1
Department of Neuroanatomy, Max-Planck-Institute for Brain Research, Frankfurt, Germany
2
Ratiopharm GmbH, Ulm, Germany
3
Department of Anatomy and Cell Biology, Wayne State University, School of Medicine, Detroit, USA
4
Department of Ophthalmology, School of Medicine, University of California, San Francisco, USA
5
Physiological Institute, Universität des Saarlandes, Homburg/Saar, Germany
6
Department of Pharmacology, The School of Pharmacy, University of London, London, UK
Glycine and γ-aminobutyric acid (GABA) are the major inhibitory neurotransmitters in the retina. Approximately half of the amacrine cells release glycine at their synapses with bipolar, other amacrine, and ganglion cells. Glycinergic amacrine cells are small-field amacrine cells with vertically oriented dendrites and comprise more than 10 different morphological types. The retinal distributions of glycine receptor (GlyR) α1, α2, α3 and α4 subtypes have been mapped with subunit-specific antibodies. GlyRs were clustered at postsynaptic hot spots which showed selective distributions for the different subunits. As a rule, only one α subunit was expressed at a given postsynaptic site. The kinetic properties of GlyRs were measured by recording spontaneous inhibitory postsynaptic currents (sIPSCs) from identified retinal neurons in wild-type, Glra1spd-ot, Glra2 and Glra3 knockout mice. From observed differences of sIPSCs in wild-type and mutant mice, the cell-type specific subunit composition of GlyRs could be defined. OFF-cone bipolar cells and A-type ganglion cells receive prominent glycinergic input with fast kinetics that is mainly mediated by α1β GlyRs (decay time constant τ ∼ 5 ms). By contrast, AII amacrine cells express α3β GlyRs with medium fast kinetics (τ ∼ 11 ms). Narrow-field (NF) and wide-field amacrine cells contain predominantly α2β GlyRs with slow kinetics (τ ∼ 27 ms). Lastly, ON-starburst, narrow-field and wide-field amacrine cells in Glra2 knockout mice express α4β GlyRs with very slow kinetics (τ ∼ 70 ms).
Glycine and GABA, the major inhibitory transmitters of the mammalian retina, are preferentially localized in different types of amacrine cells which fulfill specific roles in the processing of visual signals (Pourcho, 1996
). GABA-ergic amacrine cells are wide-field amacrine cells providing lateral interactions across the inner plexiform layer (IPL; Lin and Masland, 2006
). They are involved in the generation of receptive field surrounds (Flores-Herr et al., 2001
) and in the computation of direction selective light responses (Taylor and Vaney, 2003
). Glycinergic amacrine cells are small-field cells, whose dendrites are primarily involved in local interactions between different sublaminas of the IPL, such as the OFF- and ON-sublamina (Hsueh et al., 2008
).
Glycine has been localized in 40–50% of all retinal amacrine cells (Marc, 1989
; Pourcho, 1996
; Pow and Hendrickson, 1999
), which exist in more than 10 distinct morphological types (Badea and Nathans, 2004
; MacNeil and Masland, 1998
; Menger et al., 1998
; Vaney, 1990
). They receive synaptic input from bipolar cells at ribbon synapses and from other amacrine cells – both GABAergic and glycinergic – at conventional chemical synapses. Their output synapses contact bipolar cells, other amacrine cells and ganglion cells (Jusuf et al., 2005
; Pourcho and Owczarzak, 1991a
,b
; Sassoè-Pognetto et al., 1994
). Some glycinergic amacrine cells bear interplexiform processes ascending towards the outer plexiform layer (OPL; Kolb and West, 1977
).
The diversity of types of glycinergic amacrine cells is paralleled by the striking heterogeneity of glycine receptors (GlyRs). All four α subunits of the GlyR have been localized to specific synapses within the mammalian retina (Haverkamp et al., 2003
, 2004
; Heinze et al., 2007
; Sassoè-Pognetto et al., 1994
). To date, selective agonists or antagonists that distinguish different isoforms of synaptic GlyRs have not been identified (Betz and Laube, 2006
; Harvey and Betz, 2000
; Legendre, 2001
; Lynch, 2004
). However, mutant mice are available that have dysfunction of specific GlyR subunits and thus it became possible to study details of the glycinergic synaptic transmission in the mammalian retina (Ivanova et al., 2006
; Majumdar et al., 2007
; Weiss et al., 2008
).
It has to be emphasized that the morphological types of glycinergic amacrine cells, their circuits, and the distribution of GlyRs are closely similar when different mammalian retinas are compared (Masland, 2001
). In this review we concentrate on the mouse retina because mutants are available that express green fluorescent protein (GFP) in specific types of amacrine cells (Haverkamp et al., 2009
; Heinze et al., 2007
), and also mutants which lack specific GlyR subunits.
There are more than 10 different types of bipolar cells, at least 30 types of amacrine cells and approximately 15 types of ganglion cells in any mammalian retina (Masland, 2001
). They are involved with different retinal circuits and fulfill specific roles in visual processing. In this review it will be shown that they express different sets of synaptic GlyRs.
