Semantics in the motor system: motor-cortical beta oscillations reflect semantic knowledge of end-postures for object use
1
Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, Netherlands
2
Behavioural Science Institute, Radboud University Nijmegen, Nijmegen, Netherlands
In the present EEG study we investigated whether semantic knowledge for object use is represented in motor-related brain areas. Subjects were required to perform actions with everyday objects and to maintain either a meaningful or a meaningless end posture with the object. Analysis of the EEG data focused on the beta-frequency band, as previous studies have indicated that the maintenance of a posture is reflected in stronger beta-oscillations. Time frequency analysis indicated that the execution of actions resulting in a meaningless compared to a meaningful end posture was accompanied by a stronger beta-desynchronization towards the end of the movement and a stronger subsequent beta-rebound after posture-onset. The effect in the beta-frequency band was localized to premotor, parietal and medial frontal areas and could not be attributed to differences in timing or movement complexity between meaningful and meaningless actions. Together these findings directly show that the motor system is differentially activated during the execution and maintenance of semantically correct or incorrect end postures. This suggests that semantic object knowledge is indeed represented in motor-related brain areas, organized around specific end postures associated with the use of objects.
By brushing your teeth twice a day, at the age of 30 you have brought a toothbrush towards your mouth for more than 20,000 times! Moreover, given that on average people drink about three cups of coffee a day and assuming that it takes 10 sips to empty a cup, the number of times that you have brought a cup towards your mouth exceeds the 150,000! These numbers illustrate that throughout our lives we have profound experience with using objects. Accordingly, many of our everyday actions have become highly automatized and the cognitive processes underlying these actions are usually not given much further thought. Only in the case of action slips the importance of conceptual knowledge for action becomes unmistakably clear (Schwartz, 2006
). For instance, in the case of absent-mindedness you may end up grasping the wrong end of the toothbrush resulting in toothpaste on your hand instead of on your teeth.
The importance of conceptual knowledge for action planning is especially apparent in neuropsychological patients showing specific deficits in object-directed actions. Damage to the left inferior parietal lobule usually results in ideomotor apraxia, a disorder that is characterized by a loss of manipulation knowledge and an inability to produce and recognize gestures associated with using objects (Buxbaum, 2001
). Similarly, patients with semantic dementia are often impaired in using objects in a correct fashion (e.g. using a remote control as a telephone; Hodges et al., 2000
). These patient studies indicate that object use can be impaired at different levels and suggest that different brain areas are involved in using objects meaningfully.
Performing an action with an object involves different spatial transformations, such as coding the location of an object, generating a movement plan to grasp the object, and specifying the final posture the effectors will take after the movement. Behavioral studies suggest an automatic activation of low-level motor programs when objects are perceived (Tucker and Ellis, 2001
). In addition, several studies have shown that the mere observation of object pictures or names referring to objects results in the activation of premotor and inferior parietal brain areas (Grafton et al., 1997
; Chao and Martin, 2000
; Chao et al., 2002
; Gerlach et al., 2002
; Kellenbach et al., 2003
). These brain areas are likely involved in the visuo-motor transformations required for grasping that are mediated by a fronto-parietal network (for review, see Andersen and Cui, 2009
).
Many studies have implicated a special role for the posterior parietal cortex (PPC) in sensorimotor integration. For instance, neurons in the anterior intraparietal sulcus (AIP) are selectively involved in coding specific types of grasps directed at objects of a particular size, shape and orientation (Murata et al., 2000
). In addition, the parietal reach region is involved in planning reaching movements and the lateral intraparietal area (LIP) plans upcoming saccadic eye movements (Cohen and Andersen, 2002
). Interestingly, neuronal activity in these regions represents upcoming actions in a common eye-centered reference frame (Batista et al., 1999
) and is primarily related to the type of movement being planned (i.e. the action intention; Snyder et al., 1997
). The PPC contains massive connections to frontal lobe areas, such as the frontal eye field and the premotor cortex, thereby enabling the planning and control of object-directed actions. In sum, the workings of the fronto-parietal network underlying object-directed grasping are relatively well-established.
However, in addition to the visuo-motor transformations required for grasping objects, the meaningful use of objects requires the retrieval of semantic knowledge, specifying what to do with an object. In recent studies it was found that the categorization of objects was accompanied by functional motor activation, reflecting the motor programs most strongly associated with using the object (Masson et al., 2008
; van Elk et al., 2009
). In an object categorization task subjects were faster to respond by means of the movement most strongly associated with using the object (van Elk et al., 2009
). Subjects were faster for instance, by responding to a picture of a toothbrush by moving their arm towards their body than away from their body, suggesting that the categorization of the object was accompanied by the implicit co-activation of specific motor programs. Other studies showed that the observation of objects automatically evokes grasping gestures associated with using an object for its intended purpose (functional gestures) and gestures associated with picking up an object (volumetric gestures; Bub et al., 2008
; Masson et al., 2008
). These findings indicate that many objects are associated with specific gestures or end postures that allow the functional use of an object. Studies in monkeys indicate that these higher-level motor programs may be represented in the motor system (Graziano et al., 2002
). Graziano and colleagues observed that prolonged stimulation of the premotor cortex resulted in the elicitation of complex action sequences, such as hand grasping, mouth opening and a subsequent arm movement directed towards the mouth. Stimulation at different sites resulted in different behaviorally meaningful actions (e.g. eating, displaying fear behavior), suggesting that in primates the motor system may be organized in a posture-based fashion.
