Open and Laparo-Endoscopic Repair of Incarcerated Abdominal Wall Hernias by the Use of Biological and Biosynthetic Meshes
- 1Department of General, Visceral and Oncological Surgery, Wilhelminenspital, Vienna, Austria
- 2Department of Surgery, Center for Minimally Invasive Surgery, Vivantes Hospital, Berlin, Germany
Introduction: Although recently published guidelines recommend against the use of synthetic non-absorbable materials in cases of potentially contaminated or contaminated surgical fields due to the increased risk of infection (1, 2), the use of bio-prosthetic meshes for abdominal wall or ventral hernia repair is still controversially discussed in such cases. Bio-prosthetic meshes have been recommended due to less susceptibility for infection and the decreased risk of subsequent mesh explantation. The purpose of this review is to elucidate if there are any indications for the use of biological and biosynthetic meshes in incarcerated abdominal wall hernias based on the recently published literature.
Methods: A literature search of the Medline database using the PubMed search engine, using the keywords returned 486 articles up to June 2015. The full text of 486 articles was assessed and 13 relevant papers were identified including 5 retrospective case cohort studies, 2 case-controlled studies, and 6 case series.
Results: The results of Franklin et al. (3–5) included the highest number of biological mesh repairs (Surgisis®) by laparoscopic IPOM in infected fields, which demonstrated a very low incidence of infection and recurrence (0.7 and 5.2%). Han et al. (6) reported in his retrospective study, the highest number of treated patients due to incarcerated hernias by open approach using acellular dermal matrix (ADM®) with very low rate of infection as well as recurrences (1.6 and 15.9%). Both studies achieved acceptable outcome in a follow-up of at least 3.5 years compared to the use of synthetic mesh in this high-risk population (7).
Conclusion: Currently, there is a very limited evidence for the use of biological and biosynthetic meshes in strangulated hernias in either open or laparo-endoscopic repair. Finally, there is an urgent need to start with randomized controlled comparative trials as well as to support registries with data to achieve more knowledge for tailored indication for the use of biological meshes.
Introduction
The BioMesh Study Group has set itself the task of identifying the best way to use biological meshes for various indications. The first step (toward achieving that goal) is to compile systematic reviews of different indications on the basis of the existing literature. The available literature sources will be evaluated in accordance with the Oxford Centre for Evidence-based Medicine-Levels of Evidence (March 2009). Next, based on the review findings, corresponding Statements and Recommendations are to be formulated in a Consensus Conference for the use of biological meshes regarding different indications. The findings of the Consensus Conference will then be summarized as a joint publication. This present publication is part of the project undertaken by the BioMesh Study Group.
Although recently published guidelines recommend against the use of synthetic non-absorbable materials in cases of potentially contaminated or contaminated surgical fields due to the increased risk of infection (1, 2), the use of bio-prosthetic meshes for abdominal wall or ventral hernia repair is still controversially discussed in such cases. Especially in these indications, bio-prosthetic meshes have been recommended due to less susceptibility for infection and the decreased risk of subsequent mesh explantation. The greatest drawback of bio-prosthetics is still the high cost in comparison to synthetic non-absorbable meshes (2). Above all, there is a lack of evidence concerning the clinical efficacy of biologic over synthetic non-absorbable meshes (7). In the literature, wound infection rates after the use of biological meshes even in clean-contaminated fields are reported up to 40% (8, 9) and hernia recurrence rates up to 30%, respectively (10). On the other hand, the reports of Zafar et al. (11) regarding emergency surgery of incarcerated incisional hernia with associated bowel obstructions enrolling 60 patients by the use of permanent prosthetic meshes revealed an almost identically high percentage (31%) of wound complications in a retrospective study. The purpose of this review is to elucidate if there are any indications for the use of biological and biosynthetic meshes in incarcerated abdominal wall hernias based on the recently published literature.
