Recent Advances in Measurement and Dietary Mitigation of Enteric Methane Emissions in Ruminants
- Department of Animal Nutrition, Faculty of Veterinary and Animal Sciences, West Bengal University of Animal and Fishery Sciences, Kolkata, India
Methane (CH4) emission, which is mainly produced during normal fermentation of feeds by the rumen microorganisms, represents a major contributor to the greenhouse gas (GHG) emissions. Several enteric CH4 mitigation technologies have been explored recently. A number of new techniques have also been developed and existing techniques have been improved in order to evaluate CH4 mitigation technologies and prepare an inventory of GHG emissions precisely. The aim of this review is to discuss different CH4 measuring and mitigation technologies, which have been recently developed. Respiration chamber technique is still considered as a gold standard technique due to its greater precision and reproducibility in CH4 measurements. With the adoption of recent recommendations for improving the technique, the SF6 method can be used with a high level of precision similar to the chamber technique. Short-term measurement techniques of CH4 measurements generally invite considerable within- and between-animal variations. Among the short-term measuring techniques, Greenfeed and methane hood systems are likely more suitable for evaluation of CH4 mitigation studies, if measurements could be obtained at different times of the day relative to the diurnal cycle of the CH4 production. Carbon dioxide and CH4 ratio, sniffer, and other short-term breath analysis techniques are more suitable for on farm screening of large number of animals to generate the data of low CH4-producing animals for genetic selection purposes. Different indirect measuring techniques are also investigated in recent years. Several new dietary CH4 mitigation technologies have been explored, but only a few of them are practical and cost-effective. Future research should be directed toward both the medium- and long-term mitigation strategies, which could be utilized on farms to accomplish substantial reductions of CH4 emissions and to profitably reduce carbon footprint of livestock production systems. This review presents recent developments and critical analysis on different measurements and dietary mitigation of enteric CH4 emissions technologies.
Introduction
Greenhouse gas (GHG) emissions, largely methane (CH4) from the rumen and nitrous oxide from manure management, from livestock contribute considerably to the atmospheric GHG (1, 2). CH4 is normally produced during microbial fermentation of feeds, mainly structural carbohydrates, in the rumen by methanogenic archaea. Globally, about 95 million tones of CH4 are emitted from enteric fermentation of domestic animals in 2010 with an annual growth rate of 0.90% (2). Enteric CH4 contributes 17 and 3.3% of global CH4 and GHG emissions, respectively, which mostly arises from ruminant livestock (3). The contribution of GHG from livestock is expected to grow due to increasing populations of livestock animals triggered by an increasing demand of animal protein, especially in developing countries. Abatement of enteric CH4 emission is required to minimize the liability of livestock production for GHG emission. Mitigation strategies of enteric CH4 are considered to be less expensive than carbon dioxide (CO2) emissions (3, 4). Inhibition of CH4 emission by some technologies generally does not cause much detrimental effects on rumen fermentation, but may improve rumen fermentation efficiency. Sometimes, CH4 mitigation options are associated with improved efficiency of animal production, which is advantageous both environmentally and nutritionally. Several comprehensive reviews have been published recently, which describe a number of options and strategies to mitigate GHG from livestock production (3, 5–7). Generally, these review papers on CH4 mitigation technologies have been focused on research conducted during the past 10–15 years.
An accurate, cost-effective and repeatable measurement technique of enteric CH4 production from ruminants is required to evaluate a CH4 mitigation technology, preparation of inventory of CH4 gas emissions and assessment of carbon footprint of livestock products. Therefore, it has been an important area of research to develop new techniques, to improve accuracy of the existing methods and to compare among the methods of CH4 measurements in recent years. A number of methods are used to measure CH4 emission from ruminants, all of which differ in their application, cost, accuracy, precision, and repeatability depending on their conditions of use [e.g., Ref. (8–11)]. This paper primarily presents the latest advances in measurements and dietary mitigations of CH4 emissions from ruminants.
Measurement Methods of CH4 Emissions
A number of methods have been developed and improved in recent years, which is employed for long-term and short-term measurements in individual animals or grouped animals directly as well as indirect prediction of CH4 emissions (Figure 1). These methods are described here along with their advantages and disadvantages.
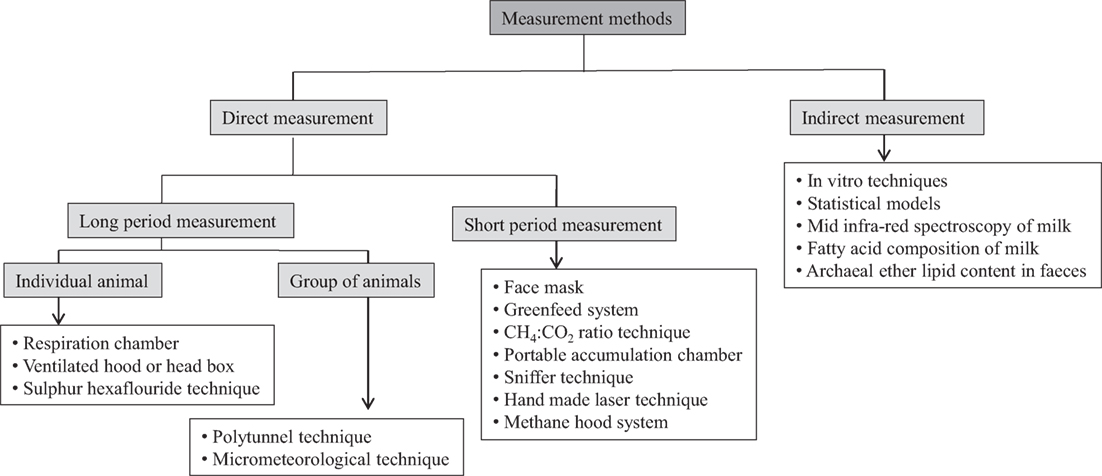
Figure 1. A schematic presentation of different CH4 measurement techniques in ruminants using different approaches.
Long-Term Measurement Methods
Respiratory Chamber Technique
Respiration chamber (RC) technique was used for determining energy balance and gaseous exchange in animals for many years [e.g., Ref. (12, 13)]. The principle of this technique is to measure the concentrations of CH4 (coming out through all avenues, i.e., mouth, nostrils, and rectum from enteric fermentation) in gas samples and total volume of air removed from the RC (10). An air pump continuously removes air from a RC through a flow meter in the open-circuit system to calculate volume of air removed. Outlet gas from the RC is continuously sampled for analysis through a duct system. The RC system is equipped with ventilation fans inside the chamber for proper mixing of expired gases and incoming air. Fresh air to the RC is directly drawn from outside or through an air conditioning system to control humidity and temperature. The RC is fitted with humidity, temperature, and barometric pressure meters to determine gas volume at standard temperature and pressure (STP) conditions (9).
The RC method for measurement of enteric CH4 production is often considered as the “gold standard” technique due to high accuracy and repeatability, and low animal-to-animal variations of CH4 measurement using this technique (14, 15). However, RC system is costly for establishment and labor intensive for operation. This method imposes restrictions on eating and other natural behaviors of the animals resulting in CH4 production that often differs from CH4 production in their normal environments. This technique also requires great technical expertise to generate accurate CH4 emission measurements. Their potential negative effects on feed intake and milk production of lactating animals while confined in the RC can be minimized with proper adaptation of the animals to the system, but must also be considered. Moreover, RC method has limited “throughput,” and thus is less suitable when CH4 measurements on large numbers of animals are required, such as screening of low CH4-producing animals for genetic selection.
The RC technique can be highly accurate and precise when used with rigor and it measures total CH4 emission, including losses from anus and rumen fistulas. This method has additional advantage of measurement of gas production or consumption of other gases (e.g., oxygen, CO2, hydrogen, ammonia). In RC, the design may also allow to investigate other nutritional evaluations, such as digestibility, nitrogen balance, and energy metabolism. The RC method can detect relatively small effects of diets and supplements on CH4 emission determined on a small number of animals (9). With repeated measurements over the course of daily CH4 production patterns, RC technique can be employed to characterize diurnal CH4 emission variations, which may provide insight into underlying mechanisms of enteric CH4 formation, including relationships with H2 production when anti-methanogenic compounds are used. Despite the better accuracy and many nutritional advantages in the RC method, this method could invite significant errors in CH4 measurement unless proper calibration procedures are regularly followed. For example, in a recent ring test of calibration of RC in different laboratories, ducting system resulted in 15.3% variations, followed by errors in mixing of air in the RC by 3.4% and analyzer by 1.3% variations (16). In conclusion, although RC technique is considered as “gold standard” in determining CH4 emission due to high accuracy and low animal and day variation of measurements, low throughput, high cost, imposing restriction on natural animal behavior, and greater technical requirements for operation limit this technique for widespread use.
Sulfur Hexafluoride Tracer Technique
The sulfur hexafluoride (SF6) tracer technique was developed by Zimmerman (17) and was first used for measurement of CH4 in grazing cattle by Johnson et al. (18). The SF6 method has been used extensively during the last two decades for measurement of CH4 emissions from ruminants. The principle behind this method is that CH4 production can be measured if SF6 gas production rate from the rumen is known (18). Small permeation tubes are filled with SF6 and are then placed into the rumen of animals. Test animals are fitted with gas sampling apparatus, which consists of a halter to support capillary tubing whose inlets to be placed close to the nose and an evacuated canister to collect gas samples (10). Representative gas samples containing respired and eructated gas are collected usually for 24 h through capillary tubing connected to an evacuated canister. The tubing regulates the gas sampling rate (19). The concentrations of SF6 and CH4 in the gas samples collected in canister are analyzed by gas chromatography. The CH4 emission is calculated from the SF6 release rate and concentrations of SF6 and CH4 in the canister gas samples in excess of background concentrations in the air using the following equation (18).
where [CH4]c and [SF6]c are the concentrations of CH4 and SF6 in the canister, respectively; while [CH4]b and [SF6]b are the CH4 and SF6 concentrations in the background air, respectively.
Unlike the RC method, the SF6 method can be employed on large numbers of animals concurrently to measure CH4 emissions from both grazing and non-grazing animals in comparatively less time and cost. Also, SF6 method can impose lesser effect on animal behaviors under typical animal management conditions (18). However, it is labor intensive and requires great technical expertise to minimize experimental errors of measuring CH4. There are concerns over the variability and repeatability of measurements (9, 20). In addition, the long-term instability of release rate of the permeation tubes remains a concern for use in studies with long period of experiments (21, 22), and background atmospheric gas concentrations can impact markedly on the success of the SF6 technique (23). Thus, the day-to-day and animal-to-animal variations in CH4 emission estimates are greater with the SF6 technique compared with the estimates derived from RC (20, 24, 25). These greater variations would induce a negative impact on the power of statistical analyses using this technique as it will require more animals (replicates) to detect treatment differences in evaluation of CH4 mitigation studies (9). The CH4 emissions measured by SF6 technique were similar to the values quantified by RC and ventilated head hood systems in some studies (14, 18, 26), but were different in other studies (14, 25, 27). Johnson et al. (18) observed that CH4 emission using the SF6 technique agreed with CH4 emission using RC technique in grazing cattle (7.3 versus 7.2% of gross energy intake). In subsequent studies (28, 29), slightly lower emissions (5–10%) were observed with the SF6 method than with the RC method for both cattle and sheep, which can partly be due to the few percent of CH4 lost via rectum. By contrast, higher values of CH4 emission were observed using the SF6 technique than RC technique (20, 27, 30). Muñoz et al. (25) also noted significantly higher mean values of CH4 emissions (gram/day) and CH4 yield (gram/kilogram DM intake) for the SF6 technique than for the RC method (443 versus 396 g/day and 26.7 versus 24.2 g/kg DM intake). Although average CH4 emissions may not be different in some studies, within- and between-animal coefficients of variations were much greater for the SF6 technique than for the RC method (14, 20, 31). Pinares-Patiño et al. (20) utilized the same animals for measurement of CH4 using with the SF6 and RC technique and reported that the within coefficients of variations were 4.7, 13.5, and 11.7% in RC technique, SF6 technique, and with SF6 technique performed inside RC, respectively. The between-animal variations were also considerably higher with the SF6 technique than with the RC technique.
