- 1Department of Psychiatry, College of Medicine, University of Ibadan, Ibadan, Nigeria
- 2Department of Behavioral Sciences, Lagos State University, Lagos, Nigeria
Objective: To determine whether screening, brief intervention, and referral for treatment can reduce the prevalence of tobacco use in rural and semi-rural settings.
Method: Design and participants: A non-randomized clinical trial with assessments at baseline and post-intervention assessments at 3 and 6 months was conducted in a rural and semi-rural district in South-West of Nigeria. A representative sample of 1203 persons consented to the study and had alcohol, smoking, and substance involvement screening test (ASSIST) administered to them by trained community health-care extension workers between October 2010 and April 2011. Follow-up participation was more than 99% at all points. Intervention: Participants received a single ASSIST-linked brief intervention (BI) and referral for treatment (RT) at entry, and a booster ASSIST BI and RT at 3 months. Main outcomes and measures: The primary outcome was self-reported scores on ASSIST.
Results: At baseline, out of 1203 respondents, lifetime prevalence and current prevalence of any tobacco products were 405 (33.7%) and 248 (20.6%), respectively. Of the current users, on the ASSIST, 79 (31.9%) scored 0–3 (low health risk), 130 (52.4%) scored 4–26 (moderate risk), and 39 (15.7%) scored 27+ (high risk). At 3 months, out of 1199 respondents, prevalence of current users was 199 (16.5%) and out of 1195 respondents, was 169 (14.1%) at 6 months. Prevalence of tobacco use reduced significantly at 3 months Z = −3.1, p = 0.01 and at 6 months when compared with baseline Z = 4.2, p = 0.001, but not at 6 months compared with at 3 months, Z = 2.1, p = 0.09. Multivariate analysis revealed that age at initiation of tobacco use, gender, marital status, setting of dwelling, and socioeconomic status were the only variables that were associated with current tobacco use at baseline, 3 and 6 months.
Conclusion: A one-time BI with a booster at 3 months had a significant effect on tobacco use in persons living in community settings. This finding suggests a need for promoting the adoption of this intervention for tobacco use in rural and semi-rural community settings.
Introduction
While globalization of the use of cigarette and other products of tobacco is a major threat to public health worldwide (1, 2), studies have noted the decline in tobacco use in high-income countries and an increase in use in middle- and low-income countries (3, 4). For instance, Guindon and Boisclair reported that from 1970 to 2000, per capita cigarette consumption reduced by 14% in the Western world while it increased by 46% in the developing nations (3).
The growth of cigarette use in developing countries might be linked to the marketing efforts of tobacco companies, loose restrictions of tobacco control policies (5), and poor surveillance of smoking prevalence in these countries (3, 6, 7). For instance, in Nigeria, cigarette imports have increased over the years from 20 million sticks in 1970, to 198 million in 1990, and 2966 in 2000 (8). In addition, Nigeria ranks third among the largest tobacco markets in Africa after Egypt and South Africa. Despite this increase, the lifetime prevalence of tobacco use is 17% (9), and the overall prevalence of tobacco use in Nigeria is 8.9% (10), which is comparatively low compared with the US prevalence rate of 16.8% (11). Thus, Nigeria appears to be in early stages of cigarette epidemic. Estimate of deaths from smoking-attributed causes in sub-Saharan Africa reaches only 5–7% for men and 1–2% for women (12). Yet, this status and the consequences of cigarette use are not likely to respond to a quick change, partly due to weak government restrictions on tobacco use or sales.
In Nigeria, the infrastructure for tobacco control is poor despite the country’s ratification of the WHO Framework Convention on Tobacco Control (13). Nigeria has not implemented regulations such as age verification for sales, sales to minors, misleading information on packaging, the amount of tar and nicotine, product constituents as confidential information, product constituents as public information, constituent disclosure by brand, and constituent disclosure by aggregate, smoking in restaurants, nightclubs, and bars (14). Specifically, the Tobacco Control Bill, which was passed in the National Assembly about a decade ago, was yet to be adopted (15). Hence, the unbridled widespread use of tobacco in Nigeria may jeopardize future improvements in longevity that could be gained by curbing the impact of AIDS, starvation, and violence (16). Of note is the fact that attributable mortality to tobacco use is rising and if the current epidemic continues, more than 70% of these deaths are expected to occur in developing countries including Nigeria (17).
Therefore, there is a need to find other strategies that might work while efforts are ongoing to firm up tobacco restrictions at the government level in Nigeria. Screening, brief intervention, and referral for treatment (SBIRT) is a public health model that is used to screen for substance abuse and also for the delivery of low-intensity substance abuse treatments in a primary health-care setting (18). SBIRT for unhealthy drug use has been described in the scientific literature for over 50 years (19), and there has been relatively robust evidence for its effectiveness for substance use, particularly unhealthy alcohol use (20). However, some studies have noted that evidence for the effectiveness of SBIRT is much more limited for other drug use and in settings other than primary care (21).
In the Western world, the provision of SBIRT as a form of smoking cessation intervention is generally toward treatment seeking smokers (22). In developing countries such as Nigeria, where the majority live in rural settings, and access to medical care is limited to urban centers, smoking cessation treatment may not be offered until tobacco-related disease is detected or until the smoker expresses interest in quitting. Within this context, SBIRT will be particularly well suited for non-treatment seeking smokers of cigarette. In other words, while much attention has focused on primary care as a setting to promote smoking cessation, community settings could be uniquely positioned for tobacco intervention efforts in resource poor settings, such as Nigeria, where access to hospital care is limited. However, given the studies that reported inefficacy or mixed results of SBIRT, SBIRT intervention should not be taken as universally effective.