Methods
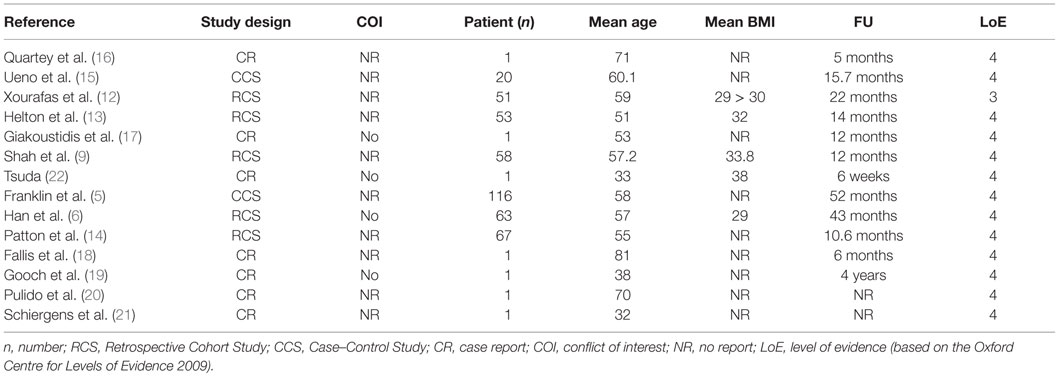
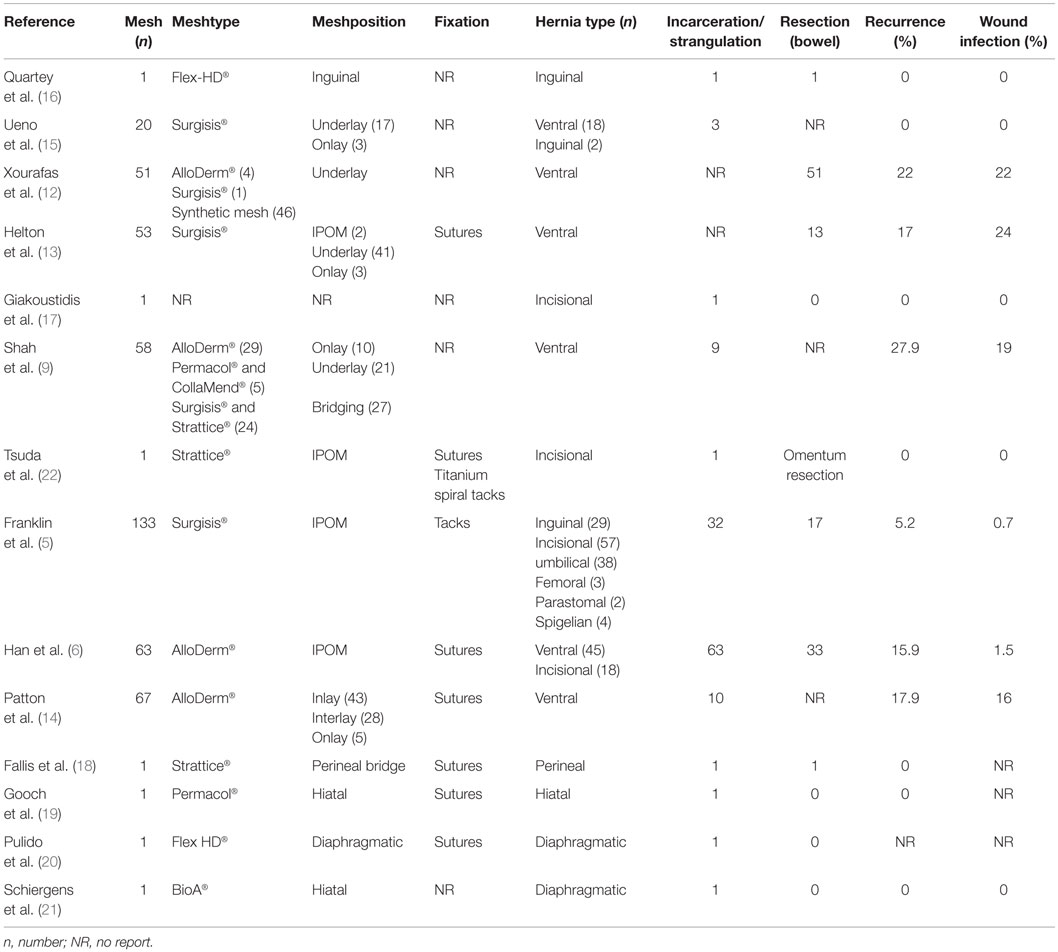
A literature search of the Medline database using the PubMed search engine, using the keywords (incarcerated hernia OR strangulated hernia OR inguinal hernia OR Groin hernia OR inguinal hernia OR ventral hernia OR incisional hernia AND biological mesh OR Biomesh OR Biological OR biosynthetic mesh AND open repair OR laparoscopic repair OR endoscopic repair) returned 486 articles up to June 2015. Titles and abstracts were searched for the use of biologic meshes in open and laparo-endoscopic repair of incarcerated/strangulated abdominal wall hernias. The full text of 486 articles was assessed, and 13 relevant papers were identified including 5 retrospective case cohort studies (9, 12–14), 2 case-controlled studies (5, 15) and 6 case reports (16–21). A summary of study demographics and characteristics is presented in Table 1 and the outcome data in Table 2. Qualitative assessment of all included studies was based on the Oxford Centre for Evidence-Based Medicine 2009 levels of evidence.
Results
In the special case of an incarcerated recurrent Amyand’s hernia, the only paper concerning the use of a biological mesh was published by Quartey et al. (16). After appendectomy in an open approach, an acellular hydrated dermis (Flex HD®) was implanted with an uneventful postoperative follow-up to 5 months. In a review regarding Amyand’s hernia, Michalinos et al. (23) concluded that in case of proper treatment, including the use of meshes, the morbidity or mortality is not increased beyond that of a typical inguinal hernia repair. Similar conclusions can be found in the review of Köckerling et al. (24) with the statement: “The use of biological meshes in inguinal hernia repair especially in potentially contaminated fields is an alternative to the use of synthetic meshes with reasonable recurrence rates.”
