Prospects and Challenges of Induced Pluripotent Stem Cells in Equine Health
- The Roslin Institute and Royal (Dick) School of Veterinary Studies, University of Edinburgh, Midlothian, UK
Pluripotent stem cells (PSCs) hold, through the capacity to differentiate into virtually all body cell types, unprecedented promise for human and animal medicine. PSCs are naturally found in the early embryo, and in rodents and humans they can be robustly harvested and grown in culture in the form of embryonic stem cells (ESCs); however, the availability of ESCs from horses is limited. ES-like cells named induced pluripotent stem cells (iPSCs) can be derived in vitro by transcription factor-mediated reprogramming of adult cells. As such, iPSCs can be generated in a patient-specific manner providing unmatched potential for tissue transplantation and in vitro disease modeling. In humans, clinical trials using iPSC-derived cells are already taking place and the use of in vitro iPSC models has identified novel mechanisms of disease and therapeutic targets. Although to a more limited extent, iPSCs have also been generated from horses, a species in which, after humans, these cells are likely to hold the greatest potential in regenerative medicine. Before a clinical use can be envisioned, however, significant challenges will need to be addressed in relation to the robust derivation, long-term culture, differentiation, and clinical safety of equine iPSCs. Toward this objective, recent studies have reported significant improvement in culture conditions and the successful derivation for the first time of functional cell types from equine iPSCs. Given the wide range of exciting applications they could have, it is hoped future research will make the biomedical promise of iPSCs a reality not only for humans but also horses.
Stem cells are defined based on their capacity for self-renewal and the ability to differentiate into specialized cell types (potency). In contrast to multipotent stem cells (including mesenchymal stem cells or MSCs), pluripotent stem cells (PSCs) are intrinsically able to self-renew indefinitely and to give rise to virtually all cell types in the body, features that provide distinct advantages in relation to regenerative medicine applications and for which PSCs have been the subject of intense research over the past 30 years. Although pluripotent cells are found naturally only in the early mammalian embryo, pluripotency can be captured in vitro in the form of embryonic stem cells (ESCs) generated from cultures of the inner cell mass, the forerunner of the embryo proper in the very early conceptus (1, 2). ESC lines that maintain their pluripotency in vivo, i.e., are able to give raise to differentiated teratomas when injected into immunodeficient mice, have been robustly derived from rodents and humans but not, to this date, from domestic species, including the horse. This is partly attributed to the relative lack of knowledge of early embryo development in domestic species, which precludes the use of optimal conditions to stably maintain embryonic cells in an undifferentiated state. Nonetheless, cultures of equine embryonic cells that lack pluripotency in vivo have been established by several groups (3, 4) and their potential in relation to veterinary regenerative medicine is being investigated (5, 6).
Generation and Characterization of Equine iPSCs
In 2006, Shinya Yamanaka’s group in Japan showed that cells equivalent to ESCs, named induced pluripotent stem cells (iPSCs), could be generated in culture from murine fibroblasts by simply inducing the expression of four genes, namely the pluripotency-associated transcription factors, Oct4, Sox2, Klf4, and Myc (7). This seminal discovery was followed shortly after by the successful generation of iPSCs from humans (8) and opened the way to the derivation, without the need to use embryos, of patient-specific PSCs that could be used for autologous tissue transplantation, thus, providing a clear advantage over ESCs. For his discoveries, Yamanaka was awarded the 2012 Nobel Prize in Medicine.
