Moreover, results from several brain imaging studies showed that, in drug addicts (63, 64), impaired performance on response inhibition tasks is associated with a hypoactivation in the anterior cingulate cortex, implicated in mechanisms of error detection, and conflict monitoring. Several studies also reported intact response inhibition in addicts [e.g., Ref. (65, 66)]. Nevertheless, this absence of behavioral difference is not necessarily indicative of intact response inhibition processes. For instance, van Holst et al. (67) observed increased dorsolateral and anterior cingulate cortex activity in pathological gamblers who exhibited a similar behavioral performance as their controls on motor response inhibition. In other words, a more effortful strategy (i.e., higher brain activation) was undertaken in pathological gamblers to perform at a similar level as their controls (67). Importantly, disruption in response inhibition processes could lead to abnormal salience attribution directed at addiction-related cues in addicts. In other words, a dysfunction of the inhibitory control system could further exacerbate automatic impulsive processes. For instance, implicit memory associations are a strong predictor of alcohol use in young adults with poor ability of motor response inhibition (68). Moreover, several studies showed that response inhibition deficits affecting drug and gambling addicted persons (69) are thought to accelerate the course of addiction by compromising abstinence from cocaine (70), gambling (71), nicotine (72), alcohol (73), aggravating problem gambling (74), and by increasing attrition from treatment (75).
The action of the “reflective” system is also crucial for choosing according to long-term, rather than short-term, outcomes (6). This function is mediated by paralimbic, medial orbitofrontal, and ventromedial prefrontal structures involved in triggering somatic states from memories, knowledge, and cognition, which allow activation of numerous affective/emotional (somatic) responses that conflict with each other; the end result is that an overall positive or negative signal emerges (76). The impact of somatic responses in addiction has been initially demonstrated in clinical research with patient populations with damage in frontal lobe regions as well as imaging studies that delineate the likely neural basis of each of these functions (76–78). After damage to the ventromedial region of the prefrontal cortex, previously well-adapted individuals become unable to observe social conventions and decide advantageously on personal matters (79). The nature of these deficits revealed that the vmPFC region serves as a link between (a) a certain category of event based on memory records in high order association cortices to (b) effector structures that produce an emotional response (77). Damage to the systems that impact emotion and/or memory compromise the ability to make advantageous decisions (79). The Iowa Gambling Task [IGT; (80)], which was initially developed to investigate the decision-making defects of neurological patients in real-life has been shown to tap into aspects of decision-making that are influenced by affect and emotion (77). This task has been regarded as the most widely used and ecologically valid measure of decision making in this clinical population. One of the reasons for this ecological validity is that performing advantageously on this task requires, as in real-life, dealing with uncertainty in a context of punishment and reward, with some choices being advantageous in the short-term (high reward), but disadvantageous in the long run (higher punishment); other choices are less attractive in the short-term (low reward), but advantageous in the long run (lower punishment). Hence, the key feature of this task is that participants have to forgo short-term benefits for long-term benefits, a process that is presumably severely hampered in drug and gambling addicts (1). Accordingly, performance on the IGT has been shown to be a sensitive measure of impaired decision-making in a diversity of neurological and psychiatric conditions (6). For instance, patients with frontal lesions [e.g., Ref. (80–82)], individuals suffering from substance [e.g., Ref. (83–87)] or non-substance addiction [e.g., Ref. (88, 89)] have demonstrated a preference for short-term gains despite larger net losses while performing the IGT.
In sum, disrupted function of the “reflective” prefrontal cortex could lead to impaired response inhibition and abnormal salience attribution toward high-short-term rewards in addictive individuals. This provides one explanation of addicts’ inability to resist substance (or addiction-related behavior) seeking and taking at the expense of non-addiction-related activities.
Neural Systems That Intensify Motivation and Weaken Control of Behavior: The Insula
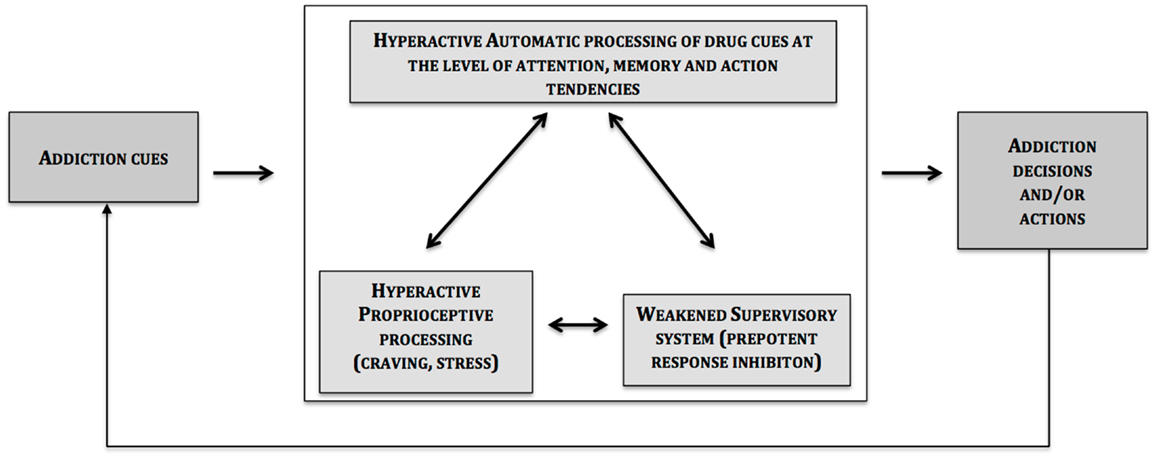
The central message of this article is that the two systems described so far are influenced by interoceptive processes (e.g., drug cravings) (see Figure 1).
At the heart of this phenomenon is the insula in which activation in craving paradigms is a reliable observation (5, 90). However, before discussing further about the specific role of the insula, one should keep in mind that the insula does not work in isolation in the brain, and that in the case of a craving for a certain drug, other regions are activated in cue-reactivity and drug-craving paradigms [for a review, see, Ref. (90)]. These data are in line with the proposal of the present article, which suggests that the insula is connected to brain-related impulsive (amygdalar striatal complex) and reflective (prefrontal cortex) systems; and it modulates their impact on decision and action (4).
The insular cortex has recently emerged as a key neural structure that plays a key role in the formation of interoceptive representation, which is crucial for subjective emotional feelings (5, 13, 91). Interoception refers to the practice of receiving, processing and integrating body-relevant signals with external stimuli of affect on-going motivated behavior (13). According to the notion of embodiment, high-level cognitive and affective processing is grounded in the organism’s sensory and motor experiences (92). Moreover, it has recently been argued that the insular cortex may contribute to the onset and maintenance of addiction by translating interoceptive signals into what one subjectively experiences as a feeling of desire, anticipation, or urge leading to approach behaviors (5, 14, 93). Indeed, highly embodied experience may overwhelm the cognitive control system by providing a highly emotional experience. But also, low levels of embodied experience may not engage the cognitive control system to adjust on-going behavior, which may result, for instance, in high risk-taking (14).
Imaging studies show activity within the insula correlating with the subjects’ rating of urge for cigarettes, cocaine, alcohol, and heroin (5, 13, 93). In addition, insula activation was closely associated with higher levels of nicotine dependence (94) and both attentional bias and the risk of relapse (95). Indeed, smokers at risk for relapse showed more insula activation while processing smoking-related words (95). In individuals with dependence to cocaine, hyperactivity to drug-specific stimuli contrasts with abnormally low activity when self-regulation processes are engaged [e.g., inhibition; (96)].