In the case cohort study of Ueno et al. (15) including 2 inguinal and 18 ventral hernias – 3 with incarceration – patients were treated with Surgisis® mesh implants. In a follow-up of 15.7 months, no infection or recurrence was detected.
The retrospective case–control study of Xourafas et al. (12) regarding the use of meshes in incarcerated ventral hernia repair with a simultaneous bowel resection included five patients (out of 51 in the mesh group) with the implantation in underlay technique using Alloderm® in four cases and Surgisis® in one case, respectively. The overall infection and recurrence rate (synthetic and biological meshes) was 22% in a follow-up of 22 months. The result of an univariate and a multivariate analysis detected a significant risk of increased postoperative infection in the mesh group, without separation regarding the type of mesh.
Helton et al. (13) reported in a retrospective case–control study of 13 patients treated with bowel resection due to incarceration or strangulation in ventral hernia by the use of Surgisis Gold® in an open approach. The wound infection rate was 24% and the recurrence rate 17% in a follow-up of 14 months.
In a retrospective study of different bio-prosthetic materials in complex ventral hernia repair by Shah et al. (9) nine patients with incarceration (out of 58) were included. Different biological meshes were used (Alloderm®, CollaMend®, Permacol®, Surgisis®, and Strattice®). The overall recurrence rate was 27.9%, and surgical wound infections were detected in 19% in a follow-up of 1 year. The 17.2% of the meshes required explantation. Non-cross-linked porcine biologics were less likely to be explanted, but had higher recurrence rates compared to cross-linked porcine biologics and a higher infection rate compared to Alloderm® (non-cross-linked human dermis).
