- 1Faculty of Medicine, University of Southampton, Southampton, UK
- 2Southampton Children’s Hospital, Southampton, UK
- 3Department of Child Health, Solent NHS Trust, Southampton, UK
Obstructive sleep apnea is a condition which affects an estimated 50% of children with Down syndrome, particularly in their early years. It can cause serious sequelae in affected children but may not be recognized by parents or health professionals. Routine screening has been recommended in some countries, but is not standard practice. There are no validated questionnaire-based tools available to screen this population of children for this particular sleep-related disorder. Using existing validated sleep questionnaire items, we have developed a questionnaire to screen children with Down syndrome up to 6 years of age for obstructive sleep apnea, which corresponds with the recommendations made in UK national guidelines. This paper describes these first steps in demonstrating content validity for a new questionnaire, which will be subject to further in-depth psychometric analysis. Relevance, clarity, and age appropriateness were rated for 33 items using a content review questionnaire by a group of 18 health professionals with expertise in respiratory pediatrics, neurodevelopmental pediatrics, and sleep physiology. The content validity index was calculated for individual items and contributed to decisions about item inclusion. Scale level content validity index for the modified questionnaire of 14 items was at an accepted level of 0.78. Two parents of children with Down syndrome took part in cognitive interviews after completing the modified questionnaire. We describe the development of this 14 item questionnaire to screen for OSA in children with DS from infancy to 6 years.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive partial (hypopnea) or complete (apnea) airway collapse during sleep despite continued respiratory effort. It is believed to affect over 50% of children with Down syndrome (DS) compared to around 1–3% of typically developing children (1–3). Causation in DS is multi-factorial with hypotonia, obesity and craniofacial anatomy all contributing to collapse of a narrow airway, further exacerbated in the pre-school years by growth of the adenotonsillar tissue. Children with DS are at increased risk of adverse complications of OSA (4, 5). Up to 60% of children with DS have congenital heart disease putting them at increased risk of developing pulmonary hypertension (4). Cognitive and behavioral sequelae of OSA seen in typically developing children, namely inattention, reduced academic performance, and daytime behavioral problems (6, 7) are likely to be more problematic in children with DS who have reduced cognitive reserve (8). Treatment options, such as adenotonsillectomy and overnight non-invasive ventilation, can reverse both physical and neurocognitive complications of OSA in typically developing children (9), although there have been no large randomized trials of such approaches in children with DS (10).
Clinical history and examination are poor at predicting OSA in both typically developing children (11–14) and children with DS. Furthermore, parents may not report OSA symptoms in their children with DS (15, 16). This may reflect lack of awareness of nocturnal symptoms on the part of parents, or failure to recognize their significance. Clinical diagnosis alone therefore has limited utility and screening is recommended. Guidelines for screening for OSA in DS vary across the world. The American Academy of Paediatrics recommends referring all children with DS for a sleep study or polysomnography by the age of 4 years (17). The UK Royal College of Paediatrics and Child Health (RCPCH) recommend annual screening of children with DS from infancy until 3–5 years old, with a minimum of pulse oximetry (18). Follow-up cardiorespiratory polygraphy studies are recommended in children with abnormalities on oximetry screening, or where clinical assessment suggests a false negative screening test (18). In effect therefore, the majority of children may require as a minimum cardiorespiratory polygraphy. The impracticality and limited availability of such technology in some parts of the world suggests a need to investigate the validity and utility of simpler screening methods.
There are few screening tools designed for clinical populations of children with developmental disorders, who may differ in their presenting symptomatology. Existing pediatric sleep questionnaires have not been designed or validated for children with DS (19). Their utility in screening for OSA in children with DS is therefore unknown. Furthermore, existing OSA screening questionnaires contain a number of behavioral questions that children with learning disabilities may score positively, even in the absence of OSA. For example, the Pediatric Sleep Questionnaire (PSQ) contains a subscale that asks questions about reduced growth rate and weight gain (20), which are common problems in children with DS that may exist independent of OSA. A questionnaire designed specifically for children with DS should have greater sensitivity and specificity and may provide a useful adjunct assessment to clinical history in screening for OSA (19).
The aim of this study was to undertake the first phase of the development of a questionnaire to be used specifically as a screening tool for OSA in children with DS aged between 6 months and 6 years old. This age range was selected as it includes the screening period recommended by the UK RCPCH (18). Here, we report the methods used and initial evidence for content validity.
Materials and Methods
This study was approved by the Faculty of Medicine research ethics committee at the University of Southampton.
Prior to testing the psychometric properties of a questionnaire, key stages of development must be completed. A detailed description of the steps required was outlined by Spruyt and Gozal in their paper on the development of pediatric sleep questionnaires (21), as well as by other authors (22). The initial steps are important in providing evidence of content validity, that is, the extent to which the questionnaire measures the intended construct and is appropriate for its intended use. The stages we used are described below.
