Section on In Vivo Neural Function, Laboratory for Integrative Neuroscience, NIAAA, NIH, Bethesda, MD, USA
The neural circuits involved in learning and executing goal-directed actions, which are governed by action-outcome contingencies and sensitive to changes in the expected value of the outcome, have been shown to be different from those mediating habits, which are less dependent on action-outcome relations and changes in outcome value. Extended training, different reinforcement schedules, and substances of abuse have been shown to induce a shift from goal-directed performance to habitual performance. This shift can be beneficial in everyday life, but can also lead to loss of voluntary control and compulsive behavior, namely during drug seeking in addiction. Although the brain circuits underlying habit formation are becoming clearer, the molecular mechanisms underlying habit formation are still not understood. Here, we review a recent study where Hilario et al. (2007)
established behavioral procedures to investigate habit formation in mice in order to investigate the molecular mechanisms underlying habit formation. Using those procedures, and a combination of genetic and pharmacological tools, the authors showed that endocannabinoid signaling is critical for habit formation.
Goal-directed actions allow us to respond in an efficient way to changing situations. However, the continuous control and attention they demand can result in an unnecessary expenditure of our cognitive resources and can thus be prejudicial in some situations. In situations where the behavior is repeated regularly for a long time without major changes in the incentive value of the outcome, or situations where we cannot manipulate the probability of obtaining an outcome irrespective of the strategy employed, rules and habits can be advantageous. However, habitual behavior when taken to an extreme is associated with loss of control and with maladaptive behavior, such as drug seeking in addiction or compulsivity. Therefore, understanding the molecular and circuit mechanisms underlying habit formation can be important to prevent or treat these disorders.
It is known that different cortico-basal ganglia circuits support the learning and execution of goal-directed actions and habits (Balleine and Dickinson, 1998
; Corbit and Balleine, 2003
; Killcross and Coutureau, 2003
; Yin and Knowlton, 2004
; Yin et al., 2004
, 2005a
,b
, 2006
). However, much less is known about the molecular bases of habit formation. In an effort to identify the molecular substrates involved in habit formation Hilario et al. (2007)
tailored behavioral paradigms to study goal-directed actions and habit formation in mice. They confirmed that different schedules of reinforcement bias mice towards goal-directed actions or habits by using devaluation by sensory-specific satiety to test for habitual behavior (Hilario et al., 2007
). They also introduced a novel assay that measures generalization of actions
to novel manipulandi similar to those where animals were trained. Using these paradigms, they investigated the role of endocannabinoid signaling through CB1 receptors in habit formation, by employing both genetic and pharmacologic tools (Hilario et al., 2007
).
Here we review the study by Hilario et al. (2007)
, starting by defining the concepts of goal-directed and habitual behavior that were used in that study, and the foundations for the experimental design adopted by the authors. We also discuss the details of the behavioral paradigms adapted to study habit formation in mice. Finally, we explore the rationale behind the hypothesis involving endocannabinoids in habit formation, and the data indicating that endocannabinoid signaling through CB1 receptors is necessary for habit formation.
The study of how we learn actions and what drives them has been the focus of neuroscience and behavioral science for some time. However, the field has struggled not only with the identification of the circuits and the cellular and molecular bases supporting actions, but also with the definitions of what goal-directed actions and habits
are (or if they differ at all). For the most part of last century learned actions were reduced to a stimulus-response (S-R) relation, and learning was perceived as a consequence of the continuous strengthening or weakening of the S-R relation by the use of reinforcements (Hull, 1943
). Even though researchers like Tolman (1948
, 1949)
proposed that animals could use information they learned flexibly and use cognitive maps, and Von Holst proposed alternatives to the dominant view of behavior as a chain of reflexes emanating from Sherrington’s work (Creed et al., 1932
; Von Holst, 1973
), for a long time behaviorists relied mostly on observational methods, and excluded intentionality, expectation or internal representation of the value of the outcome because they were considered subjective variables. However, in the later part of the 20th century Dickinson and Rescorla developed experimental tools to investigate if instrumental behavior was being performed because of its consequences or not (Adams, 1982
; Adams and Dickinson, 1981
; Colwill and Rescorla, 1985
).