Other evidence supporting the important role of the insula in addiction processes arise from human brain lesion studies. Strokes that damage the insular cortex tend to literally wipe out the urge to smoke in some individuals previously addicted to cigarette smoking (12). In this study, smokers with brain damage involving the insula were more likely to quit smoking easily and immediately, without relapse and without a persistent urge to smoke (12). These results support a novel conceptualization of one of the mechanisms by which the insula participates in maintaining addiction.
The insular cortex (and most likely the anterior insula) responds to interoceptive signals (due to homeostatic imbalance, deprivation state, stress, sleep deprivation, etc.). Besides the translation of these interoceptive signals into what may become subjectively experienced as a feeling of “urge” or “craving,” we hypothesize that the insular cortex activity increases the drive and motivation to smoke (or take drugs or to gamble) (a) by sensitizing or exacerbating the activity of the habit/impulsive system; and (b) by subverting attention, reasoning, planning, and decision-making processes to formulate plans for action to seek and procure cigarettes or drugs (97). Put differently, these interoceptive representations have the capacity to “hijack” the cognitive resources necessary for exerting inhibitory control to resist the temptation to smoke or use drugs by disabling (or “hijacking”) activity of the prefrontal (control/reflective) system.
Stress could be a critical candidate for understanding the impact of abnormal level of proprioception on impulsive and reflective systems (98, 99). Indeed, whereas at low to moderate levels of stress, the reflective system’s activity may be enhanced, abnormally high levels of stress tend to attenuate activity within the reflective system (99). This may be applied to alcohol use by successfully inducing drug craving in the laboratory through the generation of stress (e.g., 5-min individualized guided imagery exposure of each participants’ own recent stressful scenarios) (100). Research has emphasized the existing association between exposure to stress, abnormal persistent level of changes in the body state (heart rate, salivary cortisol levels), persistent craving, and use of compulsive individuals’ preferred drug [for a review, see Ref. (101)]. Interestingly, alcoholics’ prefrontal and striatal-limbic regions (including the insula) are hyper-responsive to neutral-relaxing condition, making activation pattern between neutral relaxed and stressful cues indistinguishable (102). This result suggests a dysregulated response to stress that induces craving and increases the risk of relapse in alcoholics (101).
The Impact of Poor Metacognition on Addictive Behaviors
Recent theoretical accounts (17, 103) advance that a dysfunction of the interoceptive system may also hamper self-awareness, which could take the form of failure to recognize an illness (i.e., lack of insight). Indeed, perceived need for treatment concerns only a minority of individuals suffering from addiction (104), which might reflect dysfunction in cognitive processes and the neural circuits underlying self-awareness (103). The underestimation of the addiction severity might drive these individuals’ excessive drug use, where control of use becomes exceedingly deregulated.
Impaired insight ability could be estimated through the evaluation of metacognition capacity, which refers to our ability to discriminate correct from incorrect performance. The presence of impaired metacognitive capacities in substance addiction has received support from previously identified dissociations between subjective and objective markers of behavior in addicted individuals (54, 105–111). For example, Brevers et al. (105) showed that pathological gamblers were impaired in their capacity to evaluate accurately the quality of their decisions during an artificial grammar-learning paradigm, in which the quality of choice remains uncertain throughout the task. Specifically, after each trial of this task, participants had to indicate how confident they were in their grammaticality judgments. Results showed that, by contrast with their controls, there was no correlation between pathological gamblers’ grammaticality judgments and their level of confidence, which suggests a disconnection between performance and confidence in pathological gamblers. Additional evidences for lowered metacognitive capacities in addicts comes from a study by Hester et al. (109) who observed diminished commission error awareness during a go/no-go response inhibition task in chronic cannabis users. In addition, these authors observed that the insensitivity of chronic cannabis users to detect errors was associated with the absence of right insula activity during unaware errors (when compared with aware error activity). Furthermore, lower levels of insula activity were correlated with higher levels of recent cannabis use, and higher levels of cannabis craving were associated with poor error-awareness rates. These results are in line with hypotheses suggesting that insula dysfunction may contribute to impaired interoceptive awareness and heightened experience of drug-related urges, potentially at the expense of other interoceptive signals necessary for advantageous decision-making and the ability to learn from errors (13, 112).
Other studies have also observed dissociations between subjective and objective markers of performance when they were separated in time, i.e., when subjects were asked to evaluate their overall performance after completing the experimental task in its entirety (54, 108, 111). For instance, Goldstein et al. (54, 108) observed that healthy control participants showed incentive-related performance enhancements during a sustained attention task, and that these improvements in performance correlated with their self-reports of task engagement. These same correlations did not reach significance in the cocaine subjects. Moreover, these investigators observed that healthy control subjects, but not cocaine subjects, showed significant correlations between monetary-driven P300 amplitudes and their respective behavioral performance responses (108). More recently, Moeller et al. (111) showed that, in cocaine addicted individuals, there was a poor correspondence between the most selected picture category on a probabilistic choice task (that assessed objective preference for viewing four pleasant, unpleasant, neutral, and cocaine pictures) and self-reported most chosen picture category (ascertained at the conclusion of the probabilistic task).
Finally, additional studies have also focused on the impact of diminished metacognitive capacities on addicts’ forthcoming decisions or performances (106, 107, 110). For example, Le Berre et al. (110) observed dissociations between the predictions of future recognition performance (estimated with self-rated ability to perform adequately a following word-recognition task) and actual recognition performance in alcohol-dependent individuals. More recently, Brevers et al. (106) examined metacognitive capacities in a sample of pathological gamblers by asking participants to wager on their own decisions after each choice during their performance of the Iowa Gambling Task [i.e., IGT with post-decision wagering; Ref. (113)]. These authors observed that, unlike healthy controls, pathological gamblers tend to wager high while performing poorly on the IGT (i.e., choosing option featuring high rewards but higher losses rather that options featuring low rewards and losses). This result suggests that pathological gamblers exhibited impairments not only in their ability to correctly assess risk in situations that involve ambiguity, but also in their ability to correctly express metacognitive judgments about their own performance. That is, pathological gamblers not only perform poorly, but they also erroneously estimate that their performance is much better than it actually is. In chronic cigarettes smokers, Chiu et al. (107) examined whether or not fictive prediction errors (i.e., when fictive outcomes contradict with the expectation) during a non-active gambling choice task impacted smokers’ forthcoming choice. These authors observed that the fictive prediction error was computed by chronic smokers (indexed by a corresponding signal change in the ventral striatum) but did not influence their behavioral choices (when compared with control participants). Findings from these studies (106, 107, 110) further support hypotheses advancing that a dissociation between objective and subjective markers may contribute to impaired decision-making and the ability of failure to learn from errors that underlies perseverative behavior.
This abnormal degree of dissociation found in addicted people between the “object” level and the “meta” level, raised the possibility that poor metacognition leads to poor action and decision-making monitoring and adjustment (114). However, much remains to be done in order to identify how different regions of the prefrontal cortex interact with interoceptive signals to promote accurate judgment performance and further cognitive control on decision-making, memory as well as on the sense of agency in healthy participants (115) and in addicts (17). Anatomically, the insula is a primary site for receiving interoceptive signals, but in turn the insula is connected to widespread regions of the prefrontal cortex, and hence this interoceptive-prefrontal interaction may be mediated by the insula (17, 116).
Lack of Willpower in Addicts; the Chicken or the Egg Question
Effects of Acute Drug Administration on Willpower Capacities
Evidence from cognitive and behavioral sciences has led to the idea that acute drug primes limbic incentive neurocircuitry (striatum, amygdala, insula, mesencephalon) while suppressing top-down supervisory control circuits [for a review of alcohol studies, see Ref. (117)]. Of particular interest is the altered capacity to detect and resolve errors made on a variety of cognitive tasks [for a review, see Ref. (62)] and also, when subjects are given alcohol, they tend to take more risks which parallels increased activation in the striatum to risky compared with safe choices and the neural response to notification of both wins and loses are dampened throughout NAcc, caudate, thalamus, and insula (118).
