Gait Changes Vary among Horses with Naturally Occurring Osteoarthritis Following Intra-articular Administration of Autologous Platelet-Rich Plasma
- 1Department of Veterinary Clinical Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
- 2Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
Mechanisms to reduce lameness associated with osteoarthritis (OA) are vital to equine health and performance. This study was designed to quantify response to autologous, intra-articular platelet-rich plasma (PRP) in horses with OA. Kinetic gait analysis was performed on 12 horses with unilateral forelimb lameness and OA in the same limb before and after intra-articular anesthesia (IAA). Radiographs and kinetic data were obtained before and 6 and 16 weeks after PRP administration to same joint, 4 weeks after IAA. Statistical evaluations included filtration effect on platelet concentration, relationship between kinetic variable changes after IAA versus PRP in the affected limb, and associations between response to PRP and response to IAA, platelet concentration, and radiographic OA. A positive response to IAA or PRP was defined as ≥5% improvement in peak vertical force, vertical impulse, or breaking impulse of the affected limb. Out of 10 horses that responded to IAA, 3 responded to PRP at both time points and 4 responded at one. Of the two horses that did not respond to IAA, one responded to PRP at both time points. Filtration increased platelet concentration significantly. The relationship between kinetic variable alterations of the affected limb after IAA and PRP was not significant, and response to PRP was not associated with response to IAA, platelet concentration, or radiographic OA. Changes in kinetic variables following IAA in joints with naturally occurring OA provide a custom standard to assess intra-articular therapy. Kinetic gait changes after intra-articular PRP are variable in horses with moderate to severe forelimb OA.
Introduction
Joint pain from osteoarthritis (OA) accounts for over 60% of equine lameness (1). The economic impact of lameness is substantial; annual direct and indirect costs are as high as $1 billion per year in the United States horse population of over 9.2 million (2). There is no known cure or gold standard treatment for OA. Signs are often managed with various combinations of systemic and local therapies that include non-steroidal anti-inflammatory drugs, chondroprotectants, corticosteroids, homeopathic supplements, and blood derivatives (1). Oral and injectable treatments have inconsistent results, potential side effects, and do not stop disease progression (3, 4).
Over the last few decades, platelet-rich plasma (PRP) has become increasingly popular to treat musculoskeletal damage and degeneration (5). Platelet growth factors are reported to enhance in vivo articular cartilage regeneration, but clinical efficacy is inconsistent (6). Variable response to PRP therapy has been attributed to distinct isolation and preparation methods that impact platelet concentration and quality, as well as filtrate composition (5, 6). Additionally, inconsistent treatment response may stem from differences among stages of naturally occurring OA that are distinct from each other and artificial models of joint trauma (7).
The overarching objective of this study was to evaluate the effect of autologous PRP on lameness in a population of horses with naturally, occurring forelimb OA. An initial goal was to establish a repeatable mechanism to objectively assess gait changes in the population by identifying kinetic gait variables that improved by a minimum of 5% from baseline within the majority of study subjects that exhibited reduced lameness following intra-articular anesthesia (IAA). Hence, the first hypothesis was that kinetic variables change in a consistent pattern among horses that exhibit reduced lameness following IAA in forelimb joints with naturally occurring OA (Table 1) (8, 9). The findings were applied to test the second hypothesis that kinetic gait variables change in the same magnitude and direction following IAA and autologous PRP administration in horses with naturally occurring, forelimb OA.
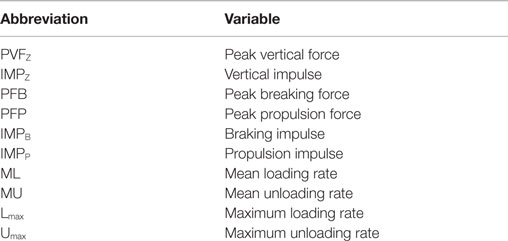
Table 1. Ground reaction force variables assessed for changes following intra-articular anesthesia and platelet-rich plasma therapy.
Materials and Methods
Inclusion Criteria
A protocol (#10-092) was approved by the Louisiana State University (LSU) Institutional Animal Care and Use Committee prior to study initiation. Horses from the LSU Equine Health Studies Program research herd were evaluated for inclusion in the study using the following criteria: (1) adult horses of either sex, (2) a subjective American Association of Equine Practitioners (AAEP) lameness grade of 2 or 3 in a single forelimb, and (3) confirmed moderate to severe radiographic OA in the fetlock or carpus of the affected forelimb. All horses received a complete physical exam, and lameness was subjectively scored by a licensed veterinarian according to the AAEP lameness scale (10). Radiographic changes in the carpus or fetlock of the affected limb were scored according to published parameters (metacarpophalangeal joint) (11) or a custom scoring system (carpometacarpal joint; Table 2) by a board certified veterinary radiologist. Horses were sedated with 5 mg/kg xylazine IV (Lloyd, Inc., Shenandoah, IA, USA) for standard, orthogonal radiographs prior to inclusion as well as 6 and 16 weeks after PRP treatment.
Study Cohorts
A comprehensive kinetic gait analysis was performed on all horses (below) to determine the effect of IAA and PRP on 10 gait variables of each of the four limbs (Table 1). The changes observed after IAA served as the study standard for comparing the changes following PRP. It was anticipated that subjects that had detectable changes in gait variables after IAA would have similar, detectable changes in gait variables after PRP therapy (responders). Those subjects that did not have changes in gait variables after IAA were not expected to have detectable alterations after PRP therapy (non-responders) (Figure 1).