Therefore, in the developing countries, such as Nigeria, where it has not been evaluated before, there is a need for evaluative studies to guide policy and interventions. The current investigation was a single arm study designed to examine the prevalence of tobacco use and evaluate the effectiveness of SBIRT in semi-rural and rural communities. We hypothesized that participants who receive SBIRT intervention would demonstrate a decrease in cigarette consumption.
Materials and Methods
Study Area
Nigeria is a developing country in West Africa. Nigeria was ranked 191st among 194 member states of the World Health Organization in terms of overall health attainment. The study site was in Ibadan, Oyo State. Ibadan is the capital of Oyo state, Nigeria, and it is the third largest city in Nigeria. The city is located in the southwestern part of the country. It has a population of over 3.5 million people and 11 local government areas (23).
Ethical approval for the study was obtained from the Ethical Review Committee of the Ministry of Health, Oyo State, Nigeria. Assent was obtained from participants between 15 and 18 years and informed consent from 18 years and above.
Study Design
A systematic stratified sampling method was used to select two local governments in Ibadan between October 2010 and April 2011. In the first stage, all 11 LGA were classified into rural or semi-rural based on government fund allocation. In the second stage, one local government was randomly chosen from each group, and in the third stage, four enumeration areas were systematically selected as clusters. The fourth stage involved the mapping and numbering of all buildings in each of the selected enumeration areas. All households within each building were serially listed in the Form specifically designed for the purpose. After getting the list of the households, simple random sampling was used to identify the households that fell within the sample. Regular households were distinguished from institutional households. All eligible respondents, who were 15 years and above in each household, were selected and were interviewed using the questionnaires including alcohol, smoking, and substance involvement screening test (ASSIST) after they gave consent/assent. The inclusion criteria for the study were both male and female tobacco users of age ≥15 years and permanent residents of study areas. The exclusion criteria were non-users of tobacco of age <15 years, not willing to get tobacco cessation intervention, and not a permanent resident of the study areas.
Intervention Training in SBIRT and Quality Control
To increase the possibility of an effect while observing a real-world feasibility in a resource poor setting, brief interventionists were recruited from community health-care extension workers (CHEWs) in participating primary health-care clinics (n = 18). The CHEWs had been involved with previous surveys and agreed to adhere to the study protocol. All interventionists were trained by Victor Olufolahan Lasebikan using a group workshop followed by individual feedback on audio-taped role plays with up to five standardized patients over the course of 5 weeks (24). Treatment fidelity was assessed by scoring of 5 audio-taped role plays using Motivational Interviewing Treatment Integrity coding system (MITI 3.0) (25). Mean global scores ranged from 4.35 to 4.64 which were well above the proficiency benchmark of 4.0. All interventionists met basic motivational interviewing proficiency training goals on at least one practice role play.
A 3 days of debriefing and review of all protocols were carried out, after a pilot survey in each of the study local governments. Each interviewer had conducted two pilot interviews in the field. All questionnaires were reviewed for completeness by field coordinators. The pilot studies were carried out in a ward unit as enumerated during the National population census in each of the study local governments. This was to assess applicability of the instruments of data collection and research adherence.
Instruments
1. A sociodemographic pro forma was specifically designed for this study to elicit the sociodemographic characteristics of the respondents. The questionnaire contained items such as age, age at initiation, frequency of tobacco use, marital status, socioeconomic class, and years of education. For the purpose of this study, tobacco use was synonymous with smoking.
2. The ASSIST was developed mainly to screen for drug use, but can be used for other substances, including alcohol and tobacco as well, particularly in high prevalence settings (26). The ASSIST is an eight-item instrument that screens for use of all substance types [tobacco products, alcohol, cannabis, cocaine, amphetamine-type stimulants (ATS), sedatives, hallucinogens, inhalants, opioids, and “other” drugs] and determines a risk score (“lower,” “moderate,” or “high”) for each substance (27). The risk scores are generated from item questions 2–7 for tobacco. The responses are scored from 0 to 6 based on the frequency of use of the specific substance. The risk scores are recorded on the ASSIST feedback report card which is used to give personalized feedback to clients by presenting them with the scores that they have obtained, and the associated health problems related to their level of risk. Asking clients if they are interested in viewing their scores allows the health worker to commence a discussion (brief intervention) with the client in a non-confrontational way and has been found to be a successful way of getting clients at moderate risk, in particular, to change their substance use (27). Scoring: for tobacco use, 0–3 (low risk), 4–26 (moderate risk), and 27+ (high risk). This instrument was used to score a representative sample of 1203 adolescents and adults (15 years and over) for the risk of hazardous and harmful consequences of tobacco and other substances.
The lifetime prevalence of tobacco use was obtained from Q1: “In your life, which of the following substances have you ever used (non-medical use only)?” We obtained current prevalence of tobacco use from Q2: “In the past 3 months how often have you used the substances you mentioned?” Responses were “never,” “once or twice,” “monthly,” “weekly,” and “daily/almost daily.” For the purpose of this study, use in the past 3 months was considered to be current use.
Procedure
For those who screened positive for unhealthy tobacco use, ASSIST-Linked SBIRT was conducted as appropriate.
Intervention
The intervention for those who had a low risk of tobacco use (score of 0–3) was general health advice, for those with moderate risk (score of 4–26), was brief intervention and a leaflet containing information about tobacco use, and those with high risk tobacco use (score of 27+) had brief intervention, information leaflet on tobacco use and were offered referral to a specialist hospital for further assessment and treatment.