Franklin et al. published a case–control study using porcine small intestinal submucosa mesh (Surgisis®) for laparoscopic IPOM repair of hernias in infected fields in the years 2002, 2004, and 2008 (3–5). In summary, 133 procedures were performed in 116 patients of which 17 (12.7%) required a bowel resection due to strangulated hernias with necrotic bowel. The overall recurrence rate was 5.2% and the infection rate 0.7% in a mean follow-up of 52 months.
Incarcerated abdominal wall hernias treated with the use of human dermal matrix (ADM®) in IPOM position by open approach in combination with vacuum wound drainage was reported by Han et al. (6) in a retrospective study. In 33 out of 63 incarcerated hernias, bowel resection was performed. In a follow-up of 43 months, 15.9% recurrences were detected and 1.6% suffered from a superficial wound infection. Multivariate analysis isolated BMI, defect size, and numbers of biological meshes used as risk factors to significantly affect recurrence rates.
Patton et al. (14) published a retrospective study of abdominal wall reconstructions with the use of acellular dermal matrix (ADM®) in complex and contaminated ventral hernias. In 51% of the repairs, the mesh was positioned as IPOM bridging with 3 cm overlap, 42% as an interlay, and 8% as an onlay. The 13 patients out of 89 were treated in case of incarcerated hernias. Overall, 16% developed wound infections, and in a follow-up of 10.6 months, 17.9% suffered from a recurrent hernia.
There are some single case reports like Giakoustidis et al. (17) reporting of a biological mesh used in an incarcerated recurrent incisional hernia as well as Tsuda (22) describing a laparoscopic repair of an incarcerated umbilical hernia using Strattice® and Fallis et al. (18) publishing an open mesh repair of a strangulated perineal hernia after abdominoperineal resection. Another single case was reported by Gooch et al. (19) concerning a transthoracic repair of an incarcerated diaphragmatic hernia with a cross-linked porcine dermal collagen (Permacol®) and finally Pulido et al. (20) who described a laparoscopic repair in a case of chronic traumatic diaphragmatic hernia containing an obstructed small bowel and gallbladder also used Permacol®.
Schiergens et al. (21) reported of an emergent laparoscopic fundoplication of acute upside-down stomach with incarceration using biocompatible gradually absorbable synthetic polymers (BioA®) in a 32-year-old male patient. The follow-up was uneventful.
Discussion/Summary
In summary, so far the data regarding the use of biological and biosynthetic meshes are very scarce and there is only one level 3 study published up to now. The results of this study of Xourafas et al. (12) comparing mesh versus mesh-free repair of ventral hernia with a simultaneous bowel resection obtained a significant risk factor for the mesh group concerning the development of an infection. On multivariate regression analysis, the risk was present irrespective of drain use, defect size, and type of bowel resection. However, the analysis of a subgroup of 10 patients treated with the use of biological meshes out of a total of 100, which underwent mesh repair, did not reveal a single infection, whereas the group of polypropylene meshes showed a 24% infection rate. There was no reported significant difference in the incidence of recurrences between the mesh- and the mesh-free group (22 versus 24%), but unfortunately no comparative analysis between synthetic and biological meshes was published.
The results of Franklin et al. (3–5) include the highest number of biological mesh repairs (Surgisis®) in infected fields by laparoscopic approach, which demonstrated a low ratio of required bowel resection (12.7%), furthermore the overall incidence of recurrence and infection was very low (5.2 and 0.7%) in a mean follow-up of 52 months. Han et al. (6) reported, in his retrospective study, the highest number of treated patients due to incarcerated hernias with bowel resection by open approach using acellular dermal matrix (ADM®) with very low rate of infection (1.6%) as well as recurrences (15.9%) in a follow-up of 43 months. Both studies achieved acceptable outcome in a follow-up of at least 3.5 years compared to the use of synthetic mesh in this high-risk population (7).
