Hippocampal network activity is transiently altered by induction of long-term potentiation in the dentate gyrus of freely behaving rats
- 1 Learning and Memory Research, Medical Faculty, Ruhr University Bochum, Germany
- 2 International Graduate School of Neuroscience, Ruhr University Bochum, Germany
A role for oscillatory activity in hippocampal neuronal networks has been proposed in sensory encoding, cognitive functions and synaptic plasticity. In the hippocampus, theta (5–10 Hz) and gamma (30–100 Hz) oscillations may provide a mechanism for temporal encoding of information, and the basis for formation and retrieval of memory traces. Long-term potentiation (LTP) of synaptic transmission, a candidate cellular model of synaptic information storage, is typically induced by high-frequency tetanisation (HFT) of afferent pathways. Taking into account the role of oscillatory activity in the processing of information, dynamic changes may occur in hippocampal network activity in the period during HFT and/or soon after it. These changes in rhythmic activity may determine or, at least, contribute to successful potentiation and, in general, to formation of memory. We have found that short-term potentiation (STP) and LTP as well LTP-failure are characterised with different profiles of changes in theta and gamma frequencies. Potentiation of synaptic transmission was associated with a significant increase in the relative theta power and mean amplitude of theta cycles in the period encompassing 300 seconds after HFT. Where LTP or STP, but not failure of potentiation, occurred, this facilitation of theta was accompanied by transient increases in gamma power and in the mean amplitude of gamma oscillations within a single theta cycle. Our data support that specific, correlated changes in these parameters are associated with successful synaptic potentiation. These findings suggest that changes in theta-gamma activity associated with induction of LTP may enable synaptic information storage in the hippocampus.
Introduction
One of the most remarkable features of neuronal networks is their oscillatory activity. In vivo, oscillations cover almost three orders of frequency (Buzsáki and Draguhn, 2004 ) and emerge already at early developmental stages (Khazipov et al., 2004 ). Oscillations dynamically reflect processes ongoing in the neuronal populations and can be considered as “windows to the brain” (Buzsáki, 2006 ). In the hippocampus, one of the most prominent patterns of oscillatory activity is theta rhythm, within the frequency range of 5–10 Hz, the power of which is highest during behavioural states such as exploration or REM sleep, that are often termed “theta-associated behaviour” (Kahana, 2006 ; Buzsáki, 2002 ; Buzsáki, 2005 ). High-frequency gamma oscillations (30–100 Hz) are closely related to theta rhythm and have the highest amplitude in the hippocampus, compared to any other region of the brain (Bartos et al., 2007 ; Csicsvari et al., 2003 ). Gamma oscillations have been implicated in binding features of sensory signals (Singer, 1993 ), consciousness (Llinas et al., 1998 ), selective attention (Fries et al., 2001 ), as well as long-term storage of acquired information (Hermann et al., 2004 ). Together, these oscillations may provide a mechanism of temporal coding of information and the basis for formation and retrieval of memory traces (Axmacher et al., 2006 ; Buzsáki, 2002 ; Csicsvari et al., 2003 ; Hasselmo et al., 2002 ; Kahana, 2006 ; Klimesch, 1999 ; Lisman and Idiart, 1995 ; Lisman, 2005 ; O'Keefe and Burgess, 1999 ; Vertes, 2005 ). Thus, oscillatory activity does not simply comprise a passive reflection of neuronal network function, but may comprise an intrinsic component of this phenomenon.
Long-term potentiation (LTP) of synaptic transmission, which is widely considered as a mechanism of learning (Abraham, 2003 ; Bliss and Collingridge, 1993 ; Lynch, 2004 ; Malenka and Nicoll, 1999 ), typically occurs following high-frequency tetanisation (HFT) of afferent pathways. LTP persists for hours in slice preparations (Frey et al., 1996 ) and days and weeks in vivo (Abraham et al., 2002 , Manahan-Vaughan et al., 1998 ). In freely moving animals, an identical HFT protocol may result in quite different alterations in synaptic strength, ranging from failure to potentiate, through short-term potentiation (STP), and LTP. Importantly, HFT has also been shown to elicit oscillatory responses in vitro (Traub et al., 1999 ; Whittington et al., 1997 ) and in vivo (Vreugdenhil et al., 2005 ). However, it is unclear whether variations in expression of oscillatory activity after tetanisation, particularly in the theta and gamma frequency ranges, may predict or determine the success of LTP induction and comprise a factor in information storage by LTP.
To address this question, we examined the relationship between HFT-induced changes in oscillatory activity and the consequences of HFT for synaptic strength potentiation in freely behaving adult rats. We compared changes in theta–gamma frequency ranges that occurred following HFT that successfully elicited persistent LTP, STP or was ineffective in inducing synaptic plasticity. Our aim was thus to establish whether any association exists between changes in theta–gamma activity and the successful induction of LTP in the hippocampus.
Materials and Methods
Surgery
Seven-to-eight week old male Wistar rats underwent stereotaxic implantation of chronic electrodes into the dentate gyrus (DG) as described previously (Manahan-Vaughan et al., 1998 ). Briefly, under sodium pentobarbitone anaesthesia (“Nembutal”, 40 mg∕kg, i.p., Serva, Germany), animals underwent implantation of a monopolar recording and a bipolar stimulating electrode (made from 0.1 mm diameter Teflon-coated stainless steel wire). A drill hole was made (1.5 mm diameter) for the recording electrode (2.8 mm posterior to, 1.8 mm lateral to the midline) and a second drill hole (1 mm diameter, 6.9 mm posterior bregma to bregma and 4.1 mm lateral to the midline) for the stimulating electrode (coordinates based on Paxinos and Watson, 1986 ). The dura was pierced through both holes and the recording and stimulating electrode lowered into the dentate gyrus (DG) granule cell layer and the medial perforant path, respectively.
To ensure the proper positioning of the electrodes, recordings of evoked field potentials via the implanted electrodes were taken throughout surgery. Potentials evoked by medial perforant path stimulation were distinguished from potentials evoked by lateral perforant path by the following criteria (Abraham and McNaughton, 1984 ): a field excitatory post-synaptic potential (fEPSP) peak latency of ca. 3.0 ms and half-width of ca. 5.0 ms and occurrence of the population spike (PS) within the first positive deflection of the fEPSP. Once verification of the location of the electrodes was complete, the entire assembly was sealed and fixed to the skull with dental acrylic (Paladur, Heraeus Kulzer GmbH, Germany). The animals were allowed between 7 and 10 days to recover from surgery before experiments were conducted. After surgery, animals were housed individually under 12 hours∕12 hours light-dark cycle with ad libitum access to water and food. Experiments were consistently conducted at the same time of day (commencing in third hour of light period). Prior to LTP experiments, basal synaptic transmission (in the absence of HFT) was monitored over a 24 hours period in all animals to confirm stability of evoked responses. At the end of the experimental series, histological verification of the electrode localisations was carried out and animals with inaccurate placements were discarded from the study.
All experimental procedures were carried out in accordance with the European Communities Council Directive 86∕609∕EEC for care of laboratory animals and were approved and authorised by the local Government Committee for Ethics and Animal Research (Bezirksamt Arnsberg).
Measurement of evoked potentials
Throughout experiments the animals could move freely and had free access to water and food. Responses were evoked by stimulating at low frequency (biphasic pulses at 0.025 Hz, 0.1 ms stimulus duration, 16 kHz sample rate). For each time-point, five evoked responses were averaged. Both fEPSP slope and PS amplitude were monitored. The amplitude of PS was measured from the peak of the first positive deflection of the evoked potential to the peak of the following negative potential. fEPSP slope was measured as the maximal slope through the five steepest points obtained on the first positive deflection of the potential. By means of input∕output curve determination the maximum PS amplitude was found for each individual animal, and all potentials employed as baseline criteria were evoked at a stimulus intensity which produced 40% of this maximum, with the same intensity used for HFT.