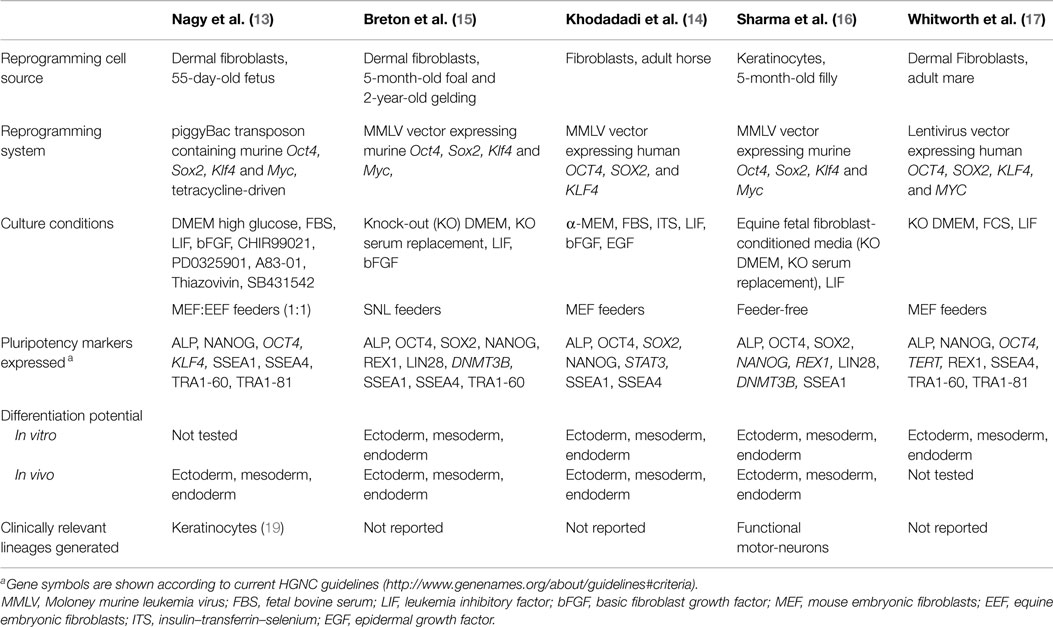
The first reports on mouse and human iPSCs in 2006–2007 led to a deluge of studies aiming to identify cell sources and gene expression systems that would allow efficient reprogramming using minimal genetic modification of the resulting iPSCs, a crucial requisite for an eventual clinical application of these cells. Studies soon extended to domestic animal species where iPSC technology was seen as a highly promising alternative to ESCs (9–12). In the horse, the prospect of a new source of stem cells for clinical use led to the first report on equine iPSCs in 2011 (13) followed by several additional publications over the following 3 years (14–17). The cells generated in these studies displayed, at various levels, features of equine embryonic cells and iPSCs from mice and humans (Table 1), including morphology, re-activated expression of molecular markers of pluripotency and the ability, in some studies, to generate differentiated teratomas in vivo, which is to-date the most stringent proof of pluripotency in the horse. Equine iPSCs were generated from fetal or adult fibroblasts and, in one of the studies, keratinocytes (Table 1); consistent with reports in humans (18), equine keratinocytes were relatively more amenable to reprogramming than fibroblasts and, in addition, once reprogrammed they had higher developmental plasticity than fibroblast-derived iPSCs as indicated by their ability to produce differentiated tissues in vivo as diverse as neurons, cartilage, muscle, lung epithelium, and gastric epithelium (16).
Reprogramming of equine cells was achieved in most studies by using viral expression vectors that mediate the integration of the reprogramming gene sequences (Oct4, Sox2, Klf4, and Myc) into the cell genome, therefore, making the reprogrammed cells not apt for clinical use. Only one study (13) used a non-integrating expression vector (piggyBac transposon) to reprogram equine cells, although this was done at the cost of reduced robustness of the resulting iPSCs, as switching off the expression of the reprogramming DNA sequences in these cells led to loss of pluripotency and rapid differentiation, indicating that re-activated expression of endogenous pluripotency genes during reprogramming was not sufficient to sustain the pluripotent state. Consistent with this observation, all other iPSC lines reported to date show clear but variable expression of the reprogramming genes, similar to observations from other domestic species (9, 11, 12). Given that silencing of the exogenous reprogramming genes is in general considered a hallmark of faithful reprogramming (20), the above findings bring into question whether equine iPSC lines reported so far are fully reprogrammed or they represent instead a partially reprogrammed cell type; potential implications of this, if any, in relation to a possible clinical application of these cells need to be established.
Potential of iPSCs in Equine Biomedicine
Induced pluripotent stem cells offer truly unprecedented potential both as a source of therapeutic cells and as a tool for in vitro disease modeling and drug discovery. The tissue regeneration potential of iPSCs has been demonstrated using different animal models of disease, including spinal cord injury (21), Parkinson (22), and retinal degeneration (23). Notably, the first human clinical trials using autologous iPSCs are already underway in Japan to treat age-related macular degeneration, and significant work is being carried out toward other eventual clinical applications. A particularly exciting possibility in this regard involves the use of iPSCs in combination with novel gene editing technologies as a new strategy for gene therapy; although a long-term clinical prospect, success using this combined approach has been reported in experimental rodent models, including sickle cell anemia (24) and limb-girdle muscular dystrophy (25).