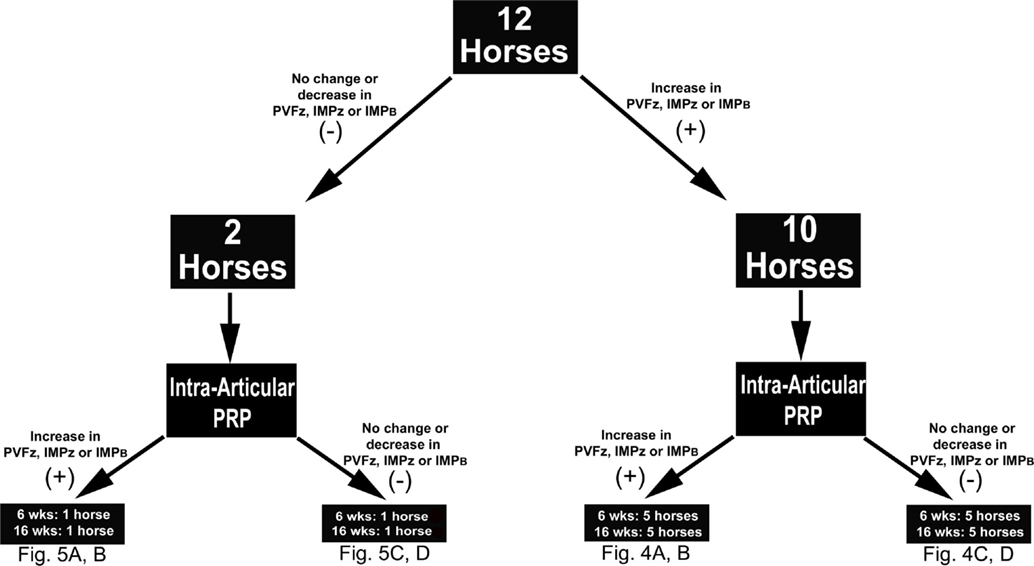
Figure 1. Study design schematic indicating the number of horses that did (+) or did not respond (−) to intra-articular anesthesia (IAA) according to changes in the indicated kinetic variables. Based on the same criteria, the number of horses that did (+) or did not (−) respond to intra-articular platelet-rich plasma (PRP), 6 or 16 weeks after administration, within each IAA cohort are also shown. Abbreviations: PVFz, peak vertical force; IMPz, vertical impulse; IMPB, breaking impulse.
Kinetic Gait Analysis
Kinetic gait data were collected as previously described (12) immediately prior to IAA, 20–25 min after IAA, 4 weeks after IAA (immediately prior to PRP administration), and 6 and 16 weeks after PRP therapy. A 900 mm × 900 mm force platform (Model #BP900900, Advanced Mechanical Technology, Inc., Watertown, MA, USA) embedded in the center of a 40-m concrete runway was used for all trials. The force platform surface is the same color and texture as the runway. An experienced handler trotted all horses for all trials. A trial was considered successful if a forelimb contacted the force platform followed by contact of the ipsilateral hind limb at a velocity of 2.00–3.80 m/s. A force of 50 N triggered data acquisition. Trials were not included if the hoof was not straight and completely on the platform or was within 5 cm of the platform edge (13). A series of five retroflective photocell sensors (Mek92-PAD, Joslyn Clark Controls, Inc., Lancaster, SC, USA) were used to determine velocity and acceleration in each trial. There is little difference in velocity between lame and sound horses (14), so horses were allowed to trot at a comfortable pace with no specific speed imposed on them for trials (12). However, because of the effect of velocity on ground reaction forces (15), the trials used for analysis were selected based on vertical force step cycle graphs (newton per kilogram versus time) that varied <5%. All trials were recorded at a rate of 1000 Hz and processed with commercially available software (Acquire v7.3, Sharon Software Inc., Dewitt, MI, USA). Four to five trials were included for the left and right limbs of each horse.
Intra-articular Anesthesia
Joints were clipped and aseptically prepared with alternating 70% isopropyl alcohol and 2% chlorhexidine scrubs. Between 5 and 10 mL of 2% mepivacaine hydrochloride (Pfizer, Inc., New York, NY, USA) was administered to each joint. Volumes varied based on joint distention. If positive pressure was experienced, fluid administration was stopped. In cases of severe joint effusion, 3–5 mL of joint fluid was removed prior to injection. After the gait trial, compressive bandages consisting of a sterile, non-adhesive pad, rolled cotton, and adhesive bandage tape were applied over the treated joints. Horses were confined to stalls for 2 days prior to bandage removal and return to pasture housing. Complete physical exams were performed every 12 h while horses were confined to stalls.
Platelet-Rich Plasma Treatment
Four weeks after IAA, autologous PRP was prepared with a commercially available kit (E-PET, Pall Corporation, Port Washington, NY, USA), according to the manufacturer’s instructions using solutions provided. Briefly, the filter system was preloaded with 9 mL of hypotonic capture solution (sterile water). Next, 55 mL of blood was aseptically collected from the jugular vein into a 60-mL syringe containing 5 mL of anticoagulant citrate dextrose solution. The blood–anticoagulant mixture was then added to the filter system that was, subsequently, gently inverted at least 10 times to mix the solutions. Using gravity flow, the mixture was filtered through the system over about 10 min. Next, 8 mL of a hypertonic harvest solution (Purecell™) was flushed through the filter into a separate sterile syringe over 2–4 s. The final isolate consisted of the filtrate in the proprietary harvest solution. Kit solutions were not individually injected into any treated joints.