The information leaflet about tobacco use contained facts about the consequences of unhealthy tobacco use, tips for reducing the risk of tobacco-related harm, and sources of support for tobacco problems (e.g., contact details of services available in the local health district). Respondents, who had an unhealthy tobacco use, were followed up and reassessed at 3 and 6 months.
A booster brief intervention and referral to treatment was given at 3 months. Interviewers used eight anchor community members to maintain contact with members of the household, while interviewers maintained contact with these anchor persons in between interviews. Tobacco use in this study is defined as cigarette smoking.
Evaluation of the Intervention
The outcome of the intervention was assessed by evaluating changes in the mean number of cigarettes smoked/day at 3 and 6 months post-intervention and the mean ASSIST scores at 3 and 6 months. This is in accordance with the application of ASSIST instrument in following up clients over time. The use of mean ASSIST score has been specifically found to be highly valuable in assessing changes in ASSIST scores over time (28).
Data Analysis
For our univariate analysis, the association between sociodemographic variables and current tobacco use was determined using the Pearson’s chi square statistics. Using the current prevalence rates at baseline, the Wilcoxon Signed-Rank test and paired t-test were used to determine significant changes in the proportion of tobacco users and the mean ASSIST scores at 3 and 6 months. For categorical data, all Chi squares were Yates corrected for all two levels comparisons and Bonferroni corrected for comparisons more than two levels. Following Bonferroni corrections, multiple pairwise comparisons were carried out using Chi square statistics.
Multivariate analyses were carried out using variables that were significant during univariate analysis to determine the association with tobacco use. This was carried out using binary logistic regression. To facilitate the interpretation of odds ratio, a reference category was always chosen for the independent variables with which other independent variables could be compared with tobacco use. This was done for the data at baseline, at 3 and 6 months. Analysis of data was carried out using the Statistical Program for Social Studies SPSS version 13.0.
Results
Enrollment and Screening
The interventionists identified a total of 1329 community dwellers as potentially eligible, of whom 1213 underwent screening. Of them, 10 were excluded because of the presence of severe general medical conditions, giving a response rate of 91.3%. The final analysis was carried out for 1203 questionnaires at baseline. At 3 months, analysis was carried out on 1199 respondents and on 1195 participants at 6 months.
Participants’ Characteristics
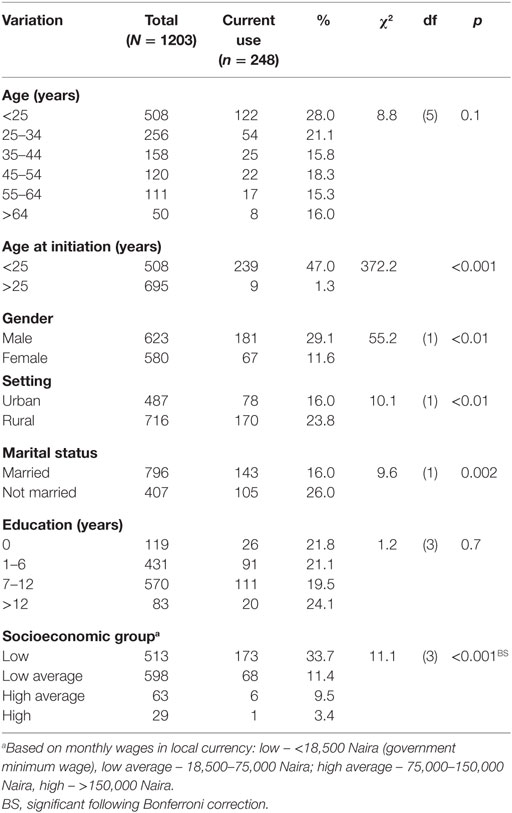
The mean age of respondents at baseline was 24.45 ± 9.23 years, 51.8% were males, 66.2% were married, 47.4% had at least some secondary education, and 49.7% were of low-average socioeconomic group. Current tobacco use was more significant among males, χ2 = 55.2, p < 0.01, unmarried, χ2 = 9.6, p = 0.002, and low socioeconomic group, χ2 = 11.1, p < 0.001 (Table 1). Mean age of initiation into smoking was 17.83 (3.23) years.
Intervention Effects
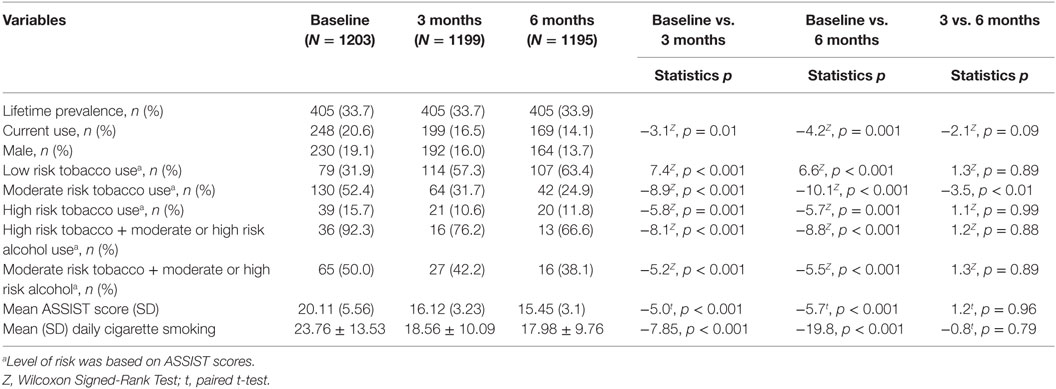
At baseline, overall lifetime prevalence and current prevalence of any tobacco products was 33.7 and 20.6%, respectively. At 3 months, prevalence of current tobacco use was 16.5% and was 14.1% at 6 months. The prevalence of tobacco use reduced significantly at 3 months Z = −3.1, p = 0.01 (RR: 0.009 < 0.04 > 0.071) and at 6 months when compared with baseline Z = −4.2, p = 0.001 (RR: 0.034 < 0.065 > 0.095), but not at 6 months compared with at 3 months, Z = −2.1, p = 0.09 (RR: −0.004 < 0.025 > 0.0536) (Table 2).