Localization of Glycine in Amacrine Cells
The first anatomical demonstration of glycine as neurotransmitter in the mammalian retina was through uptake of tritiated glycine followed by autoradiography (Ehinger and Falck, 1971
). Amacrine cells were prominently demarcated, whilst some bipolar cells were weakly labelled. More recent demonstrations of glycine in the retina applied immunolabelling with antibodies against glycine or against the glycine transporter GlyT1 (Menger et al., 1998
; Pow, 1998
; Pow and Hendrickson, 2000
). Figure 1
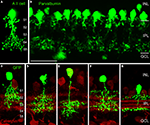
shows a vertical section through a mouse retina that was double immunolabelled for glycine and for GlyT1 (Haverkamp and Wässle, 2000
). Strong glycine immunoreactivity can be observed in amacrine cell bodies and their dendrites descending into the IPL (Figure 1
A). Weak glycine expression is also found in putative ON-cone bipolar cells in the centre of the inner nuclear layer (INL). The section was also immunolabelled for GlyT1 (Figure 1
B) which labels all glycinergic amacrine cells but not bipolar cells (Figure 1
C).
Figure 1. Glycinergic amacrine cells of the mouse retina (modified from Haverkamp and Wässle, 2000
). A vertical section was double immunolabelled for glycine (red) and the glycine transporter GlyT1 (green). (A) Strong glycine immunofluorescence is found in amacrine cells at the INL/IPL border. Weak glycine immunofluorescence is also present in the cell bodies of ON-cone bipolar cells in the center of the INL. (B) GlyT1 immunofluorescence is found in amacrine cell bodies and their dendrites descending into the IPL. (C) The superposition of (A) and (B) shows that only the amacrine cells but not the bipolar cells express GlyT1. The arrows indicate an interplexiform process ascending to the OPL (OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer; GCL; ganglion cell layer; Scale bar: 25 μm).
Bipolar cells do not express GlyT1 but they receive glycine by diffusion through electrical synapses (gap junctions) from glycinergic amacrine cells (Vaney et al., 1998
). However, there is no evidence that bipolar cells release glycine in addition to L-glutamate, the well established transmitter released by bipolar cells. In other parts of the CNS, GlyT1 has been localized to glial cells, whilst GlyT2 is now known to represent the presynaptic neuronal glycine transporter (Zafra et al., 1995
). Surprisingly, GlyT2 does not appear to be expressed in the mammalian retina (Zafra et al., 1995
). Thus, uptake of tritiated glycine, glycine immunolabelling and GlyT1 expression all indicate that half of the amacrine cells of the mammalian retina are glycinergic (Marc and Liu, 1985
; Pow and Hendrickson, 1999
; Wässle et al., 1986
) and that this is the main source of releasable glycine in the retina.
Morphological Types of Glycinergic Amacrine Cells
The most prominent and also most numerous glycinergic amacrine cell is the AII amacrine cell which transfers the light signal from rod bipolar cells into the cone pathway (Kolb and Famiglietti, 1974
). Figure 2
A shows the typical bistratified morphology of a neurobiotin injected AII amacrine cell from the mouse retina and Figure 2
B shows the dense array of AII cells immunostained for parvalbumin in the rat retina. In the outer IPL, AII cell lobular dendrites provide glycinergic, chemical output synapses onto OFF-cone bipolar cell axon terminals. In the inner IPL, AII cells receive input from rod bipolar cells and are engaged via electrical synapses (gap junctions) with ON-cone bipolar cell axon terminals. Further glycinergic, small-field amacrine cells were identified in the cat retina by combined Golgi-staining and glycine uptake (Pourcho, 1980
; Pourcho and Goebel, 1985
). In a systematic survey, Menger et al. (1998)
identified at least eight different glycinergic amacrine cells in the rat retina. More recent studies on glycinergic amacrine cells in the retina have utilised a transgenic mouse (GFP-O) which expresses green fluorescent protein (GFP) under the control of the thy-1 promoter (Feng et al., 2000
; Heinze et al., 2007
). Five such cells are illustrated in Figures 2
C–G, with double labelling for calretinin in order to reveal the different sublaminas of the IPL. Although they are all small-field amacrine cells, the level of stratification of their dendrites within the IPL is clearly different. The cells in Figures 2
C–E have small, diffuse dendritic trees confined to the outer, middle and inner IPL, respectively. By contrast, the cells in Figures 2
F,G have a bistratified appearance. More than 10 distinct types of glycinergic amacrine cells have been identified from such morphological criteria (MacNeil and Masland, 1998
) and more may be found in future studies.
Figure 2. Morphological types of glycinergic amacrine cells (modified from Heinze et al., 2007
; Weiss et al., 2008
). (A) Vertical section of an AII amacrine cell of the mouse retina that was filled with neurobiotin during patch-clamp recordings. The IPL is subdivided into 5 sublaminas of equal thickness. (B) The array of AII amacrine cells of the rat retina immunostained for parvalbumin (Scale bar: 25 μm). (C–G) Glycinergic amacrine cells expressing GFP (green fluorescent protein) in the thy1-GFP-O mouse (Feng et al., 2000
). (C) Type 2 cell. (D) Type 3 cell. (E) Type 4 cell. (F) Type 7 cell (all according to the scheme of Menger et al., 1998
). (G) A8 cell (according to Kolb et al., 1981
).