It could well be that in humans the semantic knowledge required to use objects is represented in motor-related brain areas as well, organized around specific end postures associated with using objects. This suggestion would be in line with the sensory-motor account of object knowledge, according to which object knowledge is represented in the same brain regions that are active when actually using objects (Beauchamp and Martin, 2007
). The consistent activations found in premotor and inferior parietal areas upon the observation of tools are in line with this suggestion (Grafton et al., 1997
; Chao and Martin, 2000
; Chao et al., 2002
; Gerlach et al., 2002
; Kellenbach et al., 2003
). In addition, many studies have investigated the neural correlates of preparing and executing tool use pantomimes, which is consistently associated with stronger activation in the left parietal cortex (IPS) and the left premotor and prefrontal cortex (Moll et al., 2000
; Choi et al., 2001
; Ohgami et al., 2004
; Rumiati et al., 2004
; Johnson-Frey et al., 2005
; Hermsdorfer et al., 2007
). Together, these studies show a considerable overlap in the brain regions involved in the observation of objects and in producing object-related gestures.
Previous studies on object knowledge have typically used tool-related pantomimes that involved actions within peripersonal space, such as using a pair of scissors or turning a key (e.g. Hermsdorfer et al., 2007
). However many of our everyday actions consist of attaining a specific bodily posture with an object, such as bringing a toothbrush towards the mouth or a phone to the ear. Due to our extensive experience with these type of object-directed actions, it may be expected that the motor programs associated with using these objects have become well established in motor-related brain areas, such as parietal and premotor regions. In the present study we hypothesized, that if semantic object knowledge is indeed represented at a motor-level, motor-related brain regions should be differentially involved when subjects execute and maintain meaningful or meaningless end postures with objects. Accordingly, we instructed subjects to grasp everyday objects, like a toothbrush or a cup, with a specific grip that resulted in either a meaningful end posture when the object was held at the correct goal location (i.e. a grip that allows the functional use of the object; cf. Bub et al., 2008
; Masson et al., 2008
) or a meaningless end posture (i.e. a grip that does not allow the functional use of the object, like an overhand grip applied to a cup). Subjects’ EEG was recorded while they were preparing and executing object-directed actions and while they were maintaining meaningful and meaningless end postures with objects.
Analysis of the EEG data focused mainly on the movement-related desynchronization in the beta-frequency band and the post-movement beta-rebound (Pfurtscheller et al., 1996
; Stancak and Pfurtscheller, 1996
). Desynchronization in the beta-frequency band is associated with the preparation and execution of body movements and likely reflects activation of the premotor and the primary motor cortex (Stancak and Pfurtscheller, 1996
; Caetano et al., 2007
). Termination of a movement is typically followed by synchronization in the beta frequency band (beta rebound) and initially this was thought to reflect an idling state of the motor cortex (Salmelin and Hari, 1994
; Pfurtscheller et al., 1996
). However, other studies suggest that the beta rebound reflects an active inhibition of somato-sensory processing or sensory reafference (Cassim et al., 2001
; Parkes et al., 2006
). For instance, the post-movement beta rebound was abolished after deafferentiation by ischaemic nerve block, suggesting that the beta-rebound requires sensory feedback (Cassim et al., 2001
).
Interestingly, several studies indicate that beta-oscillations play an anti-kinetic role and that they support the maintenance of an existing motor state. First, the motor cortex excitability is facilitated during beta-desynchronization and reduced during the beta-rebound, as probed with transcranial magnetic stimulation (Chen et al., 1999
). Second, during steady-state motor output typically a high cortico-muscular coherence is observed that is related to specific motor parameters and the subject’s performance (Kilner et al., 2000
; Kristeva et al., 2007
). Third, increased beta-power has been associated with the preservation of the current motor state (Gilbertson et al., 2005
). In Parkinson’s disease cortical power in the beta-frequency band is pathologically increased (Brown et al., 2001
) and in patients with Parkinson’s disease slow movements are related to delayed and deficient suppressions of the beta band local field potential oscillations in the subthalamic nucleus (Brown and Williams, 2005
). Together these findings suggest that increases in beta-power may support the maintenance of the present motor set, while decreases in beta-power may facilitate motor processing related to new movements (Gilbertson et al., 2005
).
Based on these findings, we hypothesized that the preparation and execution of actions resulting in a meaningless end posture should result in a stronger beta-desynchronization, reflecting an increased activation of the motor system to facilitate the transition to a novel and unfamiliar end posture. In addition, we expected that the maintenance of a meaningless end posture may require the active inhibition of motor-related brain areas to maintain the current posture, which should be reflected in a stronger beta-rebound. Such a finding would provide first evidence for the notion that semantic object knowledge is represented in the motor system, organized around specific end-postures associated with using objects.