To investigate if actions were habitual (governed by a S-R relation) or goal-directed they asked if the actions were dependent on the expected value of the outcome, by introducing a devaluation
test (Adams, 1982
; Adams and Dickinson, 1981
; Colwill and Rescorla, 1985
). In this devaluation test rats were trained using an operant box to get access to food rewards, and after training the expected value of the reinforcements was manipulated by decreasing the value of the food (typically food poisoning). By comparing the number of responses when the food was devalued versus when it was not, they were able to distinguish experimentally habits as behavior impervious to devaluation, and goal-directed actions as sensitive to devaluation. Another test used to investigate if actions were goal-directed examined whether the behavior was dependent on the contingency between the performance of the action and earning the outcome (Corbit et al., 2002
; Dickinson et al., 1996
; Hammond, 1980
). Briefly, if the contingency between one of the actions and the outcome was decreased (degraded), rats would decrease the performance of that action specifically. These studies established that goal-directed actions are sensitive to changes in the expected value of the outcome and the contingency between the action and the outcome (A-O); while habitual behaviors are insensitive to changes in outcome value and contingency between action and outcome, suggesting they are governed by S-R relations (Balleine and Dickinson, 1994
). These were the definitions adopted by Hilario et al. (2007)
in their study.
Adams and Dickinson noticed not only that overtraining on a particular schedule could produce a transition from goal-directed behavior to habits, but also that different schedules of reinforcement differentially predisposed for habit formation (Adams, 1982
; Adams and Dickinson, 1981
). Specifically, the use of random ratio training schedules produced goal-directed behavior in rats, while the use of random interval schedules promoted habitual behavior (Adams and Dickinson, 1981
; Dickinson, 1985
; Dickinson et al., 1983
). Balleine and Dickinson (1998)
later used these procedures to start to examine the neural circuits involved in goal-directed behavior and habits. These behavioral assays have been very useful to investigate the neural circuits and the cellular and molecular mechanisms involved in goal-directed actions and habits (Balleine and Dickinson, 1998
; Corbit and Balleine, 2003
; Corbit et al., 2003
; Coutureau and Killcross, 2003
; Faure et al., 2005
; Hilario et al., 2007
; Nelson and Killcross, 2006
; Yin et al., 2004
, 2005b
).
Genetically engineered mice can be very useful to investigate the role of specific genes in a particular behavior, and to visualize or manipulate the circuits involved in that behavior. To investigate the molecular mechanisms of habit formation Hilario et al. (2007)
adapted the experimental procedures previously used in rats (Adams, 1982
; Adams and Dickinson, 1981
; Corbit and Balleine, 2003
), and developed new ones in mice (Hilario et al., 2007
). Using an operant box where a particular action could be performed to obtain a specific outcome, they trained mice with two reinforcers: either regular “chow” pellets or sucrose. One reinforcer was delivered in the operant chamber contingent upon lever pressing (the outcome of the action of lever pressing), and the other reinforcer was presented non-contingently in their home cage and used as a control for the devaluation test (Figure 1
A). After training the mice under a continuous reinforcement schedule to establish the relation between lever pressing and outcome delivery, animals where divided into two different groups: one group was trained under a random ratio schedule while the other group under a random interval schedule of reinforcement. Mice trained under a random ratio schedule of reinforcement received one reinforcer after a certain number of presses (on average every 20 lever presses in Hilario et al., 2007)
, whereas mice trained under a random interval schedule received a reinforcer upon the first press after a certain interval had elapsed since the last reinforcer was earned (60 s on average in this study). During training, random ratio animals had a tendency to show higher rates of lever pressing than random interval animals, which is consistent with a strategy to maximize the number of reinforcers per press in the different schedules (Dickinson et al., 1983
) (Figure 2
A). For random ratio animals the more they press the more they earn, while for random interval animals the best strategy is to press at a rate matching the reinforcement rate in time. Despite the differences in pressing rate observed, Hilario et al. (2007)
matched training schedules so that the number of reinforcements, the reinforcement rate per lever press, and the reinforcement rate per time were relatively similar between ratio and interval trained animals (Figure 2
B,C).