Synaptic Localization of Glycine Receptors
The postsynaptic glycine receptor (GlyR) is a ligand-gated chloride channel composed of ligand-binding α and β subunits. The β subunits bind to the receptor clustering protein gephyrin (reviewed by Harvey and Betz, 2000
; Legendre, 2001
; Lynch, 2004
; Vannier and Triller, 1997
). Molecular cloning has revealed four genes encoding the α subunits (α1, α2, α3, α4) and only one gene encoding the β subunit (Harvey et al., 2000
). In the adult two copies of the α subunit and three copies of the β subunit form the pentameric receptor protein (Grudzinska et al., 2005
). Subunit selective antibodies have recently become available that recognize the four GlyR α subtypes (Harvey et al., 2004
; Haverkamp et al., 2003
, 2004
; Heinze et al., 2007
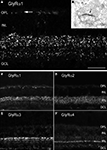
). When these antibodies were applied to lightly fixed sections of the mammalian retina, they each produce a distinct punctate immunofluorescence pattern (Figure 3
). Electron microscopy has suggested that these puncta represent clusters of GlyRs at postsynaptic sites (Figure 3
B; Sassoè-Pognetto et al., 1994
). The GlyR α1 subunit is expressed in a sparse population of puncta in the OPL, which represent synapses between glycinergic interplexiform processes and bipolar cell dendrites (Figures 3
A,C). In the outer IPL (stratum S1 and S2) GlyR α1 immunoreactivity is found in large puncta, which occur at high density. They represent synapses between AII amacrine cells and OFF-cone bipolar cells (Sassoè-Pognetto et al., 1994
). In the inner IPL (stratum S3–S5) smaller GlyR α1 immunoreactive puncta can be observed representing synapses onto ganglion cell dendrites and rod bipolar cell axons (Ivanova et al., 2006
; Majumdar et al., 2007
). The GlyR α2 subunit is more uniformly distributed across stratum 1–4 (Figure 3
D), and GlyR α2 immunoreactive puncta occur at the highest density amongst the four α subunits (Haverkamp et al., 2004
). The GlyR α3 subunit (Figure 3
E) shows four bands of higher density of puncta (Haverkamp et al., 2003
). Lastly, the GlyR α4 subunit (Figure 3
F) shows a band of high density of puncta at the border between stratum 3 and 4 (Heinze et al., 2007
). This characteristic distribution of subunits across the IPL suggests that the GlyR subtypes are expressed at different synapses and are involved with different neuronal circuits.
Figure 3. Synaptic localization of GlyR subtypes in the rodent retina (modified from Heinze et al., 2007
; Sassoè-Pognetto et al., 1994
). (A) Fluorescence micrograph of a vertical section through a rat retina that was immunostained for the GlyR α1 subunit. The arrow points to a few synaptic clusters in the OPL. (B) Electron micrograph showing a synapse (arrow) expressing the GlyR α1 subunit. The antibody (mAb2b) recognises an extracellular epitope and, therefore, the synaptic cleft is immunolabelled. (C–F) Vertical sections through the mouse retina. (C) GlyR α1 immunoreactive puncta are most prominent in the outer IPL. (D) GlyR α2 immunofluorescence is more evenly distributed across the IPL. (E) GlyR α3 expression is found in four bands. (F) GlyR α4 immunoreactivity is most prominent in a small band at the sublamina 3/4 border [Scale bar: 25 μm in (A); 65 μm in (C–F)].
Co-Localization of GlyR Subunits at Postsynaptic Sites
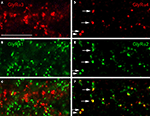
Since synaptic GlyRs are composed of 2α and 3β subunits (Grudzinska et al., 2005
) there is the possibility of two different α subunits co-existing in a single heteromeric GlyR. In addition, it is possible that two different GlyR subtypes, such as α2β and α3β GlyRs, co-distribute at the same postsynaptic sites. In both cases, the immunoreactive hot spots should coincide. However, when retinal sections were double labelled for the GlyR α1 subunit and the other three GlyR α subunits, no statistically significant coincidence rate of immunoreactive puncta was observed (Figures 4
A–C). When retinal sections were double labelled for the GlyR α2 and α3 subunits a coincidence rate of 26.7% was found (Haverkamp et al., 2004
). In retinal sections double labelled for the GlyR α3 and α4 subunits no significant coincidence rate was found (Heinze et al., 2007
). In sections double labelled for the GlyR α4 and α2 subunits, 31.5% of the α4 immunoreactive clusters also contained the α2 subunit (Figures 4
D–F). The results indicate that – as a rule – postsynaptic GlyR clusters contain only one type of α subunit. The exception is approximately one-third of synapses immunoreactive for GlyR α2 that can also contain the α3 or the α4 subunits.
Figure 4. Colocalization of GlyR subunits at postsynaptic sites (modified from Haverkamp et al., 2003
; Heinze et al., 2007
). (A) GlyR α3 immunoreactive puncta. (B) Same section as in (A), immunostained for GlyR α1. (C) Superposition of (A) and (B) shows that GlyR α3 and GlyR α1 immunoreactive puncta are not colocalized. (D) GlyR α4 immunoreactive puncta. (E) Same section as in (D), immunostained for GlyR α2. (F) superposition of (D) and (E) shows that GlyR α2 and GlyR α4 immunoreactive puncta sometimes colocalize (arrows). (Scale bar: 10 μm).