Complete blood cell counts were performed (ANTECH Diagnostics, Irvine, CA, USA) on blood and filtrate aliquots (1 mL) collected in ethylenediaminetetraacetic acid coated vacutainer tubes. Immediately after preparation, 5–10 mL of PRP was injected into joints identically to IAA administration. Bandages were applied to joints and horses housed in stalls for 2 days prior to return to pasture. One veterinarian performed all procedures and monitored joints and vital parameters every 12 h during the stall confinement.
Data Reduction
Gait variables included PVFZ, IMPZ, PFB, PFP, IMPB, IMPP, ML, MU, Lmax, and Umax (Table 1). All values were normalized to subject weight (newton per kilogram). Percent change was calculated as [(treatment value − baseline value)/baseline value] × 100. Baseline values were those collected prior to IAA or PRP, and treatment values were those immediately after IAA or 6 or 16 weeks after PRP. Responders were defined as those with an increase of ≥5% over baseline in PVFZ, IMPZ, or IMPB of the affected limb after either treatment (see Results below for additional explanation).
Statistical Analysis
Mean percent change after IAA was determined for all gait variables of each limb in all horses. Those limb variables with the highest prevalence of improvement among subjects that responded to IAA were identified. Changes in platelet concentration after filtration were evaluated with Student’s paired t-tests. Unpaired t-tests were used to compare OA scores between horses that did or did not respond to IAA and PRP. Associations between PRP response and IAA response, platelet concentration, and OA severity were determined with odds ratio tests. Relationships between kinetic variable changes after IAA versus PRP at each time point were evaluated with Pearson or Spearman’s rank order correlation tests based on the D’Agostino–Pearson omnibus normality test. All analyses were performed using commercially available software (SAS v9.4, Statistical Analysis Services, Cary, NC, USA; GraphPad Prism v6, GraphPad Software, Inc., La Jolla, CA, USA), and significance was considered at P < 0.05.
Results
Study Subjects
Seven geldings and five mares were included in the study [521.7 ± 50.1 kg; 7.5 ± 3.3 years (mean ± SD)] (Table 3). Breeds included 11 Thoroughbreds and 1 American Paint. There was no evidence of systemic or local inflammation associated with intra-articular injections based on physical examinations during stall confinement and daily assessments during pasture housing.
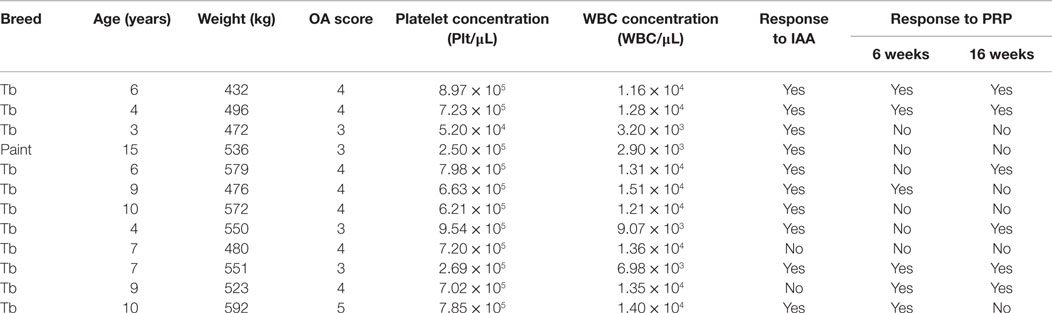
Table 3. Radiographic osteoarthritis (OA) score, platelet-rich plasma (PRP) platelet and white blood cell (WBC) concentrations, and response to intra-articular anesthesia (IAA) and PRP of study horses.
Radiographic OA
All horses had moderate to severe radiographic OA, and score did not change in any horse over the course of the study (Table 2). OA scores were not significantly different between IAA (Figure 2A) and PRP (Figure 2B) responders versus non-responders. The OA severity and response to PEP were not significantly associated.
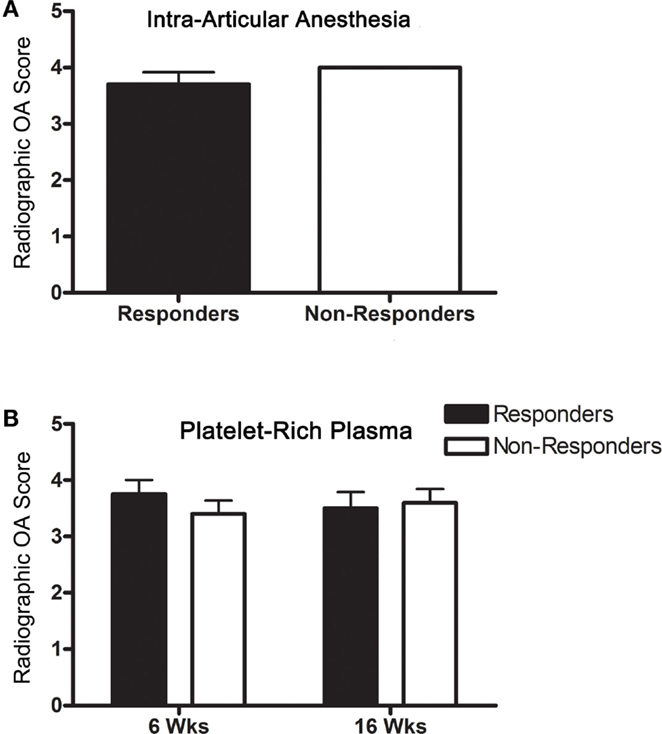
Figure 2. Radiographic OA scores (mean ± SD) for responders and non-responders (A) to IAA and (B) PRP (6 and 16 weeks).