Item Generation
Existing pediatric sleep questionnaires, which included questions focusing on OSA were reviewed by the study steering group (Catherine Tuffrey, Catherine Hill, and Hazel Evans) to identify a range of questions, which relate to symptoms relevant to identifying OSA in children with DS. Questions were selected from five existing pediatric sleep questionnaires identified through the literature review, namely the Pediatric Sleep Related Breathing Disorder Questionnaire (PSQ) (20), OSA disease specific quality of life questionnaire (OSA-18) (23), Sleep Disorders Inventory for Students-Children’s form (SDIS-C) (24), Gozal’s adaptation of Brouilettes Questionnaire (25), and the Hong Kong children’s sleep questionnaire (HK-CSQ) (26). These questionnaires have all been previously validated against objective measures, such as PSG (19). Question stems were modified to fit with the chosen response format described below. In addition, the study group devised four new questions to cover symptoms, which were not covered in existing questionnaires.
Item Response Options
Existing questionnaires were examined by the study steering group and the response format was selected that would be appropriate for all questions and that would relate to a period of time, which parents would be able to most accurately recall. A Likert response format was chosen to rate the frequency of symptoms over a week. The response format chosen was a modification of Gozal’s adaptation of Brouillette’s questionnaire (25) [Never (never in the past 6 months), Rarely (once a week or less), Occasionally (2 times a week), Frequently (three to four times a week), Almost always (more than four times a week)]. The response format was chosen to avoid forcing the respondent into choice of category. An “unsure” option was added to help avoid non-response. The addition of an “unsure” option can, however, introduce some challenges to the interpretation and scoring of responses. The response format was later reviewed for suitability as part of the expert content review and through cognitive interviews with parents.
Expert Review
Instrument development experts support the use of knowledgeable expertise to objectively measure content validity. For this to be effective, content experts must understand their expert role, the concepts being explored and the intended use of the instrument. A covering instruction sheet was included outlining the process of content review as recommended by Lynn (27). We aimed to recruit 10–20 experts from a range of relevant backgrounds to review the questionnaire for relevance, clarity, and age appropriateness. The literature suggests a minimum of 5 and a maximum of 10–20 experts for content review (22, 28). To allow for non-responders, more experts were contacted than were needed. Ninety experts from the UK Down syndrome Medical Interest Group, a network of health professionals with a specialist interest in Down syndrome, and the UK Sleep Videoconference network, an informal network of pediatric sleep centers in the UK who meet quarterly by video-conference, were emailed individually with details of the project, the content review questionnaire, and instructions for completion. No reward was offered for completion.