A number of factors, such as release rate of SF6 from permeation tubes, influence of SF6 release rate on CH4 emission rate, measurements of background concentrations of SF6, flow rate in the tubing during sample gas collection, inconsistency of CH4 measurements using RC and SF6 techniques, and within- and between-animal variations, have been implicated for low accuracy in CH4 emission measurements in the SF6 technique (9, 22, 32). A considerable research effort has been undertaken to improve the accuracy, precision, and consistency of the results in the SF6 technique in different studies [e.g., Ref. (22, 23, 33)]. The determination of SF6 release rate over time from tubes is important, which affects emission estimates (19, 34). Permeation tubes that release SF6 in greater rates may result in higher CH4 production compared with the tubes with lower release rates (34). Hence, permeation tubes with similar SF6 release rate are recommended to obtain better accuracy, especially, for the comparison of different mitigation treatments (34). Permeation tubes are regularly weighed over 1 month under laboratory conditions to obtain the release rates of SF6 (9). Only highly linear permeation tubes are used for measuring experiments (19). The SF6 release rates from permeation tubes are calculated applying zero-order kinetics (19). However, Lassey et al. (19) demonstrated that the SF6 release rates from permeation tubes kept at 39°C in a dry laboratory incubator decreased with time. Thus, the application of zero-order kinetics to predict the SF6 release rate from permeation tubes invites a considerable error in measurement of enteric CH4 outputs, particularly when an experiment continues more than 30 days after the calibration of permeation tubes (22, 35). The inconsistency in measurements of CH4 emissions using the RC and SF6 techniques increased substantially when the permeation tubes were kept in the rumen for long time (21). Researchers have shown that the curves of the rate of SF6 release from permeation tubes are curvilinear under laboratory conditions (22, 27). Therefore, Lassey et al. (19) suggested the use of quadratic equations to predict the rate of SF6 release from permeation tubes with time. This approach was suggested to be certainly much better than using zero-order kinetics (22). Recently, Moate et al. (22) demonstrated that the use of Michaelis–Menten kinetics can more accurately describe the SF6 release rate pattern from permeation tubes and this may improve the accuracy of the measurement of CH4 emissions from ruminants. Using this kinetics, measurement of CH4 emissions can be continued for even up to 800 days after insertion of the tubes into the rumen compared with the typical period of 60–90 days. Another important factor is the design of capillary-tube flow rate restrictors that can also cause appreciable errors in measurement of CH4 emissions of up to 15.6% (33). Orifice plate flow restrictors can control the gas sample collection rate into canisters and lower errors in measurement of CH4 production (33). The SF6 technique using these modifications resulted in measurements of CH4 emission to be greatly accurate with measurements taken using RC (33). In this experiment, mean CH4 yield (gram/kilogram DM intake) was 21.9 ± 1.65 and 22.3 ± 1.44 in lactating dairy cows when measured by the RC and SF6 technique, respectively; and the between-animal coefficients of variation were 7.5 and 6.5% using the RC and SF6 technique, respectively.
The background SF6 and CH4 concentrations should be corrected, but determination of representative background concentrations of these gases under field conditions may be difficult because direction and velocity of wind and other animals in the vicinity may influence the background concentrations (23). Besides, the SF6 release from permeation tubes may affect the background concentration of SF6 to which an adjacent animal is exposed (23). For this reason, Williams et al. (23) emphasized that recording of improper background concentrations of SF6 can affect the extent of CH4 emission measurements. From these observations, Lassey (36) concluded that the relationship between the estimated CH4 emission rates and the SF6 release rates from permeation tubes was merely an artifact as a result of inappropriate background SF6 concentrations. Pinares-Patiño et al. (34) suggested that this concern could be ameliorated to a certain degree by using all permeation tubes with similar rate of SF6 release in an experiment. In summary, the SF6 method can be employed without imposing much effect on natural behavior of grazing animals and has a greater throughput, but it could result in greater variations in CH4 emissions compared with the RC method. Nevertheless, the SF6 method can be used with a high level of precision when recent recommendations of this technique are followed for measurement of CH4 emissions.
Ventilated Hood Chambers or Head Box System
This system also can be used for measurement of daily CH4 emissions using the same principles as the RC method [e.g., Ref. (37, 38)]. In this technique, a box or hood is fabricated to accommodate the head of animals, and air samples drawn through the hoods are analyzed for CH4 concentrations in incoming and exhaust air (8). Unlike RC, this method does not quantify CH4 arising from the hindgut. Airflow is measured and used to calculate CH4 emission. Head chambers are typically large enough to allow the animal to move its head in an unrestricted manner and obtain feed and water. Like RC, they can be used to obtain continuous measurements over successive 24-h periods. Ventilated head hoods with different designs and sizes for small and large animals have been installed in different countries for nutritional studies [e.g., Ref. (37–40)]. The flow rate of exhaust air is important for the accuracy of gas analysis and comfort of the animals in head box (37). This system could also be employed for nutritional evaluation and energy metabolism of feeds [e.g., Ref. (40)]. Boadi et al. (26) compared ventilated head hood with the SF6 technique. The average daily CH4 production was similar for both the methods (130 and 137 L/day for head hood and SF6 method, respectively). Animal-to-animal variation was, however, significant with the SF6 method (11.7%), but not with the head hood technique (0.1%) for production. Troy et al. (41) obtained simultaneous measurements of CH4 outputs from cattle using RC and feeder-mounted hoods located within RC. It was reported that increases in concentrations of CH4 in hoods over RC background were positively correlated (r = 0.67) with daily CH4 emissions, but there was substantial variability. This technique measures CH4 emissions reliably similar to the RC method and involves low cost compared with RC, but animals are required an extensive training to become adapted to the head hood, which restricts its extensive use for screening of large numbers of animals for genetic selection purpose.
Short-Term Measurement Methods
Face Mask Method
Face-masks for “spot-sampling” of respiratory exchange and CH4 emission have been used in cattle, sheep, and goats for many years [e.g., Ref. (42)]. In this method, animals are needed to train to stay in sternal recumbency for the measurement periods (e.g., 30 min) repeated over the course of 24-h periods (8). This method presents a greater animal and day variation and only provides a short-term emission rate (43). The CH4 emission values using this technique are highly dependent upon the number and timing of respiratory exchange measurements taken with respect to diurnal patterns of feeding cycle and CH4 emission. However, if sufficient data are collected from several animals with greater regularity in sample collection throughout the measurement period of 24 h, a typical CH4 emission pattern can be calculated (11). Face mask can be useful for short-term measurements of CH4 emission rate for screening of large numbers of animals, but may cause marked discomfort and distress and change behaviors of the animals, and consequently, can affect the gas measurements.
Portable Accumulation Chamber
Portable accumulation chamber (PAC) system is essentially a RC without airflow. In this technique, PAC acts to trap all exhaled gases (CH4, CO2, and other gases), while oxygen depletes during the collection period of 1–2 h, and a single CH4 or other gas measurement is taken at the end of the collection period (44, 45). Emission of CH4 is calculated as the concentration of CH4 (corrected for background) multiplied by net chamber volume, adjusted for STP, divided by time of measurement (44). The time period of use should be restricted to avoid negative effects of increased chamber CO2 concentration, and accordingly the PAC is essentially a short-term respiration measurement. Moderate repeatability (correlation of 0.33–0.43) of measurements of CH4 emission by individual sheep using PAC was reported in studies at different sites (46). This technique could be useful for screening of low CH4-producing animals from large herds for genetic improvement purpose.
CH4/CO2 Ratio Technique
The CH4:CO2 ratio method, which was conceptualized by Madsen et al. (47), determines CH4 emission from individual animals based on the calculated CO2 emission and CH4 and CO2 concentrations measured using a gas analyzer. Their method relies on analyzing air samples for CH4 and CO2 simultaneously with a gas analyzer that is based on Fourier transform infrared (FTIR) detection, and uses CO2 from the breath of animals as the tracer gas. Emission of CO2 can be predicted based on estimates of energy metabolism, heat production, and respiratory quotient (RQ), or carbon balance (47).
where [CH4]BS and [CO2]BS are the CH4 and CO2 concentrations in the breath samples, respectively; while [CH4]BG and [CO2]BG are the CH4 and CO2 concentrations in the background air, respectively.
The repeatability of the CH4:CO2 ratio was reported to be 0.39 for Holsteins and 0.34 for Jerseys (48). Hellwing et al. (49) compared predicted CH4 emissions in lactating dairy cows using the CH4:CO2 ratio technique with CH4 emissions in the RC method and reported a positive relationship (r = 0.55) between the two methods, but the CH4:CO2 ratio technique significantly underestimated the CH4 production (412 versus 345 g/day). This difference may be due to an error in the prediction of within-day variation in CO2 emission, which needs to be improved to obtain better individual animal CH4 emission estimates (49). Several factors, such as diurnal variations in the CH4:CO2 ratio resulted from differences in digestive and metabolic activity and rumen fermentation pattern associated with feed intake and feeding frequency, and source of gas samples (e.g., exhaled air, flatus and fermentation of manure or bedding) could affect CH4 emission measurement (47, 50). Therefore, adequate numbers of measurements in different times of the days should be considered to account for diurnal and postprandial variation in CH4 and CO2 emissions in animals. Nonetheless, this technique could be employed to generate large-scale data for genetic evaluation of CH4 production.
GreenFeed System
GreenFeed (GF) system (C-lock Inc., Rapid City, SD, USA) has recently been patented by Zimmerman (51) for measurement of CH4, CO2, and H2 production from animals. This system includes an automatic baiting system, measurements of air flow and gas concentration systems, electronics and communication devices, a gas tracer device, and an animal detection system during visit of an animal to the unit. A detailed description and visualization of the system is provided in Hristov et al. (52). In GF system, CH4 emission is measured for a short period when animals visit to the system to consume feeds that are used as enticement. The concept of the GF system is that numerous short-term CH4 emission values from an individual animal measured in different times within a day for many days can be aggregated to estimate an average daily CH4 emission from the animal. Software function allows investigators to control the timing of feed availability and to allocate CH4 measurements across various times of the day. The animals entering an automatic feeding system are recognized and concentrations of CH4 are measured at that particular time. Air is constantly pumped out through the automatic feeding system to measure flow rate and thereby CH4 emission during feeding period. Daily CH4 emission is calculated using the same principle as in RC method, whereby CH4 emission rate is calculated using volumetric air flow rate adjusted to STP and corrected for capture rate, as detailed by Huhtanen et al. (50):
where Cp(i) is the fractional capture rate of air at time i; [CH4]c(i) and [CH4]b(i) are the concentrations of captured gas (ppm) and background gas of CH4 (ppm), respectively, time i; and Fair(i) is the volumetric air flow rate (L/min) measured on a dry-gas basis at time i.
The GF system was compared with the RC and SF6 techniques to assess the accuracy and suitability of the GF system for measurement and detection of treatment difference for CH4 emissions in different studies (53, 54). The mean value of CH4 emission by growing dairy cattle in GF system was similar to measurements taken in the RC system, but was lower than the values obtained using the SF6 technique (53). Dorich et al. (54) noted that the average CH4 production from lactating dairy cows measured using the GF and SF6 techniques were similar (468 versus 467 g/day), but the GF method resulted in smaller coefficients of variation (14.1–22.4 versus 16.0–111%) for CH4 emissions, and higher relationship (0.65 versus 0.41) between CH4 (gram/day) and dry matter (DM) intake compared with GF system. The authors attributed this higher variability for SF6 measurements to the high concentration of background gases combined with poor barn ventilation. Also, Arbre et al. (55) analyzed repeatability estimates and noted that 3-day periods were necessary for the SF6 technique and 17-day periods for the GF system to achieve the repeatability of 0.70 for CH4 yield (gram/kilogram DM intake). The repeatability did not increase after 4-day periods for the SF6 method (repeatability = 0.73), but increased for the GF method until 45-day periods (repeatability = 0.90). Hammond et al. (53) conducted three experiments (two experiments on indoor animals and one experiment on grazing animals) in which Holstein heifers were fed various diets to compare the GF system with the RC or SF6 method. Daily CH4 emissions (gram/day) and CH4 yield (gram/kilogram DM intake) were similar between the GF (198 g/day and 26.6 g/kg DM intake in experiment 1; and 208 g/day and 27.8 g/kg DM intake in experiment 2) and RC (218 g/day and 28.3 g/kg DM intake in experiment 1; and 209 g/day and 27.7 g/kg DM intake in experiment 2) methods in both indoor experiments. In experiment 3, CH4 emissions and yields determined using the SF6 technique were, however, greater than the values measured using the GF system during grazing (186 versus 164 g/day and 21.6 versus 18.8 g/kg DM intake). Moreover, CH4 production quantified by the GF technique was not concordant (r = 0.10) with CH4 production determined by the RC method, but was only in moderate agreement (r = 0.60) with CH4 production measured by the SF6 technique. Significant treatment and individual animal differences in CH4 emission were detected using both RC and SF6 techniques, but were unable to detect using the GF method (53). This was attributed to a limited number of measurements obtained with the GF system in grazing animals and the timing of the measurements relative to daily patterns of CH4 emission, highlighting the importance of obtaining sufficient numbers of observations using the GF system. However, Velazco et al. (56) reported that GF and RC methods produced similar CH4 emissions (209.7 versus 215.1 g CH4/day) and also CH4 yield (22.7 versus 23.7 g CH4/kg of DM intake).