Very early steps have been given to test the clinical efficacy and safety of PSCs in the horse. Guest et al. (5) injected equine ESC-like cells into damaged tendon of live horses and showed superior cell survival and migration to damaged tissue compared to transplanted bone marrow-derived MSCs. A more recent study showed intradermal injection of equine allogeneic iPSCs to be relatively well-tolerated and without noticeable long-term effects (26). These reports are encouraging and further studies will need to test the potential of iPSCs in relation to specific disorders in the horse. Clinically, equine iPSCs may be most useful in the first instance as an alternative to current MSC-based musculoskeletal therapies. In this regard, significant benefits can be gained from progress made with humans for which different protocols are already available to derive MSC-like cells from iPSCs (27–29), moreover, the potential of such cells for tissue regeneration has been clearly demonstrated using mouse models of limb ischemia (27, 29). Although, in principle, iPSCs could be derived from any equine patient, the time required for the generation and pre-clinically testing of such cells will prevent their therapeutic use early during the disease course, making autologous application a suitable option only for some non-acute disorders. Indeed, equine iPSCs will have greatest clinical potential as an off-the-shelf source of therapeutic, in vitro-produced mesenchymal precursors, a possibility that holds particular attractive at a moment when allogeneic cell therapies are being increasingly considered in the horse. Off-the-shelf iPSC-derived cells could have applications not only in musculoskeletal repair but also for other common conditions, including external wounds, ischemic/inflammatory processes such as laminitis or gastro-intestinal disease, autoimmune disorders, and even neuro-regeneration (16, 19).
Despite their enormous clinical promise, given present concerns about overall clinical safety (discussed below), disease modeling is seen as the application for which iPSCs, in general, will be most useful in the short term. In that regard, the differentiation of human patient-derived iPSCs into functional cells capable of recapitulating specific disease phenotypes has provided a truly unmatched tool for studying diseases in vitro. Disorders successfully modeled in this way include neurological, cardiovascular, hematological and metabolic (30); such studies have unraveled novel molecular mechanisms of disease as well as led to identification of promising therapeutic targets. Most notably, elegant studies [reviewed in Ref. (31)] with human iPSC-derived cardiomyocytes and neurons have elucidated novel genetic mechanisms in the pathogenesis of long-QT syndrome and familial dysautonomia, respectively. Moreover, using neurons from patients with familial dysautonomia or Rett syndrome led to the identification of novel therapeutic compounds that are now being used in early-stage clinical trials (31).
A similar potential for in vitro modeling of disease as well as normal tissue development is held by equine iPSCs. This includes the possibility, using appropriate iPSC-derived cell types, of studying the effects of different genetic backgrounds or horse breeds on disease resistance and the responses to specific therapies. This information could be extremely valuable for developing new therapies or improving current approaches for equine patients. The limited knowledge, both basic and applied, on developmental/stem cell biology and molecular aspects of disease in the horse will certainly pose significant challenges to such application. These include the need to establish robust differentiation protocols to produce cells phenotypically equivalent to the cell type(s) under study, and to appropriately identify and measure phenotypes that relate to the diseases of interest, which may be species specific. Diseases for which a phenotype is most likely to be obtained in vitro and for which iPSCs may be most useful are early-onset, cell-autonomous defects caused by known, high penetrance mutations. Several monogenic diseases with a defined causal mutation have been identified in horses (http://omia.angis.org.au). Some, such as hyperkalemic periodic paralysis and exertional rhabdomyolysis, have a relatively high, breed-specific prevalence and could be readily targeted. In that case, the phenotype of myocytes derived from iPSCs from affected and healthy animals would be compared to understand the cellular mechanisms underlying the disease(s) and establish the responses to potential therapeutic agents; alternatively, myocytes could be derived from healthy iPSCs in which the causal gene mutation would have been previously induced and would be compared to myocytes generated from non-mutated iPSCs (30). Advances in sequencing of the equine genome, together with the development over the past few years of readily available technologies for precise genome editing will significantly facilitate such undertakings toward making real therapeutic progress in relation to those and many other equine diseases.