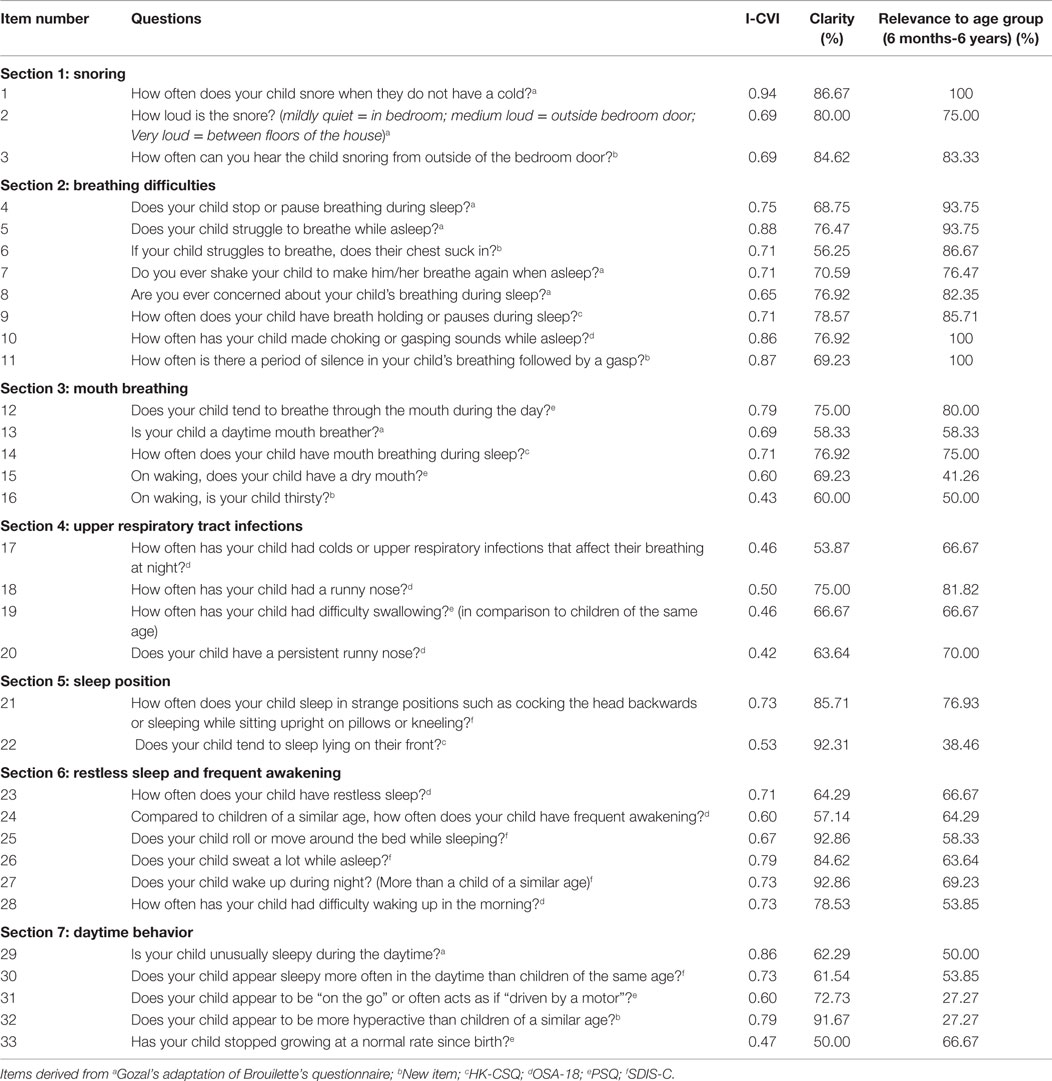
Expert opinion was collated using a structured feedback form in the form of a content review questionnaire (22). Questions were arranged into symptom subgroups under the headings; snoring, breathing difficulties, mouth breathing, upper respiratory tract infections, sleep position, restless sleep and frequent awakening, and daytime behavior. Of the 33 items included in the content review questionnaire, 3 items corresponded to snoring, 8 items to breathing difficulties, 5 items to mouth breathing, 4 items to upper respiratory tract infections, 2 items to sleep position, 6 items to restless sleep and frequent awakening, and 5 items to daytime behavior. No indication was given as to the source questionnaire of each item in order to minimize bias. For the purpose of content review, there was intentional over inclusion of questions so that experts could decide which phrasing or symptom construct was most appropriate. Experts were asked to rate the relevance of each item on a four-point scale, where 1 indicated “not relevant” and 4 indicated “relevant.” In addition, experts were asked whether the question was clear (yes or no) and age appropriate (yes or no). Free text comments were invited for each item. Further questions were asked concerning the overall comprehensiveness of the questionnaire and the suitability of the response options.
The content validity index (CVI) was calculated for each item and for the questionnaire as a whole (28). CVI is defined as the proportion of experts who rated a question relevant and is an index of inter-rater agreement. To calculate the CVI for each item, the number of experts who rated the item 3 or 4 (3 = the item needs minor revisions to be relevant, 4 = the item is relevant) is divided by the total number of experts (28).
The CVI can be calculated for individual items (I-CVI) and for the scale as a whole (S-CVI). For this latter measure, we have used the version of S-CVI where the mean of the I-CVI’s is calculated. Where a new question was suggested following review, as this would not have a CVI, this item was excluded from the S-CVI calculation. The literature recommends a CVI value of 0.78 for a question to be deemed as relevant (28). Items rated lower than this were considered for revision or deletion. It is important to understand that the CVI is only one consideration when deciding on the fate of an item and other factors such as content coverage also need to be considered. CVI does not adjust for chance agreement, but as the number of experts increase, the risk of chance agreement decreases (28).
Clarity and appropriateness were rated with a yes/no response followed by free text comments and suggestions. Proportions of experts rating an item positively for clarity and relevance were recorded.
Finally, free text comments aided the selection, rephrasing, and elimination of questions.
