Double trouble? Potential for hyperexcitability following both channelopathic up- and downregulation of Ih in epilepsy
1
Harvard Medical School, Boston, MA, USA
2
Neurology, Massachusetts General Hospital, Boston, MA, USA
3
Anatomy and Neurobiology, University of California – Irvine, Irvine, CA, USA
Studies of pathological ion channel regulation as an underlying mechanism of epilepsy have revealed alterations in the h-current in several animal models. While earlier reports indicate that downregulation of the h-current is pro-excitatory on the single neuron level, we found an upregulation of Ih in hyperexcitable CA1 pyramidal neuron dendrites following experimental febrile seizures. In addition, in several CA1 pyramidal neuron computational models of different complexity, h-current upregulation has been shown to lead to pro-excitable effects. This focused review examines the complex impact of altered h-current on neuronal resting membrane potential (RMP) and input resistance (Rin), as well as reported interactions with other ionic conductances.
The study of channelopathies, pathological changes in the expression and function of ion channels, has gained momentum in recent years in epilepsy research – several idiopathic epilepsies (inherited) have been linked to underlying mutations in channel encoding genes (for reviews see, Catterall et al., 2008
; Hirose et al., 2005
; Lerche et al., 2005
; Mulley et al., 2003
). For the study of the inherited, genetically determined pathologies, a number of experimental models reproducing human mutations and symptoms have been generated (for review see, Avanzini et al., 2007
). Similarly, the study of symptomatic epilepsy
(developed after a brain insult) in animal models has identified several acquired channelopathies
– so named because the channelopathies develop in response to the brain insult or subsequent status epilepticus (for review see, Pitkanen and Lukasiuk, 2009
). The channel types affected by the acquired channelopathies include GABAA - receptor-channels (Brooks-Kayal et al., 1998
; Sanchez et al., 2005
), voltage-dependent Na+ channels (Ellerkmann et al., 2003
; Howard et al., 2007
), Ca2+ channels (Becker et al., 2008
; Su et al., 2002
), and K+ channels (Bernard et al., 2004
; Howard et al., 2007
; Shin et al., 2008
; Shruti et al., 2008
). However, the most frequently acquired channelopathy concerns the mixed cation h-current
in a number of different epilepsy models: perinatal seizure-inducing hypoxia (Zhang et al., 2006
), the kainate model of temporal lobe epilepsy (Shah et al., 2004
), the pilocarpine model of temporal lobe epilepsy (Jung et al., 2007
; Marcelin et al., 2009
; Shin et al., 2008
), the fluid percussion injury (FPI) model of post-traumatic epilepsy (Howard et al., 2007
), and prolonged experimental febrile seizures
(Chen et al., 2001a
; Dyhrfjeld-Johnsen et al., 2008
).
In most experimental paradigms for investigation of the acquired h-channelopathies, a seizure-induced reduction in Ih was reported (Jung et al., 2007
; Marcelin et al., 2009
; Shah et al., 2004
; Shin et al., 2008
; Zhang et al., 2006
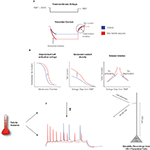
). The downregulated h-current was linked to hyperexcitability and seizures through the resulting increase in neuronal input resistance. However, our recent paper on dendritic h-channelopathy in the experimental febrile seizure model reported a ∼70% increase in the h-current density along with depolarized half-activation potential (V1/2) and slower kinetics in hyperexcitable CA1 pyramidal neuron dendrites (Figure 1
). The depolarized V1/2, along with the overall increase in h-current density, means that smaller stimuli are required to strongly activate Ih from the resting membrane potential (RMP) in CA1 pyramidal neurons following febrile seizures.
Figure 1. Febrile seizures alter Ih in CA1 pyramidal neurons. (A) Schematic of the cell-attached, dendritic recordings of Ih from CA1 pyramidal cells from control animals and from animals that were exposed to hyperthermia-induced, experimental prolonged febrile (fever-induced) seizures. The dendritic patch was held in voltage-clamp mode, a voltage command was applied, and the resulting currents were recorded. The activation and de-activation kinetics, along with the maximal currents, were derived from the recordings as indicated. (B) Analysis of current recordings showed that febrile seizures result in altered properties of Ih in the dendrites of CA1 pyramidal neurons: a depolarized half-activation potential, slower time-constants of activation and de-activation, and ∼70% increased current density (Dyhrfjeld-Johnsen et al., 2008
). (C) Whole-cell dendritic current injection experiments in the current-clamp mode showed that CA1 pyramidal dendrites are hyperexcitable following febrile seizures. However (as illustrated by the arrow with the question mark), this raised the question of how the changes in Ih parameters relate to the observed increases in excitability.