To induce LTP, HFT (200 Hz, 0.2 ms stimulus duration, 10 bursts of 15 biphasic stimuli with 10 s interburst interval) was applied 30 minutes after injection had occurred, with measurements then taken at t = 5, 10, 15 and then 15 minutes intervals (post-tetanisation) up to 4 hours, with additional measurements taken after 24 hours. In order to reduce (within- and between-group) variability, caused by the influence of behavioural state on LTP induction (Hargreaves et al., 1990 ), HFT was applied in awake immobile rats.
Analysis of network activity
The intrahippocampal electroencephalogram (EEG) was obtained in parallel with fEPSP recordings from the DG granule cell layer. EEG was sampled (0.5 or 1 kHz, gain 100 × , 0.1 Hz–20 kHz) and stored on hard disc for further off-line analysis.
To evaluate the tetanisation-induced changes of oscillatory activity in the theta (5–10Hz) and gamma (30–100Hz) frequency ranges, 4-seconds-long epochs of EEG were cut for each 10 second from five time intervals, which comprised 100 seconds of recording prior to HFT (taken as 100% for further normalisation), a period of 100 seconds during tetanisation, and a period of 300 seconds after application of HFT. Subsequently, a set of digital Finite Impulse Response filters (−3 dB points for band-stop: 48.5–54.4 Hz, band-pass: 4.5–10.5 and 29.5–99.5 Hz; transition gap 2.5 Hz) was applied to extracted epochs, and the processed EEG, as well as the filtered theta and gamma oscillations were stored in separate channels and for later analyses.
Fast Fourier analysis (Hamming window function, 1024 or 2048 frequency bins for 0.5 or 1 kHz records, respectively), as well as calculation of the trough-to-peak amplitude of filtered theta cycles, their duration and mean root mean square amplitude of filtered gamma oscillations per respective cycles was carried out for all artefact-free 4-seconds-long epochs. The absolute values of spectral power, amplitude of theta cycle and mean amplitude of gamma oscillations per theta cycle in artefact-free epochs were normalised for each individual animal to respective mean values for 100-seconds-long pre-HFT period (taken as 100%) and the relative values were used further for statistics. Generally, the results of Fourier analysis of 10 epochs were averaged for each 100-second-long time interval.
EEG data acquisition, digital filtering, Fourier analysis and calculation of the amplitudes for theta cycle and gamma oscillations, were carried out using “Spike2” software (Cambridge Electronic Devices, Cambridge, UK).
Statistical treatment and analysis of data
The statistical treatment and analysis of data included analysis of variance (ANOVA) complemented with the protected Fisher least significant difference (LSD) test. By means of two-way factorial ANOVA, we estimated the effects of tetanisation and time factors. Basing on the criterion of stability of potentiation over time, animals were selected and divided into three groups. The first group consisted of 13 rats with LTP that was stable for 4 hours after HFT and was still present 24 hours later (henceforth referred to as the LTP group). In the second group (six animals), potentiation lasted for 2–3 hours after HFT (STP group). The third group consisted of seven rats that demonstrated failure of potentiation, i.e., potentiation lasting for less than 30 minutes (failed potentiation group). Thus, the effect of the “tetanisation” factor included three levels. Generally, the effect of time included five levels for five periods prior to (100 seconds), during (100 seconds) and after HFT (300 seconds).
The results of analysis were expressed as mean % pre-HFT values ± standard error of the mean (SEM), otherwise mentioned. All statistical treatments and analyses were carried out in STATISTICA data analysis software system (StatSoft, Inc., Tulsa, OK, USA). The probability level interpreted as statistically significant was p < 0.05 (*).
Results
The consequences of HFT on synaptic plasticity in the dentate gyrus
In order to analyse the relationship between changes in hippocampal network oscillations and potentiation of synaptic transmission, we made first sure that the HFT stimulation protocol can induce long-term increases in synaptic efficacy in the DG. Indeed, in the LTP group HFT, at 200 Hz induced robust LTP of a magnitude that remained stable over the recorded period (for both PS amplitude and fEPSP slope) (Figure 1 , filled circles). In the STP group, the same stimulation protocol resulted in a potentiation that endured for approximately 3 hours after HFT (Figure 1 , grey diamonds), whereas in the failed potentiation (FP) group no persistent increase in synaptic transmission was seen (Figure 1 , open squares).
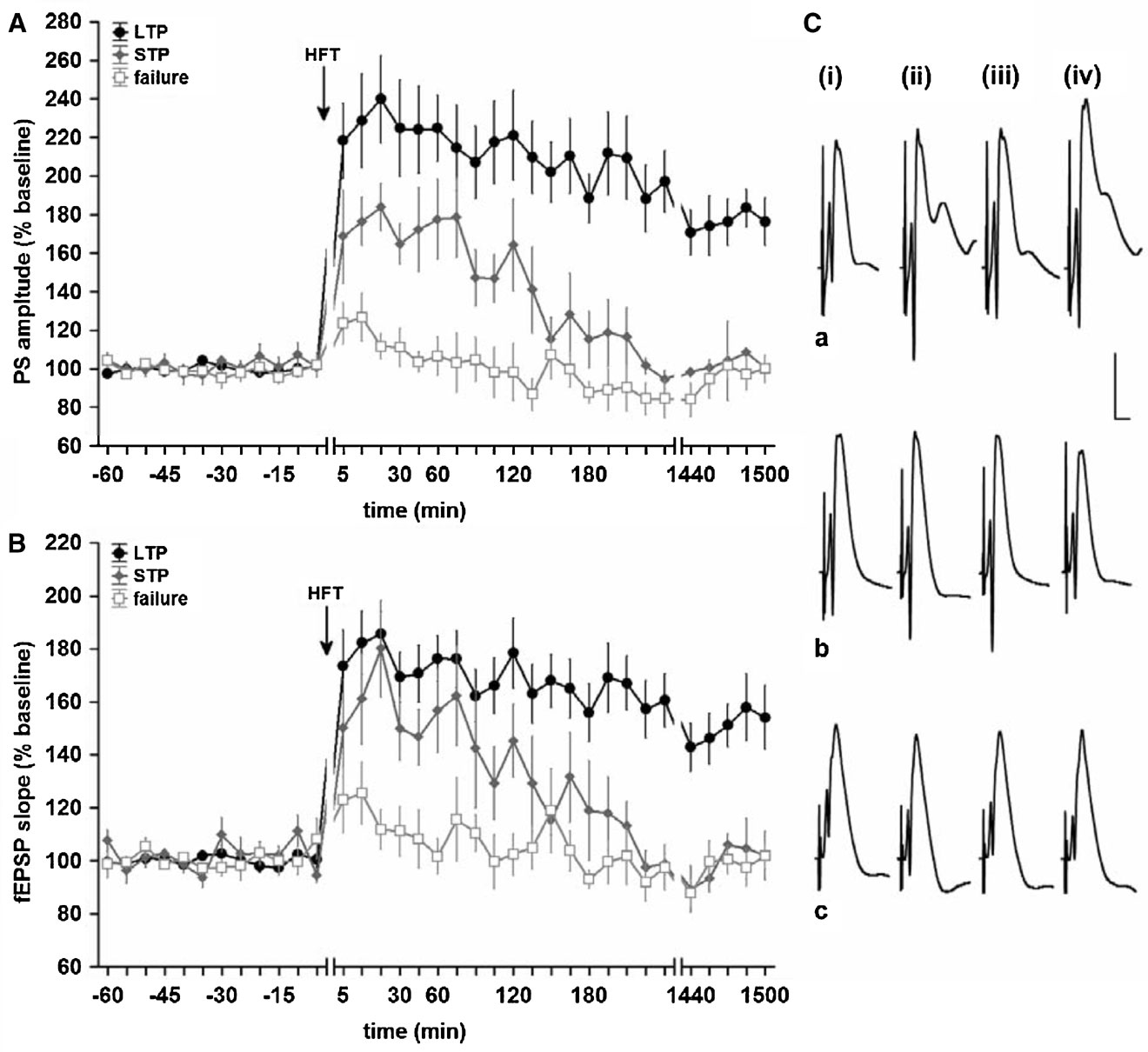
Figure 1. High-frequency tetanisation (200 Hz) results in robust LTP that persists for over 24 hours, short-term potentiation or failure to produce synaptic plasticity. Data are presented as mean ± SEM for PS amplitude (A) and fEPSP slope (B). (C) Original analogue traces showing the field potentials evoked in the DG: (i) pre-HFT, (ii) 5 minutes, (iii) 60 minutes, and (iv) 24 hours following HFT in animals that expressed (a) persistent LTP, (b) STP or (c) no change in synaptic strength. Vertical scale bar corresponds to 5 mV, horizontal scale bar corresponds to 5 ms.