Cognitive Interviews
Cognitive interviews are a recommended method of pre-testing or evaluating questionnaires and focus on the cognitive processes of the respondent in answering questions (29). A sample similar to the final user population is recommended and we therefore interviewed parents of children with DS aged <7 years old. Parents were invited to participate via a local DS parent support group. Cognitive interviews were conducted individually using “Thinking aloud” and “Probing” techniques (29). Parent volunteers were asked to read the items selected for inclusion in the questionnaire and comment as they answered each question. “Thinking aloud” has the benefit of being free from interviewer-based bias and it allows the subject to elaborate on their thought processes. “Thinking aloud,” however, suits some personality types more than others; some subjects may struggle to elaborate on their thought processes, while others may deviate from the subject topic in their answers. “Probes” allow the interviewer to focus the interview around areas of potential response error and prompt the subject into giving their opinions. Examples of probes are; “How did you get the answer of …. nights a week?” and “What does the phrase ‘restless sleep’ mean to you?” Probes can focus the respondent to think about their understanding of the question, recall of information, and degree of estimation needed to answer the question. Use of “probes” has the potential for bias therefore care to avoid leading questions should be taken (21, 29). Parents were asked additional questions concerning; the response format, time frame, clarity of questions, and general layout. Parents were also asked if their child had been investigated for, or diagnosed with, OSA. Comments received from parents during this phase guided the final phrasing of questions, questionnaire completion instructions, and changes to the layout. Ideally, sampling should continue to data saturation, however, the time constraints of this study only permitted one round of interviews. No financial incentives were offered to parents to participate.