In principle, this technique requires sufficient numbers of measurements over time to obtain accurate estimates of daily emission, and relies on animals voluntarily visiting the unit (56). Some animals may not visit the GF unit for sufficient times despite feed restriction (57). Compared with the RC and SF6 techniques, GF system requires more time and animals, when a study is planned for comparison of CH4 production among the treatments, due to higher within-day and within-animal variance (58). The use of GF system requires a feed supplement, which may also introduce between-day variation with respect to supplement consumption and interact with the actual treatments. Taken together, CH4 emissions quantified using GF method may show high variability compared with the emissions measured by the SF6 and RC methods; the GF method, however, offers a lower cost alternative as an automated method for measurement of CH4 emissions from individual animal than SF6 and RC methods both in indoor and grazing conditions.
Sniffer Method
This technique was first conceptualized by Garnsworthy et al. (59). In this technique, a sampling inlet is placed in the feed manger of an automatic milking system to collect air eructed by cattle during milking (often called the “sniffer” technique). As described by Garnsworthy et al. (59), air in the manger is constantly sampled, analyzed, and logged at 1-s intervals using data loggers to measure CH4 and CO2 concentrations in close proximity to the muzzle of the animal. Information on eructation frequency and CH4 released per eructation are used to estimate CH4 emission rate by individual animal during milking. Garnsworthy et al. (59) reported a good relationship (r = 0.79) between the measurements of CH4 emission using the RC technique and CH4 emission rate using this method. By contrast, Huhtanen et al. (50) compared the measurements of eructated CH4 concentration with CH4 emissions determined using the GF system in lactating dairy cows in two experiments. They found between-cow coefficient of variation (11.0–17.6 versus 17.5–28.0%) was smaller for the GF system compared with the sniffer method. There was weak relationship (R2 = 0.09) between the CH4 measurements (gram/day) using GF system and concentrations of CH4 recorded by the sniffer method, which may be attributed to the inconsistent air-mixing conditions within the feeding troughs influenced by the geometry of feed troughs, muzzle movement, and muzzle position (50). Thus, further research is needed if this type of sniffer method could be employed for quantification of CH4 with some consistency.
Hand Laser CH4 Detector
The hand laser CH4 detector (LMD) technique (60, 61) measures exhaled CH4 concentrations in the air near the nose or mouth of an animal in normal environment. The data consist of a series of peaks representing the animal’s respiratory cycle. Only peaks reflecting the increase in CH4 concentrations due to exhalation or eructation are used in the analysis (61). As the measurements are made in the air close to the animal’s nostrils, and measurements may not be affected by head position unlike sniffer method of Garnsworthy et al. (59). In a study with dairy cattle, a relatively strong correlation between CH4 measurements using the LMD with those determined in the RC (r = 0.80) was noted (62). The LMD can also strongly detect periods of high-enteric CH4 concentration and avoid misclassifying periods of low-enteric CH4 concentration (60). However, in a subsequent study, weak relationships (r = 0.22–0.28) between RC and LMD methods in CH4 measurements (61) have been reported. This technique allows measurements of CH4 in same animal repeatedly in their normal environments, while measurements are restricted during milking and feeding periods for the sniffer and GF techniques. However, the LMD system is labor intensive and meteorological factors, such as wind speed and direction, temperature, humidity, and atmospheric pressure, may influence the accuracy and precision of the measurements, with wind speed being a major factor for grazing studies and outdoor measurements (63). Like the sniffer technique, this technique also requires further improvements if LMD could be suitable for quantification of CH4 from large numbers of animals in normal management conditions for screening of animals on farms.
Methane Hood System
A novel method to quantify CH4 emissions during feeding has been designed recently by Troy et al. (64). This method can be used to measure CH4 output from individual animals in a group housed environment. In principle, this method is similar to GF system except that there is no requirement to provide extra feed supplements for enticement to visit an animal into the measurement area as required for GF system. Methane hood system measures CH4 concentrations in a hood designed to partially enclose the volume above a feed bin. Number of visits could be higher in methane hood than GF system, which may provide high accuracy in methane hood system. Troy et al. (64) compared this system with RC for measurement of CH4 emission employing nitrate as a CH4 mitigation option and found a comparable results between the RC method and this technique and detected significant treatment differences in CH4 production. Preliminary results suggest that this system could be a better alternative choice to quantify CH4 emissions from individual animals housed in a group in “natural” environments.
Herd Scale Measurement Technique
Polytunnel Method
Polytunnel method requires a large tunnel made up of polyethylene fitted with end wall and large diameter port (43). As described by Lockyer and Jarvis (43), measure volume of air is continuously blown into and drawn from the large tunnel containing grazing animals, concentration of CH4 between the incoming and outgoing air is regularly measured, and temperature and humidity are monitored. Lockyer and Jarvis (43) and Lockyer (65) conducted two experiments using a polytunnel system of 4.3 m wide × 9.9 m long × 2.1 m height with an approximate volume of 66 m3 in which different numbers of sheep and calves were enclosed for up to 10 days. The recovery percentage of added CH4 in the tunnel was 104%. Average CH4 production was 13–14 and 74.5 g/day for sheep and calves, respectively. However, CH4 emissions decreased with increasing time of grazing, perhaps due to declining in available forage mass in the pasture as a result of very high stocking rates. Murray et al. (66) carried out two experiments to evaluate polytunnel system in comparison with the RC system for CH4 emissions from sheep. In both the system, the sheep were fed at maintenance levels of either fresh cut grass or dried grass pelleted diets. The results showed that CH4 production using the RC technique was greater (31.7 L/kg DM intake) than the tunnel technique (26.9 L/kg DM intake). The recoveries of the added CH4 in both the systems were similar (95.5–97.9% for tunnel versus 89.2–96.7% for RC). This system is suitable for measuring CH4 in semi-normal grazing conditions in individual or small group of animals. The operation of this method is simpler and portable, but there is difficulty in controlling temperature and humidity inside the tunnel.
Micrometeorological Techniques
During the last 10–15 years, a number of CH4 emission measurement techniques based on micrometeorological variables from whole farms, feedlots, and paddocks have been developed (67, 68). Micrometeorological methods involve measuring fluxes and concentrations of gases in the free atmosphere of a large area containing animals, and relating these fluxes and concentrations to calculate gas emissions from animals. Micrometeorological dispersion methods cannot measure emissions from individual animals as well as indoor housed animals. Furthermore, the scale of micrometeorological techniques makes their use difficult for testing mitigation options (69).
The micrometeorological methods involve measurements of CH4 concentration and wind speed, but the number of points of measurement and the assumption utilized to compute emission rates vary depending upon the methods. In the external tracer ratio technique, a tracer gas is released in the paddock or barn area, and the tracer gas and CH4 concentrations are measured in the surrounding areas (68). This category of methods also includes a mass balance technique in enclosed barns, where CH4 emissions are determined from ventilation rate and concentrations in inlet and outlet air (10). While it is relatively easy to estimate emission rates from mechanically ventilated closed barns, naturally ventilated buildings are problematic because of difficulties with measuring air exchange rates (10, 70). Air exchange rates in the naturally ventilated buildings depend upon the temperature gradient, temperature humidity index, and the wind speed. The release rates of gases may also depend upon outside environment, such as wind speed, humidity, and the other parameters (10).
A considerable development in micrometeorological techniques has improved CH4 measurement accuracy using inverse dispersion method (71). Inverse dispersion technique has been employed with success in many feedlot gas emission studies (69, 71, 72). This method has some advantages, such as non-interference of natural behaviors of animals and estimation of carbon footprint over large areas (73). However, there are also many limitations of inverse dispersion method, including wind conditions and the need for source homogeneity (73).
Emissions of CH4 from grazing animals are measured in field experiments using paddock-scale micrometeorological methods (74). The paddock-scale techniques analyze the patterns of transportation and dispersion of CH4 emitted from animals by the wind (74). Consequently, the CH4 emission rates are computed from measurements of wind speed, wind direction and characteristics of turbulent airflow, and CH4 concentrations in the direction and against the direction of wind (74). The paddock-scale methods estimate CH4 emissions using flux-gradient method, mass-budget approach, and gas dispersion models (9, 74). A comparison between RC and this method show similar CH4 yield, i.e., 30.1 versus 29.7 g/kg DM intake (75). Accuracy in measurement is dependent upon certain meteorological and landscape conditions, such as wind velocity and direction, topography of the land, and location of animals in the paddock (74). The micrometeorological methods are expensive and require sensitive instruments to analyze CH4 concentration (9, 11). Because several meteorological factors influence the accuracy of CH4 outputs, further developments and documentations for obtaining consistent results are needed, but the methods are valuable in evaluating CH4 emissions and carbon footprint in whole farm systems and interactions between animals and landscape.
Indirect Measurements
In vitro Measurements
The in vitro rumen fermentation techniques have been extensively used for assessment of nutritive value of feeds for many years (76) and the techniques have been improved to simulate the rumen conditions. In this technique, feeds are fermented for long term [rumen simulation technique (77)] and short term [gas production methods (78)] under controlled laboratory conditions by rumen microbial activities. The volume of total gas production during incubation is determined and CH4 concentration in the gas is analyzed to obtain volume of in vitro CH4 production. With this system, the maximum level of total gas production and CH4 production can be determined, as well as the kinetics of gas production. Gas volumes are measured in different techniques (8) either directly by determining its volume at atmospheric pressure, e.g., Hohenheim gas production method or Menke’s method (78) and liquid displacement system (79) or by determining pressure changes due to accumulation of gas in a fixed volume container using a manometric device (80), a pressure transducer device with computerized (81) and manual (82) recordings, and a combination of pressure transducer and gas release device (83). Factors affecting the gas production in in vitro rumen fermentation system have been described in details by Rymer et al. (84). Recently, it has been shown that several other factors, such as bicarbonate concentrations in media and headspace gas composition (85), closed versus vented rumen batch culture system (86), and substrate dispersed in the medium versus kept in filter bags (87), influence the CH4 production in this technique. For diets containing different fiber concentrations and digestibility, CH4 production was close to that measured in RC method (88). Although several factors affect gas and CH4 production in the in vitro techniques, a fast screening of feedstuffs and additives for CH4 production is possible using these cost-effective simple techniques.
Modeling Enteric CH4 Production
Measurement of CH4 emissions in animals is difficult and labor intensive, and requires sophisticated and expensive equipments. Mathematical models predict CH4 emissions from ruminants without undertaking extensive and costly experiments. Therefore, prediction models are widely used for estimating national or global emissions from animals. The models used can be categorized as statistical models, which estimate CH4 production from nutrient intake directly [e.g., Ref. (2, 89)], or dynamic mechanistic models, which predict CH4 emissions using mathematical descriptions of rumen fermentation biology [e.g., COWPOLL model (90); MOLLY model (91)].