In addition to the effects in the beta-frequency band, action preparation and execution have been associated with desynchronization in the mu-frequency band as well (Salmelin and Hari, 1994
; Hari, 2006
; Caetano et al., 2007
). It has been suggested that whereas the beta-rhythm reflects activation in motor-related brain regions, the mu-rhythm originates primarily from more posterior somatosensory areas (Salmelin et al., 1995
; Hari et al., 1997
; Ritter et al., 2009
). Accordingly, an interesting question is whether the approach and maintenance of meaningful and meaningless postures also results in a differential mu-desynchronization. Such a finding would implicate that somatosensory areas, such as S1, are involved in representing action semantics as well. Alternatively, if the difference between meaningful and meaningless actions only becomes apparent in a modulation of the beta-frequency band, this would support the notion that action semantic knowledge is specifically represented in motor-related brain areas. Therefore, both the mu (8–12 Hz) and the beta frequency band (16–24 Hz) were tested for statistical significance.
Subjects
Twenty-three healthy, right-handed undergraduate students (20 females and 3 males), with no known neurological impairments and normal or corrected-to-normal vision, participated in this experiment. The mean age of the subjects was 22.6 years (SD = 2.3). Subjects were recruited from the student population of the Radboud University Nijmegen. Before the experiment started, subjects were informed about the experimental procedure and gave informed consent according to the Declaration of Helsinki. The experiment was approved by the local ethics committee.
Eleven subjects were discarded from further data analyses, due to excessive movement- or eye-movement-related artifacts, leaving 12 subjects in the final analysis. The relatively high number of subjects that was discarded from analysis was due to the fact that: (1) the paradigm elicited a lot of movement-related artifacts, due to muscular tension or movement of the head, (2) the actions often resulted in electrode-drifts and (3) the relatively long interval required for analysis (5 s) was often contaminated by ocular artifacts. No subjects were excluded based on any other criterion than inspection of the raw EEG signal, prior to frequency and statistical analyses.
Experimental Setup
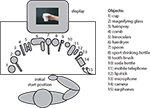
The experiment was conducted in an electrically- and sound-shielded experimental room. A schematic overview of the experimental setup in the experimental room is represented in Figure 1
. The subject was comfortably seated behind a table. A buttonbox was placed on the subject’s lap. All 15 objects were placed in front of the subject on the table. A monitor was placed in front of the subject for presenting the stimuli.
Figure 1. Overview of the experimental setup. The subject was seated behind a table on which 15 different objects were placed. A buttonbox was placed on the subject’s lap from which all movements were initiated. A picture on the screen instructed subjects by which grip they were required to grasp the object. Grips could result in either a meaningful end posture or a meaningless end posture when the object was moved towards the correct goal location.
Stimuli
Fifteen different familiar objects were used as stimuli, which are represented in Figure 1
. For each object two different pictures were made in which the handgrip applied to the object was manipulated (see Figure 2
). Half of all pictures represented a grip that would result in a meaningful end posture when the object was moved towards the correct goal location (e.g. full grip applied to a drinking bottle allows one to drink). The other half of all pictures represented a grip that would result in a meaningless end posture when the object was moved towards the correct goal location (e.g. pressing grip applied to a drinking bottle does not allow one to drink). Grips resulting in meaningless end postures were obtained by applying the meaningful grip from one object to a different object so that it would result in a meaningless end posture (e.g. applying the ‘pressing grip’ for the hairdryer to the water bottle, does not allow one to drink when the bottle is moved towards the mouth; see Figure 2
). In this way the kinematic complexity of grips was counterbalanced between meaningful and meaningless conditions.
Figure 2. Picture stimuli used in the experiment. For each object, one picture indicated a grip that would result in a meaningful end posture (left column). By crossing the grips between objects, for each object a grip was specified that would result in a meaningless end posture when the object was brought to the typical goal location (right column).
Procedure
The experimental paradigm is schematically represented in Figure 3
. Each trial started after the subjects held their finger pressed on the central button of the button box. After 4000 ms a picture appeared on the screen, which indicated how to grasp one of the 15 objects placed in front of the participant. Subjects were required to release the button box as soon as they were prepared to grasp the object that was presented on the picture with the grip specified. After the button box was released the picture disappeared from the screen and was replaced by a fixation cross in the center of the screen. Subjects grasped the object in the manner that was indicated by the picture on the screen and brought the object towards the prototypical goal location of the object (e.g. mobile telephone to the ear; cup to the mouth). When subjects had reached the end posture with the object they were required to maintain this position for 4 s and to minimize head and eye movements during the posture interval. After 4 s an instruction cue appeared on the screen, instructing the subject to put the object back on the table and to press the central button of the button box again, upon which the next trial was initiated.
Figure 3. Overview of experimental procedure. (A) Each trial started with a central fixation cross which was presented for 3000–4000 ms, while the subject was holding the hand at the button box. (B) A picture on the screen indicated by which grip subjects were required to grasp an object. (C) Upon initiating the movement the picture was removed from the screen and the subject grasped the indicated object with the pre-specified grip. (D) When the subject had reached the final end posture with the object they were required to maintain this posture for 4 s. (E) An instruction cue on the screen instructed the subject to put the object back on the table and to return to the starting position, upon which the next trial was initiated.
Importantly, subjects always moved objects towards the prototypical goal location, irrespective of the type of grip by which the object was grasped. Thus, a cup was always brought towards the mouth and a phone was always brought towards the ear. The only difference was that for meaningful end postures the way in which the object was grasped allowed the functional use of the object (e.g. drinking or phoning) and the end posture was familiar for the subject. In contrast, for meaningless end postures the way in which the object was grasped did not allow the functional use of the object (e.g. it would be difficult to drink from a cup when grasped with an overhand grip) and the end posture was unfamiliar to the subject.