Readability
Following expert review and cognitive interviews, selected and modified questions were subject to the Flesch Reading Ease test (30). This analyses the number of syllables per word and number of words per sentence to produce a readability score. A score of 60–70 equates to a reading age of 13- to 15-year-old students.
Results
Expert Review
Content Review Questionnaire
Eighteen experts from a range of healthcare backgrounds participated in the content review stage of questionnaire development. The majority (n = 10) of respondents were respiratory pediatricians; the others were two neurodevelopmental pediatricians, two sleep physiologists, two specialist nurses, a respiratory physician and a neurophysiologist.
Content Validity Index
The CVIs calculated for each item (n = 33) ranged from 0.43 to 0.94. Eight items scored a CVI greater than the accepted level of 0.78. The breathing difficulties questions had the highest CVIs, while the upper respiratory tract infections subgroup had the lowest. Full details are given in Table 1.
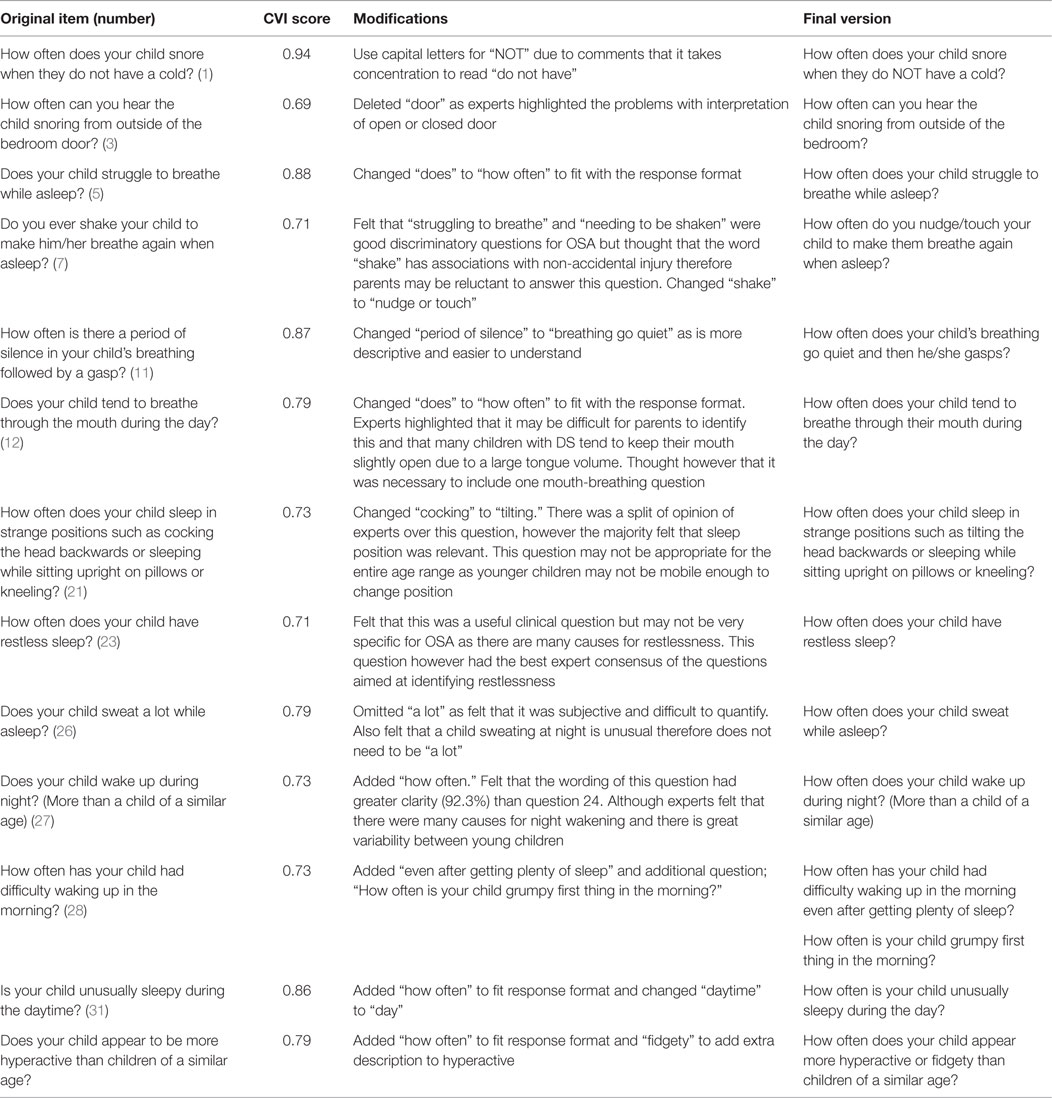
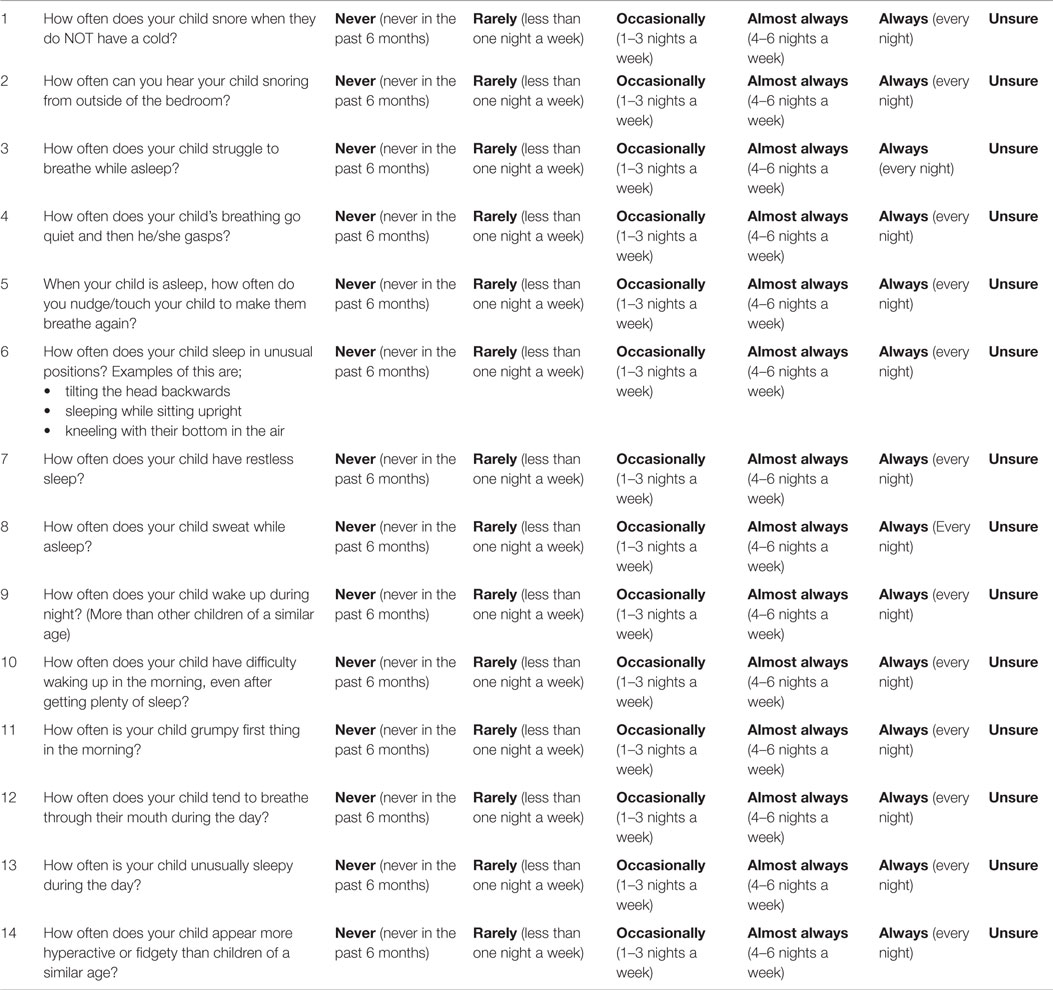
From the 33 items in the content review questionnaire (see Table 1), 13 items were included in the final selection with 1 further question added; 2 items corresponded to snoring, 3 items to breathing difficulties, 1 item to mouth breathing, 1 item to sleep position, 4 existing items, and 2 items to daytime behavior. Following comments from experts on clarity and content of item 28 on morning wakening, an additional item was written. No items from the upper respiratory tract infection subgroup were selected. Six questions with CVI scores <0.78 (0.69–0.73) were included to ensure the questionnaire had adequate content coverage prior to testing of its psychometric properties in a clinical sample. These questions were revised from their original format according to experts’ comments and are shown in Table 2. The CVIs for included items ranged between 0.69 and 0.94 and the S-CVI for the 13 items where a CVI could be calculated is 0.78.