Expression of GlyRs by Identified Neurons
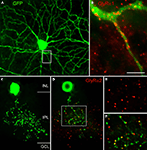
In order to reveal the involvement of selected GlyR subtypes with different retinal circuits, identified neurons were immunostained for the different GlyR α subunits. Figure 5
A shows an EGFP labelled A-type ganglion cell in a whole mount of the thy-1 GFP-O mouse retina (Majumdar et al., 2007
). The retina was also immunolabelled for the GlyR α1 subunit (Figure 5
B) demonstrating that many GlyR α1 immunoreactive puncta decorate the dendrites of this A-type ganglion cell. This suggests the cell receives glycinergic input through synapses that contain the GlyR α1 subunit. A-type ganglion cells were also double labelled for the other α subunits (data not shown), and a small number of puncta coincided with the A-type dendrites. Quantification of these results showed that the predominant input is through GlyR α1 containing synapses. However, there is also a small but significant input through synapses expressing the other GlyR α subunits (Majumdar et al., 2007
).
Figure 5. Expression of GlyRs by identified neurons (modified from Majumdar et al., 2007
). (A) This A-type ganglion cell expressed EGFP in a whole mount of the thy1 GFP-O mouse (Feng et al., 2000
). The whole mount was also immunostained for GlyR α1. (B) The boxed area from (A) is shown at higher magnification and the dendrite of the A-type ganglion cell is decorated by GlyR α1 immunoreactive puncta. (C) Vertical view of a Type 3 amacrine cell from the thy1 GFP-O mouse retina (collapsed stack of confocal sections). (D) Single confocal section of the cell in (C) also immunostained for the GlyR α2 subunit. The boxed area is shown at higher magnification in (E) and (F). (E) GlyR α2 immunoreactive puncta. (F) Dendritic varicosities and GlyR α2 immunoreactive puncta superimposed [Scale bar: 60 μm in (A), 10 μm in (B), 17 μm in (C) and (D), 10 μm in (E) and (F)].
Double labelling approaches also enable the identification of the presynaptic partner of synaptic GlyRs in the retina. Figure 5
C shows a vertical view of a Type 3, glycinergic amacrine cell in the thy-1 GFP-O mouse retina. A single optical section through this EGFP-expressing cell together with GlyR α2 immunostaining is shown in Figure 5
D. Many GlyR α2 immunoreactive puncta coincide with dendritic varicosities of the Type 3 cell. Since this cell is a glycinergic amacrine cell, the puncta may represent input synapses the cell receives from other, glycinergic amacrine cells or output synapses the cell makes onto other, non-labelled neurons. As can be seen from the magnified micrographs in Figures 5
E,F, the red GlyR α2 immunoreactive puncta are always slightly displaced from the green varicosities. We interpret this result as indicating synaptic GlyR α2 clusters which are expressed by unknown neurons that are postsynaptic to this Type 3 cell.
The two examples of cells presented in Figure 5
suggest a correlation between the morphological type of a given neuron and the molecular signature of the glycinergic synapse it receives or makes. In this context one interesting question is whether the presynaptic neuron instructs the postsynaptic cell to express certain GlyR subunits or whether a given postsynaptic neuron expresses an exclusive GlyR subtype. We addressed this question by carrying out a detailed physiological characterisation of selected synaptic GlyRs.
Glycine Receptors Expressed by Bipolar Cells
Bipolar cells receive glutamatergic, synaptic input from photoreceptors in the OPL and provide synaptic output onto ganglion and amacrine cells in the IPL. Bipolar cell axons in the IPL receive synaptic input from both GABAergic and glycinergic amacrine cells. There are about 10 different types of cone bipolar (CB) and one rod bipolar (RB) cell in the mammalian retina (reviewed by Wässle et al., 2009
). The major subdivision is into OFF- and ON-CB cells, which are hyperpolarized and depolarized, respectively, by a light stimulus. RB cells are ON bipolar cells. Axons of OFF-CB cells terminate in the outer half of the IPL, those of ON-CB and RB cells in the inner half (Wässle, 2004
).
Patch-clamp recordings performed from bipolar cells in slices of the mouse retina (Ivanova et al., 2006
) enabled the study of GlyRs by the application of exogenous glycine, and by recording and analyzing glycinergic spontaneous inhibitory postsynaptic currents (IPSCs) in the presence and absence of selected antagonists. The patch electrode was filled with a solution containing neurobiotin and Alexa 488 which diffused into the bipolar cells during the recordings. The slices were immunostained after the experiments and thus the bipolar cell type could be identified unequivocally. The glycinergic IPSCs measured in wild-type mouse retinas were also compared with those from two mutant mouse retinas, one deficient in the GlyR α1 subunit (Glra1spd-ot, “oscillator”, Buckwalter et al., 1994
; Kling et al., 1997
) and one deficient in the GlyR α3 subunit (Glra3−/−, Harvey et al., 2004
; Haverkamp et al., 2003
). Mice deficient in the GlyR α3 subunit did not show an apparent phenotype (Harvey et al., 2004
; Haverkamp et al., 2003
). Homozygous Glra1spd-ot mice die at about 3 weeks of age (Buckwalter et al., 1994
) and juvenile Glra1spd-ot mice, of the age of 16–18 days, were used for the experiments. The results were compared to measurements in wildtype mice of the same age.