Mechanistic models (e.g., MOLLY and COWPOL) have advantages over the empirical statistical models in that CH4 mitigation technologies adopted at a farm or national level can be evaluated for their efficacy. Empirical models can evaluate the changes in CH4 emissions only in relation to changes in numbers of animals and feed intake. Diet-specific mechanistic models can more accurately predict CH4 emissions in ruminants (92). However, due to complexities of the mechanistic models, preparation of national inventory of CH4 estimates may not be straightforward. The Intergovernmental Panel on Climate Change (93) and Food and Agricultural Organization (1) publishes guidelines that are usually employed for official estimates of CH4 emissions in different countries. However, accuracy of these models to predict CH4 emissions has been challenged in different studies with cattle, buffaloes, sheep, and goats (2, 89, 94–96). The IPCC (93) developed methodologies to estimate enteric CH4 emissions with the use of CH4 conversion factor (Ym). However, Ym does not directly represent variations in CH4 emissions resulted from the ruminal fermentation characteristics affected by different carbohydrates, dietary nutrient composition, and feeding levels. Thus, the utility of Ym-based models in predicting enteric CH4 emissions and assessing the dietary CH4 mitigation strategies has been criticized (94). The low predictive ability of the Ym approach may invite substantial inaccuracy in preparation of enteric CH4 emission inventory (89, 94). Moreover, the IPCC and FAO models are generally developed based on the inputs from cattle. There was no model for predicting CH4 emission from buffaloes, goats, tropical cattle, and sheep. Recently, several statistical models have been developed for buffaloes, goats, sheep, and tropical cattle (Table 1). These newly fitted models performed better than the IPCC (93) and FAO (1) models as the recently developed equations had lower RMSPE values compared with these extant models (95). These new models should be considered for accurate preparation of enteric CH4 emission inventories for buffaloes, goats, sheep, and tropical cattle. For example, Patra and Lalhriatpuii (95) showed that the estimates of CH4 emission by goats were 5.23 and 5.15 kg/goat annually (actual CH4 production was 5.22 kg/goat/year) using the equations based on gross energy intake and digestible energy intake for goats, respectively. The estimate of CH4 emission using FAO (1) was 6.78 kg/goat/year, which was substantially greater than actual CH4 production. IPCC (93) suggested a CH4 emission factor of 5 kg/goat/year, which underestimated emissions. Similarly, based on the IPCC (93) tier II model, total enteric CH4 emission from buffaloes in India was estimated to be 4584 Gg/year in 2007 (5, 97). However, the estimate of enteric CH4 production from buffaloes using the equation based on DM intake (2, 89) was 4203 Gg/year, which was 8.3% lower than IPCC (93) model-based estimate (2, 89).
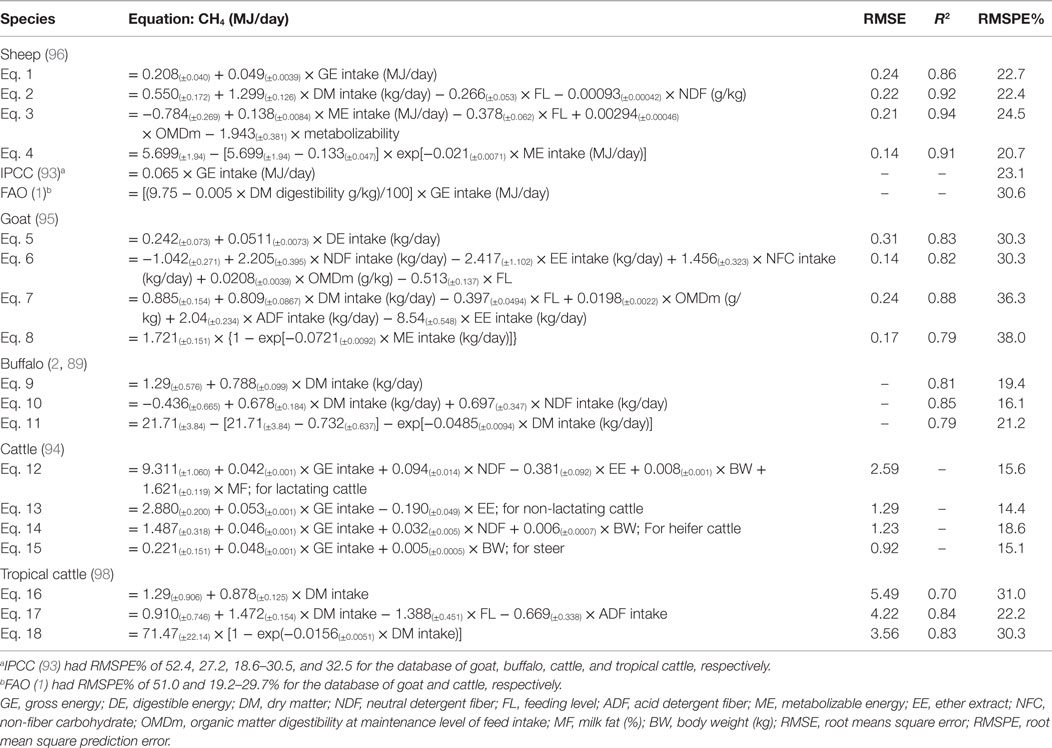
Table 1. List of developed linear and non-linear statistical models used to predict CH4 production (MJ/day) from buffaloes, sheep, goats, and tropical cattle.
Static empirical models have advantages in that they are usually based on a small number of variables (e.g., DM intake, feeding level, dietary lipid%, dietary fiber%, etc.), they can be performed in a simple spreadsheet, and they are transparent and can be easily tested on a variety of datasets (35). The disadvantage of static models is that they do not rely on an understanding of the biology and biochemistry of methanogenesis in the rumen, and they are, therefore, of limited use to study new CH4 mitigation strategies. Static empirical models are also restricted in predicting CH4 production beyond the data used for their development. The national GHG inventory in many countries uses a static empirical model to estimate emissions of CH4 from ruminant livestock. Nevertheless, prediction models are the strong base for estimating national or global emissions from animals.
Proxy Measures of CH4 Emissions
A considerable research effort has been directed toward development of proxy measures for predicting enteric CH4 production from composition of milk and feces. Several studies examined the concentrations of certain fatty acids in milk as predictors of CH4 production from dairy animals (99, 100). The assumption in this approach is that specific fatty acids in milk or feces are correlated with the composition of feeds or the amount of rumen methanogenic archaea, which would influence CH4 emissions in the rumen (100). Williams et al. (101), however, observed weak correlations between CH4 production and the concentrations of specific fatty acids in milk fat. Few studies (100, 102) indicated some correlations between milk fatty acid profiles and CH4 emissions. Milk mid-infrared spectra (influenced by milk fatty acid composition) measured using FTIR analysis apparatus could directly better predict CH4 emission (103) compared with fatty acid composition.
The use of archeol (2,3-diphytanyl-O-sn-glycerol), which is a membrane lipid ubiquitous in methanogens has been explored as a potential molecular proxy for methanogenesis in cattle (104). A significant correlation between fecal archeol concentration and CH4 production measured using the SF6 and RC technique in cattle fed either a grass- or concentrate-based diet was observed, but relationships between individual measurements within dietary treatments were weak to moderate, possibly due to selective retention of archaea in the rumen and degradation of the archeol during gut transit, differences in the CH4 producing capability per cell and post excretion (104, 105). These methods could be useful to predict the individual CH4 production to identify low-CH4-emitting animals, but further research is needed to improve the predictability of CH4 emissions using these proxy methods.
Other Potential Technology
Intra-Ruminal Gas Sensor
An intra-ruminal device, which measures the concentrations of CH4 and CO2 dissolved in rumen fluid, but does not measure flux (emission), has recently been fabricated (106). The rumen environmental conditions may be specifically unfavorable for an electronic device, which may cause corrosion of electrical circuits. In addition, the dissolved gases in rumen fluid must permeate quickly through the membrane of the intra-ruminal device in order to dynamically analyze the concentrations of gases (35). Information on internal rumen pressure, rumen size, and eructation pattern can be integrated to estimate the gas production rates (11). Thus, further research would be required to develop an approach to measure CH4 production from individual animals from the in situ measurements of gas concentrations in the rumen. The measurement of CO2 and CH4 concentrations in rumen and breath (respiratory and eructated) at the same time would be advantageous to assess the feasibility of using CO2 as a tracer gas and this could guide to the use of low-cost handheld systems to estimate CH4 production (11).
Dietary Strategies to Mitigate CH4 Emissions
Several mitigation options and strategies have been explored, which involve intervention at the animal level, dietary composition of animals, modulation of rumen fermentation, and inhibition of methanogenic archaea (Figure 2). Methanogen-specific inhibitors could be potentially effective mitigation agents if they utilize the evolutionary distinctiveness of methanogenic archaea (35). Archaea are evolutionarily distinct from other rumen microorganisms (bacteria, protozoa, fungi, and viruses), and all methanogenic archaea share a similar biochemical pathway of methanogenesis (107). Therefore, the inhibitors of this methanogenesis pathway may distinctively inhibit only methanogens without directly influencing other beneficial microorganisms in the rumen (35, 108). Several reviews on CH4 mitigation strategies and options have been published recently (3, 6, 97). In this section, further recent advances in dietary CH4 mitigation technologies are described here.
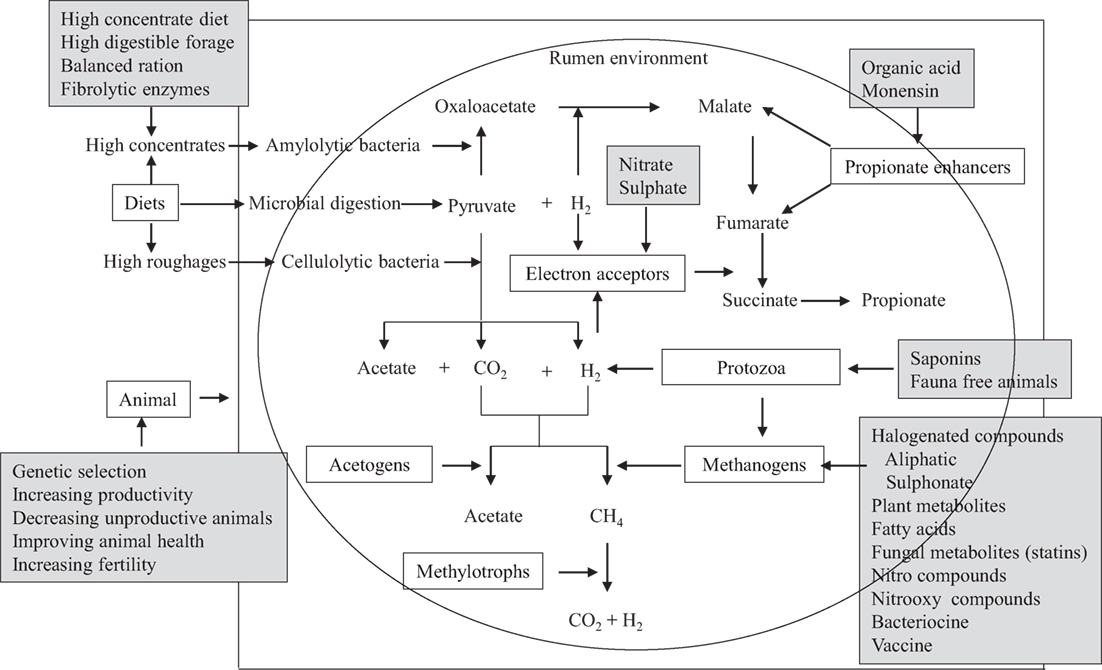
Figure 2. A schematic presentation of the potential targets of decreasing CH4 emissions from ruminants. Boxes without dark could be the targets for suppressing CH4 emissions and boxes with dark shade are the options that have been studied in vitro or in vivo to decrease CH4 production [adapted with modification from Patra (5, 97)].
Lipid Supplementation
Several studies have confirmed that addition of fats or fatty acid to the diets of ruminants can decrease enteric CH4 emissions [e.g., Ref. (2, 89, 101, 109)]. Each percentage increase in supplemental dietary fat decreases CH4 emission by 4.30% (109). Fat concentrations of up to 6% of diet DM may also increase milk production and lower enteric CH4 emissions appreciably (15%) in cattle (109), which is a win–win situation. Fat concentration beyond this concentration may decrease production efficiency due to adverse effect on rumen fermentation. Among fatty acids, C12:0, C18:3, and poly unsaturated fatty acids have more marked CH4 suppressing effects, whereas saturated long-chain fatty acids are less effective for decreasing CH4 production in cattle (109, 110). The by-products containing high concentration of lipids (e.g., brewers grains, grape marc, hominy meal, etc.) appear to be promising to mitigate CH4 emissions cost-effectively (101). Grainger et al. (24) showed that supplementary feeding of whole cottonseed to dairy cows could cause a substantial decrease in CH4 emissions without adversely affecting milk production. Moate et al. (111) compared brewers grains, cold-pressed canola, and hominy meal for their CH4 mitigation potential and found that all three by-products could substantially reduce enteric CH4 emissions from dairy cows. The feeding of red grape marc to dairy cows decreased CH4 emissions and CH4 yield by 20% (32). In addition to the presence of fat, grape marc also contains many secondary compounds such as tannins, p-coumaric acid, and resveratrol, which may also inhibit enteric methanogenesis (32).