Challenges for the Application of iPSCs in Equine Biomedicine
In spite of their enormous potential, several challenges related to clinical safety will first need to be overcome before a therapeutic use of equine iPSCs can be considered. The most important concern at present is the possibility of tumor formation following transplantation of iPSCs into patients. The capacity to generate a wide variety of unwanted tissue types after transplantation, including malignant tumors derives from the inherent development plasticity of all pluripotent cells. In addition, the reprogramming process itself together with the repeated passaging of the reprogrammed cells in culture can lead to significant genetic and epigenetic instability and an increased propensity for tumor formation (32). Another very important consideration is that iPSCs are typically generated by inducing the expression of gene sequences (Oct4, Sox2, Klf4, and c-Myc) which by themselves can be tumorigenic. As already pointed out, reprogramming is most efficiently achieved by the use of viral vectors carrying the reprogramming genes that become permanently integrated into the cell’s genome, resulting in reprogrammed cells that harbor foreign, potentially tumorigenic DNA. In addition, the continued expression of these genes can impinge on the ability of the iPSCs to later differentiate properly into adult cell types (consistent with the notion that silencing of the reprogramming genes is a hallmark of successful reprogramming), thus, reducing their clinical applicability (21). Efforts to circumvent these issues have resulted in the development of several non-integrating expression systems (33, 34), which can now be used routinely to generate human iPSCs with reasonable efficiency. Based on results (13) using a piggyBac transposon system to reprogram equine fibroblasts and on several reports in other domestic species [reviewed in Ref. (35)], it is likely that significant optimization of reprogramming and culture conditions will be necessary to efficiently derive robust equine iPSCs using available non-integrating expression systems. Such efforts will be essential for any eventual clinical application of these cells. In addition, thorough molecular and functional characterization and testing of individual iPSC lines intended for clinical use will be mandatory to completely rule out genetic or phenotypic traits indicative of tumorigenic capacity. Despite all these challenges, studies in other species have already reported the successful transplantation of iPSC-derived cells without any resulting tumorigenicity or immunogenicity (21).
Related to the point above, a significant challenge toward an eventual clinical use of equine iPSCs will be the development of robust protocols for unidirectional and efficient differentiation to specific mature and functional cell types. Significant progress has already been made in human, rodent, and even pig (30, 36), iPSCs from which species can now be differentiated into cells resembling, with variable fidelity, many different body cell types; a remaining limitation in that regard is that iPSC-derived cells often present immature, fetal-like phenotypes that may not be suitable for tissue transplantation, requiring future refinement of cell differentiation protocols. In the case of the horse, an additional limitation is the relatively lack of knowledge of normal cellular and molecular mechanisms of development, the recapitulation of which in vitro may be required to robustly produce cell lineages of interest. Despite this, progress has already been made with the successful generation for the first time of functional cells from iPSCs, namely 1) functional motor-neurons (16) and 2) cells that both expressed keratinocyte markers and were able to epithelize wounds in vitro (19). These achievements have provide important proof of concept of the biomedical potential of equine iPSCs and should encourage further exploratory studies.
The immunogenicity of transplanted iPSC derivatives will also need to be addressed. Although not tested in the horse, studies in mice and primates (37, 38) have shown that transplantation of autologous iPSC-derived cells induces minimal immune response, in contrast to the substantial activation and infiltration of immune cells leading to reduced survival of transplanted allografts. In that regard, although encouraging, the recent report that intradermal injection of allogeneic equine iPSCs produced only a moderate immune response at the site of injection needs to be interpreted with caution as the long-term fate of the injected cells was not established (26). As a clinical use of iPSCs in the future is more likely to occur in an allogeneic context, specific strategies to induce immune tolerance may need to be developed. The creation of human iPSC haplobanks from individuals homozygous at the major HLA antigens has been proposed as a route to provide immuno-compatible cells for a large proportion of the human population (39); whether such an ambitious approach would be necessary, and feasible, in the horse remains to be seen.
Finally, in addition to safety considerations, the allogeneic use of equine iPSC derivatives would likely come under regulatory scrutiny, including Good Manufacture Practice guidelines for their derivation, storage, and application in patients. While steps already being given in the human field could be followed, the implementation of such measures would require a much better understanding of the nature of equine iPSCs and a significant optimization and validation of the procedures currently used for their generation, culture, and differentiation.