Kinetic Gait Analysis
Ground reaction force variables for each of the four limbs did not change in a detectable pattern among horses that showed reduced lameness after IAA (8, 9); however, increases in PVFZ, IMPZ, and IMPB of ≥5% of the affected limb were most prevalent. For purposes of this study, a positive response to IAA or PRP was defined as ≥5% increase in one or more of the variables (PVFZ, IMPZ, or IMPB) of the affected limb. The relationships between gait variable changes of the affected limb after IAA and PRP were not significant 6 or 16 weeks after PRP treatment.
Intra-articular Anesthesia
Using the definition of a positive response above, 10/12 horses responded to IAA (Figure 3).
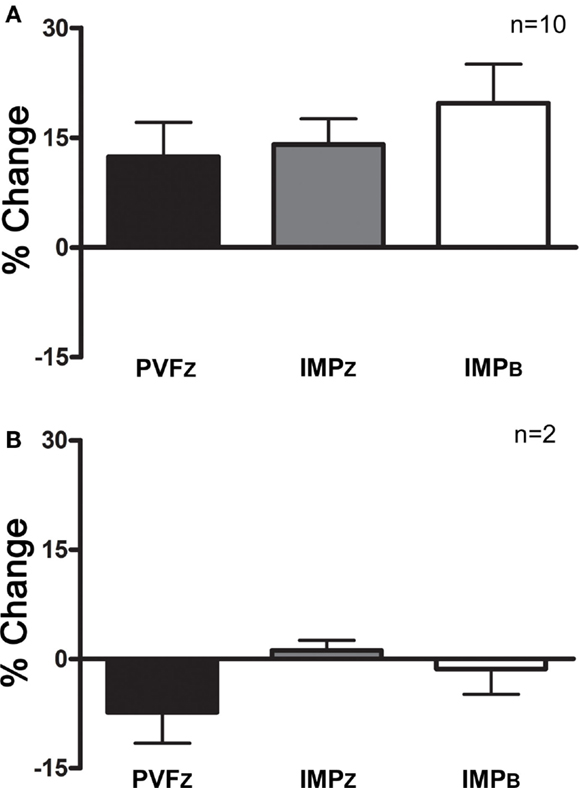
Figure 3. Percent change (mean ± SD) in PVFZ, IMPZ, and IMPB of the affected limb for (A) horses that responded to IAA and (B) horses that did not respond to IAA.
Platelet-Rich Plasma Treatment
Within those horses that responded positively to IAA (n = 10), three responded positively to PRP 6 and 16 weeks after administration, and four responded at one time point only, either 6 or 16 weeks (Figure 4). Of the horses that did not respond to IAA, one responded to PRP treatment at both time points (Figure 5). The association between response to IAA and response to PRP was not significant.
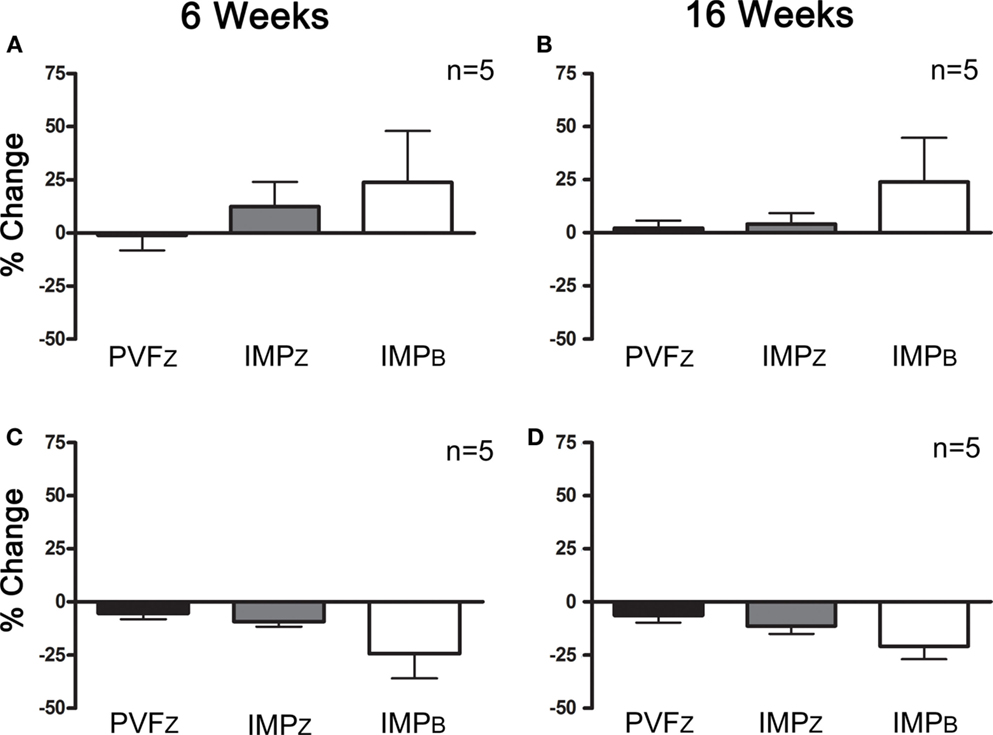
Figure 4. Percent change (mean ± SD) in PVFZ, IMPZ, and IMPB of the affected limb for horses that responded to IAA and (A) PRP after 6 weeks, (B) PRP after 16 weeks, (C) did not respond to PRP after 6 weeks, and (D) did not respond to PRP after 16 weeks.