Plant Secondary Compounds
Several plant secondary metabolites present in forages and plant extracts have been identified to be potential for CH4 inhibition in the rumen (108). Many forage plants rich in tannins and saponins have shown to be promising to reduce CH4 production in ruminants (112, 113). Tannins may decrease CH4 directly by inhibiting methanogenic bacteria, and indirectly by decreasing hydrogen production as a result of decreased fiber digestion and protozoal population in the rumen (108).
A recent investigation with different forage brassicas have shown that most of the brassicas species fed to sheep resulted in similar CH4 yields, However, swedes and forage rape significantly decreased CH4 yield (~20%) compared with ryegrass and other brassicas, such as turnips and kale (114). Although the mechanism of CH4 inhibition is not clearly known, these forages may contain S-containing plant metabolites, which may be responsible for inhibition of CH4 production (108). Additional research is required to examine the CH4 mitigation effects of these forage brassicas fed to ruminants for long time.
The studies on flavonoid compounds on rumen methanogenesis are limited although other metabolites, such as tannins, saponins, and essential oils, have extensively been studied (108). A recent study reported that inclusion of flavone, myricetin, naringin, rutin, quercetin, and kaempferol significantly decreased in vitro CH4 production from 8.6 to 5.7, 4.9, 6.3, 7.2, 6.2, and 5.3 mL/g DM, respectively. The inhibitory activities of flavonoids used in this experiment toward methanogenesis were in the following descending order as follows: myricetin ≥ kaempferol ≥ flavone > quercetin ≥ naringin > rutin ≥ catechin (115). Catechin decreased CH4 production both in vitro (116) and in vivo (117). Catechin causes direct inhibition of methanogens (115) as well as may act as hydrogen sinks during degradation by rumen microbes via cleavage of ring structures and reductive dehydroxylation reactions (116).
Nitrate Supplementation
Nitrate decreases methanogenesis acting as electron sinks and directly inhibiting the methanogens (85, 118). Use of nitrate has two advantages – (1) it decreases CH4 production and (2) it provides ammonia to the rumen microbial growth resulting in decreased dietary protein requirement. Nitrates as a dietary supplement fed to dairy cows in low nitrogen containing diet have been shown to reduce CH4 emissions (119). However, dietary nitrate supplementation may increase the risk of nitrite toxicity, particularly for the forages containing high concentrations of nitrate and crude protein.
Halogenated Compounds
Halogenated compounds had been investigated as inhibitors of CH4 production in the rumen over 40 years ago (5, 97). Some marine plants, such as the red seaweed, algae, and fungi, contain bromoform and other halogenated compounds at high concentrations (120), which have been exploited recently to inhibit CH4 production. In a recent in vitro experiment, red seaweed, Asparagopsis taxiformis, was shown to reduce CH4 production by 99% when it was used at 2% of organic matter substrate (121). Thus, the supplementation of ruminant diets with red sea weed may offer a natural means of CH4 mitigation. In vivo experiments are required to decide optimum dose levels and to study the toxic effect of the weed, if any.
Nitrooxy Compounds
Novel inhibitors 3-nitrooxypropanol (NOP) and ethyl-3NOP have been shown to have specific anti-methanogenic properties. NOP interferes with the methyl-coenzyme M reductase of the methanogens, which is the last step in the formation of CH4, thus lowering CH4 production and inhibition of the growth of methanogens (122, 123). Reynolds et al. (124) noted a 7–10% lower in CH4 production when NOP was administered directly into the rumen of cattle through a rumen cannula at a daily dose of 0.50 or 2.5 g per cow (i.e., 25 or 125 mg/kg DM). Digestibility decreased at high dose in this study. However, in subsequent studies, Haisan et al. (125) noted 60% decrease in CH4 emission in cattle fed NOP at a dose of 2.5 g/day mixing with the feed to ensure continuous intake of this compound throughout the day. Feeding of NOP at 40–80 mg/kg diet in dairy cattle was also associated with decreased CH4 production by 30% (126). In beef cattle diet also, 3NOP added to the diets at 2.0 g/day decreased CH4 yield by 59% up to 112 days of experiment without much affecting feed intake, nutrient digestibility, and total volatile fatty acid (VFA) concentrations (127). In this study, the numbers of methanogens were reduced, but protozoal numbers were increased by 3NOP. These studies suggested that 3NOP needs to be continuously supplied to the diet to get optimal inhibitor effect of CH4 production. The results have been confirmed in other study (128) where CH4 yield was lowered by 37% due to feeding of 3NOP at 2.5 g/day in dairy cow. In sheep also, 3NOP at 0.5 g/animal per day decreased CH4 production by 29% without adversely affecting digestion and rumen fermentation (123). It appears that 3NOP is a CH4 inhibitor, which has potential for reducing carbon footprint of livestock products without affecting nutrient utilization and performance in ruminants.
Fungal Metabolites
Lovastatin is a secondary metabolite of idiophase of the fungi, which inhibits the key enzyme of cholesterol biosynthesis, such as enzyme 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase (129). Aspergillus terreus fungi fermented rice straw extract containing lovastatin significantly reduced CH4 production and number of methanogens, but increased few fiber degrading bacteria (129). Ether-linked long-chain isoprenoid alcohol is a central component in archeal cell membrane lipid, which is produced from a key precursor, mevalonate. Mevalonate is synthesized by reduction of HMG-CoA catalyzed by HMG-CoA reductase and it is an intermediate rate limiting reaction in synthesis of cholesterol in human (108). Lovastatin is an inhibitor of HMG-CoA reductase, which inhibits isoprenoid alcohol synthesis, and consequently archeal cell membrane formation and growth of methanogens could be retarded (129). Strains of saprophytic fungi Mortierella wolfii were also promising as an inhibitor of methanogenesis without also reducing overall fermentation (130). In another study in sheep fed Monascus-fermented rice, CH4 emission was decreased by 30% by fungal metabolites (possibly pravastatin and mevastatin) produced by Monascus spp., which was associated with lower ruminal acetate to propionate ratio and decreased numbers of methanogens in the rumen (131).
Microalgae
An effort has been started to screen microalgae to inhibit rumen methanogenesis. Machado et al. (121) screened several marine microalgae in vitro and reported that Asparagopsis not only strongly decreased CH4 production by 99% at a dose of as low as 2% of total substrate organic matter, but also decreased the production of VFA. Oedogonium was less effective with doses ≥50% OM significantly decreasing the production of CH4. A combination of Asparagopsis (2% OM) and Oedogonium (25 and 50% OM) continued to suppress the production of CH4, independent of the inclusion rate of Oedogonium. The brown algae (Cystoseira trinodis and Dictyota bartayresii) were also identified as a potent CH4-inhibiting agent in vitro (132). One algal meal containing 20% docosahexaenoic acid fed to cows up to 375 g/cow per day of algal meal corresponding up to 75 g of docosahexaenoic acid/cow per day did not affect CH4 production in vivo, which was probably due to the low concentration of this fatty acid to inhibit CH4 production (133).
Use of Combination of CH4 Inhibitors
A number of CH4 inhibitors have been frequently evaluated, primarily individually, to lower CH4 production in ruminants. However, they generally exert detrimental effects on digestion of feeds and rumen fermentation when they are added at high concentration in order to obtain substantial effect on CH4 inhibition (5, 97, 134, 135). Some of these inhibitors also cause toxicity to animals when used at large doses (97). These adverse effects on rumen fermentation and toxicity problems to animals can be overcome at low doses, but substantial effect on inhibition to methanogenesis is not noted at low doses. Combinations of inhibitors with complementary modes of actions may synergistically or additively lower CH4 emission without exerting any detrimental effects on digestion of feeds or rumen fermentation at low doses (134). Indeed, this hypothesis has been confirmed in some studies (134–138). In a study, a binary combination of nitrate and quillaja saponin inhibited methanogenesis additively in an in vitro rumen culture [by 32% at 5 mM nitrate and 0.6 g/L saponins, and by 58% at 10 mM nitrate and 1.2 g/L saponins (134)]. Binary combination of nitrate and saponins might act additively to decrease methanogenesis in a multipronged manner: (1) saponins inhibit rumen protozoa, lowering hydrogen production by protozoa and reducing the abundance of protozoa-associated methanogens (139), (2) nitrate functions as a strong electron sink that outcompetes CO2 for electrons, and (3) nitrite, the first intermediate of nitrate reduction, exerts direct toxicity to methanogens. However, binary combination of high doses of nitrate and saponins decreased fiber degradability (85). Garlic oil is directly inhibitory to rumen methanogens acting through impairment of lipid synthesis (140). Combination of saponin + nitrate + sulfate, garlic oil + nitrate, and garlic oil + nitrate + saponin resulted in additive effects on methanogenesis (118, 136, 140). Hops extract (Humulus lupulus; containing β- and α-acids) and yucca saponin decreased CH4 in an additive manner when applied in combination (138). Additive effects of combinations of CH4 inhibitor have been tested a little in vivo. A recent in vivo study demonstrated that nitrate (3% calcium nitrate; 22% reduction) and linseed oil (4% of the diet; 17% reduction) in combination (32% reduction) for decreased methanogenesis additively in cows without altering diet digestibility (141). It appear that combinations of CH4 inhibitors with complementary mechanism of actions could be useful technology to substantially decrease CH4 production without adverse effect on nutrient utilization, but more in vivo research is required to identify the suitable dose combinations for practical on farm application.
Conclusion and Future Research Challenges
Many existing methods have been employed to measure CH4 production with different purposes, such as nutritional evaluation of feeds and feed additives and screening of animals for genetic selection, and have been investigated to improve accuracy of measurements. Many new techniques are being developed to overcome the constraints of the existing methods. However, no method is suitable in all conditions for reliable measurement of CH4 emissions. Every method has its advantages and limitations, and a method is useful in particular conditions of a study for CH4 measurement and mitigation. Most consistent RC method is only a “gold standard” when this is used with adequate rigor and technical expertise. The SF6 method can be employed with lesser effect on animal behavior and has a higher throughput relative to time and cost. With recent recommendations for the technique, the SF6 method can be used with a high level of precision in grazing animal studies for long time.
The short-term measurement techniques have advantages as it is relatively cheaper, simpler, and mobile compared to other techniques, such as SF6 or RC. All short-term measurements invite variations at several points, such as measurement time relative to feed intake and level of activity before measurement (45). The higher within and between animal-to-animal and day-to-day variations in CH4 emissions would require more number of animals and measurement days to obtain significant differences of CH4 emissions between treatments using short-term measurement techniques. Daily CH4 emission patterns of ruminants generally show a diurnal pattern in relation to the feeding time and level of feed intake (58, 142). The diurnal profiles associated with feed intake exhibit a continuous rise to a peak followed by a period of a linear decline. Therefore, for short-term measurements of CH4 emissions, such as sniffer method (59) taken twice during the daily feeding and milking time, estimation of daily CH4 production based on measurement of short-term emission rates will be diet dependent (45). For systems such as GF and methane hood where short-term emission rates are measured throughout 24 h, daily CH4 emission may be estimated without scaling up (45). For systems such as for PACs and LMD where small numbers of measurements for emission rates are taken and feed intake information of an animal is not known, scaling up to daily CH4 emission is not currently possible (45). Short-term measures of CH4 emission may strongly correlate with daily CH4 emission depending on the time after feeding at which the short-term measurements are made. Thus, short-term measuring techniques can be applied to very large numbers of animals under normal management conditions to collect information on low CH4-producing animals required for genetic selection purpose. Further research efforts are needed to improve the accuracy of all the new methods.