The exogenous application of glycine elicited large-amplitude glycinergic currents in all OFF-CB and RB cells, whilst ON-CB cells exhibited only very small, if any, glycinergic currents (Eggers and Lukasiewicz, 2006
; Ivanova et al., 2006
). Co-application of the selective GlyR antagonist strychnine (3 μM) blocked these glycine-induced currents as expected. Wang and Slaughter (2005)
have also shown that the GABAA receptor antagonists bicuculline and gabazine are also competitive antagonists of homomeric α1 and α2 subunit GlyRs expressed in HEK293 cells or on retinal neurons at high micromolar IC50s. However, glycine-induced currents recorded from bipolar cells were not affected by either bicuculline (100 μM) or gabazine (3 μM) (Ivanova et al., 2006
). It has also been reported (Han et al., 2003
) that DCKA (5,7 dichlorokynurenic acid), an antagonist of the glycine-binding site of NMDA receptors, blocks the slowly desensitizing glycine-induced current in the tiger salamander retina. Picrotoxinin is also a specific blocker of GlyRs in recombinant expression systems (Pribilla et al., 1992
). Application of picrotoxinin (50 μM) reduced the peak currents in bipolar cells to 93% but application of DCKA (500 μM) did not inhibit glycine-induced currents on bipolar cells. These results suggest that bicuculline, gabazine, picrotoxinin and DCKA are not useful pharmacological tools for differentiating the types of GlyRs expressed by bipolar cells.
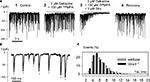
Studies using knockout mice were more revealing. While there was no significant difference between glycine-induced currents from bipolar cells in wild-type and Glra3−/− mice, glycine-induced currents could not be elicited in any bipolar cell in oscillator mice (Glra1spd-ot) even when glycine was applied at a concentration as high as 10 mM. A more detailed analysis of bipolar cell GlyRs involved the study of sIPSCs. Figure 6
A shows a recording of spontaneous IPSCs (sIPSCs) from an OFF CB cell at low temporal resolution. When GABAAR (3 μM gabazine) and the GABACR (100 μM TPMPA) antagonists were applied (Figure 6
A, 2) sIPSCs remained and the frequency was slightly increased. However, when strychnine was co-applied with both GABAR antagonists (Figure 6
A, 3) the sIPSCs completely disappeared, demonstrating that they represent glycinergic sIPSCs. The trace in Figure 6
B shows the IPSCs at higher magnification, enabling the kinetics of single sIPSCs to be analysed. Superimposed events were excluded from the analysis, and only those with monotonic rising phase, lacking inflections and returning to the baseline without contamination from subsequent events were selected (marked by X in Figure 6
B). We found that the decay phase could be described by a single exponential function with a decay time constant τ. In a total of 17 OFF CB cells studied in retinal slices from wild-type mice, the sIPSCs had a mean amplitude of –70 ± 38 pA, a mean rise time of 1.1 ± 0.3 ms and a mean decay time constant τ = 5.9 ± 1.4 ms (total number of OFF CB cells n = 17, total number of IPSCs analysed: N = 2296). Glycinergic sIPSCs were also recorded from OFF-CB cells in retinal slices from Glra3−/− mice. The IPSCs had a mean amplitude of –81.9 ± 34 pA, a mean rise time of 1.1 ± 0.2 ms and a mean decay time constant τ = 5.1 ± 1.0 ms (n = 15, N = 3273). The histogram in Figure 6
C compares the decay time constants of glycinergic IPSCs recorded from wild-type and Glra3−/− mice. No significant difference between the two distributions was found (Kolmogorov-Smirnov test, p > 0.05). The result suggests that the GlyR α3 subunit is not an essential component of GlyRs on OFF-CB cells. We also recorded 17 OFF-CB cells from the retinas from Glra1spd-ot mice, and did not observe glycine-induced currents or glycinergic sIPSCs. By contrast, amacrine and ganglion cells in Glra1spd-ot mice showed both glycine responses and glycinergic sIPSCs (Majumdar et al., 2007
; Weiss et al., 2008
). The total lack of glycine responses in Glra1spd-ot mice suggests that the GlyR α1 subunit is an essential component of GlyRs on OFF CB cells.
Figure 6. Recordings of spontaneous IPSCs from OFF-CB cells of the mouse retina (modified from Ivanova et al., 2006
). (A) At a holding potential of VH = −50 mV, large sIPSCs were recorded (1). Co-application of the GABAR antagonists gabazine and TPMPA (1,2,5,6 tetrahydrpyridine-4-yl-methylphosphonic acid) did not block the IPSCs (2). However, co-application of GABAR antagonists and strychnine (Stry) abolished the IPSCs (3). (B) IPSCs from trace 2 in (A) are shown at higher temporal resolution. IPSCs with monotonic rising and decay phase are marked by X and their decay phase was approximated by a single exponential function with the time constant τ (white lines superimposed on black traces). (C) The histogram represents the relative frequencies calculated from 17 cells recorded in wild-type mice (light bars), and 15 cells recorded from Glra3−/− mice (dark bars).