Of the current users, 79 (31.9%) scored between 0 and 3 on the ASSIST (at low health risk), 130 (52.4%) scored between 4 and 26 on the ASSIST (at moderate health risk), and 39 (15.7%) scored 27+ on the ASSIST (at high health risk). The mean ASSIST score significantly reduced at 3 and 6 months, compared with baseline measure, t = 5.0, p < 0.001 and t = −5.7, p < 0.001, respectively (Table 2).
Referral to Treatment and Engagement
Thirty-nine (15.7%) participants had ASSIST scores ≥27 and were referred for treatment. At 3 months follow-up, 21 (10.6%) participants were referred for treatment and 20 (11.8%) at 6 months follow-up (Table 2).
Of the 39 current users at high risk of health problems, 36 (92.3%) were also at either moderate or high risk of alcohol. Sixty-five (50.0%) of the 130 current users at moderate risk of health problems were either at high or moderate risk of health problems from alcohol (Table 2).
There was a significant reduction in the mean number of cigarettes smoked per day at 3 months compared with baseline, t = −7.85, p < 0.001 and also at 6 months compared with baseline, t = −19.8, p < 0.001 (Table 2).
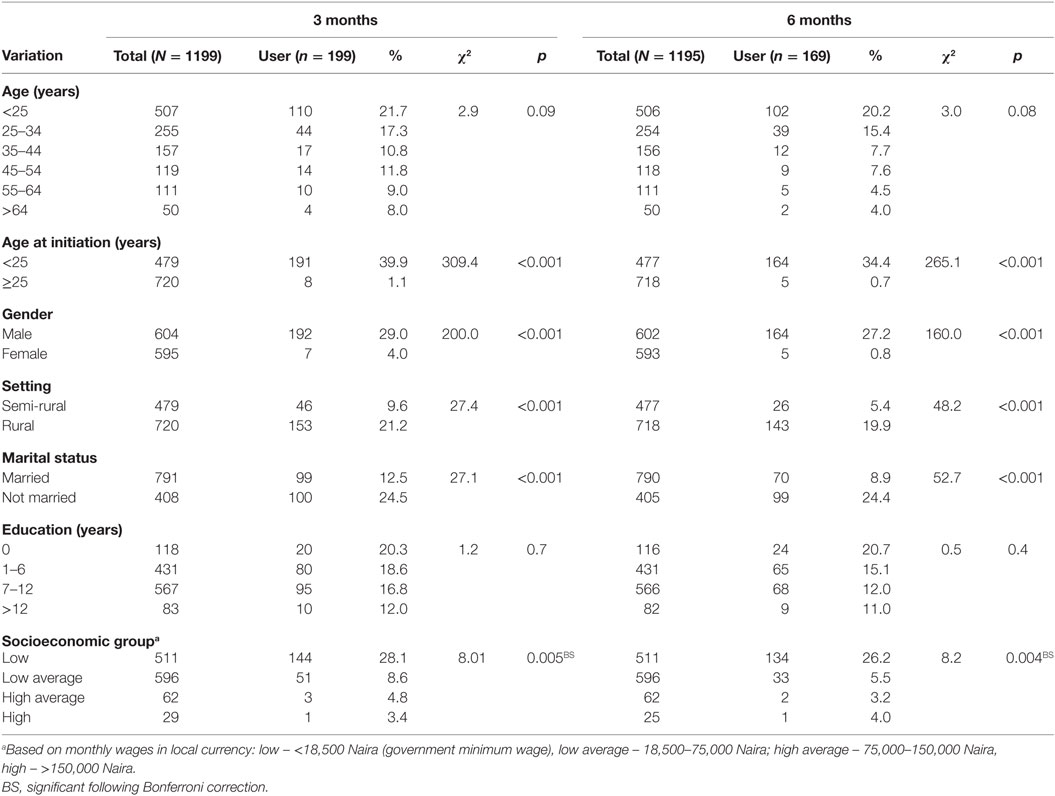
At 3 months, a significantly higher proportion of respondents whose age of initiation into tobacco use was <25 years were current users compared with those whose age at initiation into tobacco use was ≥25 years, χ2 = 309.4, p < 0.001. Also, a higher proportion of respondents, who were of male gender were current tobacco users compared with the female gender, χ2 = 200.0, p < 0.001. A significantly higher proportion of respondents who were rural dwellers were current tobacco users compared with the semi-rural dwellers, χ2 = 27.4, p < 0.001. A significantly higher proportion of respondents who were unmarried were current tobacco users compared with those who were married, χ2 = 27.1, p < 0.001. There was also a significant difference in the prevalence of current tobacco use across the socioeconomic status of these respondents χ2 = 8.01, p = 0.005. Post hoc multiple comparisons show that this difference was due to a higher current tobacco use among the low socioeconomic group, compared with the low average group χ2 = 28.2, p < 0.001, on the one hand, a higher current tobacco use among the low socioeconomic group compared with the high average group FE p < 0.001 and a higher current tobacco use among the low socioeconomic group compared with the high socioeconomic group FE p < 0.001, on the other hand.