Several new CH4 mitigation technologies have been explored, but only a few of them are practical and cost-effective, which can be adopted on farms to achieve substantial mitigation of total CH4 emissions. A combination of different CH4 mitigation strategies should be adopted in farm levels to substantially decrease CH4 emission from ruminants. The CH4 mitigation options that show both nutritional and environmental advantages would likely to be better adopted by the farmers. For example, fat supplementation could decrease CH4 production as well as improve productivity of animals. Similarly, nitrate supplementation could reduce the expensive protein meals in diets. If some mitigation technologies could be employed to improving the nutritional values of forages, they have immense practical importance in tropical feeding situations. For example, if saprophyte fungi, which produce metabolites against methanogens, could be grown on low-quality forages, such as straws, this technology would be feasible for decreasing CH4 production in ruminants. Toxicities and residues in milk and meat associated with CH4 inhibitors must be assessed with long-term trials in animals. Many fungi, for example, may produce toxic metabolites in some growth conditions, which must be avoided for practical feeding of animals (143). Nitrate supplementation may cause toxicities in animals if it is not used in proper doses with relation to the nitrate content in forages. Future mitigation research should focus for the developments of both the short-term strategies, such as dietary strategies and animal management as well as long-term strategies focusing on plant and animal breeding, and rumen microbial modulation in order to profitably decrease carbon footprint and strengthen the future sustainable and environment friendly livestock production systems.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. FAO. Greenhouse Gas Emissions from the Dairy Sector. Rome: Food and Agriculture Organization of the United Nations (2010).
2. Patra AK. Trends and projected estimates of GHG emissions from Indian livestock in comparisons with GHG emissions from world and developing countries. Asian-Australas J Anim Sci (2014) 27:592–9. doi:10.5713/ajas.2013.13342
3. Knapp JR, Laur GL, Vadas PA, Weiss WP, Tricarico JM. Invited review: enteric methane in dairy cattle production: quantifying the opportunities and impact of reducing emissions. J Dairy Sci (2014) 97:3231–61. doi:10.3168/jds.2013-7234
4. Gerber PJ, Steinfeld H, Henderson B, Mottet A, Opio C, Dijkman J, et al. Tackling Climate Change Through Livestock – A Global Assessment of Emissions and Mitigation Opportunities. Rome: Food and Agriculture Organization of the United Nations (FAO) (2013).
5. Patra AK. Enteric methane mitigation technologies for ruminant livestock: a synthesis of current research and future directions. Environ Monitor Assess (2012) 184:1929–52. doi:10.1007/s10661-011-2090-y
6. Hristov AN, Oh J, Firkins JL, Dijkstra J, Kebreab E, Waghorn G, et al. Mitigation of methane and nitrous oxide emissions from animal operations: I. A review of enteric methane mitigation options. J Anim Sci (2013) 91:5045–69. doi:10.2527/jas.2013-6583
7. Pacheco D, Waghorn G, Janssen PH. Decreasing methane emissions from ruminants grazing forages: a fit with productive and financial realities? Anim Prod Sci (2014) 54:1141–54. doi:10.1071/AN14437
8. Bhatta R, Enishi O, Kurihara M. Measurement of methane production from ruminants. Asian-Australas J Anim Sci (2007) 20:1305–18. doi:10.5713/ajas.2007.1049
9. Storm IMLD, Hellwing ALF, Nielsen NI, Madsen J. Methods for measuring and estimating methane emission from ruminants. Animal (2012) 2:160–83. doi:10.3390/ani2020160
11. Hill J, McSweeney C, Wright ADG, Bishop-Hurley G, Kalantar-zadeh K. Measuring methane production from ruminants. Trends Biotechnol (2016) 34:26–35. doi:10.1016/j.tibtech.2015.10.004
14. Grainger C, Clarke T, McGinn SM, Auldist MJ, Beauchemin KA, Hannah MC, et al. Methane emissions from dairy cows measured using the sulphur hexafluoride (SF6) tracer and chamber techniques. J Dairy Sci (2007) 90:2755–66. doi:10.3168/jds.2006-697
15. Williams SRO, Clarke T, Hannah MC, Marrett LC, Moate PJ, Auldist MJ, et al. Energy partitioning in herbage-fed dairy cows offered supplementary grain during an extended lactation. J Dairy Sci (2013) 96:484–94. doi:10.3168/jds.2012-5787
16. Gardiner TD, Coleman MD, Innocenti F, Tompkins J, Connor A, Garnsworthy PC, et al. Determination of the absolute accuracy of UK chamber facilities used in measuring methane emissions from livestock. Measurement (2015) 66:272–9. doi:10.1016/j.measurement.2015.02.029
17. Zimmerman PR. System for Measuring Metabolic Gas Emissions from Animals. Patent no. 5 265 618. Alexandria, VA: United States Patent and Trademark Office (1993).
18. Johnson K, Huyler M, Westberg H, Lamb B, Zimmerman P. Measurement of methane emissions from ruminant livestock using a SF6 tracer technique. Environ Sci Technol (1994) 28:359–62. doi:10.1021/es00051a025
19. Lassey KR, Walker CF, McMillan AMS, Ulyatt MJ. On the performance of SF6 permeation tubes used in determining methane emission from grazing livestock. Chemosphere (2001) 3:367–76. doi:10.1016/S1465-9972(01)00017-4
20. Pinares-Patiño CS, Lassey KR, Martin RJ, Molano G, Fernandez M, MacLean S, et al. Assessment of the sulphur hexafluoride (SF6) tracer technique using respiration chambers for estimation of methane emissions from sheep. Anim Feed Sci Technol (2011) 16(6–167):201–9. doi:10.1016/j.anifeedsci.2011.04.067
21. Deighton MH, O’Loughlin BM, Williams SRO, Moate PJ, Kennedy E, Boland TM, et al. Declining sulphur hexafluoride permeability of fluoropolymer membranes causes overestimation of calculated ruminant methane emissions using the tracer technique. Anim Feed Sci Technol (2013) 183:86–95. doi:10.1016/j.anifeedsci.2013.04.021
22. Moate PJ, Deighton MH, Ribaux BE, Hannah MC, Wales WJ, Williams SRO. Michaelis-Menten kinetics predict the rate of SF6 release from permeation tubes used to estimate methane emissions from ruminants. Anim Feed Sci Technol (2015) 200:47–56. doi:10.1016/j.anifeedsci.2014.12.001
23. Williams SRO, Moate PJ, Hannah MC, Ribaux BE, Wales WJ, Eckard RJ. Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: a critical evaluation of the SF6 procedure. Anim Feed Sci Technol (2011) 170:265–76. doi:10.1016/j.anifeedsci.2011.08.013
24. Grainger C, Williams R, Clarke T, Wright ADG, Eckard RJ. Supplementation with whole cottonseed causes long term reduction of methane emissions from lactating dairy cows offered a forage and cereal grain diet. J Dairy Sci (2010) 93:2612–9. doi:10.3168/jds.2009-2888
25. Muñoz C, Yan T, Will DA, Murray S, Gordon AW. Comparison of the sulphur hexafluoride tracer and respiration chamber techniques for estimating methane emissions and correction for rectum methane output from dairy cows. J Dairy Sci (2012) 95:3139–48. doi:10.3168/jds.2011-4298
26. Boadi DA, Wittenberg KM, Kennedy AD. Validation of the sulphur hexafluoride (SF6) tracer gas technique for measurement of methane and carbon dioxide production by cattle. Can J Anim Sci (2002) 82:125–31. doi:10.4141/A01-017
27. Ulyatt MJ, Baker SK, McCrabb GJ, Lassey KR. Accuracy of SF6 tracer technology and alternatives for field measurements. Aust J Agric Res (1999) 50:1329–34. doi:10.1071/AR99003
28. McGinn SM, Beauchemin KA, Iwaasa AD, McAllister TA. Assessment of the sulfur hexafluoride (SF6) tracer technique for measuring enteric methane emissions from cattle. J Environ Qual (2006) 35:1686–91. doi:10.2134/jeq2006.0054
29. Pinares-Patiño CS, D’Hour P, Jouany JP, Martin C. Effects of stocking rate on methane and carbon dioxide emissions from grazing cattle. Agric Ecosyst Environ (2007) 121:30–46. doi:10.1016/j.agee.2006.03.024
30. Pinares-Patiño CS, Holmes CW, Lassey KR, Ulyatt MJ. Measurement of methane emission from sheep by the sulphur hexafluoride tracer technique and by the calorimetric chamber: failure and success. Animal (2008) 2:141–8. doi:10.1017/S1751731107000857
31. Vlaming JB, Lopez-Villalobos N, Brookes IM, Hoskin SO, Clark H. Within- and between-animal variance in methane emissions in non-lactating dairy cows. Aust J Exp Agric (2008) 48:124–7. doi:10.1071/EA07278
32. Moate PJ, Williams SRO, Torok VA, Hannah MC, Ribaux BE, Tavendale MH, et al. Grape marc reduces methane emissions when fed to dairy cows. J Dairy Sci (2014) 97:5073–87. doi:10.3168/jds.2013-7588
33. Deighton MH, Williams SRO, Hannah MC, Eckard RJ, Boland TM, Wales WJ, et al. A modified sulphur hexafluoride tracer technique enables accurate determination of enteric methane emissions from ruminants. Anim Feed Sci Technol (2014) 197:47–63. doi:10.1016/j.anifeedsci.2014.08.003
34. Pinares-Patiño CS, Machmuller A, Molano G, Smith A, Vlaming JB, Clark H. The SF6 tracer technique for measurements of methane emission from cattle – effect of tracer permeation rate. Can J Anim Sci (2008) 88:309–20. doi:10.4141/CJAS07117
35. Moate PJ, Deighton MH, Williams SRO, Pryce JE, Hayes BJ, Jacobs JL, et al. Reducing the carbon footprint of Australian milk production by mitigation of enteric methane emissions. Anim Prod Sci (2016). doi:10.1071/AN15222
36. Lassey KR. On the importance of background sampling in applications of the SF6 tracer technique to determine ruminant methane emissions. Anim Feed Sci Technol (2013) 180:115–20. doi:10.1016/j.anifeedsci.2012.11.012
37. Takahashi J, Chaudhry AS, Beneke RG, Young BA. An open-circuit hood system for gaseous exchange measurements in small ruminants. Small Rumin Res (1999) 32:31–6. doi:10.1016/S0921-4488(98)00163-1
38. Place SE, Pan Y, Zhao Y, Mitloehner FM. Construction and operation of a ventilated hood system for measuring greenhouse gas and volatile organic compound emissions from cattle. Animal (2011) 1:433–46. doi:10.3390/ani1040433
39. Suzuki T, Phaowphaisal I, Pholson P, Narmsilee R, Indramanee S, Nitipot T, et al. In vivo nutritive value of pangola grass (Digitaria eriantha) hay by a novel indirect calorimeter with a ventilated hood in Thailand. JARQ (2008) 42:123–9. doi:10.6090/jarq.42.123
40. Patra AK, Puchala R, Animut G, Gipson TA, Sahlu T, Goetsch AL. Effects of acclimatization on energy expenditure by meat goats. Small Rumin Res (2009) 81:42–54. doi:10.1016/j.smallrumres.2008.11.002
41. Troy S, Rooke JA, Duthie CA, Ross D, Hyslop JJ, Roehe R, et al. Measurement of methane from finishing cattle fed either a forage-based or high concentrate diet from both feeder-mounted samplers and respiration chambers. Adv Anim Biosci (2013) 4:551.
42. Washburn LE, Brody S. “Growth and development with special reference to domestic animals. 42. Methane, hydrogen, and carbon dioxide production in the digestive tract of ruminants in relation to the respiratory exchange,” In Univ Missouri Coll Agric Agric Exp Stat Res Bull, Vol. 263 (1937). p. 614.