In addition, emotional processing could be dramatically altered after the ingestion of some drinks as illustrated by some recent studies showing a lack of amygdala (119) and of insula (120) recruitment by threat-indicating faces during alcohol intoxication; thus making threatening and uncertain outcomes in social context less of a moderator in inebriated participants.
Together, research on the acute effects of drugs on brain and cognitive processes is compatible with the idea that it compromises one or more of these brain systems discussed in the present paper, a disruption that may prevent the efficient exercise of willpower. However, in most of the studies, these brain abnormalities are not associated with abnormal behavioral pattern (e.g., low working memory performance) [but see Ref. (121) for abnormal both pattern of activation and behavior consecutively of alcohol ingestion], thus raising the question of the significance of these hypo-/hyper-activation found in neuroimaging studies [see (117) for a discussion of this issue]. Although not demonstrated yet, one could expect that these brain changes would be more sensitive measures than behavioral ones (122), which is likely to become abnormal on more demanding tasks.
Vulnerabilities in the Decision Process and the Risk of Addiction
Vulnerability to substance use and gambling disorders encompasses multiple dimensions of the individual’s life, ranging from biological factors to broader environmental and cultural variables [e.g., Ref. (123)]. Particularly sensitive is the period of adolescence starting with the biological changes of puberty and ending with the time at which the individual attains a stable, independent role in society. During this period decision-making is increasingly independent of adult influences as well as more vulnerable because of an enhanced taste for risk together with impulsive actions and decisions that can sometimes lead to serious consequences. This is a context that makes peers influence more powerful (for better or for worse) and novel experiences more attractive. Thus one would not be surprised to observe that adolescence is an experimentation period with a number of exciting experiences, e.g., alcohol, drugs, and gambling. Note that initiation is not a pathological process per se but sometimes it may evolve into severe disorders (i.e., compulsive behaviors) (124). At the extreme, this state of “inflexibility” exemplified by drug and non-drug addictive behaviors has been thought to reflect impaired “basic” behavioral learning processes, poor self-regulation, and impaired decision-making [e.g., Ref. (4)]. As a consequence, “willpower” may be too limited. Thus, one characteristic of adolescence is that the power of positive short-term consequences of choice and behaviors may exceed self-regulation capacities, which can be risky to health, yet might be normal within adolescent social development [Ref. (125); for a review, see (126)]. Individual motivational and regulatory responses are thought to increase the risk of alcohol and gambling misuse in adolescents. However, individual differences presented far before the onset of puberty are also important to understand the elevation of risk [e.g., low capacities of delay of gratification in children at age 4 predict cocaine/crack use in individuals vulnerable to psychosocial maladjustment, (127)]. As another example, higher levels of impulsive behavior as early as age 3 predict aggressive behavior and drug use in adolescence (128). Thus, the quality of pre-adolescents’ cognitive and affective functioning (e.g., resisting immediate temptations) is important to take into account in order to investigate the addictive risk in adolescents (129). However, during adolescence, demanding context (e.g., peer influence) and sensation seeking are rising dramatically; thus encouraging experimentation with novel behaviors, which also require “willpower” to prevent a number of deleterious consequences.
It remains difficult to know whether these abnormalities are a direct result of the toxic effects of alcohol on the brain (i.e., the consequence of use) or whether pre-existing brain conditions (antecedent to the initiation of use) are present that differentiate those who begin using at a young age form those who do not (130). The “chicken or the egg” question has been addressed in different ways. For instance, in adolescent girls, more drinking days in the past year predicted a greater reduction in performance on visuospatial memory tasks, whereas in boys, more hangover symptoms in the year before follow-up testing predicted worsening of sustained attention (131). These results suggest that due to neurotoxic effects, alcohol-use disorders during adolescence (including associated withdrawal symptoms) may interfere with normal brain maturation. However, despite increased frequency of use over a 4-year follow-up period in a community-based sample or early adolescents (132), working memory among participant remained stable over the 4-year period suggesting that the detrimental influence of increased drinking frequency on working memory is marginal between early and mid-adolescence.
Another way to address this issue was to compare adolescents who have no or minimal alcohol/drug exposure, but who have a positive family history (FH) for alcoholism (FH+), to age-matched adolescents who have a negative FH− for alcoholism. The FH+ group is an ideal genetic-risk model, as a positive history of alcoholism is associated with an earlier onset and higher magnitude of use as well as with a higher prevalence of alcohol-use disorders in adolescents and young adults. The neuropsychological literature found mixed results regarding cognitive impairments in FH+ prior to the initiation of alcohol consumption. Evidence shows that FH+ youth have deficits in abstract reasoning and planning, and they have lower IQ scores, poorer academic performance, and slower trajectories in cognitive improvement at 1-year follow-up when compared to FH− youth (133). However, other studies failed to document differences between FH+ and FH− youth. Interestingly, deficits in FH+ youth were predominantly found in children of antisocial alcoholics (134), which may constitute a critical and poorly controlled factor to explain discrepancy across studies. In addition, the number of relatives determining the criteria for a positive FH of substance (a single parent in some studies, numerous in others) is also likely to explain differences across studies. Indeed, a greater family loading of alcoholism may be associated with a greater genetic susceptibility for developing a substance-use disorder than a lesser degree of family loading.
From a neurobiological perspective, FH+ youth exhibit smaller overall total brain volumes than their FH− counterparts and less inhibitory frontal activation during the performance of a go/no-go functional magnetic resonance imaging task, compared to FH− comparison subjects (135). At the structural brain level, information processing speed was correlated significantly with white matter volume in FH− females only (136), which may indicate that subtle abnormal cognitive/brain relationship could represent a risk factor for substance abuse in female adolescents who have not yet initiated drug use.
A great number of researches support the idea that higher impulsivity, which could be defined as “actions which are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable consequences” (137), elevates the risk of addiction in young population. For instance, slower rates of development of behavioral self-control strongly and specifically predict early initiation of drug use at 14-year-old and higher number of drug-related problems at 17-years old (138). In line with this result, impulsive/hyperactive symptoms of ADHD in children were the stronger predictors in a prospective study for initiation of tobacco, alcohol, and illicit drug use at 14 (139). In another group with genetic risk of addiction, the adolescent offspring of substance-use disorder parents showed elevated prevalence of alcohol-dependency and stimulant use (140). This relationship is hypothesized to be due to impulsivity-related endophenotype (141). In the same vein, impulsivity measures (personality dimension of constraint) during adolescence also significantly predict problem gambling behavior at a follow-up assessment [e.g., Ref. (142)].
In sum, during childhood and adolescence, decrements in cognitive abilities, associated with familial history of substance abuse (and antisocial personality component and multiplex FH of alcoholism), may be present prior to cognitive impairments that result from early initiation and continued use of alcohol and drugs.