In conclusion, although significant challenges in relation to an eventual safe therapeutic application of iPSCs remain ahead, through their demonstrated clinical and in vitro modeling potential, iPSCs provides an opportunity to make unprecedented strides toward understanding and treating a variety of equine diseases. Such perspectives should stimulate work by the equine scientific community toward a better understanding of the nature and biomedical potential of these cells.
Author Contributions
Both authors have contributed to the writing of this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
Equine stem cell research in the author’s laboratory has been funded by the Horse Betting Levy Board, the Pet Plan Charitable Trust and the Royal College of Veterinary Surgeons Trust. The Roslin Institute receives strategic funding from the Biotechnology and Biological Science Research Council.
References
1. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature (1981) 292:154–6. doi: 10.1038/292154a0
2. Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science (1998) 282:1145–7. doi:10.1126/science.282.5391.1145
3. Li X, Zhou SG, Imreh MP, Ahrlund-Richter L, Allen WR. Horse embryonic stem cell lines from the proliferation of inner cell mass cells. Stem Cells Dev (2006) 15:523–31. doi:10.1089/scd.2006.15.523
4. Saito S, Sawai K, Minamihashi Am Ugai H, Yokoyama K. Derivation, maintenance, and induction of the differentiation in vitro of equine embryonic stem cells. Methods Mol Biol (2006) 329:59–79. doi:10.1385/1-59745-037-5:59
5. Guest DJ, Smith MRW, Allen WR. Equine embryonic stem-like cells and mesenchymal stromal cells have different survival rates and migration patterns following their injection into damaged superficial digital flexor tendon. Equine Vet J (2010) 42:636–42. doi:10.1111/j.2042-3306.2010.00112.x
6. Barsby T, Bavin EP, Guest DJ. Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng Part A (2014) 20:2604–13. doi:10.1089/ten.TEA.2013.0457
7. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell (2006) 126:663–76. doi:10.1016/j.cell.2006.07.024
8. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell (2007) 131:861–72. doi:10.1016/j.cell.2007.11.019
9. Ezashi T, Telugu BPVL, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A (2009) 106:10993–8. doi:10.1073/pnas.0905284106
10. Shimada H, Nakada A, Hashimoto Y, Shigeno K, Shionoya Y, Nakamura T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol Reprod Dev (2010) 77:2. doi:10.1002/mrd.21117
11. Li Y, Cang M, Lee AS, Zhang K, Liu D. Reprogramming of sheep fibroblasts into pluripotency under a drug-inducible expression of mouse-derived defined factors. PLoS One (2011) 6:e15947. doi:10.1371/journal.pone.0015947
12. Sumer H, Liu J, Malaver-Ortega LF, Lim ML, Khodadadi K, Verma PJ. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J Anim Sci (2011) 89:2708–16. doi:10.2527/jas.2010-3666
13. Nagy K, Sung H-K, Zhang P, Laflamme S, Vincent P, Agha-Mohammadi S, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev Rep (2011) 7:693–702. doi:10.1007/s12015-011-9239-5
14. Khodadadi K, Sumer H, Pashaiasl M, Lim S, Williamson M, Verma PJ. Induction of pluripotency in adult equine fibroblasts without c-MYC. Stem Cells Int (2012) 2012:429160. doi:10.1155/2012/429160
15. Breton A, Sharma R, Diaz AC, Parham AG, Graham A, Neil C, et al. Derivation and characterization of induced pluripotent stem cells from equine fibroblasts. Stem Cells Dev (2013) 22:611–21. doi:10.1089/scd.2012.0052
16. Sharma R, Livesey MR, Wyllie DJ, Proudfoot C, Whitelaw CB, Hay DC, et al. Generation of functional neurons from feeder-free, keratinocyte-derived equine induced pluripotent stem cells. Stem Cells Dev (2014) 23:1524–34. doi:10.1089/scd.2013.0565
17. Whitworth DJ, Ovchinnikov DA, Sun J, Fortuna PR, Wolvetang EJ. Generation and characterization of leukemia inhibitory factor-dependent equine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev (2014) 23:1515–23. doi:10.1089/scd.2013.0461