43. Lockyer DR, Jarvis SC. The measurement of methane losses from grazing animals. Environ Pollut (1995) 90:383–90. doi:10.1016/0269-7491(95)00009-G
44. Goopy JP, Woodgate R, Donaldson A, Robinson DL, Hegarty RS. Validation of a short-term methane measurement using portable static chambers to estimate daily methane production in sheep. Anim Feed Sci Technol (2011) 16(6–167):219–26. doi:10.1016/j.anifeedsci.2011.04.012
45. Hegarty RS. Applicability of short-term emission measurements for on-farm quantification of enteric methane. Animal (2013) 7:401–8. doi:10.1017/S1751731113000839
46. Goopy J, Robinson D, Woodgate R, Donaldson A, Oddy H, Vercoe P, et al. Estimates of repeatability and heritability of methane production in sheep using portable accumulation chambers. Anim Prod Sci (2016) 56:116–22. doi:10.1071/AN13370
47. Madsen J, Bjerg BS, Hvelplund T, Weisbjerg MR, Lund P. Methane and carbon dioxide ratio in excreted air for quantification of the methane production from ruminants. Livest Sci (2010) 129:223–7. doi:10.1016/j.livsci.2010.01.001
48. Lassen J, Løvendahl P, Madsen J. Accuracy of non invasive breath methane measurements using Fourier transform infrared methods on individual cows. J Dairy Sci (2012) 95:890–8. doi:10.3168/jds.2011-4544
49. Hellwing ALF, Madsen J, Weisbjerg MR, Lund P. What affects CH4/CO2 ratio in cow’s breath. Adv Anim Biosci (2013) 4:557.
50. Huhtanen P, Cabezas-Garcia EH, Utsumi S, Zimmerman S. Comparison of methods to determine methane emissions from dairy cows in farm conditions. J Dairy Sci (2015) 98:3394–409. doi:10.3168/jds.2014-9118
51. Zimmerman PR. Method and System for Monitoring and Reducing Ruminant Methane Production. Patent no. 7966971B2. Alexandria, VA: United States Patent and Trademark Office (2011).
52. Hristov AN, Oh J, Giallongo F, Frederick T, Weeks H, Zimmerman PR, et al. The use of an automated system (GreenFeed) to monitor enteric methane and carbon dioxide emissions from ruminant animals. J Vis Exp (2015) 103:e52904. doi:10.3791/52904
53. Hammond KJ, Humphries DJ, Crompton LA, Green C, Reynolds CK. Methane emissions from cattle: estimates from short-term measurements using a GreenFeed system compared with measurements obtained using respiration chambers or sulphur hexafluoride tracer. Anim Feed Sci Technol (2015) 203:41–52. doi:10.1016/j.anifeedsci.2015.02.008
54. Dorich CD, Varner RK, Pereira ABD, Martineau R, Soder KJ, Brito AF. Short communication: use of a portable automated open-circuit gas quantification system and the sulfur hexafluoride tracer technique for measuring enteric methane emissions in Holstein cows fed ad libitum or restricted. J Dairy Sci (2015) 98:1–6. doi:10.3168/jds.2014-8348
55. Arbre M, Rochette Y, Guyader J, Lascoux C, Gómez LM, Eugène M, et al. Repeatability of enteric methane determinations from cattle using either the SF6 tracer technique or the GreenFeed system. Anim Prod Sci (2016) 56:238–43. doi:10.1071/AN15512
56. Velazco JI, Mayer DG, Zimmerman S, Hegarty RS. Use of short-term breath measures to estimate daily methane production by cattle. Animal (2016) 10:25–33. doi:10.1017/S1751731115001603
57. Waghorn GC, Jonker A, Macdonald KA. Measuring methane from grazing dairy cows using GreenFeed. Anim Prod Sci (2016) 56:252–7. doi:10.1071/AN15491
58. Hammond KJ, Waghorn GC, Hegarty RS. The GreenFeed system for measurement of enteric methane emission from cattle. Anim Prod Sci (2016) 56:181–9. doi:10.1071/AN15631
59. Garnsworthy PC, Craigon J, Hernandez-Medrano JH, Saunders N. On-farm methane measurements during milking correlate with total methane production by individual dairy cows. J Dairy Sci (2012) 95:3166–80. doi:10.3168/jds.2011-4605
60. Chagunda MGG. Opportunities and challenges in the use of the laser methane detector to monitor enteric methane emissions from ruminants. Animal (2013) 7:394–400. doi:10.1017/S1751731113000724
61. Ricci P, Chagunda MGG, Rooke J, Houdijk JG, Duthie CA, Hyslop J, et al. Evaluation of the laser methane detector to estimate methane emissions from ewes and steers. J Anim Sci (2014) 92:5239–50. doi:10.2527/jas.2014-7676
62. Chagunda MGG, Yan T. Do methane measurements from a laser detector and an indirect open-circuit respiration calorimetric chamber agree sufficiently closely? Anim Feed Sci Technol (2011) 165:8–14. doi:10.1016/j.anifeedsci.2011.02.005
63. Chagunda MGG, Ross D, Rooke J, Yan T, Douglas JL, Poret L, et al. Measurement of enteric methane from ruminants using a hand-held laser methane detector. Acta Agric Scand A Anim Sci (2013) 63:68–75. doi:10.1080/09064702.2013.797487
64. Troy SM, Duthie CA, Ross DW, Hyslop JJ, Roehe R, Waterhouse A, et al. A comparison of methane emissions from beef cattle measured using methane hoods with those measured using respiration chambers. Anim Feed Sci Technol (2016) 211:227–40. doi:10.1016/j.anifeedsci.2015.12.005
65. Lockyer DR. Methane emissions from grazing sheep and calves. Agric Ecosyst Environ (1997) 66:11–8. doi:10.1016/S0167-8809(97)00080-7
66. Murray PJ, Moss A, Lockyer DR, Jarvis SC. A comparison of systems for measuring methane emissions from sheep. J Agric Sci (1999) 133:439–44. doi:10.1017/S0021859699007182
67. Lassey KR. Livestock methane emission: from the individual grazing animal through national inventories to the global methane cycle. Agric Forest Meteorol (2007) 142:120–32. doi:10.1016/j.agrformet.2006.03.028
68. Harper LA, Denmead OT, Flesch TK. Micrometeorological techniques for measurement of enteric greenhouse gas emissions. Anim Feed Sci Technol (2011) 16(6–167):227–39. doi:10.1016/j.anifeedsci.2011.04.013
69. McGinn SM, Turner D, Tomkins N, Charmley E, Bishop-Hurley G, Chen D. Methane emissions from grazing cattle using point-source dispersion. J Environ Qual (2011) 40:22–7. doi:10.2134/jeq2010.0239
70. Derno M, Elsner HG, Paetow EA, Scholze H, Schweigel M. Technical note: a new facility for continuous respiration measurements in lactating cows. J Dairy Sci (2009) 92:2804–8. doi:10.3168/jds.2008-1839
71. Flesch TK, Wilson JD, Harper LA, Todd RW, Cole NA. Determining ammonia emissions from a cattle feedlot with an inverse dispersion technique. Agric Forest Meteorol (2007) 144:139–55. doi:10.1016/j.agrformet.2007.02.006
72. Loh Z, Chen D, Bai M, Naylor T, Griffith D, Hill J, et al. Measurement of greenhouse gas emissions from Australian feedlot beef production using open-path spectroscopy and atmospheric dispersion modelling. Aust J Exp Agric (2008) 48:244–7. doi:10.1071/EA07244
73. van Haarlem RP, Desjardins RL, Gao Z, Flesch TK, Li X. Methane and ammonia emissions from a beef feedlot in western Canada for a twelve-day period in the fall. Can J Anim Sci (2008) 88:641–9. doi:10.4141/CJAS08034
74. Laubach J, Kelliher FM, Knight TW, Clark H, Molano G, Cavanagh A. Methane emissions from beef cattle – a comparison of paddock and animal-scale measurements. Aust J Exp Agric (2008) 48:132–7. doi:10.1071/EA07256
75. Tomkins NW, McGinn SM, Turner DA, Charmley E. Comparison of open-circuit respiration chambers with a micrometeorological method for determining methane emissions from beef cattle grazing a tropical pasture. Anim Feed Sci Technol (2011) 166:240–7. doi:10.1016/j.anifeedsci.2011.04.014
76. McBee RH. Manometric method for the evaluation of microbial activity of rumen with application to utilization of cellulose and hemicellulose. Appl Microbiol (1953) 1:106–10.
77. Czerkawski JW, Breckenridge G. Design and development of a long-term rumen simulation technique (Rusitec). Br J Nutr (1977) 38:371–83. doi:10.1079/BJN19770102
78. Menke KH, Raab L, Salewski A, Steingass H, Fritz D, Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor. J Agric Sci (1979) 93:217–22. doi:10.1017/S0021859600086305
79. Beuvink JMW, Spoelstra SF, Hogendorp RJ. An automated method for measuring time-course of gas production of feedstuff incubated with buffered rumen fluid. Neth J Agric Sci (1992) 40:401–7.
80. Waghorn GC, Stafford KJ. Gas production and nitrogen digestion by rumen microbes from deer and sheep. N Z J Agric Res (1993) 36:493–7. doi:10.1080/00288233.1993.10417750
81. Pell AN, Schofield P. Computerised monitoring of gas production to measure forage digestion. J Dairy Sci (1993) 76:1063–73. doi:10.3168/jds.S0022-0302(93)77435-4
82. Theodorou MK, Williams BA, Dhanoa MS, McAllan AB, France J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim Feed Sci Technol (1994) 48:185–97. doi:10.1016/0377-8401(94)90171-6
83. Devies DR, Theodorou MK, Baughan J, Brooks AE, Newbold JR. An automated pressure evaluation system (APES) for determining the fermentation characteristics of ruminant feeds. Ann Zootech (1995) 44:36–46. doi:10.1051/animres:19950506
84. Rymer C, Huntington JA, Williams BA, Givens DI. In vitro cumulative gas production techniques: history, methodological considerations and challenges. Anim Feed Sci Technol (2005) 123:9–30. doi:10.1016/j.anifeedsci.2005.04.055
85. Patra AK, Yu Z. Effects of gas composition in headspace and bicarbonate concentrations in media on gas and methane production, degradability, and rumen fermentation using in vitro gas production techniques. J Dairy Sci (2013) 96:4592–600. doi:10.3168/jds.2013-6606
86. Cattani M, Tagliapietra F, Maccarana L, Hansen HH, Bailoni L, Schiavon S. Technical note: in vitro total gas and methane production measurements from closed or vented rumen batch culture systems. J Dairy Sci (2014) 97:1736–41. doi:10.3168/jds.2013-7462
87. Ramin M, Krizsan SJ, Jančík F, Huhtanen P. Short communication: measurements of methane emissions from feed samples in filter bags or dispersed in the medium in an in vitro gas production system. J Dairy Sci (2013) 96:4643–6. doi:10.3168/jds.2013-6556
88. Bhatta R, Enishi O, Takusari N, Higuchi K, Nonaka I, Kurihara M. Diet effects on methane production by goats and a comparison between measurement methodologies. J Agric Sci (2008) 146:705–15. doi:10.1017/S0021859608007983
89. Patra AK. Prediction of enteric methane emission from buffaloes using statistical models. Agric Ecosyst Environ (2014) 195:139–48. doi:10.1016/j.agee.2014.06.006
90. Dijkstra J, Neal HD, Beever D, France J. Simulation of nutrient digestion, absorption and outflow in the rumen: model description. J Nutr (1992) 122:2239–55.