From Neurocognitive Theory to Clinical Practice
Based upon the model proposed in this article, a number of clinical and cognitive interventions are thought to improve treatment outcomes of addictive behaviors. These interventions generally aim at targeting one subsystem at a time from the triadic neural system model of willpower presented in this article. One strategy may be fooling the impulsive system by desensitizing automatic attention toward addiction cues, positive implicit memory associations with addiction cues, and approach tendencies toward addictive object. Boosting self-regulation control along with boosting metacognition and self-awareness of the cognitive limitation, and reducing interoceptive signaling of homeostatic disturbances are also key strategies to alter addiction-related choices and actions. Applications of some of these strategies are already taking place, such as boosting the capacity of the reflective system through physical exercise and mindfulness, through which researchers aim to modulate how an individual processes and integrates afferent sensing from the inside of the body (14). Although interesting, we argue that clinical interventions rooted in the qualitative description of abnormal interactions between the three above mentioned systems could lead to more robust and significant changes. Importantly, our defended clinical approach considers the necessity to adapt clinical strategies to every addicted individual. Indeed, there is evidence showing that these participants are highly heterogeneous with respect to cognitive and brain determinants of their addictive behaviors [for pathological gamblers, see Ref. (143); for polysubstance abusers, see Ref. (144)]. For instance, the principle of equifinality (a give end state – e.g., compulsive drug use – can be reached by many potential pathways) is particularly clear in case of impulsivity, a concept that encompasses a number of aspects including impulsive, reflective and proprioceptive determinants (14, 145).
Strategies Aimed at “Fooling” the Impulsive System
Based on an early remarkable proposition made by MacLeod et al. (146), one of the most widely used cognitive bias modification targets attention approach. In this article, the detection of the probe appearing distally from the negative information results in attenuating the intensity of anxiety and depression reactions to subsequent laboratory stressor as well as to stressful life events [see, Ref. (147)] in healthy participants. When applied to alcohol abuse disorders, it has recently been demonstrated that, when randomly assigned to either the attending (i.e., directing attention toward the location of alcohol-related cues) or avoiding (i.e., directing attention away from the location of alcohol-related cues) condition of a modified probe task, only alcohol-dependent participants who were required to systematically avoid alcohol cues showed swifter therapeutic progress with discharge from the program gaining 28 days on average and reduced risk of relapsing (time to relapse was significantly 1.25 months longer) following the attentional bias modification training intervention (148).
Similar findings were obtained with attempts to change approach tendencies toward alcohol cues (149, 150). Specifically, studies have shown that training abstinent individuals with alcoholism to make avoidance movements in response to alcohol pictures induced better treatment outcomes (decreased alcohol relapse rate at 1 year follow-up) (149, 150). Importantly, older patients and patients with a strong approach-bias profited most from this training sessions (149). Of note, in individuals with alcoholism, alcohol relapse rates increase as the relative tendency to avoid alcohol increases, thus suggesting that an avoidance orientation toward alcohol can potentially be harmful in clinical samples (51).
With regard to implicit memory bias toward addiction-related cues (e.g., good/bad; sedative/arousal), Houben et al. (151) showed that, following an evaluative conditioning session (in which alcohol-related cues were consistently paired with negative stimuli), problem drinkers showed stronger negative implicit attitudes toward alcohol and diminished their alcohol consumption within a week after the evaluative conditioning session.
Strategies Aimed at Boosting the “Reflective” System
Experimental procedures aimed at improving self-regulatory processes could also have been used to modify substance-taking behaviors. For instance, by experimentally priming either disinhibited (i.e., participants were told that they should try to inhibit responding to “Stop” stimuli if possible, but that this was less important than rapid responding to the “Go” stimuli) or restrained behaviors (i.e., participants were told that they should try to respond quickly to “Go” stimuli if possible, but that this was less important than successful inhibition on “Stop” trials) while participants completed a Stop-Signal task, Jones et al. (152) found that increased consumption of beer during a test phase in the “disinhibition group,” as compared to the “inhibition group.” Similar results have been found when comparing alcohol use during a taste phase after experimentally building an association between a “go” response and alcohol cues (by using an alcohol version of the go/go-go task) compared with a condition in which the “no-go” response was associated with alcohol cues (153).
As another recent important finding, Verbruggen et al. (154) demonstrated that proactive inhibitory control may have a direct impact on risk-taking. In this study, participants were presented with six free-choice options on every trial. Each option was associated with a certain amount to win; however, participants were informed that the higher the amount, the less probable a win. In some blocks (i.e., stop condition), in addition to gambling choice, participants were required to stop the planned manual choice response when an occasional signal occurred. Results indicated that participants reduced risky gambling in the stop condition when compared to non-stop condition. Hypothetically, the stop condition induced a proactive motor responding, that is, a general state of cautiousness that may have enhanced cognitive control and in turn reduced risky decision-making. In other words, when individuals are preparing to stop, people make proactive adjustments and become more cautious in executing motor responses (155–158), which in turn may diminish the influence of automatic, emotionally driven processes associated with high and uncertain rewards. Altogether, these studies advance the evidence for a “control transfer” between cognitive domains, which could open new avenues for intervention aiming at reducing addictive behaviors in a clinical population. Unfortunately, a recent study found that the gain on cautiousness up to 2 h when making decisions becomes negligible 24 h later (159). This finding underlines the need to find better parameters of inhibition training to achieve clinical efficacy.
Strategies Aimed at Controlling “Urges” and Interoceptive Signaling
In this paper, we emphasized that the increased liability for substance use may also emerge from a highly embodied experience through the insular cortex. This process may overwhelm the cognitive control system by providing a highly emotionalized experience (e.g., intense state of substance-related craving) and by sensitizing substance abusers to the conditioning of interoceptive drug effects (5, 93). Therefore, these individuals might benefit from interoception-modifying techniques, such as mindfulness exercises, biofeedback, or interoceptive exposure therapy, in order to train reappraisal of the significance of bodily feedback triggered by addiction-related cues (93). In addition, because dysfunction of the interoceptive system may also hamper self-awareness (17, 103), individuals suffering from addiction might also benefit from intervention aiming at enhancing insight. For instance, in recently abstinent alcohol-dependent patients, Jung et al. (160) showed that a brief insight-related intervention (five sessions in 2 weeks; 15 min per session aiming at promoting conscious awareness of symptoms associated with alcohol use) enhanced participants’ level of motivation to change their alcohol-related behavior. In another study, Kim et al. (161) showed that the rate of (1-year) abstinence among alcohol-dependent patients appears to be significantly heightened by their post-cure level of insight. These results suggest that a high level of insight has a positive effect on the diagnostic process, motivation for treatment, and substance-related behavioral change, and may play a critical role in the recovery process.
Triadic Model-Based Strategies
As mentioned above, the equifinality concept of addictive behaviors suggests the need to investigate a personalized clinical approach instead of the standard diagnostic-based view. Concretely, it is plausible that based on a cognitive assessment investigating the strength of approach tendencies triggered by either negative emotions (negative reinforcement), positive emotions (positive reinforcement) or both and given the individual’s efficiency of supervisor functions and the contribution of a particular state of feeling leading to drug use or behavioral addictions, one may benefit more from boosting response inhibition control under stress-induced and craving conditions than another who will gain more control over his/her compulsive behaviors by undergoing positive emotion induction while exercising inhibitory control. For these subjects with high levels of reported craving for drugs or gambling, it sounds reasonable to include mindfulness sessions, which obviously alter the subjective experience, possibly by disconnecting craving-related regions such as the insula and the ventral striatum [for smoking craving, see Ref. (162)].
It is obvious that the assumed necessity to work on the association of systems rather than on one single system needs a lot of development and empirical supports. At this point, our suggestion is supported by (1) a vast research literature showing that maladaptive processes to drug and non-drug behaviors are numerous and effect bottom-up/automatic as well and top-down/intentional registers; (2) the high heterogeneity of clinical profiles (positive reinforcement, negative reinforcement, habits, impulsiveness, craving intensity, automatic cue-induced reactivity, and so on) and of cognitive determinants of these learning processes; (3) the idea that supervisory function fluctuate over time and context (e.g., drug or gambling use, stress, cue exposure) [see for a discussion, Ref. (163)].