This focused review concentrates on recent data on mechanisms of h-current regulation and re-examines the long-standing dichotomy (Poolos, 2004
) of potential for both channelopathic upregulation and downregulation of Ih resulting in hyperexcitability.
In the studies of acquired h-current channelopathies mentioned above, alterations of maximal current levels as well as altered activation properties and kinetics have been reported. Below, we summarize a number of processes that are known to affect hyperpolarization-activated cation (HCN) channel properties, expression levels, and trafficking with an emphasis on activity-dependent mechanisms.
In addition to the relatively well-established modulation of HCN channel activation by intracellular pH (Munsch and Pape, 1999
) and cAMP (DiFrancesco, 1993
; Wainger et al., 2001
), recent years have seen a number of additional mechanisms impacting the half-activation voltage of Ih. This includes allosteric gating by the membrane phospholipid phosphatidylinositol-4,5 biphosphate (PIP2) (Zolles et al., 2006
) as well as activation of the p38 mitogen-activated protein kinase (p38 MAPK) (Poolos et al., 2006
) and diacylglycerol (DAG) (Fogle et al., 2007
) signaling pathways who strongly modulate the half-activation voltage of Ih.
In plasticity studies, protocols commonly used to induce long-term potentiation (LTP) and long-term depression (LTD) were shown to modulate intrinsic neuronal excitability. Specifically, increased HCN channel protein synthesis resulted in decreased neuronal excitability following the LTP induction (Fan et al., 2005
; see also van Welie et al., 2004
). This process was shown to depend on calcium influx through NMDA-receptors following action potential (AP) back-propagation and a subsequent, calcium/calmodulin-dependent protein kinase II (CamKII) activation (Fan et al., 2005
). Conversely, a downregulation of the h-current leads to increased neuronal excitability following the LTD induction, through the activation of group 1 metabotropic glutamate receptors and the protein kinase C (PKC) pathway (Brager and Johnston, 2007
).
Recent investigations of h-current regulation following kainate-induced seizures in vivo and in organotypic slice cultures have shown a profound downregulation of HCN1 channel expression (McClelland et al., 2008
). The reduced expression was linked to a seizure-induced upregulation of neuron-restrictive silencing factor (NRSF), which binds strongly to the HCN1 encoding gene and restricts transcription. Blocking NRSF function prevents seizure-induced reduction of HCN1 expression.
In the experimental febrile seizure model, a lasting increase in the maximal h-current is accompanied by a depolarized half-activation potential and slower time-constants (Chen et al., 2001a
; Dyhrfjeld-Johnsen et al., 2008
), while the HCN1 subunit expression is decreased (Brewster et al., 2002
). These complex alterations could potentially be explained by a seizure-induced increase in the formation of HCN1/HCN2 heteromeric channels with different properties than homomeric channels (Brewster et al., 2005
; Chen et al., 2001b
), through increased glycosylation of HCN1 subunits (Zha et al., 2008
).
Not only the density and properties of Ih, but also the specific subcellular distribution of HCN-channels are subject to activity-dependent regulation: in CA1 pyramidal cells, the characteristic increasing h-current density along the apical dendrites of CA1 pyramidal neurons (Dyhrfjeld-Johnsen et al., 2008
; George et al., 2008
; Lorincz et al., 2002
; Magee, 1998
) is established and maintained by excitatory input from the enthorinal cortex (Shin and Chetkovich, 2007
). Blockade of excitatory neurotransmission results in an even distribution of HCN-channels throughout the neuronal compartments. In recent years, live-imaging of CA1 pyramidal neurons transfected with GFP-tagged HCN1 channels has revealed an immediate and a strong decrease in HCN channel mobility following bath application of glutamate. The decreased mobility resulted from a >2-fold increase in the fraction of surface expressed HCN-channel proteins (Noam et al., 2008
). These results indicate the potential for rapid activity-dependent regulation of Ih by fast removal or insertion of existing HCN channel proteins. A candidate for such regulation, the chaperone protein TRIP8b, co-localizes with HCN1 subunits in pyramidal neurons (Santoro et al., 2004
). Interestingly, a disruption of the interaction between the HCN1 subunits and TRIP8b has been reported as a mechanism underlying the channelopathic mislocation of h-channels in the kainate model of temporal lobe epilepsy (Shin et al., 2008
). This suggests that altered h-current densities in epilepsy may not only be due to altered levels of channel proteins, but also depend on altered trafficking and localization of neuronal structures.