During the experiment a stationary camera was focused at the setup to monitor the subjects’ performance. The actions of the subject were carefully observed and coded online by the experimenter by means of an external button box. When the subject reached for the object and grasped it, the experimenter pressed a button on the external button box to code for an object-grasping event. When the subject reached the end posture with the object, the experimenter pressed another button on the external button box to code for an end posture event.
The experiment was controlled by a computer running Presentation 11.0.03 (Neurobehavioral Systems, Albany, NY, USA). Markers for the different experimental events (e.g. stimulus-onset, response-onset, object-grasping and end-posture) were sent to the EEG computer. In total, the experiment consisted of four blocks of 60 trials. In half of all blocks the stimuli instructed subjects to grasp the object with a grip resulting in a meaningful end posture, in the other half of all blocks the pictures instructed subjects to grasp the objects with a grip resulting in a meaningless end posture. Meaningful and meaningless action blocks were conducted in an alternating fashion and block order was counterbalanced between participants. The rationale for presenting meaningful and meaningless actions in separate blocks was provided by studies on action imitation, showing that the intermingled presentation of meaningful and meaningless actions causes subjects to use a direct visuo-motor route rather than a semantic route for planning their actions (Tessari and Rumiati, 2004
; Tessari et al., 2007
). As we were clearly interested in the representation of semantic object knowledge, we required subjects to perform meaningless and meaningless actions in separate blocks. Within blocks pictures of the different objects were presented in random order.
EEG Recording
Brain electrical activity was recorded from Ag/AgCl electrodes, referenced to the linked earlobes. Electrodes were mounted in an elastic cap configured according to the M10-equidistant 61-channel arrangement (Easycap, Herrsching-Breitbrunn, Germany). The inter electrode distance of this arrangement is 37 ± 3 mm (given a head circumference of 58 cm). The arrangement is constructed out of triangles, which are measured on the three-dimensional head surface and arranged around Cz. Horizontal electro-oculograms were recorded via two Ag/AgCl electrodes positioned 1 cm lateral to the outer canthus of each eye, respectively. Vertical electro-oculograms were recorded with two Ag/AgCl electrodes, one above and one below the right eye of the subject. The ground electrode was positioned on the clavicle. All electrode impedances were kept below 5 kΩ. Electrical activity from all channels was sampled at 500 Hz, using a multi-channel DC recording system (Brainproducts, Munich, Germany).
Behavioral Analysis
Behavioral analysis focused on reaction times (time between stimulus-cue and movement onset), object reach times (time between movement onset and grasping of the object), end posture times (time between grasping the object and arriving at the goal location/begin of the posture interval) and return times (time between the end of the posture interval and returning to starting position). Trials in which the reaction or movement times exceeded the subject’s mean by more than 2 standard deviations were discarded from further analysis. Reaction and movement times were calculated separately for meaningful and meaningless action conditions.
Electrophysiological Analysis
All data processing was conducted using Vision Analyzer (Brainproducts, Munich, Germany) and Fieldtrip open source software
1
. Two separate analyses were conducted, one that focused on the interval during which the subject prepared and executed the action with the object and one that focused on the interval during which the subject maintained the end posture with the object for 4 s. The interval of 4 s was chosen based on previous studies indicating that the beta-rebound after movement-termination takes about 1–2 s to develop (Caetano et al., 2007
; Koelewijn et al., 2008
). In addition we did not want to contaminate the posture-interval with processes related to movement preparation due to the following upcoming action (i.e. placing the object back on the table).
For the first analysis, the EEG data was segmented from −200 to 4200 ms after stimulus onset. Inspection of the reaction and movement times confirmed that this interval encompassed movement onset, object grasping and that the end of the interval concurred with the onset of the end posture. For the second analysis, EEG data was segmented from −200 to 4200 ms after posture onset. EEG trials with muscle artifacts, eye blinks or amplifier artifacts were removed from the data-analysis on the basis of careful visual inspection.
For both intervals, the time frequency representation (TFR) was computed for every trial by convolving Morlet wavelets with the signal with a width of seven cycles. Average TFRs were computed by averaging the power over single trials for every condition and subject separately. The resulting subject-averaged power changes were expressed as a relative change from the reference interval, which ranged from −200 to 0 ms before stimulus- or posture-onset. Main analysis focused on differences in the mu frequency band (8–12 Hz) and the beta frequency band (16–24 Hz) between actions resulting in a meaningful posture and actions resulting in a meaningless posture.
To test for statistical significance grand averaged data in the mu frequency band (8–12 Hz) and the beta frequency band (16–24 Hz) were exported in 50 ms bins for a cluster of three adjacent electrodes overlying the left and right motor cortex. The selected electrodes corresponded to electrode positions FC3, C3 and CP3, and FC4, C4 and CP4 of the standard 10/20 system. Statistical analysis was conducted using a repeated measures ANOVA with the factors Condition (Meaningful vs. Meaningless posture) and Hemisphere (Left vs. Right hemisphere). The significance criterion for each individual time-bin was set at p = 0.05. As 80 intervals were tested, for each factor there is a chance of 80 × 0.05 = 4 that one of the intervals shows an effect. If the criterion of four consecutive significant intervals is used, this chance is reduced to (80 × 0.054) = 0.0005, a value lower than significance criterion p = 0.05. Accordingly, to correct for false positives a criterion of four consecutive intervals showing a significant effect (p < 0.05) was adopted (for a similar statistical approach to EEG/MEG data, see Lange et al., 1999
; Koelewijn et al., 2008
).