Cognitive based interventions have already proven their efficacy in reducing craving and alcohol use, delaying relapse, and improving therapeutic commitment. It is not clear yet which particular combination between pharmacotherapy (e.g., naltrexone, acamprosate), brain stimulation, and cognitive training best fit the different forms of addiction profiles (e.g., sensation seekers/positive reinforcement vs. harm avoidant/negative reinforcement).
Based on the proposed triadic model, predictions of an improved treatment strategy would be to deliver the magnetic brain stimulation in such a way to (1) downregulate (or block) the insula; (2) upregulate (or stimulate) the prefrontal cortex. Upregulation of the dorsolateral prefrontal cortex by high frequency transmagnetic brain stimulation has already demonstrated its positive outcomes on nicotine dependence [for a review, see Ref. (164)]. For instance, this strategy reduces the power of smoking-related cues to elicit craving. However, a number of other brain regions could be indirectly altered and further studies are needed to better characterizing those motivational-related brain regions altered by upregulation of the dorsolateral prefrontal cortex.
Conclusion
The discovery of the insula as a important brain structure specifically in smoking addiction does not undermine the seminal work generated to date on the roles of other components of the neural circuitry implicated in addiction, and impulse control disorders in general, especially the mesolimbic dopamine system (incentive habit system), and the prefrontal cortex (executive control system). Addressing the role of the insula only complements this prior work, leading researchers to investigate how inter-connected activities between the prefrontal cortex, the striatum-amygdala system and the striatum accounts for distinct dimensions of clinical phenomenology of addictive behaviors. At the cognitive and behavioral level, various aspects of sensitized automatic stimulus-driven processing associated with poor self-control capacities elevate the likelihood that a state of addiction be perpetuated, particularly in the context of stress and of addiction-cue exposure.
With respect to clinical interventions, our recommendation is to adapt treatments to each individual’s triadic system configuration by adopting a multidimensional approach, focusing on the interactions between automatic, intentional, and proprioceptive cognitive processes involved in the risk of developing an addictive behavior (in vulnerable populations), in the maintenance of such behaviors (in addicted people) or in the risk of relapse (in patients). While neuropsychological testing has advanced tremendously with respect to brain areas such as the prefrontal cortex (or measuring executive functions), there is still a great lag in neuropsychological evaluation of those state-dependent systems (such as the insula). Measuring strengths or weaknesses in these systems could be the next great advancement in neuropsychological evaluation.
This effort will undoubtedly allow the discovery of novel therapeutic approaches for treating several impulse control disorders, including breaking the cycle of addiction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The primary research that supports the conceptual framework described in this article was supported by grants to Dr. Antoine Bechara from the National Institute on Drug Abuse (R01 DA023051), the National Institute of Neurological Disorders and Stroke (P50 NS19632), and the National Cancer Institute (R01CA152062). Dr. Xavier Noël is Research Associate at the Belgium Fund for Scientific Research (F.R.S./FNRS). Dr. Damien Brevers is a Post-Doctoral Research Scholar supported by the National Institute of NIDA R01 DA16708 (Antoine Bechara).
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. Arlington, VA: American Psychiatric Publishing (2013).
2. George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev (2010) 35:232–47. doi:10.1016/j.neubiorev.2010.05.002
3. Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci (2001) 2:119–128. doi:10.1038/35053570
4. Noël X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol (2013) 23:632–8. doi:10.1016/j.conb.2013.01.018
5. Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci (2009) 32:56–67. doi:10.1016/j.tins.2008.09.009
6. Bechara A. Decision-making, impulse control, and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci (2005) 8:1458–63. doi:10.1038/nn1584
7. Evans JT. Dual-processing accounts of reasoning, judgment and social cognition. Annu Rev Psychol (2008) 58:255–78. doi:10.1146/annurev.psych.59.103006.093629
8. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica (1979) 47:263–91. doi:10.2307/1914185
9. Strack F, Deutsch R. Reflective and impulsive determinants of social behaviour. Pers Soc Pscyhol Rev (2004) 8:220–47. doi:10.1207/s15327957pspr0803_1
10. Stacy AW, Wiers RW. Implicit cognition and addiction: a tool for explaining paradoxical behaviour. Annu Rev Clin Psychol (2010) 6:551–75. doi:10.1146/annurev.clinpsy.121208.131444
11. Noël X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, et al. Supervisory attentional system in nonamnesic male alcoholic subjects. Arch Gen Psychiatry (2001) 58:1152–8. doi:10.1001/archpsyc.58.12.1152
12. Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science (2007) 315:531–4. doi:10.1126/science.1135926
13. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci (2009) 10:59–70. doi:10.1038/nrn2555
14. Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology (2014) 76:342–50. doi:10.1016/j.neuropharm.2013.07.002
15. Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol (2005) 490:101–18. doi:10.1002/cne.20660
16. Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev (2013) 38:1–16. doi:10.1016/j.neubiorev.2013.10.013
17. Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci (2011) 12:652–69. doi:10.1038/nrn3119
18. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci (2005) 8:1481–9. doi:10.1038/nn1579
19. Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev (1993) 18:247–91. doi:10.1016/0165-0173(93)90013-P
20. Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res (2009) 199:89–102. doi:10.1016/j.bbr.2008.09.027
21. Wise R. Brain reward circuitry: insight from unsensed incentives. Neuron (2002) 36:229–40. doi:10.1016/S0896-6273(02)00965-0
22. Lucantonio L, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal cortex dysfunction on cocaine addiction. Nat Neurosci (2012) 15:358–66. doi:10.1038/nn.3014
23. Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci (2007) 30:259–88. doi:10.1146/annurev.neuro.28.061604.135722
24. Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron (2011) 69:603–17. doi:10.1016/j.neuron.2011.02.014
25. Franken IA. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry (2003) 27:563–79. doi:10.1016/S0278-5846(03)00081-2
26. Franken IA, Booij J, van den Brink W. The role of dopamine in human addiction: from reward to motivated attention. Eur J Pharmacol (2005) 526:199–206. doi:10.1016/j.ejphar.2005.09.025
27. Dickinson A, Balleine B, Watt A, Gonzales F, Boakes RA. Motivation control after extended instrumental training. Anim Learn Behav (1995) 23:197–206. doi:10.3758/BF03199935
28. Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top- down cognitive control. Neuron (2011) 69:680–94. doi:10.1016/j.neuron.2011.01.020
29. Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron (2008) 57:432–41. doi:10.1016/j.neuron.2007.12.019
30. Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol (2012) 22:545–51. doi:10.1016/j.conb.2011.09.009
31. Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science (2010) 328:1709–12. doi:10.1126/science.1187801
32. Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci (2006) 26:3805–12. doi:10.1523/JNEUROSCI.4305-05.2006
33. De Houwer J, Teige-Mocigemba S, Spruyt A, Moors A. Implicit measures: a normative analysis and review. Psychol Bull (2009) 135:347–68. doi:10.1037/a0014211
34. Marteau TM, Hollands GJ, Fletcher PC. Changing human behaviour to prevent disease: the importance of targeting automatic processes. Science (2012) 337:1492–5. doi:10.1126/science.1226918
35. Bargh JA, Chen M, Burrows L. Automaticity of social behavior: direct effects of trait construct and stereotype-activation on action. J Pers Soc Psychol (1996) 71:230–44. doi:10.1037/0022-3514.71.2.230
36. Rozin P, Scott S, Dingley M, Urbanek JK, Jiang H, Kaltenbach M. Nudge to nobesity I: minor changes in accessibility decrease food intake. Judgm Decis Mak (2011) 6:323–32.