In this study, we did not focus on analysis of the behavioural effects of HFT, but we observed nonetheless that tetanisation does not immediately affect behaviour, although locomotory activity during stimulation varied in general from behavioural arrest and complete immobility to active exploration of the recording chamber. Nevertheless, exploratory behaviour comprising rearings and sniffing, followed by stereotypical behaviour, was generally more typical for the post-HFT period. Consistent with previous reports (Rick and Milgram, 1996 ), the HFT protocol used in the present study evoked no behavioural signs of either focal or secondary-generalised seizure activity, such as head nodding, excessive grooming, “wet dog shake”, etc.
HFT-induced facilitation of theta and gamma oscillations reflects successful potentiation
HFT induces a wide variety of effects and influences the properties of the neuronal membrane, as well as triggering metabolic changes, which require a certain amount of time to happen (Bliss and Collingridge, 1993 ). In order to estimate the prolonged effects of HFT on oscillatory activity, we analysed 4 seconds long epochs pooled for five time intervals covering a period of 100 seconds prior to HFT, a period of 100 seconds for HFT itself and a period of 300 seconds after tetanisation.
Recordings of field network activity revealed that in most animals, HFT bursts were followed by a transient decrease in the amplitude of oscillations (Figure 2 ). In some animals, transient gamma oscillations, comprising a spectral peak of around 70 Hz and a duration up to 350 ms, occurred during this suppression of network activity (Figure 2 B). These gamma oscillations appear to be not exclusively, but nonetheless predominantly associated with LTP, because such activity was observed in five rats from LTP group and one rat from STP group, while it was absent in cases of failed potentiation. Thus, evoked gamma oscillations can be considered as indication or, alternatively, one of the conditions for successful potentiation.
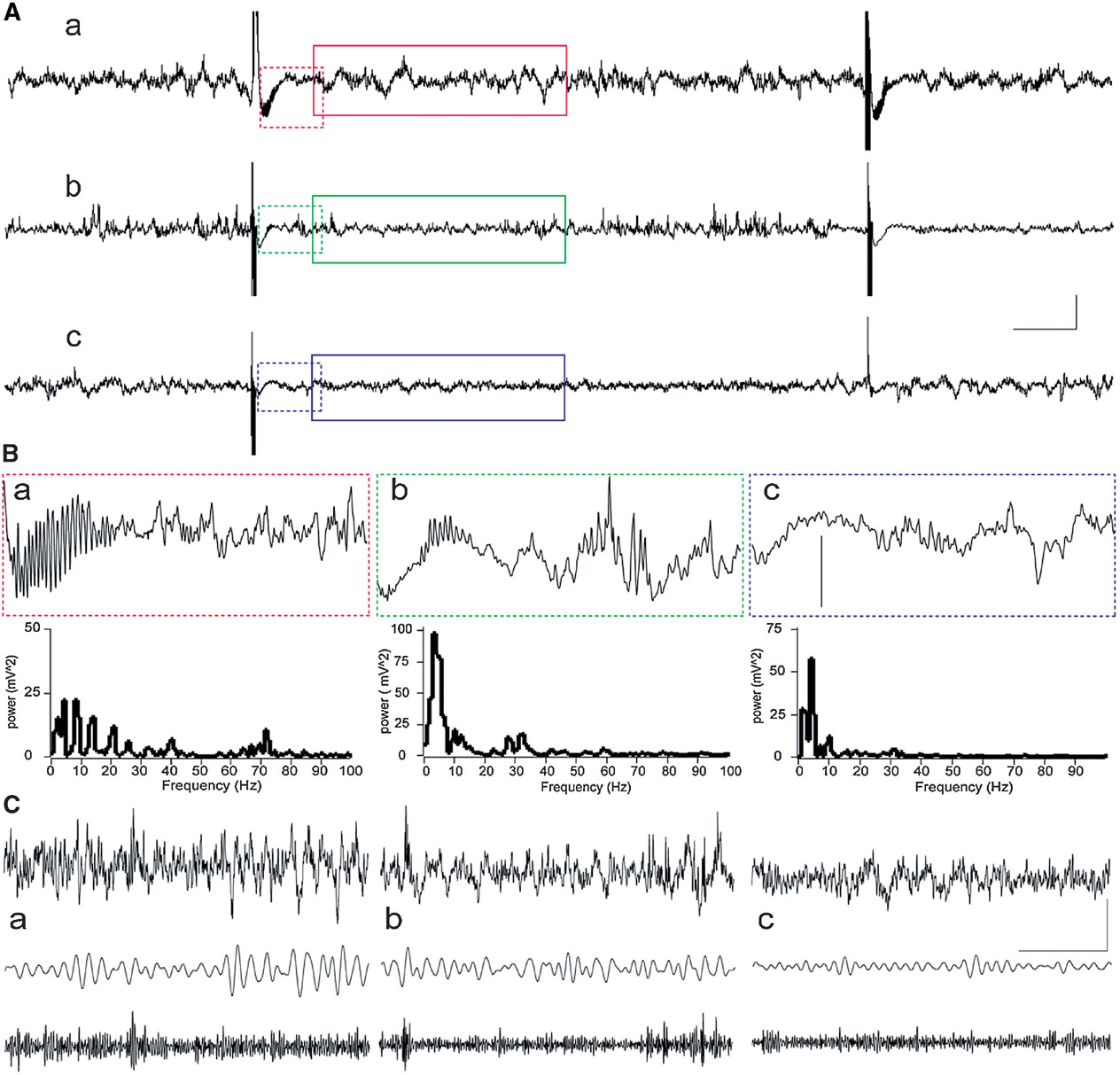
Figure 2. Examples of network activity in the DG during HFT. Raw records with two HFT bursts are shown resulted in LTP (a), STP (b) or failure of potentiation (c). (A) Examples of raw EEG containing two HFT trains delivered at a 10 second interval in an animal that showed further LTP (a), STP (b) or failure of potentiation (c). Scale bars: vertical 0.25 mV; horizontal 1 second. (B) Representative examples of 1-second epochs cut immediately after HFT train and their power spectra (frequencies higher than 100 Hz are not shown). Note a burst of evoked gamma oscillations, which are most prominent in case of LTP (a) than in STP rat (b), and are absent in FP rat (c). Vertical scale bar: 0.25 mV. (C). An example of 4-seconds-long EEG epoch (upper trace), filtered activity in theta (5–10 Hz) and gamma (30–100 Hz) frequency ranges. Scale bars: vertical 0.25 mV; horizontal 1 second.
HFT-induced changes included a substantial decrease in the relative spectral power of theta oscillations, when compared to pre-HFT values. This decrease was observed during HFT in all groups. The conclusion of HFT was followed by recovery and∕or increase in the relative theta power (Figure 3 A). The results of two-way ANOVA demonstrated significant effects for both time (F4,1204 = 15.56, p < 0.001) and tetanisation (F2,1204 = 5.48, p < 0.01) factors, but not for factorial interaction. Cross-group comparisons showed that only the difference between the LTP and failed potentiation groups was significant (p < 0.001, LSD test), reflecting a significantly higher level of the relative theta power in the LTP group to in the period after HFT. The overall difference in theta power between the STP and failed potentiation groups just failed to reach significance (p < 0.063).