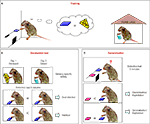
Figure 1. Investigating goal-directed actions and habit formation in mice. (A) Mice were trained with two reinforcers. In the figure, the task is exemplified with one of the reinforcers, cheese, being delivered in the operant box contingent upon lever pressing, while the other reinforcer, sugar water, is being delivered freely to the mouse in the home cage. The types of reinforcers used in the figures are for illustrative purposes only. (B) Devaluation is performed in two days: Day 1, the mouse is given the reinforcer, cheese, previoulsy earned by lever pressing (devalued condition); Day 2, the mouse receives the reinforcer, sugar water, previously freely available in its home cage (valued condition). The order of the conditions is randomized. Immediately after each feeding session, which last 1 h, the mouse goes through a 5-min extinction test in the operant chamber, with the training lever extended. The number of presses on the training lever under the valued and the devalued conditions are compared. If the mouse presses more under the valued versus devalued condition, then the behavior is goal-directed behavior. However, if the mouse presses both levers equally his behavior is classified as habitual. (C) The generalization test. Two levers are presented in a 5-min extinction test: If the mouse pressed the training lever more than the novel lever, it is discriminating/exploiting. However, if the mouse presses both levers equally then there is significant generalization/exploration. Training lever is in blue and a novel lever is in pink.
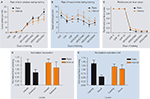
Figure 2. Different schedules of reinforcement produce different predisposition to habit formation in C57Bl6/J mice. (A) Acquisition of the lever pressing task in animals trained on random ratio and random interval schedules. The rate of lever pressing (per minute) for each daily session is depicted. (B) Average rate of head entry throughout training for the random interval and random ratio groups. (C) Rate of reinforcement per lever press throughout training for the random interval and random ratio groups.
(D) Lever pressing during the valued versus the devalued condition for the different training schedules, normalized to the lever pressing of the last day of training. (E) Lever pressing on the training lever versus a novel lever for the different training schedules, normalized to the lever pressing of the last day of training.
To determine if lever pressing in mice trained under different schedules was goal-directed or habitual, the effects of devaluation by sensory-specific satiety were examined during tests in extinction (Figure 1
B). During this type of devaluation test, the outcome that was earned contingent upon lever pressing was devalued by satiating the animals with it before the extinction test (devalued), and the performance of the animal was compared to the control situation in which the animals were satiated with the reinforcer they got for free in their home cage (valued). This test allowed them to examine how much the lever pressing action was dependent upon the expected value of the outcome that was earned contingently upon lever pressing, and controled for the motivational effects of general satiety. As expected, during the devaluation test, random ratio-trained animals responded significantly less during the devalued condition than during the valued condition. Conversely, random interval-trained animals were insensitive to changes in value during the test, and pressed equally during the valued and devalued conditions, indicating that they were habitual (Figure 2
D). Because random interval trained animals pressed less during training and during the test, they examined if the different sensitivity to devaluation of animals trained on ratio and interval schedules could be explained by a floor effect, i.e. that the random interval trained animals would not show devaluation because they could not decrease their lever pressing further. This was not the case, since when the performance was normalized to the amount of pressing during the last training day the same results were observed. Furthermore, no correlation was found between lever pressing during training or testing and the amount of devaluation for each of the training schedules. On the contrary, there was a significant negative correlation between the total number of lever presses during devaluation and the amount of devaluation in interval schedule trained animals, indicating that animals that pressed less were the ones that devalued more. Therefore, Hilario et al. (2007)
confirmed previous observations in rats that random interval schedules favor habit formation while random ratio schedules favor goal-directed behavior (Adams, 1982
; Dickinson, 1985
; Dickinson et al., 1983
), and showed that these schedules of reinforcement can be used to study habit formation in mice.
Hilario et al. (2007)
also introduced a new assay which investigates how much the animals explore or generalize to a novel lever. This test was designed based on the assumption that the shift from goal directed responding to habitual responding corresponds to a shift from actions being driven by the expected value of the outcome and the contingency between action and outcome (A-O relation) to actions being elicited by antecedent stimuli (S-R relation) (Balleine and Dickinson, 1994
) (Figure 1
C). They reasoned that if in habitual animals the response is being elicited by antecedent stimuli, then if they would be given a choice between pressing the training lever or a novel lever that is similar to the training lever but just in a different location, the mouse will show a tendency to generalize and thus press the novel lever. Conversely if goal-directed actions are being driven by the relation between the action and the outcome, the mouse should press more on the training lever and very little on the novel lever, which was never paired with the outcome. They showed for the first time that random interval schedules known to promote habit formation favor relatively more exploration of a novel lever in relation to those mice trained under random ratio schedules, which favored discrimination of the actions and exploitation of the reinforced lever (Figure 2
E).