In the conclusion of a critical review of biologic mesh use in ventral hernia repairs under contaminated conditions by Primus and Harris (7) as well as in the systematic review by Lee et al. (25), a similar statement can be found: “The available evidence is limited, but does not support the superiority of biologic over synthetic non-absorbable prosthetics in contaminated fields. Due to a lack of scientific evidence concerning the use of biologic mesh in case of laparoscopic treatment in incarcerated/strangulated ventral hernias (in potentially contaminated field) no recommendation or suggestion can be stated.”
Taking into account that there is a significantly increasing rate of emergent incisional hernia repair in the group of older men (>65 years) when analyzing the years 2001 to 2010 in the United States (26), the importance to treat this growing population with an appropriate method including the selection of mesh type and material should be addressed in further studies and registries. The results of a survey of practicing surgeons (members of American College of Surgeons) concerning the use of biological meshes in abdominal reconstructions (27) revealed a lack of consensus in terms of indication, surgical techniques, as well as type of biological mesh.
Looking to the different Guidelines based on consensus conferences of the European Association for Endoscopic Surgery (EAES), International Endo Hernia Society (IEHS), European Hernia Society (EHS), and the World Society of Emergency Surgery (WSES) (28–33), we can only find a recommendation of the WSES in terms of the question which kind of mesh should be used in incarcerated/strangulated hernia. The WSES guideline based on a Consensus Meeting in 2013 (29) recommends the use of biological meshes as a valid option in case of emergency hernia repair in potentially contaminated surgical field for patients with intestinal strangulation and/or concurrent bowel resection [grade 2C recommendation GRADE (34)]. In case of stable patients with strangulated obstruction and peritonitis by bowel perforation (contaminated-dirty surgical field), direct tissue suture is recommended when the hernia defect is small; in the events that direct tissue suture is not possible, biological mesh repair may be suggested (grade 2C recommendation). The choice between cross-linked and non-cross-linked biological mesh should be evaluated depending on the defect size and degree of contamination (grade 2C recommendation).
Without any doubt, currently there is a very limited evidence for the use of biological and biosynthetic meshes in strangulated hernias in open as well as in laparo-endoscopic repair. Finally, there is an urgent need to start with randomized controlled comparative trials as well as to support registries with data to achieve more knowledge for tailored indication for the use of biological meshes.
Author Contributions
RF main authorship, corresponding author. AH support in selection of papers of the review, composing tables of the manuscript. CM support in composing of the manuscript, proof reading. FK final proof.
BioMesh Study Group
Ferdinand Köckerling (Chairman), Stavros Antoniou, René H Fortelny, Frank A. Granderath, Markus Heiss, Franz Mayer, Marc Miserez, Agneta Montgomery, Salvador Morales-Conde, Filip Muysoms, Alexander Petter-Puchner, Rudolph Pointner, Neil Smart, Marciej Smietanski, Bernd Stechemesser.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery (2010) 148:544–58. doi: 10.1016/j.surg.2010.01.008
2. Bachman S, Ramshaw B. Prosthetic material in ventral hernia repair: how do I choose? Surg Clin North Am (2008) 88:101–12. doi:10.1016/j.suc.2007.11.001