Source Analysis
To identify sources of oscillatory activity, a beam-forming approach was used (Dynamic Imaging of Coherent Sources; DICS). For source reconstruction the data from all electrodes with respect to an average reference was used. The DICS technique uses adaptive spatial filters to localize power in the entire brain (Gross et al., 2001
; Liljestrom et al., 2005
). For the source reconstruction in the beta band, the interval between 1.2 and 4.0 s after posture-onset was chosen, as this was the time-interval in which a significant difference in beta power between meaningful and meaningless conditions was observed
2
. A multitaper method (Mitra and Pesaran, 1999
) was applied as part of the Fourier transformation in 0.5 s time windows using three tapers, resulting in ±4 Hz frequency smoothing. The multitaper approach was used since it effectively allows to control the spectral concentration over a desired frequency range. Note that the analysis was done with respect to a frequency of 20 Hz. As a consequence, the ±4 Hz frequency smoothing resulted in a 16–24 Hz range, which is the same range used in the other analyses. Cross-spectral density matrices were calculated from the Fourier transformed data following the tapering. For all subjects a standard multisphere forward model was used, based on the standard MNI/SPM brain. The brain volume was discretized to a grid with a 0.5 cm resolution and using the cross-spectral density matrices and the forward model, spatial filters were constructed for each grid point. These filters were applied to the Fourier transformed data and the spatial distribution of power was estimated for each condition.
Differences between meaningless and meaningful action conditions were tested for significance, using the cluster-level randomization procedure at a voxel level (Maris and Oostenveld, 2007
). Prior to averaging and statistical analysis, the source estimates of the individual subjects’ functional data were overlaid on the standard MNI template [Montreal Neurological Institute (MNI), Montreal, Quebec, Canada]
3
.
Behavioural Results
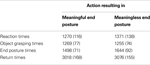
The behavioral analysis focused on object grasping times (time between movement onset and grasping the object), end posture times (time between grasping the object and bringing the object to the correct goal location) and end movement times (time between starting to place the object back on the table and returning to starting position). Reaction and movement times were averaged across all objects and are represented in Table 1
. Differences between meaningful and meaningless conditions were tested for significance using paired-samples t-tests. No significant difference was observed between reaction times in meaningful (1270 ms) and meaningless conditions (1371 ms), t(11) = −1.3, p = 0.22. For object grasping times, also no significant difference was found between meaningful (1269 ms) compared to meaningless (1255 ms) action conditions, t(11) = 0.25, p = 0.81. A significant difference was found only for end posture times, indicating that subjects were slower in bringing the object to the correct goal location in meaningless (1644 ms) compared to meaningful action conditions (1498 ms), t(11) = −2.2, p < 0.05. For return times, no significant difference was found between meaningful (3018 ms) and meaningless conditions (3076 ms), t(11) = −0.68, p = 0.51.
Table 1. Behavioral data. Reaction and movement times for actions resulting in a meaningful end posture or in a meaningless end posture. Standard errors are represented between brackets.
TFR Results during Movement Preparation and Execution
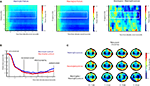
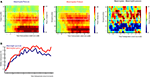
As can be seen in Figure 4
, the onset of the picture cue resulted in a gradual suppression in both the mu power (8–12 Hz; mu ERD) and the beta power (16–24 Hz; beta ERD) that was found maximal around the time of object grasping. Towards the end of the movement a difference in beta power between meaningful and meaningless conditions became apparent, reflected in a stronger beta-desynchronization for meaningless compared to meaningful actions. This difference was found maximal around bilateral electrodes located above central brain areas (electrodes corresponding to C3 and C4 of the 10/20 system). Statistical analysis of the difference in the beta frequency band showed a stronger beta-suppression for meaningless compared to meaningful actions from 1250 to 1400 ms, from 1800 to 2000 ms and from 3500 to 4000 ms, F(1,11) > 5.6, p < 0.05. An interaction with Hemisphere, indicated that the effect was lateralized to the right hemisphere in the interval from 1600 to 1950 ms F(1,11) > 5.2, p < 0.05.
Figure 4. Beta-desynchronization during movement preparation and execution. EEG data time-locked to the onset of the picture instructing the subject by which grip they were required to grasp the object. (A) Time frequency representations (TFRs) for actions resulting in a meaningful end posture (left graph), a meaningless end posture (middle graph) and the difference between meaningless and meaningful action conditions (right graph). The time-interval and frequency range used for statistical analysis are marked in white. (B) Relative beta-power (16–24 Hz) for actions resulting in a meaningful end posture (blue lines) or a meaningless end posture (red lines). (C) Topographical maps of the beta-power (16–24 Hz) for meaningful actions (upper panel), meaningless actions (middle panel) and the difference between meaningless and meaningful action conditions (lower panel). Electrodes that were used for statistical analysis and for plotting the TFRs are marked in black.