In the study of voltage-gated channel alterations in neurological disorders, a key question is how the plasticity of intrinsic properties affects single neuron excitability (Beck and Yaari, 2008
). With the multitude of potential mechanisms for regulating Ih expression and characteristics described above, it is perhaps not surprising that different acquired h-channelopathies have been discovered in different animal models of epilepsy. However, an apparent contradiction exists between the studies suggesting that single neuron hyperexcitability results from a downregulation (Jung et al., 2007
; Shah et al., 2004
) or an upregulation (Chen et al., 2001a
; Dyhrfjeld-Johnsen et al., 2008
) of the h-current.
Unique Properties of the h-Current
To explore the dichotomy, it is necessary to bear in mind some unique properties of Ih: the h-current is hyperpolarization-activated and non-inactivating with a reversal potential between −25 and −40 mV (Robinson and Siegelbaum, 2003
), making the current an inward or depolarizing (hence, per definition, an “excitatory”) current with respect to the resting potential. Ih is tonically active at the RMP of most neurons (Kaupp and Seifert, 2002
), resulting in a contribution to both the neuronal RMP and input resistance (Rin) as demonstrated, e.g., by the hyperpolarization accompanied by increased Rin following h-current blockade (Magee, 1998
) or h-channel deletion (Nolan et al., 2007
). By contributing to both the neuronal RMP and Rin, the h-current plays a dual role in determining neuronal excitability by influencing the resting distance from the firing threshold of the cell as well as the amount of depolarization caused by excitatory currents.
Hanging in the Balance: Ih Effects on RMP and Rin
When assessing the effect of altered Ih, a common practice dictates that recordings are made from a common holding potential. This ensures that other voltage-gated conductances and the membrane voltage relative to firing threshold remain the same in control and altered h-current condition. However, as emphasized in combined computational and experimental studies (Dyhrfjeld-Johnsen et al., 2008
; George et al., 2008
), this practice masks the excitatory effects of Ih by negating the impact on the RMP, and also on the amount of depolarization required to reach firing threshold.
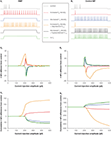
An increased h-current density leads to a depolarized RMP closer to the firing threshold, but also a decreased Rin due to the increased number of HCN-channels open at rest (Figure 2
A). Conversely, a decreased h-current density results in a hyperpolarized RMP further away from the firing threshold, but an increased input resistance due to a decreased number of HCN-channels open at rest (Figure 2
A). Injecting a constant current to hold a neuron with altered Ih at the control RMP (Figure 2
B) creates a situation in which increased h-current density only leads to decreased Rin, while decreased Ih only leads to increased Rin. The effects are further pronounced (Figure 2
) when the altered h-current density is accompanied by changes in the half-activation potential: a depolarized V1/2, along with upregulated Ih density, further increases the h-current at more depolarized membrane potentials (Chen et al., 2001a
; Dyhrfjeld-Johnsen et al., 2008
), while a hyperpolarized V1/2 accompanying downregulated Ih density further decreases the h-current even at hyperpolarized membrane potentials (Jung et al., 2007
).
Figure 2. Effects of altered Ih on RMP and Rin. (A) Increased h-current density depolarizes the neuronal RMP (bringing it closer to firing threshold) but decreases the Rin (reducing the effect of inputs). Decreased h-current density hyperpolarizes the neuronal RMP (bringing it further away from firing threshold) but increases the Rin (increasing the effect of inputs). The effects on the RMP are further increased when the h-current V1/2 is depolarized or hyperpolarized in addition to density changes. (B) Holding neurons with altered Ih at the control resting membrane potential (control MP) abolishes the depolarizing or hyperpolarizing effects on the RMP, but further accentuates the respective decrease or increase of Rin by increased or decreased h-current density.