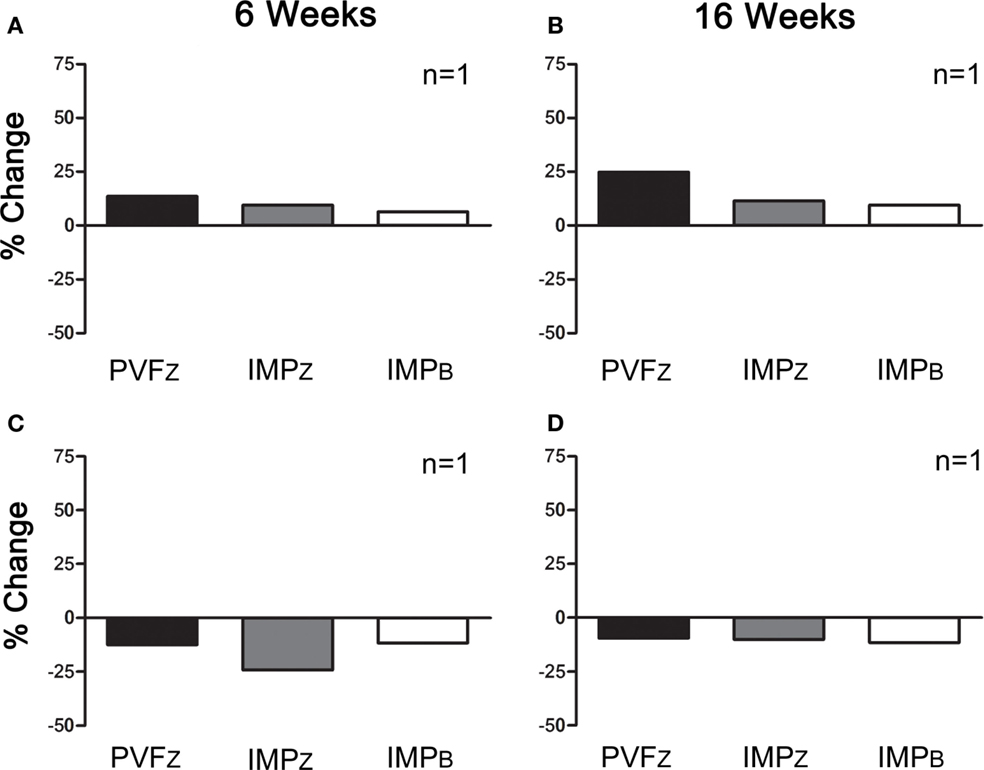
Figure 5. Percent change (mean ± SD) in PVFZ, IMPZ, and IMPB of the affected limb for horses that did not respond to IAA and (A) responded to PRP after 6 weeks, (B) responded to PRP after 16 weeks, (C) did not respond to PRP after 6 weeks, and (D) did not respond to PRP after 16 weeks.
Effect of Platelet Concentration
Post-filtration platelet concentration ranged from 5.2 × 104 to 9.5 × 105 platelets/μL, a 1.3- to 9.1-fold increase. Mean platelet concentration was significantly higher after filtration (Figure 6A; P < 0.0001). Platelet concentrations were not significantly different between horses that had a positive response to PRP versus those that did not respond at either time point (Figure 6B). There was no significant association between platelet concentration and response to PRP.
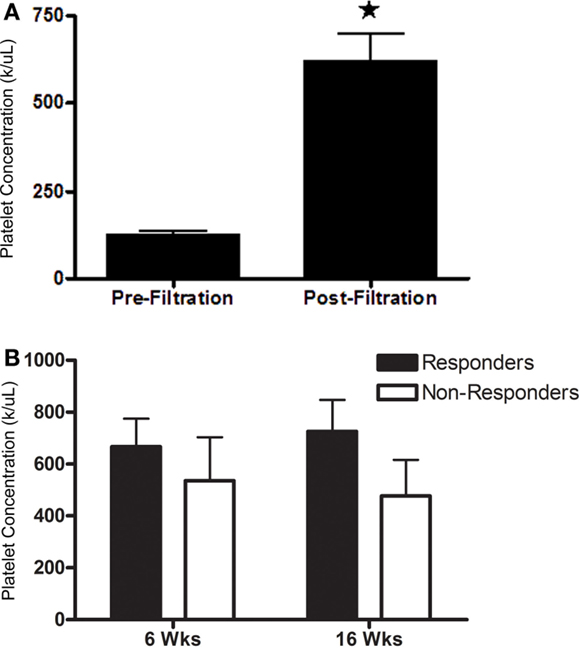
Figure 6. Platelet concentration (mean ± SD) (A) prior to and after filtration and (B) administered to horses that responded (responders) or did not respond (non-responders) to intra-articular PRP 6 or 16 weeks after administration. Columns with different symbols within graphs are significantly different from each other (P < 0.05).