As can be seen in Figure 4
, at posterior sites a strong difference in beta-power between meaningless and meaningful postures was observed as well. However, visual inspection of this difference indicated that the effect was not restricted to the beta-frequency band, but extended to higher frequencies in the gamma-range as well. Given the sensitivity of peripheral electrodes and higher frequency ranges to muscular artifacts (Pope et al., 2009
), these effects were likely related to muscular tension in the neck.
For the mu frequency band (8–12 Hz) no differences were observed between meaningful and meaningless actions.
TFR Results during the Posture Interval
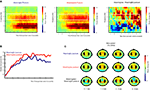
As can be seen in Figure 5
, the onset of the end posture with the object resulted in a gradual increase in beta power (16–24 Hz; post-movement beta rebound) that reached its maximum around 1500 ms after termination of the movement. Differences between meaningful and meaningless postures became apparent in a stronger beta-rebound for meaningless compared to meaningful postures. This difference was found maximal around bilateral electrodes located above central brain areas (electrodes corresponding to C3 and C4 of the 10/20 system). Statistical analysis of the difference showed a stronger beta-rebound for meaningless compared to meaningful actions from 500 to 1000 and from 1200 to 4000 ms after onset of the posture, F(1,11) > 5.0, p < 0.05. An interaction with Hemisphere, indicated that the effect was slightly lateralized to the right hemisphere from 1650 to 1950 ms, F(1,11) > 5.3, p < 0.05.
Figure 5. Beta-rebound during posture maintenance. EEG data time-locked to the onset of the end posture. (A) Time frequency representations for actions resulting in a meaningful end posture (left graph), a meaningless end posture (middle graph) and the difference between meaningless and meaningful action conditions (right graph). The time-interval and frequency range used for statistical analysis are marked in white. (B) Relative beta-power (16–24 Hz) for actions resulting in a meaningful end posture (blue lines) or a meaningless end posture (red lines). (C) Topographical maps of the beta-power (16–24 Hz) for meaningful actions (upper panel), meaningless actions (middle panel) and the difference between meaningless and meaningful action conditions (lower panel). Electrodes that were used for statistical analysis and for plotting the TFRs are marked in black.
In addition to the effects observed in the beta-frequency band, a strong suppression and rebound in the mu-frequency band (8–12 Hz) was observed in association with the approach and maintenance of meaningful and meaningless end postures as well. However, statistical analysis of the mu-frequency band did not yield significant differences between meaningful and meaningless action conditions during the posture interval.
Source Analysis of the Beta-Rebound during the Posture Interval
The sources accounting for the modulation in the beta band during the posture interval were characterized by comparing the 16–24 Hz (20 ± 4 Hz smoothing) activity during the interval from 1200 to 4000 ms after the onset of the posture, between meaningless and meaningful actions. The single subject functional data were aligned with a standard brain, averaged across subjects and thresholded for statistical significance using the cluster-level randomization procedure (Maris and Oostenveld, 2007
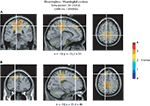
). The strongest modulation in the beta band for meaningless compared to meaningful actions was observed in BA 6 in the superior parietal cortex (x = −10, y = −35, z = 50; see Figure 6
A) and in BA 9 in the superior frontal gyrus (x = −18, y = 35, z = 46; see Figure 6
B).
Figure 6. Source analysis of the beta-rebound. Source reconstructions accounting for the stronger beta-rebound for meaningless compared to meaningful actions from 1200 to 4000 ms relative to the onset of the end posture. The strongest beta modulations were observed in the superior parietal cortex [BA 6 (A)] and in the superior frontal gyrus [BA 9 (B)]. Source activation is projected on a standard brain.
Control for Timing Differences between Meaningful and Meaningless Actions
One concern might be that the differences in beta-power partly reflect differences in the duration of the movements between meaningful and meaningless actions. Although for reaction times no significant difference was observed, overall end posture times were slower for meaningless action conditions (1644 ms) compared to meaningful action conditions (1498 ms). Accordingly, the stronger desynchronization in the beta-frequency band for meaningless actions during action execution and the stronger beta-rebound for meaningless actions in the posture interval could partly reflect differences in movement duration.
To control for this possible confound, for each object the average reaction time and end posture time were calculated, averaged across subjects. Next, for meaningful actions we excluded seven objects from the analysis, to which subjects responded relatively fast, as reflected in overall faster reaction and end posture times. In contrast, for meaningless actions we removed seven objects to which subjects responded relatively slow, as reflected in overall slower reaction and posture times. Care was taken that when matching for timing differences between conditions, objects were matched for posture as well, like in the original set. In this way, meaningful and meaningless actions could be matched for movement duration.
Behavioral results after exclusion of objects
Analysis of the behavioral data indicated that removal of these objects successfully eliminated the overall slower movement times for meaningless compared to meaningful actions. No significant difference was observed between reaction times in meaningful (1316 ms, SE = 126 ms) and meaningless conditions (1298 ms, SE = 130 ms), t(11) = 0.21, p = 0.84. For object grasping times, also no significant difference was found between meaningful (1313 ms, SE = 86 ms) compared to meaningless (1229 ms, SE = 76 ms) action conditions, t(11) = 1.2, p = 0.25. For end posture times no significant difference was found between meaningful (1610 ms, SE = 83 ms) and meaningless action conditions (1533 ms, SE = 80 ms), t(11) = 1.7, p = 0.13. For return times, no significant difference was found between meaningful (2867 ms) and meaningless conditions (2974 ms), t(11) = −0.31, p = 0.77. These behavioral findings indicate that removal of these objects successfully eliminated timing differences between meaningful and meaningless action conditions.