Glycine Receptors on AII and Narrow-Field Amacrine Cells
Glycinergic amacrine cells comprise at least 10 different morphological types (Figure 2
). The GlyRs expressed by AII amacrine cells and by the narrow-field (NF) amacrine cells Types 5/6 and Type 7 (Menger et al., 1998
) were studied by patch-clamp recordings in mouse retinal slices. During the recordings, the cells were filled with a fluorescent marker (Figures 7
A) to aid morphological identification. Application of exogenous glycine induced Cl−-currents both in AII and NF amacrine cells that were blocked by the co-application of strychnine (3 μM) and reduced by the co-application of picrotoxinin to 73.6 ± 1.8% in AII cells and to 69.9 ± 1.8% in NF cells. Glycine-induced currents were also measured in Glra1spd-ot, Glra2−/− (Weiss et al., 2008
) and Glra3−/− mouse retina. Comparable to Glra3−/− mice, no obvious phenotype was observed in Glra2−/− mice (Weiss et al., 2008
; Young-Pearse et al., 2006
). In the case of NF amacrine cells the currents elicited in the three mutant mouse lines were not different from those measured in wild-type mice. In AII amacrine cells the currents were significantly reduced in Glra3−/− mice but not in the other two mutants.
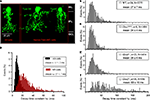
Figure 7. Glycinergic sIPSCs of small-field amacrine cells of the mouse retina (modified from Weiss et al., 2008
). (A) Collapsed stack of confocal sections through an AII amacrine cell (left), a Type 5/6 cell (center) and a Type 7 cell (right). They are referred to as narrow-field (NF) amacrine cells. (B) Frequency histogram of decay time constants of glycinergic sIPSCs recorded from AII cells in wild-type mice (black histogram). The brown histogram shows the sIPSCs recorded from NF cells in wild-type mice. (C) Decay time constants τws of glycinergic sIPSCs recorded from NF amacrine cells of wild-type mice. (D) τws recorded from Glraspd-ot mice. (E) τws recorded from Glra3−/− mice. (F) τws recorded from Glra2−/− mice.
The analysis of sIPSCs revealed significant differences between AII and NF amacrine cells (Figure 7
B). Glycinergic sIPSCs recorded from AII amacrine cells could be approximated by a single decay time constant. The histogram of decay time constant τ from AII amacrine cells showed a peak at ∼10 ms and a mean of 11.2 ± 2.0 ms (n = 33; N = 2407). Glycinergic sIPSCs recorded from NF amacrine cells could be approximated in 80% of the cells by a single exponential function, in 20% of the cells a bi-exponential fit had to be applied from which a single weighted decay time constant was calculated. The histogram of decay time constants τw of NF amacrine cells in Figure 7
C shows a peak at ∼20 ms and a mean of 27 ± 7 ms (n = 24; N = 3275). The difference of the decay time constants between AII cells and NF cells was statistically significant (Weiss et al., 2008
).
In order to reveal the GlyR subunits that are responsible for these differences, sIPSCs were also recorded in Glra1spd-ot, Glra2−/− and Glra3−/− mutant mice. In the case of AII cells, the decay time constants measured in wild-type, Glra1spd-ot and Glra2−/− mice were not significantly different. We also attempted to record glycinergic sIPSCs from AII cells in Glra3−/− mice, however, in a total of n = 50 cells recorded, no glycinergic sIPSCs could be measured. This suggests that the GlyR α3 subunit is a necessary constituent of synaptic GlyRs on AII cells. In the case of NF cells, sIPSCs were measured in all three mutant mice (Figures 7
C–F). No significant differences in the distribution of decay time constant τw were observed when results from wild-type (Figure 7
C), Glra1spd-ot (Figure 7
D) and Glra3−/− (Figure 7
E) mice were compared. By contrast, decay time constants measured from Glra2−/− mice (Figure 7
F) were significantly longer. This difference was highly significant (Kolmogorov-Smirnov p < 0.01) and suggests that the α2 subunit shapes the kinetics of GlyRs in NF amacrine cells.
Glycine Receptors Expressed by other Retinal Neurons
Glycine induced currents and sIPSCs were also recorded from displaced wide-field, putative GABAergic amacrine cells (Majumdar et al., 2009
). These GlyRs had slow kinetics (mean τ > 25 ms; Majumdar et al., 2009
; Veruki et al., 2007
) comparable to NF amacrine cells. ON-starburst (cholinergic amacrine cells) had sIPSCs with extremely long decay time constants (mean τ ∼ 70 ms), which did not differ between wild-type and the three mutant mice (Majumdar et al., 2009
). Since GlyR α4 immunoreactive puncta (Figure 3
F) occur at higher density along the dendrites of ON-starburst amacrine cells, it is possible that GlyRs of ON-starburst cells are dominated by the α4 subunit. This would in turn suggest that GlyRs containing the α4 subunit have slow kinetics.
There are approximately 15 different types of ganglion cells in any mammalian retina. The GlyRs expressed by A-type ganglion cells of the mouse retina were also investigated both in wild-type and mutant mice (Majumdar et al., 2007
). In the wild-type retina, glycinergic sIPSCs of A-type ganglion cells have fast kinetics (mean τ = 3.9 ± 2.5 ms). Glycinergic sIPSCs recorded from Glra2−/− and Glra3−/− mice did not differ from those of wild-type mice. However, the number of glycinergic sIPSCs was significantly reduced in Glra1spd-ot mice and the remaining sIPSCs had slower kinetics. These results show that A-type ganglion cells receive preferentially kinetically fast glycinergic inputs, mediated by GlyRs containing α1 subunits.