Discussion
The comprehensive gait analysis performed before and after IAA in this investigation established a baseline to assess response to PRP in a population of horses with naturally occurring, forelimb OA. Given the inherent variability among horses with a naturally occurring condition, we sought to identify a reproducible mechanism and a standard to compare response to intra-articular therapy based on current understanding of gait modifications associated with progressive, degenerative joint disease. Subjective lameness evaluations are limited by inter- and intra-observer error (10, 16), and there is evidence of overestimation of changes in lameness after treatment (17). Kinetic gait analysis is an established mechanism to overcome many of the limitations (14). Analgesia from IAA used for comparison in this study was not necessarily an achievable standard for intra-articular therapy since the pain relief 6 and 16 weeks after administration is unlikely to reach that of complete tissue desensitization. As an additional comparison, true negative controls, or horses that did not respond to joint desensitization and would therefore be unlikely to respond to joint pain relief associated with PRP, were included in the study. Based on objective gait analysis of horses with forelimb OA that did and did not respond to IAA, response to intra-articular PRP varied among horses with naturally occurring, forelimb OA.
Quantification of kinetic gait changes in multiple limbs makes it possible to detect compensatory gait changes (9). Evidence supports that it is necessary to quantify numerous gait variables to assess complex kinetic gait alterations (2). These considerations were the rationale to evaluate multiple ground reaction force variables in all limbs. However, the horses in this study did not respond to IAA with a consistent pattern of gait changes as expected from responses of horses with pain in only one limb. This is not surprising given the age of the population and the presence of significant OA in a forelimb joint. While horses were subjectively lame in one forelimb, it is likely that they experienced other sources of discomfort. As such, the limb and gait variables that consistently reflected improved gait in the population were selected to compare gait alterations following PRP. Although peak breaking force and braking impulse are less commonly used to quantify equine lameness, they correlate well to lameness severity (2) and superficial digital flexor tendonitis pain (18).
The equine population used in this study had joint changes that were likely beyond those typically considered consistent with continued use. Several available studies support that PRP treatment appears to be more effective in younger patients, human and equine, with mild cases of OA (19, 20). The population in this study was selected with the goal to ensure consistent forelimb lameness across trials to reduce potential variability or alternating lameness that could obscure the ability to discern treatment response. There are many horses with comparable OA that are “pasture sound” or appropriate for light work. These horses could benefit from a long-lasting, intra-articular therapy to reduce pain associated with OA. Hence, while the population was not consistent with high-performance horses, they represent a significant patient population.
Joint changes associated with iatrogenic injury are often used to evaluate therapeutic efficacy of autologous products (21). Such models are more closely representative of acute, traumatic OA in an otherwise healthy joint environment (7). The majority of clinical cases are progressive OA (1), a scenario that is best replicated through naturally occurring disease (22). Additionally, gait is often assessed with one kinetic gait variable in a single limb or via subjective evaluation (19, 23). These distinctions, among others, could account for the differences in results among studies. Additionally, a challenge of naturally occurring OA is that cartilage damage and joint environments can vary widely among joints with comparable radiographic changes (24, 25). Synovial fluid analysis may have provided additional information about joint inflammation; however, direct observation is required to assess differences in degenerative joint changes (26). Hence, variable responses among horses with seemingly similar radiographic OA may be attributable to significant differences in the local joint environment.
The duration of autologous platelet treatments is variable. Some reports indicate that treatment effectively reduces lameness in horses up to 8 months (23). Others suggest a decline in efficacy between 12 and 52 weeks after therapy, with most significant improvement between 7 and 14 days (19). Likely, the duration of response is directly related to the joint condition, including articular and periarticular tissue changes. As such, it is possible that the peak effect of the treatment was not captured at the 6- and 16-week time points in this study. The initial assessment point was selected to replicate reasonable therapeutic expectations in that more frequent injections, within 6 weeks, may not be physically reasonable or financial feasible. The highly inflamed joint environment, consistent with advanced OA, may shorten the effect of PRP, so earlier assessment points may have made it possible to identify a response that was not detected in this study. The 16-week time point was included to evaluate the duration of detectable effects in individual animals to help guide treatment strategies. More frequent assessments over the course of the study might have made it possible to determine a window during which treatment was effective for a greater number of horses.
A potential advantage of the system evaluated in this study is the fact that PRP can be isolated without the need for centrifugation, making “stall side” PRP preparation possible. Overall platelet concentrations were significantly higher after filtration, but numbers varied among preparations. There is no therapeutic standard for platelet concentration in PRP preparations (5). A proposed target concentration is around 1 × 106 platelets/μL or a threefold to fivefold increase over whole blood, though studies have shown successful treatments with both higher and lower concentrations (27, 28). The potential effect of the white blood cell (WBC) concentration on PRP benefits is also a contemporary question with no clear answer. At present, a maximum concentration of 3 × 103 WBC/μL is thought to avoid inflammatory cytokine accumulation (5). The majority of platelet concentrations in this study was consistent with current standards and reports using the same filtration kit (29), and there was no detectable effect of platelet concentration on treatment response.
Use of platelet products in combination with cell isolates has been tested both in vitro (30) and in vivo (31). Platelet lysate reportedly increases proliferation of equine adult multipotent stromal cells (MSCs) from adipose tissue in vitro (30), potentially due to high concentrations of growth factors (32). However, at high concentrations, platelet lysate decreases equine cord blood MSC proliferation in vitro (33). An investigation to evaluate the impact of autologous platelet-enriched fibrin scaffold with or without bone marrow-derived multipotent stromal cells (BMSCs) on equine stifle cartilage repair did not show a benefit of BMSCs (31). Another showed improved healing and lower inflammatory infiltrate in experimental equine tendon lesions treated with adipose-derived MSCs in platelet concentrate versus phosphate-buffered saline alone (34). The information highlights ongoing efforts to test and institute novel treatment options using progenitor cells and platelets to augment the efficacy of either alone.