3. Franklin ME Jr, Gonzalez JJ Jr, Michaelson RP, Glass JL, Chock DA. Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia (2002) 6(4):171–4. doi:10.1007/s10029-002-0078-9
4. Franklin ME Jr, Gonzalez JJ Jr, Glass JL. Use of porcine small intestinal submucosa as a prosthetic device for laparoscopic repair of hernias in contaminated fields: 2-year follow-up. Hernia (2004) 8(3):186–9. doi:10.1007/s10029-004-0208-7
5. Franklin ME Jr, Treviño JM, Portillo G, Vela I, Glass JL, González JJ. The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc (2008) 22(9):1941–6. doi:10.1007/s00464-008-0005-y
6. Han JG, Pang GY, Wang ZJ, Zhao Q, Ma SZ. The combined application of human acellular dermal matrix and vacuum wound drainage on incarcerated abdominal wall hernias. Int J Surg (2014) 12(5):452–6. doi:10.1016/j.ijsu.2014.03.019
7. Primus FE, Harris HW. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia (2013) 17:21–30. doi:10.1007/s10029-012-1037-8
8. Diaz JJ Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, et al. Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg (2009) 144:209–15. doi:10.1001/archsurg.2009.12
9. Shah BC, Tiwari MM, Goede MR, Eichler MJ, Hollins RR, McBride CL, et al. Not all biologics are equal! Hernia (2011) 15:165–71. doi:10.1007/s10029-010-0768-7
10. Rosen MJ, Krpata DM, Ermlich B, Blatnik JA. A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg (2013) 257:991–6. doi:10.1097/SLA.0b013e3182849871
11. Zafar H, Zaidi M, Qadir I, Memon AA. Emergency incisional hernia repair: a difficult problem waiting for a solution. Ann Surg Innov Res (2012) 6(1):1. doi:10.1186/1750-1164-6-1
12. Xourafas D, Lipsitz SR, Negro P, Ashley SW, Tavakkolizadeh A. Impact of mesh use on morbidity following ventral hernia repair with a simultaneous bowel resection. Arch Surg (2010) 145(8):739–44. doi:10.1001/archsurg.2010.144
13. Helton WS, Fisichella PM, Berger R, Horgan S, Espat NJ, Abcarian H. Short-term outcomes with small intestinal submucosa for ventral abdominal hernia. Arch Surg (2005) 140(6):549–60. doi:10.1001/archsurg.140.6.549
14. Patton JH Jr, Berry S, Kralovich KA. Use of human acellular dermal matrix in complex and contaminated abdominal wall reconstructions. Am J Surg (2007) 193(3):360–3. doi:10.1016/j.amjsurg.2006.09.021
15. Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg (2004) 8(1):109–12. doi:10.1016/j.gassur.2003.09.025
16. Quartey B, Ugochukwu O, Kuehn R, Ospina K. Incarcerated recurrent Amyand’s hernia. J Emerg Trauma Shock (2012) 5(4):344–6. doi:10.4103/0974-2700.102407
17. Giakoustidis A, Morrison D, Neofytou K, Giakoustidis D, Mudan S. Emergency open incarcerated hernia repair with a biological mesh in a patient with colorectal liver metastasis receiving chemotherapy and bevacizumab uncomplicated wound healing. Case Rep Emerg Med (2014) 2014:848030. doi:10.1155/2014/848030
18. Fallis SA, Taylor LH, Tiramularaju RM. Biological mesh repair of a strangulated perineal hernia following abdominoperineal resection. J Surg Case Rep (2013) 2013(4):ii:rjt023. doi:10.1093/jscr/rjt023
19. Gooch B, Smart N, Wajed S. Transthoracic repair of an incarcerated diaphragmatic hernia using hexamethylene diisocyanate cross-linked porcine dermal collagen (Permacol). Gen Thorac Cardiovasc Surg (2012) 60(3):145–8. doi:10.1007/s11748-011-0786-0
20. Pulido J, Reitz S, Gozdanovic S, Price P. Laparoscopic repair of chronic traumatic diaphragmatic hernia using biologic mesh with cholecystectomy for intrathoracic gallbladder. JSLS (2011) 15(4):546–9. doi:10.4293/108680811X13176785204472
21. Schiergens TS, Thomas MN, Hüttl TP, Thasler WE. Management of acute upside-down stomach. BMC Surg (2013) 13:55. doi:10.1186/1471-2482-13-55
22. Tsuda S. Laparoscopic repair of complicated umbilical hernia with Strattice Laparoscopic− reconstructive tissue matrix. Int J Surg Case Rep (2014) 5(12):1167–9. doi:10.1016/j.ijscr.2014.11.007