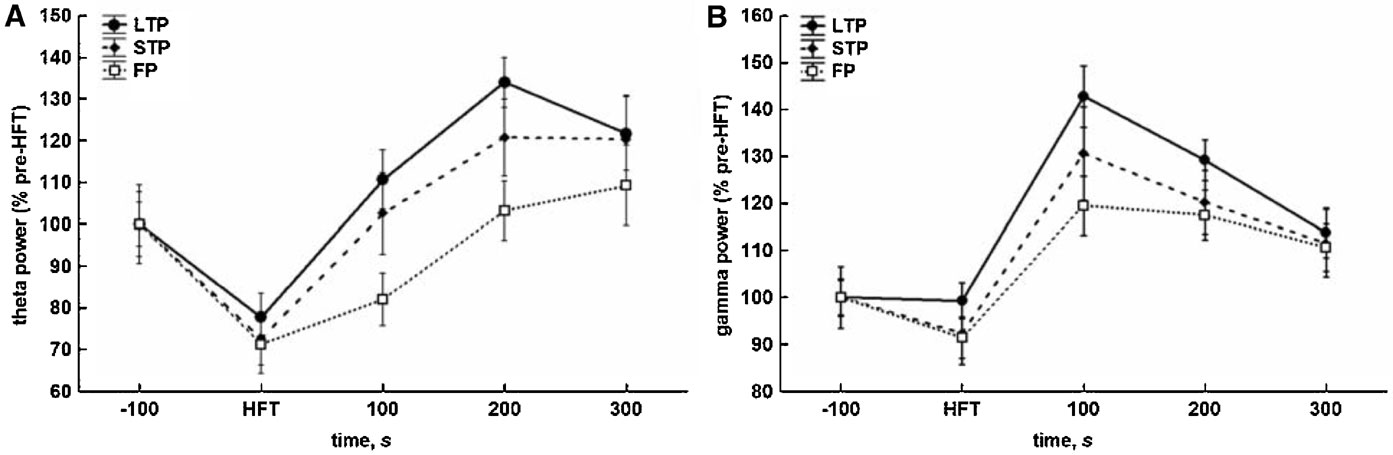
Figure 3. Different consequences of tetanisation are characterised by distinct profiles of changes in theta (5–10 Hz) and gamma (30–100 Hz) spectral power in the DG in vivo. (A) HFT-induced increase in theta spectral power is more prominent in LTP and STP, but not the failed potentiation group. (B) HFT induces an increase in gamma spectral power with the peak within first 100 seconds after stimulation. The results were pooled for 10 4-second-long epochs for each of five 100-seconds-long periods and are expressed as Mean ± SEM.
As for gamma oscillations, no suppression during HFT was found, although there was a trend towards a decrease in the relative gamma power in the STP and failed potentiation groups (Figure 3 B). Within 100 seconds after tetanisation, the power of gamma activity increased and reached a peak followed by a gradual decrease to approximately the same level in all groups. ANOVA revealed significant effects for the temporal factor (F4,1204 = 19.35, p < 0.001), confirming the similarity of patterns of HFT-induced changes of the relative spectral power in all groups, and for the potentiation factor (F2,1204 = 3.70, p < 0.05), but not for factorial interaction. The results of the LSD test confirmed the lack of suppression of gamma oscillations during tetanisation, and a significantly higher level of gamma power in the periods of 100, 200 and 300 seconds after HFT, when compared to pre-HFT values (p < 0.001, p < 0.001 and p < 0.01, respectively) or the period during HFT (all at p < 0.001). In addition, an overall comparison showed that the relative gamma power in the LTP group was significantly higher in comparison with failed potentiation group (p < 0.01).
Thus, the effect of HFT on theta power in the DG comprises two phases: (1) an initial suppression and (2) a subsequent increase. The generally less prominent suppression of the relative gamma power, when compared with theta, was also followed by a transient increase, with the peak occurring in the period of 100 seconds immediately after tetanisation.
Stable potentiation of synaptic transmission is associated with an increased amplitude of the theta cycle as well as an increased mean amplitude of gamma oscillations within a single theta cycle
The results of analysis of the averaged theta cycle amplitude and of the mean amplitude of gamma oscillations per respective theta cycle confirmed and extended the outcomes of Fourier analysis. Accordingly, distinct profiles of the amplitude changes for theta and gamma oscillations were found in the LTP, STP and failed potentiation groups (Figure 4 ). Again, the difference between the groups was particularly prominent in the period encompassing 100-200 seconds after HFT.
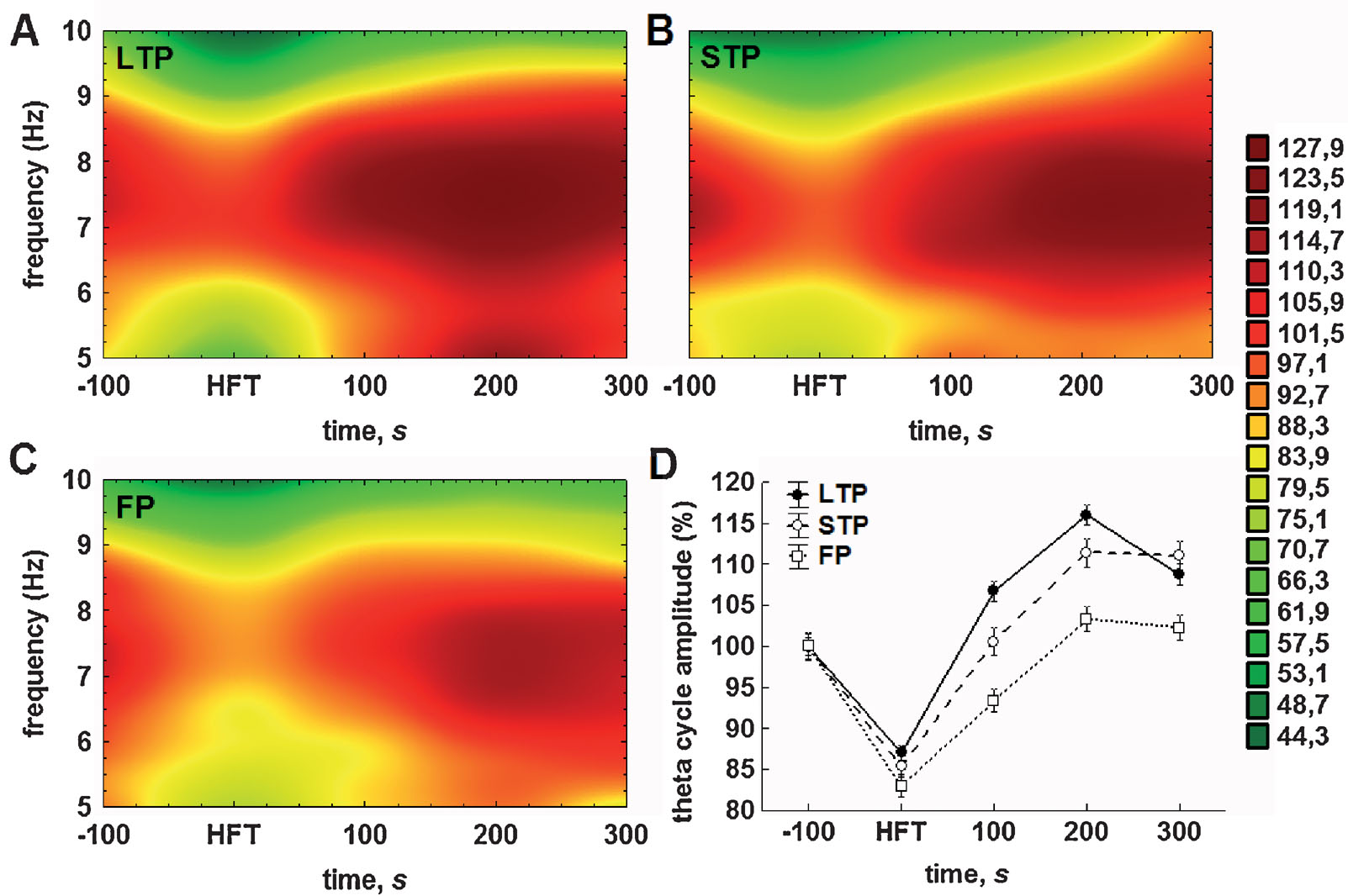
Figure 4. Either STP or LTP of synaptic strength is associated with relatively prolonged increases in the theta cycle amplitude. (A–C) Colour surface plots represent the mean amplitude of individual theta cycles plotted against frequency within the theta (5–10 Hz) range and time to 200 Hz HFT (distance weighted least squares fitting). Colour scale represents percents to the mean pre-HFT value. (D) Whisker plot represents mean ± SEM for LTP (filled circles), STP (open circles) and failed potentiation (open squares) groups.