This comprehensive evaluation of autologous PRP therapy in a population of horses with naturally occurring, forelimb OA provides unique information owing to the study design. Horses selected for inclusion based on specific criteria were treated, assessed, and housed under identical conditions throughout the study period. Additionally, gait changes were evaluated with quantifiable, kinetic measures. Potential kinetic changes were compared not only to a pretreatment baseline but also to a “gold standard” of joint anesthesia for each horse. Finally, horses that did and did not show kinetic gait changes associated with joint anesthesia were treated with PRP to test the potential for improvement unrelated to pain reduction in the treated joint. Based on the study findings, the first hypothesis is rejected. Kinetic gait variables did not change consistently in horses with forelimb OA following IAA. Nonetheless, meaningful kinetic variables for individual assessment of gait changes 6 and 16 weeks after PRP administration were identified for the study population. Similarly, the second hypothesis is rejected since kinetic gait variables changed in the same magnitude and direction in less than half of the horses after IAA and PRP administration. The outcomes suggest that there may be a potential benefit of autologous, intra-articular PRP therapy for forelimb OA in some horses. Additional work, including double-blind, randomized studies with large numbers of participants and more frequent observations over longer durations, is required to determine specific characteristics of degenerative joint disease that may influence the clinical effects.
Conclusion
Together, the results of this study indicate variable changes in kinetic gait parameters following intra-articular administration of autologous PRP in horses with naturally occurring, forelimb OA.
Ethical Considerations
This study was approved by the Louisiana State University Institutional Animal Care and Use Committee (protocol #10-092).
Author Contributions
ML – contributed to the study design, data collection, data reduction, data analysis, data interpretation, preparation of the manuscript, and gave final approval of the manuscript. MM and PB – contributed to the study design, data collection, preparation of the manuscript, and gave final approval of the manuscript. HR and MK – contributed to data reduction, data analysis, preparation of the manuscript, and gave final approval of the manuscript. NR – contributed to data collection, data reduction, data interpretation, preparation of the manuscript, and gave final approval of the manuscript.
Conflict of Interest Statement
Research funding and platelet filtration kits were provided by Pall Corporation. The authors declare that the funding partners approved the design prior to study initiation but did not participate in data collection, reduction, analysis, or interpretation. The manuscript contents, including the conclusion, are solely those of the authors.
Acknowledgments
The authors thank members of the Laboratory for Equine and Comparative Orthopedic Research for data collection assistance.
Funding
Funding and platelet filtration kits were provided by Pall Corporation.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/article/10.3389/fvets.2016.00029
Figure S1. Radiographs of the treated joint (two views) of each study subject.
References
1. Orth MW, Schlueter AE. Equine osteoarthritis: a brief review of the disease and its causes. Equine Comp Exerc Physiol (2004) 1:221–31. doi: 10.1079/ecp200428
2. Keegan KG. Evidence-based lameness detection and quantification. Vet Clin North Am Equine Pract (2007) 23:403–23. doi:10.1016/j.cveq.2007.04.008
3. Baek SH, Kim SY. Pharmacologic treatment of osteoarthritis. J Korean Med Assoc (2013) 56:1123–31. doi:10.5124/jkma.2013.56.12.1123
4. Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse – a review. Vet J (2006) 171:51–69. doi:10.1016/j.tvjl.2004.07.008
5. McLellan J, Plevin S. Does it matter which platelet-rich plasma we use? Equine Vet Educ (2011) 23:101–4. doi:10.1111/j.2042-3292.2010.00185.x
6. Hildner F, Albrecht C, Gabriel C, Redl H, van Griensven M. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med (2011) 5:e36–51. doi:10.1002/term.386
7. Ameye LG, Young MF. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol (2006) 18:537–47. doi:10.1097/01.bor.0000240369.39713.af
8. Maliye S, Voute LC, Marshall JF. Naturally-occurring forelimb lameness in the horse results in significant compensatory load redistribution during trotting. Vet J (2015) 204:208–13. doi:10.1016/j.tvjl.2015.03.005
9. Weishaupt MA. Adaptation strategies of horses with lameness. Vet Clin North Am Equine Pract (2008) 24:79–100. doi:10.1016/j.cveq.2007.11.010
10. Keegan KG, Dent EV, Wilson DA, Janicek J, Kramer J, Lacarrubba A, et al. Repeatability of subjective evaluation of lameness in horses. Equine Vet J (2010) 42:92–7. doi:10.2746/042516409X479568
11. Verwilghen D, Busoni V, Gangl M, Franck T, Lejeune JP, Vanderheyden L, et al. Relationship between biochemical markers and radiographic scores in the evaluation of the osteoarticular status of Warmblood stallions. Res Vet Sci (2009) 87:319–28. doi:10.1016/j.rvsc.2009.02.002
12. Xie L, Spencer ND, Beadle RE, Gaschen L, Buchert MR, Lopez MJ. Effects of athletic conditioning on horses with degenerative suspensory ligament desmitis: a preliminary report. Vet J (2011) 189:49–57. doi:10.1016/j.tvjl.2010.06.010
13. Williams GE, Silverman BW, Wilson AM, Goodship AE. Disease-specific changes in equine ground reaction force data documented by use of principal component analysis. Am J Vet Res (1999) 60:549–55.