TFR results during the posture interval after exclusion of objects
After meaningful and meaningless action conditions were matched for length of the actions, an additional time-frequency analysis was conducted on the posture interval, ranging from −200 to 4200 ms relative to posture onset
4
. As can be seen in Figure 7
, the onset of the posture resulted in a comparable pattern as was observed for the entire object set, reflecting a gradual increase in beta power (16–24 Hz; post-movement beta rebound) that reached its maximum around 1500 ms after termination of the movement. Differences between meaningful and meaningless postures became apparent in a stronger beta-rebound for meaningless compared to meaningful postures. Statistical analysis of the difference showed a stronger beta-rebound for meaningless compared to meaningful actions from 750 to 1400 ms, from 1900 to 2600 ms, and from 3200 to 3450 ms after posture onset, F(1,11) > 5.1, p < 0.05. An interaction with Hemisphere, indicated that the effect was lateralized to the right hemisphere from 1250 to 1400 ms F(1,11) > 7.5, p < 0.05. Together these findings replicate the main finding and suggest that the stronger beta-rebound for meaningless actions cannot be attributed to differences in the duration of the movements.
Figure 7. Beta-rebound during posture maintenance after exclusion of objects. EEG data time-locked to the onset of the end posture after exclusion of objects contributing to slower responding in Meaningless action conditions and objects contributing to faster responding in Meaningful action conditions. (A) Time frequency representations for actions resulting in a meaningful end posture (left graph), a meaningless end posture (middle graph) and the difference between meaningless and meaningful action conditions (right graph). The time-interval and frequency range used for statistical analysis are marked in white. (B) Relative beta-power (16–24 Hz) for actions resulting in a meaningful end posture (blue lines) or a meaningless end posture (red lines).
The main finding of the present study was that the approach and maintenance of meaningless end postures compared to meaningful end postures resulted in a stronger beta-desynchronization and a stronger subsequent beta-rebound, that was localized to the central sulcus adjacent to the superior parietal cortex and the superior frontal gyrus. These findings indicate that motor-related brain areas are differentially activated during the approach and maintenance of meaningful and meaningless end postures supporting object use. The functional significance of the modulations in the beta-frequency band will be discussed below.
Previous studies have indicated that the desynchronization in the beta-frequency band reflects activation of the premotor and the primary motor cortex (Stancak and Pfurtscheller, 1996
; Caetano et al., 2007
) whereas the beta rebound reflects an active inhibition of somato-sensory processing (Cassim et al., 2001
; Parkes et al., 2006
). More specifically, increased beta-power has been associated with the maintenance of a posture (Brown and Williams, 2005
; Gilbertson et al., 2005
) and patients with Parkinson’s disease, who are characterized by postural stiffness, show a pathological increase in the beta-frequency band (Brown et al., 2001
). Together these findings suggest that increases in beta-power may support the maintenance of the present motor set, while decreases in beta-power may facilitate motor processing related to new movements (Gilbertson et al., 2005
). Accordingly, the stronger beta-desynchronization for meaningless actions may reflect increased activation of the motor system to facilitate the transition to a novel and unfamiliar end posture. The stronger beta-rebound for meaningless actions may reflect an active inhibition of motor-related areas to maintain the current end posture. In other words: for the preparation of meaningless actions the motor system needs to be ‘forced’ in a novel state that subsequently is actively maintained.
The strongest sources accounting for the difference in the beta-rebound between meaningless and meaningful actions were identified in the medial superior parietal cortex (BA 5) and in the superior frontal gyrus (BA 9). The superior parietal cortex is a multi-sensory integration area related to the planning and execution of eye and hand movements (Rizzolatti et al., 1998
; Battaglia Mayer et al., 2006
). More specifically, subpopulations of neurons in BA 5 encode the current position of the arm in body-centered coordinates, by combining both visual and somatosensory signals (Lacquaniti et al., 1995
; Graziano et al., 2000
). It has been suggested that BA 5 is involved in actively maintaining a representation of the current postural state of the body (Wolpert et al., 1998
). In line with this suggestion, damage to the superior parietal lobule results in specific difficulties with applying the appropriate hand posture required for using objects (Sirigu et al., 1995
) and in optic ataxia, a disorder that is characterized by severe deficits in the online control of movements, such as pointing and grasping (Glover, 2003
). The left superior frontal gyrus is associated with the planning of movement sequences (Rowe et al., 2000
; Shima and Tanji, 2000
; Rushworth et al., 2004
) and the planning of actions with respect to the intended goal location (Majdandzic et al., 2007
). Lesions to the left superior frontal gyrus have been associated with deficits in performing object-directed actions, such as ideomotor apraxia, as well (Haaland et al., 2000
). In addition, anatomical studies have shown that connections between the superior parietal cortex and prefrontal motor-related areas comprise a fronto-parietal network, supporting complex grasping and reaching movements (Rizzolatti et al., 1998
). In the present study, both meaningful and meaningless action conditions likely required a coding of the intended end posture in terms of body-centered coordinates. Accordingly, the difference between meaningless and meaningful postures in BA 5 and BA 9 may indicate that the maintenance of a meaningless posture requires the active inhibition of input to somatosensory- motor related areas, reflected in a stronger beta-rebound for meaningless actions (Cassim et al., 2001
).