The analysis of the relationship between the amplitude and duration of theta cycles revealed that the majority of theta cycles exhibited a frequency of between 7 and 8 Hz (Figures 4 A–4 C). Consistent with the results of the Fourier analysis (Figure 3 A) and pooled data for the amplitudes of theta cycle (Figure 4 D), we found that tetanisation resulted in a prominent increase in the theta cycle amplitude, which was generally higher in the LTP and STP groups, when compared with the failed potentiation group. Additionally, we found that the distribution of the mean amplitude of individual theta cycles within the theta frequency range generally fits to a normal distribution in all groups, although the second component can be seen, particularly in the LTP group, near to the lower limit of the range in the post-tetanisation period.
The results of the two-way ANOVA for the relative theta cycle amplitude demonstrated highly significant effects for both time (F4,23592 = 137.27, p < 0.001) and potentiation (F2,23592 = 36.91, p < 0.001) factors, as well as for their interaction (F8,23592 = 5.24, p < 0.001). The results of the subsequent LSD test also suggest the particular importance of the first 100 seconds after HFT for the outcome of synaptic plasticity. Regardless of the success of potentiation, the application of HFT led to a highly significant decrease in amplitude of the individual theta cycle, when compared to pre-HFT levels (all p < 0.001). Within the period of 100 seconds after HFT, the theta cycle amplitude in all three groups increased significantly, but to a different extent. In the failed potentiation group, it remained at a level that was markedly lower than baseline (p < 0.001); in the LTP group it was significantly higher than prior to HFT (p < 0.001); whereas in the STP group it returned to baseline levels (Figure 4 D). All cross-group differences were significant. This trend towards an increase in the relative amplitude of the theta cycle, as well as the significance of differences between groups, was preserved in the next time interval. Here, in the failed potentiation group it reached baseline levels, while in the STP group it was significantly higher in comparison to baseline, but lower than in the LTP group. Finally, 5 minutes after application of HFT, no increase in the amplitude of the theta cycle was seen in any group. Moreover, in the LTP, but not the STP group, it decreased significantly and the difference between these groups became non-significant, but remained at markedly higher level, when compared to respective baseline or to the failed potentiation group (both p < 0.001). One should note that no significant increase in the theta cycle amplitude over pre-HFT levels was seen in the latter group at all, hence suggesting that the lack of modulation∕increase in theta cycle amplitude may predict the failure of persistent changes in synaptic strength.
With regard to the mean amplitude of gamma oscillations, the HFT-induced changes and their subsequent stabilisation required a shorter period of time, than for the theta cycle amplitude. The amplitude of gamma oscillations was significantly lower during HFT in the STP and failed potentiation groups, but not in the LTP group, and reached peak values in the first 100 seconds after HFT (Figure 5 ).
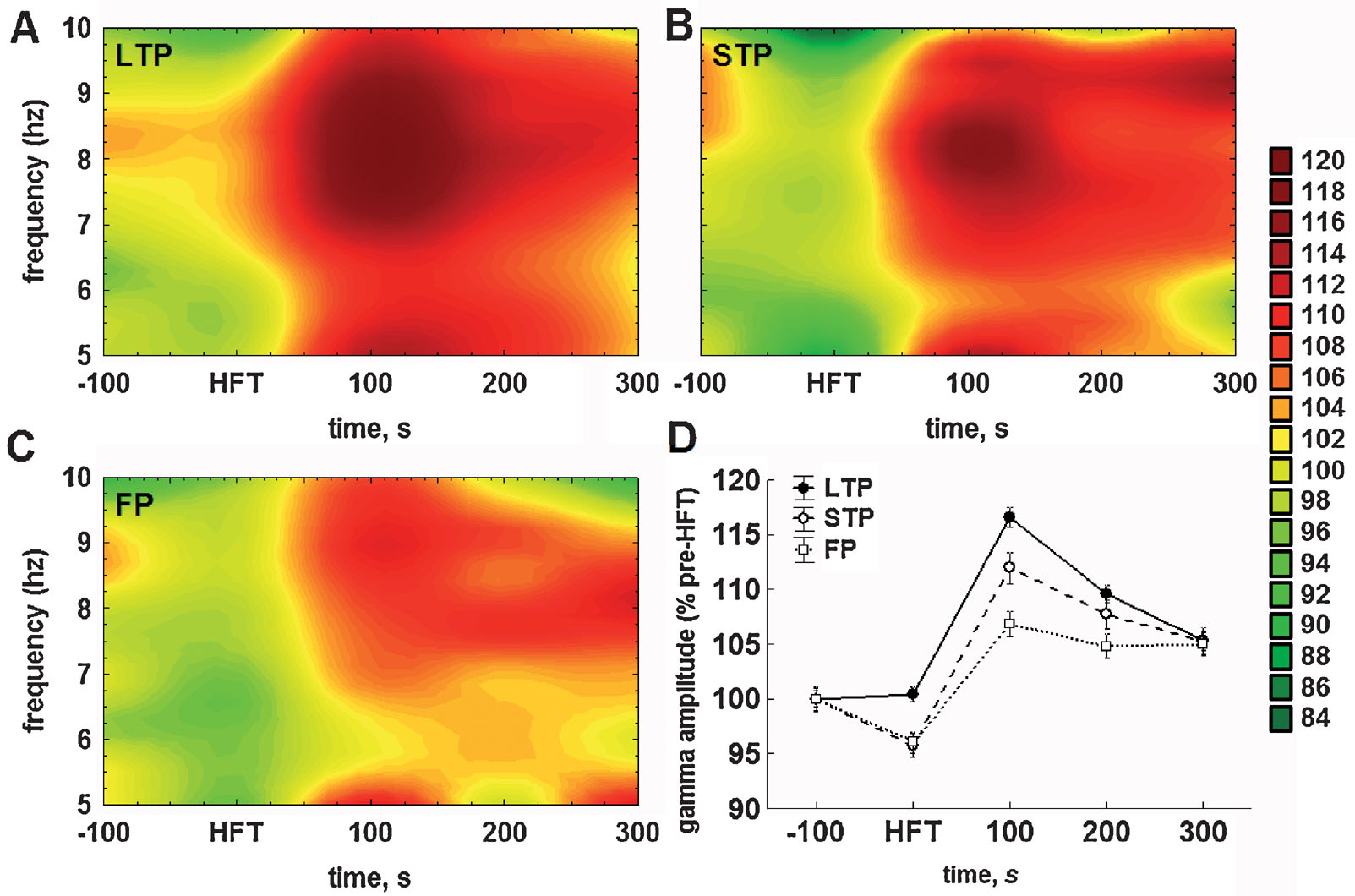
Figure 5. Application of HFT to the medial perforant path increases the mean relative amplitude of gamma oscillations per individual theta cycle in the DG of freely moving animals. (A–C) Colour surface plots represent the mean amplitude of gamma oscillations per one theta cycle plotted against frequency of the respective theta cycle (Y-axis) and time to HFT (X-axis) (distance weighted least squares fitting). Colour scale represents percents to the mean pre-HFT value. (D) Whisker plot represents mean ± SEM for LTP (filled circles, solid line), STP (open circles, dashed line) and FP (open squares, dotted line) groups.