14. Merkens HW, Schamhardt HC. Evaluation of equine locomotion during different degrees of experimentally induced lameness. I: lameness model and quantification of ground reaction force patterns of the limbs. Equine Vet J Suppl (1988) 20:99–106. doi:10.1111/j.2042-3306.1988.tb04655.x
15. McLaughlin RM Jr, Gaughan EM, Roush JK, Skaggs CL. Effects of subject velocity on ground reaction force measurements and stance times in clinically normal horses at the walk and trot. Am J Vet Res (1996) 57:7–11.
16. Hammarberg M, Egenvall A, Pfau T, Rhodin M. Rater agreement of visual lameness assessment in horses during lungeing. Equine Vet J (2016) 48:78–82. doi:10.1111/evj.12385
17. Arkell M, Archer RM, Guitian FJ, May SA. Evidence of bias affecting the interpretation of the results of local anaesthetic nerve blocks when assessing lameness in horses. Vet Rec (2006) 159:346–9. doi:10.1136/vr.159.11.346
18. Clayton HM, Schamhardt HC, Willemen MA, Lanovaz JL, Colborne GR. Kinematics and ground reaction forces in horses with superficial digital flexor tendinitis. Am J Vet Res (2000) 61:191–6. doi:10.2460/ajvr.2000.61.197
19. Bertone AL, Ishihara A, Zekas LJ, Wellman ML, Lewis KB, Schwarze RA, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res (2014) 75:141–51. doi:10.2460/ajvr.75.2.141
20. Filardo G, Kon E, Pereira Ruiz MT, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: single- versus double-spinning approach. Knee Surg Sports Traumatol Arthrosc (2012) 20:2082–91. doi:10.1007/s00167-011-1837-x
21. Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res (2007) 68:290–6. doi:10.2460/ajvr.68.3.290
23. Carmona JU, Arguelles D, Climent F, Prades M. Autologous platelet concentrates as a treatment of horses with osteoarthritis: a preliminary pilot clinical study. J Equine Vet Sci (2007) 27:167–70. doi:10.1016/j.jevs.2007.02.007
24. Miosge N, Hartmann M, Maelicke C, Herken R. Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem Cell Biol (2004) 122:229–36. doi:10.1007/s00418-004-0697-6
25. Lopez MJ, Lewis BP, Swaab ME, Markel MD. Relationships among measurements obtained by use of computed tomography and radiography and scores of cartilage microdamage in hip joints with moderate to severe joint laxity of adult dogs. Am J Vet Res (2008) 69:362–70. doi:10.2460/ajvr.69.3.362
26. Brandt KD, Fife RS, Braunstein EM, Katz B. Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum (1991) 34:1381–6. doi:10.1002/art.1780341106
27. Kon E, Filardo G, Di Martino A, Marcacci M. Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc (2011) 19:516–27. doi:10.1007/s00167-010-1306-y
28. Brossi PM, Moreira JJ, Machado TS, Baccarin RY. Platelet-rich plasma in orthopedic therapy: a comparative systematic review of clinical and experimental data in equine and human musculoskeletal lesions. BMC Vet Res (2015) 11:98. doi:10.1186/s12917-015-0403-z
29. Hessel LN, Bosch G, van Weeren PR, Ionita JC. Equine autologous platelet concentrates: a comparative study between different available systems. Equine Vet J (2015) 47:319–25. doi:10.1111/evj.12288
30. Del Bue M, Ricco S, Conti V, Merli E, Ramoni R, Grolli S. Platelet lysate promotes in vitro proliferation of equine mesenchymal stem cells and tenocytes. Vet Res Commun (2007) 31(Suppl 1):289–92. doi:10.1007/s11259-007-0099-z
31. Goodrich LR, Chen AC, Werpy NM, Williams AA, Kisiday JD, Su AW, et al. Addition of mesenchymal stem cells to autologous platelet-enhanced fibrin scaffolds in chondral defects: does it enhance repair? J Bone Joint Surg Am (2016) 98:23–34. doi:10.2106/JBJS.O.00407
32. Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost (2004) 91:4–15. doi:10.1160/TH03-07-0440
33. Russell KA, Koch TG. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture – too much of a good thing? Equine Vet J (2016) 48:261–4. doi:10.1111/evj.12440
Keywords: kinetics, joint, platelet, equine, lameness, animal, cell therapy
Citation: Mirza MH, Bommala P, Richbourg HA, Rademacher N, Kearney MT and Lopez MJ (2016) Gait Changes Vary among Horses with Naturally Occurring Osteoarthritis Following Intra-articular Administration of Autologous Platelet-Rich Plasma. Front. Vet. Sci. 3:29. doi: 10.3389/fvets.2016.00029
Received: 08 January 2016; Accepted: 24 March 2016;
Published: 13 April 2016
Edited by:
Fausto Cremonesi, Università degli Studi di Milano, ItalyReviewed by:
Francisco M. Sanchez Margallo, Minimally Invasive Surgery Centre Jesus Uson, SpainRuchi Sharma, Axol Bioscience, UK
Copyright: © 2016 Mirza, Bommala, Richbourg, Rademacher, Kearney and Lopez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mandi J. Lopez, mlopez@lsu.edu
 Mustajab H. Mirza
Mustajab H. Mirza Prakash Bommala1
Prakash Bommala1
 Heather A. Richbourg
Heather A. Richbourg Mandi J. Lopez
Mandi J. Lopez