37. Field M, Munafò MR, Franken IA. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull (2009) 135:589–607. doi:10.1037/a0015843
38. Browning M, Holmes EA, Harmer CJ. The modification of attentional bias to emotional information: a review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci (2010) 10:8–20. doi:10.3758/CABN.10.1.8
39. Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin Psychol Rev (2010) 30:203–16. doi:10.1016/j.cpr.2009.11.003
40. Raymond JE, Shapiro K, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform (1992) 18:849–60. doi:10.1037/0096-1523.18.3.849
41. Brevers D, Cleeremans A, Tibboel H, Bechara A, Kornreich C, Verbanck P, et al. Reduced attentional blink for gambling-related stimuli in problem gamblers. J Behav Ther Exp Psychiatry (2011) 42:265–9. doi:10.1016/j.jbtep.2011.01.005
42. Tibboel H, De Houwer J, Field M. Reduced attentional blink for alcohol-related stimuli in heavy social drinkers. J Psychopharmacol (2010) 24:1349–56. doi:10.1177/0269881109106977
43. Brevers D, Cleeremans A, Bechara A, Laloyaux C, Kornreich C, Verbanck P, et al. Time course of attentional bias for gambling information in problem gambling. Psychol Addict Behav (2011) 25:675–82. doi:10.1037/a0024201
44. Greenwald AG, Banaji MR. Implicit social cognition: attitudes, self-esteem, and stereotypes. Psychol Rev (1995) 102:4–27. doi:10.1037/0033-295X.102.1.4
45. Greenwald AG, McGhee DE, Schwartz JK. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol (1998) 74:1464–80. doi:10.1037/0022-3514.74.6.1464
46. Wiers RW, Ames SL, Hofmann W, Krank M, Stacy AW. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Front Psychopathol (2010) 1:144. doi:10.3389/fpsyg.2010.00144
47. Thush C, Wiers RW. Explicit and implicit alcohol-related cognitions and the prediction of current and future drinking in adolescents. Addict Behav (2007) 32:1367–83. doi:10.1016/j.addbeh.2006.09.011
48. Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction (2003) 98:825–36. doi:10.1046/j.1360-0443.2003.00392.x
49. Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend (2006) 85:75–82. doi:10.1016/j.drugalcdep.2006.03.018
50. Field M, Kiernan A, Eastwood B, Child R. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psychiatry (2008) 39:209–18. doi:10.1016/j.jbtep.2007.06.001
51. Spruyt A, De Houwer J, Tibboel H, Verschuere B, Crombez G, Verbanck P, et al. On the predictive validity of automatically activated approach/avoidance tendencies in abstaining alcohol-dependent patients. Drug Alcohol Depend (2013) 127:81–6. doi:10.1016/j.drugalcdep.2012.06.019
52. Robinson TE, Berridge KC. Addiction. Annu Rev Psychol (2003) 54:25–53. doi:10.1146/annurev.psych.54.101601.145237
53. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry (2002) 159:1642–52. doi:10.1176/appi.ajp.159.10.1642
54. Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry (2007) 164:43–51. doi:10.1176/appi.ajp.164.1.43
55. Bühler M, Vollstädt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, et al. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry (2010) 67:745–52. doi:10.1016/j.biopsych.2009.10.029
56. Reichel CM, Bevins RA. Competition between the conditioned rewarding effects of cocaine and novelty. Behav Neurosci (2008) 122:140–50. doi:10.1037/0735-7044.122.1.140
57. Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci (2001) 115:683–694. doi:10.1037/0735-7044.115.3.683
58. Aigner TG, Balster RL. Choice behaviour in rhesus monkeys: cocaine versus food. Science (1978) 201:534–5. doi:10.1126/science.96531
59. Dougherty DM. Laboratory Behavioural Measures of Impulsivity: General Information Manual. Houston: University of Texas Health Science Center (2003).
60. Newman JP, Widom CS, Nathan S. Passive avoidance in syndromes of disinhibition: psychopathy and extraversion. J Pers Soc Psychol (1985) 48:1316–27. doi:10.1037/0022-3514.48.5.1316
61. Brevers D, Noël X. Pathological gambling and the loss of willpower: a neurocognitive perspective. Socioaffect Neurosci Psychol (2013) 3:21592. doi:10.3402/snp.v3i0.21592
62. Noël X, Bechara A, Brevers D, Verbanck P, Campanella S. Alcoholism and the loss of willpower: a neurocognitive perspective. J Psychophysiol (2010) 24:240–8. doi:10.1027/0269-8803/a000037
63. Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, et al. Impaired response inhibition function in abstinent heroin dependence. Neurosci Lett (2008) 438:322–6. doi:10.1016/j.neulet.2008.04.033
64. Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci (2004) 24:11017–22. doi:10.1523/JNEUROSCI.3321-04.2004
65. Grant JE, Chamberlain SR, Schreiber LRN, Odlaug BL, Kim SW. Selective decision-making deficits in at-risk gamblers. Psychiatry Res (2011) 189:115–20. doi:10.1016/j.psychres.2011.05.034
66. Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Impulsivity and response inhibition in alcohol dependence and problem gambling. Psychopharmacology (2009) 207:163–72. doi:10.1007/s00213-009-1645-x
67. van Holst RJ, Van Holstein M, Van den Brink W, Veltman DJ, Goudriaan AE. Response inhibition during cue reactivity in problem gamblers: an fMRI study. PLoS One (2012) 7:e30909. doi:10.1371/journal.pone.0030909
68. Houben K, Wiers RW. Response inhibition moderates the relationship between implicit associations and drinking behaviour. Alcohol Clin Exp Res (2009) 33:626–33. doi:10.1111/j.1530-0277.2008.00877.x
69. Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (2012) 219:469–90. doi:10.1007/s00213-011-2550-7
70. Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev (2007) 17:337–45. doi:10.1007/s11065-007-9034-x
71. Goudriaan AE, Oosterlaan J, De Beurs E, Van Den Brink W. The role of self-reported impulsivity and reward sensitivity versus neurocognitive measures of disinhibition and decision-making in the prediction of relapse in pathological gamblers. Psychol Med (2008) 38:41–50. doi:10.1017/S0033291707000694
72. Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioural impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend (2007) 88:79–82. doi:10.1016/j.drugalcdep.2006.09.006
73. Bowden-Jones H, McPhillips M, Rogers R, Hutton S, Joyce E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci (2005) 17:417–20. doi:10.1176/appi.neuropsych.17.3.417
74. Brevers D, Cleeremans A, Goudriaan AE, Bechara A, Kornreich C, Verbanck P, et al. Decision making under ambiguity but not under risk is related to problem gambling severity. Psychiatry Res (2012) 200:568–74. doi:10.1016/j.psychres.2012.03.053
75. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend (2006) 81:313–22. doi:10.1016/j.drugalcdep.2005.08.003
76. Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci (2005) 9:159–64. doi:10.1016/j.tics.2005.02.002
77. Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn (2004) 55:30–40. doi:10.1016/j.bandc.2003.04.001
78. Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology (2009) 56:48–62. doi:10.1016/j.neuropharm.2008.07.035
79. Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science (1997) 275:1293–5. doi:10.1126/science.275.5304.1293
80. Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition (1994) 50:7–15. doi:10.1016/0010-0277(94)90018-3
81. Bechara A, Tranel D, Damasio H. Characterization of the decision-making impairment of patients with bilateral lesions of the ventromedial prefrontal cortex. Brain (2000) 123:2189–202. doi:10.1093/brain/123.11.2189
82. Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain (2002) 125:624–39. doi:10.1093/brain/awf049
83. Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia (2001) 39:376–89. doi:10.1016/S0028-3932(00)00136-6
84. Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia (2000) 38:1180–7. doi:10.1016/S0028-3932(99)00158-X
85. Noël X, Van der Linden M, d’ Acremont M, Bechara A, Dan B, Hanak C, et al. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology (2007) 192:291–8. doi:10.1007/s00213-006-0695-6
86. Petry NM, Bickel WK. Polydrug abuse in heroin addicts: a behavioral economic analysis. Addiction (1998) 93:321–35. doi:10.1046/j.1360-0443.1998.9333212.x
87. Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, et al. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend (2004) 76:107–11. doi:10.1016/j.drugalcdep.2004.04.009
88. Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in disorder gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cogn Brain Res (2005) 23:137–51. doi:10.1016/j.cogbrainres.2005.01.017
89. Brevers D, Cleeremans A, Verbruggen F, Bechara A, Kornreich C, Verbanck P, et al. Impulsive action but impulsive choice determines problem gambling severity. PLoS One (2012) 7:e50647. doi:10.1371/journal.pone.0050647
90. Garavan H. Insula and drug cravings. Brain Struct Funct (2010) 214:593–601. doi:10.1007/s00429-010-0259-8
91. Damasio AR. How the brain creates the mind. Sci Am (1999) 281:112–7. doi:10.1038/scientificamerican1299-112
92. Winkielman P, Niedenthal P, Oberman LM. Embodied perspective on emotion-cognition interactions. In: Pineda J editor. Mirror Neuron Systems. New York: Humana Press (2009). p. 235–57.
93. Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev (2012) 36:1857–69. doi:10.1016/j.neubiorev.2012.05.007
94. Goudriaan AE, De Ruiter MB, Van den Brink W, Oosterlaan J, Veltman DJ. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addict Biol (2010) 15:491–503. doi:10.1111/j.1369-1600.2010.00242.x
95. Janes AC, Pizzagalli DA, Richardt S, deB FrederickB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry (2010) 67:722–9. doi:10.1016/j.biopsych.2009.12.034
96. Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a go/no-go task as revealed by event-related functional magnetic resonance imaging. J Neurosci (2003) 23:7839–43.
97. Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, et al. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (2012) 221:701–8. doi:10.1007/s00213-011-2617-5
98. Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol (2009) 14:84–98. doi:10.1111/j.1369-1600.2008.00134.x
99. Metcalfe J, Mischel W. A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychol Rev (1999) 106:3–19. doi:10.1037/0033-295X.106.1.3
100. Sinha R, O’Malley SS. Craving for alcohol: findings from the clinic and the laboratory. Alcohol Alcohol (1999) 34:223–30. doi:10.1093/alcalc/34.2.223
102. Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev (2007) 26:25–31. doi:10.1080/09595230601036960
103. Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, et al. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci (2009) 13:372–80. doi:10.1016/j.tics.2009.06.004
104. Substance Abuse and Mental Health Services Administration. (2009). Results from the 2008 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434). Rockville.
105. Brevers D, Cleeremans A, Bechara A, Kornreich C, Verbanck P, Noël X. Impaired metacognitive capacities in problem gamblers. J Gambl Stud (2013) 29 (1):119–29. doi:10.1007/s10899-012-9292-2
106. Brevers D, Cleeremans A, Bechara A, Greisen M, Kornreich C, Verbanck P, et al. Impaired self-awareness in pathological gamblers. J Gambl Stud (2013) 29:119–29. doi:10.1007/s10899-012-9292-2
107. Chiu PH, Lohrenz TM, Montague PR. Smokers’ brains compute, but ignore, a fictive error signal in a sequential investment task. Nat Neurosci (2008) 11:514–20. doi:10.1038/nn2067
108. Goldstein RZ, Parvaz MA, Maloney T, Alia-Klein N, Woicik PA, Telang F, et al. Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology (2008) 45:705–13. doi:10.1111/j.1469-8986.2008.00670.x
109. Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology (2009) 34:2450–8. doi:10.1038/npp.2009.67
110. Le Berre AP, Pinon K, Vabret F, Pitel AL, Allain P, Eustache F, et al. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res (2010) 34:1888–98. doi:10.1111/j.1530-0277.2010.01277.x
111. Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, et al. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain (2010) 133:1484–93. doi:10.1093/brain/awq066
112. Paulus MP. Decision-making dysfunctions in psychiatry – altered homeostatic processing? Science (2007) 318:602–6. doi:10.1126/science.1142997
113. Persaud N, McLeod P, Cowey A. Post-decision wagering objectively measures awareness. Nat Neurosci (2007) 10:257–61. doi:10.1038/nn1840
114. Nelson TO, Narens L. Metamemory: a theoretical framework and new findings. Psychol Learn Motiv (1990) 26:125–73. doi:10.1016/S0079-7421(08)60053-5
115. Fleming SM, Dolan RJ. The neural basis of metacognitive ability. Philos Trans R Soc Lond B Biol Sci (2012) 367:1338–49. doi:10.1098/rstb.2011.0417
116. Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci (2002) 3:655–66. doi:10.1038/nrn894
117. Bjork JM, Gilman JM. The effects of acute alcohol administration on the human brain: insights from neuroimaging. Neuropharmacology (2013). doi:10.1016/j.neuropharm.2013.07.039
118. Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addict Biol (2012) 17:465–78. doi:10.1111/j.1369-1600.2011.00383.x
119. Sripada CS, Angstadt M, McNamara P, King AC, Phan KL. Effects of alcohol on brain responses to social signals of threat in humans. Neuroimage (2011) 55:371–80. doi:10.1016/j.neuroimage.2010.11.062
120. Padula CB, Simmons AN, Matthews SC, Robinson SK, Tapert SF, Schuckit MA, et al. Alcohol attenuates activation in the bilateral anterior insula during an emotional processing task: a pilot study. Alcohol Alcohol (2011) 46:547–52. doi:10.1093/alcalc/agr066
121. Ridderinkhof KR, de Vlugt Y, Bramlage A, Spaan M, Elton M, Snel J, et al. Alcohol consumption impairs detection of performance errors in mediofrontal cortex. Science (2002) 298(5601):2209–11. doi:10.1126/science.1076929
122. Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci (2004) 5:67–73. doi:10.1038/nrn1302
123. Swendsen J, Le Moal M. Individual vulnerability to addiction. Ann N Y Acad Sci (2011) 1216:73–85. doi:10.1111/j.1749-6632.2010.05894.x
124. Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry (2003) 160:1041–52. doi:10.1176/appi.ajp.160.6.1041
125. Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, et al. Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics (2008) 121:S273–89. doi:10.1542/peds.2007-2243C
126. Noël X. Why adolescents are at risk of misusing alcohol and gambling. Alcohol Alcohol (2013). doi:10.1093/alcalc/agt161
127. Ayduk O, Mendoza-Denton R, Mischel W, Downey G, Peake PK, Rodriguez M. Regulating the interpersonal self: strategic self-regulation for coping with rejection sensitivity. J Pers Soc Psychol (2000) 79:776–92. doi:10.1037/0022-3514.79.5.776
128. Caspi A, Silva PA. Temperamental qualities at age three predict personality traits in young adulthood: longitudinal evidence from a birth cohort. Child Dev (1995) 66:486–98. doi:10.2307/1131592
129. Romer D. Adolescent risk taking, impulsivity, and brain development: implications for prevention. Dev Psychobiol (2010) 52:263–76. doi:10.1002/dev.20442
130. Silveri MM. Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry (2012) 20:189–200. doi:10.3109/10673229.2012.714642
131. Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav (2009) 23:715–22. doi:10.1037/a0016516
132. Khurana A, Romer D, Betancourt LM, Brodsky NL, Giannetta JM, Hurt H. Working memory ability predicts trajectories of early alcohol use in adolescents: the mediational role of impulsivity. Addiction (2013) 108:506–15. doi:10.1111/add.12001
133. Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci (2004) 1021:363–70. doi:10.1196/annals.1308.046
134. Poon E, Ellis DA, Fitzgerald HE, Zucker RA. Intellectual, cognitive, and academic performance among sons of alcoholics, during the early school years: differences related to subtypes of familial alcoholism. Alcohol Clin Exp Res (2000) 24:1020–7. doi:10.1111/j.1530-0277.2000.tb04645.x
135. Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, et al. An fMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci (2004) 1021:391–4. doi:10.1196/annals.1308.050
136. Silveri MM, Tzilos GK, Yurgelun-Todd DA. Relationship between white matter volume and cognitive performance during adolescence: effects of age, sex and risk for drug use. Addiction (2008) 103:1509–20. doi:10.1111/j.1360-0443.2008.02272.x
137. Durana JH, Barnes PA, Johnson JL, Shure MB. A neurodevelopmental view of impulsivity and its relationship to the superfactors of personality. In: McCown WG editor. The Impulsive Client: Theory, Research and Treatment. Washington, DC: American Psychological Association (1993). p. 23–37.
138. Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, et al. Behavioural control and resiliency in the onset of alcohol and illicit drug use: a prospective study from preschool to adolescence. Child Dev (2006) 77:1016–33. doi:10.1111/j.1467-8624.2006.00916.x
139. Elkins IJ, McGue M, Iacono WG. Prospective effects of attention-deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Arch Gen Psychiatry (2007) 64:1145–52. doi:10.1001/archpsyc.64.10.1145
140. Kendler KS, Sheth K, Gardner CO, Prescott CA. Childhood parental loss and risk for first-onset of major depression and alcohol dependence: the time-decay of risk and sex differences. Psychol Med (2002) 32:1187–94. doi:10.1017/S0033291702006219
141. Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev (2008) 32:777–810. doi:10.1016/j.neubiorev.2007.11.003
142. Slutske WS, Caspi A, Moffitt TE, Poulton R. Personality and problem gambling: a prospective study of a birth cohort of young adults. Arch Gen Psychiatry (2005) 62:769–75. doi:10.1001/archpsyc.62.7.769
143. Billieux J, Lagrange G, Van der Linden M, Lançon C, Adida M, Jeanningros R. Investigation of impulsivity in a sample of treatment-seeking pathological gamblers: a multidimensional perspective. Psychiatry Res (2012) 198:291–6. doi:10.1016/j.psychres.2012.01.001
144. Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia (2002) 40:1690–705. doi:10.1016/S0028-3932(02)00016-7
145. Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol (1999) 13:180–92. doi:10.1177/026988119901300211
146. MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol (2002) 111:107–23. doi:10.1037/0021-843X.111.1.107
147. MacLeod C, Bridle R. The reduction of vulnerability through the modification of attentional bias: a real world study using a home-based cognitive bias modification procedure. J Abnorm Psychol (2009) 118:65–75. doi:10.1037/a0014377
148. Schoenmakers TM, de Bruijn M, Lux IM, Goertz AG, van Kerkhof DT, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend (2010) 109:30–6. doi:10.1016/j.drugalcdep.2009.11.022
149. Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker E, Lindenmeyer J. Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci (2013) 4:38–51. doi:10.1016/j.dcn.2012.11.002
150. Wiers RW, Eberl C, Rinck M, Becker E, Lindenmeyer J. Re-training automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci (2011) 22:490–7. doi:10.1177/0956797611400615
151. Houben K, Schoenmakers TM, Wiers RW. I didn’t feel like drinking but I don’t know why: the effects of evaluative conditioning on alcohol-related attitudes, craving and behaviour. Addict Behav (2010) 35:1161–3. doi:10.1016/j.addbeh.2010.08.012
152. Jones A, Guerrieri R, Fernie G, Cole J, Goudie A, Field M. The effects of priming restrained versus disinhibited behaviour on alcohol-seeking in social drinkers. Drug Alcohol Depend (2011) 113:55–61. doi:10.1016/j.drugalcdep.2010.07.006
153. Houben K, Nederkoorn C, Wiers RW, Jansen A. Resisting temptation: decreasing alcohol-related affect and drinking behaviour by training response inhibition. Drug Alcohol Depend (2011) 116:132–6. doi:10.1016/j.drugalcdep.2010.12.011
154. Verbruggen F, Adams R, Chambers CD. Proactive motor control reduces monetary risk taking in gambling. Psychol Sci (2012) 23:805–15. doi:10.1177/0956797611434538
155. Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry (2011) 69:55–68. doi:10.1016/j.biopsych.2010.07.024
156. Jahfari S, Stinear CM, Claffey M, Verbruggen F, Aron AR. Responding with restraint: what are the neurocognitive mechanisms? J Cogn Neurosci (2010) 22:1479–92. doi:10.1162/jocn.2009.21307
157. Liddle EB, Scerif G, Hollis CP, Batty MJ, Groom MJ, Liotti M, et al. Looking before you leap: a theory of motivated control of action. Cognition (2009) 112:141–58. doi:10.1016/j.cognition.2009.03.006
158. Verbruggen F, Logan GD. Models of response inhibition in the stop-signal and the stop-change paradigms. Neurosci Biobehav Rev (2009) 33:647–61. doi:10.1016/j.neubiorev.2008.08.014
159. Verbruggen F, Adams RC, van’t Wout F, Stevens T, McLaren IP, Chambers CD. Are the effects of response inhibition on gambling long-lasting? PLoS One (2013) 26(8):e70155. doi:10.1371/journal.pone.0070155
160. Jung JG, Kim JS, Kim GJ, Oh MK, Kim SS. The role of alcoholics’ insight in abstinence from alcohol in male Korean alcohol dependents. J Korean Med Sci (2011) 22:132–7. doi:10.3346/jkms.2007.22.1.132
161. Kim JS, Park BK, Kim GJ, Kim SS, Jung JG, Oh MK, et al. The role of alcoholics’ insight in abstinence from alcohol in male Korean alcohol dependents. J Korean Med Sci (2007) 22:132–7. doi:10.3346/jkms.2007.22.1.132
162. Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci (2011) 8:73–84. doi:10.1093/scan/nsr076
163. Jones A, Christiansen P, Nederkoorn C, Houben K, Field M. Fluctuating disinhibition: implications for the understanding and treatment of alcohol and other substance use disorders. Front Psychiatry (2013) 22(4):140. doi:10.3389/fpsyt.2013.00140
Keywords: addiction, self-regulation, impulsive system, interoception, decision making
Citation: Noël X, Brevers D and Bechara A (2013) A triadic neurocognitive approach to addiction for clinical interventions. Front. Psychiatry 4:179. doi: 10.3389/fpsyt.2013.00179
Received: 31 August 2013; Accepted: 12 December 2013;
Published online: 27 December 2013.
Edited by:
Antonio Verdejo-García, Universidad De Granada, SpainReviewed by:
Giovanni Martinotti, Catholic University of Rome, ItalyAgnes J. Jasinska, National Institute on Drug Abuse, USA
Copyright: © 2013 Noël, Brevers and Bechara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xavier Noël, Psychological Medicine Laboratory, Faculty of Medicine, Brugmann-Campus, Université Libre de Bruxelles, 4 Place Van Gehuchten, 1020 Brussels, Belgium e-mail: xnoel@ulb.ac.be
 Xavier Noël
Xavier Noël Damien Brevers
Damien Brevers Antoine Bechara
Antoine Bechara