At 6 months, a significantly higher proportion of respondents whose age at initiation into tobacco use was <25 years were current users compared with those whose age at initiation into tobacco use was ≥25 years, χ2 = 265.1, p < 0.001. Also, a higher proportion of respondents who were of male gender were current tobacco users compared with the female gender, χ2 = 160.0, p < 0.001. A significantly higher proportion of respondents who were rural dwellers were current tobacco users compared with the semi-rural dwellers, χ2 = 48.2, p < 0.001. A significantly higher proportion of respondents who were unmarried were current tobacco users compared with those who were married, χ2 = 52.7, p < 0.001. There was also a significant difference in the prevalence of current tobacco use across the socioeconomic status of these respondents χ2 = 8.2, p = 0.004. Post hoc multiple comparisons show that this was due to a higher current tobacco use among the low socioeconomic group compared with the low average group χ2 = 59.3, p < 0.001, on the one hand, a higher current tobacco user among the low socioeconomic group compared with the high average group FE p < 0.001 and a higher current tobacco user among the low socioeconomic group compared with the high socioeconomic group FE p < 0.001, on the other hand (Table 3).
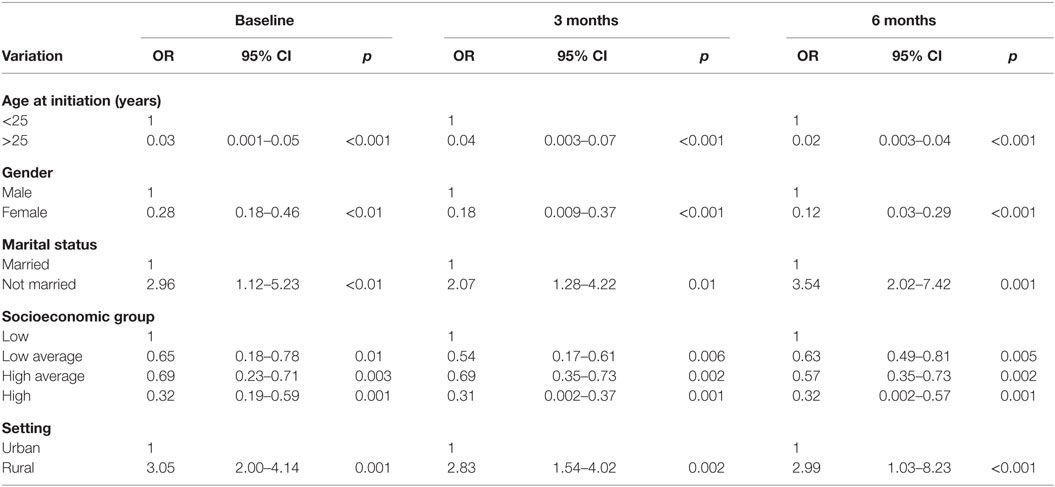
Multivariate analysis reveals that at baseline, significant factors that remained associated with current tobacco use were age at initiation into tobacco use OR = 0.03, 95% CI (0.001–0.05), p < 0.001, female gender OR = 0.28, 95% CI (0.18–0.46), p < 0.01, being unmarried OR = 2.96, 95% CI (1.12–5.23), p < 0.01, high socioeconomic status OR = 0.32, 95% CI (0.19–0.59), p = 0.001, high average socioeconomic status, OR = 0.69, 95% CI (0.23–0.71), p = 0.003, low average socioeconomic status, OR = 0.65, 95% CI (0.18 = 0.78), p = 0.01, and being a rural dweller OR = 3.05, 95% CI (2.00–4.14), p = 0.001.
At 3 months, significant factors that remained associated with current tobacco use were age at initiation into tobacco use OR = 0.04, 95% CI (0.003–0.07), p < 0.001, female gender OR = 0.18, 95% CI (0.009–0.37), p < 0.001, being unmarried OR = 2.07, 95% CI (1.28–4.22), p < 0.01, high socioeconomic status OR = 0.31, 95% CI (0.002–0.37), p = 0.001, high average socioeconomic status, OR = 0.69, 95% CI (0.35–0.73), p = 0.002, low average socioeconomic status, OR = 0.54, 95% CI (0.17–0.61), p = 0.006, and being a rural dweller OR = 2.83, 95% CI (1.54–4.02), p = 0.002.
At 6 months, significant factors that remained associated with current tobacco use were age at initiation into tobacco use OR = 0.02, 95% CI (0.003–0.04), p < 0.001, female gender OR = 0.12, 95% CI (0.03–0.29), p < 0.001, being unmarried OR = 3.54, 95% CI (2.02–7.423), p = 0.001, high socioeconomic status OR = 0.32, 95% CI (0.002–0.57), p = 0.001, high average socioeconomic status, OR = 0.57, 95% CI (0.35–0.73), p = 0.002, low average socioeconomic status, OR = 0.63, 95% CI (0.49–0.81), p = 0.005, and being a rural dweller OR = 2.99, 95% CI (1.03–8.23), p < 0.001 (Table 4).
Discussion
This study is most probably the first in sub-Saharan Africa that aimed to determine in semi-rural and rural community settings, the prevalence and correlates of tobacco use, as well as the effectiveness of ASSIST-Linked SBIRT in unhealthy tobacco users among these communities dwellers.