The data on clarity and appropriateness were also used to contribute to decisions about changes needed and whether or not a question was retained.
Of the finally selected questions, four were modified from Gozal’s adaptation of Brouillettes questionnaire, three items were adaptation of the SDIS-C, two items were sourced from the OSA-18, one item was from the PSQ, and four items were new questions. None were chosen from the HK-CSQ, which may reflect the fact that this was developed for an older age group. The fact that questions retained came from a number of different sources suggests no systematic selection bias.
Cognitive Interviews
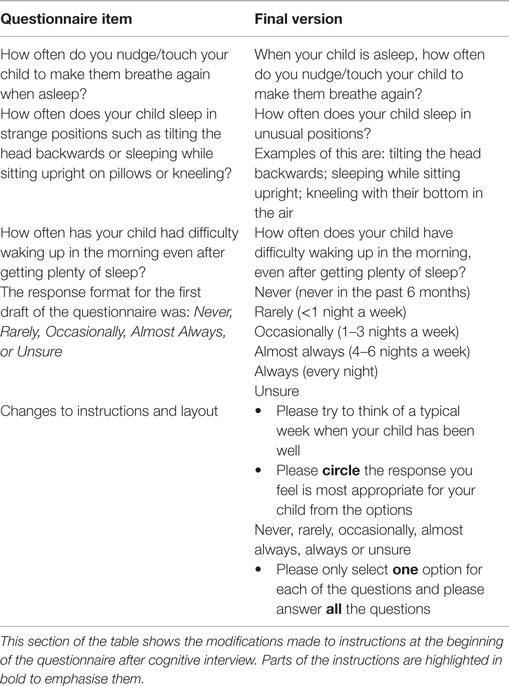
The two parents interviewed had young children with DS previously investigated for OSA. They were members of a local DS support group and were health professionals. They suggested revision of the phrasing of 3 of the 14 selected questions as well as improvements to the instructions at the beginning of the questionnaire (see Table 3). Importantly, they felt that a typical week would be easier to recall than the last typical week in the last 6 weeks. They also recommended extending the number of options in the response format.
As a consequence of the cognitive interviews, changes to the instructions were made, the phrasing of some questions was modified to improve readability and “always” was added to the response format.
Readability
The Flesch Reading Ease score for the final question selection was appropriate at 66.
Discussion
This is the first parent reported questionnaire designed to screen young children with DS for obstructive sleep apnea. Using questions from previously validated questionnaires developed for other populations, we have used a structured approach involving both health professionals and parents of children with DS to adapt items and construct the new questionnaire.
Using an expert panel of relevant health professionals from a range of backgrounds and disciplines enabled a transparent process of assessment of items. The structured format in which the potential questions were presented to experts, enabled views to be obtained in a rigorous manner. Specifically, it enabled comment to be made on item relevance, clarity, and age appropriateness as well as content coverage of the items combined.
We included between two and eight items for each symptom for consideration by the expert panel, which enabled the best items to be selected for relevance. Children with DS have other medical problems, which may present with symptoms similar to those attributed to OSA in otherwise healthy children with normal development. This was reflected in the low rating for relevance of some items included in other questionnaires such as “Has your child stopped growing at a normal rate since birth?” Although growth restriction may result from OSA in children with DS, parents may interpret this question as referring to the different growth pattern of a child with DS, compared to their normally developing peers.
However, another example where similar concerns might apply, is “How often does your child appear more hyperactive or fidgety than children of a similar age?” This was rated relevant by the experts, emphasizing the importance of a range of professional views rather than simply relying on a small group of researchers who may have a biased view.
User input into questionnaire design is important in maximizing face validity. The views of parents in this study provided valuable additional feedback to improve clarity, and provided further evidence of this aspect of content validity.
Limitations of the Study
Although expert panels allow for a wide range of opinion to be expressed and a level of agreement reached, there may be difficulties where some items receive CVI scores of less than the “acceptable” level of 0.78. It was felt necessary to include such items to ensure adequate content coverage such that questionnaire length was not unduly reduced prior to further psychometric testing. We have therefore included some questions for these reasons, which in their original wording, were not rated by all experts as highly relevant. Although the scale level CVI was adequate, this could be seen as weakening the case for content validity, but conversely, removing too many items too early in the development process is recognized as potentially problematic. A second round of content review could have been carried out by the expert group using the reworded questions in order to reassess the CVI, but, given that the items would still need to be tested more rigorously with psychometric analysis, this was not judged to be a useful exercise.