These results suggest that, in ratio trained animals, behavior is governed by the action-outcome relation, while in random interval trained animals, behavior is governed more by a stimulus-response relation. Hilario et al. (2007)
concluded that the reinforcement schedules could be presented as useful tools in studying the molecular, cellular, and circuit mechanism of goal-directed actions and habit formation in mice. Furthermore, they suggested that the generalization/exploration test could be a complement to the devaluation test in mutant animals that may have different metabolism, different sensitivities to satiety, or different sensitivities to food reward. However, it still remains to be determined if the processes and the neural substrates underlying generalization/exploration in the two-lever choice test and the insensitivity to changes in value in the devaluation test, are similar or different.
The neuroanatomical circuits that support goal-directed actions have been shown to differ from those supporting habitual behavior. Parallel cortico-basal ganglia loops seem to be critical for learning actions in a different manner. While the limbic loops that stream through the Nucleus Accumbens seem to mediate responses in relation to specific stimuli (stimulus outcome relations or pavlovian to instrumental transfer), loops that course through the dorsal striatum seem to be more involved in operant behavior (Parkinson et al., 2002
; Setlow et al., 2002
; Wiltgen et al., 2007
; Yin et al., 2004
, 2005b
). Although the dorsal striatum in rodents is not divided clearly into caudate and putamen, it does have a medial-lateral gradient of connectivity which is similar (but not identical) to the caudate (ventromedial), and putamen (dorsolateral) connectivity in primates (McFarland and Haber, 2000
; Voorn et al., 2004
). The medial portion of the dorsal striatum, which extends ventrally to the limits of accumbens has been shown to receive most of its input from the associative areas of the cortex, (like the caudate), while the dorsolateral striatal region receives input from the sensorimotor areas of the cortex (like the putamen) (Voorn et al., 2004
). The associative cortico-basal ganglia circuits involving the dorsomedial striatum (Yin et al., 2005a
, b
), the pre-limbic cortex (Balleine and Dickinson, 1998
; Corbit and Balleine, 2003
), and the mediodorsal thalamus (Corbit et al., 2003
) have been shown to support the learning and performance of goal-directed behavior, but do not affect habit formation. In contrast, the dorsolateral or sensorimotor striatum (Yin et al., 2004
) and the infralimbic cortex (Killcross and Coutureau, 2003
) have been shown to support the formation of habits (Figure 3
A). Interestingly, the different corticostriatal loops interact with each other (Kasanetz et al., 2008
). Given this, the shift from goal-directed behavior to habitual behavior in interval trained animals has been proposed to reflect a competition between the dorsomedial and the dorsolateral striatum (Yin et al., 2006
), which are involved in these different types of learning respectively.
Figure 3. Gradients of function across the striatum. (A) Scheme depicting the striatal regions shown to be involved in goal-directed actions (A-O) and habits (S-R). DMS-dorsomedial striatum; DLS-dorsolateral striatum. (B) Representation of the striatal areas innervated by dopaminergic neurons from the VTA and the SNc in the rat. (C) The number of dendritic spines in medium spiny neurons increases in DLS and decreases in DMS after chronic exposure to methamphetamine. (D) Gradient of expression of CB1 receptors in the striatum. The references for each panel are given.
Cocaine self-administration in primates has been shown to progressively activate the limbic, associative and sensorimotor areas of the striatum (Porrino et al., 2004
), and administration of cocaine in rats induced a shift in task-related activity from ventromedial to dorsolateral striatum (Takahashi et al., 2007
). Interestingly, the projection of dopaminergic neurons to striatum also follows an interesting gradient with dopaminergic neurons projecting from the substantia nigra pars compacta (A9) targeting more the dorsolateral striatum, and dopaminergic neurons projecting from the ventral tegmental area (A10) targeting more the ventromedial striatum, nucleus accumbens (Moore et al., 2001
), and frontal cortices (Figure 3
B). Consistently, lesions of the nigrostriatal input to the dorsolateral striatum (Faure et al., 2005
), and infusion of dopamine into the ventral medial prefrontal cortex seem to impair habits and favor goal-directed behavior (Hitchcott et al., 2007
).