Taking into account the close relationship between theta and gamma oscillations in the DG (Bragin et al., 1995 ; Csicsvari et al., 2003 ) we also analysed the distribution of gamma amplitudes depending on the frequency of ongoing theta oscillation (i.e., the duration of the respective cycle). This analytical approach led us to an interesting finding. Figures 5 A–5 C represent the mean amplitude of gamma oscillations plotted against the frequency of the theta cycle carrying those oscillations. In all three groups, tetanisation increased the gamma amplitude, but predominantly in the upper half of theta range. Owing to the fact that no preferential enhancement of the theta cycle amplitude over the theta frequency range was found (Figures 4 A–4 C), it is especially surprising to see that the increase in gamma amplitude was specific for theta cycles of higher frequency.
Hence, there are at least two components in the distribution of gamma amplitudes, one of which is more prominent, is associated with faster theta cycles, and expressed to different extents in the three groups (LTP > STP > failures). The second distinguishable component of the increase in gamma amplitude is linked to the lower half of the theta range and is particularly small in the failed potentiation group (Figure 5 C). Taken together, this demonstrates that HFT-induced enhancement of gamma oscillations depends not only on the amplitude, but also on the frequency of the ongoing theta rhythm.
Similarly to theta, the two-way ANOVA demonstrated highly significant effects with regard to the mean amplitude of gamma oscillations for both factors (time: F4,23587 = 88.86, p < 0.001; tetanisation: F4,23587 = 19.99, p < 0.001), as well as for factorial interaction (F4,23587 = 4.52, p < 0.001). During tetanisation, the mean amplitude of gamma oscillations was markedly lower in the STP and failed potentiation groups, when compared to the LTP group (both p < 0.01) or appropriate baseline levels (both p < 0.05). Within the next hundred seconds, the increase in gamma amplitude was evident in all groups, with the difference being significant in comparison with either the respective baseline (all p < 0.001), or to gamma amplitude in the same group during HFT (all p < 0.001). Thus, the increase in gamma amplitude following HFT seems to be a common phenomenon. However, the magnitude of this increase was significantly different in all cross-group comparisons: gamma amplitude was higher in the LTP group (Figure 5 A), than in the STP (p < 0.01) (Figure 5 B) or in the failed potentiation group (p < 0.001) (Figure 5 C), and higher in the STP group, when compared with cases of failed potentiation (p < 0.01).
In the period of 200 seconds after HFT, the mean gamma amplitude decreased in both the LTP (p < 0.001) and STP (p < 0.05), but not in the failed potentiation group. Nevertheless, in all three groups gamma amplitude remained at a significantly higher level than that prior to HFT (LTP: p < 0.001; STP: p < 0.001; failed potentiation: p < 0.01). Finally, in the period of 300 seconds after HFT, the amplitude of gamma oscillations per theta cycle decreased significantly in the LTP group only, when compared with previous time intervals (p < 0.001). In the other groups no significant changes were found. Furthermore, no significant cross-group difference was found in this period, reflecting the similarity of values in the three groups. Nevertheless, in the period of 300 seconds after HFT the mean gamma amplitude was significantly higher in all groups, when compared with the pre-HFT period (LTP: p < 0.001; STP and failed potentiation: p < 0.01).
In summary, we found that the LTP, STP and failed potentiation groups are characterised by distinct profiles of changes in the mean amplitude of both the theta cycle and gamma oscillations per one theta cycle. In animals that showed LTP, the tetanisation-induced suppression of the theta cycle amplitude was less prominent and no suppression of gamma amplitude was found. In contrast, in both the STP and failed potentiation groups a significant suppression of gamma amplitude after HFT was revealed in both frequency ranges. Moreover, the LTP group was characterised by a rapid recovery and further increase in both theta and gamma amplitudes within first 100 seconds after HFT. The same trend was seen in the STP group, but the increase was expressed less prominently. In animals with failed potentiation, the recovery of theta cycle amplitude after the HFT-induced suppression required a substantially longer period of time and did not increase above the baseline values. Thus, these results show that LTP is preceded, accompanied and facilitated by corresponding changes of theta and gamma amplitudes. These changes comprise a transient increase in the mean amplitude of gamma oscillations at least in the period of 100 seconds after HFT, supported by a rapid recovery and steady increase in theta cycle amplitude.
Discussion
In the present study, we demonstrated that different consequences, for synaptic efficacy, of HFT are characterised by distinct profiles of oscillatory activity in the theta and gamma frequency ranges. The main findings of the present study are as follows: three groups representing different outcomes of HFT, namely LTP, STP and failed potentiation, diverged in the period after HFT in terms of the degree of increase found for both theta and gamma power. In the LTP group, we observed a rapid and significant increase in both theta and gamma power in the period after HFT, which was less prominent in the STP group and absent in the failed potentiation group. Furthermore, potentiation of synaptic transmission in the DG was associated with a significant and coordinated increase in the amplitude of theta cycles and the mean amplitude of gamma oscillations within a single theta cycle. Thus, our data demonstrate that the increase in theta and gamma power in the DG that occurs within first 100-200 seconds after HFT predicts and may in fact enable LTP.
Induction of LTP in the DG is known to depend critically upon calcium entry through NMDA receptors and voltage-gated calcium channels (Bliss and Collingridge, 1993 ; Lynch, 2004 ). We previously showed that the transition of STP into LTP in the DG of freely behaving rats is mediated by activation of group I metabotropic glutamate receptors (mGluRs) (Manahan-Vaughan and Reymann, 1996 ). Furthermore, the maintenance of LTP beyond an initial period of minutes was shown to require activation of mGluRs (Anwyl, 1999 ; Bortolotto et al., 1999 ; Wilsch et al., 1998 ), while persistent LTP that endures for hours and days requires de novo gene expression and protein synthesis (Frey et al.,1993 ; Frey et al.,1996 ; Krug et al., 1984 ). Taking into account that HFT elicited LTP, STP or failed potentiation in our study, the occurrence of STP may have derived from an insufficient activation of group I mGluRs by the tetanus, whereas the failure of potentiation is likely to have occurred due to an inadequate activation of NMDA or voltage-gated calcium channels (Manahan-Vaughan et al., 1998 ).
Besides the activation of different subsets of receptors following HFT, LTP induction may also be regulated by oscillatory network activity. Early reports demonstrated that the stimulation protocols mimicking natural theta rhythm are optimal for LTP induction (Diamond et al., 1988 ; Larson et al., 1986 ). Furthermore, stimulation delivered at the peak of ongoing theta cycle has been shown to result in potentiation of synaptic transmission both in vitro and in vivo (Huerta and Lisman, 1993 ; Huerta and Lisman, 1995 ; Pavlides et al., 1988 ), which in turn can be depotentiated by stimulation delivered at the trough of theta cycle (Hölscher et al., 1997 ). However, our data offer the first description of a relationship between the expression of HFT-induced changes in the theta and gamma oscillatory activity and the subsequent modification of synaptic strength. This finding is particularly intriguing given the known relationship between theta and gamma oscillations and information processing in the form of learning and memory formation in the hippocampus (Axmacher et al., 2006 ; Buzsáki, 2002 ; Buzsáki, 2005 ; Csicsvari et al., 2003 ; Hasselmo et al., 2002 ; Kahana, 2006 ; Lisman and Idiart, 1995 ; Lisman, 2005 ; O'Keefe and Burgess, 1999 ; Vertes, 2005 ). However, further studies incorporating selective manipulations of theta and/or gamma oscillations will be required, in order to conclusively demonstrate the direct link between hippocampal theta and gamma oscillations and LTP.