The parents who contributed to the cognitive interviews were active members of DS support groups, from healthcare backgrounds with a relatively high educational level and may not represent the whole population of parents looking after children with DS in the UK. Although their opinions and confidence in speaking aloud helped to modify the questions, different responses may have been obtained from using a wider group. Ideally, cognitive interviews would have been conducted with more families across a broader range of educational ability and cultural background. However, we were limited by the parents who volunteered to participate within the time period of the study. Nonetheless, the Flesch Reading Ease score shows that the language used should be accessible to parents of lower reading ability (31).
Further Work
The items used in this questionnaire were taken from other validated scales. However, evidence for validity must be acquired afresh when the items are used in a different tool and for a different patient group to the original. Although we have started this process, work now needs to be done to provide evidence of construct validity, internal consistency, and test–retest reliability as well as sensitivity to change. This work has commenced with the use of the questionnaire in a large study of young children aged 6 months to 6 years with DS (30).
Conclusion
Pediatric sleep questionnaires use parents’ observations of their child’s sleep to formulate a view of sleep behavior that can be used in a clinical context, in this case to screen for OSA in children with DS. Questionnaires should aid clinical decision making and reduce errors of judgment. In this context, the questionnaire should describe a symptom or behavior associated with OSA to the parent observer in a clear manner. This ensures the perception of a symptom or behavior between clinician and parent is the same. Where parents observe clinically significant signs in their DS children, such as apnea, they do not always report this to their child’s pediatrician (15), and similarly, physicians may overlook OSA in a child assessed during the daylight waking hours. The use of a questionnaire should therefore reduce bias and errors associated with human judgment (19). We hope to have achieved the first important step in the production of an unbiased questionnaire using a systematic approach to optimize content validity and clarity of presentation.
Author Contributions
CH and HE conceived the idea for the study, CH, HE, and CT designed the work, and ES carried out the data collection. All authors were involved with data analysis and interpretation, all were involved with drafting and revising the paper, and all have approved the final draft.
Conflict of Interest Statement
Dr. Catherine Mary Hill has received honoraria from Janssen Pharmaceuticals by way of speaker’s fees and a study grant from Respironics UK (2006) to attend an overseas course. The remaining co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the members of our expert panel and the parents who were interviewed for this study. ES undertook this work as part of her BMedSc undergraduate thesis at the University of Southampton.
References
1. Primhak R, O’Brien C. Sleep apnoea. Arch Dis Child Educ Pract Ed (2005) 90:87–91. doi: 10.1136/adc.2005.072975
2. Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med (2001) 164:16–30. doi:10.1164/ajrccm.164.1.2008171
3. de Miguel-Diez J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep (2003) 26:1006–9.
4. Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatrics (1991) 88:132.
5. Stores RJ, Stores G. The significance of aspects of screening for obstructive sleep apnoea in children with Down syndrome. J Intellect Disabil Res (2014) 58(4):381–92. doi:10.1111/jir.12033
6. Owens JA. Neurocognitive and behavioural impact of sleep disordered breathing in children. Pediatr Pulmonol (2009) 44:417–22. doi:10.1002/ppul.20981
7. Churchill SS, Kieckhefer GM, Bjornson KF, Herting JR. Relationship between sleep disturbance and functional outcomes in daily life habits of children with Down syndrome. Sleep (2015) 38(1):61–71. doi:10.5665/sleep.4326
8. Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol (2014) 56:657–64. doi:10.1111/dmcn.12376
9. Marcus CL, Moore RH, Rosen CL, Giordani B, Garetz SL, Taylor HG, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med (2013) 368:2366–76. doi:10.1056/NEJMoa1215881
10. Ng DK, Chan CH, Cheung JM. Children with Down syndrome and OSA do not necessarily snore. Arch Dis Child (2007) 92:1047–8.