The lifetime prevalence of tobacco use among our participants was 33.7% and this is not much lower than the 44% lifetime use among those 15 years and older in Canada (29). We also found that the current prevalence of tobacco use was 20.6% at baseline. This is higher than the 17% prevalence reported among Nigerian adults in 2007 in a nationally representative sample (9), but is similar to the 16 and 20% prevalence of tobacco use among persons who were 15 years and above in Canada and America, respectively. It is also similar to the overall tobacco use prevalence of 21% in India (30). However, compared with our estimates, the 2012 Global Adult tobacco Survey (GATS) that was conducted in 16 countries found higher current tobacco use prevalence in North America, Europe, and South Asia (31). Nonetheless, our finding suggests that tobacco use might be assuming epidemic proportions in people who live in rural and semi-rural settings in Nigeria.
Another key finding in our study is the group of correlates of tobacco use among current users. Those who used tobacco were more likely to have started around 17 years of age, to be males, unmarried, of low socioeconomic status and live in a rural setting. With respect to the age at initiation of tobacco use, this is similar to the age at smoking initiation that is before the age of 18 years in Western countries (32). We acknowledge that the average age at tobacco initiation varies by country, income, education, and age cohort (31, 33–35). In addition, we note that differences in age at initiation by country may reflect variation in stages of tobacco use epidemic between countries or the complexity of tobacco control measures implemented (36). We cautioned above that tobacco use in the rural and semi-rural settings in Nigeria might be assuming epidemic proportions. In line with this, we situate our current finding of lower age at initiation of tobacco use as highlighting a significant problem that could be faced in the future of the health consequences of tobacco use and dependency on tobacco.
From the foregoing, one could justifiably ask “what could make youths especially in rural settings use tobacco at an earlier age?” Our data deductively serve to guide and stimulate additional research. In addition, priority needs to be given to the development of country specific tobacco control programs for adolescents and youths. Furthermore, given the public health importance of tobacco-related diseases such as CVD and other CVD risk factors (e.g., diabetes, hypertension) (37, 38), it is critical to track tobacco use within specific contexts (i.e., rural vs. semi-rural settings) in Nigeria and to characterize tobacco use patterns in terms of populations that could be vulnerable to tobacco use.
Concerning other correlates, our study aligns with prior research (30, 38–40). In both India and Pakistan, some predictors of tobacco use are male gender, low socioeconomic status, and rural geographic location (30, 41). We confirm that male gender is a correlate of tobacco use in low-income countries, in contrast to middle- and high-income countries where the male preponderance is blurring (42). Similar to the male gender contrast, our findings as regards the association between tobacco use and rural dwelling is in line with low-income countries (43) but dissimilar to findings in middle- and high-income countries where tobacco use correlates with dwellers in large cities (44). However, our result with respect to a positive relationship between tobacco use and low socioeconomic status is in agreement with Western findings (44) as well as findings from other developing nations (30, 38, 39). It is possible that unawareness of the health risks of tobacco use might be the factor that ties these associations together. Further research is needed to tease out the possible moderating influence of lack of information on the health risk of tobacco use.
Our observation of an associated alcohol-related health risk among respondents with tobacco-related health risks is illustrative of the co-use of both tobacco and alcohol. Studies have found that smokers are much more likely to use alcohol and vice versa (45). Also, tobacco and alcohol share a similar psychological mechanism as subjective mood altering chemicals that are socially learned and are used by some individuals as coping mechanisms (46). Moreover, repeated use acts as positive reinforcement (47); the pharmacological dependence of nicotine usually increases the probability of alcohol use, usually in social settings because of the social acceptability of alcohol.
A major finding in this study is that it underscores the usefulness and applicability of SBIRT in the hands of CHEW and the positive impact of SBIRT delivered through CHEW on tobacco use as well as unhealthy use in a semi-rural community setting. This current study is important in three ways: (1) it focused on tobacco, and not alcohol, (2) SBIRT was deliverable by CHEW rather than by clinicians only, and (3) it enrolled people in the community with poor access to orthodox medicine and who might not seek treatment rather than those who went to the hospital or were admitted in emergency settings.
In rural and semi-rural community settings, we investigated the usefulness of a single session of brief intervention with a booster session in reducing tobacco use. A major finding in this assessment was that the rate of tobacco use reduced significantly between baseline and 3 and 6 months, respectively. There were significant shifts from high risk to moderate and low risk use of tobacco.
Our study was limited by a number of factors. First, we did not stratify the users into different stages of change. In other words, we could not assess the impact of different stages of change in unhealthy tobacco use in our study population. This is very relevant considering reports indicating that psychosocial interventions, that target behavioral change often do not yield a significant effect (48). We therefore recommend further studies to explore the possible influence of stages of change in tobacco use reduction. Second, we did not assess tobacco cessation and tobacco cessation in relation to SBIRT. The main objective of SBIRT being cessation from substance use. Future studies are required to access tobacco cessation following SBIRT, because the reduction in the tobacco use rate does not equate cessation. Third, we did not include a control group. This has greatly limited the interpretation of the effect of the intervention. Fourth, all our analysis was based on self-reports. Future works require, including a toxicological screen to their methodology. Fifth, we also did not allocate any diagnosis to the tobacco users; therefore, it was difficult to determine if the effect of the intervention was on sparing users or long-term users.
In conclusion, while tobacco use is reaching epidemic proportions in rural and semi-rural settings in Nigeria and is associated with male gender, early age at initiation into use, low socioeconomic status, and living in rural areas, SBIRT promises to have implementation potentials in delivering the intervention for the reduction of tobacco use and unhealthy tobacco use in semi-rural community in Nigeria.