Using a modified version of a previously published CA1 pyramidal neuron compartmental model (Dyhrfjeld-Johnsen et al., 2008
; Golding et al., 2001
), the direct effect of altered Ih on AP firing in response to the steady-state dendritic current injection can be assessed (Figure 3
).
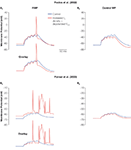
Figure 3. Altered h-current changes neuronal excitability. The response of the modified control CA1 pyramidal neuron model (Dyhrfjeld-Johnsen et al., 2008
; Golding et al., 2001
) and models with altered Ih to a 1000 ms depolarizing current injection from RMP (left column) and held at the common holding potential equal to the control RMP (right column). (A1, B1) Example traces from current injections with 210 pA amplitude. (A2, B2) The number of APs fired in models with altered Ih that differs from the control model for each current injection amplitude. Positive numbers indicate hyperexcitability, while negative numbers indicate hypoexcitability. (A3, B3) The cumulative number of APs fired in models with altered Ih that differs from the control model illustrates the impact of different combinations of h-current alterations.
When current injections are performed at the “free-floating” RMP with control or altered h-current levels, an increase in the number of APs fired is seen in the models with increased h-current density, but not in the decreased Ih (Figures 3
A1–A3). Conversely, when the models are held at a common membrane potential before the current injection, only the models with decreased Ih fire more APs than the control model (Figures 3
B1–B3). Interestingly, even complete removal of the h-current from the model does not result in hyperexcitability from the RMP.
Similarly, excitability-enhancing effects of increased Ih were also obtained using dendritic EPSP summation as outcome measure, in both a relatively simple (Figures 4
A1, A2) and a more complex (Figures 4
B1, B2) model of CA1 pyramidal cells (Dyhrfjeld-Johnsen et al., 2008
). In both the models, increased Ih accompanied by a depolarized V1/2 leads to decreased temporal summation of EPSP inputs due to the decreased Rin (see overlays in Figures 4
A1,B1). However, models with increased Ih are closer to threshold and fire APs in response to the simulated synaptic input due to the depolarized RMP. From a common control RMP, only the decreased temporal summation due to the decreased Rin remains in the models with increased Ih (Figures 4
A2,B2). Therefore, it is important to allow the effects of the altered h-current on both RMP and Rin to come into play when assessing the direct impact on neuronal excitability.
Figure 4. Effect of increased Ih on summation and AP firing. The effect of five small EPSPs from the RMP (A1, B1) and the common holding potential at control RMP (A2, B2) in two CA1 pyramidal neuron models (A1, A2, Poolos et al., 2002
; B1, B2, Poirazi et al., 2003
) with control Ih (blue) and increased Ih density with depolarized V1/2 (red). Note that the increased Ih leads to less summation, but firing of APs is abolished when the depolarization of the RMP is eliminated by holding the cells at a common control RMP.
Interactions with Other Conductances
The h-current does exert effects on neuronal excitability not only by influencing the RMP and Rin, but also through interactions with other voltage-gated channels. Recent data show that the shunting effect of Ih is achieved through the activation of non-inactivating, voltage-gated potassium conductances (George et al., 2008
). These results showed that RMP depolarization by Ih leads to a steady-state activation of a K+ channel, which may produce dual excitatory and inhibitory effects of Ih depending on the input strength. Computational and experimental data suggested that a physiological candidate for the K+ current was the M-current, whose regulation through neuromodulation may switch the role of Ih in signal integration from inhibitory to excitatory (George et al., 2008
). In the febrile seizure model, we reported a downregulation of a presumed persistent potassium current (Dyhrfjeld-Johnsen et al., 2008
), suggesting a reduction of such an interaction as an additional mechanism for increased excitability following the h-current upregulation.
Increased Ih may also interact with inhibitory synaptic inputs resulting in post-inhibitory rebound firing in CA1 pyramidal cells after febrile seizures (Chen et al., 2001a
), and a similar effect is well-known to occur in thalamo-cortical projection neurons (Crunelli and Leresche, 1991
; Soltesz et al., 1991
). Additionally, depolarized dendritic membrane potentials could facilitate the propagation of distal dendritic Ca2+-spikes to the soma (Jarsky et al., 2005
). Conversely, the membrane hyperpolarization following the reduction of Ih has been shown to release constraints on distal dendritic Ca2+ spikes (Tsay et al., 2007
), suggesting a potential pro-excitatory role for downregulation of the h-current in epilepsy.