92. Kebreab E, Johnson A, Archibeque L, Pape D, Wirth T. Model for estimating enteric methane emission from United States dairy and feedlot cattle. J Anim Sci (2008) 86:2738–48. doi:10.2527/jas.2008-0960
93. IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Hayama, Kanagawa: IGES (2006). Available from: http://www.ipcc-nggip.iges.or.jp/support/Primer_2006GLs.pdf
94. Moraes LE, Strathe AB, Fadel JG, Casper DP, Kebreab E. Prediction of enteric methane emissions from cattle. Glob Chang Biol (2014) 20:2140–8. doi:10.1111/gcb.12471
95. Patra AK, Lalhriatpuii M. Development of statistical models for prediction of enteric methane emission from goats using nutrient composition and intake variables. Agric Ecosyst Environ (2016) 215:89–99. doi:10.1016/j.agee.2015.09.018
96. Patra AK, Lalhriatpuii M, Debnath B. Predicting enteric methane emission in sheep using linear and non-linear statistical models from dietary variables. Anim Prod Sci (2016) 56:574–84. doi:10.1071/AN15505
97. Patra AK. Estimation of methane and nitrous oxide emissions from Indian livestock. J Environ Monitor (2012) 14:2673–84. doi:10.1039/c2em30396e
98. Patra AK. Prediction of enteric methane emission from cattle using linear and non-linear statistical models in tropical farming systems. Mitig Adap Strat Glob Chan (2016). doi:10.1007/s11027-015-9691-7
99. Chilliard Y, Martin C, Rouel J, Doreau M. Milk fatty acids in dairy cows fed whole crude linseed, extruded linseed, or linseed oil, and their relationship with methane output. J Dairy Sci (2009) 92:5199–211. doi:10.3168/jds.2009-2375
100. Dijkstra J, van Zijderveld SM, Apajalahti JA, Bannink A, Gerrits WJJ, Newbold JR, et al. Relationships between methane production and milk fatty acid profiles in dairy cattle. Anim Feed Sci Technol (2011) 16(6–167):590–5. doi:10.1016/j.anifeedsci.2011.04.042
101. Williams SRO, Moate PJ, Deighton MH, Hannah MC, Wales WJ. Methane emissions of dairy cows cannot be predicted by the concentrations of C8:0 and total C18 fatty acids in milk. Anim Prod Sci (2014) 54:1757–61. doi:10.1071/AN14292
102. Montoya JC, Bhagwat AM, Peiren N, De Campeneere S, De Baets B, Fievez V. Relationships between odd- and branched-chain fatty acid profiles in milk and calculated enteric methane proportion for lactating dairy cattle. Anim Feed Sci Technol (2011) 166–167:596–602. doi:10.1016/j.anifeedsci.2011.04.080
103. Dehareng F, Delfosse C, Froidmont E, Soyeurt H, Martin C, Gengler N, et al. Potential use of milk mid-infrared spectra to predict individual methane emission of dairy cows. Animal (2012) 6:1694–701. doi:10.1017/S1751731112000456
104. Gill FL, Dewhurst RJ, Evershed RP, McGeough E, O’Kiely P, Pancost RD, et al. Analysis of archaeal ether lipids in bovine faeces. Anim Feed Sci Technol (2011) 166:87–92. doi:10.1016/j.anifeedsci.2011.04.006
105. McCartney CA, Bull ID, Yan T, Dewhurst RJ. Assessment of archaeol as a molecular proxy for methane production in cattle. J Dairy Sci (2013) 96:1211–7. doi:10.3168/jds.2012-6042
106. CSIRO. System, Method and Device for Measuring a Gas in the Stomach of a Mammal. Patent publication number W020213/003892 A1 (2014).
107. Hedderich R, Whitman WB. Physiology and biochemistry of the methane-producing archaea. 4th ed. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes. Berlin: Springer (2013). p. 635–62.
108. Patra AK, Saxena J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry (2010) 71:1198–222. doi:10.1016/j.phytochem.2010.05.010
109. Patra AK. The effect of dietary fats on methane emissions, and its other effects on digestibility, rumen fermentation and lactation performance in cattle: a meta-analysis. Livest Sci (2013) 155:244–54. doi:10.1016/j.livsci.2013.05.023
110. Patra AK, Yu Z. Effects of coconut and fish oils on ruminal methanogenesis, fermentation, and abundance and diversity of microbial populations in vitro. J Dairy Sci (2013) 96:1782–92. doi:10.3168/jds.2012-6159
111. Moate PJ, Williams SRO, Grainger G, Hannah MC, Ponnampalam EN, Eckard RJ. Influence of cold-pressed canola, brewers grains and hominy meal as dietary supplements suitable for reducing enteric methane emissions from lactating dairy cows. Anim Feed Sci Technol (2011) 166–167:254–64. doi:10.1016/j.anifeedsci.2011.04.069
112. Li XX, Durmic Z, Liu SM, McSweeney CS, Vercoe PE. Eremophila glabra reduces methane production and methanogen populations when fermented in a rusitec. Anaerobe (2014) 29:100–7. doi:10.1016/j.anaerobe.2013.10.008
113. Pal K, Patra AK, Sahoo A, Kumawat PK. Evaluation of several tropical tree foliages for methane production potential, degradability and rumen fermentation in vitro. Livest Sci (2015) 180:98–105. doi:10.1016/j.livsci.2015.07.011
114. Sun XZ, Waghorn GC, Hoskin SO, Harrison SJ, Muetzel S, Pacheco D. Methane emissions from sheep fed fresh brassicas (Brassica spp.) compared to perennial ryegrass (Lolium perenne). Anim Feed Sci Technol (2012) 176:107–16. doi:10.1016/j.anifeedsci.2012.07.013
115. Oskoueian E, Abdullah N, Oskoueian A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. Biomed Res Int (2013) 2013:349129. doi:10.1155/2013/349129
116. Becker PM, van Wikselaar PG, Franssen MC, de Vos RC, Hall RD, Beekwilder J. Evidence for a hydrogen-sink mechanism of (+) catechin-mediated emission reduction of the ruminant greenhouse gas methane. Metabolomics (2014) 10:179–89. doi:10.1007/s11306-013-0554-5
117. Aemiro A, Hanada M, Umetsu K, Nishida T. The effect of sunphenon 30S-O on methane emission, nutrient intake, digestibility and rumen fermentation. Anim Feed Sci Technol (2016) 214:34–43. doi:10.1016/j.anifeedsci.2016.02.007
118. Patra AK, Yu Z. Effects of adaptation of in vitro rumen culture to garlic oil, nitrate and saponin and their combinations on methanogenesis, fermentation, and abundances and diversity of microbial populations. Front Microbiol (2015) 6:1434. doi:10.3389/fmicb.2015.01434
119. van Zijderveld SM, Gerrits WJJ, Dijkstra J, Newbold JR, Hulshof RBA, Perdok HB. Persistency of methane mitigation by dietary nitrate supplementation in dairy cows. J Dairy Sci (2011) 94:4028–38. doi:10.3168/jds.2011-4236
120. Burreson BJ, Moore RE, Roller PP. Volatile halogen compounds in the alga Asparagopsis taxiformis (Rhodophyta). J Agric Food Chem (1976) 24:856–61. doi:10.1021/jf60206a040
121. Machado L, Magnusson M, Paul NA, de Nys R, Tomkins N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One (2014) 9:e85289. doi:10.1371/journal.pone.0085289
122. Duval S, Kindermann M. Use of Nitrooxy Organic Molecules in Feed for Reducing Enteric Methane Emissions in Ruminants, and/or to Improve RUMINANT Performance. International patent application WO 2012/084629 A1, World Intellectual Property Organization (2012).
123. Martínez-Fernández G, Abecia L, Arco A, Cantalapiedra-Hijar G, Martín-García AI, Molina-Alcaide E, et al. Effects of ethyl-3-nitrooxy propionate and 3-nitrooxypropanol on ruminal fermentation, microbial abundance, and methane emissions in sheep. J Dairy Sci (2014) 97:3790–9. doi:10.3168/jds.2013-7398
124. Reynolds CK, Humphries DJ, Kirton P, Kindermann M, Duval S, Steinberg W. Effects of 3-nitrooxypropanol on methane emission, digestion, and energy and nitrogen balance of lactating dairy cows. J Dairy Sci (2014) 97:3777–89. doi:10.3168/jds.2013-7397
125. Haisan J, Sun Y, Guan LL, Beauchemin KA, Iwaasa A, Duval S, et al. The effects of feeding 3-nitrooxypropanol on methane emissions and productivity of Holstein cows in mid lactation. J Dairy Sci (2014) 97:3110–9. doi:10.3168/jds.2013-7834
126. Hristov AN, Oh J, Giallongo F, Frederick TW, Harper MT, Weeks HL, et al. An inhibitor persistently decreased enteric methane emission from dairy cows with no negative effect on milk production. Proc Natl Acad Sci U S A (2015) 112:10663–8. doi:10.1073/pnas.1504124112
127. Romero-Perez A, Okine EK, McGinn SM, Guan LL, Oba M, Duval SM, et al. Sustained reduction in methane production from long-term addition of 3-nitrooxypropanol to a beef cattle diet. J Anim Sci (2015) 93:1780–91. doi:10.2527/jas.2014-8726
128. Haisan J, Sun Y, Guan L, Beauchemin KA, Iwaasa A, Duval S, et al. The effects of feeding 3-nitrooxypropanol at two doses on milk production, rumen fermentation, plasma metabolites, nutrient digestibility, and methane emissions in lactating Holstein cows. Anim Prod Sci (2016). doi:10.1071/AN15219
129. Jahromi FM, Liang JB, Ho YW, Mohamad R, Goh YM, Shokryazdan P, et al. Lovastatin in Aspergillus terreus: fermented rice straw extracts interferes with methane production and gene expression in Methanobrevibacter smithii. Biomed Res Int (2013) 2013:604721. doi:10.1155/2013/604721
130. Cosgrove GP, Muetzel S, Skipp RA, Mace WJ. Effects of endophytic and saprophytic fungi on in vitro methanogenesis. N Z J Agric Res (2012) 55:293–307. doi:10.1080/00288233.2012.693106
131. Morgavi DP, Martin C, Boudra H. Fungal secondary metabolites from Monascus spp. reduce rumen methane production in vitro and in vivo. J Anim Sci (2013) 91:848–60. doi:10.2527/jas.2012-5665
132. Dubois B, Tomkins NW, Kinley RD, Bai M, Seymour S, Paul NA, et al. Effect of tropical algae additives on rumen in vitro gas production and fermentation characteristics. Am J Plant Sci (2013) 4:34–43. doi:10.4236/ajps.2013.412A2005
133. Moate PJ, Williams SRO, Torok VA, Hannah MC, Eckard RJ, Auldist MJ, et al. Effects of feeding algal meal high in docosahexanoic acid on feed intake milk production and methane emissions in dairy cows. J Dairy Sci (2013) 96:3177–88. doi:10.3168/jds.2012-6168
134. Patra AK, Yu Z. Effective reduction of enteric methane production by a combination of nitrate and saponin without adverse effect on feed degradability, fermentation, or bacterial and archaeal communities of the rumen. Bioresour Technol (2013) 148:352–60. doi:10.1016/j.biortech.2013.08.140
135. Grainger C, Beauchemin KA. Can enteric methane emissions from ruminants be lowered without lowering their production? Anim Feed Sci Technol (2011) 166–167:308–20. doi:10.1016/j.anifeedsci.2011.04.021
136. Patra AK, Yu Z. Combinations of nitrate, saponin, and sulfate additively reduce methane production by rumen cultures in vitro while not adversely affecting feed digestion, fermentation or microbial communities. Bioresour Technol (2014) 155:129–35. doi:10.1016/j.biortech.2013.12.099
137. Patra AK, Yu Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J Appl Microbiol (2015) 119:127–38. doi:10.1111/jam.12819
138. Narvaez N, Wang Y, McAllister T. Effects of extracts of Humulus lupulus (hops) and Yucca schidigera applied alone or in combination with monensin on rumen fermentation and microbial populations in vitro. J Sci Food Agric (2013) 93:2517–22. doi:10.1002/jsfa.6068
139. Patra AK, Saxena J. The effect and mode of action of Saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev (2009) 22:204–19. doi:10.1017/S0954422409990163
140. Patra AK, Yu Z. Effects of essential oils on methane production and fermentation by, and abundance and diversity of, rumen microbial populations. Appl Environ Microbiol (2012) 78:4271–80. doi:10.1128/AEM.00309-12
141. Guyader J, Eugène M, Meunier B, Doreau M, Morgavi DP, Silbergerg M, et al. Additive methane-mitigating effect between linseed oil and nitrate fed to cattle. J Anim Sci (2015) 93:3564–77. doi:10.2527/jas.2014-8196
142. Wang M, Wang R, Sun X, Chen L, Tang S, Zhou C, et al. A mathematical model to describe the diurnal pattern of enteric methane emissions from non-lactating dairy cows post-feeding. Anim Nutr (2015) 1:329–38. doi:10.1016/j.aninu.2015.11.009
143. Gallo A, Giuberti G, Frisvad JC, Bertuzzi T, Nielsen KF. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins (2015) 7:3057–111. doi:10.3390/toxins7083057
Keywords: methane, ruminants, measurement method, mitigation technology, diet
Citation: Patra AK (2016) Recent Advances in Measurement and Dietary Mitigation of Enteric Methane Emissions in Ruminants. Front. Vet. Sci. 3:39. doi: 10.3389/fvets.2016.00039
Received: 25 February 2016; Accepted: 02 May 2016;
Published: 20 May 2016
Edited by:
Stephen Brent Smith, Texas A&M University, USACopyright: © 2016 Patra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amlan K. Patra, patra_amlan@yahoo.com
 Amlan K. Patra
Amlan K. Patra