Author Contributions
VL conceived the idea and was responsible for study design, analysis, and manuscript writing. BO was responsible for data collection and was also involved in manuscript writing. Both authors gave a substantial contribution to the study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Acknowledgment is given to the Director of Planning, Research and Statistics, Oyo state Ministry of Health, Ibadan for granting ethical approval for this study. Acknowledge with thanks is also given to Dr. O. Aremu (RRSH), Dr. O. Amoran (OOUTH), and Christianah Alabi (NWPSH) for providing support during data collection. I thank the coordinating staff members of the two local governments where the study took place and all participants.
Funding
New World Specialists Hospitals, Ibadan, Nigeria.
References
2. Yach D, Bettcher D. Globalisation of tobacco industry influence and new global responses. Tob Control (2000) 9(2):206–16. doi:10.1136/tc.9.2.206
3. Guindon G, Boisclair D. Past, current and future trends in tobacco use. Health, nutrition and population (HNP). Discussion Paper (Economics of Tobacco Control Paper No. 6): Nutrition and Population (HNP) Discussion Paper. Washington (2003).
4. Mackay J, Eriksen M, Shafey O. The tobacco atlas. Paper Presented at the American Cancer Society. Atlanta, GA (2006).
5. Warner K. The economics of tobacco: myths and realities. Tob Control (2000) 9(1):78–89. doi:10.1136/tc.9.1.78
6. Barisa E, Brigdena LW, Prindivellea J, Da Costa e Silvab VL, Chitanonhc H, Chandiwanad S. Research priorities for tobacco control in developing countries: a regional approach to a global consultative process. Tob Control (2000) 9(2):217–23. doi:10.1136/tc.9.2.217
7. Sasco AJ. Africa: a desperate need for data. Tob Control (1994) 3(3):281–281. doi:10.1136/tc.3.3.281
8. WHO. Tobacco Economy. (2009). Available from: http://www.who.int/tobacco/media/en/Nigeria.pdf
9. Gureje O, Degenhardt L, Olley B, Uwakwe R, Udofia O, Wakil A, et al. A descriptive epidemiology of substance use and substance use disorders in Nigeria during the early 21st century. Drug Alcohol Depend (2007) 91(1):1–9. doi:10.1016/j.drugalcdep.2007.04.010
10. WHO. WHO Report on the Global Tobacco Epidemic. (2015). Available from: http://www.who.int/tobacco/surveillance/policy/country_profile/nga.pdf
11. CDC. Current cigarette smoking among adults in the United States. MMWR Morb Mortal Wkly Rep (2015) 64(44):1233–40. doi:10.15585/mmwr.mm6444a2
12. Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control (2004) 13(4):388–95. doi:10.1136/tc.2003.005215
13. WHO FCTC. The World Health Organization Framework Convention on Tobacco Control. Geneva: WHO (2005).
14. Iyiola D. 10 Years of tobacco control: Nigeria fails domestication hurdles. The Nation. (2015). Available from: http://thenationonlineng.net/10-years-of-tobacco-control-nigeria-fails-domestication-hurdles/
15. Yishau O. Who wants tobacco bill dead? The Nations. (2012). Available from: http://www.thenationonlineng.net/2011/index.php/news/47664-who-wants-tobacco-control-bill-dead.html
16. Yach D, McIntyre D, Saloojee Y. Smoking in South Africa: the health and economic impact. Tob Control (1992) 1(4):272–80. doi:10.1136/tc.1.4.272
17. Peto R, Chen Z, Boreham J. Tobacco the growing epidemic. Nat Med (1999) 5(1):15–7. doi:10.1038/4691
18. Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abus (2007) 28(3):7–30. doi:10.1300/J465v28n03_03
19. Chafetz ME. A procedure for establishing therapeutic contact with the alcoholic. Q J Stud Alcohol (1961) 22:325–8.
20. Kaner EF, Dickinson HO, Beyer F, Pienaar E, Schlesinger C, Campbell F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev (2009) 28(3):301–23. doi:10.1111/j.1465-3362.2009.00071.x
21. Saitz R, Palfai TP, Cheng DM, Alford DP, Bernstein JA, Lloyd-Travaglini CA, et al. Screening and brief intervention for drug use in primary care: the assessing screening plus brief intervention’s resulting efficacy to stop drug use (ASPIRE) randomized trial. Addict Sci Clin Pract (2013) 8(Suppl 1):A61–61. doi:10.1186/1940-0640-8-S1-A61
22. Fiore MC, Jaen CR, Baker TB, Bailey WC, Bennet G, Benowitz NL, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: P. H. S. Department of Health and Human Services (2008).
23. Ruaf Foundation. Ibadan (Nigeria) (1999). Available from: http://www.ruaf.org/node/1517
24. Baer JS, Rosengren DB, Dunn CW, Wells EA, Ogle RL, Hartzler B. An evaluation of workshop training in motivational interviewing for addiction and mental health clinicians. Drug Alcohol Depend (2004) 73(1):99–106. doi:10.1016/j.drugalcdep.2003.10.001
25. Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat (2005) 28(1):19–26. doi:10.1016/j.jsat.2004.11.001
26. World Health Organization. The ASSIST-Linked Brief Intervention for Hazardous and Harmful Substance Use Manual for Use in Primary Care. (2010).
27. Humeniuk RE, Ali RA, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the alcohol smoking and substance involvement screening test (ASSIST). Addiction (2008) 103(6):1039–47. doi:10.1111/j.1360-0443.2007.02114.x
28. Humeniuk RE, Dennington V, Ali RL. The Effectiveness of a Brief Intervention for Illicit Drugs Linked to the ASSIST Screening Test in Primary Health Care Settings: A Technical Report of Phase III Findings of the WHO ASSIST Randomised Controlled Trial. Geneva: World Health Organization (2008).