All four GlyR α subunits are clustered in synaptic hot spots (Figure 3
) that show characteristic distributions across the IPL of the mouse retina (Heinze et al., 2007
). Gephyrin is responsible for clustering GlyRs to postsynaptic sites by linking the GlyR β subunit to the cytoskeleton (Kirsch et al., 1993
; Vannier and Triller, 1997
). In gephyrin deficient mouse retinas no GlyR clusters could be detected (Fischer et al., 2000
) which suggests that synaptic GlyRs in the retina are always heteromeric, i.e. composed of α and β subunits. In the adult, two copies of the α subunit and three copies of the β subunit form the pentameric receptor protein (Grudzinska et al., 2005
). Thus, it is theoretically possible that two different α subunits are present in a heteromeric GlyR. From double-labelling experiments using subunit-specific antibodies we found that – as a rule – only one type of α subunit is present in a given receptor. However, colocalization of two GlyR α subunits within the same synaptic cluster has also been observed. In the case of the GlyR α2 and GlyR α3 subunits we found a coincidence rate of 26.7% (Haverkamp et al., 2004
), whilst in the case of GlyR α2 and GlyR α4 subunits 31.5% of the GlyR α4 clusters also contained GlyR α2 (Heinze et al., 2007
).
To date, selective agonists or antagonists that distinguish different isoforms of synaptic GlyRs have not been identified (Betz and Laube, 2006
; Harvey and Betz, 2000
; Legendre, 2001
; Lynch, 2004
). However, mutant mice with specific knockouts or spontaneous mutations of GlyR α1, α2 or α3 subunit genes have proven to be useful tools for the analysis of GlyRs expressed by different retinal neurons. If a given retinal cell type expresses exclusively α1β, α2β or α3β synaptic receptors, one would imagine that both glycine-induced currents and glycinergic sIPSCs should be abolished in the corresponding mutant which is the case for bipolar cells of Glra1spd-ot mice. However, the situation is usually more complex, because given retinal neurons express more than one type of synaptic GlyR (Majumdar et al., 2007
, 2009
; Weiss et al., 2008
). These synaptic receptors might be localised at the same postsynaptic site and small glycine-containing vesicles released from the presynaptic terminal could activate both receptors simultaneously. Hence the sIPSC will be composed of the pooled responses of both receptors. However, it is also possible that GlyRs are composed of α2α3β or α2α4β subunits and such receptors may have unusual and characteristic kinetics. Moreover, there may be a compensatory up-regulation of expression of other GlyR subunit genes, ameliorating the loss of the subunit affected by the knockout/mutation (Heinze et al., 2007
). Keeping these provisos in mind, GlyRs of the mammalian retina have the following properties:
The GlyR α1 subunit is clustered in large synaptic hot spots in the OFF-sublamina (Figure 3
A), which represent synapses from AII amacrine cells onto OFF-bipolar cell axon terminals (Sassoè-Pognetto et al., 1994
). GlyR α1 immunoreactive puncta in the inner IPL (Figures 3
A and 5
B) are located on ganglion cell dendrites (Majumdar et al., 2007
). Rod bipolar cells receive input along their axons descending through the IPL, while their axon terminals do not coincide with GlyR α1 hot spots (Ivanova et al., 2006
). Studies with recombinant GlyRs have shown that the expression of the GlyR α1 subunit results in channels with fast kinetics (Betz and Laube, 2006
; Harvey et al., 2000
; Legendre, 2001
; Lynch, 2004
). This is consistent with GlyR-mediated synaptic currents (sIPSCs) recorded from the adult brain stem, where GlyR α1-containing synapses show a fast decay time constant (τ ∼ 6 ms; Singer et al., 1998
). OFF-cone bipolar, rod bipolar and A-type ganglion cells of the retina have GlyR α1-containing synapses which also have fast decay time constants (τ bip ∼ 5.9 ms, τ gang ∼ 3.9 ms; Ivanova et al., 2006
; Majumdar et al., 2007
).
GlyR α2 immunoreactive synapses (Figure 3
D) are distributed more evenly across the IPL and are the most frequent type of glycinergic synapses in this region (Haverkamp et al., 2004
). GlyR α2 is not expressed by bipolar cells (Ivanova et al., 2006
), but is confined to amacrine and ganglion cells. Since in the neonatal brain stem and spinal cord GlyR α2 is the predominant subunit (Becker et al., 1988
; Malosio et al., 1991
; Singer et al., 1998
; Smith et al., 2000
; Takahashi et al., 1992
) it was possible to study the kinetics of synapses containing α2 GlyRs. Spontaneous IPSCs recorded from neonatal GlyRs had slow decay time constants (τ ∼ 14 ms; Singer et al., 1998
). In the retina, narrow-field (NF) amacrine cells receive their predominant glycinergic input through synapses containing GlyR α2 (Figure 7
) and spontaneous IPSCs recorded from these synapses also have slow decay time constants (τ ∼ 27 ms). A-type ganglion cells (Majumdar et al., 2007
) also received a small portion of their glycinergic input through synapses expressing GlyR α2. This was demonstrated both by physiological recordings and immunostaining. In other ganglion cell classes this input through GlyR α2 expressing synapses was more prominent (Majumdar, unpublished results).