18. Aasen T, Izpisua Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc (2010) 5:371–82. doi:10.1038/nprot.2009.241
19. Aguiar C, Therrien J, Lemire P, Segura M, Smith LC, Theoret CL. Differentiation of equine induced pluripotent stem cells into a keratinocyte lineage. Equine Vet J (2015). doi:10.1111/evj.12438
20. Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo HG, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol (2009) 27:1033–U1100. doi:10.1038/nbt.1580
21. Okano H, Nakamura M, Yoshida K, Okada Y, Tsuji O, Nori S, et al. Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res (2013) 112:523–33. doi:10.1161/CIRCRESAHA.111.256149
22. Wernig M, Zhao J-P, Pruszak J, Hedlund E, Fu D, Soldner F, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A (2008) 105:5856–61. doi:10.1073/pnas.0801677105
23. Tucker BA, Park I-H, Qi SD, Klassen HJ, Jiang C, Yao J, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One (2011) 6:e18992. doi:10.1371/journal.pone.0018992
24. Hanna J, Wernig M, Markoulaki S, Sun C-W, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science (2007) 318:1920–3. doi:10.1126/science.1152092
25. Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, et al. Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med (2012) 4:140ra189. doi:10.1126/scitranslmed.3003541
26. Aguiar C, Theoret C, Smith O, Segura M, Lemire P, Smith LC. Immune potential of allogeneic equine induced pluripotent stem cells. Equine Vet J (2014) 47(6):708–14.
27. Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation (2010) 121:1113–23. doi:10.1161/CIRCULATIONAHA.109.898312
28. Waese EYL, Stanford WL. One-step generation of murine embryonic stem cell-derived mesoderm progenitors and chondrocytes in a serum-free monolayer differentiation system. Stem Cell Res (2011) 6:34–49. doi:10.1016/j.scr.2010.08.007
29. Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation (2012) 125:87–99. doi:10.1161/CIRCULATIONAHA.111.048264
30. Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature (2012) 481:295–305. doi:10.1038/nature10761
31. Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol (2012) 13:713–26. doi:10.1038/nrm3448
32. Mayshar Y, Ben-David U, Lavon N, Biancotti J-C, Yakir B, Clark AT, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell (2010) 7:521–31. doi:10.1016/j.stem.2010.07.017
33. O’Malley J, Woltjen K, Kaji K. New strategies to generate induced pluripotent stem cells. Curr Opin Biotechnol (2009) 20:516–21. doi:10.1016/j.copbio.2009.09.005
34. Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science (2013) 341:651–4. doi:10.1126/science.1239278
35. Telugu BP, Ezashi T, Roberts RM. The promise of stem cell research in pigs and other ungulate species. Stem Cell Rev (2010) 6:31–41. doi:10.1007/s12015-009-9101-1
36. Zhou L, Wang W, Liu Y, De Castro JF, Ezashi T, Telugu BPVL, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells (2011) 29:972–80. doi:10.1002/stem.637
37. Morizane A, Doi D, Kikuchi T, Okita K, Hotta A, Kawasaki T, et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Reports (2013) 1:283–92. doi:10.1016/j.stemcr.2013.08.007
38. Kaneko S, Yamanaka S. To be immunogenic, or not to be: that’s the iPSC question. Cell Stem Cell (2013) 12:385–6. doi:10.1016/j.stem.2013.03.008
Keywords: horses, stem cells, induced pluripotent stem cells, regenerative medicine, cell differentiation
Citation: Donadeu FX and Esteves CL (2015) Prospects and Challenges of Induced Pluripotent Stem Cells in Equine Health. Front. Vet. Sci. 2:59. doi: 10.3389/fvets.2015.00059
Received: 02 September 2015; Accepted: 02 November 2015;
Published: 19 November 2015
Edited by:
Fausto Cremonesi, Università degli Studi di Milano, ItalyReviewed by:
Mario Baratta, University of Turin, ItalyCharlotte Beerts, Global Stem Cell Technology, Belgium
Copyright: © 2015 Donadeu and Esteves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F. Xavier Donadeu, xavier.donadeu@roslin.ed.ac.uk
 F. Xavier Donadeu
F. Xavier Donadeu Cristina L. Esteves
Cristina L. Esteves