29. Canadian Tobacco Use Monitoring Survey (CTUMS). (2012). Available from: http://www.hc-sc.gc.ca/hc-ps/tobac-tabac/research-recherche/stat/ctums-esutc_2012-eng.php
30. Chockalingam K, Vedhachalam C, Rangasamy S, Sekar G, Adinarayanan S, Swaminathan S, et al. Prevalence of tobacco use in urban, semi urban and rural areas in and around Chennai City, India. PLoS One (2013) 8(10):e76005. doi:10.1371/journal.pone.0076005
31. Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet (2012) 380(9842):668–79. doi:10.1016/s0140-6736(12)61085-x
32. Warren CW, Riley L, Asma S, Eriksen MP, Green L, Blanton C, et al. Tobacco use by youth: a surveillance report from the Global Youth Tobacco Survey project. Bull World Health Organ (2000) 78(7):868–76.
33. Gilman SE, Abrams DB, Buka SL. Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation. J Epidemiol Community Health (2003) 57(10):802–8. doi:10.1136/jech.57.10.802
34. La Vecchia C, Decarli A, Pagano R. Patterns of smoking initiation in Italian males and females from 1955 to 1985. Prev Med (1995) 24(3):293–6. doi:10.1006/pmed.1995.1047
35. Schulze A, Mons U. Trends in cigarette smoking initiation and cessation among birth cohorts of 1926-1970 in Germany. Eur J Cancer Prev (2005) 14(5):477–83. doi:10.1097/01.cej.0000174777.98518.7e
36. Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med (2013) 368(4):351–64. doi:10.1056/NEJMsa1211127
37. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. Differences in risk factors, atherosclerosis and cardiovascular disease between ethnic groups in Canada: the study of health assessment and risk in ethnic groups (SHARE). Indian Heart J (2000) 52(7 Suppl):S35–43.
38. Berg CJ, Ajay VS, Ali MK, Kondal D, Khan HM, Shivashankar R, et al. A cross-sectional study of the prevalence and correlates of tobacco use in Chennai, Delhi, and Karachi: data from the CARRS study. BMC Public Health (2015) 15:483. doi:10.1186/s12889-015-1817-z
39. Ahmad S, Zhao W, Renstrom F, Rasheed A, Samuel M, Zaidi M, et al. Physical activity, smoking, and genetic predisposition to obesity in people from Pakistan: the PROMIS study. BMC Med Genet (2015) 16(1):114. doi:10.1186/s12881-015-0259-x
40. McCabe CT, Woodruff SI, Zúñiga M. Sociodemographic and substance use correlates of tobacco use in a large, multi-ethnic sample of emergency department patients. Addict Behav (2011) 36(9):899–905. doi:10.1016/j.addbeh.2011.04.002
41. Angermeyer MC, Carta MG, Matschinger H, Millier A, Refai T, Schomerus G, et al. Cultural differences in stigma surrounding schizophrenia: comparison between Central Europe and North Africa. Br J Psychiatry (2016) 208(4):389–97. doi:10.1192/bjp.bp.114.154260
42. WHO, editor. WHO report on the global tobacco epidemic. The MPOWER Package. Geneva: World Health Organization (2008).
43. Khan MH, Khan A, Kraemer A, Mori M. Prevalence and correlates of smoking among urban adult men in Bangladesh: slum versus non-slum comparison. BMC Public Health (2009) 9:149. doi:10.1186/1471-2458-9-149
44. Kaleta D, Makowiec-Dabrowska T, Dziankowska-Zaborszczyk E, Fronczak A. Prevalence and socio-demographic correlates of daily cigarette smoking in Poland: results from the Global Adult Tobacco Survey (2009-2010). Int J Occup Med Environ Health (2012) 25(2):126–36. doi:10.2478/s13382-012-0016-8
45. Ellickson PL, Hays RD, Bell RM. Stepping through the drug use sequence: longitudinal scalogram analysis of initiation and regular use. J Abnorm Psychol (1992) 101:441–51. doi:10.1037/0021-843X.101.3.441
46. Aaro LE, Laberg JC, Wold B. Health behaviours among adolescents: towards a hypothesis of two dimensions. Health Educ Res (1995) 10:83–93. doi:10.1093/her/10.1.83
47. Cottraux J, Schbath J, Messy P, Mollard E, Juenet C, Collet L. Predictive value of MMPI scales on smoking cessation programs outcomes. Acta Psychiatr Belg (1986) 86(4):463–9.
Keywords: screening, brief intervention, community, tobacco, smoking
Citation: Lasebikan VO and Ola BA (2016) Community-Based Screening, Brief Intervention, and Referral for Treatment for Unhealthy Tobacco Use: Single Arm Study Experience and Implementation Success in Rural and Semi-Rural Settings, South-West Nigeria. Front. Psychiatry 7:134. doi: 10.3389/fpsyt.2016.00134
Received: 29 March 2016; Accepted: 19 July 2016;
Published: 02 August 2016
Edited by:
Thomas Heffernan, Northumbria University, UKReviewed by:
Janice Bartholomew, Teesside University, UKJonathan Ling, University of Sunderland, UK
Copyright: © 2016 Lasebikan and Ola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor Olufolahan Lasebikan, victorlash@yahoo.com
 Victor Olufolahan Lasebikan
Victor Olufolahan Lasebikan Bolanle Adeyemi Ola
Bolanle Adeyemi Ola