Although the strongest sources accounting for the difference between meaningless and meaningful actions were localized to the left hemisphere, in the scalp EEG the strongest difference in beta-power between both conditions was observed over the ipsilateral (right) hemisphere. One possibility is that the relatively superficially located sources in the right hemisphere resulted in a more focal distribution of the beta-effect in the scalp-recorded EEG. In contrast, the deeper medial sources observed in the left hemisphere likely resulted in the spreading of activation and in a less focal scalp distribution. The notion that motor-related areas ipsilateral to the moving limb may play a role in controlling complex movements is in line with previous studies (Shibasaki et al., 1993
; Sadato et al., 1996
; Chen et al., 1997
; Tinazzi and Zanette, 1998
; Verstynen et al., 2005
; Avanzino et al., 2008
). For instance, single-cell studies in monkeys show that neuronal activation in ipsilateral motor cortex is especially involved in the fine control of unimanual hand movements (Tanji et al., 1988
; Aizawa et al., 1990
). Because meaningless postures were more unfamiliar than meaningful postures, the execution of meaningless actions likely required an increased process of motor control, encompassing activation in both hemispheres.
It is known from previous studies that beta-oscillations can be modulated by the kinematic properties of the movement, such as timing or force (Kilner et al., 2000
), but importantly the difference in beta-power found in the present study cannot be attributed to low-level differences between meaningful and meaningless actions. First, for both meaningful and meaningless actions the grip applied to the object was balanced between conditions, so that both action conditions involved comparable kinematics. Secondly, even after exclusion of those objects that contributed most strongly to timing differences between meaningful and meaningless action conditions, still a stronger beta-rebound was observed for meaningless compared to meaningful postures. Rather than being attributable to low-level kinematic features, we suggest that the effects in the beta-frequency band reflect the semantic correctness or incorrectness of the subject’s end posture. Apparently, motor-related brain areas are differentially activated during the execution and maintenance of semantically correct or incorrect end postures.
This finding is in line with the sensory-motor account of conceptual knowledge, according to which our semantic knowledge about objects is represented in the motor system (Beauchamp and Martin, 2007
). In addition, the present findings fit well with a distributed account of cortical memory, according to which memories are represented in distributed networks over the cortex (Allport, 1985
; Fuster, 2000
, 2009
). These networks are hierarchically organized, ranging from low-level sensory-motor structures at the bottom to high-level association areas at the top of the network. In line with the proposed role of experience in shaping these memory representations (Fuster, 2009
), the present study showed that the familiarity of an action influenced the involvement of motor-related brain regions during action execution.
In contrast to previous studies that have tested object knowledge using an observation paradigm (Grafton et al., 1997
; Chao and Martin, 2000
; Gerlach et al., 2002
) or using action pantomimes (Rumiati and Tessari, 2002
; Rumiati et al., 2005
), the present study directly shows the involvement of motor-related brain areas in the actual use of everyday objects. Furthermore, the present results fit nicely with the idea that our semantic knowledge about objects is organized around specific end postures associated with using the object (e.g. Rosenbaum, 1991
). For example, in monkeys prolonged stimulation of parietal areas resulted in the elicitation of complex movements directed at a specific end-posture (Cooke et al., 2003
) and in humans the identification of objects was found accompanied by the retrieval of functional motor information (van Elk et al., 2009
). The stronger beta-desynchronization and subsequent beta-rebound for meaningless postures suggests that the motor system is differentially activated during the approach and maintenance of semantically correct and incorrect end postures. Thereby the present study suggests that our real-life experiences with objects have become well-established in motor-related brain areas, organized around specific end postures associated with using the object.
Conclusions
In the present study we investigated the neural dynamics of using objects with which we have profound experience. It was found that the execution of actions resulting in a meaningless end posture resulted in a stronger beta-desynchronization towards the end of the movement and a stronger subsequent beta-rebound during the maintenance of the end posture. These findings show that the motor system is differentially activated during the execution and maintenance of semantically correct and incorrect actions, thereby providing direct support for the hypothesis that action semantics are represented in motor-related brain areas, organized around specific end postures associated with using objects.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the Netherlands Organization for Scientific Research (grants 453-05-001 awarded to HB).
- ^ http://fieldtrip.fcdonders.nl/
- ^ Source analysis of the beta-effects during action preparation and execution proved to be difficult, due to contamination of the EEG signal with muscle artifacts at posterior sites, likely caused by tension in the subject’s neck.
- ^ http://www.bic.mni.mcgill.ca/brainweb
- ^ The time-frequency analysis could not be conducted on the action preparation interval, because of insuffi cient artifact-free trials after exclusion of half of the objects.
Shibasaki, H., Sadato, N., Lyshkow, H., Yonekura, Y., Honda, M., Nagamine, T., Suwazono, S., Magata, Y., Ikeda, A., Miyazaki, M., Fukuyama, H., Asato, R., and Konishi, J. (1993). Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain 116 (Pt 6), 1387–1398.