11. Dyken ME, Lin-Dyken DC, Poulton S, Zimmerman MB, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in Down syndrome. Arch Pediatr Adolesc Med (2003) 157:655–60. doi:10.1001/archpedi.157.7.655
12. Sproson EL, Hogan AM, Hill CM. Accuracy of clinical assessment of paediatric obstructive sleep apnoea in two English centres. J Laryngol Otol (2009) 22:1–8. doi:10.1017/S0022215109005532
13. Brietzke SE, Katz ES, Roberson DW. Can history and physical examination reliably diagnose pediatric obstructive sleep apnea/hypopnea syndrome? A systematic review of the literature. Otolaryngol Head Neck Surg (2004) 131:827–32. doi:10.1016/j.otohns.2004.07.002
14. Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest (1995) 108:610–8. doi:10.1378/chest.108.3.610
15. Lin SC, Davey MJ, Horne RS, Nixon GM. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr (2014) 165:177–122. doi:10.1016/j.jpeds.2014.02.032
16. Rosen D, Lombardo A, Skotko B, Davidson EJ. Parental perceptions of sleep disturbances and sleep-disordered breathing in children with Down syndrome. Clin Pediatr (Phila) (2011) 50:121–5. doi:10.1177/0009922810384260
17. Bull MJ. Health supervision for children with Down syndrome. Pediatrics (2011) 128:393–406. doi:10.1542/peds.2011-1605
18. Royal College of Paediatrics and Child Health. Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood, Standards for Services for Children with Disorders of Sleep Physiology Report (2009). pp 23–33.
19. Spruyt K, Gozal D. Paediatric Sleep Questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev (2011) 15:19–32. doi:10.1016/j.smrv.2010.07.005
20. Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med (2000) 1:21–32. doi:10.1016/S1389-9457(99)00009-X
21. Spruyt K, Gozal D. Development of pediatric sleep questionnaires as diagnostic tools: a brief review of dos and don’ts. Sleep Med Rev (2011) 15:7–17. doi:10.1016/j.smrv.2010.06.003
22. Slocumb EM, Cole FLA. Practical approach to content validation. Appl Nurs Res (1991) 4:192–5. doi:10.1016/S0897-1897(05)80097-7
23. Constantin E, Tewfik T, Brouillette R. Can the OSA-18 quality-of-life questionnaire detect obstructive sleep apnea in children? Pediatrics (2010) 125:e162–8. doi:10.1542/peds.2009-0731
24. Luginbuehl M, Bradley-Klug KL, Ferron J, Anderson WM, Benbadis SR. Pediatric sleep disorders: validation of the sleep disorders inventory for students. School Psych Rev (2008) 37:409–31. doi:10.1016/j.sleep.2014.03.021
25. Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics (1998) 102:616–20. doi:10.1542/peds.102.3.616
26. Li AM, Cheung A, Chan D, Wong E, Ho C, Lau J, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol (2006) 41:1153–60. doi:10.1002/ppul.20505
27. Lynn MR. Determination and quantification of content validity. Nurs Res (1986) 35:382–6. doi:10.1097/00006199-198611000-00017
28. Polit DF, Beck CT, Owen SV. Is the CVI an acceptable indicator of content validity? Appraisal and recommendations. Res Nurs Health (2007) 30:459–67. doi:10.1002/nur.20199
29. Willis G. Cognitive Interviews – A Tool for Improving Questionnaire Design. Thousand Oaks, CA: SAGE (2005).
30. Hill CM, Ashworth A, Elphick H, Evans H, Farquhar M, Gavlak J, et al. Prevalence of obstructive sleep apnoea in young children with Down syndrome: a domiciliary cardiorespiratory study. Abstract, Biennial Meeting of the European Sleep Research Society, Tallinn, Estonia, 16-20 September (2014).
Appendix
Final Questionnaire
Sleep Questionnaire for Children with Down syndrome
Please try to think of a typical week when your child has been well.
Please circle the response you feel is most appropriate for your child from the options;
Never, Rarely, Occasionally, Almost Always, Always or Unsure.
Please only select one option for each of the questions and please answer all the questions.
Child’s name………………………………………………………………………………
Child’s age………………… years ……………….months
Date completed questionnaire………/………/……….
Keywords: obstructive sleep apnea, Down syndrome, sleep disorders, pediatrics, screening, measurement
Citation: Sanders E, Hill CM, Evans HJ and Tuffrey C (2015) The development of a screening questionnaire for obstructive sleep apnea in children with Down syndrome. Front. Psychiatry 6:147. doi: 10.3389/fpsyt.2015.00147
Received: 05 March 2015; Accepted: 24 September 2015;
Published: 20 October 2015
Edited by:
Karen Spruyt, Vrije Universiteit Brussel, Belgium; Jiaotong University, ChinaReviewed by:
Jean-Paul Praud, Université de Sherbrooke, CanadaAnneke Maas, Radboud University Nijmegen, Netherlands
Silvia Miano, Civic Hospital of Lugano, Switzerland
Copyright: © 2015 Sanders, Hill, Evans and Tuffrey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Tuffrey, tuffrey@yahoo.co.uk
†Hazel Jean Evans and Catherine Tuffrey are joint last authors.
 Emma Sanders
Emma Sanders Catherine Mary Hill
Catherine Mary Hill Hazel Jean Evans
Hazel Jean Evans Catherine Tuffrey
Catherine Tuffrey