Another interesting aspect of the relationship between theta rhythm and LTP is a theta phase reset that is caused by an afferent stimulus and occurs immediately after its delivery (Givens, 1996 ; Huerta and Lisman, 1993 ; Huerta and Lisman, 1995 ; Pavlides et al., 1988 ). In some respects, the suppression of theta oscillations resembles the phenomenon of theta phase reset. For a weak stimulus, phase reset can be considered as a means of selective amplification, whereas a strong stimulus not only can reset the oscillatory cycle, but can shift a large assembly of neurons towards a different dynamic state (Buzsáki, 2006 ). The latter seems to be the case for HFT. Being a relatively strong signal, the burst of tetanic stimulation not only resets the phase of ongoing theta activity, but introduces a new component into the concerted activity of principal cells and interneurons. As a consequence, a suppression of the previous (theta) activity occurs for the period of time required to “update” the dynamic state of the system. In our study, the character of such post-reset alterations of the system varied in different animals. Tetanisation was sufficiently strong to affect the ongoing theta oscillation in all rats, but in some of them the system did not shift to a new dynamic state, as would be characterised by a new pattern of weak and strong synaptic connections between spatially distributed principal cells and interneurons. Thus, failed synaptic potentiation was characterised by a suppression of theta power, followed by its slow recovery to the same level as compared with pre-HFT period. Conversely, in cases of successful potentiation of synaptic transmission, after an initial suppression of extracellular theta, a rapid increase in theta power to a significantly higher level occurred. Together with facilitated gamma oscillations, this heightened level of theta may reflect a novel state of the system, modified by acquired sensory information. At the same time, enhanced oscillatory activity represents a necessary component of this new state, which also can be dynamically shifted∕changed by another signal, which is properly timed and∕or strong enough to do so.
The facilitation of theta oscillations in the period after HFT may be triggered by several mechanisms, such as increased septal GABAergic input targeted exclusively to interneurons (Freund and Antal, 1988 ), activation of CA3-CA1 theta generator that receives septal cholinergic input and increases activity after release from suppressing entorhinal theta drive (Bragin et al., 1995 ; Kocsis et al., 1999 ), theta suppression- induced activation of “antitheta” cells (Buzsáki et al., 1983 ; Mizumori et al., 1990 ), and∕or increased firing of granule cells and following commissural activation of basket cells and hilar interneurons with perforant path axon projections (Freund and Buzsáki, 1996 ). Supporting the idea that theta enhancement favours LTP, the theta phase reset was reported to regulate the probability of LTP induction in the DG: HFT delivered at the peak of theta after reset induced LTP, whereas HFT that coincided with the trough of theta cycle did not (McCartney et al., 2004 ). Taking into account the proposed roles of the peak and the trough of the theta cycle in information encoding and retrieval, respectively (Hasselmo et al., 2002 ), and regulation of the probability of NMDARs activation by the theta phase (Vertes, 2005 ), one can conclude that the increase in theta power after individual HFT bursts, and in the period after tetanisation may serve as a mechanism that optimises the conditions for induction of LTP and encoding of afferent signals. This is in line with data showing a higher power of theta in the “aroused” brain state when acquisition and∕or encoding of sensory information should be facilitated (Bragin et al., 1995 ). Importantly, a disruption of hippocampal theta oscillations was found to retard acquisition in eyeblink conditioning tasks in rabbits (Kaneko and Thompson, 1997 ). Moreover, rabbits completed this task twice as effectively when pairings of stimuli were presented during prominent theta activity in CA1 (in comparison to animals that were stimulated during non-theta periods), suggesting a facilitatory effect of theta oscillations early in training (Seager et al., 2002 ). However, our results demonstrate that the increase in theta power should be accompanied with enhanced gamma power to enable or mediate the plastic changes in synaptic transmission and, more generally, to enable encoding of sensory information and formation of memory traces. Furthermore, our results show the importance of a short time interval, such as 100 seconds after HFT, for this correlated increase in theta and gamma power to occur.
Two gamma generators have been identified in the hippocampus, one of which resides in the DG and the second of which in CA3-CA1 regions (Bragin et al., 1995 ; Csicsvari et al., 2003 ). In the DG, the amplitude of gamma oscillations is driven by the entorhinal input (Bragin et al., 1995 ) and strongly depends on the activity of basket cells (Bartos et al., 2001 ; Penttonen et al., 1998 ; Vida et al., 2006 ). Gamma oscillations seen in the present study in the periods immediately after HFT bursts resemble, and have a similar duration as, those tetanically induced in vitro, and also require participation of both principal cells and interneurons (Colling et al., 1998 ; Traub et al., 1999 ; Whittington et al., 1997 ). The mechanisms of tetanically induced oscillations in vitro involve a slow and large depolarisation, lasting for hundreds of milliseconds, in both cell types and, at least in principal cells, activation of mGluRs (Traub et al., 2004 ; Whittington et al., 1997 ). The facilitation of gamma activity in a prolonged time scale, i.e., within hundreds of seconds after HFT, may also involve activation of GABAA receptors and mGluRs. Activation of group I mGluRs, in pharmacologically isolated interneuronal networks in vitro induce gamma oscillatory activity, which is abolished by bicuculline (Whittington et al., 1995 ). Furthermore, in hippocampal interneurons the activation of group I mGluRs induces a large-amplitude inward current in O-LM (str. oriens-lacunosum moleculare) cells and inward currents of lower amplitude in basket cells. mGluR activation also causes high-frequency firing of basket cells (van Hooft et al., 2000 ), which is in line with previous reports that basket cells maintain high-frequency firing under depolarised conditions (Ylinen et al., 1995 ). Thus, NMDARs and mGluRs seem to have a dual impact on hippocampal LTP. Firstly, their activation mediates the elevation of intracellular calcium concentration, which is necessary to trigger gene expression and protein synthesis, and, subsequently, determines the longevity of plastic changes in synaptic transmission. Secondly, together with fast GABAA-mediated inhibition, activation of both ionotropic and mGluRs can regulate neuronal oscillatory activity, hence shaping the optimal conditions for potentiation, and mediating the encoding and incorporation of the newly acquired information on the network level.
Conclusion
In conclusion, we analysed, in this study, the association between oscillatory evoked responses and LTP in the DG following high-frequency simulation of the perforant path in freely moving rats. We found that successful potentiation, in the form of STP or LTP, was associated with a correlated transient increase in the power of both extracellular theta and gamma oscillations, whereas the absence of such an enhancement of oscillatory activity was indicative for the failure of synaptic plasticity. Taken together, our results suggest that an increase in both theta and gamma power shortly after tetanisation is characteristic of LTP and, in general, of long-term synaptic information storage. These findings link LTP as a plasticity mechanism related to learning and memory, with oscillatory activity in theta and gamma frequency ranges, and supports the role of network activity as a mechanism of encoding∕retrieval of memory traces in the hippocampus.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This work was supported by a Deutsche Forschungsgemeinschaft grant (Ma1843) to D. Manahan-Vaughan. We are grateful to Jens Klausnitzer for technical assistance.
References
Abraham, W. C. (2003). How long will long-term potentiation last? Philos. Trans. R. Soc. Lond., B, Biol. Sci. 358, 735–744.
Abraham, W. C., and McNaughton, N. (1984). Differences in synaptic transmission between medial and lateral components of the perforant path. Brain Res. 303, 251–260.
Abraham, W. C., Greenwood, J. M., Logan, B. L., Mason-Parker, S. E., and Dragunow, M. (2002). Induction and experience-dependent reversal of stable LTP lasting months in the hippocampus. J. Neurosci. 22, 9626–9634.
Anwyl, R. (1999). Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res. Rev. 9, 83–120.
Axmacher, N., Mormann, F., Fernandez, G., Elger, C. E., and Fell, J. (2006). Memory formation by neuronal synchronization. Brain Res. Rev. 52, 170–182.
Bartos, M., Vida, I., Frotscher, M., Geiger, J. R., and Jonas, P. (2001). Rapid signaling at inhibitory synapses in a dentate gyrus interneuron network. J. Neurosci. 21, 2687–2698.
Bartos, M., Vida, I., and Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci. 8, 45–56.
Bliss, T. V., and Collingridge, G. L. (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39.
Bortolotto, Z. A., Fitzjohn, S. M., and Collingridge, G. L. (1999). Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr. Opin. Neurobiol. 9, 299–304.
Bragin, A., Jando, G., Nadasdy, Z., Hetke, J., Wise, K., Buzsáki, G. (1995). Gamma (40-100Hz) oscillation in the hippocampus of the behaving rat. J. Neurosci. 15, 47–60.