The dopamine transporter (DAT), the main target of cocaine, is highly expressed in the dorsolateral striatum, and less expressed in more medial and ventral regions of the striatum and in the pre-frontal cortex, where Catechol-O-methyl transferase (COMT) is more prevalent (Arbuthnott and Wickens, 2007
; Matsumoto et al., 2003
). Sensitization with amphetamine, which also acts on the dopamine transporter, can increase dendritic spine density in medium spiny neurons (MSNs) in the dorsolateral striatum (Jedynak et al., 2007
), which is necessary for habit formation, and at the same time decrease spine density in the dorsomedial striatum, which is critical for goal-directed instrumental behavior (Figure 3
C). Consistently, amphetamine sensitization favors a shift from goal-directed to habitual behavior (Nelson and Killcross, 2006
; Nordquist et al., 2007
).
In addition, LTP was found to occur more easily in the dorsomedial striatum, while LTD has been shown to be easier to induce in the dorsolateral striatum (Partridge et al., 2000
). Interestingly, striatal LTD was found to depend on CB1 receptor activation, the primary molecular target in the brain of endocannabinoids (Gerdeman and Lovinger, 2001
; Gerdeman et al., 2002
). Endocannabinoid release in the striatum has been shown to be modulated by dopamine signaling (Giuffrida et al., 1999
; Kreitzer and Malenka, 2005
; Yin and Lovinger, 2006
). Intriguingly, recent studies have shown that amphetamine sensitization depends on endocannabinoid signaling through CB1 receptors in the dorsal striatum (Corbille et al., 2007
), which raises the possibility that the effects of amphetamine in predisposing for habit formation could be mediated by endocannabinoid signaling. Furthermore, the expression of CB1 receptors across the striatum displays a medial-lateral gradient of increased expression, with the highest expression in the dorsolateral striatum (Gerdeman et al., 2003
; Herkenham et al., 1991
), which has been shown to be necessary for habit formation (Figure 3
D). Moreover, signaling through the cannabinoid receptor type 1 (CB1) has been implicated in reward and addiction (Caille et al., 2007
; Casadio et al., 1999
; Cossu et al., 2001
; De Vries et al., 2001
; Di Marzo et al., 2001
; Gerdeman et al., 2003
; Hansson et al., 2007
; Houchi et al., 2005
; Sanchis-Segura et al., 2004
; Wang et al., 2003
). This long line of evidence may suggest a possible role of endocannabinoid signaling in habit formation.
To study if habit formation is dependent upon endocannabinoid signaling, Hilario et al. (2007)
employed mice with genetically targeted mutations in the CB1 gene (Zimmer et al., 1999
). Three groups of mice, wild-type (WT), CB1+/−, and CB1−/− littermates, were trained on a random interval schedule, previously shown by the authors to promote habitual behavior. Hilario et al. (2007)
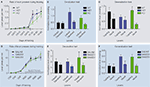
demonstrated that, independent of the genotype, all animals were capable of learning to press for reinforcements in a similar manner (Figure 4
A). However, when tested on the devaluation test, while WT mice showed insensitivity to change in value of the outcome and thus habitual behavior, both CB1+/−, and CB1−/− mutants showed sensitivity to sensory-specific satiety, suggesting that their actions were still goal-directed (Figure 4
B). These results were further confirmed using the exploration/generalization test. During the choice test, WT mice pressed equally the training lever and a novel lever similar to the training lever (generalization/exploration) suggesting that their actions were habitual. However, CB1−/− mutant mice pressed preferentially the training lever suggesting that their actions were driven by the relation between action and outcome (discrimination/exploitation) (Figure 4
C).