Finally, in the post-traumatic, hyperexcitable dentate gyrus, mossy cells exhibit extensive modifications in Na+, K+, and h-currents, without altered I–F and I–V curves (Howard et al., 2007
). The importance of the opposing, apparently coordinated and homeostatic-like changes in several conductances of single neurons was elucidated computationally in a realistic large-scale model of the dentate gyrus (Dyhrfjeld-Johnsen et al., 2007
; Howard et al., 2007
), demonstrating that individually the ion channel perturbations could significantly affect network activity.
In CA1 pyramidal cell models, only an increased functional Ih appears to be underlying direct pro-excitatory effects on single neuron firing, as previously demonstrated in three different CA1 neuron computational models of widely differing complexity (Dyhrfjeld-Johnsen et al., 2008
). However, as the result depends on the balance between the effects on RMP and Rin, the conclusion could be affected by other intrinsic properties determined by neuron type, developmental stage, and neuromodulation. Furthermore, through interaction with other voltage-gated and ligand-gated conductances (Dyhrfjeld-Johnsen et al., 2008
; George et al., 2008
; Howard et al., 2007
; Tsay et al., 2007
), normal and altered h-current may modify neuronal excitability in a complex fashion.
Additionally, the h-current is involved in determining resonance frequencies of neurons (Hu et al., 2002
; Wang et al., 2006
) and likely to play a significant role in pathological network oscillatory behavior in epilepsy. In recent years, the response of CA1 pyramidal neurons to the hippocampal theta rhythm has been shown to be impaired due to the downregulated h-current in the pilocarpine model of temporal lobe epilepsy (Marcelin et al., 2009
). This finding suggests additional roles for h-channelopathies in impaired learning and memory (Nolan et al., 2003
, 2004
). Finally, neurons with high Ih have recently been implicated in the initiation of highly excitable, network-wide UP states (Kang et al., 2008
).
While this focused review concentrates on channelopathic alteration of the h-current in pyramidal neuron dendrites, Ih is also expressed in axonal terminals (Bender et al., 2007
; Lujan et al., 2005
) and inhibitory interneurons (Aponte et al., 2006
; Lupica et al., 2001
; Maccaferri and McBain, 1996
). Along with the complex effects on pyramidal neuron excitability discussed above, it is emphasized that factors ranging from direct effects on RMP and Rin to interactions with other ion channels, h-channel localization, and neuronal subtype must be taken into account when judging whether upregulation or downregulation of Ih leads to hyperexcitability in a given animal model.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work is supported by the Epilepsy Foundation/Milken Family Foundation (EFA-36656 to JDJ) and the NIH (NS38580 to IS).
Epilepsy: A family of neurological seizure disorders characterized by abnormal electrical discharges in the brain, resulting in behavioral symptoms ranging from staring spells to intense convulsions and loss of consciousness. Idiopathic epilepsies do not result from an identifiable external cause and are presumed to be genetic, while symptomatic epilepsies have an identifiable cause such as severe head trauma.
Bender, R. A., Kirschstein, T., Kretz, O., Brewster, A. L., Richichi, C., Ruschenschmidt, C., Shigemoto, R., Beck, H., Frotscher, M., and Baram, T. Z. (2007). Localization of HCN1 channels to presynaptic compartments: novel plasticity that may contribute to hippocampal maturation. J. Neurosci. 27, 4697–4706.
McClelland, S., Richichi, C., Dube, C., Zha, Q., and Baram, T. Z. (2008). Activity-dependent increase of NRSF/REST levels suppresses expression of the hyperpolarization activated cyclic-nucleotide gated (HCN) channel. Poster 33. 7/D65 presented at The Annual Meeting of the Society for Neuroscience 2008.
Nolan, M. F., Malleret, G., Dudman, J. T., Buhl, D. L., Santoro, B., Gibbs, E., Vronskaya, S., Buzsaki, G., Siegelbaum, S. A., Kandel, E. R., and Morozov, A. (2004). A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 119, 719–732.
Nolan, M. F., Malleret, G., Lee, K. H., Gibbs, E., Dudman, J. T., Santoro, B., Yin, D., Thompson, R. F., Siegelbaum,S. A., Kandel, E. R., and Morozov, A. (2003). The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 115, 551–564.