Buzsáki, G. (2005). Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827–840.
Buzsáki, G., and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929.
Buzsáki, G., Leung, L., and Vanderwolf, C. H. (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain Res. Rev. 6, 139–171.
Colling, S. B., Stanford, I. M., Traub, R. D., and Jefferys, J. G. R. (1998). Limbic gamma rhythms. I. Phase-locked oscillations in hippocampal CA1 and subiculum. J. Neurophysiol. 80, 155–161.
Csicsvari, J., Jamieson, B., Wise, K. D., Buzsáki, G. (2003). Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37, 311–322.
Diamond, D. M., Dunwiddie, T. V., and Rose, G. M. (1988). Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J. Neurosci. 8, 4079–4088.
Freund, T. F., and Antal, M. (1988). GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336, 170–173.
Frey, U., Huang, Y. Y., and Kandel, E. R. (1993). Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science 260, 1661–1664.
Frey, U., Frey, S., Schollmeier, F., and Krug, M. (1996). Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J. Physiol. 490, 703–711.
Fries, P., Neuenschwander, S., Engel, A. K., Goebel, R., and Singer, W. (2001). Rapid feature selective neuronal synchronization through correlated latency shifting. Nat. Neurosci. 4, 194–200.
Givens, B. (1996). Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport 8, 159–163.
Hargreaves, E. L., Cain, D. P., and Vanderwolf, C. H. (1990). Learning and behavioral-long-term potentiation: importance of controlling for motor activity. J. Neurosci. 10, 1472–1478.
Hasselmo, M. E., Bodelon, C., and Wyble, B. P. (2002). A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 14, 793–817.
Hermann, C. S., Munk, M. H., and Engel, A. K. (2004). Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn. Sci. 8, 347–355.
Hölscher, C., Anwyl, R., and Rowan, M. J. (1997). Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J. Neurosci. 17, 6470–6477.
Huerta, P. T., and Lisman, J. E. (1993). Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364, 723–725.
Huerta, P. T., and Lisman, J. E. (1995). Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron 15, 1053–1063.
Kahana, M. J. (2006). The cognitive correlates of human brain oscillations. J. Neurosci. 26, 1669–1672.
Kaneko, T., and Thompson, R. F. (1997). Disruption of trace conditioning of the nictitating membrane response in rabbits by central cholinergic blockade. Psychopharmacology (Berl.) 131, 161–166.
Khazipov, R., Sirota, A., Leinekugel, X., Holmes, G. L., Ben-Ari, Y., Buzsáki, G. (2004). Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432, 758–761.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195.
Kocsis, B., Bragin, A., Buzsáki, G. (1999). Interdependence of multiple theta generators in the hippocampus: a partial coherence analysis. J. Neurosci. 19, 6200–6212.
Krug, M., Lössner, B., and Ott, T. (1984). Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res. Bull. 13, 39–42.
Larson, J., Wong, D., and Lynch, G. (1986). Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 368, 347–350.
Lisman, J. (2005). The theta∕gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15, 913–922.
Lisman, J. E., and Idiart, M. A. (1995). Storage of 7∕2 short-term memories in oscillatory subcycles. Science 267, 1512–1515.
Llinas, R., Ribary, U., Contreras, D., and Pedroarena, C. (1998). The neuronal basis for consciousness. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 353, 1841–1849.
Malenka, R. C., and Nicoll, R. A. (1999). Long-term potentiation—a decade of progress? Science 285, 1870–1874.
Manahan-Vaughan, D., and Reymann, K. G. (1996). Metabotropic glutamate receptor subtype agonists facilitate long-term potentiation within a distinct time window in the dentate gyrus in vivo. Neuroscience 74, 723–731.
Manahan-Vaughan, D., Braunewell, K. H., and Reymann, K. G. (1998). Subtype-specific involvement of metabotropic glutamate receptors in two forms of long-term potentiation in the dentate gyrus of freely moving rats. Neuroscience 86, 709–721.
McCartney, H., Johnson, A. D., Weil, Z. M., and Givens, B. (2004). Theta reset produces optimal conditions for long-term potentiation. Hippocampus 14, 684–687.
Mizumori, S. J., Barnes, C. A., and McNaughton, B. L. (1990). Behavioral correlates of theta-on and theta-off cells recorded from hippocampal formation of mature young and aged rats. Exp. Brain Res. 80, 365–373.
O'Keefe, J., and Burgess, N. (1999). Theta activity, virtual navigation and the human hippocampus. Trends Cogn. Sci. 3, 403–406.
Pavlides, C., Greenstein, Y. J., Grudman, M., and Winson, J. (1988). Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 439, 383–387.
Paxinos, G., and Watson, C. (1986). The rat brain in stereotaxic coordinates(San Diego, Academic Press).
Penttonen, M., Kamondi, A., Acsady, L., Buzsáki, G. (1998). Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur. J. Neurosci. 10, 718–728.
Rick, J. T., and Milgram, N. W. (1996). Frequency dependence of long-term potentiation and depression in the dentate gyrus of the freely moving rat. Hippocampus 6, 118–124.
Seager, M. A., Johnson, L. D., Chabot, E. S., Asaka, Y., and Berry, S. D. (2002). Oscillatory brain states and learning: impact of hippocampal theta-contingent training. Proc. Natl. Acad. Sci. USA 99, 1616–1620.
Singer, W. (1993). Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 55, 349–374.
Traub, R. D., Whittington, M. A., Buhl, E. H., Jefferys, J. G. R., and Faulkner, H. J. (1999). On the mechanism of the gamma −> beta frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J. Neurosci. 19, 1088–1105.
Traub, R. D., Bibbig, A., LeBeau, F. E. N., Buhl, E. H., and Whittington, M. A. (2004). Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu. Rev. Neurosci. 27, 247–278.
van Hooft, J. A., Giuffrida, R., Blatow, M., and Monyer, H. (2000). Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J. Neurosci. 20, 3544–3551.
Vertes, R. P. (2005). Hippocampal theta rhythm: a tag for short-term memory. Hippocampus 15, 923–935.
Vida, I., Bartos, M., and Jonas, P. (2006). Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49, 107–117.
Vreugdenhil, M., Bracci, E., and Jefferys, J. G. R. (2005). Layer-specific pyramidal cell oscillations evoked by tetanic stimulation in the rat hippocampal area CA1 in vitro and in vivo. J. Physiol. 562, 149–164.
Whittington, M. A., Traub, R. D., and Jefferys, J. G. R. (1995). Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612–615.
Whittington, M. A., Stanford, I. M., Colling, S. B., Jefferys, J. G. R., and Traub, R. D. (1997). Spatiotemporal patterns of g frequency oscillations tetanically induced in the rat hippocampal slice. J. Physiol. (Lond.) 502, 591–607.
Wilsch, V. W., Behnisch, T., Jäger, T., Reymann, K. G., and Balschun, D. (1998). When are class I metabotropic glutamate receptors necessary for long-term potentiation? J. Neurosci. 18, 6071–6080.
Keywords: hippocampus, synaptic plasticity, LTP, theta, gamma, oscillations, tetanisation
Citation: Arthur Bikbaev and Denise Manahan-Vaughan (2007). Hippocampal network activity is transiently altered by induction of long-term potentiation in the dentate gyrus of freely behaving rats. Front. Behav. Neurosci. 1:7. doi: 10.3389/neuro.08/007.2007
Received: 15 November 2007;
Paper pending published: 3 December 2007;
Accepted: 4 December 2007;
Published online: 30 December 2007.
Edited by:
Carmen Sandi, Ecole Polytechnique Federale de Lausanne, SwitzerlandReviewed by:
Carmen Sandi, Ecole Polytechnique Federale de Lausanne, SwitzerlandCopyright: © 2007 Bikbaev and Manahan-Vaughan. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Denise Manahan-Vaughan, Learning and Memory Research, Medical Faculty, Ruhr University Bochum, Universitaetsstrasse 150, FNO 01116, 44780 Bochum, Germany. e-mail: denise.manahan-vaughan@rub.de