Figure 4. Decreased predisposition for habit formation in CB1 mutant mice and in C57Bl6/J mice injected with CB1 antagonists during random interval training. (A) Acquisition of the lever pressing task in WT, CB1+/− and CB1−/− mice trained in a random interval schedule. The rate of lever pressing (per minute) for each daily session is depicted. (B) Normalized lever pressing during the valued versus the devalued condition for WT, CB1+/− and CB1−/− mice. (C) Lever pressing (normalized) on the training lever versus a novel lever in WT, CB1+/− and CB−/− mice. (D) Acquisition of the lever pressing task for animals injected with saline, 3 mg/kg AM251 or 6 mg/kg AM251. The rate of lever pressing (per minute) for each daily session is depicted. Note that animals were only injected during RI-30 and RI-60 training. (E) Normalized lever pressing during the valued versus the devalued condition for mice injected with saline, 3 mg/kg AM251 or 6 mg/kg AM251. (F) Lever pressing (normalized) on the training lever versus a novel lever in mice injected with saline, 3 mg/kg AM251 or 6 mg/kg AM251. The devaluation and generalization tests were performed without drug treatment.
CB1 receptors have been shown to be important for development, feeding behavior, and reward (Caille et al., 2007
; Di Marzo et al., 2001
; Sanchis-Segura et al., 2004
). To prevent conclusions that could be confounded by possible chronic developmental or behavioral abnormalities in the CB1 knockout mice, Hilario et al. (2007)
ran another set of experiments using acute pharmacological blockade of CB1 receptors. CB1 receptors were blocked specifically during the random interval schedule training sessions with two different doses of the CB1 receptor antagonist AM251 (Figure 4
D). The devaluation and generalization tests that followed were performed in the absence of drug. Hilario et al. (2007)
showed that the mice injected with the CB1 antagonist during training were still sensitive to manipulations of outcome value and displayed a higher tendency to exploit the trained lever, while animals injected with saline during training were habitual in both tests (Figure 4
E,F). These results indicate that CB1 activation is necessary during training but not during testing, and that the decreased predisposition observed in CB1 knockout mice is not likely attributable to developmental abnormalities or altered CB1 signaling during feeding on the devaluation test.
To summarize, genetic knockout and pharmacological blockade of CB1 receptors consistently impaired habit formation and the development of a stimulus-response behavioral pattern, providing evidence for the critical role of endocannabinoid signaling in habit formation.
Hilario et al. (2007)
showed that endocannabinoid signaling through CB1 receptors is critical for habit formation. This finding opens new lines of questioning, such as where and how CB1 signaling operates to promote habit formation. Endocannabinoids in the brain can function as retrograde messengers, modulating the release of different neurotransmitters, and producing short-term and long-term depression of excitatory and inhibitory transmission (Gerdeman et al., 2002
; Kreitzer and Regehr, 2001
; Wilson and Nicoll, 2001
; Yin and Lovinger, 2006
). Although CB1 receptors are one of the most-abundant G-protein coupled receptors in the brain and are expressed almost ubiquitously, we have already described the dorsolateral striatum as a good candidate for the “where” question. In the dorsolateral striatum CB1 receptors could serve to decrease “competing” glutamatergic inputs to MSNs by inducing depression at these synapses (Gerdeman et al., 2002
; Huang et al., 2001
). However, CB1 receptor activation is also important for the depression of inhibitory inputs in the dorsolateral striatum (Adermark and Lovinger, 2007
), suggesting it could potentially reduce lateral inhibition between MSNs or reduce inhibition of MSNs by fast-spiking interneurons. Interestingly, a combination of depression of “competing” excitatory inputs and reduction in lateral inhibition could facilitate the firing of groups of neurons that are preferentially connected, like a cell assembly (Carrillo-Reid et al., 2008
), with less interference from the cortex and competing cell assemblies in the striatum. CB1 mediated long-term depression in the striatum is expressed by a decrease in presynaptic release probability, which is manifested by a decrease in amplitude of spontaneous excitatory postsynaptic currents, but also by an increase in paired pulse facilitation (a second afferent stimulation given within a certain time window of the first produces a larger response). Therefore, another interesting possibility is that endocannabinoid signaling through CB1 receptors acts as a filter to increase signal to noise, since after the induction of pre-synaptic depression the postsynaptic neuron would listen preferentially to bursts of inputs rather than single inputs.