GlyR α3 expressing synapses have been described in the dorsal horn of the spinal cord (Harvey et al., 2004
), but their kinetic parameters are not yet known in detail (Heindl et al., 2007
). Synapses containing GlyR α3 in the mouse retina are aggregated in four sublayers of the IPL (Figure 3
E) and their density is reduced along the two sublayers where the dendrites of starburst amacrine cells are found. It was found that dendrites of AII amacrine cells in sublamina 1/2 express GlyR α3 immunoreactive puncta (Haverkamp et al., 2003
). Haverkamp et al. (2003)
observed also GlyR α3 immunoreactive puncta on the axon terminals of OFF-cone bipolar cells. The physiological results, however, did not confirm a glycinergic input of cone bipolar cells that would be mediated by the GlyR α3 subunit (Ivanova et al., 2006
). It is, therefore, possible that the GlyR α3 labeled puncta were not localized on the bipolar cell axon terminals but on nearby amacrine cells processes. A-type ganglion cells too expressed GlyR α3 immunoreactive puncta along their dendrites (Majumdar et al., 2007
). Physiological recordings have shown that AII amacrine cells have glycinergic synapses containing the GlyR α3 subunit (Weiss et al., 2008
). Spontaneous IPSCs recorded from AII cells had decay time constants (τ ∼ 11 ms) which were slower than those of synapses containing GlyR α1 but faster than those harbouring GlyR α2. In the rat retina sIPSCs of AII amacrine cells also exhibited fast kinetics (Gill et al., 2006
).
To date, synapses containing GlyR α4 have only been described in the mammalian retina (Heinze et al., 2007
). They are sparsely distributed across the IPL with a significantly higher density within the sublamina where the processes of ON-starburst amacrine cells ramify. Double labeling experiments with antibodies against choline acetyltransferase and GlyR α4 suggested that processes of ON-starburst cells express GlyR α4 in synaptic hot spots (Heinze et al., 2007
). The kinetics of sIPSCs of synapses containing GlyR α4 can only be estimated from circumstantial evidence. As mentioned above, sIPSCs of NF amacrine cells receive their predominant glycinergic input through synapses harbouring GlyR α2. However, in the Glra2−/− mouse NF amacrine cells still received a small number of slow glycinergic sIPSCs, which are most likely to represent synapses containing GlyR α4 (Figure 7
F). The decay time constants of these slow sIPSCs (τ ∼ 70 ms) were much slower than those recorded from synapses containing GlyR α2 (Weiss et al., 2008
). Recent recordings of glycinergic sIPSCs from ON-starburst cells which are decorated by GlyR α4 immunoreactive puncta have shown that GlyRs of ON-starburst cells in Glra2−/− mice also have slow kinetics (τ ∼ 70 ms). Although they did not differ between wild-type, GlyR α1, and GlyR α3 subunit mutant mice, the final proof that such slow GlyRs contain the α4 subunit will require the generation of Glra4−/− mice.
In conclusion, more insights into the functional role of GlyRs in retinal processing can only come from measurements of light responses. Early experiments in the intact cat eye, involving extracellular recordings from ganglion cells and iontophoretic application of glycine and its antagonist strychnine, showed that the light responses of all ganglion cells become more sustained upon the application of strychnine (Bolz et al., 1985
). With the same approach it was shown that in the dark adapted retina light responses of OFF-ganglion cells were completely blocked by the application of strychnine (Müller et al., 1988
). This confirms that the signal transfer from AII amacrine cells to OFF-cone bipolar cells involves a glycinergic synapse. More recently light responses were studied in retinal slices and retinal whole mounts. Recordings from mouse rod bipolar cells showed that they receive three light driven inhibitory inputs: a fast input mediated by GABAA receptors, an intermediate input through GlyRs and a slow input through GABAC receptors (Eggers and Lukasiewicz, 2006
). Modulating the relative proportions of these inhibitory inputs will change the characteristics of rod bipolar cell output. Recordings from A-type ganglion cells of the mouse retina (Manookin et al., 2008
; Murphy and Rieke, 2008
; van Wyk et al., 2009
) demonstrated that the light driven responses of OFF-A type ganglion cells were mediated by direct glycinergic inhibitory inputs. The light responses of local edge detector (LED) ganglion cells of the rabbit retina are dominated by inhibitory inputs mediated by glycinergic amacrine cells (van Wyk et al., 2006
). These examples show that glycinergic inhibition not only modulates the light responses of retinal neurons but it is also instrumental for creating specificity, preferentially by crossover inhibition between the ON- and the OFF-channels.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Dr. Ulrike Müller, Dr. G. A. O’Sullivan and Dr. Heinrich Betz for providing the Glra3−/− and the Glra2−/− mice, respectively. We thank Brigitte Marshallsay and Brigitte Sinke for excellent technical assistance and Irmgard Odenthal for typing the manuscript.
Harvey, R. J., Depner, U. B., Wässle, H., Ahmadi, S., Heindl, C., Reinold, H., Smart, T. G., Harvey, K., Schutz, B., Abo-Salem, O. M., Zimmer, A., Poisbeau, P., Weltzl, H., Wolfer, D. P., Betz, H., Zeilhofer, H. U., and Müller, U. (2004). GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887.