23. Michalinos A, Moris D, Vernadakis S. Amyand’s hernia: a review. Am J Surg (2014) 207(6):989–95. doi:10.1016/j.amjsurg.2013.07.043
24. Köckerling F, Alam NN, Narang SK, Daniels IR, Smart NJ. Biological meshes for inguinal hernia tepair – review of the literature. Front Surg (2015) 2:48. doi:10.3389/fsurg.2015.00048
25. Lee L, Mata J, Landry T, Khwaja KA, Vassiliou MC, Fried GM, et al. A systematic review of synthetic and biologic materials for abdominal wall reinforcement in contaminated fields. Surg Endosc (2014) 28(9):2531–46. doi:10.1007/s00464-014-3499-5
26. Beadles CA, Meagher AD, Charles AG. Trends in emergent hernia repair in the United States. JAMA Surg (2015) 150(3):194–200. doi:10.1001/jamasurg.2014.1242
27. Harth KC, Krpata DM, Chawla A, Blatnik JA, Halaweish I, Rosen MJ. Biologic mesh use practice patterns in abdominal wall reconstruction: a lack of consensus among surgeons. Hernia (2013) 17(1):13–20. doi:10.1007/s10029-012-1029-8
28. Agresta F, Ansaloni L, Baiocchi GL, Bergamini C, Campanile FC, Carlucci M, et al. Laparoscopic approach to acute abdomen from the Consensus Development Conference of the Società Italiana di Chirurgia Endoscopica e nuove tecnologie (SICE), Associazione Chirurghi Ospedalieri Italiani (ACOI), Società Italiana di Chirurgia (SIC), Società Italiana di Chirurgia d’Urgenza e del Trauma (SICUT), Società Italiana di Chirurgia nell’Ospedalità Privata (SICOP), and the European Association for Endoscopic Surgery (EAES). Surg Endosc (2012) 26(8):2134–64. doi:10.1007/s00464-012-2331-3
29. Sartelli M, Coccolini F, van Ramshorst GH, Campanelli G, Mandalà V, Ansaloni L, et al. WSES guidelines for emergency repair of complicated abdominal wall hernias. World J Emerg Surg (2013) 8(1):50. doi:10.1186/1749-7922-8-50
30. Bittner R, Arregui ME, Bisgaard T, Dudai M, Ferzli GS, Fitzgibbons RJ, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)]. Surg Endosc (2011) 25(9):2773–843. doi:10.1007/s00464-011-1799-6
31. Bittner R, Montgomery MA, Arregui E, Bansal V, Bingener J, Bisgaard T, et al. Update of guidelines on laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia (International Endohernia Society). Surg Endosc (2015) 29(2):289–321. doi:10.1007/s00464-014-3917-8
32. Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia (2009) 13(4):343–403. doi:10.1007/s10029-009-0529-7
33. Miserez M, Peeters E, Aufenacker T, Bouillot JL, Campanelli G, Conze J, et al. Update with level 1 studies of the European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia (2014) 18(2):151–63. doi:10.1007/s10029-014-1236-6
Keywords: incarceration, strangulation, groin hernia surgery, abdominal wall hernia, biological mesh, incisional hernia, ventral hernia, bio-resorbable mesh
Citation: Fortelny RH, Hofmann A, May C, Köckerling F and BioMesh Study Group (2016) Open and Laparo-Endoscopic Repair of Incarcerated Abdominal Wall Hernias by the Use of Biological and Biosynthetic Meshes. Front. Surg. 3:10. doi: 10.3389/fsurg.2016.00010
Received: 15 January 2016; Accepted: 05 February 2016;
Published: 25 February 2016
Edited by:
Hakan Kulacoglu, Rize University, TurkeyReviewed by:
Gabriel Sandblom, Karolinska University Hospital, SwedenPremkumar Balachandran, Apollo Hospitals, India
Copyright: © 2016 Fortelny, Hofmann, May, Köckerling and BioMesh Study Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: René H. Fortelny, rene.fortelny@wienkav.at
†Members of the BioMesh Study Group are listed at the end of the article.
 René H. Fortelny
René H. Fortelny Anna Hofmann
Anna Hofmann Christopher May
Christopher May Ferdinand Köckerling
Ferdinand Köckerling BioMesh Study Group†
BioMesh Study Group†