CB1 is also expressed heavily in the distal terminals of the MSNs from the direct and indirect pathway, which synapse onto the substantia nigra pars reticulate and the globus pallidus, respectively (Sanudo-Pena et al., 1999
). Therefore, since MSNs are inhibitory projection neurons, it is possible that endocannabinoid signaling through CB1 receptor activation is necessary to disinhibit basal ganglia nuclei downstream of the striatum. Another intriguing possibility is that CB1 mediated signaling modulates the strength of excitatory and inhibitory synaptic inputs onto dopaminergic neurons (Lupica and Riegel, 2005
; Szabo et al., 2002
). It has been shown that endocannabinoids are released in response drugs of abuse (Caille et al., 2007
), and that the transient increases in dopamine release by drugs of abuse are mediated by CB1 receptors (Cheer et al., 2007
). Since CB1 receptor blockade diminishes the effects of several drugs of abuse on dopamine release (Cheer et al., 2007
), one possibility is that endocannabinoid-mediated inhibition of GABA release onto dopamine neurons is necessary for dopaminergic neurons to increase firing and release dopamine onto downstream targets like the dorsolateral striatum, where dopamine has been shown to be necessary for habit formation (Faure et al., 2005
; Nelson and Killcross, 2006
; Szabo et al., 2002
).
Hilario et al. (2007)
demonstrated that endocannabinoid signaling is necessary for the development of habitual behavior. Precisely how endocannabinoids modulate striatal information processing in vivo and interact with other neurotransmitter systems, such as glutamate, acetylcholine, and dopamine, is still a matter for much needed research. If endocannabinoids are indeed involved in the balance of the neural mechanisms that underlie our vulnerability to develop habits, drug seeking behaviors, compulsions, or even other striatal-based pathologies, their understanding is of the utmost importance to the formulation of more adequate treatments. Because current research has suggested that the endocannabinoid system can control the dopamine system and vice versa, the blockade of CB1 receptors has been targeted as a potential therapeutic approach for pathological conditions that involve dopamine-related imbalances. The drug Rimonabant, a CB1 antagonist, has been employed in the treatment of addiction (Cahill and Ussher, 2007
), and has been proposed to function by reducing the levels of dopamine in the motivation centers of the brain, which are triggered by addictive drugs. This drug class has been shown to induce a decrease in drug rewarding effects, to reduce the influence of drug-associated stimuli, and to lower the relapse rates of drugs such as opioids, cocaine, nicotine, ethanol and amphetamine (De Vries et al., 2001
; Le Foll et al., 2008
). It has also been proposed that manipulations of endocannabidoing signaling through CB1 could be beneficial in other striatal involving disorders like Parkinson’s disease (Garcia-Arencibia et al., 2008
; Kreitzer and Malenka, 2007
). In the future, it will be important to investigate the brain region and cell types where CB1 signaling is required for its effects, to not only define how endocannabinoids contribute to normal behavior, but to also understand how therapies can be customized to specific pathologies.
Hilario et al. (2007)
demonstrate that training paradigms using different reinforcement schedules are useful tools for studying the molecular, cellular, and circuit mechanisms of goal-directed actions and habit formation in mice. Furthermore, they introduced a novel experimental behavioral tool, the generalization/exploration assay, which can be used complementarily with devaluation and contingency degradation assays to measure behavioral changes during habit formation. Using these paradigms for examining habit formation in mice, the authors showed using genetic and pharmacological tools that endocannabinoid signaling through CB1 receptors is necessary at the time of training for habit formation to occur.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank C. Gremel and E. Dias-Ferreira for helpful comments on this manuscript. This work was supported by the NIAAA DICBR.
Hansson, A. C., Bermudez-Silva, F. J., Malinen, H., Hyytia, P., Sanchez-Vera, I., Rimondini, R., Rodriguez de Fonseca, F., Kunos, G., Sommer, W. H., and Heilig, M. (2007). Genetic impairment of frontocortical endocannabinoid degradation and high alcohol preference. Neuropsychopharmacology 32, 117–126.
Parkinson, J. A., Dalley, J. W., Cardinal, R. N., Bamford, A., Fehnert, B., Lachenal, G., Rudarakanchana, N., Halkerston, K. M., Robbins, T. W., and Everitt, B. J. (2002). Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav. Brain Res. 137, 149–163.